Abstract
Aurora kinase A (Aurora-A) is a cell cycle-associated serine–threonine kinase that is overexpressed by various types of cancer and is highly associated with poor prognosis. Since the expression of Aurora-A in normal tissues has been shown to be significantly lower as compared to tumor cells, this protein is being considered as a potential tumor-associated antigen for developing immunotherapies. The goal in the present study was to identify CD4 helper T lymphocyte (HTL) epitopes for Aurora-A for the design of T cell-based immunotherapies against Aurora-A-expressing tumors. Synthetic peptides corresponding to potential HTL epitopes were identified from Aurora-A and used to stimulate CD4 T lymphocytes in vitro to generate antigen-specific HTL clones that were evaluated for antigen specificity, MHC restriction and for their ability to interact with Aurora-A-expressing tumor cells. The results show that two peptides (Aurora-A161–175 and Aurora-A233–247) were effective in generating HTL responses that were restricted by more than one MHC class II allele (i.e., promiscuous responses). The CD4 HTL clones were able to directly recognize Aurora-A-expressing tumor cells in an antigen-specific and MHC class II-restricted manner and some of the clones displayed cytolytic activity toward Aurora-A + tumor cells. Both of these peptides were capable of stimulating in vitro T cell responses in patients with bladder cancer.
Keywords: Aurora kinase A, Immunotherapy, Tumor antigens, Major histocompatibility complex class II, CD4 helper T lymphocytes
Introduction
Effective anti-tumor immune responses require the active collaboration between CD8 cytotoxic T lymphocytes (CTLs) and CD4 helper T lymphocytes (HTLs). CTLs and HTLs recognize peptide epitopes derived from tumor-associated antigens (TAAs) which are processed and presented on tumor cell surface in association with major histocompatibility complex (MHC) molecules [29]. These TAAs can be either cell surface molecules or intracellular proteins that, due to protein turnover, generate peptides that associate with MHC molecules that are transported to the cell surface for presentation to the T cell receptor. Among the numerous potential TAAs, those that are involved in tumorigenesis and are only expressed at low levels in normal tissues could serve as ideal targets for developing effective cancer immunotherapy. Transformation events leading to alterations in the regulation of the cell cycle are considered critical events of cancer development and persistence [21]. Considerable research has focused on gene products that are intimately involved in the regulation of the cell cycle such as cell cycle-associated kinases with the goal of developing new anti-cancer therapies. Because overexpression of these kinases is frequently observed in many tumor types, it is expected that these therapies will mostly affect malignant cells and not normal proliferating cells [5, 6, 19].
Aurora kinase A (Aurora-A, aka STK15 or BTAK) is a serine–threonine kinase that regulates the progression of mitosis during the late G2 to M cell cycle phase [23]. The Aurora-A gene is frequently amplified and overexpressed in various malignancies such as breast, colon, bladder, ovarian, pancreatic, prostate and renal cell carcinomas [2, 16, 18–20, 30, 31]. Aurora-A overexpression is associated with genetic instability [7] and poor histological differentiation [35], leading to poor prognosis and outcome [17]. Transfection and overexpression of Aurora-A in fibroblasts induces malignant transformation, indicating that this kinase can behave as a classical oncogene product [2, 36]. Although high expression of Aurora-A occurs in a broad range of cancer types, it is also found to be expressed, but at much lower levels, in normal dividing cells [9, 12, 26]. Thus, it is possible that this protein could be utilized as a therapeutic target. Indeed, several small molecule Aurora-A inhibitors that block the ATP-binding site have been developed and are currently being tested in the clinic [22, 32, 33]. Due to its controlled expression pattern, Aurora-A has been recently examined as a target for developing T cell-based anti-tumor immunotherapy. A recent study described that Aurora-A-specific MHC class I-restricted CTLs were able to recognize and kill leukemic cells overexpressing Aurora-A [26]. Although CD8 CTLs are considered to be the most effective components of the adaptive immune system capable of eradicating tumor cells, increasing evidence from both human and murine studies indicates that CD4 HTLs are required for full induction, activation and persistence of CTLs [13]. Moreover, in some instances, HTLs are known to exhibit anti-tumor effector function and are capable of eliminating tumors on their own without the participation of CTLs [25, 27]. In view of this, we believe that only those vaccines capable of stimulating both TAA-reactive CTLs and HTLs will be effective in generating immune responses that will provide clinical benefit. In the present study, we describe novel peptide epitopes capable of inducing HTL responses against Aurora-A in an MHC-II-restricted manner. Most notably, the Aurora-A-specific CD4 T cells exhibited high immunological activity against MHC-II positive, Aurora-A-expressing tumors cells and little, if any, against non-transformed lymphoid cells. These results have important implications for the development of new immunological therapies against cancer.
Materials and methods
Cell lines
Epstein-Barr virus (EBV)-transformed lymphoblastoid cells (EBV-LCL) were produced from peripheral blood mononuclear cells (PBMCs) of HLA-typed volunteers using culture supernatant from the EBV-producing B95-8 cell line, obtained from the American Type Culture Collection (ATCC, Manassas, VA). Mouse fibroblasts cell lines (L-cells) transfected and expressing individual human MHC-II molecules were kindly provided by Dr. Robert W. Karr (Karr Pharma, St. Louis, MO) and Dr. Takehiko Sasazuki (International Medical Center of Japan, Tokyo, Japan). The following tumor cell lines were purchased from the ATCC: LNCaP and PC3 (prostate); MCF7 and SKBR3 (breast); UMUC3 (bladder) and Jurkat (T cell lymphoma). MT2 is an HTLV-1-transformed T cell line [24]. The bladder cancer cell lines 5637 and renal cell carcinoma cell lines SW839 and Caki-1 were supplied by the Cell Resource Center for Biomedical Research Institute of Development, Aging and Cancer (Tohoku University, Sendai, Japan). All cell lines were maintained in tissue culture as recommended by the supplier.
Synthetic peptides
Potential HLA-DR-restricted CD4+ T cell epitopes were selected from the amino acid sequence of Aurora-A using the peptide/MHC binding predictions for four common HLA-DR alleles (DRB1*0101, DRB1*0401, DRB1*0701 and DRB1*0901) using the computer-based algorithms found in the Immune Epitope Database Analysis Resource (http://tools.immuneepitope.org/main/index.html) [4]. The predicted peptide epitopes were synthesized by solid phase organic chemistry and purified by high-performance liquid chromatography (HPLC). The purity (>80%) and identity of peptides were assessed by HPLC and mass spectrometry, respectively. The following synthetic peptides were used throughout this work: Aurora-A161–175 (LKVLFKAQLEKAGVE), Aurora-A204–218 (TRVYLILEYAPLGTV), Aurora-A233–248 (TATYITELANALSYCH), WT1124–138 (QARMFPNAPYLPSCL), and the Pan DR Epitope [1] “PADRE” (aKXVAAWTLKAAa, where is a d-alanine, and X is l-cyclohexylalanine).
