Abstract
Hepatocellular carcinoma (HCC) and colorectal carcinoma with hepatic metastases (mCRC) are cancers with poor prognosis and limited therapeutic options. New approaches are needed and adoptive immunotherapy with Vγ9Vδ2 T lymphocytes represents an attractive strategy. Indeed, Vγ9Vδ2 T cells were shown to exhibit efficient lytic activity against various human tumor cell lines, and in vitro Vγ9Vδ2 T expansion protocol based on single phosphoantigen stimulation could be easily performed for healthy donors. However, a low proliferative response of Vγ9Vδ2 T cells was observed in about half of the cancer patients, leading to an important limitation in the development of Vγ9Vδ2 T cell-based immunotherapy. Here, for the first time in the context of cancer patients, Vγ9Vδ2 T cell expansions were performed by co-culturing peripheral blood mononuclear cell (PBMCs) with autologous dendritic cells (DCs) pretreated with aminobisphosphonate zoledronate. For patients not responding to the conventional culture protocol, co-culture of PBMC with zoledronate-pretreated DCs induced strong cell expansion and allowed reaching a minimal rate of purity of 70% of Vγ9Vδ2 T cells. The potent immunostimulatory activity of zoledronate-treated DCs was associated with higher amount of isopentenyl pyrophosphate (IPP) in the culture and was correlated with better ability to activate Vγ9Vδ2 T cells as measured by IFN-γ production. Moreover, we demonstrated that the cytotoxic level of Vγ9Vδ2 T cells against freshly autologous tumor cells isolated from patients could be significantly increased by pretreating the tumor cells with zoledronate. Thus, this method of generating Vγ9Vδ2 T cells leads eligible for Vγ9Vδ2 T cell adoptive immunotherapy the HCC and mCRC patients.
Keywords: Colorectal carcinoma, Hepatocarcinoma, Immunotherapy, Aminobisphosphonate, γδ T cell, Isopentenyl pyrophosphate
Introduction
Hepatocellular carcinoma (HCC) and colorectal carcinoma with hepatic metastases (mCRC) are cancers that show poor prognosis. Today, only surgical resection, realized in the first course of the disease, represents a curative treatment [1, 2]. Recently, better understanding of tumor immunity has opened new perspectives to treat human malignant tumors [3]. Immunological approaches using innate antitumor effector cells are currently being evaluated in clinical trials [4]. γδ T cells are a T cell subset displaying a non-MHC restricted lytic activity against a broad panel of tumors including colon, kidney, liver, esophageal, small lung cancers and myeloma [5–8]. The presence of tumor-infiltrating γδ T cells in the liver of patients with HCC supports their potential role in tumor immunosurveillance and indicates them as candidates for liver cancer immunotherapy [9, 10].
Most of the circulating γδ T cells belong to the Vγ9Vδ2 subset, which recognizes small nonpeptidic phosphorylated antigens (phosphoantigens, P-Ags) such as isopentenyl pyrophosphate (IPP) produced in mammalian cells through the mevalonate pathway [11]. The mevalonate pathway is enhanced in tumor cells and mevalonate metabolites have been proposed as a danger signal that activates the immune response [12]. Aminobisphosphonates (ABP), such as zoledronate, used in the management of bone metastases, have shown direct antitumor properties. They also increase the γδ T cell number in peripheral blood [13–16]. Zoledronate inhibits the farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, leading to an accumulation of IPP [12]. An interesting therapeutic approach consists of expanding γδ T cells in vitro from patients’ peripheral blood mononuclear cells (PBMCs) using ABP (zoledronate, Zometa™, Novartis) or a synthetic analog of IPP, i.e., bromohydrin pyrophosphate (BrHPP, Phosphostim™, Innate Pharma), to infuse a large number of highly cytolytic γδ T cells into the patient. However, the first clinical trials revealed that this strategy was hindered by the unsuccessful γδ T cell amplification that occurs in about 30–50% of cancer patients [17–19]. Thus, better understanding of the γδ T cell activation and design of new expansion protocol is needed to allow the development of such strategy. Dendritic cells (DCs), the most efficient professional antigen-presenting cells, are known to behave as cellular bridges between innate and adaptive immunity. Reciprocal influence has been described between DCs and γδ T cells [20–22]. Mimicking this interaction in vitro in a co-culture model could be useful to improve γδ T cell activation.
The aim of this work was both to establish a new expansion protocol and to evaluate the interest for γδ T cell-based immunotherapy in HCC and mCRC. We documented that zoledronate-pretreated DCs overcame failure of γδ T cell amplification observed in cancer patients. The potent immunostimulatory activity of zoledronate-treated DCs was associated with the presence of higher amount of isopentenyl pyrophosphate in the culture and was correlated with better ability to activate Vγ9Vδ2 T cells as measured by IFN-γ production. Moreover, we evidenced that pretreatment of tumor cells with zoledronate significantly enhanced cell lysis by γδ T cells. The data led to conclude that, with this new expansion protocol, HCC and mCRC patients could be eligible for γδ T cell adoptive immunotherapy.
Materials and methods
Patients
Patients with HCC (n = 20) and mCRC (n = 22) were enrolled in this preclinical study at the time of removal surgery (Table 1). HCC tumor size was 4 ± 3 cm and a cirrhotic base was present in half of the cases. Hepatic metastases from CRC were 2 ± 1 cm and occurred on normal hepatic environment. Normal hepatic cells were obtained from the adjacent normal tissue removed during the surgery. Tumor or normal status was attested by an anatomopathologist. The study was approved by the regional ethics committee (Comité de Protection des Personnes de Rennes) and was in accordance with the 1964 Declaration of Helsinki.
Table 1.
