Abstract
Introduction
Amino-bisphosphonates are potent activators of human γδ T cells. The aim of our study was to evaluate the immunomodulating properties of a single-dose of zoledronic acid (ZA) on γδ T cells in a select group of disease-free breast cancer patients with osteopenia.
Materials and methods
Blood samples were obtained, from 23 patients, before and 7, 28, 56, 90 and 180 days after a single-dose (4 mg) of ZA and analyzed by flow cyometry.
Results
A significant decrease of the different γδ T cell subsets was observed: Naïve (CD3+/Vdelta2+/CD45RA+/CD27+) after 180 days (P < 0.01); Central Memory (CD3+/Vdelta2+/CD45RA-CD27+) after 28 (P < 0.05), 90 (P < 0.01) and 180 days (P < 0.01); and Effector Memory (CD3+/Vdelta2+/CD45RA-/CD27-) after 56 (P < 0.01) and 90 (P < 0.05) days. Based on the observed γδ T cells kinetics patients could be divided in two groups: “responders” that showed a significant decrease in total numbers of γδ T cells and “non-responders” that showed no significant change. However, in vitro phosphoantigen stimulation of patients cells did not show significant differences in terms of IFN-γ response by Vδ2 T cells.
Conclusion
We describe for the first time a long-lasting activation of effector subsets of γδ T cells in disease-free breast cancer patients after a single-dose of ZA. Our results highlight the need to further investigate the clinical significance of the immunomodulating properties of N-BPs.
Keywords: γδ T cells, Zoledronic acid, Bisphosphonates, Cancer patients
Introduction
Bisphosphonates (BPs) are analogs of endogenous pyrophosphates (PPi) in which a carbon atom replaces the central oxygen atom. Based on their chemical structure they are traditionally divided into two pharmacological classes with distinct molecular mechanisms of action: nitrogen-containing (N-) and non-nitrogen (non-N) containing bisphosphonates. Zoledronic acid (ZA) is a newer amino-bisphosphonate with a tertiary amino group included within a ring structure. It can be considered the most potent and widely used intravenous bisphosphonate with the broadest clinical activity in terms of prevention or delayed onset of skeletal complications and also treatment of hypercalcemia of malignancy [1, 2]. ZA is approved for the treatment of patients with bone metastases from breast cancer, hormone-refractory prostate cancer, as well as, other solid tumors and multiple myeloma [3–5].
Amino-bisphosphonates (N-BPs) act at molecular level by inhibiting the mevalonate pathway [6] with a consequent accumulation of phosphoantigen isopentenyl-diphosphate (IPP) and inhibition of GTPases prenylation [7].
Extensive in vitro evidence suggests a direct antitumor effect of BPs exerted at different levels [8]. Another interesting aspect concerns the immunomodulating properties of BPs on γδ T cells. γδ T cells constitute a separate lineage of T lymphocytes (about 1–5% of peripheral blood T cells). More than 70% of γδ T cells in the peripheral blood and lymphoid organs use the TCR region pair Vγ9-Vδ2 [9]. Antigen recognition by γδ T cells is TCR mediated and crucially lead to their activation [10]. A variety of non-peptide antigens, including N-BPs have been shown to activate Vγ9/Vδ2 T cells [11].
In vivo studies have demonstrated the presence of γδ T cells among tumor-infiltrating lymphocytes of a variety of tumors, including lung cancer [12], renal cell carcinoma [13], seminoma [14] and breast cancer [15]. Vγ9/Vδ2 T cell are able to recognize several tumor-associated ligands such as HSP-60 [16] and those derived from Daudi Burkitt’s lymphoma [17] and glial cells [18]. Vγ9/Vδ2 T cells have been shown to exert tumor cytotoxicity as demonstrated by their ability to lyse glioblastoma [19], neuroblastoma [20], colon [21] and lung [22] carcinoma cells. More recently, Kunzmann et al. demonstrated that N-BPs are potent activators of human γδ T cells both in vitro [23] and in vivo [24]. In fact, N-BPs such as alendronate, ibandronate and pamidronate were able to induce a dose-dependent activation and expansion of γδ T cells in primary peripheral blood mononuclear cell (PBMC) cultures of healthy donors while non-N-BPs were unable to do so [25]. Dieli et al. reported that ZA is able to activate γδ T cell effector functions in patients with metastatic solid tumors. In this report, the authors found that in vivo treatment with ZA induced Vγ9/Vδ2 cells toward an IFNγ-producing effector phenotype, which might induce a more effective antitumor response [26]. Based on the diverse phenotype as well as effector functions, Dieli et al. identified different subsets of human γδ T cells: Central Memory [CM] γδ T cells which are proliferative; Effector Memory [EM] γδ T cells are IFN-γ producing and Terminal Effectors [TE] γδ T cells have cyotoxic functions. In addition, upon in vitro culture with IPP, in the presence of IL-2 for 12 days, a differentiation pathway from naїve to CM and EM (CD45RA+CD27+→ CD45RA–CD27+→ CD45RA–CD27–) has been demonstrated for Vγ9 Vδ2 T lymphocytes [27].
