Abstract
The prognosis of malignant gliomas remains dismal and alternative therapeutic strategies are required. Immunotherapy with dendritic cells (DCs) pulsed with tumour antigens emerges as a promising approach. Many parameters influence the efficacy of DC-based vaccines and need to be optimised in preclinical models. The present study compares different vaccine schedules using DCs loaded with tumour cell lysate (DC-Lysate) for increasing long-term survival in the GL26 orthotopic murine glioma model, focusing on the number of injections and an optimal way to recall antitumour immune response. Double vaccination with DC-Lysate strongly prolonged median survival compared to unvaccinated animals (mean survival 87.5 daysvs. 25 days; p < 0.0001). In vitro data showed specific cytotoxic activity against GL26. However, late tumour relapses frequently occurred after 3 months and only 20% of mice were finally cured at 7 months. While one, two or three DC injections gave identical survival, a boost using only tumour lysate after initial DC-Lysate priming dramatically improved long-term survival in vaccinated mice, compared to the double DC-Lysate group, with 67.5% of animals cured at 7 months (p < 0.0001). In vitro data showed better specific CTL response and also the induction of specific anti-GL26 antibodies in the DC-Lysate/Lysate group, which mediated Complement Dependent Cytotoxicity. These experimental data may be of importance for the design of clinical trials that currently use multiple DC injections.
Keywords: Vaccination, Brain tumours, Dendritic cells, Immunotherapy, Gliomas
Introduction
Malignant gliomas represent about one-third of all adult primary brain tumours. The prognosis of human malignant glioma remains poor with an overall 2-year survival rate of less than 10% and a median survival time of about 1 year for higher grade tumours such as glioblastoma [1]. Alternative therapeutic approaches to surgery, radiotherapy and chemotherapy are required to improve patient survival. Recent insights into neuroimmunology provide evidence of the immunocompetence of the brain even in the case of tumour development [2, 3], thus opening new hopes for immunotherapy. Potential antigen presenting cells, that is, microglia and clonally expanded CD8+ cytotoxic T lymphocytes (CTL) mostly tumour-specific, have been described in glioma [2–5]. Glioma cells do not express MHC class II molecules, but weakly express MHC class I molecules and are sensitive to Fas/fas ligand pathway [2]. Over the past three decades, the stimulation of the immune system by systemic or local intratumoral injections of cytokines (TNF α, IFN β, IL-2...) [6, 7] or adoptive transfer of effector cells [lymphokine-activated-killer cells (LAK)] [8, 9] were investigated in glioma patients without demonstrating reproducible clinical benefit. Neither did experiments involving vaccination strategies [10, 11]. Successful manipulation of the host immune response to glioma tumours is hampered by multiple mechanisms of immune dysregulation such as the secretion of inhibitory factors (TGF β, prostaglandin E2 and IL10), the poorly antigenic character of these tumours, the inefficiency of tumour antigen presentation, the absence of CD4+ helper response [2–4, 12].
Dendritic cells (DCs) play a crucial role during the priming of antitumour-specific immune response [13, 14]. Accumulating evidence suggests that DC functions are altered in cancer patients and that peripheral vaccination with in vitro generated DCs loaded with tumour antigen can be a promising approach to overcome the local immunosuppressive context. Preclinical and clinical observations have shown that DC-based immunotherapy may be efficient to control cancer development in melanoma [15, 16], or renal cell carcinoma [17] and even in glioma [18–24]. However, clinical trials have produced contrasted results and no definitive conclusion can be drawn about the real efficacy of this approach [25, 26]. Basic questions, concerning the design of optimal vaccine protocols for DC therapy, are currently unresolved. The present study compares vaccine protocols using DCs pulsed with tumour cell lysate in an orthotopic murine glioma model (GL26). We especially focused on the number of DC vaccinations and on the optimal way to recall in vivo antitumour immune response. Our results strongly suggest that multiple vaccinations with DCs are efficient but do not elicit optimal long-term survival. In contrast, one injection of DCs for the priming, followed by a boost with tumour cell lysate alone, generates the most effective antitumour effect. This protocol allowed a better CTL response and also triggered an antitumour humoral response through complement-mediated cell lysis.
Materials and methods
Animals
Female C57Bl/6 mice purchased from Charles River laboratories (l’Arbresle, France) were bred under pathogen-free conditions at the animal facility of our institute. Animals were treated in accordance with the European Union guidelines and French laws for the laboratory animal care and use. All mice used in this experimental study were 5–6-week old at the time of tumour implantation.
Tumour cell line
Glioma cell line GL26 was kindly provided by the National Cancer Institute, USA. GL26 glioma is highly tumorigenic in syngenic C57Bl/6 mice and is an analogue to the GL261 cell line [27]. The GL26 cells express MHC class I but not MHC class II molecules (data not shown). The expression of two melanoma-associated antigens, tyrosinase-related protein-2 (TRP2) and gp100 have been recently described in the GL26 glioma [28].
Implantation of brain tumour
Prior to injection, GL26 cells were harvested, washed twice in PBS, counted, and adjusted to 105 cells in 5 μl of PBS in a 25-gauge Hamilton syringe. Six-week-old syngenic C57Bl/6 mice were anaesthetised by intraperitoneal (i.p.) injection of 200 μl (0.019 ml/g of weight) of a mixture of xylazine (2.2%) and ketamine (6.5%). For intracranial (i.c.) implantation of GL26, the needle was positioned 2 mm to the right of the bregma and 4 mm below the surface of the skull using a stereotactic frame (Kopf instruments for small animals, Phymep, France), and cells were implanted into the right frontal lobe in a total volume of 5 μl. The injection was done very slowly (1 μl/min), then the needle was left in place for 2 min before removal to avoid liquid reflux. Animals were then followed and euthanised for ethical reasons on the occurrence of characteristic symptoms such as hunched posture, reduced mobility and visible body weight loss. In this model, the appearance of these signs reliably predicted the time of death within 2 days of anticipation. Therefore, this end point was used for survival studies.
DC generation and phenotypic analysis
The Dendritic cells were generated in vitro from syngeneic mouse bone marrow precursors as described previously, with minor modifications [29]. Briefly, murine bone marrow cells were flushed from femurs and tibias of C57Bl/6 mice or nude mice euthanized at 5 weeks. The cells were depleted of red blood cells by hypotonic treatment with 0.9% ammonium chloride for 5 min at room temperature. After washing, B and T lymphocytes, MHC class II+ cells and granulocytes were labelled using rat IgG monoclonal antibodies (mAbs) specific for murine CD4, CD8, CD45/B220, class II-Iab, GR1 (Becton-Dickinson, Pont de Claix, France), then depleted using immunomagnetic beads coated with sheep anti-rat IgG (Dynal, Compiegne, France). Enriched bone marrow progenitors were cultured in 24-well plates at 105 cells/ml of RPMI 1640 medium supplemented with 5% foetal calf serum (FCS), 20 ng/ml of murine recombinant GM-CSF (PeproTech Inc., Rocky Hill, NJ, USA), and 20 ng/ml of murine recombinant IL4 (PeproTech Inc.). Human recombinant Flt3-ligand at 5 ng/ml (PeproTech Inc.) was added for the initial 3 days of culture to enhance the proliferation rate of DC precursors. On day 3, aggregates of proliferating DC precursors were harvested and re-plated in 24-well plates at 2×105 cells/ml with complete fresh medium containing 20 ng/ml of mGM-CSF and mIL4. On day 6, relatively immature DCs were obtained as loosely adherent aggregates. These DCs were able to mature after 6 h of incubation with 1 μg/ml of Ribomunyl (Pierre Fabre Medicament, Boulogne, France) and 10 ng/ml of γIFN (RD Systems, UK).
The phenotype of harvested DCs was assessed by flow cytometric analysis using FITC-conjugated or phycoerythrin-conjugated mAbs to mouse MHC class II-Iab, CD11c, CD11b, CD80, CD86 (Becton Dickinson). Cells were pre-incubated with 2.4G2 mAbs (rat anti-mouse Fcγ-RIII/II receptor mAbs) for 10 min at 4°C to block non-specific FcγR binding of labelled antibodies, then incubated with the relevant mAbs for 30 min at 4°C. Isotype-matched mAbs were used in parallel as negative controls. Prior to vaccination, flow cytometric analysis was performed on a FACScan system (Becton Dickinson) and data were analysed with CellQuest-pro software. The quality of the DCs was determined by the relative percentages of double CD11c+/CD11b+ cells among day-7 cultured bone marrow cells. The maturity of the DCs was assessed according to the percentage of expression of co-stimulatory molecules CD80 and CD86 and the level of expression of MHC class II-Iab.