Patients’ medical status
| HCC (n = 20) | mCRC (n = 22) | |
|---|---|---|
| Sex | 92% men | 75% men |
| Age | 65 ± 12 | 58 ± 11 |
| Tumor histology | 56% well differentiated, 35% moderately differentiated, 9% undifferentiated | 82% adenocarcinoma, 18% colloid adenocarcinoma |
| Tumor size (cm) | 4 ± 3 | 2 ± 1 |
| Liver metastases | 1–5 | |
| Associated hepatic pathologies (SA successful amplification) | Alcoholic cirrhosis (n = 8; 4 SA) Hemochromatosis (n = 5; 4 SA) | |
| Previous chemotherapy | Chemoembolization (n = 2; 0 SA) Epirubicin/cisplatin/5FU (n = 1; 1 SA) |
Folfox (n = 4; 3SA) Folfiri (n = 4; 1 SA) 5-FU (n = 1; 1 SA) |
γδ T lymphocyte culture
PBMCs were isolated by density gradient separation (UniSep®, Novamed, Jerusalem, Israel) from blood samples of 21 healthy donors (Etablissement Français du Sang, Rennes) and cancer patients (Département de Chirurgie Viscérale, CHU de Rennes). PBMCs were resuspended at 2 × 106/ml in RPMI 1640 (Eurobio, Les Ullis, France) supplemented with 10% fetal calf serum (Gibco Invitrogen Life Technologies, Cergy Pontoise, France), 1% l-glutamine, 50 μg/ml streptomycin and 50 IU/ml penicillin, referred to elsewhere as complete medium, and cultured according to the protocol previously described by Bennouna et al. [17]. Briefly, cells were treated once on day 0 with 3 μM BrHPP (kind gift of Innate Pharma, Marseille, France) or 1 μM zoledronate (Zometa®, Novartis, France) and cultured in the presence of 400 IU IL-2/ml (Proleukin® Chiron, Suresnes, France) for 2 weeks. Every 3 days, fresh complete medium with additional 400 IU IL-2/ml was added. A γδ T cell rate of 70% after in vitro amplification was considered by our team and other groups as the minimal rate of purity to investigate the therapeutic effect of γδ [17]. γδ T cell amplification index = number of recovered γδ T cells/number of seeded γδ T cells. Concentrations of BrHPP and zoledronate used in this study were defined in dose scaling studies ranging from 300 nM to 30 μM for BrHPP and from 1 to 10 μM for zoledronate.
DC differentiation
PBMCs were isolated by density gradient separation and plated in complete medium for 2 h. Adherent cells were cultured in complete medium supplemented with 1,000 IU/ml GM-CSF (Leukine®, Berlex, USA) and 400 IU/ml IL-4 (R&D Systems, Minneapolis, USA). On days 3 and 5, fresh complete medium containing the same amount of GM-CSF and IL-4 was added. On day 5, immature monocyte-derived DCs were treated overnight with 30 μM BrHPP or 10 μM zoledronate (Novartis, France) or in some experiments with 25 μM mevastatin (Sigma, France) plus 10 μM zoledronate. Pretreated DCs were washed and then incubated with autologous PBMC in a 1:10 ratio in the presence of 400 IU IL-2. For ELISA assays, DCs were plated at 0.5 × 106 cells/ml and cytokine levels were evaluated after 48 h in the supernatant using IL-10, IL-12 and IL-15 OptEIA sets from Becton–Dickinson (Mountain View, USA) and IL-7 duoset from R&D systems.
Tumor cell lines and primary cultures
CRC cell lines (SW620, SW403, HT29, Colo205) were obtained from American Type Cell Collection. HCC cell lines (HepG2, HuH7, BC2) were a gift from C. Guillouzo (INSERM U522, Rennes, France). Two cell lines were established in the laboratory from patients with CRC (C181, C187). Tumor and normal hepatic cells from patients were isolated by enzymatic digestion with 28 Wünsch units/ml collagenase (Liberase blendzyme3™, Roche, Indianapolis, USA) and 32 U/ml DNase (Pulmozyme®, Roche, Neuilly/Seine, France) immediately after surgery and used in cytotoxic assays without the in vitro culture step.
Analysis of isopentenyl pyrophosphate production
To extract the analytes from the cell samples, ice-cold acetonitrile (300 μL) was added to the cell pellet subsequent to the addition of ice-cold water (200 μL) containing phosphatase inhibitors (0.25 mM NaF and Na3VO4). Cell extracts were transferred to microcentrifuge tubes and suspended by pipette mixing. Precipitated macromolecules were separated by centrifugation (13,000×g, 2 min, 4°C). Samples were analyzed by liquid chromatography coupled to tandem mass spectrometry, as previously described [23].
Flow cytometry analysis
γδ T cells were stained with conjugated monoclonal antibodies (mAbs) against pan-γδ, Vγ9 and Vδ2 from Immunotech (Marseille, France). mAbs against CD40, CD80, CD86, CD83 and HLA-DR used for DC phenotype were purchased from Immunotech. Isotype-matched murine fluorochrome-conjugated immunoglobulins from the corresponding manufacturer were used as negative controls. Data were analyzed on FACScalibur cytometer (Becton–Dickinson).
Cytotoxicity assays
Cytotoxic activity of expanded γδ T cells was analyzed by a 4 h chromium (51Cr) release assay. Target cells were allogeneic established cell lines and autologous freshly isolated tumor cells. Normal hepatocytes were used as control targets. As much as 5 × 103 target cells, labeled with 51Cr sodium chromate (0.2 mCi/106 cells, Amersham, Saclay, France), were co-cultured in complete medium in 96 U-bottomed well plates for 4 h with γδ T cells. 51Cr release was assessed in culture supernatants, using a top-count gamma counter (Packard Instrument). Specific lysis, expressed as percentage, was calculated using the standard formula: [(mean experimental cpm − mean spontaneous cpm)/(mean maximum cpm − mean spontaneous cpm)] × 100. Results are the mean of assays performed in triplicate. In some experiments, tumor cell lines were pretreated overnight with 10 μM zoledronate and then washed extensively before the assay.
IFN-γ production by γδ T cells
PBMCs from healthy donors and non-responding patients were incubated for 10 h in complete medium with 400 IU IL-2 in the presence of 3 μM BrHPP or with DCs pretreated or not with 10 μM zoledronate. Monensin (3 μM, Sigma) was added 1 h after the beginning of the assay. Cells were fixed with 4% paraformaldehyde, stained with anti-δ2 TCR and anti-IFN-γ mAbs and analyzed on a FACScalibur cytometer.
Statistical analyses
Statistical analyses were performed using the Mann–Whitney non-parametric ranking test.
