Abstract
The present paper shows, for the first time, the membrane expression of the dendritic cell maturation marker CD83 on tumor cells from lung cancer patients. CD83 was also detected on freshly cultured fibroblast-like cells from these tissues and on several adherent human tumor cell lines (lung adenocarcinomas P9, A459 and A549, melanomas A375 and C81-61, breast adenocarcinomas SKBR-3 and MCF-7 and colon carcinoma AR42-J), but not in the non-adherent MOT leukemia cell line. CD83 may have immunosuppressive properties and its expression by cancer cells could have a role in facilitating tumor growth.
Keywords: CD83, Lung cancer, Human tumor cell lines, Cancer immunosuppression
Introduction
Since its discovery by Zhou [15] CD83 has been widely used as a marker of human mature dendritic cell (DC). CD83 is a member of the immunoglobulin (Ig) superfamily and consists of an extracellular domain similar to Ig, a highly glycosylated transmembrane domain and an intracellular domain composed of 39 amino acids [16]. Expression of CD83 is believed to be restricted to activated DC, activated B and T lymphocytes and neutrophils [4, 14–16], whereas the yet uncharacterized CD83 ligand (s) are expressed by monocytes, a subset of activated T cells and DC populations [9, 11]. Although expression of CD83 by Hodgkin’s cells has been reported [10], little is known concerning its expression by other malignant populations. Recent studies have shown that recombinant soluble CD83 inhibits both DC maturation and DC-dependent T cell responses [9, 12] and that the inoculation of recombinant CD83 suppresses in vivo anti-tumor responses. In contrast, immobilized or cell surface recombinant CD83 enhances T cell responses. These studies provide strong evidence that membrane CD83 is important in cellular immunity and that soluble CD83 has an immunosuppressive role. In agreement with an immunosuppressive role of soluble CD83, Zinser et al. [17] have shown that soluble CD83 may prevent and treat mice with experimental autoimmune encephalomyelitis (EAE). Hock et al. [5] have previously demonstrated that both activated DC and B cells release soluble CD83 and that low levels of soluble CD83 are present in normal sera. On the other hand, Hirano et al. [3] have shown that, engagement with CD83 enables the long-term survival of antigen-specific T-cell cultures by inducing proliferation and inhibiting apoptosis. It is worth mentioning that some viruses such as HSV-1 [8], HIV-1 [7], vaccinia, [1, 6], measles virus [2] and HCMV [13] appear to interfere with the CD83 expression or induce the shedding of this molecule by DCs, affecting, therefore, the activation of T lymphocytes by DCs.
We demonstrate here, for the first time, CD83 membrane expression by tumor cells freshly obtained from lung carcinomas as well as by several human tumor cell lines (fresh and established lines).
Materials and methods
Preparation of single-cell suspension from lung tissues
Lung tissues (tumor affected and non-affected) were obtained from patients who underwent surgical resection for primary tumor removal. Written informed consent was obtained from all subjects, and the protocol was approved by the Ethical Committee of the Centro de Tratamento e Pesquisa Hospital do Câncer A C Camargo (HCACC) (n°742/05) and the Institute of Biomedical Sciences (n°676/CEP). Tissue samples, aseptically obtained from surgical resection, were minced and single-cell suspensions were generated by digestion with collagenase type VIII (0.56 mg/ml; Sigma, St Louis, MO, USA) during 120 min incubation at 37°C.
Cell lines
Human lung adenocarcinomas cell lines A459 and A549, human melanomas A375 and C81-61, human breast adenocarcinomas SKBR-3 and MCF-7 and human colon carcinoma AR42-J were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS).
Labeling of single-cell suspension for flow cytometry
Cell suspensions (2 × 105 cells) were labeled with each of the various specific fluorescent antibodies (CD1a, CD14, CD83, CD123, CCR7 and HLA-DR) (Caltag Laboratories, Burlingame, California or BD Bioscience, Chicago, Illinois or R&D Systems, Minneapolis, Minnesota, USA), and analyzed in a FACSCalibur cytometer (Becton Dickinson, San Jose, California, USA) with the CellQuest software (Becton Dickinson, San Jose, California, USA).