Mixed lymphocyte reaction (MLR)
Primary MLR were performed using day-6 cultured DCs from C57Bl/6 mice, unloaded (DCs only) or loaded overnight with GL26 tumour cell lysate (DC-Lysate) and allogeneic purified T cells. Purified T cells were obtained from splenocytes of naive Balb-c mice by selective immunomagnetic depletion using anti-CD19, anti-CD11b, anti-Gr1, anti-MHC-class II-Iad as described above. The DCs were irradiated (30 greys) then added as stimulator cells in graded doses (103 –5×104) in 96-well round-bottomed culture plates (Nunc, Rockilde, Denmark) with 105 T cells per well. After 4 days of incubation at 37°C in 5% CO2, cell proliferation response was assessed by tritium-thymidine incorporation during the last 18 h of culture. Tests were carried out in triplicates and results expressed as mean counts per minute (cpm ± SD). The levels of 3 H-TdR uptake by stimulator cells alone were constantly below 150 cpm.
Tumour antigen DC loading
The GL26 tumour cell suspension was adjusted to the concentration of 15×106 cells/ml of PBS. The GL26 tumour cell lysate was obtained by three cycles of freezing–thawing, filtrated through a 0.20 μm filter and stored at −20°C until used. Day-6 cultured DCs were loaded with tumour cell lysate at a ratio of three lysed tumour cells for one DC, then incubated overnight at 37°C.
Vaccination with DCs
Day-7 cultured DCs, either unloaded or loaded overnight with DC-Lysate, were collected, washed, and resuspended in PBS at a concentration of 106 cells/200 μl. Animals (n = 5 per group) were vaccinated intraperitoneally twice by injections of 106 DC-Lysate (gp DC-Lysatex2) on days 14 and 7 before i.c. stereotactic tumour implantation (day 0). Control groups (n = 5 per group) were either injected with 200 μl of PBS (gp CTR), or with tumour cell lysate alone (corresponding to 106 GL26 cells) (gp Lysatex2) or with unloaded DCs (gp DCx2) using the same schedule. Mice were followed for survival and then euthanised using previously described guidelines. Autopsy was performed systematically on each euthanised mouse to confirm that brain tumour growth was responsible for symptoms. Four experiments were carried out using the same methodology to confirm the results.
Treatment of pre-established i.c. GL26 tumours by i.p. injections of DC-Lysatex2
A total of 105 GL26 tumour cells were injected stereotactically into the right frontal lobe (day 0). Mice were treated on days 2 and 9 by i.p. injections of 106 DC-Lysate, then followed for survival.
Treatment of pre-established i.c. GL26 tumours by IV adoptive transfer of splenocytes from vaccinated mice
Mice were vaccinated twice (once a week) with unloaded DCs or with DCs loaded with tumour lysate. Seven days after the last vaccination, mice were euthanised, splenocytes were harvested and pooled for each group of mice (n = 5 per group). Red blood cells were removed by hypotonic ammonium chloride osmotic chock, then washed in PBS and counted. An adoptive transfert of 30×106 whole splenocytes/mouse was injected into the tail vein of mice bearing a pre-established 6-day intracranial GL26 tumour (i.c. implantation, day 0). This transfer was followed by two i.p. injections with DC-Lysate (day 8) and with tumour lysate (day 15). Mice were followed for survival.
Modification of the vaccination schedule
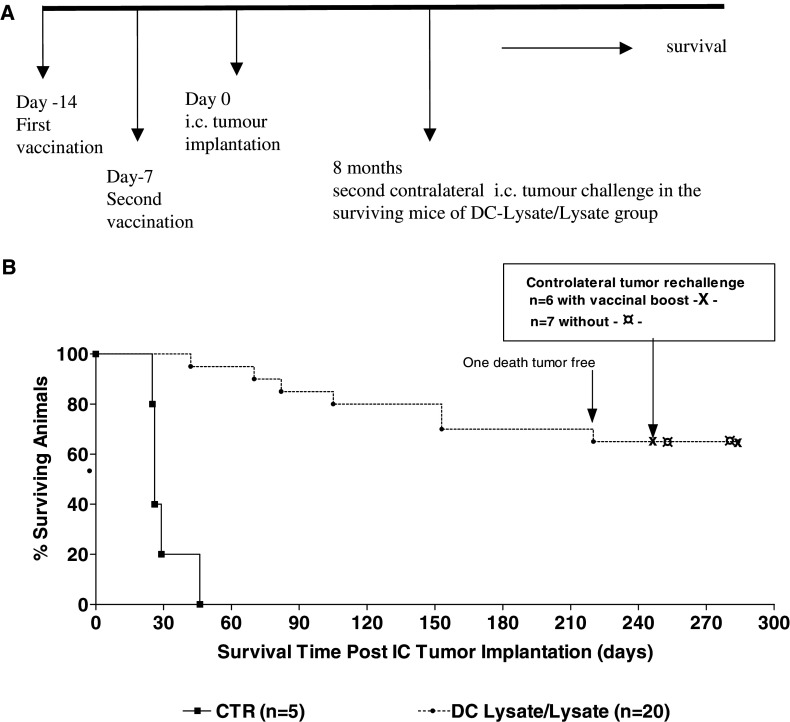
We compared the protective effect of different protocols using one (14 days before i.c. tumour implantation), two (14 and 7 days before i.c. tumour implantation) or three (14 and 7 days before and 30 days after i.c. tumour implantation) i.p. injections of DC-Lysate. Lastly, we studied the response to a simplified vaccine protocol using DC-Lysate only for the priming (14 days before i.c. tumour implantation) followed 7 days later by a boost with tumour cell lysate alone corresponding to 106 cells (DC-Lysate/Lysate group). Mice were followed for survival as previously described. Experiments were repeated three times with the same methodology to confirm the results obtained. In one experiment, mice still alive 8 months after i.c. tumour implantation were rechallenged in the contralateral frontal lobe with 105 GL26 cells, with or without previous i.p. recall with tumour cell lysate 7 days before the second challenge. Mice were followed for survival.
FACS flow cytometer analysis of splenocytes and peripheral blood leukocytes (PBL) according to the different schedules of vaccination and after IC tumour implantation
In one experiment, naive mice or mice vaccinated (three mice per group) with unloaded DC, DC-Lysatex2 and DC-Lysate/Lysate were i.c. implanted with GL26 cells as described previously (day 0). At day 14, mice were euthanised to collect spleen and blood. Samples from all mice in each group were pooled. Red blood cells were removed by hypotonic osmotic chock. Splenocytes or PBL were pre-incubated with 2.4G2 mAbs (rat anti-mouse Fcγ-RIII/II receptor mAbs) for 10 min at 4°C to block non-specific FcγR binding of labelled antibodies, then incubated 30 min at 4°C with specific fluorescent antibodies (from Becton-Dickinson) against CD4/CD62L, CD8/CD62L, CD4/CD25, CD8/CD25, CD19, DX5 (for NK cells), CD11b/Gr1, CD11c/CD11b, for FACS analysis. Isotype-matched mAbs were used in parallel as negative controls. Flow cytometry acquisition and analysis were performed on a FACScan flow cytometer using CellQuestPro software (Becton-Dickinson).
In vitro cytotoxic assay
The cytolytic activity of splenocytes from naive or vaccinated groups of mice (three mice per group) was tested in vitro against GL26 tumour cells in a standard 51CR release assay. Mice were vaccinated, using DC-Lysate for priming and DC-Lysate or lysate alone for boosting 7 days later. Single-cell suspensions of red blood cell-depleted splenocytes from individual mice were harvested 7 days after the last vaccination and resuspended in 10% FCS-RPMI 1640 medium at a concentration of 106/ml, then transferred to 24-well plates. GL26 cells were irradiated at 75 greys then added to the culture at a concentration of 105/ml in the presence of 20 UI/ml of human IL-2 (Eurocetus, Amsterdam, The Netherlands). Five days after culture initiation, cells were harvested and pooled for each group. Activated splenocytes were mixed with 104 GL26 or γIFN-treated GL26 targets labelled with 51CR, in 96-well round-bottomed plates at different effector/target ratios (range from 0.1/1 to 40/1). The 51CR-release in the supernatants was measured after 4 h of incubation at 37°C. All determinations were made in triplicates and the percentage of lysis was calculated using the following formula:
 |
Determination of anti-GL26 humoral response
Mice were vaccinated twice by unloaded DCs, DC-Lysate or with a prime-boost by DC-Lysate followed by a boost by tumour lysate alone at days 14 and 7 before i.c. tumour implantation (Day 0). The detection of antigen-specific antibodies against GL26 in the sera of vaccinated mice was assessed 21 days and 3 months after the first vaccination in the surviving mice. The GL26 cells were incubated with serum dilutions (1/20) from naive or vaccinated groups of mice (three mice per group) for 30 min at 4°C, washed, then incubated with a rat anti-mouse IgG1κ-FITC-conjugated antibody (Becton-Dickinson) and analysed on a FACS flow cytometer. The GL26 cells incubated without serum but with anti-mouse IgG1κ-FITC-conjugated antibody were used as negative control.