Results
Autologous γδ T cells efficiently lyse zoledronate-pretreated hepatic CRC metastases, but not adjacent normal cells
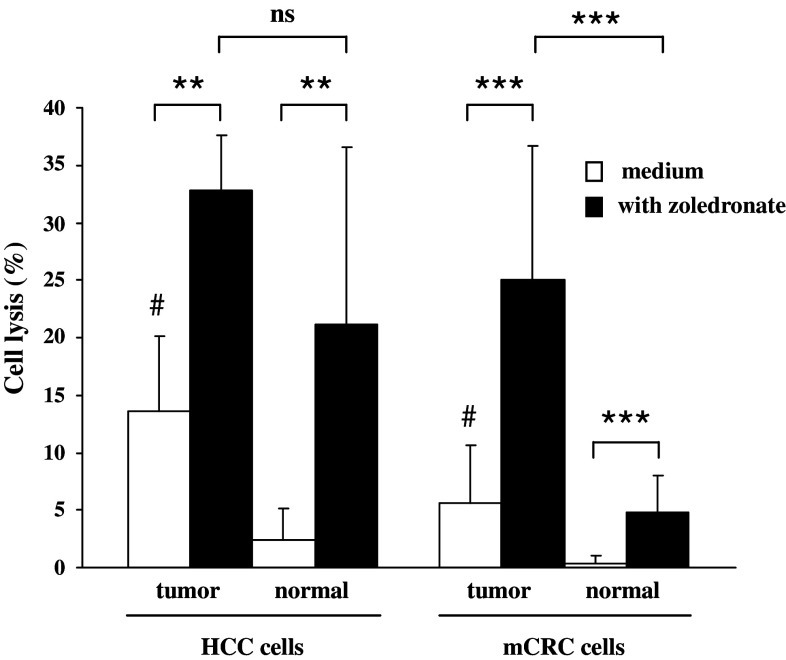
PBMCs from patients with HCC and mCRC were stimulated in vitro with a single dose of phosphoantigen (BrHPP) and cultured in the presence of IL-2. Cytotoxic activity was first studied in the allogeneic context against HCC and CRC long-term established cell lines. γδ T cells displayed high lytic capacities on HCC (47 ± 23%, n = 8) and CRC (36 ± 17%, n = 20) cell lines (Fig. 1). Then, we evaluated lytic properties of γδ T cells against freshly dissociated cells from patients’ tumor biopsies. To address this point, PBMCs from patients were stimulated 2 weeks before HCC or mCRC surgical resection. Then, cytotoxicity of expanded γδ T cells was evaluated against autologous freshly isolated tumor and adjacent normal hepatic cells. Lysis of HCC or mCRC tumor cells was significantly higher than that of normal adjacent cells (Fig. 2). No auto-reactivity was observed against adjacent normal cells from CRC metastases. Conversely, hepatic cells surrounding HCC tumors were found sensitized to autologous γδ T effectors cells likely due to the underlying cirrhosis and hemochromatosis (Fig. 2). Cpm for spontaneous and maximum 51Cr releases were 1.925 ± 1.750 and 13.350 ± 8.500 for cell lines, and 4.700 ± 3.200 and 21.800 ± 14.400 for freshly isolated cells, respectively.
Fig. 1.
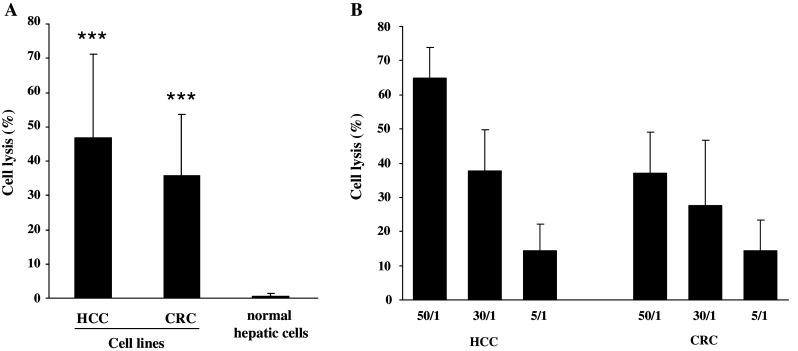
γδ T cells from patients efficiently lyse allogeneic tumor cell lines. PBMCs from HCC and mCRC patients were treated at day 0 with 3 μM BrHPP and cultured in the presence of 400 IU IL-2/ml for 2 weeks. Expanded γδ T cells (rate >70%) were incubated for 4 h with 5 × 103 target cells labeled with 51Cr. a Hepatic cell lines (HuH7, HepG2, BC2, n = 8) were used in three independent experiments and CRC cell lines (SW620, SW403, HT29, Colo205, n = 20) in five independent experiments; ratio of effector to target (E/T) was 30/1. ***Statistically significant (p < 0.001) compared to normal hepatic cells. b Effectors/targets titration lysis of hepatic and CRC cell lines was evaluated at 50/1, 30/1 and 5/1 E/T ratios (n = 3)
Fig. 2.
Zoledronate pretreatment sensitizes tumor cells to lysis by γδ T cells. PBMCs from HCC and mCRC patients were treated at day 0 with 3 μM BrHPP and cultured in the presence of 400 IU IL-2/ml for 2 weeks. Expanded γδ T cells (rate >70%) were incubated for 4 h with 5 × 103 target cells labeled with 51Cr in a ratio effector to the target (E/T) of 30/1. Target cells were freshly isolated cells from HCC or hepatic CRC metastases and normal hepatic cells. They were pretreated overnight (filled bars) or not (open bars) with 10 μM zoledronate. Data are from 5 experiments for HCC and 11 experiments for hepatic CRC metastases. #Statistically significant compared to normal hepatic adjacent cells with p < 0.001; **statistically significant with p < 0.01; ***Statistically significant with p < 0.001. As attested by trypan blue exclusion test and similar maximum/spontaneous ratio in the chromium release assay, 10 μM zoledronate was not toxic for tumor cells
A high proportion of cancer patients did not respond to conventional phosphoantigen stimulation
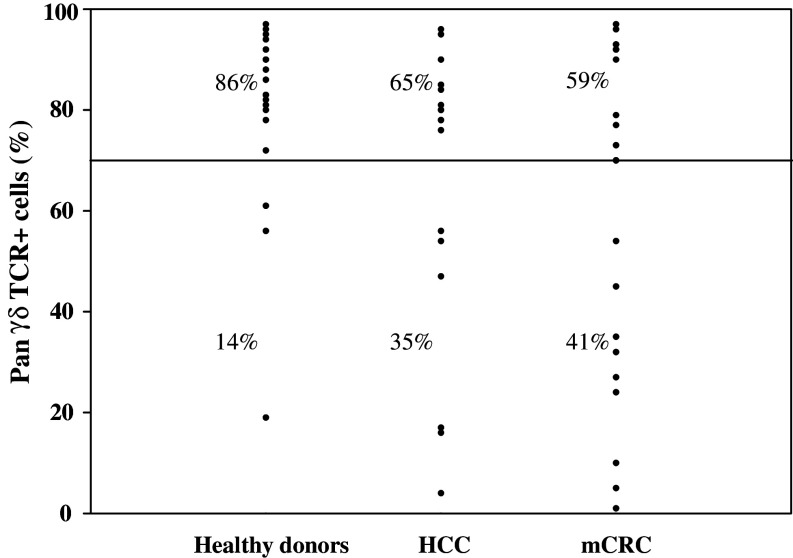
γδ T cell culture was considered as successful if the γδ T cell rate reached 70%, associated with an amplification index suitable for immunotherapy. In healthy donors, single phosphoantigen (BrHPP) stimulation allowed a strong amplification of γδ T cells (amplification index = 723 ± 505) and a γδ T cell rate of 81 ± 17% after 2 weeks of culture (n = 21). Culture was unsuccessful for only 3/21 healthy donors (Fig. 3). In successful expansion, phenotypic analysis of cell subsets showed 86 ± 12% Vγ9Vδ2+ cells, 12 ± 7% pan αβ TCR+ cells and 2 ± 1% CD3−/CD56+ cells (n = 18). Inversely, expected γδ T cell rate was not achieved in 35 and 41% of the HCC and mCRC patients, respectively. An increase of the BrHPP and zoledronate concentrations until 30 and 10 μM, respectively, was inefficient to improve γδ T cell rate (data not shown). The ratio of neutrophil granulocytes/lymphocytes, but not the initial percentage of γδ T cells, in the peripheral blood was predictive of a successful γδ T cell culture (Table 2). No correlation was found between anatomopathological criteria or prior chemotherapy treatment and the outcome of the culture.
