Abstract
The proinflammatory cytokine tumor necrosis factor-alpha (TNFα) exists naturally in two forms, a 26 kDa transmembrane form (TM-TNFα), and a 17 kDa secretory form (S-TNFα). The biological roles for each of these forms of TNFα in tumor killing have not been completely elucidated. Therefore, in this study, three different recombinant retroviral vectors, wild-type TNFα, solely secretable TNFα mutant, and uncleavable transmembrane TNFα mutant, were constructed by molecular techniques and stably transfected into a murine hepatic carcinoma cell line (H22). TNFα, either secreted in cell culture supernatants by secretable TNFα mutant- or wild-type TNFα-producing tumor cells, or as a treansmembrane form expressed on the cell surface of uncleavable TNFα mutant- or wild-type TNFα-synthesizing tumor cells, was demonstrated to be cytotoxic against the TNF sensitive L929 cell line. The H22 cells transfected with the three different forms of TNFα were shown to kill parental H22 cells in an in vitro cytotoxicity assay [effect/target (E/T) ratio-dependent manner], and their maximal killing rates were ~38–43% at E/T ratio of 5:1. The injection of total 2.5×105 mixed cells containing transfected and parental H22 tumor cells at different ratios into syngeneic mice resulted in the inhibition of tumor growth with a maximal inhibition rates of ~57~72% at E/T ratio of 5:1. A transient weight loss was found in mice bearing solely secretable TNFα mutant producing tumors, whereas no obvious side effects were seen in mice bearing uncleavable TNFα mutant or wild-type TNFα expressing tumors. Finally, we demonstrate that tumors secreting S-TNFα promoted the subsequent infiltration of CD4+ T cells, and to a lesser extent CD8+ T cells, to the tumor site. The TM-TNFα expressing tumors up-regulated Fas (CD95) expression and inhibited the expression of tumor metastasis associated molecule CD44v3. These results suggest that S-TNFα and TM-TNFα kill cancer cells in vivo through different mechanisms of action. We conclude that the non-secreted form of TNFα may be an ideal candidate for cancer gene therapy due to its therapeutic potential and lowered side effect profile.
Keywords: Transmembrane TNFα, Secretory TNFα, Cytokine antitumor effects, Gene therapy, Biological Immunotherapy
Introduction
Tumor necrosis factor-alpha (TNFα) is a pleotrophic cytokine produced by a wide variety of cell types including macrophages, T lymphocytes and endothelial cells. TNFα exists in two bioactive forms, a 26 kDa transmembrane TNFα (TM-TNFα) form, and a 17 kDa secretory TNFα (S-TNFα) form [1]. Human TNFα is initially synthesized as a 26 kDa type II transmembrane molecule. Upon cellular activation, TM-TNFα can be cleaved into 17 kDa secretory form by membrane-bound metalloproteases, including TNFα converting enzyme (TACE) [2]. The biological function(s) of both forms of TNFα can be signaled by two distinct TNF receptors: TNFR1 (55–60 kDa) and TNFR2 (75–80 kDa). TNFR1 is constitutively expressed in nearly all tissues, while TNFR2 is more restricted and tightly regulated in expression [3].
Recent studies have shown that TM-TNFα has biological activities distinct from S-TNFα. For example, TM-TNFα can kill various types of tumor cells, which are resistant to S-TNFα [4], showing a wider tumoricidal spectrum than S-TNFα. S-TNFα plays a crucial role in the development of endotoxic shock whereas TM-TNFα transgenic mice were completely protected from endotoxic shock [5]. Furthermore, a critical involvement of TM-TNFα signaling has been revealed in adaptive immune responses, including the interactions between T and B lymphocytes [6], and the release of numerous cytokines (including IL-12), and enhanced macrophage responses towards leishmania [7].