In vitro induction of antigen-specific HTLs with synthetic peptides
The procedure utilized for the generation of Aurora-A-reactive HTL lines using peptide-stimulated lymphocytes from PBMCs of normal human volunteers has been described in detail [15]. Briefly, dendritic cells (DCs) were produced from purified CD14 monocytes (using antibody-coated magnetic microbeads from Miltenyi Biotech, Auburn CA) that were cultured for 7 days at 37°C in a humidified CO2 (5%) incubator in the presence of 50 ng/ml GM-CSF and 1,000 IU/ml IL-4. Peptide-pulsed DCs (3 μg/ml for 2 h at room temperature) were irradiated (4,200 rad) and co-cultured with autologous purified CD4 T cells (Miltenyi Biotech Inc, Auburn, CA) in 96-flat-bottomed well culture plates. One week after peptide stimulation, the CD4 T cells were restimulated in individual microcultures with peptide-pulsed irradiated autologous PBMC and 2 days later, recombinant human IL-2 was added at a final concentration of 10 IU/ml. One week later, the T cells were tested for antigen reactivity using a cytokine-release assay as described below. Those microcultures exhibiting a significant response of cytokine-release to peptide (at least 2.5-fold over background) were cloned by limiting dilution and expanded in 24-well plates by weekly restimulation with peptides and irradiated autologous PBMCs. Complete culture medium for all procedures consisted of AIM-V medium (Invitrogen/GIBCO, Carlsbad, CA) supplemented with 3% human male AB serum. All blood samples were obtained after the appropriate informed consent.
Measurement of antigen-specific responses with HTL clones
CD4 T cells (3 × 104 per well) were mixed with irradiated antigen-presenting cells (APCs) in the presence of various concentrations of antigen in 96-well culture plates. APCs consisted of either autologous PBMC (1 × 105 per well), HLA-DR-expressing L-cells (3 × 104 per well), MHC typed EBV-LCL, CD40-stimulated B cells, Jurkat T cell lymphoma, prostate, bladder, renal cell or breast tumor cell lines (3 × 104 per well). Prostate, bladder, renal cell and breast tumor cell lines were previously treated with IFN-γ at 500 units/ml for 48 h to enhance MHC class II expression. To generate CD40-stimulated B cells, purified CD19+ cells (using antibody-coated magnetic microbeads from Miltenyi Biotech) were cultured for 7–10 days with 10 μg/ml of an anti-human CD40 mAb (BD Pharmingen) in the presence of 1,000 IU/ml IL-4 in complete medium consisting of Iscove’s Modified Dulbecco’s medium (Invitrogen) supplemented with 10% FCS. The expression of HLA-DR molecules on tumor cells was evaluated by flow cytometry using anti-HLA class II monoclonal antibody (mAb) TU39 conjugated with fluorescein isothiocyanate (BD Biosciences, San Jose, CA). Culture supernatants of the T cell activation assays were collected after 48 h for measuring antigen-induced lymphokine (IFN-γ or GM-CSF) production by the HTL using ELISA kits (BD Pharmingen, San Diego, CA). To demonstrate antigen specificity and MHC restriction, blocking of antigen-induced responses was assessed by adding anti-HLA-DR mAb L243 (IgG2a, prepared from supernatants of the hybridoma HB-55 obtained from the ATCC), anti-HLA-DQ mAb SPV-L3 (IgG2a, Beckman Coulter Inc, Fullerton, CA) or anti-HLA-A/B/C mAb W6/32 (IgG2a, ATCC) at 10 μg/ml throughout the 48-h antigen-stimulation period. All ELISA determinations were carried out in triplicates and results correspond to the mean values with the standard deviation (SD) of the means.
Western blot analyses
One million cells (tumor or non-transformed) were washed in PBS and lysed in NuPAGE LDS sample buffer (Invitrogen). The cell lysate was subjected to electrophoresis in a 4–12% NuPAGE bis–Tris SDS–PAGE gel (Invitrogen) under reducing condition and then transferred to Immobilon-P (Millipore, Bedford, MA) membrane. The membrane was then blocked in PBS containing 0.01% Tween 20 and 5% nonfat dry milk for 1 h at room temperature and incubated with rabbit anti-human Aurora-A mAb (1:2,000 in blocker; Epitomics, Burlingame, CA) or anti-β-actin (C4, Santa Cruz Biotechnology, Santa Cruz, CA), as the control, for 18 h at 4°C. After washing, the membrane was incubated with horseradish peroxidase-labeled sheep anti-rabbit IgG and subjected to the enhanced chemiluminescence assay using the ECL detection system (Amersham, Buckinghamshire, UK).
Cell-mediated cytotoxicity assays
Cytotoxic activity of HTLs was measured using a colorimetric CytoTox 96 assay (Promega, Madison, WI). This system quantifies the release of lactate dehydrogenase (LDH) from target cells. T cells were mixed with 2 × 104 targets at different effectors to target (E:T) ratios in 96-round-bottomed-well plates. After 6–9 h incubation at 37°C, a 50 μl sample of supernatant was collected from each well to measure LDH content. To correct for spontaneous LDH release from effector cells, LDH levels were measured for each individual effector cell concentration used in the experimental setup (effector spontaneous). All measured values were assayed in triplicate and corrected for the culture medium LDH background. The percentage of specific LDH release was determined as % cytotoxicity = [(experimental − effector spontaneous − target spontaneous)/(target maximum − target spontaneous)] × 100.
Measurement of peptide-specific responses in bladder cancer patients
Peripheral blood mononuclear cells were isolated from fresh heparinized blood by Ficoll-Hypaque centrifugation and washed twice with RPMI 1640. PBMCs were resuspended in complete medium and placed at 1.5 × 105 cells per well and cultured in triplicate in 96-well round-bottomed plates in the presence of 10 μg/ml peptide. Negative controls, in the absence of peptide, were done in six replicate samples. One week later, the cultures were restimulated with peptide-pulsed (10 μg/ml) irradiated autologous PBMCs (5 × 104 cells per well). After 7 days of restimulation, supernatants were harvested for examining the ability of peptide-induced lymphokine production by the patient’s PBMCs. The institutional Ethics Committee had approved the study protocol and the appropriate informed consent for blood donation was obtained from all patients before blood sampling.