Fig. 3.
γδ T cell culture fails in 1/3 of patients with HCC or hepatic CRC metastases. PBMCs from healthy donors (n = 21) as well as HCC (n = 20) and mCRC (n = 22) patients were treated at day 0 with 3 μM BrHPP and cultured in the presence of 400 IU IL-2/ml for 2 weeks. Plots represent the rate of pan-γδ-TCR-positive cells evaluated by flow cytometry. A successful culture was defined by a pan-γδ-TCR rate higher than 70%. Percentages of successful and unsuccessful cultures are indicated
Table 2.
Correlation between the γδ T cell amplification and the ratio neutrophil granulocytes/lymphocytes
| Successful amplification γδ T cell rate >70% (n = 18) | Unsuccessful amplification γδ T cell rate <70% (n = 11) | |
|---|---|---|
| Ratio of γδ T cells/PBMCs at day 0 | 2.6 ± 2.7 | 1.2 ± 0.6 |
| Ratio of neutrophil granulocytes/lymphocytes in peripheral blood | 2.7 ± 1.5 | 5.3 ± 3.5** |
** Statistically significant with p < 0.01
Co-culture with zoledronate-pretreated DCs allows efficient amplification of γδ T cells in cancer patients
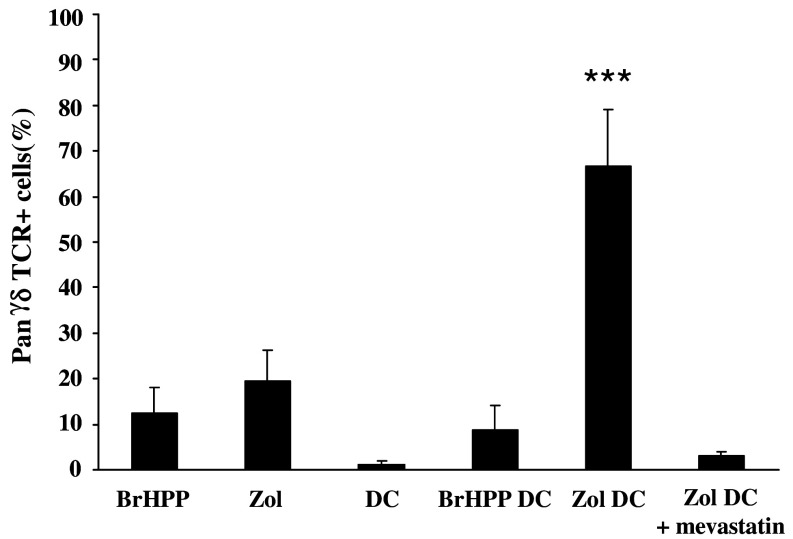
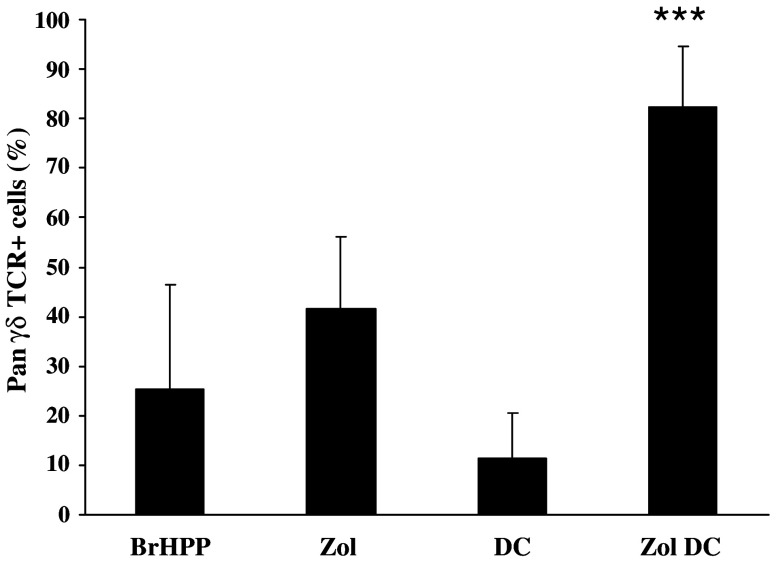
PBMCs from a non-responding healthy donor were first used to establish a new method to overcome the weak response of cancer patients’ PBMC to phosphoantigens. As shown in Fig. 4, conventional phosphoantigen stimulation with either BrHPP or zoledronate did not produce a sufficient rate of purity. Inversely, when immature DCs were pretreated overnight by zoledronate and then co-cultured with autologous PBMC, a suitable γδ T cell rate could be achieved (Fig. 4). The use of DCs either alone or pretreated with BrHPP was not efficient. Interestingly, when applied to non-responding patients with HCC or mCRC, this protocol produced the expected rate of purity (Fig. 5) and increased the γδ T cell amplification index (amplification index = 3588 ± 1904, n = 6).
Fig. 4.
Zoledronate-pretreated DCs allow amplification of γδ T cells from a BrHPP non-responding donor. PBMCs isolated from a BrHPP non-responding donor were stimulated with 3 μM BrHPP, 1 μM zoledronate (Zol), DCs alone (DC), DCs pretreated overnight with 30 μM BrHPP (BrHPP DC), DCs pretreated with 10 μM zoledronate (Zol DC) or DCs pretreated with both 10 μM zoledronate and 25 μM mevastatin (Zol DC + mevastatin). After 2 weeks of culture with 400 IU IL-2, the percentage of cells expressing the pan-γδ-TCR was evaluated by flow cytometry. Data are from six experiments except for mevastatin assays (n = 3). ***Statistically significant with p < 0.001. BrHPP and zoledronate do not affect dendritic cell viability as assessed by trypan blue exclusion test
Fig. 5.