Based on these studies and on the immunomodulating role of bisphosphonates [28], we conducted an observational perspective study to evaluate the immunomodulating properties of a single-dose of ZA on γδ T cells in early breast cancer patients without any evidence of macroscopic disease.
Materials and methods
Study design
Patients received a single-dose of ZA 4 mg in 100 mL Normal Saline (15 min infusion). Peripheral blood was drawn previous to and after 7, 28, 56, 90 and 180 days ZA treatment.
The primary end point of this study was to determine γδ T lymphocyte absolute numbers and percentage modifications after a single-dose of ZA. The secondary objective was to evaluate modifications induced by a single-dose of ZA on the different subsets of Vδ2 T lymphocytes. After zoledronic acid treatment absolute number and percentage of the following lymphocyte subsets were also evaluated: CD3+, CD4+, CD8+ T lymphocytes; CD19+ B lymphocytes, CD56+ Natural Killer lymphocytes.
Patient’s characteristics
Twenty-three patients were enrolled in the study from April 2005 to May 2006. The clinical features of these patients are listed in Table 1. The median age was 66 years (range 47–85 years) and the median time for patient enrolment was 37 months after breast surgery (range 12–125 months). Breast cancer was classified according to the TNM staging system.
Table 1.
Main clinical characteristics of the enrolled patients
| Characteristics | No patients (%) |
|---|---|
| Total number | 23 |
| Age (years) | |
| Median, range | 66, 47–85 |
| Tumor staging | |
| T in situ | 2 (8.7) |
| T1 | 15 (65.2) |
| T2 | 4 (17.4) |
| T3 | 1 (4.3) |
| T4 | 1 (4.3) |
| N0 | 16 (69.6) |
| N+ | 7 (31.4) |
| M0 | 23 (100) |
| M+ | 0 (0) |
| Enrollment time (months) | |
| Median, range | 37, 12–125 |
| Acute phase reaction | |
| Fever ≥ 38º | 8 (34.8) |
| Myalgia/arthralgia. | 16 (69.5) |
The study was approved by the local Ethical Committee and informed consent was obtained by each patient. The inclusion criteria consisted of: radical surgery for breast cancer, no evidence of any sign of macroscopic tumor disease, osteopenia demonstrated by DEXA (Total T score ≤ −2), clinical and biochemical menopause (for at least 1 year), treatment with aromatase inhibitors for at least 1 year (but less than 2 years), normal renal and liver functions, normal blood cell count (Platelets > 100 × 109/L, Leukocytes > 1.5 × 109/L). All patients were included at least 1 year after the last day of chemotherapy, radiotherapy, immunotherapy or use of haematopoietic growth factors, at least 6 months after corticosteroid treatment and at least 2 weeks after any sign of infection (fever > 38ºC). The exclusion criteria consisted of: acute or chronic infective disease, acute or chronic inflammatory disease, disease relapse, any therapy modifications, a prior treatment with any BPs.
Multiparameter flow cytometric analysis
Anticoagulated (EDTA) peripheral whole blood samples were used to perform the analysis. For lymphocyte subpopulations a panel of four murine monoclonal antibodies (Cyto-Stat Tetrachrome, Beckman-Coulter) each conjugated to a specific fluorochrome (CD45-FITC/CD4-RD1/CD8-ECD/CD3-PC5) and (CD45-FITC/CD56-RD1/CD19-ECD/CD3-PC5) was used. Cell staining was done by adding 10 μL of Cyto-Stat Tetrachrome reagent to 100 μL of whole blood followed by a 20 min incubation at room temperature. Red blood cells were lysed with Ammonium Chloride (8 g/L H2O). Samples were analyzed with the aid of Coulter Epics XL-MCL Flow Cytometry Systems (Beckman-Coulter). For either reagent a two-parameter CD45-FITC (FL1 LOG) versus Side Scatter histogram to identify lymphocytes (Gate A) was used. Additional histograms were used to determine the percentage of positively stained cells. The frequencies of CD45+/CD3+ (pan T lymphocytes), CD45+/CD3+/CD4+ (lymphocytes Th), CD45+/CD3+/CD8+ (Tc plus Ts), CD45+/CD3−/CD19+ (B lymphocytes), CD45+/CD3−/CD56+ (NK cells) were evaluated. For γδ T lymphocyte subsets, 50 μL of peripheral blood collected in EDTA (anticoagulant) tubes were stained with 10 μL of either a mixture of Vδ2-FITC/CD27-PE/CD45RA-CY5/CD3−APC or Vδ1-FITC/CD27-PE/CD45RA-CY5/CD3-APC all from Becton-Dickinson and then incubated for 20 min at room temperature according to the manufacturer’s instructions. Red blood cells were lysed using 1 mL of Lysis Buffer (Becton-Dickinson). Samples were analyzed using a FACS-Calibur (Becton-Dickinson) flow cytometry system. Frequency of CD3+/Vδ1+(Vδ1+ T lymphocytes), CD3+/Vδ2+ (Vδ2+ T lymphocytes) and the following Vdelta2 subsets CD3+/Vδ2+/CD45RA+/CD27+ (Naïve [N]), CD3+/Vδ2+/CD45RA−CD27+ (Central Memory [CM]), CD3+/Vδ2+/CD45RA−/CD27− (Effector Memory [EM]), CD3+/Vδ2+/CD45RA+/CD27− (Terminal Effectors [TE]) were evaluated. Absolute numbers of the different T lymphocytes subsets is based on the total lymphocyte count from the peripheral blood.