Statistical analysis
Results were checked for normality by the Kolmogorov–Smirnov test and comparisons between results obtained from nonaffected and tumor-affected lung in the same patient were made by a paired t test.
Results and discussion
Patients’ characteristics
The patients’ age, gender, pathological (pTNM) and clinical stage (CS) are shown in Table 1.
Table 1.
Demographic and clinical characteristics of the patients
| Patient | Age | Sex | Diagnosis | pTNMa | CSb |
|---|---|---|---|---|---|
| 1 | 81 | M | SCC | T1N0M0 | 1A |
| 3 | 58 | M | NC | T2N0M0 | 1B |
| 4 | 62 | F | NSCLC | T2N0M0 | 1B |
| 5 | 58 | M | S CC | T4N2M1 | 4 |
| 6 | 70 | M | ADC | T2N2M0 | 3A |
| 7 | 83 | M | LCC | T2N0M0 | 1B |
| 8 | 68 | M | ADC | T1N1M0 | 2A |
| 9 | 49 | F | ADC | T1N1M0 | 2A |
| 10 | 62 | F | ADC | T4N1M1 | 4 |
| 11 | 52 | F | ADC | T1N0M0 | 1A |
| 12 | 43 | F | ACC | T2N0M0 | 1B |
F Female, M male, ADC adenocarcinoma, ACC adenoid cystic carcinoma, LCC large cell carcinoma, NC neuroendocrine carcinoma, NSCLC non-small cell lung carcinoma, SCC squamous cell carcinoma
aPathological tumor-node metastasis classification
bClinical stage
CD83 expression on cells from lung cancer patients
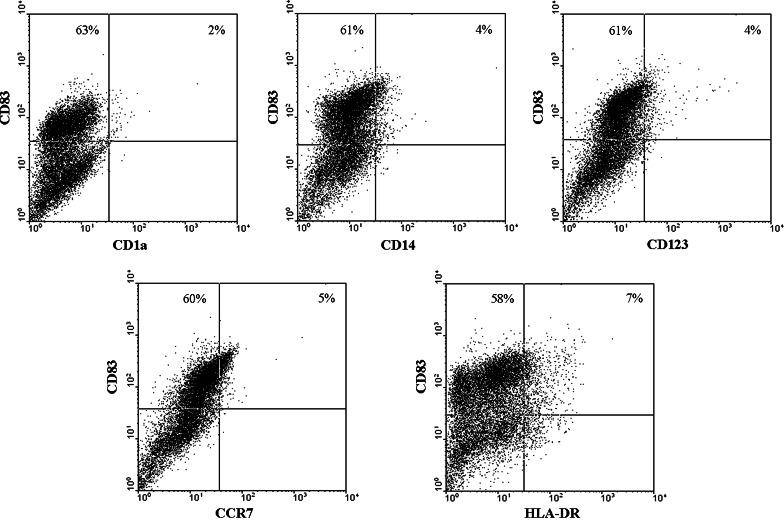
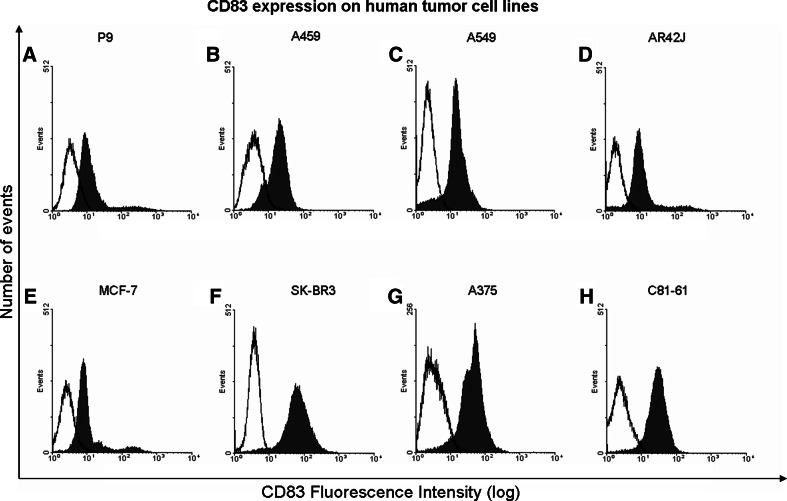
After enzymatic digestion, the cell suspension derived from tumor-affected lung or non-affected lung were stained with fluorescent monoclonal antibodies against CD1a, CD14, CD83, CD123, CCR7 and HLA-DR and their expression was analyzed by flow cytometry. The average frequency of cells positive for these markers varied from 11% for CD14 in non-affected lung and 13% in tumor samples to 30% for HLA-DR in non-affected lung and 35% in tumor samples. Surprisingly, we noticed a high percentage of cells expressing CD83 (Table 2) in 10 of 11 patients analyzed. It is noteworthy that the high expression of CD83 was not simultaneous with the expression of the other myeloid cell markers analyzed (CD1a, CD14, CD123, CCR7 and HLA-DR), as illustrated in Figs. 1 (tumor) and 2 (lung). Furthermore, both affected and nonaffected lung tissues had similar levels of CD83+ cells expressing similar levels of the antigen, as judged by the mean fluorescence intensity of the positive cells (Table 2). Also, the percentage of CD4+ and CD8+ cells in these tissues was very low (data not shown), indicating