Zoledronate-pretreated DCs allow amplification of γδ T cells from BrHPP non-responding patients. PBMCs isolated from six non-responding patients were stimulated with 3 μM BrHPP, 1 μM zoledronate (Zol), DCs alone (DC) or DCs pretreated overnight with 10 μM zoledronate (Zol DC). After 2 weeks of culture with 400 IU IL-2, the percentage of cells expressing the pan-γδ-TCR was evaluated by flow cytometry. ***Statistically significant with p < 0.001. Zoledronate does not affect dendritic cell viability as assessed by trypan blue exclusion test
Co-culture of PBMC/zoledronate-pretreated DCs displays high IPP level and enhances IFN-γ production by the γδ T cells
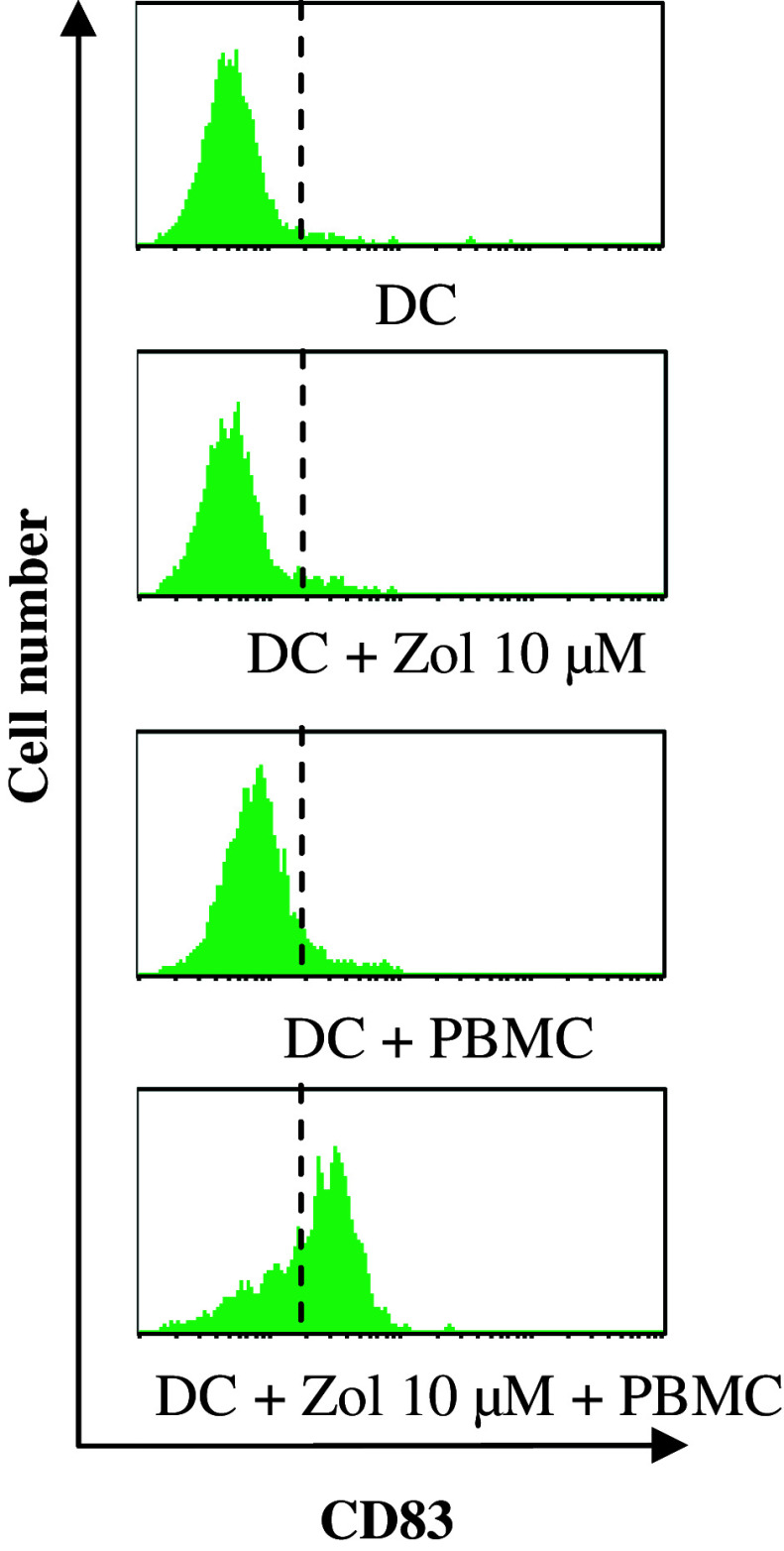
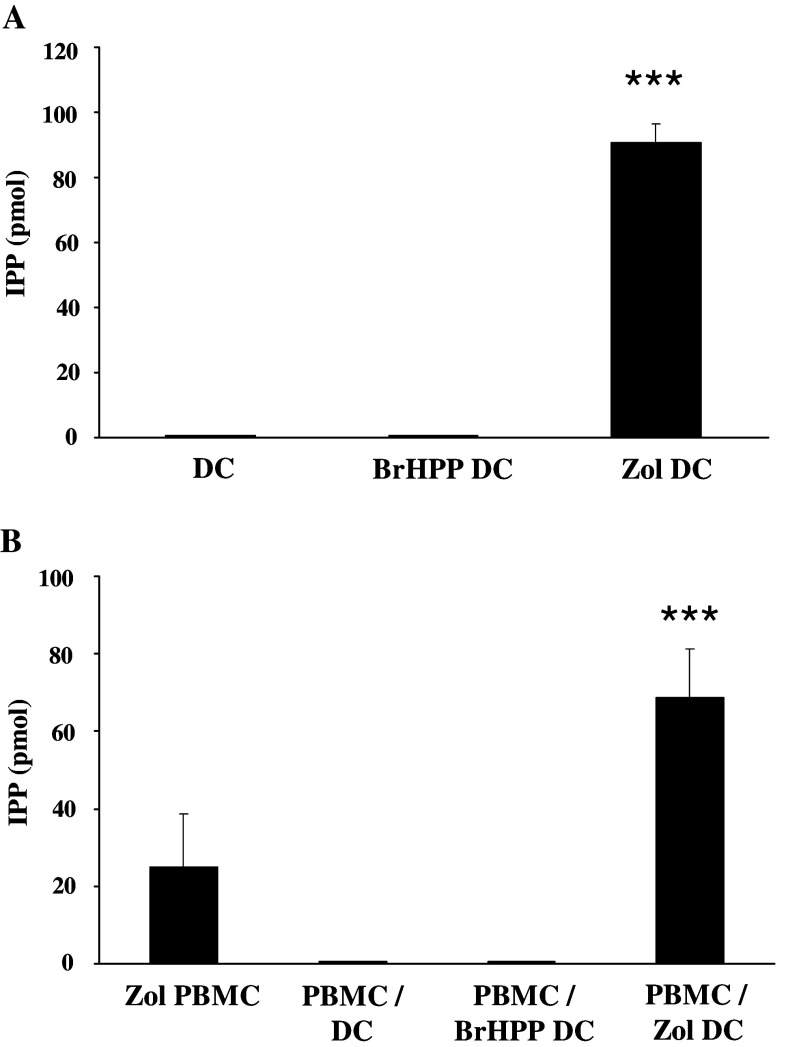
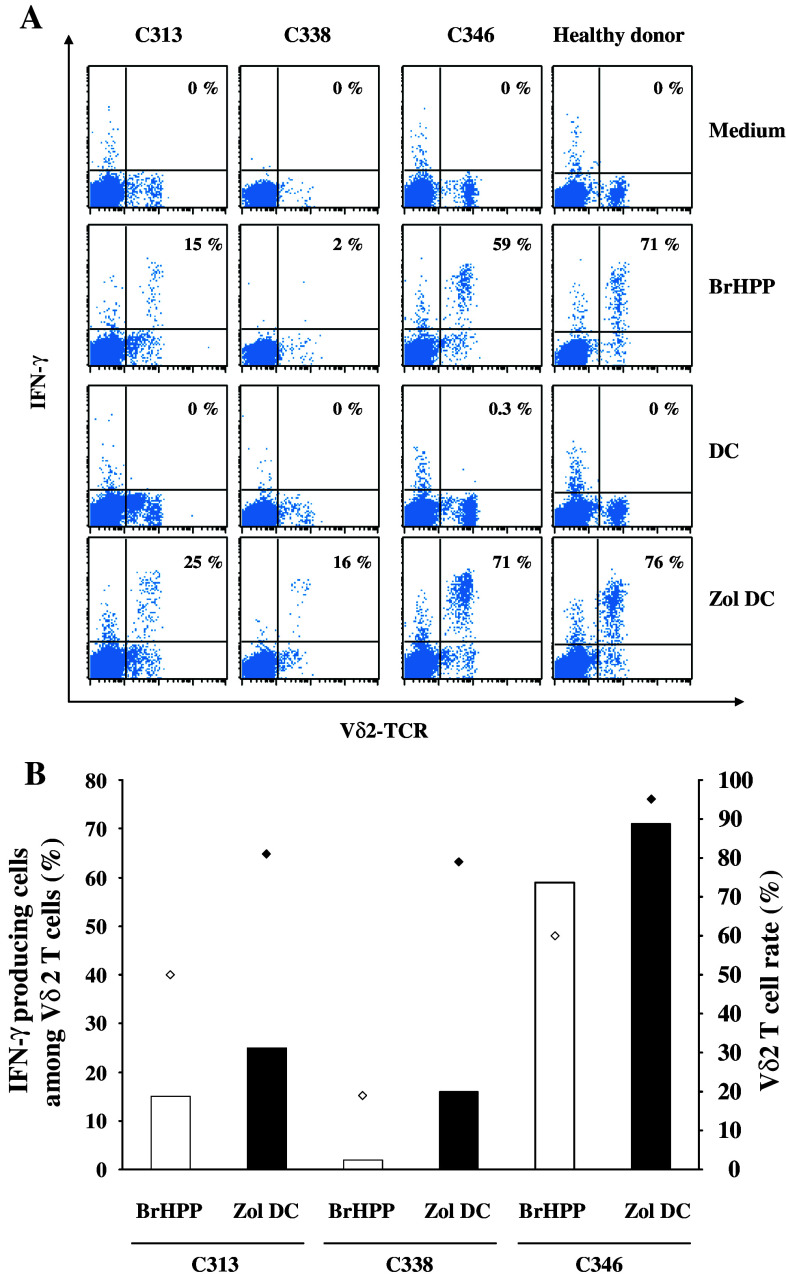
We found that DC, treated or not with zoledronate, expressed low levels of CD40, CD80 and CD86 costimulatory molecules and low IL-7, IL-10, IL-12 and IL-15 secretions (level <100 pg/ml), characterizing an immature state. DC maturation occurred during co-culture with PBMC as attested by CD83 expression (Fig. 7), but this phenotypic maturation was not associated with an induction of the secretion of the indicated cytokines. Of note, zoledronate-pretreated DCs displayed high IPP level (Fig. 6a). As a result, PBMC/zoledronate-pretreated DCs co-cultures are also characterized by a high level of IPP, a mevalonate pathway metabolite that is likely involved in the PBMC activation (Fig. 6b). In keeping with this hypothesis, addition of mevastatin, an inhibitor of the mevalonate pathway, during the DC pretreatment by zoledronate completely abrogated the γδ T cell amplification (Fig. 4). Moreover, zoledronate-pretreated DC displayed a better ability to induce IFN-γ production by the γδ T cells, an early event in the activation process of the T lymphocytes, in comparison with BrHPP signal (Fig. 8). Interestingly, in the non-responding patients’ C313 and C338, IFN-γ production improvement led to successful γδ T cell amplification (Fig. 8).
