Abstract
Prostatic acid phosphatase (PAP) is a prostate cancer tumor antigen and a prostate-specific protein shared by rats and humans. Previous studies indicated that Copenhagen rats immunized with a recombinant vaccinia virus expressing human PAP (hPAP) developed PAP-specific cytotoxic T cells (CTL) with cross reactivity to rat PAP (rPAP) and evidence of prostate inflammation. Viral delivery of vaccine antigens is an active area of clinical investigation. However, a potential difficulty with viral-based immunizations is that immune responses elicited to the viral vector might limit the possibility of multiple immunizations. In this paper, we investigate the ability of another genetic immunization method, a DNA vaccine encoding PAP, to elicit antigen-specific CD8+ T cell immune responses. Specifically, Lewis rats were immunized with either a plasmid DNA-based (pTVG-HP) or vaccinia-based (VV-HP) vaccine each encoding hPAP. We determined that rats immunized with a DNA vaccine encoding hPAP developed a Th1-biased immune response as indicated by proliferating PAP-specific CD4+ and CD8+ cells and IFNγ production. Rats immunized with vaccinia virus encoding PAP did not develop a PAP-specific response unless boosted with a heterologous vaccination scheme. Most importantly, multiple immunizations with a DNA vaccine encoding the rat PAP homologue (pTVG-RP) could overcome peripheral self-tolerance against rPAP and generate a Th1-biased antigen-specific CD4+ and CD8+ T cell response. Overall, DNA vaccines provide a safe and effective method of generating prostate antigen-specific T cell responses. These findings support the investigation of PAP-specific DNA vaccines in human clinical trials.
Keywords: Prostatic acid phosphatase, DNA vaccine, Prostate cancer, Rat
Introduction
Prostate cancer is the most common malignancy and the second leading cause of cancer-related deaths in American men. Over the last two decades, several vaccine strategies with the goal of eliciting anti-tumor immunity have been investigated in animal models and clinical trials in patients with prostate cancer [1]. These studies have provided evidence of the immunogenicity of the target antigens and, more importantly, possible clinical efficacy for the treatment of prostate cancer [2, 3]. However, the search for target antigen(s) and vaccination method(s) capable of generating more robust destructive immune responses to malignant prostate cells continues.
Prostatic acid phosphatase (PAP), a prostate-specific protein, has been explored as a target antigen for prostate cancer vaccines. Peshwa and colleagues [4] identified several HLA-A2-restricted epitopes from PAP, suggesting these could be explored as vaccine antigens. Similarly, we have reported the identification of PAP-derived CD4+ epitopes [5]. We have also previously shown that approximately 10% of patients with prostate cancer have preexisting PAP-specific T cell proliferative responses, suggesting that tolerance to this protein can be overcome in vivo [6]. Fong and investigators at Dendreon Corporation have studied dendritic cell vaccines targeting PAP, and results from phase I and II trials conducted in patients with metastatic prostate cancer from these groups suggest that immune responses to PAP can be elicited by means of these vaccines, with possible clinical benefit in terms of delay in disease progression and overall survival [3, 7–9]. A phase III confirmatory trial by Dendreon Corporation using a similar approach is currently underway [3].
Fong and colleagues [10] have also reported a vaccinia virus vaccine approach targeting human PAP in a Dunning rat model. Specifically, they demonstrated that Copenhagen rats immunized with a recombinant vaccinia virus expressing human PAP developed cross-reactive PAP-specific cytotoxic T cells (CTL) and evidence of prostate tissue inflammation. The use of viral vaccine approaches, and vaccinia virus in particular, has been widely studied as a means of eliciting antigen-specific CD8+ T cell responses, and has entered clinical testing. Hodge and colleagues [11] demonstrated that a recombinant vaccinia virus encoding human PSA was safe and effective in a primate model in eliciting antigen-specific immunity. Several phase I clinical trials have been conducted and have been shown to be safe in patients with prostate cancer, and capable of eliciting a low-level PSA-specific T cell response [12–14]. Given the immunogenicity of the vaccinia virus itself, and concerns for repeated booster immunizations with this construct, a multi-institutional phase II trial was conducted in which patients with early metastatic prostate cancer were immunized with recombinant vaccinia virus or fowlpox virus expressing PSA, in various prime-boost sequences. In this study, PSA-specific T cells were generated in some patients; one cohort immunized with the recombinant vaccinia virus showed a prolonged progression-free survival [2]. At the time of this writing, a phase III clinical trial testing this approach is about to open to accrual.
The promising clinical results using vaccinia virus-based vaccines in patients with prostate cancer, and the preclinical work of Fong [10], suggest that vaccinia virus-based vaccines targeting PAP could be further explored. As suggested in clinical trials, however, vaccinia-based immunization strategies are necessarily limited by pre-existing immunity to vaccinia in some subjects and the generation of a strong virus-specific immune response precluding multiple immunizations and possibly limiting the generation of a “self” antigen-specific response. For these reasons, we and others have focused on another type of genetic immunization method, plasmid DNA-based vaccines. Because plasmid DNA can be taken up by antigen presenting cells at the site of immunization, with presentation of processed MHC class I and class II epitopes, many groups have shown this to be an effective means of eliciting antigen-specific cellular immunity, and CTL in particular [15, 16]. This response is qualitatively similar to that elicited by viral vaccines, but without the concurrent immunization with foreign viral proteins. This fact suggests that an advantage of DNA vaccines might be the possibility for multiple immunizations without the need for heterologous vaccine strategies. Consequently, in this study, we hypothesized that immunization with a DNA vaccine targeting PAP would be a means of eliciting PAP-specific CD8+ T cells. To test this, Lewis rats were immunized with either a DNA vaccine or vaccinia virus-based vaccine targeting PAP, using a similar model to that established by Fong and colleagues [10]. We report that male Lewis rats immunized with vaccinia virus encoding hPAP did not develop a PAP-specific response unless boosted with a heterologous vaccination scheme with either hPAP protein or a plasmid DNA vaccine encoding hPAP. In contrast, plasmid DNA encoding hPAP alone was sufficient to immunize rats, with the generation of PAP-specific CD4+ and CD8+ T cells, with a Th1-biased immune response. In addition, we report that multiple immunizations with the plasmid DNA encoding the autologous rPAP generated an autoimmune T cell response. Overall, these results suggest that DNA vaccines encoding PAP could be studied in human clinical vaccine trials as a potential treatment for prostate cancer.