Tumor necrosis factor-alpha was originally identified in the late 1970s as a mediator of necrosis for various murine and human tumor cells and cell lines. Importantly, it has been shown that TNFα is able to kill many kinds of tumor cells in vivo as well as in vitro [8, 9]. The clinical applications of the soluble trimeric form of TNFα, however, have been hampered because therapeutic doses of this potent cytokine have been accompanied by serious systemic toxic side effects [10]. It is, therefore, very important to develop newer strategies in cancer immunotherapy to alleviate these potentially life-threatening side effects and, in addition, to simultaneously improve the antitumor activities of this cytokine in the patient.
TM-TNFα acts locally by cell-cell contact and is able to kill susceptible tumor cells that may be either sensitive or resistant to S-TNFα. It has been suggested that the expression of non-secreted TM-TNFα within a tumor may serve to reduce systemic toxicity while retaining effective antitumor activities. Importantly, results from previous research studies in regards to the antitumor effects of TM-TNFα in vivo have been controversial. Studies by Karp and colleagues [11] have shown that TM-TNFα expressing tumors grow progressively over time in a mouse model of tumorigenesis, while Marr and colleagues [12] have reported that treatment of mice with an adenovirus vector containing the TM-TNFα gene induced partial, and in some cases, permanent tumor regression.
The goal of our present study was to delineate the different biological mechanisms of action responsible for the cytotoxic and tumoricidal activities of two physiological forms of human TNFα. In an attempt to separate the functions of these two TNFα forms, we genetically generated two mutant forms of the wild-type TNFα cDNA that could encode both uncleavable form of 26 kDa TNFα and a solely secretable form of 17 kDa TNFα. Three retroviral vectors were constructed which expressed TNFα in different physiological forms. These vectors were subsequently transfected into the murine liver carcinoma cell line H22, and the cytotoxicity and tumorigenicity of these transfected cells were assessed in vitro and in vivo. The mechanisms of these TNFα forms involved in the regression of tumors were also compared. Our results suggest that there are striking biological mechanisms of action differences between the transmembrane and secretory forms of TNFα in our model systems
Materials and methods
Tumors and cell lines
The L929 cell line, a murine fibroblast line sensitive to either murine or human TNFα, was used for TNF bioassay [13]. The virus packaging cell line PA317 [14] and the murine hepatic carcinoma H22 [15], which do not express detectable TNFα, were kindly provided by Professor Xuetao Cao (Department of Immunology and Shanghai Brilliance Biotechnology Institute, Second Military Medical University, Shanghai, People’s Republic of China). All cell lines were cultured in RPMI-1640 medium (GIBCO, Grand Island, NY, USA) supplemented with 10% heat inactivated (56°C, 30 min), pyrogen-free FCS, 1.0 mM sodium pyruvate, 2.0 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin, and 5×10−5 M 2-ME.
Gene transfer
pLXSN is a Moloney leukemia virus based retrovirus. The 5′ viral LTR of pLXSN contains promoter/enhancer sequences that control the expression of the interested gene in the multiple cloning site. The SV40 early promoter (P SV40e) controls expression of the neomycin resistance gene (Neor), which allows antibiotic selection in eukaryotic cells. The pLW-TNF is a vector in which the human cDNA for wild-type TNF (wt-TNFα) was cloned into the HpaI (blunt end) and XhoI (stick end) cloning site of LXSN just 5′ of the SV40 promoter. The TNFΔ1-12 sequence encoding a mutant transmembrane TNFα molecule, which has 12 amino acids (residues 1~12 of TNFα, as the cleavage site) deleted to prevent cleavage of the membrane 26 kDa TNFα into the secretory TNFα form [16, 17], was also cloned into LXSN. Similarly cloned into LXSN was TNF-IL-2SIG, which has the region coding for the TNFα signal peptide (amino acids –76 to -1) replaced with a sequence coding for the IL-2 signal peptide (amino acids –20 to -1) to express a solely secretable form of 17 kDa TNFα. All the expression constructs were sequenced on an ABI DNA sequencer to confirm the integrity and preciseness of the described mutations. Packaging of the viral particles was achieved by transfecting the expression plasmids into PA317 cells with lipofectin (GIBCO, Grand Island, NY, USA). Positive clones were selected in medium containing 800 μg/ml G418 (GIBCO, Grand Island, NY, USA) and the viral titers released from theses clones were determined. Supernatant from packing cells was collected after 48 h incubation and filtered through sterile 0.45 μm syringe filters. H22 cells (2×105) were infected with 1 ml of retroviral supernatant and 8 μg/ml polybrene for 4 h at 37°C, followed by addition of 20% FCS RPMI 1640 into the culture to dilute polybrene to 2 μg/ml. Then 48 h post-transduction, cells were selected in 800 μg/ml G418. The selections lasted about 2 weeks and the bulk-transduce cell lines were subcloned by limiting dilution at 0.3 cells /100 μl/well in 96-well microtiter plates and maintained thereafter in 200 μg/ml G418.