Fig. 7.
Expression of CD83 by the dendritic cells. PBMCs isolated from three healthy donors were differentiated into DCs in complete medium supplemented with 1,000 IU/m GM-CSF and 400 IU/ml IL-4. On day 5, DCs were treated overnight with 10 μM zoledronate. Pretreated DCs were washed and then incubated with autologous PBMC in a 1:10 ratio, in the presence of 400 IU IL-2. On day 7, cells were stained by anti-CD1a and anti-CD83 mAbs and analyzed on a FACScalibur cytometer. Histograms represent the expression of CD83, the main marker of DC maturation, among the dendritic cell population (CD1a+ cells) and are from three independent experiments
Fig. 6.
Isopentenyl pyrophosphate production in PBMC/zoledronate-pretreated DCs co-cultures. IPP production was analyzed by liquid chromatography coupled to tandem mass spectrometry. a. DCs (3 × 106) differentiated from three donors were not cultured or cultured in the presence of 3 μM BrHPP or 1 μM zoledronate. IPP production was analyzed 48 h after the addition of BrHPP or zoledronate. ***Statistically significant with p < 0.001. b. PBMCs (3 × 106) isolated from three donors were cultured in the presence of 1 μM zoledronate (PBMC Zol) or co-cultured at a 10:1 ratio with DCs (PBMC/DC), DCs pretreated overnight with 30 μM BrHPP (PBMC/BrHPP DC) or 10 μM zoledronate (PBMC/Zol DC). IPP production was analyzed 48 h after the addition of zoledronate or 48 h after the initiation of the co-culture. ***Statistically significant with p < 0.001 compared to PBMC Zol
Fig. 8.