In vitro stimulation with phosphoantigen
In order to assess the Vδ2 peripheral T cells responsiveness to phosphoantigens, an in vitro stimulation assay by using a phosphoantigen (Picostim) was performed.
Anticoagulated (EDTA) peripheral whole blood samples were used and red blood cells were lysed with lysing buffer (BD). Samples were washed twice in PBS and once in RPMI 1,640 media supplemented with 10% CFS and antibiotics.
Cells were dispensed in flat-bottomed 96 wells microplates (200 μL/well). Wells were set-up in presence of Brefeldin (Sigma, 10 mg/mL) and the following stimuli were added: culture medium (tc), phosphoantigen (Picostim, pic, 20 nM), ZA (zol 2 μM), and Phorbol Myristic Acetate (PMA)+Ionomycin (PMA, Sigma, 50 ng/mL, 1 mg/mL). The plates were kept overnight in a CO2 incubator at 37°C, washed and stained (15 min at 4°C in dark) using the following monoclonal antibodies against cell surface antigens: Vdelta2 (clone B6, FITC, BD), CD27 (Clone M-T271, PE, BD), CD45RA (Clone HI100, Cy-5, BD). For intracytoplasmic for IFN-γ expression-cells were first permeabilized using saponin (0.5% Saponin, Sigma) and stained with IFN-γ APC (Clone K3, BD). Samples were acquired by a FACS-Calibur instrument (BD) by using CellQuest software (BD). At least 200,000 lymphocyte events were collected.
Statistical analysis
The statistical analysis was performed using GraphPad Prism Ver.4.0 (GraphPad Software, Inc., San Diego, CA, USA). Wilcoxon Matched-Pairs Signed-Ranks Test was used to assess differences among means regarding lymphocyte kinetics. The differences between patient groups were compared using Mann–Whitney test. The level of significance was set at less than 0.05.
Results
Effects of ZA on total lymphocyte subsets
We evaluated the kinetics of the different lymphocyte subsets previous to and after 7, 28, 56, 90 and 180 days ZA treatment. The absolute numbers of total lymphocytes were assessed based on the total white blood cell count and that of the different subsets was determined based on the percentage expression of the cell surface markers (CD3+ for T lymphocytes, CD4+ for T helper lymphocytes, CD8+ for T cytotoxic and suppressor lymphocytes, CD19+ for B lymphocytes, CD56+ for Natural Killer lymphocytes). Treatment with a single-dose of ZA in vivo did not cause significant modifications either in the absolute number of total lymphocytes or of the different subsets at any time-point compared with the basal value.
Effects of ZA on γ/δ T lymphocytes
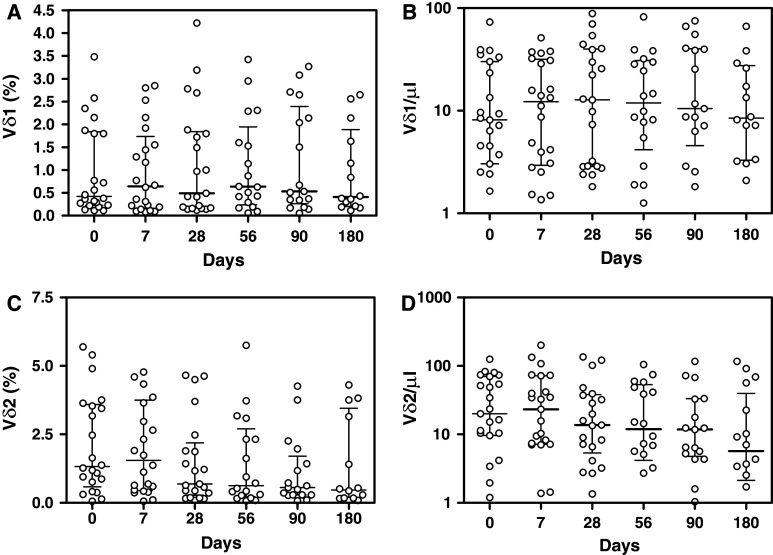
We next analyzed the changes in the percentages and absolute numbers of Vδ2 T lymphocytes before and 7, 28, 56, 90 and 180 days after ZA treatment. We did not find significant variations after ZA treatment at any timepoint compared with the basal value. Figure 1 shows kinetics of percentage and absolute numbers of Vδ1 and Vδ2 T lymphocytes after the single-dose of ZA.