Results
Induction of Aurora-A peptide-specific CD4 HTL clones
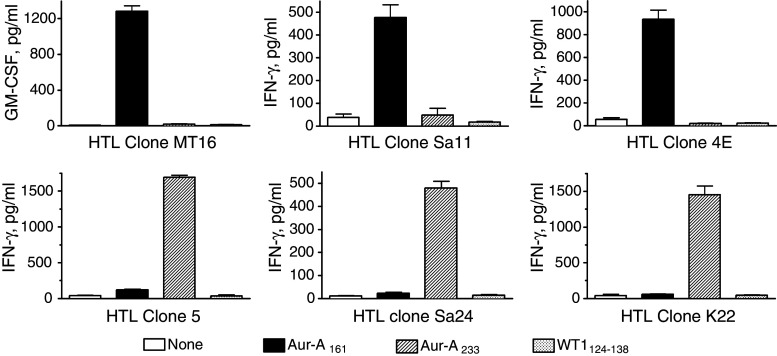
Candidate HTL epitopes predicted to bind to several HLA class II alleles (promiscuous MHC-II binders) from Aurora-A were identified using in silico algorithms for four common HLA class II molecules, HLA-DR1, DR4, DR7 and DR9 [4]. Based on these predictions, we selected three peptide sequences, Aurora-A161–175 (LKVLFKAQLEKAGVE), Aurora-A204–218 (TRVYLILEYAPLGTV) and Aurora-A233–248 (TATYITELANALSYCH) as potential promiscuous CD4 HTL epitopes against Aurora-A-expressing tumor cells. Purified CD4 T lymphocytes from four MHC-II typed healthy volunteers (HLA-DR1/15, DQ5/6; HLA-DR4/15, DQ1/4; HLA-DR4/9, DQ7/8 and HLA-DR9/14, DQ7/9) were stimulated with peptide-pulsed autologous DCs as described in “Materials and methods”. After three rounds of stimulation with peptide-pulsed autologous APCs, the CD4+ T cell cultures were tested for their ability to respond to the peptide antigenic challenge. In these experiments, two of three peptides (Aurora-A161–175 and Aurora-A233–247) were able to elicit HTL responses (data not presented). T cell clones were isolated from those cultures containing peptide-reactive T cells for further characterization (antigen specificity, MHC restriction and ability to recognize tumors expressing Aurora-A). Three Aurora-A161–175-reactive clones (MT16 from the DR9/14, DQ7/9 donor; 4E from the DR4/9, DQ7/8 donor; Sa11 from the HLA-DR4/15, DQ1/4 donor) and three Aurora-A233–247-reactive clones (K22 from the DR1/15, DQ5/6 donor; clone 5 from the DR4/9, DQ7/8 donor; Sa24 from the DR4/15, DQ1/4 donor) were isolated and expanded for further analyses. As shown in Fig. 1, all six HTL clones responded well to the Aurora-A peptide that was originally used to select them and did not react with irrelevant peptides (the other Aurora-A epitope or a peptide from the WT1 antigen). Furthermore, peptide titration curves showed that the antigen-induced lymphokine production (IFN-γ or GM-CSF) in these HTL clones was dependent on the peptide concentration (Fig. 2). In some instances, as with clones MT16, Sa11, K22 and clone 5, significant production of cytokine was observed with low amounts of peptide (0.01 μg/ml) indicating that these HTLs exhibit high avidity for antigen, which is critical for the recognition of tumor cell-derived antigen.
Fig. 1.
Antigen specificity of Aurora-A-reactive HTL clones. The specificity of the T cell clones was determined by their capacity to produce cytokine (GM-CSF or IFN-γ) using autologous irradiated PBMCs as APCs in the presence of various synthetic peptides (used at 10 μg/ml). HTL clones MT16, Sa11 and 4E (top panels) responded only to the Aurora-A161–175 peptide (Aur-A161) and clones 5, Sa24 and K22 (bottom row) only recognized peptide Aurora-A233–247 (Aur-A233). None of the clones displayed a significant response to the irrelevant aurora-A peptide or to an irrelevant peptide from the WT1 antigen (WT1124–138). Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean
Fig. 2.
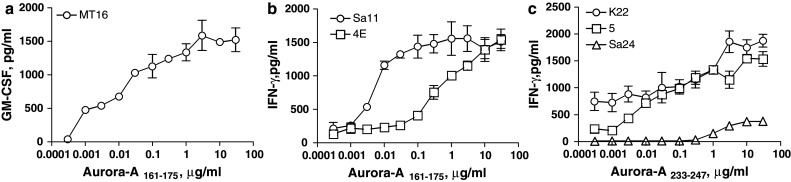
CD4 T cell responses against predicted peptide epitopes derived from Aurora-A. a Production of GM-CSF by HTL clone MT16 (from a DR9/14, DQ7/9 donor) when stimulated with various doses of peptide Aurora-A161–175 (this clone did not produce IFN-γ when stimulated with antigen). b Peptide Aurora-A161–175 induces production of IFN-γ by HTL clones Sa11 (DR4/15, DQ1/4 donor) and 4E (DR4/9, DQ7/8 donor). c Production of IFN-γ by HTL clones K22 (DR1/15, DQ5/6 donor), clone 5 (DR4/9, DQ7/8 donor) and Sa24 (DR4/15, DQ1/4 donor) as the result of stimulation with peptide Aurora-A233–247. In all instances, autologous irradiated PBMCs were used as APCs. Points means of triplicate determinations, bars SD. Points without bars had SD < 10% the value of the mean. Results are representative of two experiments that were done with the same samples
Peptides Aurora-A161–175 and Aurora-A233–247 function as promiscuous HTL epitopes
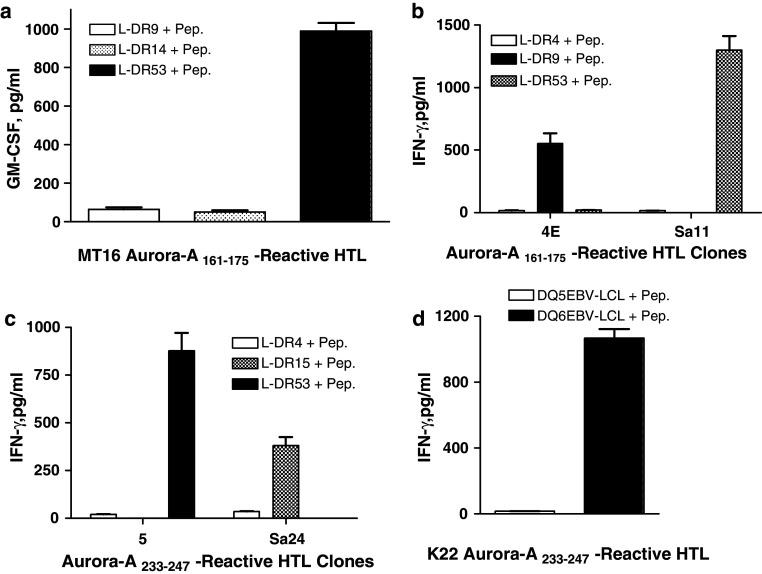
Next, we performed HLA class II restriction analysis of the Aurora-A peptide-specific HTL clones. When antibody-blocking experiments using mAbs specific for either HLA class I (W6/32) or HLA class II molecules, HLA-DR (L243) or HLA-DQ (SPV-L3), were carried out, it was apparent that all three Aurora-A161–175-specific HTL clones recognized antigen in the context of HLA-DR (Fig. 3a, b). In case of the Aurora-A233–247-reactive HTL clones, peptide recognition by HTL clones 5 and Sa24 was inhibited by the anti-HLA-DR mAb but not by the anti-HLA-DQ mAb demonstrating that these clones were also restricted by HLA-DR (Fig. 3c). On the other hand, the recognition of antigen by HTL clone K22 was inhibited by addition of the anti-HLA-DQ mAb indicating that this clone recognizes antigen in the context of an HLA-DQ molecule (Fig. 3c). To specifically identify the MHC class II restriction elements presenting the Aurora-A peptides to the HTLs, the responses to antigen were evaluated using a panel of HLA-DR-transfected mouse fibroblasts (L-cells) and EBV-LCLs (homozygous for HLA-DQ). The results presented in Fig. 4a and b demonstrate that HTL clones MT16 and Sa11 recognized Aurora-A161–175 presented by HLA-DR53, while clone 4E recognized the same peptide in the context of HLA-DR9. HTL clone 5 recognized Aurora-A233–247 presented by HLA-DR53 and HTL clone Sa24 recognized the same peptide in the context of HLA-DR15 (Fig. 4c). Lastly, the HLA-DQ-restricted HTL clone K22 recognized peptide Aurora-A233–247 in the context of HLA-DQ6 (Fig. 4d). These results indicate that peptides Aurora-A161–175 and Aurora-A233–247 behave as classic promiscuous CD4 HTL epitopes because they can be presented to T cells by more than one MHC class II allele.