Materials and methods
Materials
Complete (CFA) and incomplete Freund’s adjuvant (IFA) were purchased from Sigma (St. Louis, MO, USA). Recombinant rat GM-CSF was purchased from Peprotech, Inc. (Rocky Hill, NJ, USA) or Research Diagnostics, Inc. (RDI, Flanders, NJ, USA).
Animals
Two to 3-month-old male Lewis rats were purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were housed in a facility maintained by the Laboratory Animals Resources of the University of Wisconsin Medical School, and all treatments and euthanasia were conducted under an institutional animal care and use committee-approved protocol (IACUC).
Viral and DNA constructs
The recombinant vaccinia virus was constructed by first cloning the cDNA encoding the entire length of human PAP, including the secretory signal, into a vaccinia viral shuttle vector, pSC11. This vector contains a multiple cloning site downstream of the p7.5 viral promoter, and flanked by sequence from the vaccinia thymidine kinase gene. Recombinant virus was then generated by homologous recombination, selecting for disruption of the thymidine kinase gene, by standard methodology. Individual plaques were isolated, purified, and a clonal population that produced the highest concentration of PAP by immunoblot after transduction of a reporter cell line was used for the immunization studies.
The pTVG-HP and pTVG-RP constructs were derived from the pTVG4 immunization vector previously described [17]. Into this construct was cloned the cDNA encoding the entire length of hPAP (pTVG-HP) or rPAP (pTVG-RP) protein, including the secretory signal for each protein.
cDNAs encoding the green fluorescent protein (GFP), hPAP or rPAP were cloned into the pRRL.PPT.PGK.W.SIN lentiviral expression vector. Replication-deficient lentivirus were then prepared as previously described [18]. The production of GFP, hPAP or rPAP by these constructs were confirmed by ELISA, PAP activity [19], and/or flow cytometric analysis following transduction of reporter cell lines, including rat primary dendritic cells (data not shown).
Immunization studies
Male, Lewis rats were immunized subcutaneously at 14-day intervals with 25 μg human PAP protein (hPAP) in Freund’s adjuvant, 107 pfu wild type vaccinia virus (VVwt), or 107 pfu vaccinia virus expressing hPAP (VV-HP). Additional groups were immunized with 107 pfu vaccinia virus expressing hPAP followed 14 days later with a booster immunization of PAP protein with Freund’s adjuvant. Animals were euthanized 2 weeks after the second immunization. Spleens and blood were collected for immunological analysis.
Male, Lewis rats were immunized subcutaneously with 107 pfu VV-HP and then boosted with 100 μg of a plasmid DNA encoding hPAP (pTVG-HP), or immunized with 100 μg pTVG-HP or the vector (pTVG4) alone. All DNA immunizations were performed intradermally at 14-day intervals with 5 μg rat GM-CSF co-administered as a vaccine adjuvant. In each case, animals were euthanized 2 weeks after the second immunization. Spleens and blood were collected for immunological analysis.
Male, Lewis rats were immunized six times with 100–500 μg of plasmid DNA encoding hPAP (pTVG-HP), rPAP (pTVG-RP), or vector (pTVG4). Again, all DNA immunizations were performed intradermally at 14-day intervals with 5 μg rat GM-CSF co-administered as a vaccine adjuvant. Two weeks after the last immunization, animals were euthanized with collection of spleens and blood for immunological analyses.
Separation of rat dendritic cells (DCs)
Splenocytes from naïve male Lewis rats were collected by centrifugation over a Histopaque 1083 (Sigma) gradient and washed with Click’s medium (Sigma) supplemented with 10 mM l-glutamine, 2% penicillin–streptomycin, 50 μM β-mercaptoethanol and 10% fetal calf serum (FCS, BioWhittaker, Walkersville, MD, USA). Cells were then incubated with a saturating concentration of OX62 antibody (Serotec, Oxford, UK) for 15 min. After a wash with phosphate-buffered saline (PBS)–2% FCS–1 mM EDTA, cells were mixed with anti-PE microbeads and the DCs were separated according to manufacturer’s instructions (StemCell Technologies, Vancouver, BC, Canada). Purity of isolated DCs was determined by flurochrome-labeled anti-rat CD11c (BD Pharmingen) and anti-rat OX62 staining, and analyzed by flow cytometry.
Transduction of rat dendritic cells with lentivirus expressing rPAP, hPAP or GFP
Rat dendritic cells were transduced with lentivirus expressing either rPAP, hPAP, or GFP at a multiplicity of infection (MOI) of 10. After 48 h, transduced DCs were tested for the expression of GFP by fluorescent microscopy or flow cytometry, and PAP expression was evaluated by ELISA or tartrate-inhibited phosphatase activity [19].
In vitro stimulation conditions and T cell proliferation assays
Lymphocytes from immunized animals were collected by centrifugation over a Histopaque 1083 (Sigma) gradient and resuspended in Click’s medium (Sigma) supplemented with 10 mM l-glutamine, 2% penicillin–streptomycin, 50 μM β-mercaptoethanol and 10% FCS (BioWhittaker). T cell proliferation was assessed as previously described [17]. Specifically, 2 × 105 splenocytes were cultured in individual wells of 96-well culture plates with 2 μg/ml purified hPAP protein (hPAP, Chemicon Int., Temecula, CA, USA), 2 μg/ml purified ovalbumin (OVA, ICN Biomedicals, Aurora, OH, USA), 2 × 104 pfu irradiated wild-type vaccinia virus (VVwt), 2 × 104 lentivirus-transfected DC (for cytokine release studies), or media only. PHA (10 μg/ml), a T cell mitogen, was used as a nonspecific positive control. Of note, different lots of PAP protein were used for the immunization experiments and the immunological assays to control for possible co-contaminants. All cultures were incubated at 37°C in an atmosphere of 5% CO2 for 72 h and then pulsed with 1 μM BrdU (BD Biosciences, San Jose, CA, USA) for the last 8–12 h. CD4+ and CD8+ T cells proliferating in response to antigen stimulation (BrdU+) were detected using an intracellular flow cytometric staining method (BD Flow kit, BD Biosciences) according to the manufacturer’s standard protocol. All antibodies (anti-rat CD4+-Cy-Chrome (clone OX-35), anti-rat CD8+-PE (clone OX-8), anti-BrdU-FITC (clone B44), and fluorochrome-labeled isotype controls) were purchased from BD Biosciences. 2 × 104 events for each culture/antigen condition were collected using a FACSCalibur flow cytometer (Beckton Dickinson), and the results were analyzed as the percent of CD4+ or CD8+ cells co-staining with anti-BrdU compared with a FITC-labeled isotype control. A positive CD4 or CD8 immune response to PAP was defined as a significant (P ≤ 0.05) antigen-specific response by comparing the percentage of CD4+/BrdU+ or CD8+/BrdU+ events following PAP antigen stimulation with those populations following media (no antigen) stimulation only (chi square analysis). Mean and standard error (SE) of proliferation results from animals within each experimental group were tabulated, as shown. Of note, and as we have previously reported, the proliferation of rat CD8+ T cells following PHA stimulation was not consistently observed under these culture conditions [17].