Fig. 1.
Percentage (a) and absolute number (b) of CD3+/Vδ1 T lymphocytes and percentage (c) and absolute number (d) of CD3+/Vδ2 T lymphocytes before and 7, 28, 56, 90 and 180 days after treatment with zoledronic acid. Columns indicate median and interquartil-range (IQR) of each group. Wilcoxon Matched-Pairs Signed-Ranks Test did not show significativity for any parameter considered compared with the basal value
Effects of ZA on Vδ2 T lymphocyte differentiation
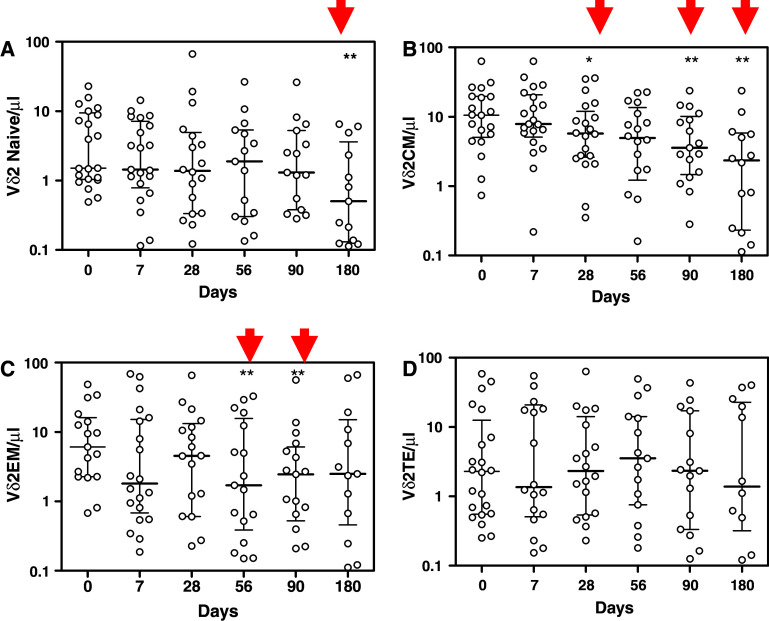
To investigate the ability of ZA to induce activation of γδ T cells and their differentiation from proliferative (CD45RA+/CD27+ Naïve and CD45RA-/CD27+ Central Memory γδ T cells) to effector subsets (CD45RA-/CD27—Effector Memory and CD45RA+/CD27—Terminal Effectors γδ T cells), we evaluated the changes in the different subsets 7, 28, 56, 90 and 180 days after ZA treatment compared with the basal value. Treatment with a single-dose of ZA in vivo caused a significant decrease of Naïve Vδ2 T lymphocytes after 180 days (P < 0.01; Fig. 2a) and a significant progressive decrease of Central Memory subset after 28 (P < 0.05), 90 (P < 0.01) and 180 days (P < 0.01; Fig. 2b) compared with the basal value. These results support the previous findings that ZA is able to induce in vivo redistribution of γδ T cell subsets [26]. Moreover, ZA treatment leads to a significant transient reduction of Effector Memory Vδ2 T lymphocytes after 56 (P < 0.01) and 90 days (P < 0.05; Fig. 2c). This result is indicative of the ability of ZA to activate effector subsets that can carry out their functions migrating into tissues. No significant changes were observed with Vδ2 Terminal Effectors T cells (Fig. 2d).
Fig. 2.