Fig. 3.
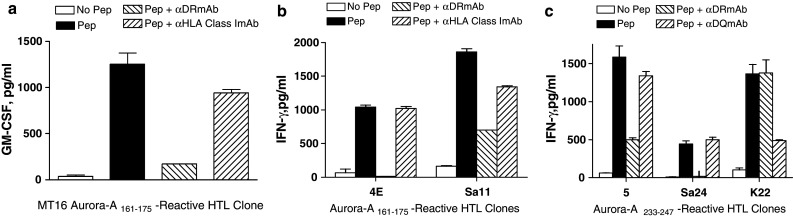
The presentation of Aurora-A peptides to CD4 T cells is in the context of HLA-DR or HLA-DQ molecules. MHC class II restriction molecules were identified using antibody-blocking assays: anti-HLA-DR (clone L243), anti-HLA-DQ (clone SPV-L3) or anti-HLA-A, -B, -C (clone W6/32 used as negative control) were all used at 10 μg/ml. Experiments were done using irradiated peptide-pulsed autologous PBMCs as APCs. Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean. Results are representative of two similar experiments that were done under the same conditions
Fig. 4.
Definition of restricting MHC class II alleles of the Aurora-A-specific HTL clones. Lymphokine production of the Aurora-A161–175-reactive HTL clones (a, b) and Aurora-A233–247-reactive HTL clones (c, d) was evaluated using L-cells transfected with individual HLA-DR molecules or semi-allogenic EBV-LCLs homozygous for HLA-DQ molecules as APCs. No significant responses (<20 pg/ml) were observed in the absence of peptide (not shown, for simplicity). Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean. Results are representative of two experiments
Assessment of recognition of naturally processed antigen by Aurora-A-specific HTL clones
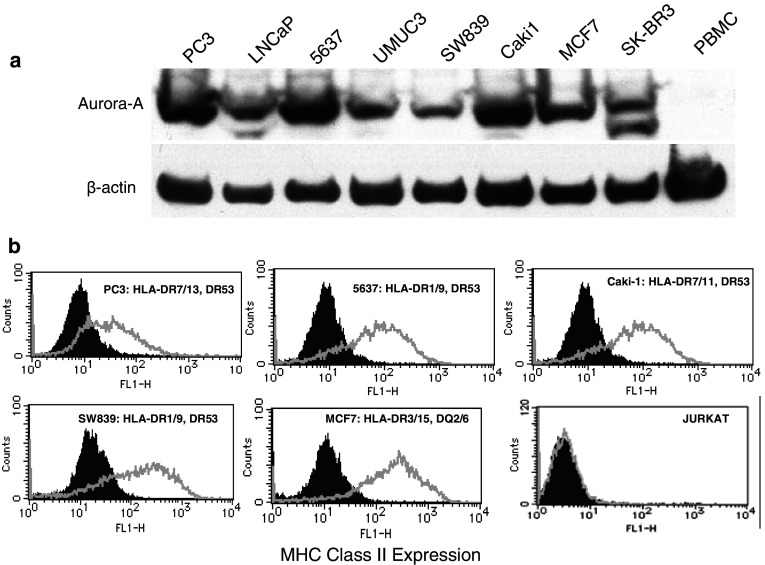
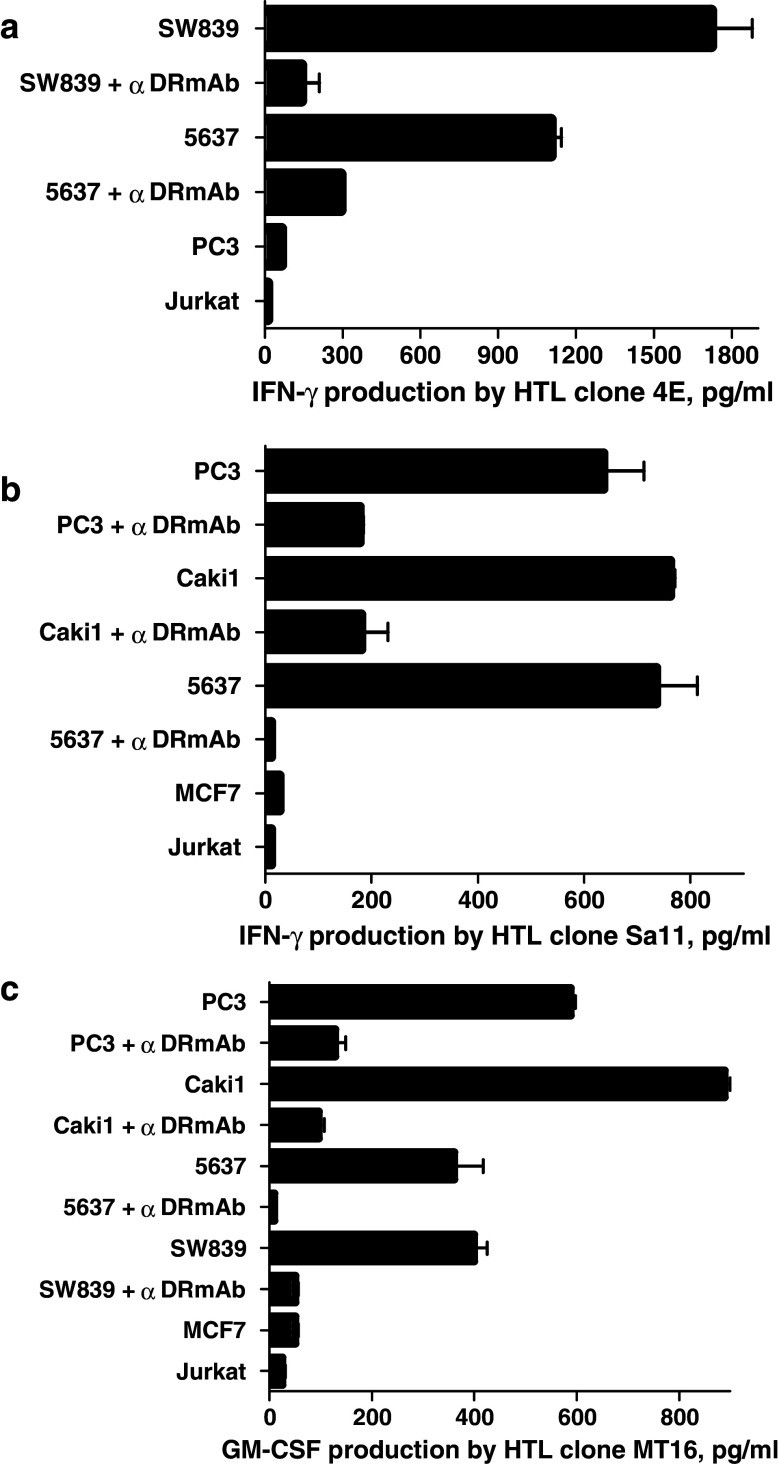
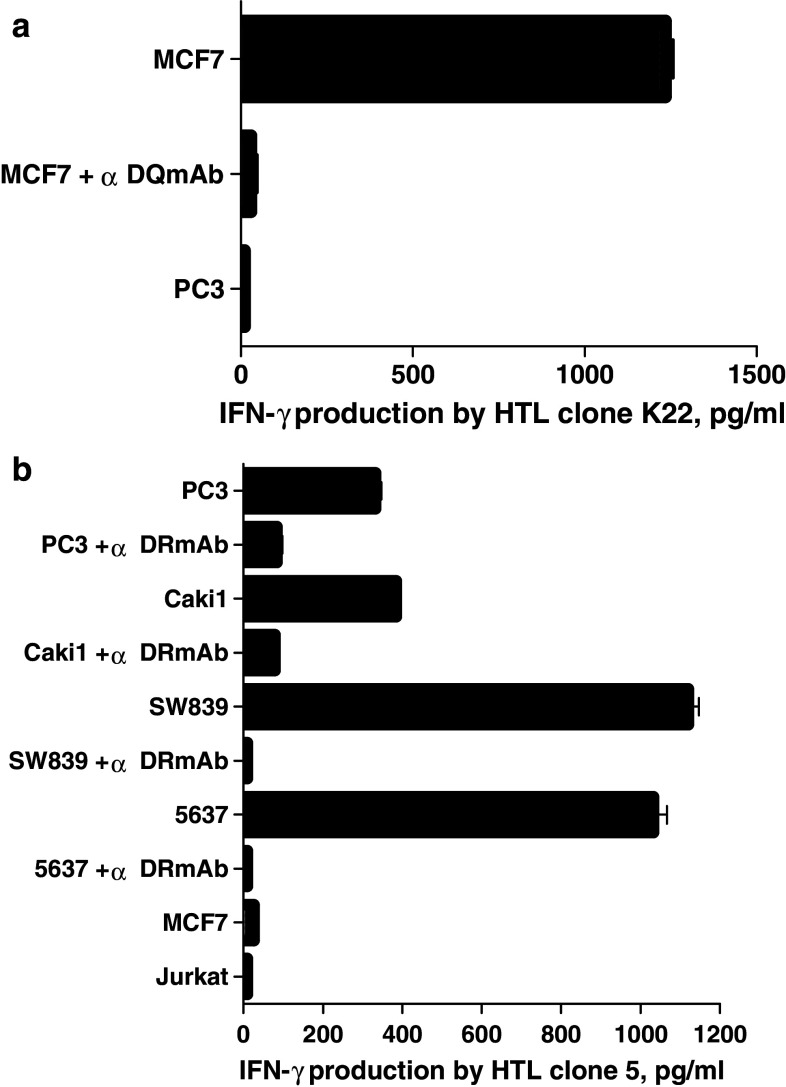
The next step was to assess whether the Aurora-A-specific HTLs were capable of recognizing the naturally processed epitopes on MHC class II molecules expressed by tumor cells. First, we assessed the expression of Aurora-A and MHC class II molecules on several tumor cell lines that would be used for these studies. As shown in Fig. 5a, the Aurora-A protein was detected by Western blot analysis in various tumor lines from different histological origins such as PC3, LNCaP (prostate), 5637, UMUC-3 (bladder), SW839, Caki-1 (renal), MCF7 and SK-BR3 (breast). On the other hand, Aurora-A was undetectable in normal (non-proliferating) PBMCs. Based on these results and using the information gathered by the MHC restriction analysis of the HTL clones, we selected several tumor cell lines (previously typed for their MHC alleles) to examine the expression of cell surface MHC class II molecules. As shown in Fig. 5b, all five of the selected Aurora-A positive tumor cells (PC3, 5637, Caki-1, SW839 and MCF7) expressed significant levels of cell surface MHC class II after treatment with IFN-γ. We then proceeded to test the capacity of the MHC-II + Aurora-A + tumor cells to stimulate the responses of the peptide-reactive HTL clones. As shown in Fig. 6a, clone 4E (DR9-restricted, Aurora-A161–175-reactive) recognized two Aurora-A+/DR9 tumors (5637 and SW839). HTL clones Sa11 and MT6 (DR53-restricted, Aurora-A161–175-reactive) recognized several Aurora-A+/DR53 tumors (PC3, 5637, SW839 and Caki-1) in an antigen-specific manner (Fig. 6b, c). Similarly, clone K22 (DQ6-restricted, Aurora-A233–247-reactive) recognized the Aurora-A+/DQ6-expressing MCF7 breast tumor cells (Fig. 7a). Lastly, as shown in Fig. 7b, clone 5 (DR53-restricted, Aurora-A233–247-reactive) recognized several Aurora-A+/DR53 tumors (PC3, 5637, Caki-1 and SW839). On the other hand, the DR15-restricted, Aurora-A233–247-reactive HTL clone Sa24 was unable to react with any Aurora-A-expressing tumor (data not shown). Direct tumor recognition in all cases was antigen-specific and MHC-restricted since tumor cell lines not expressing either Aurora-A or the appropriate MHC allele failed to stimulate the responses of the HTL clones. Moreover, the capacity of the Aurora-A-expressing tumor cells to activate the HTL clones was significantly inhibited by the addition of either anti-MHC class II mAbs confirming that the peptide epitopes were presented through MHC class II molecules.