Southern blots
Total genomic DNA was isolated, and digested with KpnI. The DNA samples were loaded on a 0.8% agarose gel. After electrophoresis, DNAs were transferred to a nylon membrane, and hybridized to a DIG-labeled cDNA-specific for human TNFα (Boerhinger Mannheim, Germany).
Cell surface TNF expression was determined by FACS analysis
Transfected H22 cells were harvested with PBS, washed and incubated with a mouse anti-human TNF monoclonal antibody (DAKO, Denmark). A goat anti-mouse FITC-conjugated IgG was used for staining and analysis on a FACScan flow cytometer (Becton Dickenson).
ELISA
1×106 cells of each clone were cultured for 24 h in 1 ml of complete medium, and the supernatants were then collected and tested for secretory TNFα by ELISA (Bangding Biotech Co. Ltd. China). A mouse mAb specific for human TNFα was used to coat the tissue culture plates. After incubation with either the samples or rHuTNFα standard, a polyclonal goat antibody against TNFα, conjugated to horseradish peroxidase, was added. The ELISA kit has previously been shown to have no cross-reaction to murine TNFα.
TNFα bioassay
The biological activity of TNFα released and expressed on the cell surface of transfected H22 cells was detected by measuring cytotoxicity against the parental H22 tumor cells at different E/T ratios (1:1, 1:5, 5:1), respectively. The mixed cells (4×105/100 μl/well) were seeded in 96-well microtiter plates and incubated at 37°C, 5% CO2 for 48 h. Viability of cells was measured by staining for 4 h with 30 mM glucose-PBS containing 0.45 mg/ml of MTT (Sigma), followed by lysis of cells with 0.1 ml of 100% DMSO. The photometric measurement was performed at 570 nm on a microplate-autoreader (Titertek Multiskan Scanner). The cytotoxicity of TNFα was calculated by the following formula: Cell death rate (%)=(1-OD sample/OD control) ×100%. Specificity of TNFα determinations was assessed by the neutralization of TNFα by a highly specific mouse anti-human TNFα monoclonal antibody. The cytotoxicity of TNFα released in supernatants and expressed on cell surface of 4% paraformaldehyde fixed tumor cells transfected with TNFα and its mutants was detected against the TNF-sensitive L929 cells as described previously [18].
In vivo murine tumor model
H22 cells expressing TNFα and its mutants and parental H22 cells were mixed at different ratios, and a total of 2.5×105 mixed cells were injected S.C. into syngeneic BALB/C mice (six per group). Tumor dimensions were measured with calipers every 3 days. Mean tumor size was calculated by taking width2×length×0.52 [19]. All measurements were performed in a coded, blinded fashion.