that the CD83+ cells did not express these markers either. Such pattern of expression indicates that cells expressing CD83 are neither myeloid nor lymphoid cells. These cellular suspensions were maintained in culture for at least 2 months in serum-free medium (RPMI 1640) after which their cytokeratin expression was evaluated by immunocytochemistry. Most cell lines had a fibroblast-like appearance and were cytokeratin negative (data not shown), but expressed CD83 (Fig. 3). Interestingly, the cell line established from patient 9 (P9), was cytokeratin positive and positive to CD83 (Fig. 4a). These data suggest that the expression of CD83 is a characteristic of lung tissues, but also of tumor cells from this origin.
Table 2.
Percentage and mean fluorescence intensity (MFI) of CD83 positive cells detected by flow cytometry of cells freshly obtained from nonaffected lung and tumor-affected lung tissue
| Patient | Percentage of positive cells | MFI | ||
|---|---|---|---|---|
| Non-affected lung | Tumor-affected lung | Non-affected lung | Tumor-affected lung | |
| 1 | 73.2 | 41.4 | 84.26 | 46.2 |
| 3 | 60.1 | 79.9 | 71.8 | 55.7 |
| 4 | –a | 84.6 | –a | 60.7 |
| 5 | 54.0 | 97.3 | 38.8 | 58.2 |
| 6 | 27.1 | 28.3 | 8.9 | 29.6 |
| 7 | 64.1 | 33.7 | 18.2 | 12.1 |
| 8 | 49.9 | 64.8 | 49.2 | 59.3 |
| 9 | 46.0 | 41.0 | 16.1 | 28.5 |
| 10 | 61.0 | 79.0 | 23.1 | 31.5 |
| 11 | 15.0 | 22.2 | 25.9 | 40.3 |
| 12 | 0.0 | 0.0 | 0.0 | 0.0 |
| Mean | 43.0 | 51.8 | 33.6 | 38.4 |
| P value | 0.5378 | 0.6723 | ||
aLost material
Fig. 1.
Expression of myeloid/lymphoid markers CD1a, CD14, CD123, CCR7 and HLA-DR on CD83+ cells obtained from tumor-affected lung. Lung tissues were obtained from patients who underwent surgical resection of primary lung cancer. Tissue samples were minced and single-cell suspensions generated by digestion with collagenase type VIII (0.56 mg/ml; Sigma, St Louis, MO, USA) during 120 min incubation at 37°C. Cell suspensions (2 × 105 cells) were labeled with each of the various specific fluorescent antibodies (CD1a, CD14, CD83, CD123, CCR7 and HLA-DR) (Caltag Laboratories, Burlingame, California or BD Bioscience, Chicago, Illinois or R&D Systems, Minneapolis, Minnesota, USA), and analyzed in a FACSCalibur cytometer (Becton Dickinson, San Jose, California, USA) with the CellQuest software (Becton Dickinson, San Jose, California, USA)
Fig. 2.