Cytokine release enzyme-linked immunosorbent assays (ELISA)
Splenocytes from immune rats were prepared by Histopaque centrifugation, as described above, and cultured with antigens as described above for 72 h (for IFNγ detection) and 96 h (for IL-10 detection). Fifty microlitres of culture media were then removed and assessed for IFNγ or IL-10 concentration by direct ELISA. In brief, 5 μg/ml mouse anti-rat IFNγ monoclonal antibody (clone DB1, RDI) or anti-rat IL-10 monoclonal antibody (BD Pharmingen) was adsorbed to individual wells of Immulon-4 polystyrene plates (Dynex Technologies Inc., Chantilly, VA, USA) in 50 mM sodium carbonate buffer (pH 9.6) overnight at 4°C. Plates were then blocked with PBS–1% bovine serum albumin (BSA) for 3 h at room temperature, and then washed with PBS–0.1% Tween-20. Fifty microlitres of culture media were then added to experimental wells in triplicate, and plates were incubated overnight at 4°C. After incubation, plates were washed and the detection of IFNγ or IL-10 was performed with a biotinylated rabbit anti-rat IFNγ polyclonal antibody (RDI) or biotinylated mouse anti-rat IL-10 antibody (BD Pharmingen), followed by a peroxidase–conjugated streptavidin. The plates were washed and developed with TMB peroxidase substrate (Kierkegard and Perry Laboratories, Gaithersburg, MD, USA) according to the manufacturer’s instructions. Reactions were stopped with addition of HCl to 0.5 N concentration and the plates were then read at OD450. Concentrations were calibrated based on wells containing known concentrations of purified recombinant rat IFNγ (RDI) or IL-10 (BD Pharmingen). Replicate results following antigen stimulation were compared with results following media only stimulation by a Student’s T test.
Enzyme-linked immunosorbent assays
IgG antibodies specific for PAP were detected by ELISA as previously described [17, 20]. Results are reported as a ΔOD = (OD450-antigen)−(OD450-blank) for each sera and sera concentration. Mean and standard error (SE) of ΔOD results from animals within each experimental group were tabulated, as shown.
Results
Immunization of male Lewis rats with vaccinia virus-encoding hPAP did not elicit PAP-specific T cell immunity unless boosted by a different means of antigen delivery
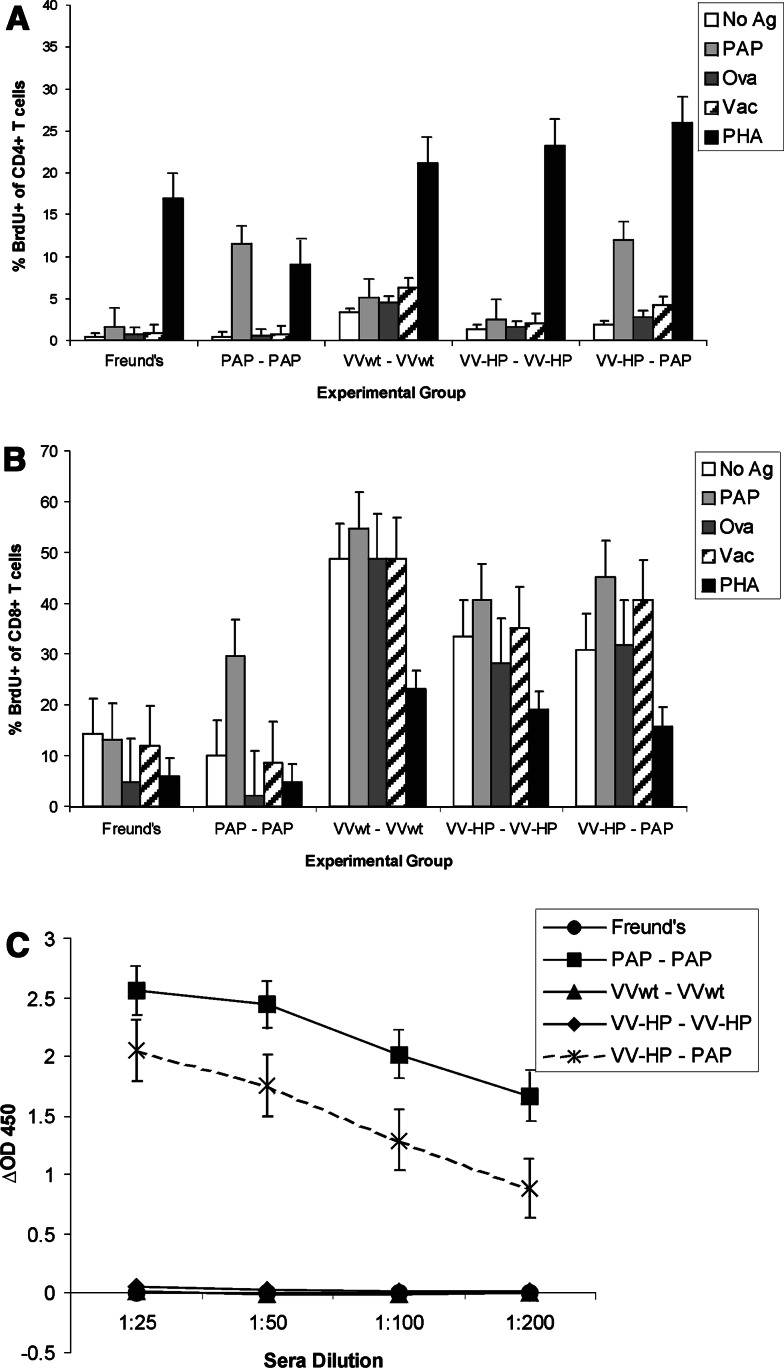
Fong and colleagues [10] previously demonstrated that Copenhagen rats could be immunized with vaccinia virus encoding hPAP with the generation of a cross-reactive immune response to the rat PAP protein. We wished to replicate these findings in the Lewis rat strain, which is better characterized than the Copenhagen rat strain with respect to MHC class I and class II epitope determinants. Two-to-3-month-old male Lewis rats were immunized once with 107 pfu of recombinant vaccinia virus encoding human PAP (VV-HP) versus wild type vaccinia virus (VVwt). Control groups received 25 μg purified hPAP protein administered subcutaneously in Freund’s adjuvant versus Freund’s adjuvant only. After 14 or 28 days, splenocytes and blood were removed and assessed for PAP-specific CD4+ or CD8+ T cell proliferation or PAP-specific IgG responses. In four replicate experiments, no PAP-specific CD4+ or CD8+ T cell immune responses could be detected in any group, although low-titer PAP-specific IgG could be detected in approximately 1–3 of animals immunized once with hPAP protein in adjuvant after 28 days (data not shown). These findings suggested that a booster immunization was necessary to effectively immunize animals. Consequently, separate groups of male Lewis rats were immunized twice with VVwt, VV-HP, hPAP protein in Freund’s adjuvant, or with VV-HP followed by a booster immunization with hPAP protein in adjuvant. As indicated in Fig. 1, hPAP-specific CD4+ and CD8+ proliferative T cells could be detected in animals immunized twice with hPAP protein, and in animals immunized first with VV-HP and boosted with hPAP protein, but not in animals immunized twice with VV-HP. Similarly, PAP-specific IgG could be detected in the sera of animals immunized with hPAP protein as well as in animals that received VV-HP vaccination followed by hPAP protein as a booster immunization, but not in animals immunized twice with VVwt or VV-HP (Fig. 1c). As shown, high background (non-specific) CD8+ T cell proliferation was observed in all animals immunized with vaccinia virus, and this finding was observed in all of multiple replicate experiments.