Complement-dependent cell cytotoxicity assay (CDC)
The ability of antigen-specific antibodies against GL26 found in the sera of surviving vaccinated mice at 90 days was assessed for CDC. The CDC assay was based on previous work [30] adapted by a new non-radioactive flow cytometric PKH-26 assay [31]. The GL26 target cells were stained with PKH-26 fluorescent dye (incubation at 2 μM for 5 min in specific buffer; Sigma, MO, USA) that was stably integrated into the cell membrane. The GL26-labelled target cells were washed three times and plated in a 96-well conic-bottom microplate at a concentration of 105 cells/well and incubated with serum dilutions (1/10, 1/20 and 1/40) from groups of naive or vaccinated mice (pooled from three mice per group) for 30 min at 4°C. Low-Tox-M Rabbit complement at final 1/10 dilution (Cedarlan, Hornby, Canada) was added and the plate was incubated for 18 h at 37°C in 5% CO2 incubator. Cells were washed in PBS, then resuspended in high calcium annexin V-binding buffer and stained with 5 μl annexin V-FITC (Becton Dickinson) for 10 min in the dark at room temperature . Annexin V-FITC staining allowed to discriminate between apoptotic and non-apoptotic target cells using FACS analysis. Data analysis was performed by first gating on PKH-26 positive target cells, followed by analysis of the annexin V-FITC positive subpopulation. The percentage of specific CDC in the PKH-26 gated GL26 cells was calculated by subtracting unspecific annexin V-FITC positive target cells (resulting from spontaneous apoptosis during the 18 h of incubation), measured in appropriate controls without serum and with Lox-Tox Rabbit complement. This method correlates well with standard 51Cr release assays [31].
Antibody-dependent cell cytotoxicity assay (ADCC)
The same serum that was used for CDC assay were also tested for ADCC. This ADCC assay was also based on a non-radioactive flow cytometric PKH-26 assay [30]. The GL26 target cells were stained with PKH-26 fluorescent dye as described above for the CDC assay. The GL26-labelled target cells were plated in a 96-well conic-bottom microplate at a concentration of 105 cells/well and incubated with serum dilutions (1/10, 1/20 and 1/40) from groups of naive or vaccinated mice (pooled from three mice per group) for 30 min at 4°C. Effector cells at an E/T ratio of 20 were then added for ADCC. Effector cells were peritoneal exudate cells obtained from the peritoneal cavity of naive mice, 5 days after i.p. injection of 1 ml of thioglycolate broth [30]. After 18 h of incubation at 37°C in 5% CO2, cells were labelled with Annexin-V as described for CDC, in the presence of an anti-CD45-cyt antibody (Becton Dickinson) to exclude apoptotic macrophages. Annexin V-FITC staining allowed to discriminate between apoptotic and non-apoptotic target cells using FACS analysis. Data analysis was performed by first gating on PKH-26 positive target cells, followed by analysis of the annexin V-FITC positive subpopulation with the exclusion of CD45-Cyt positive cells. The percentage of cytotoxicity in the PKH-26 gated cell population was calculated by subtracting the percentage of spontaneous apoptosis of GL26 during the 18 h incubation time, as measured in appropriate controls incubated with effector cells but without serum. This method correlates well with standard 51Cr release assays [31].
Statistical analysis
Survival and median survival were estimated using the Kaplan and Meier method. A Wilcoxon’s log-rank test was used to determine the statistical difference between survival rates of mice in various experimental and control groups, using the SPSS 10.1 package SPSS 11.5.1 (SPSS 2002 Inc, Chigaco). A p value less than 0.05 was considered significant.
Results
DC phenotype and functions
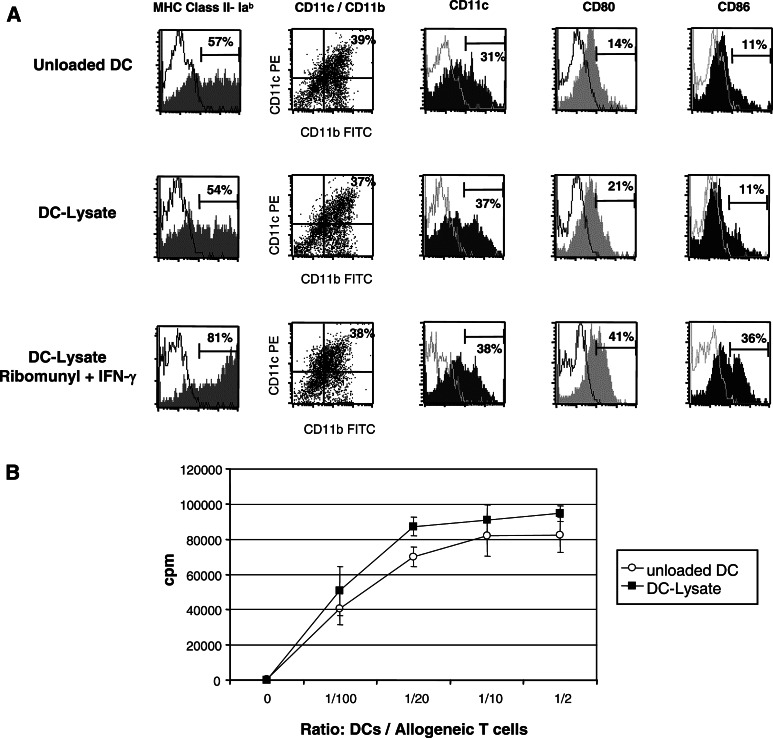
After 6 days of culture, 35–40% of the cells generated from bone marrow progenitors cultured with mGM-CSF, mIL4, and hFLT3-L were myeloid DCs, as assessed by the co-expression of CD11c+ and CD11b+. They displayed a relative immature phenotype with intermediate level of MHC class II expression and only low expression of CD80 and CD86 co-stimulatory molecules, which allowed the up-take and the processing of antigens (Fig. 1a). These culture conditions (Flt3-ligand for 3 days) have been reported to generate DCs with very potent in vivo antitumour effect similar to that obtained after CD40L stimulation [32]. The addition of tumour cell lysate for 12 h did not alter their phenotype and their capability to maturate in the presence of ‘danger signals’ such as ‘ribomunyl’ (ribosomal RNA from bacteria) and γIFN, as shown by the upregulation of co-stimulatory molecules CD80 and CD86 and MHC-class II molecules (Fig. 1a).
Fig. 1.
In vitro generated DCs loaded or not with tumour cell lysate display the same phenotype with an intermediate level of maturation, are able to maturate in response to ‘danger signals’ such as ribomunyl/γIFN and induce a strong proliferation of purified allogeneic T cells. Bone marrow DCs were generated in vitro with Flt3-L, GM-CSF, and IL4 as described in ‘Materials and methods’. On day 6, DCs were either not loaded (unloaded DC) or loaded overnight with GL26 tumour cell lysate (DC-Lysate). a Cell surface expression of MHC Class II-Iab, CD11c/CD11b, CD80, and CD86 of unloaded DCs, DC-Lysate, and DC-Lysate incubated for 6 h with ribomunyl and γIFN. Myeloid DCs were defined as double-positive CD11c+/CD11b+ cells representing 30–40% of loosely adherent harvested cells. b Functionality of unloaded DCs (open circle) and DC-Lysate (filled square) was tested in an MLR assay. Graded numbers of DCs (0, 103, 5×103, 104 and 5×104) were coincubated with a fixed number of allogeneic purified T cells (105 T cells from Balb-c mice) for 4 days at 37°C, followed by 18 h pulse with [3 H] thymidine. The DCs alone did not yield counts greater than the background obtained with T cells alone (fewer than 150 cpm). Average 3 H-thymidine uptakes, expressed in cpm, are shown for each DCs/T ratio. Error bars represent the SD of triplicate values. Results are representative of two independent experiments
The functionality of day-7 DCs generated from C57Bl6 mice H-2b was analysed in an MLR assay. As shown in Fig. 1b, these DCs were highly efficient stimulators for purified T cells from the spleen of Balb-c mice (H-2d), even at the lowest ratio of 1/1000, thus demonstrating their high antigen-presenting capacity. The DC pulsed overnight with GL26 tumour cell lysate displayed the same functionality.
