Abstract
Primary tumors developing in immunocompetent hosts escape immunosurveillance by acquiring immune evasive properties. This raises the prospect that metastases derived from such tumors will also evade immunity. To investigate whether immune surveillance plays a role in preventing metastases, we studied a murine model which mimics the clinical progression of osteosarcoma: primary tumor growth in the lower extremity, amputation, minimal residual disease followed by the development of overt metastases. K7M2 implants readily escaped immune surveillance since normal BALB/c mice, T cell deficient SCID and T/NK cell deficient SCID-bg mice showed no difference in the rate of growth of primary osteosarcomas. However, both SCID and SCID-bg mice had higher rates of metastases than immunocompetent mice. Similarly, immune reconstitution following transfer of naive T cells to SCID or SCID-bg mice did not impact primary tumor growth, but significantly diminished metastatic recurrence. T cells in osteosarcoma bearing mice produced IFNγ in response to tumor and IFNγ production by immune reconstituting T cells was required to prevent metastases. These results demonstrate an important role for T cell based immune surveillance in preventing metastases, even when metastases develop from tumors that adeptly evade immunosurveillance. The results further suggest that T cell depleting cancer therapies may eliminate beneficial immune responses and that immune reconstitution of lymphopenic cancer patients could prevent metastatic recurrence of solid tumors.
Keywords: Osteosarcoma, Minimal Residual Disease, Immune Surveillance, Immune Reconstitution, Primary Tumor Growth
Introduction
Most cancer deaths result from metastases rather than from primary tumor growth [2, 9]. Therefore, two major goals of cancer research are to identify biological factors that predispose to metastases and to develop new approaches to prevent metastases. Murine studies have convincingly demonstrated that innate and adaptive immune surveillance can prevent growth of some primary tumors [3, 12, 16, 33, 36, 37]. Nonetheless, most tumors arise in immunocompetent humans and tumors can be readily induced in immunocompetent mice, illustrating that cancer also adeptly evades immunity. Recent models reconcile these observations by emphasizing that immune pressure exerted during early stages of oncogenesis contributes to immune evasion by selecting immunoresistant cancer cells through a process described as “immunoediting” [6, 7]. Since metastases seed from primary tumors which have evaded immune surveillance, the immunoediting hypothesis predicts that metastases will readily escape immune surveillance [26]. Conversely, several current models emphasize a role for the “seed” and the “soil” in the multistep process of invasion, proliferation and angiogenesis required for metastatic spread [13, 20, 38, 43], thus potentially implicating host immunity in modulating metastatic spread. This study used a previously described model of spontaneous metastasis of osteosarcoma to assess the role that immune surveillance plays in preventing metastatic disease [18–20]. Surgically implanted cryopreserved osteosarcoma tumor fragments, which retain both the malignant osteosarcoma cells as well as the surrounding stroma, generate primary osteosarcomas in the extremity of mice. Following amputation of the extremity, a reproducible frequency of animals will develop pulmonary metastases, mimicking the clinical scenario in osteosarcoma patients. Using this model we demonstrate that there is no difference in the rate of growth of primary osteosarcomas when normal animals are compared to T cell depleted hosts, however metastatic disease is substantially increased in T cell depleted hosts. These results demonstrate that T cell based immune responses can prevent spontaneous metastases, even in hosts wherein primary tumors evade immune surveillance.
Materials and methods
Mice
BALB/c, SCID and SCID-bg, were purchased from the Animal Production Unit, National Cancer Institute, Frederick, MD. IFNγ − mice were kindly provided by Dr. Jon Wigginton. All mice were fully backcrossed to a BALB/c background and were used between 8–16 weeks of age. Mice were housed in a strict pathogen-free environment at the NCI animal facility at the NIH in Bethesda, MD. The NCI Animal Care and Use Committee approved all protocols. Where noted, at 4–5 weeks of age, thymectomized (TXY) mice underwent vacuum suction removal of the thymus according to standard protocol and T cell depletion (TCD) was accomplished using affinity purified rat anti-mouse anti-CD4 (clone GK1.5) and anti-CD8 (clone 2.43) antibodies as previously described [10].
Tumor model
K7M2 is a subclone of the spontaneously occurring K12 osteosarcoma, selected for high metastatic capacity as previously described [19]. To generate cell suspensions for injection, K7M2 cells were maintained in culture at 37°C in 5% CO2 in DMEM with 10% heat-inactivated FCS, 1% HEPES buffer, 1% non-essential amino acids, 1% sodium pyruvate, 1% penicillin/streptomycin, 1% L-glutamine (Gibco BRL), and 2-mercaptoethanol 50 μM (Sigma). In studies of the impact of immunization of tumorigenicity, exponentially growing tumor cells were prepared as a single cell suspension, irradiated to 10,000 cGy, and injected subcutaneously at a dose of 1 × 106 cells/mouse. K7M2 immunized and non-immunized animals were challenged subcutaneously with 1 × 106 tumor cells 14 days later.