Fig. 1.
Immunization of male Lewis rats with vaccinia virus-encoding hPAP did not elicit PAP-specific T cell immunity unless boosted by a different means of antigen delivery. Male Lewis rats were immunized subcutaneously twice 14 days apart with Freund’s adjuvant, 25 μg hPAP protein (PAP–PAP), 107 pfu wild type vaccinia virus (VVwt–VVwt), or 107 pfu vaccinia virus expressing hPAP (VV-HP–VV-HP) as indicated. An additional group was immunized with 107 pfu VV-HP followed by a booster immunization 2 weeks later of 25 μg PAP protein (VV-HP–PAP). Two weeks after the second immunization animals were euthanized with collection of spleens and blood. Splenocytes were cultured in the presence of 2 μg/ml hPAP protein, 2 μg/ml ovalbumin, 104 pfu irradiated wild type vaccinia virus, 10 μg/ml PHA, or media only for 72 h. Eight–12 h before termination of culture, cells were pulsed with 1 μM BrdU, and the % of CD4+ T cells (a) or % of CD8+ T cells (b) costaining for BrdU uptake was determined by flow cytometry (BrdU Flow kit). The data show the mean and standard error (SE) of independent analyses for each of three animals per experimental group. The data is representative of four independent, replicate experiments for a total of 12 animals per treatment group. Sera from immunized animals were evaluated by ELISA for hPAP-specific IgG (c). Similarly, the data show the mean and SE of the ΔOD450 obtained for each of three animals per experimental group, and are representative of four independent, replicate experiments
Immunization of male Lewis rats with plasmid DNA encoding hPAP elicits PAP-specific T cell immunity without the need for heterologous immunization strategies
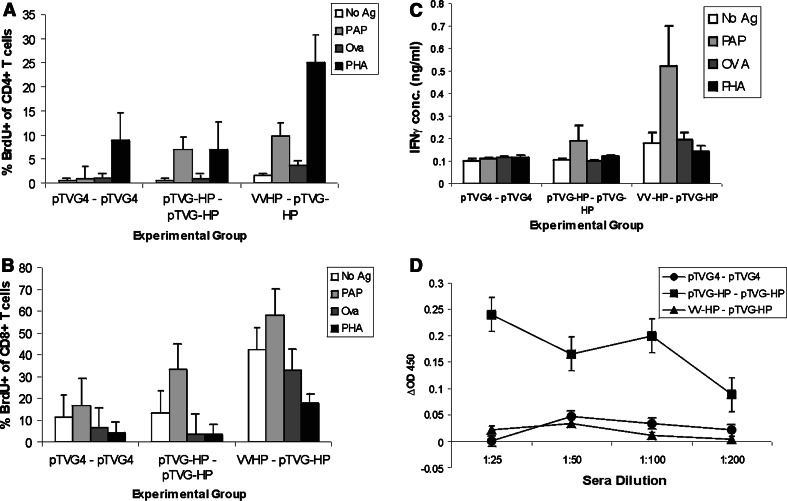
Male Lewis rats were then immunized twice with plasmid DNA encoding hPAP (pTVG-HP), or DNA following a priming immunization with VV-HP, or with vector DNA (pTVG4). Fourteen days after the second immunization, animals were assessed for CD4+ or CD8+ T cell proliferation, or antibody responses specific for PAP, as described above (Fig. 2). As shown, animals immunized with pTVG-HP twice, or following VV-HP immunization, had detectable PAP-specific CD4+ and CD8+ T cells (Fig. 2a, b). Supernatants from these cell cultures were assayed for IFNγ, and PAP-specific IFNγ release was detected following two immunizations with pTVG-HP or with VV-HP followed by pTVG-HP immunization (Fig. 2c). No antigen-specific IL-10 release was detectable (data not shown). Low titer PAP-specific IgG were detected after two immunizations with plasmid DNA encoding PAP, but not after a single immunization with VV-HP followed by a booster immunization with pTVG-HP (Fig. 2d). IgG subtype analysis showed this to be a mixed response, predominantly IgG1, but also IgG2a and IgG2b (data not shown). At least two immunizations with pTVG-HP appeared necessary to elicit a T cell immune response, as a single immunization did not elicit a detectable antigen-specific T cell response (Fig. 3).
Fig. 2.
Immunization of male Lewis rats with plasmid DNA encoding hPAP elicits PAP-specific T cell immunity without the need for heterologous immunization strategies. Male Lewis rats were immunized intradermally twice at a 14-day interval with 100 μg pTVG4 or pTVG-HP. An additional group was immunized subcutaneously with 107 pfu VV-HP and boosted with 100 μg pTVG-HP (VV-HP–pTVG-HP). Five micrograms GM-CSF protein was co-administered with plasmid DNA as a vaccine adjuvant. CD4+ T cell proliferation (a), CD8+ T cell proliferation (b), and IgG responses specific for PAP (d), were evaluated as in Fig. 1. Supernatants from the splenocyte-antigen cultures were evaluated for IFNγ by quantitative ELISA (c). The data show the mean ± SE of independent analyses for each of three animals per experimental group from a single study, and are representative of 4 independent, replicate experiments for a total of 12 animals per each treatment group
Fig. 3.