Vaccination effect of two i.p. injections of DC-Lysate against intracranial GL26 challenge
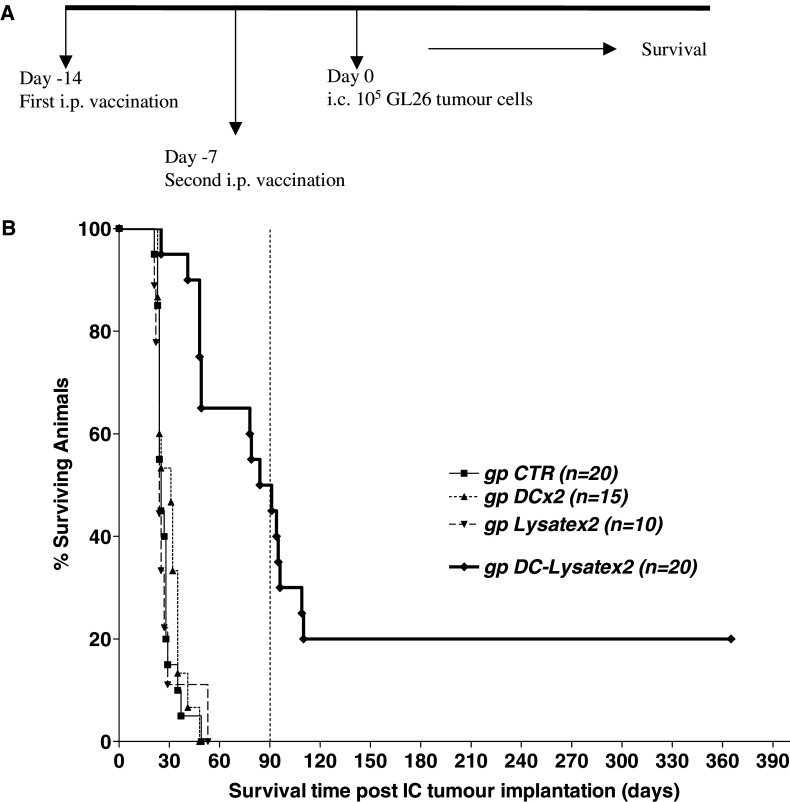
In order to define the antitumour efficacy of DCs loaded with tumour lysate in the GL26 model, we first analysed the protective effect of two i.p. injections of DC-Lysate against lethal intracranial tumour challenge. Four successive experiments were performed using the same methodology; cumulative results are shown in Fig. 2. Mice were either not vaccinated (gp CTR) or vaccinated twice (days 14 and 7) with i.p. injections of unloaded DCs (gp DCx2), tumour cell lysate (gp Lysatex2) or DCs loaded with tumour lysate (gp DC-Lysatex2) before intracranial tumour implantation (Day 0). The median survival times of the CTR, unloaded DCx2 and Lysatex2 groups were similar: 25, 31, 24 days, respectively (no statistical difference between groups). In contrast, in the DC-Lysatex2 group, the median survival was strongly enhanced to 87.5 days (p < 0.0001 compared to the CTR group) and 50% of the animals were still alive 90 days after brain tumour implantation. However, a delayed tumour recurrence (after 3 months) was observed in 30% of the mice who initially responded to treatment. Finally, less than 20% of the animals were cured in 1 year.
Fig. 2.
Vaccination twice by DCs loaded with tumour lysate significantly prolonged median survival and cured 20% of animals. However, late deaths from tumour relapse after 3 months were observed. a Mice were not vaccinated (gp CTR) or intraperitoneally vaccinated twice (days 14 and 7) with unloaded DCs (gp DCx2), with tumour cell lysate (gp Lysatex2) or DCs loaded with tumour lysate (gp DC-Lysatex2) before intracranial implantation of 105 GL26 tumour cells (day 0). The mice were then followed for survival using the ethical guidelines described in materials and methods. b Survival curve represents the cumulative results of four experiments using the same methodology
Adoptive transfer of splenocytes from mice immunised with DC-Lysatex2 has a curative effect against pre-established intracranial GL26 tumours
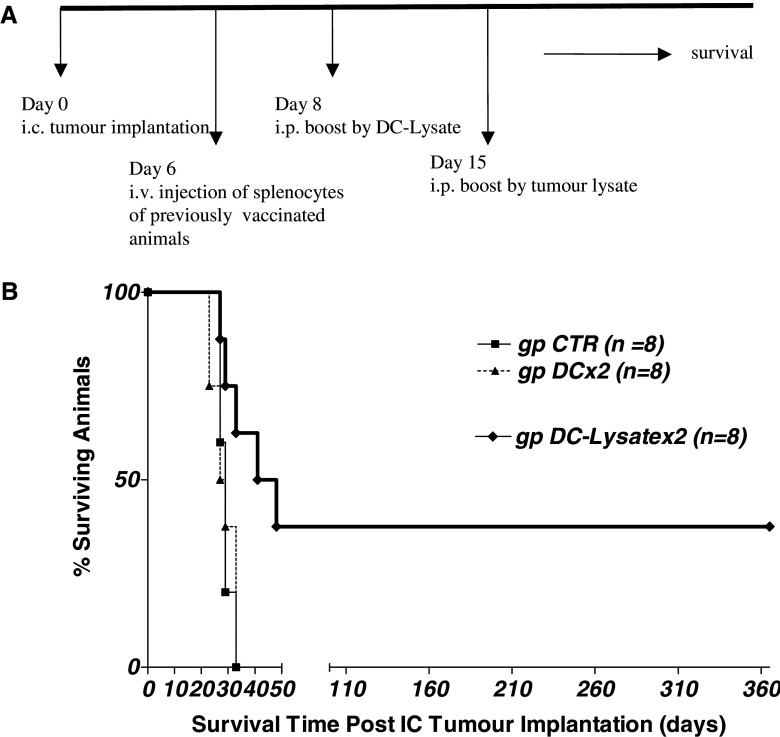
The therapeutic effect of two injections of DC-Lysate was then assessed against pre-established intracranial GL26 tumours. No curative effect was observed after i.p. injection of DC-Lysate, 2 and 9 days after i.c. tumour implantation (data not shown). These negative results may be attributed to the very rapid growing capacity of GL26 tumours, which induced animal death in about 30 days and did not allow enough time for the induction of an efficient immune response. To test this hypothesis, we investigated the effect of adoptive transfer of splenocytes from vaccinated mice. A significant curative effect against day-6 pre-established intracranial tumours was obtained after intravenous adoptive transfer of 30×106 splenocytes from mice vaccinated with DC-Lysatex2. As shown in Fig. 3, 37.5% of mice transferred with splenocytes from this group were cured, compared to 0% for the CTR group transferred with splenocytes from unvaccinated mice (mean survival of 44.5 days vs. 29 days, respectively; p = 0.0190).
Fig. 3.
Adoptive transfer of splenocytes from mice immunized by double DC-Lysate vaccination cured pre-established i.c. tumours in 37.5% of animals. a Study design: mice were vaccinated twice (once a week) with unloaded DCs or with DCs loaded with tumour lysate. Seven days after the last vaccination, mice were euthanized, splenocytes were harvested and pooled for each group of mice. An adoptive transfer of 30×106 whole splenocytes/mouse was injected into the tail vein of mice bearing a pre-established 6-day intracranial GL26 tumour (i.c. implantation, day 0). This transfer was followed by two i.p. injections with DC-Lysate (day 8) and with tumour lysate (day 15). b Survival curve of mice bearing 6-day i.c. tumours after adoptive transfer of splenocytes from vaccinated mice. One-third of animals bearing i.c. tumours treated with splenocytes from vaccinated mice by DC lysate were cured
Modification of the vaccination schedule and of the nature of the second boost to improve long-term survival
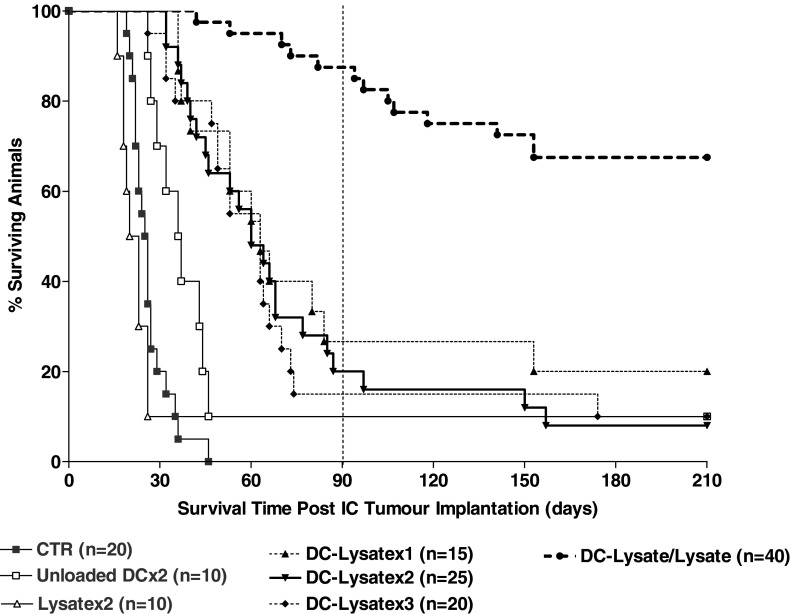
To improve long-term survival and avoid late tumour relapse, we tested the efficiency of multiple DC-Lysate injections and also investigated the effect of a prime/boost protocol using DC-Lysate for priming and tumour cell lysate alone for boosting. Cumulative results of three independent experiments using the same methodology are presented (Fig. 4). Interestingly, the vaccination with one (day-14), two (days-7 and 14) or three (day-14, day-7, and day +30) i.p. injections gave similar results in terms of survival (mean survival: 63, 60 and 63 days, respectively). In contrast, survival was dramatically improved in the group of mice vaccinated with DC-Lysate (day 14) and boosted with tumour cell lysate alone (day 7) (DC-Lysate/Lysate), compared to the reference group DC-Lysatex2 (mean survival: >105 days vs. 60 days; p < 0.0001). In the DC-Lysate/Lysate group, 87.5 % of the mice were still alive 3 months post challenge and 67.5% were cured with a follow-up of 7 months, compared to 20% and 8% at 3 and 7 months in the reference group DC-Lysatex2 (Fig. 4).