To generate tissue fragments for implantation, cell suspensions of exponentially growing tumor cells were generated as described above and 1 × 106 cells were injected into the caudal gastrocnemius muscle, shown previous to reproducibly generate local tumors which are prone to metastasize [19]. Following development of bulky primary tumors (0.8–1.2 cm3), mice were euthanized, tumors were collected en bloc, then dissected in small fragments (≤3 mm diameter) under sterile conditions. Tumor fragments were snap frozen in 20% FCS and 10% DMSO (Sigma) and stored at −80°C until implantation. Frozen fragments were thawed at 37°C and promptly implanted in a muscular pocket surgically created in the cranial tibial muscle, following a surgical technique previously described [19]. Tumor volume was monitored serially using a digital caliper and calculated according to the formula: volume = π(D) × (d)2, where D represents the largest diameter and d the smaller diameter. To create a setting of minimal residual disease, amputation of the involved extremity was performed when the tumor volume attained 0.8 cm3. Amputation at this volume reproducibly led to metastases in 50% of normal, immunocompetent BALB/c mice.
The development of metastases in this model was readily apparent by examination of the mice, which typically developed constitutional symptoms of weight loss, ruffled fur and/or hunched posture. Animals with pulmonary metastases showed evidence for respiratory distress and animals with intraabdominal metastases developed palpable masses. According to the animal study protocol and for humane reasons, animals were euthanized when signs and symptoms of tumor recurrence interfered with normal activity, which typically occurred within 1 week of the onset of clinical signs of illness. Necropsy was performed in all euthanized animals to confirm the presence of metastatic osteosarcoma and on all animals who survived until the end of the experiment to confirm the absence of metastatic osteosarcoma.
Lymphocyte injection and T cell selection
To acquire T cells for immune reconstitution, lymph node cells were harvested from the axillary and inguinal lymph nodes (LNs) of tumor naïve, BALB/c mice or IFNγ − mice. The nodes were teased apart to obtain a single cell suspension, cells were passed through nylon mesh, then resuspended in PBS + 0.5% BSA (Sigma). Where indicated, single cell suspensions of whole lymph node cells were injected into the tail vein of recipients using the cell doses and timing noted. For experiments using purified populations of LN derived T cells or T cell subpopulations, positive selection of T cell subpopulations was achieved with MACS microbeads conjugated to monoclonal rat anti-mouse CD4 (L3T4) antibodies (clone GK1.5) and rat anti-mouse CD8α (Ly-2) antibodies (clone: 53–6.7) (Miltenyi Biotec) according to the manufacturer’s instructions. Purity of selected T cells, CD4 + T cells or CD8 + T cells was evaluated by flow cytometry prior to injection, and routinely were > 97%. Purified cells were maintained in ice and injected i.v. at appropriate concentrations in PBS + 0.5% BSA.
Assessment of immune reactivity to K7M2 in vitro
To identify direct evidence for immune reactivity by tumor bearing mice toward K7M2 osteosarcoma, Elispot assays for IFNγ production were used. Plates with a high protein binding immobilon-P membrane (Millipore Corporation) were coated overnight at 4°C with mouse IFNγ capture mAb (clone R4–6A2 Pharmingen) at a concentration of 2.5 μg/ml. Plates were then blocked for 1 h with 1% BSA (Sigma) in PBS and washed. Splenocytes were isolated from tumor naïve BALB/c and K7M2 bearing BALB/c mice. Splenocytes were resuspended in HL-1 serum-free media (BioWhittaker) supplemented with 1% penicillin/streptomycin/L-glutamine and were plated at 1 × 106/well in triplicate.
To evaluate IFNγ production in response to tumor, irradiated K7M2 cells (10,000 cGy) or irradiated splenocytes as a control were used as stimulators at a 1:1 effector:stimulator ratio. IFNγ production was also studied in response to syngeneic dendritic cells co-incubated in vitro with K7M2 tumor lysate (and splenocyte lysate as a control) at 10:1 effector:stimulator ratio. Effectors and stimulators were plated in triplicate and incubated at 37°C in 5% CO2 for 24 h. After incubation, cells were removed and the plates were washed four times with PBS + 0.05% Tween 20. A detection mAb, anti-IFN-γ (clone biotin-XMG1.2 Pharmingen), was added at 1 μg/ml, and plates were incubated overnight at 4°C. The plates were washed, developed with streptavidin-alkaline phosphatase (DAKO) at a 1/2,000 dilution for 2 h, then washed and spots colored with BCIP membrane phosphatase substrate (KPL) for 5–10 min. Finally, plates were washed once with dH20 and allowed to dry at room temperature. Image analysis of ELISPOT plates was performed as already described [17] with Series 1 Immunospot Satellite Analyzer (Cellular Technology). Briefly, digitized images were analyzed for areas in which the color density exceeded that of the background by a preset factor based on the comparison of control wells and experimental wells. Specific spots were calculated by subtracting the mean number of spots formed in control wells from the mean number of spots formed in each experimental well.