Fig. 5.
Evaluation of the expression of Aurora-A protein and cell surface MHC class II molecules by various human tumor cell lines. a Western blot analysis was done using an Aurora-A-specific mAb and β-actin-specific mAb as the control as described in “Materials and methods”. The Aurora-A protein has a mass of approximately 46 kDa. b Expression of cell surface MHC class II molecules on tumor cells evaluated by flow cytometry using anti-HLA class II mAb Tu39 conjugated with fluorescein isothiocyanate (thick line open histograms). Staining with the isotype-negative control (filled histograms). For each tumor, the previously determined MHC class II typing is shown
Fig. 6.
Direct recognition of Aurora-A-expressing tumor cells by Aurora-A161–175-reactive HTL clones. DR9-restricted HTL clone 4E (a) and DR53-restricted HTL clones Sa11 (b) and MT16 (c) were tested for their capacity to recognize antigen directly on Aurora-A+ tumor cells that are either matched or mismatched for the restricting MHC class II alleles. The MHC class II-negative Jurkat T cell lymphoma cells were also used as negative controls. The antigen specificity of these responses was demonstrated by blocking tumor recognition with anti-HLA-DR mAb (L243). Columns without bars had SD < 10% the values of the mean. Results are representative of two separate experiments
Fig. 7.
Direct recognition of Aurora-A-expressing tumor cells by Aurora-A233–247-reactive HTL clones. DQ6-restricted HTL clone K22 (a) and DR53-restricted HTL clone 5 (b) were tested for their capacity to recognize antigen directly on Aurora-A+ tumor cells that are either matched or mismatched for the restricting MHC class II alleles. The antigen specificity of these responses was demonstrated by blocking tumor recognition with anti-HLA-DR mAb (L243) or anti-HLA-DQ (SPV-L3) mAbs. Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean. Results are representative of two separate experiments
HTL epitopes Aurora-A161–175 and Aurora-A233–247 are naturally processed and presented by transformed cells but not by normal proliferating cells
We have showed that Aurora-A-specific HTL clones can directly recognize Aurora-A-expressing tumor cells in an MHC-II-dependent manner. It is known that Aurora-A is ubiquitously expressed in normal dividing cells and that it regulates mitotic cell division. Nevertheless, the expression level of Aurora-A in normal proliferating cells is significantly lower as compared with malignant proliferating cells [9, 26]. In agreement, we observed that Aurora-A is expressed in much higher amounts in aberrant mitotic cells such as T cell lymphoma (Jurkat, MT2) and EBV-LCLs as compared to normal proliferating lymphocytes such as phytohemagglutinin (PHA)-stimulated PBMCs and CD40-activated B cells (Fig. 8a). Notwithstanding these results, we assessed whether the Aurora-A-reactive HTLs were capable of responding to normal dividing cells that express low levels of Aurora-A. As shown in Fig. 8b, HTL clones (4E, 5 and Sa11) did not react significantly to either autologous PHA blasts or CD40-activated B cells unless exogenous peptide was added. On the other hand, the HTL clones were able to respond to autologous EBV-LCLs, which express higher amounts of Aurora-A as compared to normal proliferating cells, and as expected the addition of peptide increased these responses. Thus, it appears that the Aurora-A-specific HTLs are able to discriminate between malignant cells and normal proliferating cells, perhaps on the basis of the amount of antigen that is being produced.