IFN-γ production by γδ T cells from non-responding mCRC patients. a PBMCs isolated from one healthy donor and three mCRC patients (C313, C338, C346) were incubated for 10 h with complete medium, 3 μM BrHPP, DC alone (DC) or DC pretreated overnight with 10 μM zoledronate (Zol DC) in the presence of 400 IU IL-2. Monensin (3 μM) was added 1 h after the start of the experiment. Then, cells were fixed with 4% paraformaldehyde, stained with anti-Vδ2 TCR and anti-IFN-γ mAbs and analyzed on a FACScalibur cytometer. The percentage of IFN-γ+ cells among the Vδ2 TCR+ population is indicated on each cytogram. b Relation between the IFN-γ production by Vδ2 TCR+ cells 10 h after stimulation by BrHPP (bars) and the percentage of Vδ2 TCR+ cells after 2 weeks of culture (diamonds)
Discussion
The rationale for using γδ T cells in cancer immunotherapy is provided by studies showing their potent ability to kill in vitro human tumor cells from many cancer types such as colon, kidney, esophageal cancers and myeloma [5, 6, 8]. In this study, we demonstrate that HCC and mCRC cell lines are efficiently lysed by γδ T cells. Moreover, autologous tumor cells, dissociated from the patients’ tumors but not the adjacent normal cells, are recognized by the γδ T cells. Interestingly, we show that zoledronate significantly sensitizes tumor cells from mCRC biopsies to cell lysis without affecting the viability of normal surrounding hepatic cells. So, the clinical-grade ABP zoledronate may be an attractive adjuvant to reinforce the clinical efficacy of γδ T-based immunotherapy for mCRC cancer. Conversely, our results indicate that zoledronate increases the cytotoxicity against hepatic cells isolated from normal tissue adjacent to HCC. This cytotoxicity may be linked to the cirrhotic basis of HCC. Indeed, cirrhosis is associated with important alterations in lipid metabolism [24, 25]. Cells isolated from normal tissue adjacent to HCC may have a disregulated mevalonate pathway and, thus, display a higher sensitivity to zoledronate. In addition, we report here that 1/3 patients with HCC or mCRC cancer show a poor in vitro γδ T cell amplification following stimulation by BrHPP or zoledronate. In our cohort of patients (n = 42), this functional defect could not be linked to the initial percentage of γδ T cells in peripheral blood of non-responding patients. Instead, the ratio of neutrophil granulocytes/lymphocytes in peripheral blood appeared discriminant and could thus be useful to select patients likely to benefit from this therapy. Similarly, other groups have described in clinical trials that a high proportion (50%) of patients with metastatic renal cell carcinoma, prostate cancer or myeloma displayed a poor γδ T cell amplification in response to BrHPP [17–19]. This concern is likely a major limitation to the use of γδ T cells in therapy protocols. It emphasizes the need for new culture protocol to promote optimal expansion of γδ T cells for these advanced cancer patients. Previous studies have shown a reciprocal stimulation between γδ T cells, αβ T cells and DCs [22, 26]. To our knowledge, the role of DCs in the γδ T cell activation in the specific context of cancer patients has not been investigated. Interestingly, our findings reveal that co-culture of PBMC with zoledronate-pretreated DCs induces a strong expansion of γδ T cells in all enrolled non-responding patients, attesting to a powerful stimulation ability. Based on our γδ T cell amplification index, 100 ml of peripheral blood would be sufficient to obtain several billions of γδ T cells, a suitable number for an adoptive therapy [27–29]. Effect on γδ T cell amplification is specific to the treatment of DCs by zoledronate, since untreated DCs or DCs incubated with BrHPP are ineffective. Moreover, the stimulating ability of zoledronate-treated DCs, associated with high IPP level, is abrogated by mevastatin indicating that the DC–mevalonate pathway is involved in the γδ T cell activation. Phenotypic characterization of DCs show that zoledronate does not by itself induce the maturation of DCs, but maturation occurs during the co-culture probably due to the secretion by the γδ T cells of cytokines such as IFN-γ and TNF-α. Stimulation of γδ T cells by zoledronate-treated DCs results in a higher IFN-γ production than the stimulation by BrHPP or zoledronate. This stronger immunostimulatory ability is associated with a successful expansion of γδ T cells, suggesting that the proliferative defect observed in some patients results from a low responsiveness of γδ T lymphocytes that can be overcome by an enhanced IPP level during the activation process. The mechanisms underlying the proliferative defect of γδ T cells are still unknown and need further investigations. Nevertheless, it is likely that in some cancer patients, a chronic immunocompromised environment may impair γδ T cell functions. In agreement with this hypothesis, several studies have reported a reduced capacity of γδ T cells to proliferate in patients with HIV infection, a pathology associated with a severe immunodeficiency [30].
In conclusion, this innovative ex vivo expansion protocol leads eligible for γδ T cell adoptive immunotherapy the HCC and mCRC patients. A combination of γδ T cell and zoledronate infusions in clinical trials is a promising approach to strengthen γδ T responses in mCRC patients. Conversely, our results also prompt avoiding this association in HCC developed from a cirrhotic etiology, since zoledronate could sensitize non-tumoral hepatic cells. For HCC, an interesting option could be to focus γδ T cells on the tumor via a local hepatic infusion in the hepatic artery.
Acknowledgments
The authors thank C. Guillouzo (INSERM U522, Rennes) for providing HCC cell lines and expert assistance in the culture of normal hepatocytes, L. Jégu (Département de Chirurgie Hépatobiliaire et Digestive, CHU de Rennes) for assistance in patient inclusion and Innate Pharma (Marseille) for providing bromohydrin pyrophosphate (BrHPP, Phosphostim™). This study was supported by funds from the Comité Grand Ouest de la Ligue contre le Cancer and the Institut National du Cancer (PL075).
Conflict of interest statement
None.