Immunization of male Lewis rats with a single DNA immunization did not elicit antigen-specific immune responses. Male Lewis rats (n = 3) were immunized once with 100 μg pTVG4 or pTVG-HP and splenocytes were collected 28 days later. Antigen-specific CD4+ (a) or CD8+ T cell (b) proliferation were evaluated as in Fig. 1. Supernatants from the splenocyte-antigen cultures were evaluated for IFNγ by quantitative ELISA (c). The data show the mean ± SE of independent analyses for each of three animals per experimental group from a single study, and are representative of two independent replicate experiments
Multiple immunizations of DNA vaccines encoding the rPAP antigen elicit PAP-specific CD4+ and CD8+ T cells
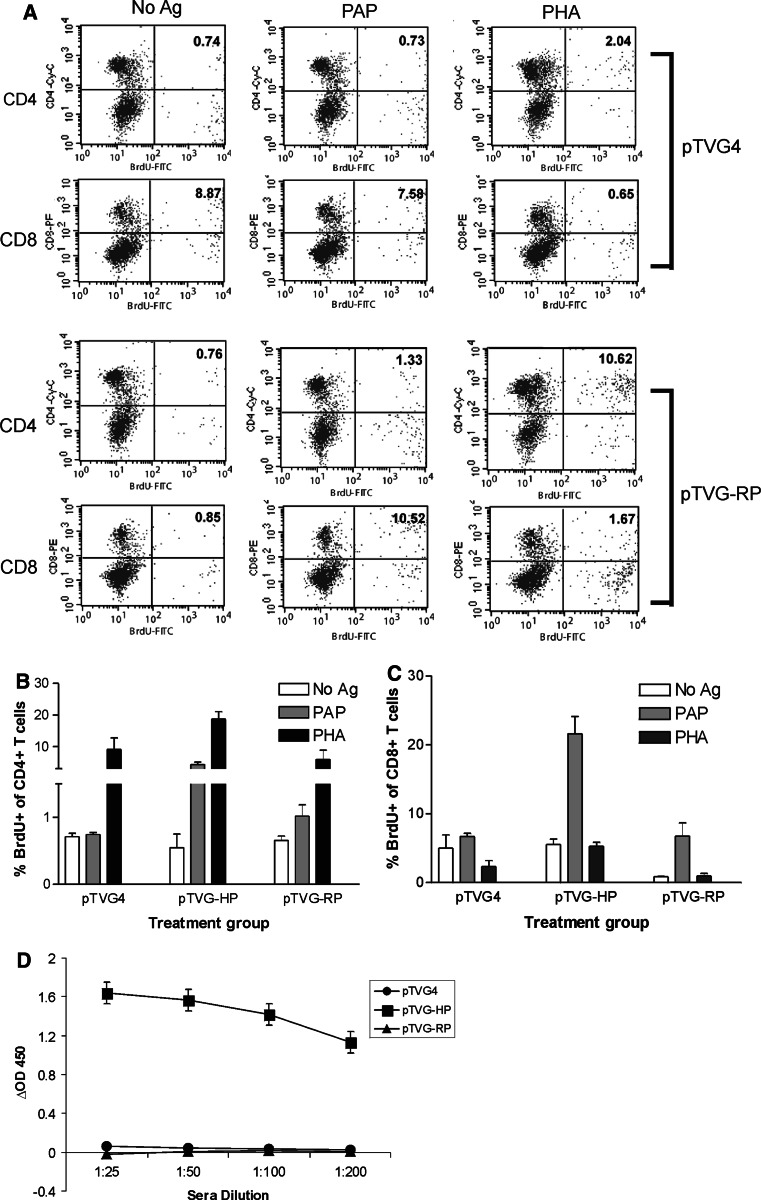
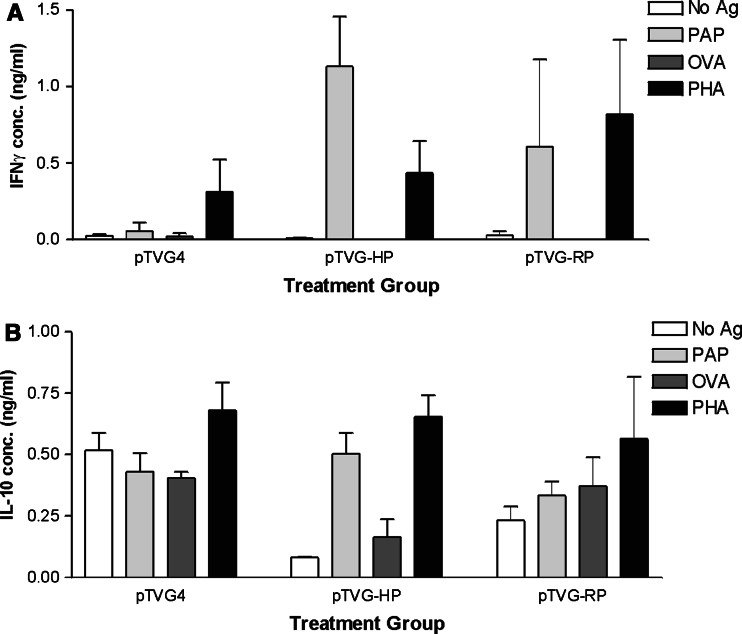
The studies above used the human homologue of PAP (hPAP), which is approximately 80% identical to the rat homologue (rPAP) at the amino acid level. Therefore, we next questioned whether T cell responses to PAP could similarly be elicited by immunization with the DNA plasmid encoding the native rPAP antigen, without the need for immunization with the human xenoantigen. In multiple experiments, we could not detect PAP-specific T cell responses or IgG responses in animals immunized twice with an identical DNA construct encoding rPAP, pTVG-RP (data not shown). However, with six repetitive immunizations at 2-week intervals with pTVG-RP, we detected significant CD4+ and CD8+ proliferative T cell responses specific for the hPAP antigen in approximately half (15 of 27) of the immunized animals (Fig. 4, and data not shown). Notably, high-titer IgG antibodies specific for hPAP could only be detected in animals immunized with pTVG-HP, but not pTVG-RP (Fig. 4d). However, low-titer IgG antibodies could be detected in a few animals immunized multiple times with pTVG-RP (not shown). The response following immunization with pTVG-RP was found to be predominantly Th1 in type, as assessed by IFNγ-specific release, rather than IL-10 (Fig. 5). Of note, repetitive immunization with the DNA construct encoding hPAP did elicit a mixed T cell response, with antigen-specific IFNγ and IL-10 secretion observed, as shown in Fig. 5a and b, and as we have previously demonstrated [17]. The response observed following immunization with pTVG-RP did not appear to be strictly dose-dependent, as similar frequencies of T cell or antibody responses were seen with multiple immunizations with 100 or 500 μg of plasmid DNA (data not shown).