Dendritic cells were generated from bone marrow (BM) by modifying the technique of Morelli et al. [25]. Briefly, marrow was flushed from tibias and femurs of BALB/c mice using a 25-gauge needle, red blood cells were lysed with ammonium chloride lysis buffer (BioWhittaker), and committed erythroid precursors, T and B lymphocytes, granulocytes and MHC-II + cells were depleted using a cocktail of monoclonal antibodies (anti-TER-119, anti CD3e, anti B220, anti-Ly6G, anti-I-Ab) (all from Pharmingen) followed by guinea pig complement. Cells were cultured at 37°C, 5% CO2 for 7 days in media supplemented with rmGM-CSF 1,000 U/ml and rmIL-4 1,000 U/ml (Peprotech), on days 1, 3 and 5. On day 6, tumor lysate or control lysate (approximate ratio of cell equivalents in tumor lysate:DC = 2:1) was added to the DC culture for 18 h. Lysate pulsed DCs were collected by gentle pipetting on day 7 and used for Elispot. Tumor lysate was prepared following a technique already described [32]. Briefly, confluent cultures of K7M2 cells and control cells were detached by incubation with trypsin-EDTA (Gibco BRL), washed twice with PBS, resuspended at 5 × 106/ml in serum free media then frozen at –80°C. The cells then underwent four freeze–thaw cycles, followed by centrifugation for 10 min at 300 g. The supernatant was collected and filtered through a 0.2 μm filter. The amount of tumor or control lysate used for incubation with the DCs approximated the lysate generated from twice the number of dendritic cells pulsed.
Statistical analysis
Statistical tests were performed using GraphPad Prism version 4.0a for Macintosh (GraphPad Software, San Diego, CA). The last tumor volume recorded for each mouse at the time of euthanasia was used to calculate mean tumor volume at each time point for each group. Survival curves were generated by the method of Kaplan–Meier, and differences in survival were computed with the log-rank test. Animals that died due to surgical complications or were euthanized but showed no evidence of metastatic disease at necropsy (<10%) were censored for survival analyses. Significant differences when comparing two groups was determined by two-tailed Mann–Whitney test. One-way ANOVA/Bonferroni post-test was used to assess statistical differences between cumulative tumor volumes in selected pair of groups. P values were considered significant if < 0.05.
Results
Lymphopenic mice sustain a higher rate of osteosarcoma metastases than normal mice
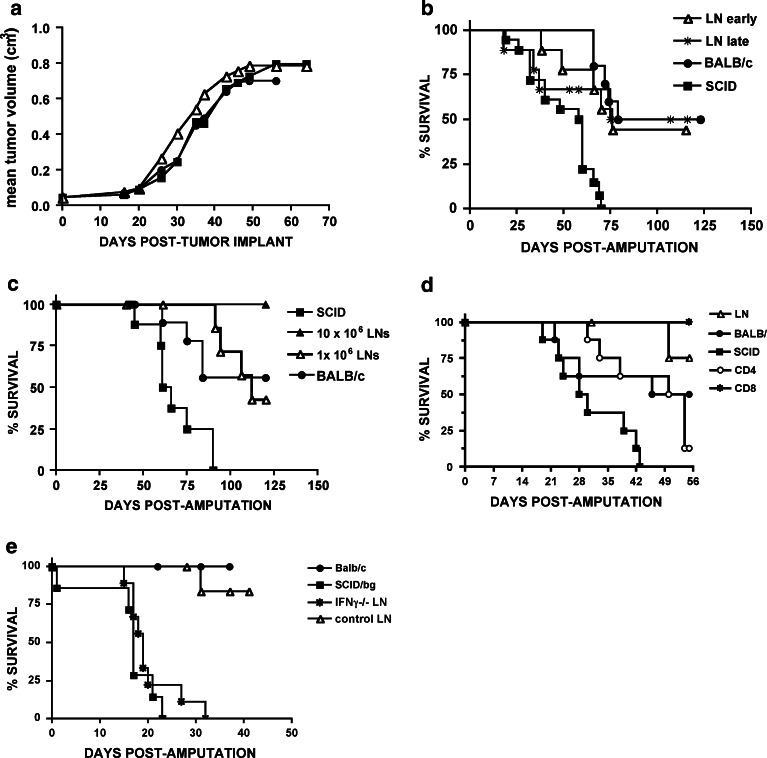
K7M2 is a subline of the spontaneous murine K12 osteosarcoma selected for high metastatic capacity. As reported previously, amputation of primary K7M2 tumors results in a period of minimal residual disease followed by a high rate of metastatic recurrence [18–20, 41]. To assess whether immune surveillance contributed to control of primary and/or metastatic osteosarcoma, we used this model to evaluate primary and metastatic tumor growth in immunocompetent versus immunodeficient mice. Implantation of thawed K7M2 tumor fragments measuring ≤3 mm in diameter (harvested previously from immunocompetent BALB/c mice) gave rise to primary tumors which readily escaped immunosurveillance, with no difference between BALB/c mice, SCID mice and NK-deficient SCID-bg mice in the time to develop palpable tumors (median 14d BALB/c, 12d SCID and 12 SCID-bg, P = NS), or the time required for tumors to reach the predetermined amputation volume of 0.8 cm3 (median 41d BALB/c, 33d SCID and 32.5d SCID-bg, P = NS) (Fig. 1a). Similarly, adult thymectomized (TXY) mice subjected to T cell depletion with anti-CD4, anti-CD8 or both moAbs showed no difference in the time to amputation compared to sham thymectomized animals (median 46d Sham TXY, 39d TXY, 46d TXY + anti-CD4 + anti-CD8, 40d TXY + anti-CD4, 41d TXY + anti-CD8).