Fig. 4.
Vaccination with DC-Lysate followed by a boost with tumour lysate alone dramatically increases the long-term survival of mice. C57Bl/6 mice were vaccinated intraperitoneally either by one (14 days before tumour implantation: gp DC-Lysatex1), two (14 and 7 days before tumour implantation: gp DC-Lysatex2) or three (14, 7 days before and 30 days after tumour implantation: gp DC-Lysatex3) i.p. injections of DCs loaded with tumour lysate. One group was vaccinated with DC-Lysate for the priming (day 14) and with tumour cell lysate only for the boost (day 7) (gp DC-Lysate/Lysate). Cumulative results of three independent experiments conducted with the same methodology are shown. A 67.5% of animals in the DC-Lysate/Lysate group were considered as cured compared to 8% in the reference group DC-Lysatex2 (median survival of the DC-Lysate/Lysate group >105 days vs. 60 days for the DC-Lysatex2 group; p < 0.0001, Log-rank test)
We analysed the long-term memory of the antitumour immune response induced by such prime/boost vaccination in one experiment. Cured mice were challenged a second time by implantation of tumour cells in the contralateral frontal lobe, 8 months after the first i.c. tumour challenge. One group of cured mice (n = 7) was directly rechallenged, whereas one group (n = 6) was first injected with i.p. GL26 lysate 7 days before the second tumour challenge to recall antitumour immune memory. All the mice from these two responding groups equally survived the second i.c. challenge, compared to none of the mice from a control group (naive mice of the same age) (Fig. 5). These results suggest that the initial antitumour response induced by DC-Lysate priming followed by lysate boost generated long-lasting antitumour immune response, and that animals did not need further peripheral recall to reject subsequent tumour challenge.
Fig. 5.
Long-term specific immune response was observed with DC-Lysate/Lysate vaccination. a Study design: in one experiment with DC lysate/lysate vaccine protocol, mice still alive 8 months after the first i.c. tumour implantation were rechallenged in the controlateral hemisphere in the same manner with or without an i.p. vaccine boost by tumour lysate. b Survival curve demonstrated that all animals were still protected against tumour rechallenge
The protective effect of DC-Lysate/Lysate vaccination was T cell mediated
FACS phenotype analysis of spleen and peripheral blood samples following different vaccination schedules
In order to have a better understanding of the mechanism responsible for survival improvement in the DC-Lysate/Lysate group, splenocytes and PBL of mice unvaccinated and vaccinated by unloaded DCs, DC-Lysatex2 and DC-Lysate/Lysate were analysed on a FACS flow cytometer 14 days after i.c. tumour implantation.
In the spleen, no significant difference in the percentage of cells (T, B lymphocytes, NK cells and Monocytes-Macrophages) was observed (data not shown). However, the total number of cells counted in each spleen sample greatly increased following vaccination, with a higher number for the DC-Lysate/Lysate group (respectively, 24.5×106 cells for naive mice; 17.5×106 for CTR mice bearing tumour; 35.5×106 for the unloaded-DC group; 48×106 for the DC-Lysatex2 group, and 68×106 for the DC-Lysate/Lysate group). These results demonstrate a global stimulation of the immune system, especially in the DC-Lysate/Lysate group.
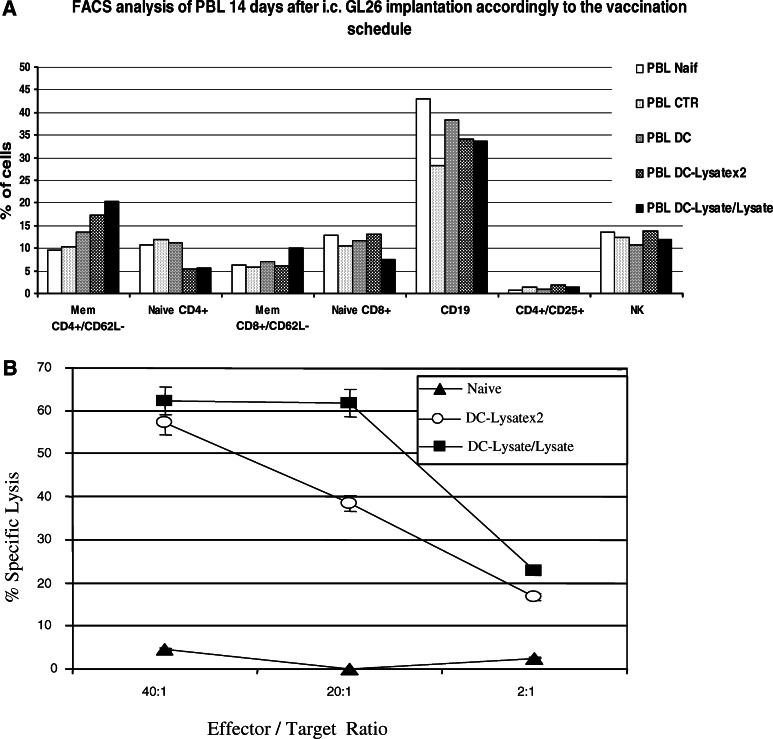
In the PBL (Fig. 6a), no difference was observed in the percentage of NK and B lymphocytes between the different groups. No modification of regulatory T cells defined as CD4+/CD25+ T lymphocytes was noted. In contrast, an increase of the memory pool of T helper lymphocytes defined as CD4+/CD62L—was observed in the vaccinated groups. The pool of memory CD8+/CD62L-T lymphocytes increased only in the DC-Lysate/Lysate group. The DC-Lysate/Lysate protocol thus induced a better T-cell response.
Fig. 6.