Fig. 4.
Immunization of male Lewis rats with DNA vaccines encoding rPAP elicits PAP-specific CD4+ and CD8+ T cells. Splenocytes were obtained from animals immunized six times at 2-week intervals with pTVG4, pTVG-HP, or pTVG-RP. Splenocytes were co-cultured with hPAP protein (2 μg/ml), PHA (10 μg/ml), or no antigen, and assessed as above for CD4+ or CD8+ T cell proliferation and cytokine secretion. In a an example of the analysis with splenocytes obtained from two animals immunized with either pTVG4 or pTVG-RP is demonstrated. Specifically, shown are plots of CD4+-Cy-chrome/BrdU-FITC or CD8+-PE/BrdU-FITC gated on live lymphocyte scatter. The numbers represent the percentage of total CD4+ or CD8+ events that costained with BrdU. A compilation of data for all animals immunized in a single study is shown in b (CD4+ T cell proliferation) and c (CD8+ T cell proliferation). Sera from immunized animals were also evaluated by ELISA for hPAP-specific IgG, as above (d). As in the previous figures, data in b, c, d show the mean ± SE of independent analyses for each of 3 animals per experimental group, and are representative of three independent, replicate experiments using 3–6 animals per experimental group for a total of 15 rats per treatment group
Fig. 5.
Multiple immunizations with a DNA vaccine encoding the rPAP antigen elicit antigen-specific IFNγ secretion. Splenocytes from animals immunized six times at 2-week intervals with pTVG4, pTVG-HP, or pTVG-RP were cultured in the presence of hPAP (PAP), ovalbumin (OVA), PHA, or media only as above. Supernatants were then evaluated by quantitative ELISA for IFNγ (a) or IL-10 (b) concentrations as indicated. The data shows the mean and standard deviation of three animals per group from a single study, and are representative of three, independent replicate experiments
The previous studies used the hPAP protein as the antigen for the in vitro analysis, given the high homology of the human and rat homologues, and the absence of a commercially available source of rPAP protein. To confirm that immunization with pTVG-RP elicited a rPAP-specific T cell response, splenocytes from immunized animals found to have T cell proliferative responses specific for hPAP were cultured in the presence of syngeneic dendritic cells transduced to express either hPAP or rPAP. As shown in Fig. 6, immunization with pTVG-RP elicited a rPAP-specific T cell response as indicated by rPAP-specific IFNγ production (P = 0.057) and a cross-reactive immune response to hPAP (P = 0.083) as shown by hPAP-specific IFNγ production. The response following immunization with pTVG-HP was predominantly to hPAP (P = 0.042) rather than rPAP (P = 0.39) as identified by IFNγ production.
Fig. 6.
Immunization with pTVG-RP elicits rPAP-specific T cell responses. Splenocytes were evaluated from animals previously determined to have an hPAP-specific immune response following six immunizations at 2-week intervals with 100 μg of pTVG-HP (n = 3) or 500 μg of pTVG-RP (n = 3). Splenocytes were also evaluated from control animals from the same experiment immunized six times at 2-week intervals with 100 μg of pTVG4 vector (n = 3). Splenocytes were assessed for IFNγ production following a 72 h co-culture with a 1:10 ratio of lentiviral-GFP-transduced DCs (DC-GFP), lentiviral-hPAP-transduced DCs (DC-hPAP), lentiviral-rPAP-transduced DCs (DC-rPAP), or 10 μg/ml PHA. Supernatants of the cultures were evaluated for IFNγ concentration by quantitative ELISA. The data indicates the mean IFNγ concentration and standard deviation from the cultures of 3 animals per group, and is representative of data from replicate experiments testing splenocytes from 15 pTVG-RP immunized rats
Discussion
At present, several tumor vaccines are entering clinical trials for patients with prostate cancer. These trials are using a variety of immunization strategies and targeting several prostate tumor antigens [1, 21]. In particular, there has been much interest in the use of genetic immunization strategies using viral vectors. Multiple clinical trials using vaccinia-based vaccines encoding human PSA have suggested that patients can develop PSA-specific T cells and may derive clinical benefit [12–14, 22]. In addition, it has previously been shown in animal studies that vaccinia encoding another prostate-specific protein, hPAP, can elicit antigen-specific CTL with evidence of autoimmune prostate tissue inflammation [10]. While vaccinia has been shown to be a potent immunization vector, some of these studies have highlighted difficulties with multiple immunizations with this vector due to the development of potent vaccinia-specific immunity, thus prompting heterologous vaccination approaches. In the current study, we evaluated another method of genetic immunization, using plasmid DNA as the vector, and compared this with a vaccinia-based method of immunization. We report that: (1) in this Lewis rat model, immunization with vaccinia virus encoding hPAP did not elicit PAP-specific immunity unless the immunization was boosted with a heterologous immunization approach, (2) immunization of Lewis rats with DNA encoding hPAP was able to induce PAP-specific CD4+ and CD8+ T cells, Th1/Tc1 in type, and (3) multiple immunizations with plasmid DNA encoding the autologous rat PAP antigen results in a PAP-specific immune response without the need for a heterologous or xenogeneic immunization approach. These findings suggest that plasmid DNA vaccines encoding PAP may have distinct advantages, and that multiple immunizations may be sufficient to elicit an immune response to this autologous antigen. Thus, DNA vaccines encoding PAP could be investigated in clinical trials for patients with prostate cancer.