Fig. 1.
Immunosurveillance does not prevent primary tumor growth but prevents metastases in murine osteosarcoma. a Rates of primary tumor growth following implantation of cryopreserved tumor fragments are shown in control BALB/c, SCID and SCID-bg mice (n = 10 mice/group, mean and SEM for each timepoint is shown, P > 0.05 one way ANOVA). Similar results were observed in three experiments. b Rates of primary tumor growth were compared following subcutaneous injection of 1 × 106 K7M2 cells in control BALB/c mice (closed circles) versus BALB/c mice immunized 1 month prior with 1 × 106 irradiated K7M2 cells (open triangles, n = 6 mice/group, P > 0.05 one way ANOVA). Similar results were observed in two experiments. c BALB/c mice with progressively growing K7M2 tumors show immune responses toward K7M2. Responders were splenocytes from (n = 5, black bars) tumor bearing BALB/c mice with bulky (>0.8–1.2 cm3) primary tumors or from (n = 6, white bars) tumor naïve BALB/c mice. Left bars denote mean spot formuing units (SFUs) via Elispot in response to irradiated K7M2 stimulators. Right bars denote mean net SFUs calculated using: SFUs in response to syngeneic marrow derived dendritic cells incubated with tumor lysate minus SFUs in response to dendritic cells incubated with normal BALB/c spleen lysate. Error bars represent the SEM generated from triplicate wells. Results are representative of two separate experiments. d Lymphopenic mice show increased death from metastases compared to normal mice. Survival of control BALB/c mice, SCID and SCID-bg mice following amputation of a tumor-involved extremity. All non-surviving animals showed evidence for metastatic osteosarcoma at necropsy. Surviving animals were euthanized on Day 125 and showed no evidence for metastatic disease. Significant differences exist between BALB/c versus SCID (P = 0.007) and BALB/c versus SCID-bg (P = 0.002). Results are representative of three separate experiments
As it is impossible to quantitate the tumor burden contained within tumor fragments, the inability for immune surveillance to impact primary tumor growth following implantation could relate to provision of tumor inocula that quantitatively overwhelms weak immune surveillance mechanisms. However, we saw a similar lack of effect of immune responses on the growth of primary K7M2 tumors following inoculation with 1 × 106 tumor cell suspensions in immunocompetent versus immunodeficient mice (data not shown), and a priori immunization with irradiated K7M2 also did not prevent or alter the pace of K7M2 tumor growth following injection of 1 × 106 K7M2 tumor cells subcutaneously (Fig. 1b). Thus, we could detect no influence of prophylactic tumor vaccination or naturally acquired immunosurveillance on the growth of primary K7M2 osteosarcoma. This is consistent with historical data demonstrating that spontaneously arising tumors [14, 21], which the parent K12 tumor represents, are not highly immunogenic and therefore readily overcome immune surveillance.
Recent studies have demonstrated that hosts with uncontrolled primary tumor growth are not ignorant of tumor antigens, but rather that immune responses generated during the incipient phase of tumorigenesis provides a selection pressure which results in the outgrowth of cells most capable of immune evasion [6, 7]. To identify evidence for immune reactivity in mice with primary progressive K7M2 osteosarcoma, we measured IFNγ production in response to K7M2 tumor cells using Elispot [17]. Splenocytes harvested from mice with K7M2 tumors showed increased IFNγ production in response to K7M2 tumor cells compared to non-tumor bearing mice (Fig. 1c, left panel). This could not be attributed entirely to non-specific activation of innate immunity and/or NK cell reactivity, since splenocytes from tumor bearing mice also produced increased IFNγ in response to tumor lysate pulsed syngeneic dendritic cells (Fig. 1c, right panel). Thus, animals with progressively growing primary K7M2 osteosarcoma show measurable immune reactivity, which coexists in the face of progressively growing primary tumors.