DC-Lysatex2 and DC-Lysate/Lysate vaccine protocols both induce cytotoxic activity against GL26. a FACS analysis of PBL performed 14 days after i.c. GL26 implantation. Naïve mice and mice vaccinated by unloaded DC, DC-Lysatex2 and DC-Lysate/Lysate (n = 3 per group) were implanted i.c. at day 0 with 105 GL26 cells. 14 days later, mice were euthanized and blood samples collected for FACS analysis as described in the ‘Materials and methods’. The only difference observed is a better response for CD4+ helper memory T cell and CD8+ memory T cell in the DC-Lysate/Lysate group compared to the DC-Lysatex2 group. b Cytolytic activity against GL26 tumour cells by vaccination with DC-Lysate/Lysate or DC-Lysatex2. Splenocytes from naive mice (- -) or mice vaccinated with DC-Lysatex2 (open circle) or with DC-Lysate/Lysate (filled square) were harvested 7 days after the last vaccination and restimulated for 5 days on irradiated GL26 with low-dose IL-2 as described in ‘Materials and methods’. These effector cells were then tested for their ability to lyse γIFN-treated GL26 targets labelled with 51CR. The 51CR-release in the supernatants was measured after 4 h of incubation at 37°C at different E/T ratios. All determinations were made in triplicates. Representative results from two independent experiments are shown. Both vaccine protocols induced cytolytic activity against GL26 cells
Cytotoxic assay
A cytotoxic assay was performed in order to demonstrate that our vaccination approach generated a specific CTL antitumour response. Splenocytes from mice vaccinated using the DC-Lysatex2 or DC-Lysate/Lysate protocols were restimulated in vitro on irradiated GL26 with low-dose IL-2, then tested for cytotoxic activity against GL26 cells pre-treated with γIFN to increase the expression of MHC class I molecules. Splenocytes from vaccinated mice specifically lysed 57% (group DC-Lysatex2) and 62% (group DC-Lysate/Lysate) of γIFN pre-treated GL26 targets (at E/T ratio of 40) (Fig. 6b). These splenocytes were also able to lyse parental GL26 cells, though with a lower efficiency, demonstrating the implication of CD8+ T cells: 38% for the DC-Lysatex2 group versus 44% for the DC-Lysate/Lysate group at E/T = 40 (data not shown). This cytotoxicity was not NK-mediated, as demonstrated by the absence of lysis of NK-sensitive YAC-1 murine target (data not shown). Moreover, the percentages of NK cells in the different groups were found identical in the brain tumour, in the spleen and in the PBL (FACS analysis 14 days after i.c. tumour implantation; for brain tumours: enzymatic digestion with collagenase, DNase and hyaluronidase was done before antibodies labelling and FACS analysis; data not shown).
The implication of T cells as antitumour effectors was also indirectly confirmed in vivo by the absence of protection against i.c. The GL26 challenge when using the DC-Lysate vaccination in nude mice (data not shown).
A specific humoral response was induced only by DC-Lysate/Lysate vaccination
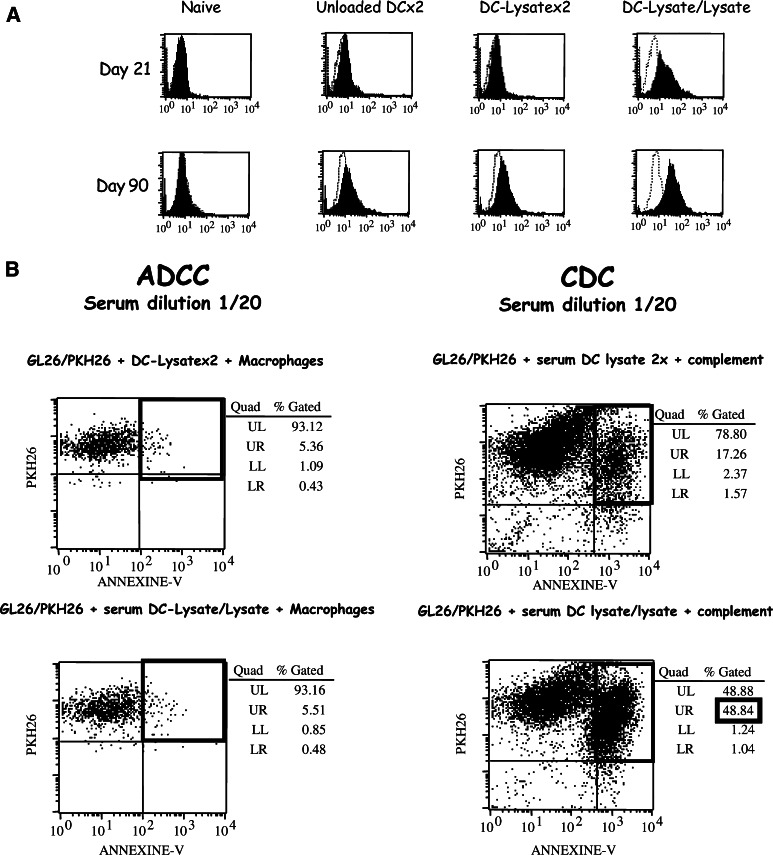
To evaluate the ability of the different vaccine protocols to induce an in vivo humoral response, specific mouse antibodies against GL26 were searched in the serum of mice vaccinated with unloaded-DCx2, DC-Lysatex2 or DC-Lysate/Lysate, as described in Materials and methods. The data in Fig. 7a show that in vivo vaccination with DC-Lysate/Lysate prime/boost protocol induced a strong humoral response specific for GL26, whereas DC-Lysatex2 produced no specific antibodies. The humoral response observed in the DC-Lysate/Lysate group was detected 21 days and 3 months post priming.
Fig. 7.
Only the DC-Lysate/Lysate protocol induces a specific humoral response mediating tumour lysis through Complement-Dependent Cytotoxicity. a Specific humoral response induced by DC-Lysate/Lysate vaccination only. Mice were vaccinated twice by unloaded DCs, DC-Lysate or with a priming by DC-Lysate followed by a boost with tumour lysate alone at days 14 and 7 before i.c. tumour implantation (Day 0). At days 21 and 90, specific mouse antibodies against GL26 were examined in the sera of mice vaccinated with unloaded-DCx2, DC-Lysatex2 or DC-Lysate/Lysate by incubation of serum dilutions (1/20) on GL26 cells. The specific binding of antibodies on GL26 cells was detected by FACS analysis after incubation with a secondary anti-mouse IgG1-FITC conjugated antibody. The in vivo vaccination with the DC-Lysate/Lysate prime/boost protocol induced a humoral response specific for GL26, detectable 21 days after the priming (the variation of mean fluorescence intensity (MFI) compared to unloaded-DC CTR was 18.1), whereas DC-Lysatex2 vaccine produced no specific antibodies (no difference of MFI compared to unloaded DC CTR). This humoral response observed in the DC-Lysate/Lysate group remained detectable 3 months post priming (variation of MFI compared to unloaded-DCx2 CTR was 42.3). b Specific anti-GL26 antibodies contribute to antitumour response through CDC and not through macrophage-mediated ADCC. Naïve mice and mice vaccinated by unloaded DCs, DC-Lysatex2, and DC-Lysate/Lysate were implanted i.c. with GL26 tumour cells (day 0). At day 90, blood samples were collected and ADCC and CDC assays were performed as described in ‘Materials and methods’ to study the mechanism of GL26 antibody toxicity. In the DC-Lysate/Lysate group, a 48.8% GL26 cell lysis (corresponding to 31.6% of specific lysis) was observed in the CDC assay compared to 17.2% of non-specific lysis in the CTR groups. No ADCC was observed
Specific GL26 antibodies found in the DC-Lysate/Lysate group induced lysis of tumor cells by CDC and not by macrophage-mediated ADCC
Antibodies can mediate tumour cell lysis by two mechanisms CDC or ADCC. The ADCC assays using thioglycolate activated macrophages as effectors and CDC assays were performed in our model using serum from the different of vaccinated groups. Whatever the dilution used for the test, no macrophage-mediated ADCC was observed (Fig. 7b, left). No significant CDC was noted in the DC-Lysatex2 group (17.2% as in control groups) but the antibodies found in the sera of the DC-Lysate/Lysate group mediated a 31.6% specific complement cell lysis (48.8% of total lysis-17.2% of aspecific lysis) (Fig. 7b, right). These specific GL26 antibodies are thus considered to contribute to a better antitumour immune response via a CDC mechanism.
Discussion
The Dendritic cell immunotherapy for inducing specific immunity against tumour has shown promising activity in both preclinical models and clinical observations. However, recent reports indicate that the efficacy of the induction or the maintenance of antitumour immune responses need to be improved before maximal effects can be achieved [33]. The aim of the present study was to define the most effective way to deliver DC immunotherapy in a preclinical orthotopic murine glioma model (GL26). Tumour cell lysate was chosen to pulse DCs because of the relative paucity of well-defined glioma-associated antigens. The use of tumour cell lysate may also provide both classes I and II epitopes and prevent immune evasion by tumour clones. Peripheral vaccination with ex vivo generated DCs was chosen to overcome the intratumoral immunosuppressive context. The i.p. route was chosen for comparison with other major glioma preclinical studies [19, 20, 23] and also to induce a systemic immune response able to reach the brain.