In the current study, we report that immunization of Lewis rats with vaccinia virus encoding hPAP did not develop PAP-specific cellular or humoral immunity after two immunizations; however, a strong vaccinia-specific immune response was elicited (data not shown). This is different from the findings of Fong and colleagues [10] who demonstrated that a single immunization of Copenhagen rats with vaccinia virus encoding hPAP was capable of eliciting PAP-specific cellular immune responses, including cross-reactivity to the rPAP protein. The difference in observed results may be due to several differences in the model systems used. For example, our vaccinia strain was a clonal strain selected based on high levels of PAP expression following infection of a reporter cell line, and therefore may differ in terms of antigen expression to the clone used in the previous study. Certainly, differences in antigen expression might account for some variation in the observed results. In addition, the route of administration of the vaccinia-based vaccine, subcutaneous immunization in our studies versus intravenous administration in the studies of Fong, might also account for differences in immune responses observed. Finally, the different rodent strains used may have influenced the end results. The studies of Fong and colleagues were performed in the Copenhagen strain. We chose to use the Lewis strain given that it has been used as a model to investigate other experimental autoimmune diseases, and because the MHC determinants of this strain are better characterized and may permit future studies of T cell epitopes specifically recognized. Certainly the variation in MHC type between the rat strains could account for the differences in antigen-specific immune responses observed due to differences in antigen processing and presentation of different epitopes. This is potentially relevant to human vaccine studies, as clearly most rodent vaccine studies are conducted in a limited number of syngeneic strains and may not be representative of human trials with subjects of diverse MHC types. Regardless, our model demonstrates that vaccinia virus, while a potent immunization strategy capable of eliciting immune responses within a short period of time, did not elicit robust antigen-specific immunity. The findings may suggest that immune responses to the vector itself overwhelmed responses to the targeted antigen. However, it is likely that immune responses to PAP were in fact elicited but below our level of detection. In support of this, our findings indicate that immunization with VV-HP followed by immunization with pTVG-HP could elicit PAP-specific T cells (Fig. 2), while a single immunization with DNA was not able to elicit a detectable immune response (Fig. 3). This suggests that, in fact, VV-HP was capable of at least priming an immune response.
Our studies further demonstrate that in the Lewis rat model immunization with plasmid DNA encoding human PAP could elicit PAP-specific CD4+ and CD8+ T cells, Th1/Tc1 in type. Unfortunately, there is no prostate cancer cell line derived from the Lewis rat strain to permit an in vivo assessment of the ability of these cells to lyse prostate cancer cells. In future studies we consequently plan to explore the ability of DNA vaccines encoding PAP to elicit CD8+ T cells capable of lysing the aggressive Mat-Lu prostate cancer cell line in the Copenhagen rat strain. However, qualitatively, the CD8+ T cell responses elicited are similar to the responses observed by Fong [10] after vaccinia-based immunization and similar to the responses we observed with vaccinia immunization followed by DNA immunization. Theoretically, a vaccine approach capable of eliciting an inflammatory type of immune response should be more effective as an anti-tumor vaccination approach. These findings are not novel, as many groups have demonstrated in rodents and humans that DNA vaccines are able to elicit antigen-specific CD8+ T cells [23–25]. Many groups are exploring the use of DNA vaccines as a means of boosting immune responses elicited by a viral-based immunization, as these approaches may well be complementary [26, 27]. Of note, however, we did observe that multiple immunizations with the DNA vaccine encoding the human homologue elicited an IL-10 response, consistent with a possible tolerizing response. This was not seen using the DNA vaccine encoding the autologous rat antigen. It is unknown at present if continued repetitive booster immunizations with the pTVG-RP would elicit a similar response. This will be an important parameter to monitor in clinical trials using DNA vaccines as anti-tumor treatments, because it would theoretically not be desirable to elicit tumor antigen-specific tolerant or regulatory T cell responses.
Overall, our studies demonstrate that multiple immunizations with plasmid DNA encoding PAP can be administered. The magnitude of the immune response, as measured by antibody titers, suggests that the immune response elicited by DNA vaccines can continue to be boosted with repetitive immunization (Figs. 2, 4) [17]. It has been suggested that DNA vaccines are less potent than viral vaccines, and that strategies need to be developed to increase their potency for their use in human application [28]. Most human trials with DNA vaccines, however, while demonstrating safety, have used a limited number of immunizations. Our findings suggest that multiple immunizations can be given to boost immune responses to the encoded antigen, and notably to the “self” rPAP antigen without requiring immunization with the foreign hPAP xenoantigen.
In conclusion, the findings in this study indicate that DNA vaccines provide a means to generate PAP-specific CD8+ T cells and that PAP-specific immune responses can be boosted with multiple immunizations of a DNA vaccine. Importantly, we found that multiple immunizations with a DNA vaccine encoding autologous rPAP lead to a Th1-type immune response. Thus in this model, multiple immunizations with a DNA vaccine encoding an autologous antigen appears to be a feasible approach to elicit a PAP-specific Th1/CD8+ T cell response. Overall, our findings would support the evaluation of DNA vaccines encoding PAP in vaccine trials for patients with prostate cancer. A pilot trial evaluating the ability of pTVG-HP to elicit IFNγ-secreting CD8+ T cells in patients with prostate cancer is currently underway [29].
Acknowledgments
This work is supported for L.E.J. by the DOD Prostate Cancer Research Program (W81XWH-04-1-0256) and for D.G.M. by NIH (K23 RR16489) and the DOD Prostate Cancer Research Program (DAMD17-03-1-0050).