Since primary K7M2 tumors grow in the face of ongoing immune responses and are therefore immune-evasive, metastatic deposits derived from primary K7M2 tumors in immunocompetent mice would also be predicted to evade immunity. As reported previously, amputation of primary K7M2 tumors results in a period of minimal residual disease followed by a high rate of metastatic recurrence [19]. Observation based assessment (respiratory distress, weight loss, ruffled fur, hunched posture and/or palpable intra-abdominal masses) can reliably identify animals with metastatic osteosarcoma in this model. To determine whether immunosurveillance prevents metastatic recurrence, amputated immunocompetent and immunodeficient mice were monitored clinically for metastases. Whereas 50% of immunocompetent BALB/c mice remained disease-free following amputation, 100% of SCID-bg mice succumbed to metastatic disease (P = 0.007). NK cells were not sufficient to mediate the immunosurveillance observed [34, 39] since 100% of SCID mice, which retain a normal NK cell compartment, also developed metastatic recurrence (P = 0.0002 vs. BALB/c, Fig. 1d). Further, adult thymectomized mice T cell depleted via opsonizing anti-CD4 and anti-CD8 moAbs had only 10% survival (data not shown). Osteosarcoma metastases were confirmed by necropsy in all groups. With regard to distribution of involvement, all of the metastases in the immunocompetent BALB/c involved the lung, whereas approximately 90% of the metastases in the SCID and SCID-bg mice involved the lung with approximately 10% of these animals showing isolated involvement of the site of amputation and/or intraabdominal metastases. These differences were not statistically significant. Furthermore, among animals that developed metastatic osteosarcoma and were euthanized, no differences in the number of metastases were observed between immunocompetent and immunodeficient hosts.
Interestingly, it did not appear that inflammation-mediated “danger” signals [23] resulting from the surgical procedure of amputation itself were responsible for the differential effects seen on primary versus metastatic tumors since there was no difference in the growth rate of primary extremity osteosarcoma between animals which did or did not undergo contralateral amputation of an unaffected extremity (50% of animals in both amputated and non-amputated groups (n = 5/group) required humane euthanasia for tumors ≥2 cm at 23 days, P = NS). Furthermore, 100% of immunocompetent animals which received intravenous injection of 1 × 106 K7M2 tumor cells via the tail vein developed pulmonary K7M2 metastases (data not shown) and [19], demonstrating that the immune surveillance network which prevents spontaneous metastasis in normal Balb/c mice in this model can be overwhelmed when large tumor burdens are presented. Thus, although T cell mediated immunosurveillance does not modulate metastatic growth of injected tumor cells or implanted primary tumor fragments, immunosurveillance plays a significant role in preventing spontaneous metastases derived from primary tumors in this model of murine osteosarcoma.
Immune reconstitution via homeostatic peripheral expansion prevents metastatic osteosarcoma
Mature T cells undergo homeostatic peripheral expansion (HPE) in lymphopenic hosts and provide an important pathway for T cell immune reconstitution [11]. To determine whether immune reconstitution via HPE can prevent metastases, we transferred lymph node cells from tumor naive BALB/c mice to syngeneic tumor bearing SCID mice. Because host–tumor interactions during primary tumor growth could either enhance immunosurveillance by priming immune effectors or diminish immunosurveillance by editing the tumor to become immune evasive, results were compared when cells were administered on the day of tumor implantation (designated “early” in Fig. 2), versus on the day following amputation, (designated “late” in Fig. 2). Early adoptive transfer performed on the day of tumor implantation did not alter primary tumor growth (Fig. 2a), but substantially diminished metastatic recurrence (Fig. 2b). Similarly, if the immune reconstituting inocula was administered late, on the day following amputation, we also saw diminished metastatic recurrence (Fig. 2b). Since there was no difference in metastatic recurrence between groups receiving cells “early” versus “late”, we conclude that immune priming during primary tumor growth is not required for immunosurveillance, nor does immunoediting of the primary tumor diminish the effectiveness of immunosurveillance to prevent metastases in this model.
Fig. 2.