Percentage of Naïve (CD45RA+/CD27+; a), Central Memory (CM; CD45RA−/CD27+; b), Effector Memory (CD45RA−/CD27−; c), Terminal Effectors (CD45RA+/CD27−; d) before and 7, 28, 56, 90 and 180 days after treatment with zoledronic acid. Columns indicate median and interquartil-range (IQR) of each group. Results of Wilcoxon Matched-Pairs Signed-Ranks Test compared with the basal value: * P < 0.05, ** P < 0.01
Effects of ZA on γδ T cells in two different patient subsets
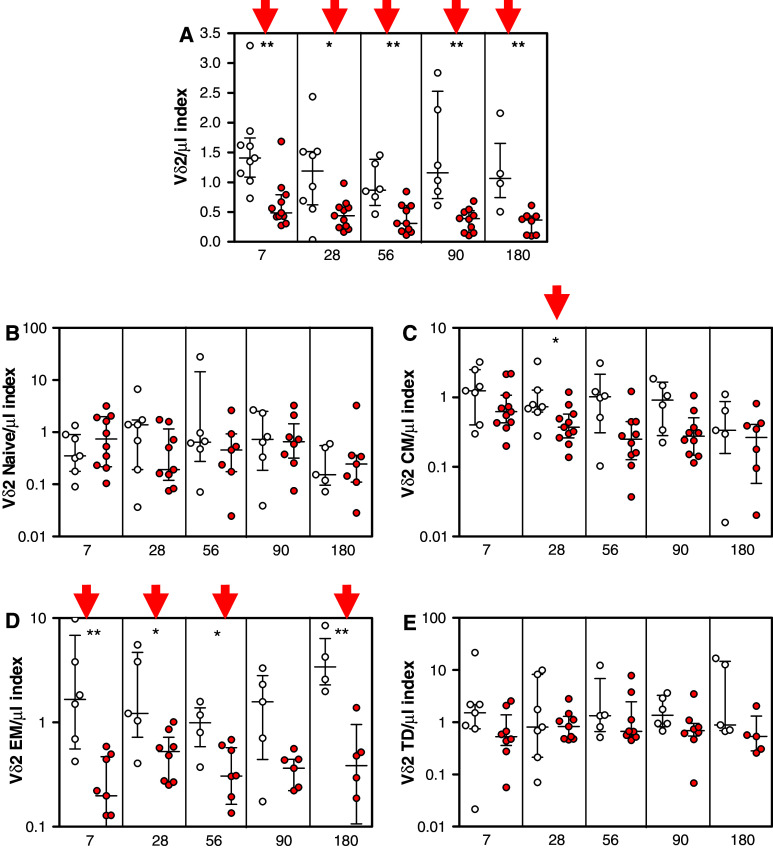
We next expressed our γδ kinetics data as an index of the different timepoints with respect to the baseline value obtained before ZA treatment. For each timepoint, the index refers to the actual value at that particular timepoint divided by the individual baseline (T0) value. Interestingly, we observed that the patient population could be divided into two subsets characterized by completely different γδ T lymphocyte kinetics. The first group was characterized by a progressive decrease of γδ T lymphocytes Vδ2 total number compared with the basal value while in the second subset the Vδ2 kinetics index did not significantly decrease over time. Between the two subgroups of patients we detected significant differences in terms of γδ T lymphocytes total number 7 (P < 0.01), 28 (P < 0.05), 56 (P < 0.01) and 180 (P < 0.01) days after ZA treatment (Fig. 3a).
Fig. 3.
Patients were divided in two groups: in the first one, indicated with empty circles, the number of Vδ2 T lymphocytes did not decrease after zoledronic acid treatment; in the second one, indicated with filled circles, the number of T Vδ2 lymphocytes decreased significantly after zoledronic acid treatment. The figure shows the changes in the total CD3+/Vδ2 T lymphocytes (a) and of the different subsets: Naïve (CD45RA+/CD27+; b), Central Memory (CM; CD45RA−/CD27+; c), Effector Memory (CD45RA−/CD27-; d), Terminal Effector (CD45RA+/CD27−; e) in patients of each group. Values are expressed as indexes with respect to the individual baseline values before zoledronic acid treatment. Columns indicate median and interquartil-range (IQR) of each group. Results of Wilcoxon Matched-Pairs Signed-Ranks Test compared with the basal value: * P < 0.05, ** P < 0.01
We also noticed significant differences between the two patient groups when the different γδ T lymphocyte subsets were considered, specifically the Central Memory subset after 28 days (P < 0.05) (Fig. 3c) and the Effector Memory subset after 7 (P < 0.01), 28 (P < 0.01), 56 (P < 0.05) and 180 days (P < 0.01) (Fig. 3d). No significant differences were observed in the Naïve subset (Fig. 3b) or in the Terminal Effectors (Fig. 3e) Vδ2 T lymphocyte subsets.
Picostim stimulation induced similar expansion of peripheral Vδ2 T cells in the two different patient subsets
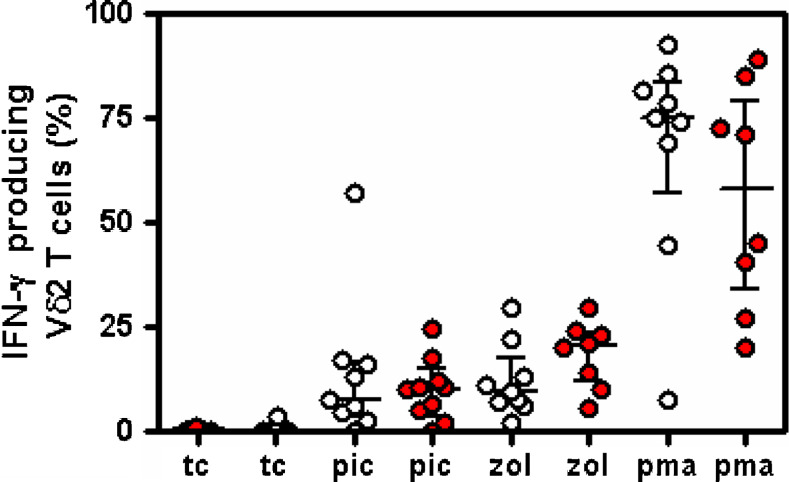
In order to assess if the different behavior observed in the two subsets could be related to a different responsiveness of Vδ2 T cells, we performed an in vitro assay by using Picostim, a phosphoantigen. As shown in Fig. 4, 20 nM of the phosphoantigen induced a similar increased proportion of γδ T cells IFNγ producing in both patient subsets. However, no significant difference in the percentage of intracytoplamic IFNγ expressing Vδ2 T cells was seen after phosphoantigen stimulation between the two different patient subsets (Fig. 4).