We investigated first the vaccination effect of two IP injections of DCs loaded with tumour cell lysate (DC-Lysatex2) before IC implantation of GL26 tumour cells. A strongly increased median survival was observed, with 50% of surviving animals at 90 days in the DC-Lysatex2 group, compared to 0% in the control groups (unvaccinated or vaccinated by unloaded DCs or tumour lysate alone). The results of experiments of nude mice showed no protection (data not shown) and the results of cytotoxic assays indicated the involvement of T cells in the antitumour effect. No curative effect was observed when animals were treated on days 2 and 9 after GL26 cells i.c. injection (data not shown). This was probably due to a too fast tumour growth preventing the initiation of an adaptive immune response. This hypothesis was confirmed by the results of adoptive transfer experiments allowing the cure of 37.5% mice bearing 6-day tumours. Those data are in agreement with previous findings obtained in the glioma model using DC vaccination, reporting 50–75% of surviving animals at 2 or 3 months post intracranial challenge [18, 20, 21]. All vaccination protocols used multiple DC injections for immune stimulation and had a short follow-up (no more than 3 months compared to 1 year in the present study). Interestingly, we report for the first time that some animals initially responding to DC-Lysatex2 vaccination later developed a lethal intracranial tumour, with less than 20% of animals ultimately cured. No sign of autoimmune disease against normal brain was observed, and late deaths were always associated with intracranial tumour recurrence. Late relapses might result from the immune selection of GL26 clones resistant to CTL lysis or from the loss of long-term immune memory. No down-regulation of MHC-class I molecules was observed on recurrent tumours (same level of expression on parental GL26 cell line, on primary untreated tumours and on late recurring tumours; data not shown).
Several options can be considered to improve DC antitumour activity: the increase of DC maturity (with γIFN and ‘ribomunyl’), the addition of helper signal (keyhole limpet hemocyanin, used KLH as an immunogenic carrier protein to elicit T cell help) or adjuvant cytokines (IL-2) [34, 35]. Such strategies were assessed in our model without any improvement on the long-term survival of mice (data not shown).
Finally, we investigated in our model the number of DC-Lysate injections as well as the nature of the vaccine used for the second boost. Surprisingly, the vaccination effect of one, two or three DC-Lysate injections gave similar results (mean survival around 60 days, compared to 25 days for the CTR group). In contrast, a vaccine schedule using DC-Lysate for the priming followed by tumour lysate only for the boost (DC-Lysate/Lysate) was dramatically beneficial for long-term survival (mean survival >105 days with 67.5% of animals cured at 7 months compared to a mean survival of 60 days and only 8% of cured mice with the DC-Lysatex2 protocol). Furthermore, all cured animals were protected against a second contralateral i.c. tumour challenge performed several months after the first one, thus demonstrating the persistence of long-term antitumour immune memory response.
To our knowledge, this study represents the first in vivo evidence that multiple DC injections are not an efficient option to obtain optimal long-term antitumour immunity. Several explanations can be provided.
The cellular immune response is improved in the DC-Lysate/Lysate group. In our model, PBL analysis according to the vaccination schedule clearly showed a better CD4+ helper T-cell response associated with a better CD8+ T-cell response in the DC-Lysate/Lysate group as compared to the DC-Lysatex2 group (Fig. 6a). As show in Fig. 6b, a stronger cytotoxic activity against GL26 was observed in the DC-Lysate/Lysate group. This was also observed in vitro by Kurokawa using PBL from patients with renal cell carcinoma who reports a clonal expansion of tumour-specific CD8+ CTL only after sequential stimulation with DC-Lysate followed by tumour cells alone [36].
In addition to cellular immunity, generation of specific antibodies may also contribute to the regression of some cancers [37]. Antibodies against oncogenic proteins or growth factors could have direct antitumour efficacy. Antibodies can also mediate tumour cell killing through antibody-dependent cell-mediated cytotoxicity (ADCC) or through complement activation (CDC) [38]. Interestingly, only the DC-Lysate/Lysate protocol induced the generation of a humoral response with GL26-specific IgG1 antibodies in the sera of mice (as shown in Fig. 7a). Similarly, Sornasse et al. have reported that a priming with antigen-pulsed-DCs followed by a boost with soluble antigens can efficiently induce a humoral antitumour response in vivo [39]. In our model, CDC (Fig. 7b) was the mechanism of cell toxicity for the GL26 antibodies observed in the DC-Lysate/Lysate group. Using peritoneal exudate consisting mostly of macrophages and low level of NK cells, we observed no ADCC (Fig. 7b). These results demonstrate that macrophage-mediated ADCC was not the mechanism o f tumour cytolysis, however NK cell mediated-ADCC cannot be ruled out. These antibodies, shown to be still present at 90 days, could explain the improvement of the long-term survival of the DC-Lysate/Lysate group associated with a better CTL response.
The number of injections for DC immunotherapy remains under debate. Interestingly in our model, DC-Lysatex1, DC-Lysatex2, and DC-Lysatex3 median survival times were identical, indicating that only the first DC injection was effective. In the study by Kurokawa mentioned above, multiple in vitro stimulations with DC-Lysate led to a predominant CD4+ T-cell response [36]. Chakraborty has also described in vitro the emergence of regulatory CD4+ T cells in response to repetitive stimulation with DCs loaded with melanoma lysate. These CD4+ T cells have a Th2 phenotype, secrete large amounts of IL4 and IL10 and block the activation of T lymphocytes [40]. In our model, no increase of CD4+/CD25+ regulatory T-cell level was noted in PBL or splenocytes of mice vaccinated with repetitive DC-Lysate injections. Frequent injections of DC-Lysate may be also detrimental by causing the activation-induced cell death of recently activated T cells [41]. Conversely, antigen-specific primed CD8+ T cells are capable to eliminate antigen-loaded DCs. This mechanism may serve as a negative feedback to limit the activity of DCs within the lymph nodes. A second injection with tumour lysate alone may implicate cross priming by host DCs which processed and presented tumour antigens, but at a lower density compared to in vitro-loaded DCs. Therefore, host DCs should be cleared less rapidly by recently activated CTL. Finally, DC reinjection can take place during the memory phase when T lymphocytes must be strongly stimulated to become operative (i.e. at 2 months from priming) [42].
Most ongoing immunotherapeutic protocols use multiple rounds of DC vaccination at fairly short intervals [15, 17, 22, 24, 43–46]. These clinical trials have demonstrated the feasibility and the safety of DC-based vaccination but have yielded only few clinical responses [47]. Despite the availability of very sensitive techniques for the immunomonitoring of vaccinated patients (i.e. ELISPOT and peptide-MHC tetramer staining), immune responses have been weak and their durability has not been clearly established. Taken together, our experimental data indicate that if DCs loaded with tumour lysate are essential for the priming, they are less effective than tumour cell lysate alone for boosting the antitumour immune response and for inducing long-term immune memory. This may be of importance for the design of clinical trials in order to reach the full potential of DC-based immunotherapy.
Acknowledgements
We thank Drs Christine Menetrier-Caux, Christophe Caux, and Nathalie Vermare-Bendriss for helpful comments on the manuscript and Dominique Reynaud for technical assistance. This study was supported, in part, by grants from the French national league against cancer, the departmental leagues against cancer of Ardèche and Drôme, the French Association for Research on Cancer (ARC) and the breast cancer research foundation (BCRF).