Immune reconstitution prevents metastatic recurrence of osteosarcoma. a Mean primary tumor volumes following implantation of tumor fragments into control BALB/c mice, SCID mice and SCID mice administered 1 × 106 tumor naive LN cells IV on the day of K7M2 implantation (LN early). Growth rates did not differ between groups (n = 6 mice/group, P > 0.05 one way ANOVA, error bars for each timepoint were <10%). b SCID mice administered 1 × 106 tumor naive LN cells IV on the day of K7M2 implantation (LN early) and SCID mice administered 1 × 106 LN cells harvested from tumor naïve BALB/c mice IV on the day of amputation (LN late) show improved survival compared to SCID mice which did not receive lymph node infusions (n = 6 mice/group, LN early versus no LN P = 0.001; LN late versus no LN P = 0.01). Similar results were observed in three separate experiments. c Survival following amputation of K7M2 extremity osteosarcoma is shown for control BALB/c, SCID, SCID mice administered 1 × 106 LN early and SCID mice administered 10 × 106 LN early (n = 8/group). Similar results were observed in two separate experiments. d Survival following amputation of K7M2 extremity osteosarcoma is shown for control BALB/c, SCID mice, SCID mice administered 10 × 106 tumor naive LN early, and SCID mice which received 5 × 106 immunomagnetically selected CD4 + LN cells or CD8 + LN cells on the day of tumor implantation. (n = 6 mice/group, LN early versus no LN and CD8 + LN versus no LN P < 0.05 whereas CD4 + LN versus no LN P > 0.05). e Survival following amputation of K7M2 extremity osteosarcoma is shown for control BALB/c, SCID-bg mice and SCID-bg mice which received 10 × 106 tumor naive LN cells IV on the day of implantation. LN cells were harvested from normal BALB/c mice (n = 8) or from IFNγ − (n = 9) BALB/c mice. Significant differences in survival are observed between SCID-bg receiving IFNγ − cells versus those receiving normal BALB/c cells (P = 0.0003). Similar results were observed in two separate experiments
We did observe a dose–response effect of lymph node cells in the protection against tumor recurrence. As shown in Fig. 2c, whereas 1 × 106 LN cells prevented metastases in 50% of the mice, transfer of 10 × 106 cells was able to protect 100% of the mice. Remarkably, the protection rendered via homeostatic peripheral expansion was greater than that observed in normal BALB/c mice, confirming results from previous studies which demonstrate that lymphopenic mice undergoing immune reconstitution via HPE exhibit increased antitumor immunity compared to normal hosts [1, 4, 5]. We further sought to assess which component of the LN cells was sufficient to mediate tumor protection. Whereas transfer of highly enriched (>97% pure) CD8 + T cells mediated complete protection against metastases, we saw no protection following transfer of similarly enriched CD4 + T cell populations (Fig. 2d). Finally, IFNγ has been shown to be a critical mediator of immunosurveillance in several models [16, 33, 35, 37]. We therefore sought to determine whether IFNγ was required for the protection from metastasis observed following immune reconstitution in this model. Indeed, as shown in Fig. 2e, mice receiving LN cells from IFNγ − mice were unable to prevent osteosarcoma metastases. We conclude therefore, that CD8 + T cells, which undergo homeostatic peripheral expansion in lymphopenic mice, can prevent the development of metastases in this model of murine osteosarcoma via a mechanism that requires the production of IFNγ.
Discussion
Recent work has convincingly demonstrated that the development of immune evasive properties is a necessary step for the growth of primary tumors in immune competent hosts [6]. This model, termed the immune editing hypothesis, predicts that metastases derived from primary tumors would also evade immune surveillance. Remarkably however, in this model of osteosarcoma metastases, T cell immune responses play a critical role in determining whether metastases developed. Whereas lymphopenic mice showed no difference in the susceptibility toward or the rate of growth of primary osteosarcomas and injections of sizable numbers of tumor via the tail vein reproducibly induced metastases, the incidence of death from spontaneous metastases derived from primary osteosarcomas was significantly reduced by T cell mediated immunity. Two other groups have recently demonstrated an increase in the effectiveness of antitumor immunity on metastases compared to primary tumors [40, 42]. While it remains possible that differences in the effects of immune surveillance on primary versus metastatic tumors reflect qualitative differences in the processes involved in primary versus metastatic tumor growth, several lines of evidence suggest that the increased susceptibility of spontaneous metastases compared to primary tumors observed in this model of immune surveillance relates, at least in part, to quantitative factors. Indeed, several model systems have demonstrated that development of spontaneous metastases is a very inefficient process [24], and thus the number of clonogenic tumor cells contained within implanted tumor fragments or within injected single cell suspensions is likely to be much greater than the individual cells which give rise to metastatic tumors. Indeed, immunocompetent animals were unable to prevent metastases when substantial numbers of tumor cells were inoculated intravenously. Therefore, on a per cell basis, it remains possible that primary tumors and metastatic tumors are equally susceptible to immune surveillance, and the findings reported here relate to the lower numbers of cells that can initiate the metastatic process. Alternatively, it is also possible that qualitative differences within the microenvironment of a primary tumor versus that of a metastatic cell that must exit the bloodstream and survive within a new microenvironment may favor immune surveillance efficiency. Regardless of whether the differential susceptibility to immune surveillance observed in this report reflects qualitative and/or quantitative differences in metastatic versus primary osteosarcomas, the data clearly demonstrate that spontaneous metastases derived from immune evasive primary tumors are remarkably susceptible to immune surveillance.
The observation that homeostatic peripheral expansion accomplished via transfer of tumor naive T cells provides a means to augment antitumor immunity is consistent with a variety of recent reports demonstrating that lymphopenic hosts which undergo immune reconstitution via this pathway demonstrate augmented antitumor effects [4, 5]. This presumably occurs due to T cell proliferation toward tumor antigens comprising weak self-antigens, which is induced by the lymphopenic environment [8]. Unfortunately, since the tumor antigens expressed by the K7M2 osteosarcoma have not been identified, we were unable to enumerate the number of truly tumor-specific T cells present in these animals. Future studies will be required to elucidate the tumor antigens expressed by K7M2 before more definitive immune characterization of this model can be pursued.