Fig. 4.
Picostim stimulation induced increased percentage of IFNγ producing gamma/delta T cells in both patient subgroups. Empty circles IFNγ producing Vdelta2 T cells (%) from patients without any significant modification of Vδ2 T lymphocytes after zoledronic acid treatment; filled circles IFNγ producing Vdelta2 T cells (%) from patients who showed a significant decrease of Vδ2 T lymphocytes after zoledronic acid treatment. tc Negative control, pic picostim, zol zoledronic acid, pma positive control
Discussion
Several in vitro studies have investigated the immunomodulating properties of zoledronic acid on γδ T lymphocytes. This aminobisphosphonate has been shown to inhibit the mevalonate pathway with a consequent accumulation of IPP, a phosphoantigen able to induce an expansion and an activation of γδ T cells [29]. ZA also induces a polyclonal expansion of naïve γδ T lymphocytes and their differentiation into different subsets via molecular mechanisms.
Several studies, carried out in vitro [25] or on cell lines obtained from patients with haematologic tumors (mainly leukemia cell lines of both lymphoid and myeloid origin) [30], have evaluated the immunomodulating properties of ZA. Dieli et al. conducted the first study to investigate the in vivo effects of zoledronic acid on γδ T cells subsets in patients affected by solid tumors [26]. Based on the results of these studies, it has been proposed that the ability to activate γδ T cells may contribute to the clinical efficacy of N-BPs therapy in tumors.
Our study is the first to prospectively evaluate, the immunomodulating properties of a single-dose of ZA on a subset of disease-free patients. We observed that ZA was not able to induce significant changes in the absolute numbers of the various lymphocyte subsets or of the γδ T cells during the treatment. However, ZA induced a redistribution of the γδ T lymphocytes subsets, in vivo, from proliferative (Naïve, Central Memory) to effector subsets (Effector Memory, Terminal Effectors). In fact, ZA caused a significant decrease of peripheral blood Naïve (after 180 days) and Central Memory (after 28, 90 and 180 days) Vδ2 T lymphocytes. Moreover, we observed a peripheral blood decrease of Effector Memory after 56 and 90 days. This subset is the most effective against tumors, because of its ability to produce IFN-γ. The Central Memory population may constitute an antigen-primed γδ population within lymph nodes able to generate effector cells upon encounter with antigen. The Effector Memory subset represents a pool of antigen-primed γδ T cells which enter the peripheral tissues having the capability to acquire the Terminal Effector phenotype and display cytotoxic activity. Our results suggest that ZA has the ability to activate effector subsets and stimulate their migration into peripheral tissues where they can perform their terminal functions.
The other interesting finding was the long-lasting immunomodulating capability of a single infusion of ZA. This aminobisphosphonate has a very short plasmatic half-life (167 h) that cannot explain its persistent effects on γδ T cells. Our hypothesis is that ZA could be constantly released from the bone where it has a longer half-life (150–200 days) and unceasingly stimulate the activation of peripheral blood γδ T cells [31]. One important characteristic of ZA is its high affinity for bone. It has been shown to accumulate in bone for prolonged periods of time while maintaining remarkably low systemic concentrations. As a consequence, the bone becomes a reservoir for this agent and the systemic effects may be related to its prolonged release from bone, a process that is governed mostly by the rate of bone remodelling [32].
On the basis of γδ T cells responsiveness to ZA, we observed that the patient population could be divided into two subsets with completely different γδ T lymphocyte kinetics. Several studies have reported a general decrease of γδ T cells after ZA treatment. However, in our study in one subset of patients there were no changes in the kinetics of the γδ T cells. It is interesting to note that the immunological unresponsiveness of our two subgroups of patients concerned mainly the EM subset, which typically produce IFNγ and display cytotoxicity, thus representing the most effective in the immunity against tumors.
Changes in the γδ T cell subset are consistent with the migration of effector cells to the tumor site.
We hypothesized that this different behavior was potentially related to some of the patients’ clinical characteristics but we did not find any significant correlation (data not shown). It might possible that patient’s follow up will show up differences in the clinical outcome between responders and non-responders patients.