Abbreviations
- HCC
Hepatocellular carcinoma
- mCRC
Colorectal cancer with hepatic metastases
- IPP
Isopentenyl pyrophosphate
- ABP
Aminobisphosphonate
- BrHPP
Bromohydrin pyrophosphate
- PBMC
Peripheral blood mononuclear cell
- DC
Dendritic cell
- mAb
Monoclonal antibody
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
- cpm
Count per minute
- P-Ag
Phosphoantigen
Footnotes
F. Cabillic and O. Toutirais contributed equally to this work.
References
- 1.Dupont-Bierre E, Compagnon P, Raoul JL, Fayet G, de Lajarte-Thirouard AS, Boudjema K. Resection of hepatocellular carcinoma in noncirrhotic liver: analysis of risk factors for survival. J Am Coll Surg. 2005;201(5):663–670. doi: 10.1016/j.jamcollsurg.2005.06.265. [DOI] [PubMed] [Google Scholar]
- 2.Thomas MB, O’Beirne JP, Furuse J, Chan AT, Abou-Alfa G, Johnson P. Systemic therapy for hepatocellular carcinoma: cytotoxic chemotherapy, targeted therapy and immunotherapy. Ann Surg Oncol. 2008;15(4):1008–1014. doi: 10.1245/s10434-007-9705-0. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabelitz D, Wesch D, He W. Perspectives of gammadelta T cells in tumor immunology. Cancer Res. 2007;67(1):5–8. doi: 10.1158/0008-5472.CAN-06-3069. [DOI] [PubMed] [Google Scholar]
- 5.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 6.Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, Caignard A. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174(3):1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 7.Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintiere C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57(4):531–539. doi: 10.1007/s00262-007-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas ML, Samant UC, Deshpande RK, Chiplunkar SV. Gammadelta T cells lyse autologous and allogenic oesophageal tumours: involvement of heat-shock proteins in the tumour cell lysis. Cancer Immunol Immunother. 2000;48(11):653–659. doi: 10.1007/s002620050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenna T, Golden-Mason L, Norris S, Hegarty JE, O’Farrelly C, Doherty DG. Distinct subpopulations of gamma delta T cells are present in normal and tumor-bearing human liver. Clin Immunol. 2004;113(1):56–63. doi: 10.1016/j.clim.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356(9232):802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 11.Bonneville M, Scotet E. Human Vgamma9Vdelta2 T cells: promising new leads for immunotherapy of infections and tumors. Curr Opin Immunol. 2006;18(5):539–546. doi: 10.1016/j.coi.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197(2):163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenson JR. Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist. 2005;10(1):52–62. doi: 10.1634/theoncologist.10-1-52. [DOI] [PubMed] [Google Scholar]
- 14.Stresing V, Daubine F, Benzaid I, Monkkonen H, Clezardin P. Bisphosphonates in cancer therapy. Cancer Lett. 2007;257(1):16–35. doi: 10.1016/j.canlet.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 15.Wada A, Fukui K, Sawai Y, Imanaka K, Kiso S, Tamura S, Shimomura I, Hayashi N. Pamidronate induced anti-proliferative, apoptotic, and anti-migratory effects in hepatocellular carcinoma. J Hepatol. 2006;44(1):142–150. doi: 10.1016/j.jhep.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Winter MC, Coleman RE. Bisphosphonates in breast cancer: teaching an old dog new tricks. Curr Opin Oncol. 2009;21(6):499–506. doi: 10.1097/CCO.0b013e328331c794. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galea C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Negrier S. Phase-I study of innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57(11):1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi H, Tanaka Y, Yagi J, Osaka Y, Nakazawa H, Uchiyama T, Minato N, Toma H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: a pilot study. Cancer Immunol Immunother. 2007;56(4):469–476. doi: 10.1007/s00262-006-0199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102(1):200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 20.Eberl M, Jomaa H, Hayday AC. Integrated immune responses to infection—cross-talk between human gammadelta T cells and dendritic cells. Immunology. 2004;112(3):364–368. doi: 10.1111/j.1365-2567.2004.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munz C, Steinman RM, Fujii S. Dendritic cell maturation by innate lymphocytes: coordinated stimulation of innate and adaptive immunity. J Exp Med. 2005;202(2):203–207. doi: 10.1084/jem.20050810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore F, Castella B, Nuschak B, Bertieri R, Mariani S, Bruno B, Pantaleoni F, Foglietta M, Boccadoro M, Massaia M. Enhanced ability of dendritic cells to stimulate innate and adaptive immunity on short-term incubation with zoledronic acid. Blood. 2007;110(3):921–927. doi: 10.1182/blood-2006-09-044321. [DOI] [PubMed] [Google Scholar]
- 23.Jauhiainen M, Monkkonen H, Raikkonen J, Monkkonen J, Auriola S. Analysis of endogenous ATP analogs and mevalonate pathway metabolites in cancer cell cultures using liquid chromatography–electrospray ionization mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(27):2967–2975. doi: 10.1016/j.jchromb.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J, Nilsson-Ehle P, Xu N. Influence of liver cancer on lipid and lipoprotein metabolism. Lipids Health Dis. 2006;5:4. doi: 10.1186/1476-511X-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez A, Colell A, Garcia-Ruiz C, Fernandez-Checa JC. Cholesterol and sphingolipids in alcohol-induced liver injury. J Gastroenterol Hepatol. 2008;23(Suppl 1):S9–S15. doi: 10.1111/j.1440-1746.2007.05280.x. [DOI] [PubMed] [Google Scholar]
- 26.Devilder MC, Maillet S, Bouyge-Moreau I, Donnadieu E, Bonneville M, Scotet E. Potentiation of antigen-stimulated V gamma 9V delta 2 T cell cytokine production by immature dendritic cells (DC) and reciprocal effect on DC maturation. J Immunol. 2006;176(3):1386–1393. doi: 10.4049/jimmunol.176.3.1386. [DOI] [PubMed] [Google Scholar]
- 27.Barkholt L, Danielsson R, Calissendorff B, Svensson L, Malihi R, Remberger M, Uzunel M, Thorne A, Ringden O. Indium-111-labelled donor-lymphocyte infusion by way of hepatic artery and radio-frequency ablation against liver metastases of renal and colon carcinoma after allogeneic hematopoietic stem-cell transplantation. Transplantation. 2004;78(5):697–703. doi: 10.1097/01.TP.0000129807.53523.97. [DOI] [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takayama T, Makuuchi M, Sekine T, Terui S, Shiraiwa H, Kosuge T, Yamazaki S, Hasegawa H, Suzuki K, Yamagata M, et al. Distribution and therapeutic effect of intraarterially transferred tumor-infiltrating lymphocytes in hepatic malignancies. A preliminary report. Cancer. 1991;68(11):2391–2396. doi: 10.1002/1097-0142(19911201)68:11<2391::AID-CNCR2820681110>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Poccia F, Boullier S, Lecoeur H, Cochet M, Poquet Y, Colizzi V, Fournie JJ, Gougeon ML. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157(1):449–461. [PubMed] [Google Scholar]