Immunohistochemistry
Several cell surface molecules, including CD4, CD8, Fas (CD95), and CD44v3 were examined in the tumor tissue by the Avidin-biotin complex method with specific primary antibodies (1:200 dilution of a goat anti-mouse CD4 antibody, 1:200 of a goat anti-mouse CD8 antibody, 1:100 of a rabbit anti-mouse Fas antibody and 1:150 of a rabbit anti-mouse CD44V3 antibody and the biotin-conjugated secondary antibodies (anti-goat IgG or anti-rabbit IgG). Frozen sections were allowed to air-dry at room temperature for 10 min, and then fixed in colder acetone for 15 min. The sections were incubated with primary antibodies for 60 min at room temperature, followed by washing five times with PBS. After a 30-min incubation with secondary antibodies (1:200), the sections were washed three times with PBS and then incubated with peroxidase labeled streptavidin for 20 min. After washing, color reaction was performed by incubation of sections in a solution contain DAB for 5 min. The sections were then washed under running water and observed under microscope. In tumor tissue, immunoreactive cells were counted by a blinded experimenter in two sections per animal and ten squares (38.4 mm2 each) per section using a Zeiss microscope (objective ×40; eyepiece ×10). Then the percentages of CD4, CD8, Fas or CD44V3 stained cells (brown) were determined. The positive cell ratio (integral optical density value/ integral area) was calculated by Tongji Qianping Image Analysis Software. Six mice per group were used for analysis. Results represent mean ± SD. All statistics were assessed using SigmaStat software for Microsoft Windows.
Results
Biological expression of secretory and/or transmembrane TNFα by transduced H22 tumor cells
It was conformed by Southern blot analysis of wt-TNF-α, TNFΔ1-12 or TNF-IL-2SIG transduced H22 tumor clones that the proviral sequences could be hybridized to a DIG-labeled probe specific for recombinant human TNFα (data not shown). While the TNFα-specific probe did not hybridize to non-transduced cells (H22) or to cells transduced with the empty vector (H22/neo).
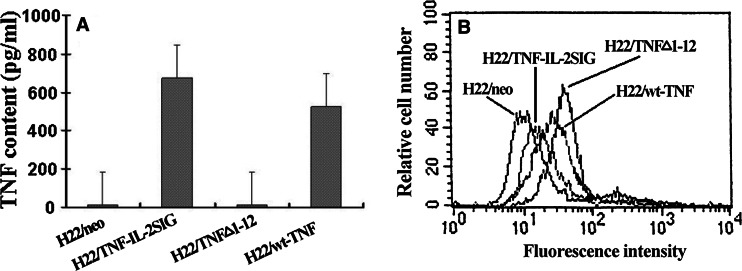
The release of human TNFα from the transduced tumor clones was measured with ELISA. As shown in Fig. 1a, neither H22/neo nor H22/TNFΔ1-12 tumor cells secreted detectable TNFα into the tissue culture supernatants. In contrast, H22/wt-TNFα cells released TNFα in 524±24.16 pg/ml/1×106 cells/24 h, H22/TNF- IL-2SIG cells produced greater amount of TNFα (674±28.51 pg/ml/1×106 cells/24 h) than H22/wt-TNFα cells.
Fig. 1.
The expression of transmembrane and secretory TNFα by transfected cells. a TNFα levels measured by ELISA from the 24 h cultured supernatants of 1×106 cells stably transfected with various TNFα constructs. b Transmembrane TNFα on cell surface of transduced H22 tumor cells was analyzed by FACS. The cultured transfected H22 tumor cells were harvested and stained with anti-TNFα mAb and FITC-labeled goat anti-mouse IgG. At least 20,000 cells were counted. Data is representative of three independent experiments
Cell surface expression of TNFα, as determined by FACS analysis, showed that H22/neo and H22/TNF-IL-2SIG tumor cells did not express human TNFα on the cell surface although H22/TNF-IL-2SIG tumor cells released high level of TNFα to the supernatants. In contrast, both H22/TNFΔ1-12 and H22/wt-TNFα cells were positively stained; the H22/TNFΔ1-12 expressed surface TNFα (97%) higher than the H22/wt-TNFα cells (51.65%) (Fig. 1b).