We performed further functional analysis on γδ T cells to better understand the reason for this difference in γδ T lymphocytes responsiveness between the two patient subgroups. One hypothesis to explain our results was that functional anergy of γδ T cells, in terms of IFNγ production, occurs. Thus, we performed a stimulation assay using a phosphoantigen to determine if Vδ2 T cells from both subgroups of patients can produce IFNγ. The assay results showed a normal IFNγ production response of circulating Vδ2 T cells in both subgroups of patients without any significant difference between either group. Functional anergy of γδ T cells in the subset of patients with a progressive decrease of total γδ T cells compared with the basal value was excluded as a possibility.
In conclusion, our data suggest that ZA can exert long-lasting immunomodulating activity on γδ T lymphocytes, but only in a subset of disease-free cancer patients.
Our results clearly highlight the need to further investigate the clinical significance of the immunomodulating properties of ZA and to understand the immunological pathogeneses of the different γδ T cell responsiveness recorded in our cohort of patients.
Acknowledgments
This work is in memory of Fabrizio Poccia. This work was supported by grants from MIUR (COFIN, 2005) and from “Giuliana Cardarelli Mazzi” cancer research foundation. Any financial/commercial conflicts have been disclosed.
Conflict of interest statement
The authors declare no financial or commercial conflicts of interest.
Abbreviations
- N-BPs
Amino-bisphosphonates
- BPs
Bisphosphonates
- ZA
Zoledronic acid
- γ/δ T Ly
γ/δ T lymphocytes
- IPP
Isopentenyl-diphosphate
- CM
Central memory
- EM
Effector memory
- TE
Terminal effectors
References
- 1.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9:745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 2.Sietsema WK, Ebetino FH, Salvagno AM, Bevan JA. Antiresorptive dose–response relationship across three generations of bisphosphonates. Drugs Exp Clin Res. 1989;15:389–396. [PubMed] [Google Scholar]
- 3.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, et al. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2004;22:1351. doi: 10.1200/JCO.2004.12.115. [DOI] [PubMed] [Google Scholar]
- 4.Saad F, Gleason D, Murray R, et al. Zoledronic acid is well tolerated for up to 24 months and significantly reduces skeletal complications in patients with advanced prostate cancer metastatic to bone. J Urol. 2003;169(suppl):394. [Google Scholar]
- 5.Rosen LS, Gordon D, Tchekmedyian NS, Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with non small cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer. 2004;100:2613–2621. doi: 10.1002/cncr.20308. [DOI] [PubMed] [Google Scholar]
- 6.Van Beek E, Pieterman E, Cohen L, Lowick C, Papapoulous S. Farnesyl pyrophosphate synthase is the molecular target of nitrogen-containing bisphosphonates. Biochem Biophys Res Commun. 1999;264:108–111. doi: 10.1006/bbrc.1999.1499. [DOI] [PubMed] [Google Scholar]
- 7.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 8.Santini D, Vespasiani Gentilucci U, Vincenzi B, Picardi A, Vasaturo F, LaCesa A, Onori N, et al. The antineoplastic role of bisphosphonates: from basic research to clinical evidence. Ann Oncol. 2003;14:1468–1476. doi: 10.1093/annonc/mdg401. [DOI] [PubMed] [Google Scholar]
- 9.Kabelitz D, Marischen L, Oberg HH, Holtmeier W, Wesch D. Epithelial defence by γ/δ T cells. Int Arch Allergy Immunol. 2005;137:73–81. doi: 10.1159/000085107. [DOI] [PubMed] [Google Scholar]
- 10.Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda D. Zoledronic acid inhibits visceral metastasis in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10:4554–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 11.Caccamo N, Meraviglia S, Ferlazzo V, Angelini D, Borsellino G, Poccia F, Battistini L, et al. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vgamma9Vdelta2 naïve, memory and effector T cell subsets. Eur J Immunol. 2005;35:1764–1772. doi: 10.1002/eji.200525983. [DOI] [PubMed] [Google Scholar]
- 12.Zocchi MR, Ferrarini M, Rugarli C. Selective lysis of the autologous tumor by delta TCS1+ gamma/delta+ tumor-infiltrating lymphocytes from human lung carcinomas. Eur J Immunol. 1990;20:2685–2689. doi: 10.1002/eji.1830201224. [DOI] [PubMed] [Google Scholar]
- 13.Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–3940. [PubMed] [Google Scholar]
- 14.Zhao X, Wei YQ, Kariya Y, Teshigawara K, Uchida A. Accumulation of gamma/delta T cells in human dysgerminoma and seminoma: roles in autologous tumor killing and granuloma formation. Immunol Invest. 1995;24:607–618. doi: 10.3109/08820139509066861. [DOI] [PubMed] [Google Scholar]