References
- 1.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/S0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 2.Parney IF, Hao C, Petruk KC. Glioma immunology and immunotherapy. Neurosurgery. 2000;46:778–791. doi: 10.1097/00006123-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Walker PR, Calzascia T, Dietrich PY. All in the head: obstacles for immune rejection of brain tumours. Immunology. 2002;107:28–38. doi: 10.1046/j.1365-2567.2002.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrin G, Schnuriger V, Quiquerez AL, Saas P, Pannetier C, de Tribolet N, Tiercy JM, Aubry JP, Dietrich PY, Walker PR. Astrocytoma infiltrating lymphocytes include major T cell clonal expansions confined to the CD8 subset. Int Immunol. 1999;11:1337–1350. doi: 10.1093/intimm/11.8.1337. [DOI] [PubMed] [Google Scholar]
- 5.Ridley A, Cavanagh JB. Lymphocytic infiltration in gliomas: evidence of possible host resistance. Brain. 1971;94:117–124. doi: 10.1093/brain/94.1.117. [DOI] [PubMed] [Google Scholar]
- 6.Yoshida J, Kajita Y, Wakabayashi T, Sugita K. Long-term follow-up results of 175 patients with malignant glioma: importance of radical tumour resection and postoperative adjuvant therapy with interferon, ACNU and radiation. Acta Neurochir (Wien) 1994;127:55–59. doi: 10.1007/BF01808547. [DOI] [PubMed] [Google Scholar]
- 7.Yung WK, Prados M, Levin VA, Fetell MR, Bennett J, Mahaley MS, Salcman M, Etcubanas E. Intravenous recombinant interferon beta in patients with recurrent malignant gliomas: a phase I/II study. J Clin Oncol. 1991;9:1945–1949. doi: 10.1200/JCO.1991.9.11.1945. [DOI] [PubMed] [Google Scholar]
- 8.Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, Pierz DM, Chen DK, Budzilovich GN, Ransohoff J. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–852. doi: 10.1002/1097-0142(19950901)76:5<840::AID-CNCR2820760519>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Merchant RE, Merchant LH, Cook SH, McVicar DW, Young HF. Intralesional infusion of lymphokine-activated killer (LAK) cells and recombinant interleukin–2 (rIL-2) for the treatment of patients with malignant brain tumor. Neurosurgery. 1988;23:725–732. doi: 10.1097/00006123-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Bloom HJ, Peckham MJ, Richardson AE, Alexander PA, Payne PM. Glioblastoma multiforme: a controlled trial to assess the value of specific active immunotherapy in patients treated by radical surgery and radiotherapy. Br J Cancer. 1973;27:253–267. doi: 10.1038/bjc.1973.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trouillas P. Immunology and immunotherapy of cerebral tumors. Current status. Rev Neurol (Paris) 1973;128:23–38. [PubMed] [Google Scholar]
- 12.Weller M, Fontana A. The failure of current immunotherapy for malignant glioma. Tumor-derived TGF-beta, T-cell apoptosis, and the immune privilege of the brain. Brain Res Brain Res Rev. 1995;21:128–151. doi: 10.1016/0165-0173(95)00010-0. [DOI] [PubMed] [Google Scholar]
- 13.Schuler G, Steinman R. Dendritic cells as adjuvants for immune-mediated resistance to tumors. Exp Med. 1997;186(8):1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young JW, Inaba K. Dendritic cells as adjuvants for class I major histocompatibility complex-restricted antitumor immunity. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg SA, Zhai Y, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Seipp CA, Einhorn JH, Roberts B, White DE. Immunizing patients with metastatic melanoma using recombinant adenoviruses encoding MART-1 or gp100 melanoma antigens. J Natl Cancer Inst. 1998;90:1894–1900. doi: 10.1093/jnci/90.24.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kugler A, Stuhler G, Walden P, Zoller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Muller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert RH. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332–336. doi: 10.1038/73193. [DOI] [PubMed] [Google Scholar]
- 18.Akasaki Y, Kikuchi T, Homma S, Abe T, Kofe D, Ohno T. Antitumor effect of immunizations with fusions of dendritic and glioma cells in a mouse brain tumor model. J Immunother. 2001;24:106–113. doi: 10.1097/00002371-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Aoki H, Mizuno M, Natsume A, Tsugawa T, Tsujimura K, Takahashi T, Yoshida J. Dendritic cells pulsed with tumor extract-cationic liposome complex increase the induction of cytotoxic T lymphocytes in mouse brain tumor. Cancer Immunol Immunother. 2001;50:463–468. doi: 10.1007/s002620100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heimberger AB, Crotty LE, Archer GE, McLendon RE, Friedman A, Dranoff G, Bigner DD, Sampson JH. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103:16–25. doi: 10.1016/S0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 21.Insug O, Ku G, Ertl HC, Blaszczyk-Thurin M. A dendritic cell vaccine induces protective immunity to intracranial growth of glioma. Anticancer Res. 2002;22:613–621. [PubMed] [Google Scholar]
- 22.Kikuchi T, Akasaki Y, Irie M, Homma S, Abe T, Ohno T. Results of a phase I clinical trial of vaccination of glioma patients with fusions of dendritic and glioma cells. Cancer Immunol Immunother. 2001;50:337–344. doi: 10.1007/s002620100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamanaka R, Yajima N, Tsuchiya N, Honma J, Tanaka R, Ramsey J, Blaese M, Xanthopoulos KG. Administration of interleukin-12 and −18 enhancing the antitumor immunity of genetically modified dendritic cells that had been pulsed with Semliki forest virus-mediated tumor complementary DNA. J Neurosurg. 2002;97:1184–1190. doi: 10.3171/jns.2002.97.5.1184. [DOI] [PubMed] [Google Scholar]
- 24.Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, Zhang W, Prins RM, Black KL. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61:842–847. [PubMed] [Google Scholar]
- 25.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nat Immunol. 2004;5:7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 26.Fong L, Engleman EG. Dendritic cells in cancer immunotherapy. Annu Rev Immunol. 2000;18:245–273. doi: 10.1146/annurev.immunol.18.1.245. [DOI] [PubMed] [Google Scholar]
- 27.Ausman JI, Shapiro WR, Rall DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–2400. [PubMed] [Google Scholar]
- 28.Prins RM, Odesa SK, Liau LM. Immunotherapeutic targeting of shared melanoma-associated antigens in a murine glioma model. Cancer Res. 2003;63:8487–8491. [PubMed] [Google Scholar]
- 29.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tutt AL, French RR, Illidge TM, Honeychurch J, McBride HM, Penfold CA, Fearon DT, Parkhouse RM, Klaus GG, Glennie MJ. Monoclonal antibody therapy of B cell lymphoma: signaling activity on tumor cells appears more important than recruitment of effectors. J Immunol. 1998;161:3176–3185. [PubMed] [Google Scholar]
- 31.Fischer K, Mackensen A. The flow cytometric PKH-26 assay for the determination of T-cell mediated cytotoxic activity. Methods. 2003;31:135–142. doi: 10.1016/S1046-2023(03)00123-3. [DOI] [PubMed] [Google Scholar]
- 32.Labeur MS, Roters B, Pers B, Mehling A, Luger TA, Schwarz T, Grabbe S. Generation of tumor immunity by bone marrow-derived dendritic cells correlates with dendritic cell maturation stage. J Immunol. 1999;162:168–175. [PubMed] [Google Scholar]
- 33.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 34.Banchereau J, Schuler-Thurner B, Palucka AK, Schuler G. Dendritic cells as vectors for therapy. Cell. 2001;106:271–274. doi: 10.1016/S0092-8674(01)00448-2. [DOI] [PubMed] [Google Scholar]
- 35.Shimizu K, Thomas EK, Giedlin M, Mule JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001;61:2618–2624. [PubMed] [Google Scholar]
- 36.Kurokawa T, Oelke M, Mackensen A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer. 2001;91:749–756. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1141>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 37.Glennie MJ, Johnson PW. Clinical trials of antibody therapy. Immunol Today. 2000;21:403–410. doi: 10.1016/S0167-5699(00)01669-8. [DOI] [PubMed] [Google Scholar]
- 38.Gelderman KA, Tomlinson S, Ross GD, Gorter A. Complement function in mAb-mediated cancer immunotherapy. Trends Immunol. 2004;25:158–164. doi: 10.1016/j.it.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Sornasse T, Flamand V, De Becker G, Bazin H, Tielemans F, Thielemans K, Urbain J, Leo O, Moser M. Antigen-pulsed dendritic cells can efficiently induce an antibody response in vivo. J Exp Med. 1992;175:15–21. doi: 10.1084/jem.175.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chakraborty NG, Li L, Sporn JR, Kurtzman SH, Ergin MT, Mukherji B. Emergence of regulatory CD4+ T cell response to repetitive stimulation with antigen-presenting cells in vitro: implications in designing antigen-presenting cell-based tumor vaccines. J Immunol. 1999;162:5576–5583. [PubMed] [Google Scholar]
- 41.Dhodapkar MV, Krasovsky J, Steinman RM, Bhardwaj N. Mature dendritic cells boost functionally superior CD8(+) T-cell in humans without foreign helper epitopes. J Clin Invest. 2000;105:R9–R14. doi: 10.1172/JCI9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hermans IF, Ritchie DS, Yang J, Roberts JM, Ronchese F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J Immunol. 2000;164:3095–3101. doi: 10.4049/jimmunol.164.6.3095. [DOI] [PubMed] [Google Scholar]
- 43.Banchereau J, Palucka AK, Dhodapkar M, Burkeholder S, Taquet N, Rolland A, Taquet S, Coquery S, Wittkowski KM, Bhardwaj N, Pineiro L, Steinman R, Fay J. Immune and clinical responses in patients with metastatic melanoma to CD34(+) progenitor-derived dendritic cell vaccine. Cancer Res. 2001;61:6451–6458. [PubMed] [Google Scholar]
- 44.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumour lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka R, Abe T, Yajima N, Tsuchiya N, Homma J, Kobayashi T, Narita M, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: results of a clinical phase I/II trial. Br J Cancer. 2003;89:1172–1179. doi: 10.1038/sj.bjc.6601268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kikuchi T, Akasaki Y, Abe T, Fukuda T, Saotome H, Ryan JL, Kufe DW, Ohno T. Vaccination of glioma patients with fusions of dendritic and glioma cells and recombinant human interleukin 12. J Immunother. 2004;27:452–459. doi: 10.1097/00002371-200411000-00005. [DOI] [PubMed] [Google Scholar]
- 47.Figdor CG, De Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]