To ensure that TNFα and its mutants secreted by or expressed on H22 cells transfected by different TNF plasmids were functional, the cytotoxic assay was performed using the TNFα sensitive murine L929 cell line as a target. Our results showed that the formaldehyde fixed H22/TNFΔ1-12 tumor cells as well as the supernatants of cultured H22/TNF-IL-2SIG tumor cells were cytotoxic against L929 cells, while both the fixed- and the supernatants of cultured-H22/wt-TNFα cells were shown to induce cytotoxicity. The cytotoxic effect of all the transfected H22 cell clones could be completely blocked by a highly specific mouse anti-human TNFα monoclonal antibody (data not shown).
Comparison of cytotoxicity between wild-type TNFα and its mutant-transfected tumor cells versus non-transfected tumor cells in vitro
In order to determine whether H22 cells transfected with TNF plasmids were cytotoxic against the parental H22 cells, transfected/parental (E/T) cells were mixed at different increasing E/T ratios. After incubation for 48 h, H22 cells transduced with different TNF plasmids showed a significant (P<0.05) cytotoxicity effect on the parental H22 cells at an E/T ratio of 5:1 (Table 1, 2). There were no significant differences between the wild-type TNFα and its mutant-transfected H22 cells in lysing the parental cells. The antitumor effect of the transductants declined along with the decrease of E/T ratios. At the E/T ratio of 1:5, the cytotoxicity of wild-type TNFα and its mutant-transfected H22 cells was ~15.79–22.5% of their effects at the E/T 5:1 (Table 2).
Table 1.
TNFα and its two mutants generated and their expression forms
| Transduced genes | Mutation | Abbreviations of transduced tumor cells | Expression forms |
|---|---|---|---|
| Wild-type TNFα | No | H22/wt-TNFα | TM-TNF and S-TNF |
| Uncleavable TNFα mutant | Deletion of TNF cleavage site between amino acid residues 1 and 12 | H22/TNFΔ1-12 | TM-TNF |
| Solely secretable TNFα mutant | Replacement of TNF signal peptide with IL-2 signal peptide | H22/TNF-IL-2SIG | S-TNF |
| Empty vector | – | H22/neo | No TNF |
Table 2.
Cytotoxicity of H22 cells transfected with TNF-α and its mutants in vitroa
| H22/neo:H22 | H22/TNFΔ1-12:H22 | H22/TNF-IL2-SIG:H22 | H22/wt-TNF:H22 | |
|---|---|---|---|---|
| E/T=5:1 | 2.0±1.22b | 43.0±6.04 | 40.0±6.98 | 38.0±5.85 |
| E/T=1:1 | 1.8±1.64 | 17.0±4.52 | 22.0±5.06 | 15.0±6.56 |
| E/T=1:5 | 1.6±0.97 | 8.2±4.38 | 9.0±4.78 | 6.0±3.02 |
| 1:5/5:1c | 18.6% | 22.5% | 15.79% |
aTNFα and its mutants transduced and non-transduced H22 tumor cells were mixed at different E/T ratios as shown in the table. The mixed cells (4×105/100 μl/well) were seeded in 96-well microtiter plates and incubated for 48 h. Viability of cells was measured by staining with MTT. Data are represented as mean ± SD
bThe numbers in columns are cytotoxicity rates (%)
cCytotoxicity of tranductant at E/T of 1:5 is the percentage of its cytotoxicity at E/T of 5:1
Tumorigenicity of wild-type TNFα and mutant-transfected tumor cells in vivo
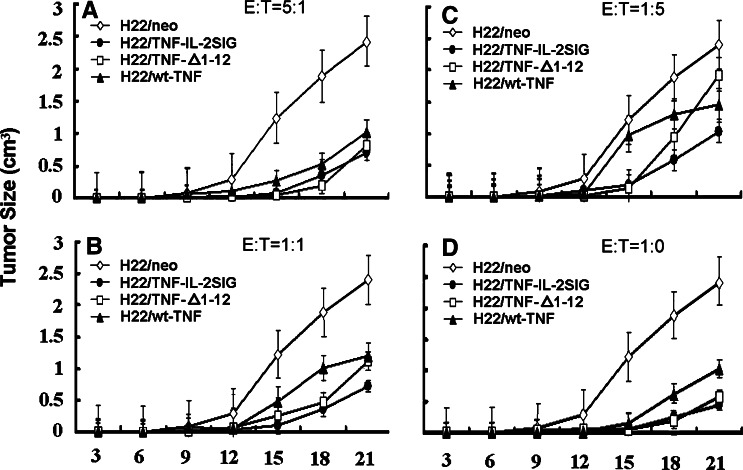