Fig. 8.
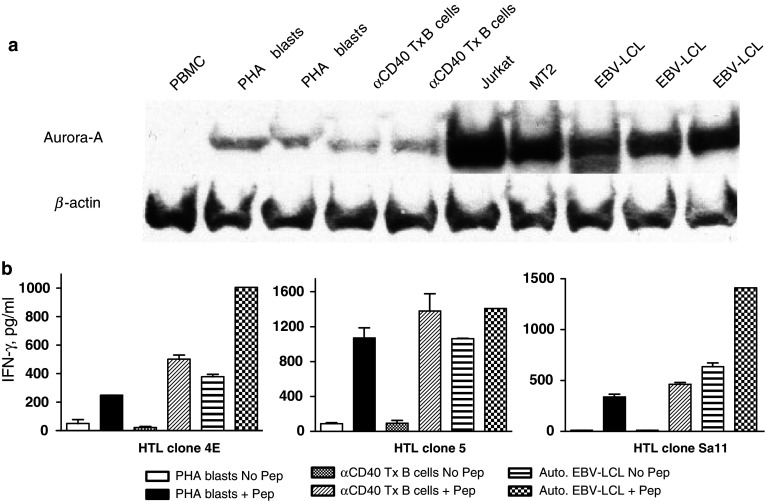
Aurora-A-reactive HTL recognize naturally processed antigen presented by transformed proliferating cells but not by normal dividing cells. a Western blot analysis to examine expression level of Aurora-A in normal PBMC, PHA-blasts, CD40-stimulated B cell blasts, T cell lymphomas (Jurkat, MT2) and EBV-LCLs. Anti-β-actin antibody was used as a loading control. b Direct recognition of Aurora-A-specific HTL clones 4E, 5 and Sa11 against CD40 activated B cells and Aurora-A-expressing EBV-LCLs with or without peptide. Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean. Results are representative of two separate experiments
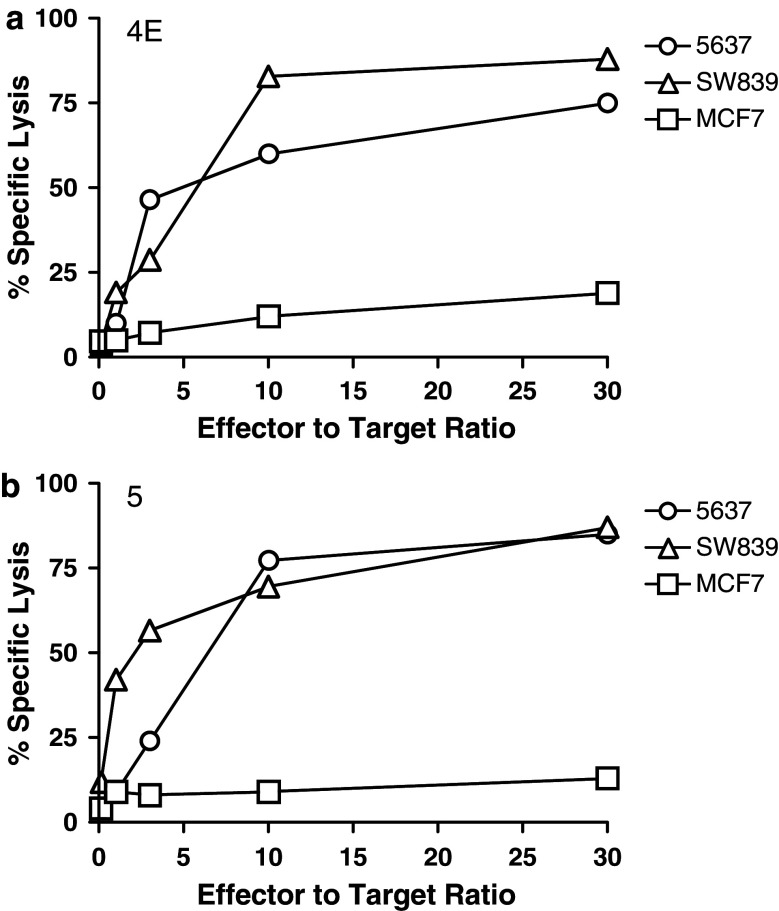
Cytotoxic activity of Aurora-A-specific HTLs
It has been observed that MHC class II-restricted CD4+ T lymphocytes can function as effector cells, sometimes capable of killing virus-infected or tumor cells [14, 27]. Therefore, we examined whether some of the Aurora-A-specific HTL clones would be able to kill Aurora-A-expressing tumor cells. As shown in Fig. 9, two of the HTL clones, 4E (DR9-restricted/Aurora-A161–175-specific) and clone 5 (DR53-restricted/Aurora-A233–247-specific), exhibited high specific cytotoxicity against Aurora-A-expressing tumors expressing the appropriate MHC-II molecule (5637 and SW839) but not toward Aurora-A positive tumor cells that express an irrelevant MHC class II allele (MCF7). On the other hand, clones MT16, Sa11 and K22 were unable to kill Aurora-A-expressing tumor cells (data not shown), although as previously shown these HTLs were fully capable of producing cytokines as the result of directly recognizing Aurora-A + tumor cells.
Fig. 9.
Assessment of cytolytic activity of Aurora-A161–175 and Aurora-A233–247-specific HTL clones. The DR9-restricted, Aurora-A161–175-specific HTL clone 4E (a) and the Aurora-A233–247-specific, DR53-restricted HTL clone 5 (b) were evaluated for their capacity to kill Aurora-A expressing, DR9+ and DR53+ tumor cells (5637 and SW839) or Aurora-A expressing, DR9 negative, DR53 negative tumor cells (MCF7, negative control). Points means of triplicate determinations, bars SD. Points without bars had SD < 10% the value of the mean
Recognition of the Aurora-A161–175 and Aurora-A233–247 HTL epitopes by PBMCs from bladder cancer patients
Lastly, we determined whether T cell responses to the Aurora-A peptides Aurora-A161–175 and Aurora-A233–247 could be detected in cancer patients. Because only small blood samples (25 ml) from these patients were available, we were unable to establish long-term T cell lines and to perform HLA typing of the patients. Direct T cell responses to these peptides were studied by culturing the patients’ PBMCs with peptide, and 1 week later, they were restimulated one more time using irradiated autologous PBMCs as APCs. Seven days after the second antigen stimulation, culture supernatants were collected for measuring lymphokines secreted (IFN-γ) as the response to antigen. The data presented in Fig. 10 show that significant T cell responses to at least one of the Aurora-A peptides and to the positive control peptide PADRE [1] were evident in all five bladder cancer patients and that 4/5 patients recognized both Aurora-A peptides. Moreover, the ability of anti-HLA-DR mAbs to inhibit the response was an indication that the T cells recognized these peptides in the context of MHC class II. These results indicate that T cell precursors reactive with the Aurora-A epitopes exist in the peripheral blood of both normal individuals and cancer patients.