Expression of myeloid/lymphoid markers CD1a, CD14, CD123, CCR7 and HLA-DR on CD83+ cells obtained from non-affected lung. Lung tissues were obtained from patients who underwent surgical resection of primary lung cancer. Tissue samples were minced and single-cell suspensions generated by digestion with collagenase type VIII (0.56 mg/ml; Sigma, St Louis, MO, USA) during 120 min incubation at 37°C. Cell suspensions (2 × 105 cells) were labeled with each of the various specific fluorescent antibodies (CD1a, CD14, CD83, CD123, CCR7 and HLA-DR) (Caltag Laboratories, Burlingame, California or BD Bioscience, Chicago, Illinois or R&D Systems, Minneapolis, Minnesota, USA), and analyzed in a FACSCalibur cytometer (Becton Dickinson, San Jose, California, USA) with the CellQuest software (Becton Dickinson, San Jose, California, USA)
Fig. 3.
Expression of CD83 on freshly cultured fibroblast-like cells from non-affected lung (a) or tumor (b). Cells, obtained after surgical resection of primary lung cancer, as described in the Figs. 1 and 2, were maintained in serum-free RPMI 1640 during 2 months and then recovered by trypsin-treatment, labeled and analyzed by flow cytometry for the expression of CD83. Results from one patient (# 8) are shown. Empty histogram isotype control and filled histogram cells labeled with anti-CD83 antibody
Fig. 4.
Expression of CD83 on human cancer cell lines: A, B, C lung adenocarcinomas, D colon carcinoma, E, F breast carcinomas and G, H melanomas. Freshly established from patient 9 (P9), and long term lung adenocarcinoma cell lines A459 and A549, human melanomas A375 and C81-61, human breast adenocarcinomas SKBR-3 and MCF-7 and human colon carcinoma AR42-J were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS). Cell suspensions (2 × 105 cells) were labeled with specific fluorescent antibodies to CD83 (BD Bioscience, Chicago, Illinois or R&D Systems, Minneapolis, Minnesota, USA), and analyzed in a FACSCalibur cytometer (Becton Dickinson, San Jose, California, USA) with the CellQuest software (Becton Dickinson, San Jose, California, USA). Empty histogram isotype control and filled histogram cells labeled with anti-CD83 antibody
CD83 expression on human tumor cell lines
Therefore, we asked whether expression of CD83 was a particular case for lung tissues or if it was a phenomenon present in different tumors. In order to investigate that, our next step was to test several human tumor cell lines for the expression of CD83 and all adherent cell lines analyzed were positive (Fig. 4a–h).
It is intriguing that a “classical” dendritic cell maturation marker such as CD83 should be expressed by tumor cells. Since CD83 secretion, by dendritic and leukemia cells, has been shown to occur and soluble CD83 is able to inhibit T cell activation by dendritic cells in vitro [4, 5, 9], it is possible to speculate that tumor cells, expressing this molecule, may also secrete it within the local microenvironment. Such phenomenon could have an inhibiting effect on the immune response against tumor antigens and facilitate tumor growth. On the other hand, since we could detect CD83 expression on adherent cells but not on a human non-adherent tumor cell lineage derived from T lymphocytes (data not shown) we can also hypothesize that CD83 may possibly play a role in cell adhesion. Nevertheless, further studies need to be performed in order to clarify the biological meaning behind CD83 expression by tumor cells.
Acknowledgments
This study was supported by grants (#05/50422-2; #04/09956-0) from the Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP).