Interestingly, the benefit observed following adoptive transfer of tumor naive cells during the setting of minimal residual disease is consistent with recent clinical data demonstrating an inverse correlation between the absolute lymphocyte count following autologous bone marrow transplantation and the risk of tumor recurrence [15, 28, 29, 31]. Furthermore, a higher lymphocyte dose in the autograft provided to patients undergoing autologous stem cell transplantation for malignancy is also associated with diminished recurrence [27, 30]. Together, this clinical data and the preclinical data contained in this report raise the possibility that lymphodepleting therapy, which is commonly administrated for a variety of solid tumors which have a propensity for metastasis [22, 44], may predispose the host to metastatic recurrence by eliminating potentially beneficial low level immune reactivity. Furthermore, this study raises the prospect that adoptive transfer of autologous T cells might diminish tumor recurrence, a hypothesis that is potentially testable in the context of a randomized clinical trial.
In summary, in a model of murine osteosarcoma wherein primary tumors readily escape immunosurveillance, metastases remain highly sensitive to naturally acquired, T cell mediated anti-tumor immunity. As standard primary therapies administered for pediatric sarcomas and other cancers routinely induce profound, prolonged T cell depletion, future clinical studies are warranted to determine whether the immune status of cancer patients correlates with susceptibility to metastatic recurrence and ultimately to determine whether immune reconstitution therapies can prevent metastatic disease.
Acknowledgments
The authors would like to thank Dr. Jon Wigginton for kindly providing the IFNγ deficient mice. This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
By acceptance of this article, the publisher or recipient acknowledges right of the U.S. Government to retain a nonexclusive, royalty-free license in and to any copyright covering the article.
The contents of this publication do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Animal care was provided in accordance with procedures outlined in the “Guide for the Care and Use of Laboratory Animals” (NIH Pub. No. 86-23, 1996). This project was funded in whole or part with funds from the National Cancer Institute, National Institutes of Health, under Contract No. NO1-CO-56000.
References
- 1.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 2.Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–575. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 3.Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ. Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol. 2002;168:1356–1361. doi: 10.4049/jimmunol.168.3.1356. [DOI] [PubMed] [Google Scholar]
- 4.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dummer W, Niethammer AG, Baccala R, Lawson BR, Wagner N, Reisfeld RA, Theofilopoulos AN. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI200215175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 8.Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/S1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- 9.Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- 10.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 11.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–1533. doi: 10.1182/blood.V97.6.1525. [DOI] [PubMed] [Google Scholar]
- 12.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 13.Gutman M, Singh RK, Xie K, Bucana CD, Fidler IJ. Regulation of interleukin-8 expression in human melanoma cells by the organ environment. Cancer Res. 1995;55:2470–2475. [PubMed] [Google Scholar]
- 14.Hewitt HB, Blake ER, Walder AS. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976;33:241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joao C, Porrata LF, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Gastineau DA, Markovic SN. Early lymphocyte recovery after autologous stem cell transplantation predicts superior survival in mantle-cell lymphoma. Bone Marrow Transplant. 2006;37:865–871. doi: 10.1038/sj.bmt.1705342. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–61. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karulin AY, Hesse MD, Tary-Lehmann M, Lehmann PV. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J Immunol. 2000;164:1862–1872. doi: 10.4049/jimmunol.164.4.1862. [DOI] [PubMed] [Google Scholar]
- 18.Khanna C, Prehn J, Yeung C, Caylor J, Tsokos M, Helman L. An orthotopic model of murine osteosarcoma with clonally related variants differing in pulmonary metastatic potential. Clin Exp Metastasis. 2000;18:261–271. doi: 10.1023/A:1006767007547. [DOI] [PubMed] [Google Scholar]
- 19.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 20.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 21.Klein G, Klein E. Immune surveillance against virus-induced tumors and nonrejectability of spontaneous tumors: contrasting consequences of host versus tumor evolution. Proc Natl Acad Sci U S A. 1977;74:2121–2125. doi: 10.1073/pnas.74.5.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackall CL, Fleisher TA, Brown MR, Magrath IT, Shad AT, Horowitz ME, Wexler LH, Adde MA, McClure LL, Gress RE. Lymphocyte depletion during treatment with intensive chemotherapy for cancer. Blood. 1994;84:2221–2228. [PubMed] [Google Scholar]