Abbreviations
- BrdU
Bromodeoxyuridine
- BSA
Bovine serum albumin
- CFA
Complete Freund’s adjuvant
- CTL
Cytotoxic T lymphocytes
- DC
Dendritic cell
- EDTA
Ethylenediaminetetraacetic acid
- ELISA
Enzyme-linked immunosorbent assay
- GFP
Green fluorescent protein
- GM-CSF
Granulocyte macrophage colony-stimulating factor
- hPAP
Human prostatic acid phosphatase
- IFA
Incomplete Freund’s adjuvant
- IFNγ
Interferon-gamma
- IgG
Immunoglobulin G
- IL-10
Interleukin-10
- MHC
Major histocompatibility complex
- MOI
Multiplicity of infection
- OD
Optical density
- PAP
Prostatic acid phosphatase
- PBS
Phosphate buffered saline
- pfu
Plaque-forming unit
- PHA
Phytohemaglutinin
- pTVG-HP
DNA vaccine encoding hPAP
- pTVG-RP
DNA vaccine encoding rPAP
- rPAP
Rat prostatic acid phosphatase
- SE
Standard error
- TMB
Tetramethylbenzidine
- VV-HP
Recombinant vaccinia virus encoding hPAP
- VVwt
Wild type vaccinia virus
References
- 1.McNeel DG (2005) Prostate cancer antigens and vaccines, preclinical developments. In: Giaccone G, Schilsky R, Sondel P (eds) Cancer chemotherapy biological response modifiers Elsevier, Amsterdam, pp 247–261 [DOI] [PubMed]
- 2.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, Whiteside TL, Schlom J, Wilding G, Weiner LM. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 3.Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 4.Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate. 1998;36:129–138. doi: 10.1002/(SICI)1097-0045(19980701)36:2<129::AID-PROS8>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 5.McNeel DG, Nguyen LD, Disis ML. Identification of T helper epitopes from prostatic acid phosphatase. Cancer Res. 2001;61:5161–5167. [PubMed] [Google Scholar]
- 6.McNeel DG, Nguyen LD, Ellis WJ, Higano CS, Lange PH, Disis ML. Naturally occurring prostate cancer antigen-specific T cell responses of a Th1 phenotype can be detected in patients with prostate cancer. Prostate. 2001;47:222–229. doi: 10.1002/pros.1066. [DOI] [PubMed] [Google Scholar]
- 7.Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk-Pavlovic S. Priming tissue-specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000;6:2175–2182. [PubMed] [Google Scholar]
- 8.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 9.Fong L, Brockstedt D, Benike C, Breen JK, Strang G, Ruegg CL, Engleman EG. Dendritic Cell-Based Xenoantigen Vaccination for Prostate Cancer Immunotherapy. J Immunol. 2001;167:7150–7156. doi: 10.4049/jimmunol.167.12.7150. [DOI] [PubMed] [Google Scholar]
- 10.Fong L, Ruegg CL, Brockstedt D, Engleman EG, Laus R. Induction of tissue-specific autoimmune prostatitis with prostatic acid phosphatase immunization; implications for immunotherapy of prostate cancer. J Immunol. 1997;159:3113–3117. [PubMed] [Google Scholar]
- 11.Hodge JW, Schlom J, Donohue SJ, Tomaszewski JE, Wheeler CW, Levine BS, Gritz L, Panicali D, Kantor JA. A recombinant vaccinia virus expressing human prostate-specific antigen (PSA): safety and immunogenicity in a non-human primate. Int J Cancer. 1995;63:231–237. doi: 10.1002/ijc.2910630215. [DOI] [PubMed] [Google Scholar]
- 12.Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, Milenic D, Panicali D, Montie JE. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/S0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 13.Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 14.Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, Michael Hamilton J. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 15.Ciernik IF, Berzofsky JA, Carbone DP. Induction of cytotoxic T lymphocytes and antitumor immunity with DNA vaccines expressing single T cell epitopes. J Immunol. 1996;156:2369–2375. [PubMed] [Google Scholar]
- 16.Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LE, Frye TP, Arnot AR, Marquette C, Couture LA, Gendron-Fitzpatrick A, McNeel DG. Safety and immunological efficacy of a prostate cancer plasmid DNA vaccine encoding prostatic acid phosphatase (PAP) Vaccine. 2006;24:293–303. doi: 10.1016/j.vaccine.2005.07.074. [DOI] [PubMed] [Google Scholar]
- 18.Chinnasamy D, Fairbairn LJ, Neuenfeldt J, Treisman JS, Hanson JP, Jr, Margison GP, Chinnasamy N. Lentivirus-mediated expression of mutant MGMTP140K protects human CD34+ cells against the combined toxicity of O6-benzylguanine and 1,3-bis(2-chloroethyl)-nitrosourea or temozolomide. Hum Gene Ther. 2004;15:758–769. doi: 10.1089/1043034041648417. [DOI] [PubMed] [Google Scholar]
- 19.Choe BK, Pontes EJ, Bloink S, Rose NR. Human prostatic acid phosphatases: I. Isolation. Arch Androl. 1978;1:221–226. doi: 10.3109/01485017808988340. [DOI] [PubMed] [Google Scholar]
- 20.McNeel DG, Nguyen LD, Storer BE, Vessella R, Lange PH, Disis ML. Antibody immunity to prostate cancer-associated antigens can be detected in the serum of patients with prostate cancer. J Urol. 2000;164:1825–1829. doi: 10.1016/S0022-5347(05)67114-5. [DOI] [PubMed] [Google Scholar]
- 21.McNeel DG, Malkovsky M. Immune-based therapies for prostate cancer. Immunol Lett. 2005;96:3–9. doi: 10.1016/j.imlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Arlen PM, Gulley JL, Parker C, Skarupa L, Panicali D, Beetham P, Palena C, Tsang KY, Schlom J, Dahut W. A pilot study of concurrent docetaxel plus PSA pox-vaccine versus vaccine alone in metastatic androgen independent prostate cancer (AIPC) Proc Am Soc Clin Oncol. 2003;22:1701. [Google Scholar]
- 23.Corr M, Lee DJ, Carson DA, Tighe H. Gene vaccination with naked plasmid DNA: mechanism of CTL priming. J Exp Med. 1996;184:1555–1560. doi: 10.1084/jem.184.4.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy MJ, Wu MS, Barr LJ, Fuller JT, Tussey LG, Speller S, Culp J, Burkholder JK, Swain WF, Dixon RM, Widera G, Vessey R, King A, Ogg G, Gallimore A, Haynes JR, Heydenburg Fuller D. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764–778. doi: 10.1016/S0264-410X(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 25.Wang R, Epstein J, Baraceros FM, Gorak EJ, Charoenvit Y, Carucci DJ, Hedstrom RC, Rahardjo N, Gay T, Hobart P, Stout R, Jones TR, Richie TL, Parker SE, Doolan DL, Norman J, Hoffman SL. Induction of CD4(+) T cell-dependent CD8(+) type 1 responses in humans by a malaria DNA vaccine. Proc Natl Acad Sci USA. 2001;98:10817–10822. doi: 10.1073/pnas.181123498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsay AJ, Kent SJ, Strugnell RA, Suhrbier A, Thomson SA, Ramshaw IA. Genetic vaccination strategies for enhanced cellular, humoral and mucosal immunity. Immunol Rev. 1999;171:27–44. doi: 10.1111/j.1600-065X.1999.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 27.Mincheff M, Tchakarov S, Zoubak S, Loukinov D, Botev C, Altankova I, Georgiev G, Petrov S, Meryman HT. Naked DNA and Adenoviral Immunizations for Immunotherapy of Prostate Cancer: a Phase I/II Clinical Trial. Eur Urol. 2000;38:208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 28.Liu MA. DNA vaccines: a review. J Intern Med. 2003;253:402–410. doi: 10.1046/j.1365-2796.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 29.Zlotocha S, Staab MJ, Horvath D, Straus J, Dobratz J, Oliver K, Wasielewski S, Alberti D, Liu G, Wilding G, Eickhoff J, McNeel DG. A phase I study of a DNA vaccine targeting prostatic Acid phosphatase in patients with stage D0 prostate cancer. Clin Genitourin Cancer. 2005;4:215–218. doi: 10.3816/CGC.2005.n.036. [DOI] [PubMed] [Google Scholar]