References
- 1.Engelmayer J, Larson M, Subklewe M, Chahroudi A, Cox WI, Steinman RM, Bhardwaj N. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism immune evasion. J Immunol. 1999;163:6762. [PubMed] [Google Scholar]
- 2.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Riossan MC, Liu YJ, Rabourdin-Combe C. Measle virus suppresses cell mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirano N, Butler MO, Xia Z, Anse´n S, von Bergwelt-Baildon MS, Neuberg D, Freeman GJ, Nadler LM. Engagement of CD83 ligand induces prolonged expansion of CD8+ T cells and preferential enrichment for antigen specificity. Blood. 2006;107:1528. doi: 10.1182/blood-2005-05-2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hock BD, Haring LF, Steinkasserer A, Taylor KG, William N, Patton WN, McKenzie JL. The soluble form of CD83 is present at elevated levels in a number of hematological malignancies. Leuk Res. 2004;28:237. doi: 10.1016/S0145-2126(03)00255-8. [DOI] [PubMed] [Google Scholar]
- 5.Hock BD, Kato M, McKenzie JL, Hart DN. A soluble form of CD83 is released from activated dendritic cells and B lymphocytes, and is detectable in normal human sera. Int Immunol. 2001;13:959. doi: 10.1093/intimm/13.7.959. [DOI] [PubMed] [Google Scholar]
- 6.Jenne L, Schuler G, Steinkasserer A. Viral vectors for dendritic cell-based immunotherapy. Trends Immunol. 2001;22:102. doi: 10.1016/S1471-4906(00)01813-5. [DOI] [PubMed] [Google Scholar]
- 7.Knight SC, Paderson S. Bone marrow-derived dendritic cells infection with human immunodeficiency virus and immunopathology. Annu Rev Immunol. 1997;15:593. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 8.Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with Herpes Simplex virus type 1 exhibit inhibited T cell stimulatory capacity. J Virol. 2000;74:7127. doi: 10.1128/JVI.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lechmann M, Krooshoop DJ, Dudziak D, Kremmer E, Kuhnt C, Figdor CG, et al. The extracellular domain of CD83 inhibits dendritic cell-mediated T cell stimulation and binds to a ligand on dendritic cells. J Exp Med. 2001;194:1813. doi: 10.1084/jem.194.12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorg UR, Morse TM, Patton WN, Hock BD, Angus HB, Robinson BA. Hodgkin’s cells express CD83, a dendritic cell lineage associated antigen. Pathology. 1997;29:294. doi: 10.1080/00313029700169125. [DOI] [PubMed] [Google Scholar]
- 11.Scholler N, Hayden-Ledbetter M, Hellstrom KE, Hellstrom I, Ledbetter JA. CD83 is a sialic acid-binding Ig-like lectin (Siglec) adhesion receptor that binds monocytes and a subset of activated CD8+ T cells. J Immunol. 2001;166:3865. doi: 10.4049/jimmunol.166.6.3865. [DOI] [PubMed] [Google Scholar]
- 12.Scholler N, Hayden-Ledbetter M, Dahlin A, Hellstrom I, Hellstrom KE, Ledbetter JA, et al. Cutting edge: CD83 regulates the development of cellular immunity. J Immunol. 2002;168:2599. doi: 10.4049/jimmunol.168.6.2599. [DOI] [PubMed] [Google Scholar]
- 13.Sénéchal Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103:4207. doi: 10.1182/blood-2003-12-4350. [DOI] [PubMed] [Google Scholar]
- 14.Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood. 2000;96:3958. [PubMed] [Google Scholar]
- 15.Zhou L, Schwarting R, Smith HM, Tedder TF. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992;149:735. [PubMed] [Google Scholar]
- 16.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821. [PubMed] [Google Scholar]
- 17.Zinser E, Lechmann M, Golka A, Lutz MB, Steinkasserer A. Prevention and treatment of experimental autoimmune encephalomyelitis by soluble CD83. J Exp Med. 2004;200:345. doi: 10.1084/jem.20030973. [DOI] [PMC free article] [PubMed] [Google Scholar]