- 23.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 24.Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- 25.Morelli AE, Zahorchak AF, Larregina AT, Colvin BL, Logar AJ, Takayama T, Falo LD, Thomson AW. Cytokine production by mouse myeloid dendritic cells in relation to differentiation and terminal maturation induced by lipopolysaccharide or CD40 ligation. Blood. 2001;98:1512–1523. doi: 10.1182/blood.V98.5.1512. [DOI] [PubMed] [Google Scholar]
- 26.Pardoll D. T cells and tumours. Nature. 2001;411:1010–1012. doi: 10.1038/35082676. [DOI] [PubMed] [Google Scholar]
- 27.Porrata LF, Ingle JN, Litzow MR, Geyer S, Markovic SN. Prolonged survival associated with early lymphocyte recovery after autologous hematopoietic stem cell transplantation for patients with metastatic breast cancer. Bone Marrow Transplant. 2001;28:865–871. doi: 10.1038/sj.bmt.1703236. [DOI] [PubMed] [Google Scholar]
- 28.Porrata LF, Litzow MR, Tefferi A, Letendre L, Kumar S, Geyer SM, Markovic SN. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 29.Porrata LF, Inwards DJ, Micallef IN, Ansell SM, Geyer SM, Markovic SN. Early lymphocyte recovery post-autologous haematopoietic stem cell transplantation is associated with better survival in Hodgkin’s disease. Br J Haematol. 2002;117:629–633. doi: 10.1046/j.1365-2141.2002.03478.x. [DOI] [PubMed] [Google Scholar]
- 30.Porrata LF, Gertz MA, Geyer SM, Litzow MR, Gastineau DA, Moore SB, Pineda AA, Bundy KL, Padley DJ, Persky D, Lacy MQ, Dispenzieri A, Snow DS, Markovic SN. The dose of infused lymphocytes in the autograft directly correlates with clinical outcome after autologous peripheral blood hematopoietic stem cell transplantation in multiple myeloma. Leukemia. 2004;18:1085–1092. doi: 10.1038/sj.leu.2403341. [DOI] [PubMed] [Google Scholar]
- 31.Porrata LF, Litzow MR, Inwards DJ, Gastineau DA, Moore SB, Pineda AA, Bundy KL, Padley DJ, Persky D, Ansell SM, Micallef IN, Markovic SN. Infused peripheral blood autograft absolute lymphocyte count correlates with day 15 absolute lymphocyte count and clinical outcome after autologous peripheral hematopoietic stem cell transplantation in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33:291–298. doi: 10.1038/sj.bmt.1704355. [DOI] [PubMed] [Google Scholar]
- 32.Schnurr M, Galambos P, Scholz C, Then F, Dauer M, Endres S, Eigler A. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445–6450. [PubMed] [Google Scholar]
- 33.Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- 34.Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, Kawano T, Pelikan SB, Crowe NY, Godfrey DI. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–668. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Street SE, Cretney E, Smyth MJ. Perforin and interferon-gamma activities independently control tumor initiation, growth, and metastasis. Blood. 2001;97:192–197. doi: 10.1182/blood.V97.1.192. [DOI] [PubMed] [Google Scholar]
- 36.Street SE, Trapani JA, MacGregor D, Smyth MJ. Suppression of lymphoma and epithelial malignancies effected by interferon gamma. J Exp Med. 2002;196:129–134. doi: 10.1084/jem.20020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, Diefenbach A, Yagita H, Godfrey DI, Smyth MJ. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med. 2004;199:879–884. doi: 10.1084/jem.20031981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dale P, Galand P. Effect of partial hepatectomy on experimental liver invasion by intraportally injected colon carcinoma cells in rats. Invasion Metastasis. 1988;8:217–227. [PubMed] [Google Scholar]
- 39.van den Broek ME, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz WK, Melief CJ, Zinkernagel RM, Hengartner H. Decreased tumor surveillance in perforin-deficient mice. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Duijnhoven FH, Aalbers RI, Rothbarth J, Terpstra OT, Kuppen PJ. A systemic antitumor immune response prevents outgrowth of lung tumors after i.v. rechallenge but is not able to prevent growth of experimental liver tumors. Clin Exp Metastasis. 2004;21:13–18. doi: 10.1023/B:CLIN.0000017162.35708.73. [DOI] [PubMed] [Google Scholar]
- 41.Wan X, Mendoza A, Khanna C, Helman LJ. Rapamycin inhibits ezrin-mediated metastatic behavior in a murine model of osteosarcoma. Cancer Res. 2005;65:2406–2411. doi: 10.1158/0008-5472.CAN-04-3135. [DOI] [PubMed] [Google Scholar]
- 42.Yang X, Chu Y, Wang Y, Guo Q, Xiong S. Vaccination with IFN-inducible T cell alpha chemoattractant (ITAC) gene-modified tumor cell attenuates disseminated metastases of circulating tumor cells. Vaccine. 2006;24:2966–2974. doi: 10.1016/j.vaccine.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Yu Y, Khan J, Khanna C, Helman L, Meltzer PS, Merlino G. Expression profiling identifies the cytoskeletal organizer ezrin and the developmental homeoprotein Six-1 as key metastatic regulators. Nat Med. 2004;10:175–181. doi: 10.1038/nm966. [DOI] [PubMed] [Google Scholar]
- 44.Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, Long LM, Bernstein D, Hill BJ, Douek DC, Berzofsky JA, Carter CS, Read EJ, Helman LJ, Mackall CL. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4 + CD25 + regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]