Before starting our in vivo experiments, we had tested the growth rates of parental cells and transfected cells in vitro. The results showed that transfection with various constructs did not affect the cell growth rate (data not shown). To evaluate the tumorigenicity of TNFα-transfected H22 cells, equal numbers (2.5×105) of H22/TNF-IL-2SIG, H22/ TNFΔ1-12, H22/wt-TNFα as well as H22/neo cells were inoculated subcutaneously into BALB/C mice (N=6 per group), respectively. The H22/neo control tumor cells grew to form a tumor at a visible size (0.5 cm3) at 10 days after inoculation, and reached the maximum allowable size (2.4 cm3) at 21 days. In comparison, the TNFα and its mutant-transfected tumor cells grew slowly to form the visible tumor size requiring ~15 days (i.e., 5 days later than control tumor). The tumor sizes were 0.5–1 cm3 at 21 days, which indicated that the tumorigenicity of TNFα and its mutant-transductants was significantly reduced (P<0.01, Fig. 2d). There were no observed differences regarding the growth rates in vivo between H22/ TNFΔ1-12 and H22/ TNF-IL-2SIG cells (Table 3).
Fig. 2.
The tumorigenicity of TNFα and its mutants transduced and non-transduced H22 tumor cells in vivo. TNFα or its mutants transduced tumor cells were mixed with non-transduced tumor cells at different E/T ratios – 5:1(a), 1:1(b), 1:5(c), 1:0(d). Syngeneic BALB/C mice (six per group) were injected s.c with 2.5×105 mixed tumor cells/100 μl. Tumor size was measured every 3 days. Data are represented as mean ± SD
Table 3.
The inhibitory rate of tumor growth by TNFα or its mutants in vivoa
| H22/neo:H22 | H22/TNFΔ1-12:H22 | H22/TNF-IL-2SIG:H22 | H22/wt-TNF:H22 | |
|---|---|---|---|---|
| E/T=1:0 | 1.5±0.40b | 83.0±8.04 | 82.0±7.96 | 73.0±6.62 |
| E/T=5:1 | 2.0±1.00 | 68.0±5.72 | 72.0±5.89 | 57.0±5.12 |
| E/T=1:1 | 1.8±0.67 | 54.0±3.87 | 71.0±6.05 | 54.0±3.76 |
| E/T=1:5 | 1.6±1.23 | 14.0±4.28 | 48.0±4.07 | 35.0±5.06 |
| 1:5/5:1c | 20.58% | 66.67% | 61.4% |
aTNF and its mutants transduced and non-transduced H22 tumor cells were mixed at different E/T ratios as shown in the table. 2.5×105 mixed tumor cells were injected s.c. to syngeneic BALB/C mice. Tumor size was measured every 3 days. The inhibitory rate of tumor growth was calculated with the following formula: (1-mean tumor size on 21 days of TNF transductants/ mean tumor size on 21 days of non-transfected control) ×100%. Data are represented as mean ± SD
bThe numbers in columns are inhibitory rates (%) of tumor growth
cThe inhibitory rate of tumor growth by TNFα or its mutants at E/T 1:5 is the percentage of their action at E/T 5:1
Figure 2a–c and Table 3 shows the tumor growth curves and the inhibitory rates of tumor growth after injection of transfected and non-transfected H22 tumor cell mixtures at different E/T ratios. The TNFα gene and its mutant-transfected tumor cells have been found to inhibit tumor growth in an E/T ratio-dependent manner (i.e., the tumor development was delayed 5 days in contrast to control tumor). At the E/T ratio of 5:1, the H22 cells transfected with TNFα and its mutants displayed the maximum effect on tumor regression. A decrease in E/T ratios correlates with the reduced activity of TNFΔ1-12 on tumor growth. The inhibitory rate of tumor growth was reduced from 68% (at E/T 5:1) to 54% (at E/T 1:1), leaving only 14% at E/T 1:5. The antitumor effect of TNF-IL-2SIG, however, was less dependent on the E/T ratio; its inhibitory rate of tumor growth remained nearly unchanged when the E/T ratio was decreased from 5:1 to 1:1 and the suppression rate was still 48% even at E/T 1:5.