Fig. 10.
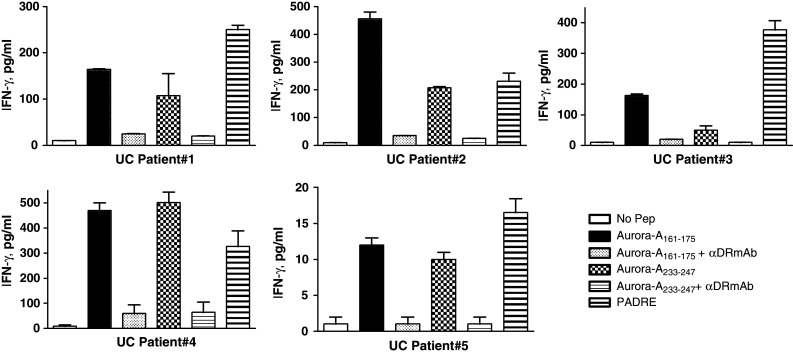
Assessment of T cell responses to the Aurora-A161–175 and Aurora-A233–247 epitopes in bladder cancer patients. PBMCs were isolated from five bladder cancer (UC 1–5) patients and were stimulated with peptides as described in “Materials and methods”. The peptide-stimulated PBMCs derived from patients produced IFN-γ, which could be inhibited by adding anti-HLA-DR mAb L243 at 10 μg/ml during cultures. Columns means of triplicate determinations, bars SD. Columns without bars had SD < 10% the values of the mean. Results are representative of at least two separate experiments
Discussion
In the present study, we were able to demonstrate for the first time that Aurora-A can function as a TAA to induce tumor-reactive CD4 T cell responses both in normal individuals and bladder cancer patients. Using an MHC-II binding prediction algorithm, we were successful in identifying two Aurora-A promiscuous MHC class II-restricted T cell epitopes. Our results show that epitope Aurora-A161–175 can be presented to HTL by either HLA-DR9 or DR53 and that epitope Aurora-A233–247 is presented in the context of either DR15, DR53 or DQ6 (Fig. 4). The HTL clones that were restricted by DR9, DR53 and DQ6 were able to recognize antigen directly by tumor cells (Figs. 6, 7), indicating that these epitopes are endogenously processed and can be presented by the tumor’s own MHC class II molecules. However, clone Sa24, which recognized Aurora-A233–247 in the context of DR15, failed to react directly with tumor cells (data not presented), possibly due to the low affinity that these T cells exhibit with their peptide, as compared to the other clones (Fig. 2). From these results, we cannot determine whether peptide Aurora-A233–247 binds weakly to HLA-DR15 or if the TCR of clone Sa24 has an intrinsic low affinity for the Aurora-A233–247/DR15 complex. Additional Aurora-A233–247-reactive HTL restricted by DR15 would have to be selected and tested against DR15-expressing tumors before it can be concluded whether this epitope can or cannot induce anti-tumor responses in the context of DR15. Regardless of this, it is clear that Aurora-A233–247 represents a potent tumor-reactive HTL epitope when presented in the context of either DR53 or DQ6.
In addition to our findings, it was recently reported that Aurora-A can also be a source of MHC class I-restricted T cell epitopes, capable of inducing CD8 CTLs that effectively killed Aurora-A-expressing leukemia cells [26]. In our hands, we observed a high cytotoxic activity of CD4 T cells against tumors that expressed Aurora-A and the corresponding restricting MHC class II molecules, indicating that CD4 T cells can also exert potent anti-tumor effector function. Furthermore, it has been observed that IFN-γ-secreting CD4 Th1 cells play an important role in orchestrating and sustaining the effector function of cytotoxic CD8+ CTLs and in recruiting eosinophils and macrophages further facilitating tumor rejection [10, 11]. Moreover, lymphokines produced by CD4 T cells (IFN-γ, TNF-α) have direct pro-apoptotic and anti-angiogenic effects and can up-regulate expression of MHC molecules on tumors, increasing their susceptibility to both CD4 and CD8 T cells [3, 28]. Our group has previously advocated the important role that CD4 T cells play enhancing the function of CD8 T cells at the tumor site by providing direct costimulation through CD70/CD27, 4-1BB/4-1BBL interactions [8]. In agreement with this, it has been observed in human colorectal cancer that the presence of IFN-γ producing tumor antigen (p53)-specific CD4 T cells was associated with a stronger CD8 T cell infiltration of the tumor [34], suggesting that the induction tumor-specific Th1 responses may enhance the efficacy of the overall anti-tumor response.
The utilization of Aurora-A as a TAA for the development of T cell-based immunotherapy offers several potential advantages. First, an Aurora-A therapeutic vaccine would be applicable to a large variety of malignant diseases, since this TAA is commonly overexpressed in many types of tumors including breast, colon, bladder, ovarian, renal, pancreatic cancers and in some hematological malignancies. Second, because Aurora-A is expressed at low levels in normal tissues, there is a suggestion that immune tolerance may not be a major hurdle to inducing effective immune responses and that the generation of autoimmune pathology will not be a major cause for concern. Third, targeting a TAA that is directly involved in the malignant function of the tumor cell could decrease the appearance and outgrowth of antigen-loss variants.
Acknowledgments
Ministry of Education, Sports, and Culture of Japan grant-in-aid 21590424 (H. Kobayashi), 20390438 (Y. Harabuchi) and supported in part by NIH grant P50CA91956 (E. Celis).