- 15.Bagot M, Heslan M, Dubertret L, Roujeau JC, Tourine R, Levy JP. Antigen-presenting properties of human epidermal cells compared with peripheral blood mononuclear cells. Br J Dermatol. 1985;113(suppl 28):55. doi: 10.1111/j.1365-2133.1985.tb15626.x. [DOI] [PubMed] [Google Scholar]
- 16.Laad AD, Thomas ML, Fakih AR, Chiplunkar SV. Human gamma delta T cells recognize heat shock protein-60 on oral tumor cells. Int J Cancer. 1990;80:709–714. doi: 10.1002/(SICI)1097-0215(19990301)80:5<709::AID-IJC14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Fisch P, Malkovsky M, Kovats S, Sturm E, Braakman E, Klein BS, Voss SD, et al. Recognition by human Vγ9/Vδ2 T cells of a GroEL homolog on Daudi Burkitt’s lymphoma cells. Science. 1990;250:1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 18.Freedman MS, D’Souza S, Antel JP. Gamma delta T-cell-human glial cell interactions In vitro induction of gamma-delta T-cell expansion by human glial cells. J Neuroimmunol. 1997;74:135–142. doi: 10.1016/S0165-5728(96)00217-2. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki Y, Fujimiya Y, Ohno T, Katakura R, Yoshimoto T. Enhancing effect of tumor necrosis factor (TNF)-alpha, but not IFN-gamma, on the tumor-specific cytotoxicity of gammadelta T cells from glioblastoma patients. Cancer Lett. 1999;140:161–167. doi: 10.1016/S0304-3835(99)00067-1. [DOI] [PubMed] [Google Scholar]
- 20.Schilbach KE, Geiselhart A, Wessels JT, Niethammer D, Handgretinger R. Human gammadelta T lymphocytes exert natural and IL-2-induced cytotoxicity to neuroblastoma cells. J Immunother. 2000;23:536–548. doi: 10.1097/00002371-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Courvasier M, Moureau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, et al. Vγ9/Vδ2 T cell response to colon carcinoma cells. J Immunol. 2005;175(8):5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 22.Ferrarini M, Ferrero E, Dagna L, Poggi A, Zocchi MR. Human gammadelta T cells: a nonredundant system in the immune surveillance against cancer. Trends Immunol. 2002;23:14–18. doi: 10.1016/S1471-4906(01)02110-X. [DOI] [PubMed] [Google Scholar]
- 23.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasmacell activity in multiple myeloma. Blood. 2000;96:384–392. [PubMed] [Google Scholar]
- 24.Kunzmann V, Bauer E, Wilhelm M. γδ T cell stimulation by pamidronate. N Engl J Med. 1999;340:737–738. doi: 10.1056/NEJM199903043400914. [DOI] [PubMed] [Google Scholar]
- 25.Dieli F, Gebbia N, Poccia F, Caccamo N, Montesano C, Fulfaro F, Arcara C, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–1. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 26.Ferlazzo V, Sferrazza C, Caccamo N, Di Fede G, Di Lorenzo G, D’Asaro M, Meraviglia S, et al. In vitro effects of aminobisphosphonates on Vgamma9Vdelta2 T cell activation and differentiation. Int J Immunopathol Pharmacol. 2006;19:309–317. doi: 10.1177/039463200601900208. [DOI] [PubMed] [Google Scholar]
- 27.Dieli F, Poccia F, Lipp M, Sireci G, Caccamo N, Di Sano C, Salerno A. Differentiation of effector/Memory Vdelta2 T cells and migratory routes in lymph nodes or inflammatory sites. J Exp Med. 2003;198:391–397. doi: 10.1084/jem.20030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galluzzo S, Santini D, Vincenzi B, Caccamo N, Meraviglia F, Salerno A, Dieli F, et al. Immunomodulating role of bisphosphonates on human gamma delta T cells: an intriguing and promising aspect of their antitumour activity. Expert Opin Ther Targets. 2007;11(7):941–954. doi: 10.1517/14728222.11.7.941. [DOI] [PubMed] [Google Scholar]
- 29.Gober HJ, Kistowska M, Angman L, Jeno P, Mori L, De Libero G. Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med. 2003;197:163–168. doi: 10.1084/jem.20021500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe N, Narita M, Yokoyama A, Sekiguchi A, Saito A, Tochiki N, Furukawa T, et al. Type I IFN-mediated enhancement of anti-leukemic cytotoxicity of gammadelta T cells expanded from peripheral blood cells by stimulation with zoledronate. Cytotherapy. 2006;8:118–129. doi: 10.1080/14653240600620200. [DOI] [PubMed] [Google Scholar]
- 31.Thompson K, Rogers MJ. Statins prevent bisphosphonate-induced gamma, delta-T-cell proliferation and activation in vitro. J Bone Miner Res. 2004;19(2):278–288. doi: 10.1359/JBMR.0301230. [DOI] [PubMed] [Google Scholar]
- 32.Rogers MJ. New insights into the molecular mechanisms of action of bisphosphonates. Curr Pharm Des. 2003;9(32):2643–2658. doi: 10.2174/1381612033453640. [DOI] [PubMed] [Google Scholar]