Mechanisms involved in the suppressive effects of TNFα and its mutants on tumor growth in vivo
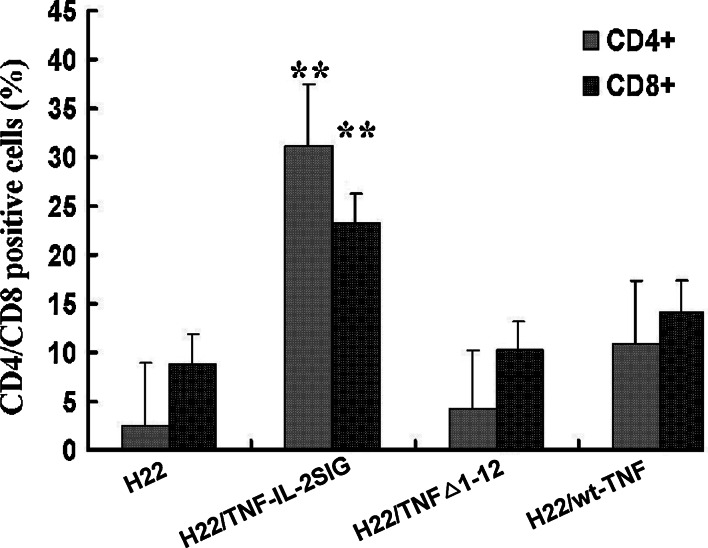
To determine how tumor cells expressing TNFα and its mutants result in the subsequent regression of tumors in vivo, fresh-frozen sections of murine tumor tissue were stained with specific monoclonal antibodies against CD4, CD8, Fas, and CD44v3, respectively. In control tumors, the infiltration of a small amount of lymphocytes, mainly CD8+ lymphocytes, was observed (Fig. 3). The tumor secreting TNF-IL-2SIG strongly enhanced lymphocytic infiltration (P<0.001). The tumor expressing wt-TNFα appeared to have reduced effects on the lymphocytic infiltration, while the TNFΔ1-12-expressing tumor failed to recruit lymphocytes to the tumor site (Fig. 3).
Fig. 3.
CD4+/CD8+ lymphocytes infiltration in TNFα and its mutants modified tumors. Indirect immunoenzymatic staining of frozen sections of tumors containing TNFα gene or its mutants transductants mixed with parental tumor cells at E/T of 5:1 was performed with specific primary antibodies (a goat anti-mouse CD4 or CD8 antibody) and the biotin-conjugated anti-goat IgG secondary antibody. Data are represented as mean ± SD. **P<0.01
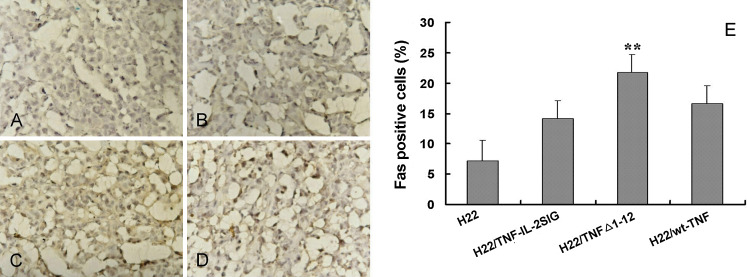
Similarly, we were interested in testing the involvement of additional TNF-family members in tumor cell killing. Importantly, the Fas/FasL pathway is known to be involved in the killing mechanisms of CD8+ CTLs and CD4+ T cells toward cancer cells. Therefore, the effect of TNFα and the two mutants on subsequent Fas expression on tumor cells was examined. As shown in Fig. 4, limited levels of Fas were expressed in the control tumor tissue (Fig. 4a), while the overexpression of Fas was observed in the tumor inoculated with mixture of H22/TNFΔ1-12 and parental H22 cells (E/T of 5:1; P<0.01, Fig. 4c). However, the S.C. injection of the same ratio of mixed H22/TNF-IL-2SIG (Fig. 4b) or H22/wt-TNFα (Fig. 4d) with the parental H22 tumor cells did not significantly affect Fas expression.