Abbreviations
- APC
Antigen-presenting cell(s)
- Aurora-A
Aurora kinase A
- DC
Dendritic cell(s)
- HTL
Helper T lymphocyte(s)
- TAA
Tumor-associated antigen(s)
References
- 1.Alexander J, Sidney J, Southwood S, Ruppert J, Oseroff C, Maewal A, Snoke K, Serra HM, Kubo RT, Sette A, et al. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity. 1994;1:751–761. doi: 10.1016/S1074-7613(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, Schryver B, Flanagan P, Clairvoyant F, Ginther C, Chan CS, Novotny M, Slamon DJ, Plowman GD. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J. 1998;17:3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 4.Bui HH, Sidney J, Peters B, Sathiamurthy M, Sinichi A, Purton KA, Mothe BR, Chisari FV, Watkins DI, Sette A. Automated generation and evaluation of specific MHC binding predictive tools: ARB matrix applications. Immunogenetics. 2005;57:304–314. doi: 10.1007/s00251-005-0798-y. [DOI] [PubMed] [Google Scholar]
- 5.Carvajal RD, Tse A, Schwartz GK. Aurora kinases: new targets for cancer therapy. Clin Cancer Res. 2006;12:6869–6875. doi: 10.1158/1078-0432.CCR-06-1405. [DOI] [PubMed] [Google Scholar]
- 6.Gautschi O, Heighway J, Mack PC, Purnell PR, Lara PN, Jr, Gandara DR. Aurora kinases as anticancer drug targets. Clin Cancer Res. 2008;14:1639–1648. doi: 10.1158/1078-0432.CCR-07-2179. [DOI] [PubMed] [Google Scholar]
- 7.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–250. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Giuntoli RL, 2nd, Lu J, Kobayashi H, Kennedy R, Celis E. Direct costimulation of tumor-reactive CTL by helper T cells potentiate their proliferation, survival, and effector function. Clin Cancer Res. 2002;8:922–931. [PubMed] [Google Scholar]
- 9.Hamada M, Yakushijin Y, Ohtsuka M, Kakimoto M, Yasukawa M, Fujita S. Aurora2/BTAK/STK15 is involved in cell cycle checkpoint and cell survival of aggressive non-Hodgkin’s lymphoma. Br J Haematol. 2003;121:439–447. doi: 10.1046/j.1365-2141.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 10.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–2368. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy R, Celis E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- 12.Kimura M, Matsuda Y, Yoshioka T, Okano Y. Cell cycle-dependent expression and centrosome localization of a third human aurora/Ipl1-related protein kinase, AIK3. J Biol Chem. 1999;274:7334–7340. doi: 10.1074/jbc.274.11.7334. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi H, Celis E. Peptide epitope identification for tumor-reactive CD4 T cells. Curr Opin Immunol. 2008;20:221–227. doi: 10.1016/j.coi.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi H, Nagato T, Takahara M, Sato K, Kimura S, Aoki N, Azumi M, Tateno M, Harabuchi Y, Celis E. Induction of EBV-latent membrane protein 1-specific MHC class II-restricted T-cell responses against natural killer lymphoma cells. Cancer Res. 2008;68:901–908. doi: 10.1158/0008-5472.CAN-07-3212. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–5236. [PubMed] [Google Scholar]
- 16.Kurahashi T, Miyake H, Hara I, Fujisawa M. Significance of Aurora-A expression in renal cell carcinoma. Urol Oncol. 2007;25:128–133. doi: 10.1016/j.urolonc.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Landen CN, Jr, Lin YG, Immaneni A, Deavers MT, Merritt WM, Spannuth WA, Bodurka DC, Gershenson DM, Brinkley WR, Sood AK. Overexpression of the centrosomal protein Aurora-A kinase is associated with poor prognosis in epithelial ovarian cancer patients. Clin Cancer Res. 2007;13:4098–4104. doi: 10.1158/1078-0432.CCR-07-0431. [DOI] [PubMed] [Google Scholar]
- 18.Lassmann S, Shen Y, Jutting U, Wiehle P, Walch A, Gitsch G, Hasenburg A, Werner M. Predictive value of Aurora-A/STK15 expression for late stage epithelial ovarian cancer patients treated by adjuvant chemotherapy. Clin Cancer Res. 2007;13:4083–4091. doi: 10.1158/1078-0432.CCR-06-2775. [DOI] [PubMed] [Google Scholar]
- 19.Lee EC, Frolov A, Li R, Ayala G, Greenberg NM. Targeting Aurora kinases for the treatment of prostate cancer. Cancer Res. 2006;66:4996–5002. doi: 10.1158/0008-5472.CAN-05-2796. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Zhu J, Firozi PF, Abbruzzese JL, Evans DB, Cleary K, Friess H, Sen S. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9:991–997. [PubMed] [Google Scholar]
- 21.Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Manfredi MG, Ecsedy JA, Meetze KA, Balani SK, Burenkova O, Chen W, Galvin KM, Hoar KM, Huck JJ, LeRoy PJ, Ray ET, Sells TB, Stringer B, Stroud SG, Vos TJ, Weatherhead GS, Wysong DR, Zhang M, Bolen JB, Claiborne CF. Antitumor activity of MLN8054, an orally active small-molecule inhibitor of Aurora A kinase. Proc Natl Acad Sci USA. 2007;104:4106–4111. doi: 10.1073/pnas.0608798104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marumoto T, Zhang D, Saya H. Aurora-A—a guardian of poles. Nat Rev Cancer. 2005;5:42–50. doi: 10.1038/nrc1526. [DOI] [PubMed] [Google Scholar]
- 24.Miyoshi I, Kubonishi I, Yoshimoto S, Akagi T, Ohtsuki Y, Shiraishi Y, Nagata K, Hinuma Y. Type C virus particles in a cord T-cell line derived by co-cultivating normal human cord leukocytes and human leukaemic T cells. Nature. 1981;294:770–771. doi: 10.1038/294770a0. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura T, Iwakabe K, Sekimoto M, Ohmi Y, Yahata T, Nakui M, Sato T, Habu S, Tashiro H, Sato M, Ohta A. Distinct role of antigen-specific T helper type 1 (Th1) and Th2 cells in tumor eradication in vivo. J Exp Med. 1999;190:617–627. doi: 10.1084/jem.190.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochi T, Fujiwara H, Suemori K, Azuma T, Yakushijin Y, Hato T, Kuzushima K, Yasukawa M. Aurora-A kinase: a novel target of cellular immunotherapy for leukemia. Blood. 2009;113:66–74. doi: 10.1182/blood-2008-06-164889. [DOI] [PubMed] [Google Scholar]
- 27.Omiya R, Buteau C, Kobayashi H, Paya CV, Celis E. Inhibition of EBV-induced lymphoproliferation by CD4(+) T cells specific for an MHC class II promiscuous epitope. J Immunol. 2002;169:2172–2179. doi: 10.4049/jimmunol.169.4.2172. [DOI] [PubMed] [Google Scholar]
- 28.Qin Z, Blankenstein T. CD4 + T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677–686. doi: 10.1016/S1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 30.Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14:2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- 31.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, Jiang F, Johnston D, Grossman HB, Ruifrok AC, Katz RL, Brinkley W, Czerniak B. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320–1329. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 32.Shi Y, Reiman T, Li W, Maxwell CA, Sen S, Pilarski L, Daniels TR, Penichet ML, Feldman R, Lichtenstein A. Targeting aurora kinases as therapy in multiple myeloma. Blood. 2007;109:3915–3921. doi: 10.1182/blood-2006-07-037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyler RK, Shpiro N, Marquez R, Eyers PA. VX-680 inhibits Aurora A and Aurora B kinase activity in human cells. Cell Cycle. 2007;6:2846–2854. doi: 10.4161/cc.6.22.4940. [DOI] [PubMed] [Google Scholar]
- 34.van der Burg SH, Menon AG, Redeker A, Franken KL, Drijfhout JW, Tollenaar RA, Hartgrink HH, van de Velde CJ, Kuppen PJ, Melief CJ, Offringa R. Magnitude and polarization of P53-specific T-helper immunity in connection to leukocyte infiltration of colorectal tumors. Int J Cancer. 2003;107:425–433. doi: 10.1002/ijc.11419. [DOI] [PubMed] [Google Scholar]
- 35.Xu HT, Ma L, Qi FJ, Liu Y, Yu JH, Dai SD, Zhu JJ, Wang EH. Expression of serine threonine kinase 15 is associated with poor differentiation in lung squamous cell carcinoma and adenocarcinoma. Pathol Int. 2006;56:375–380. doi: 10.1111/j.1440-1827.2006.01974.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, Brinkley BR, Sen S. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]