Fig. 4.
Fas expression in TNFα and its mutants modified tumors. Tissue sections from tumors induced by injection of TNF-α or its mutants transductants mixed with parental tumor cells at E/T 5:1 were stained by the avidin-biotin complex method with 1:100 of a rabbit anti-mouse Fas antibody and a biotin-conjugated anti-rabbit IgG antibody. Fas expression was in a small amount on control tumors (a), but significantly increased in tumors containing H22/TNF-Δ1-12 (c) and not markedly affected in tumors containing H22/TNF-IL-2SIG (b) or H22/wt-TNFα (d). The rates of Fas positive cells in tumors expressing TNF-α and its mutants are shown in (e). Data are represented as mean ± SD. **P<0.01
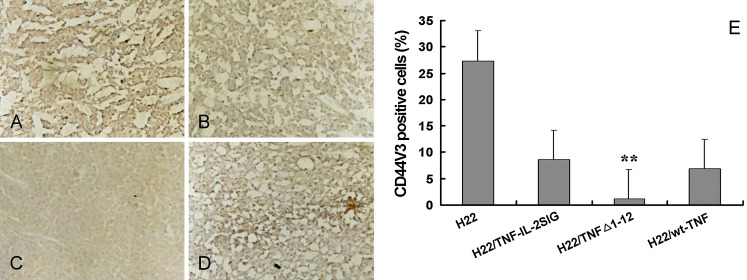
Furthermore, in contrast to the control tumor, the tumor formed by injecting a mixture of H22/TNFΔ1-12 and the parental H22 cells was covered with an intact tumor capsule that could be seen distinctly with the naked eye, suggesting the possible involvement of TM-TNFα in tumor metastasis. The expression of CD44v3, a tumor metastasis associated molecule, was detected by immunohistochemistry. CD44v3 expression was down regulated in the tumor containing H22/TNF-Δ1-12 ((Fig. 5c, P<0.01)), but was not affected in the tumor containing H22/TNF-IL-2SIG (Fig. 5b) or the H22/wt-TNFα (Fig. 5d), when compared to the control tumor (Fig. 5a).
Fig. 5.
CD44V3 expression in TNFα and its mutants modified tumors. Tissue sections of tumors containing TNF-α or its mutants transductants mixed with parental tumor cells at E/T 5:1were stained by the avidin-biotin complex method with 1:150 of a rabbit anti-mouse CD44V3 antibody and a biotin-conjugated anti-rabbit IgG secondary antibody. CD44v3 expression (brown) was at high level in the control tumors (a), but down-regulated in the tumors containing H22/TNFΔ1-12 (c) and not obviously affected in the tumors containing H22/TNF-IL-2SIG (b) or H22/wt-TNFα (d). The rates of CD44v3 positive cells in tumors expressing TNF-α and its mutants are shown in (e). Data are represented as mean ± SD. **P<0.01
Finally, we observed no obvious side effects in mice after the injection of mixed H22/TNFΔ1-12 or H22/wt-TNFα with the parental H22 cells at various E/T ratios. A slight, transient weight loss was observed within a week in the mice inoculated with a mixture of H22/TNF-IL-2SIG and parental H22 cells at E/T 5:1 or 1:0. However, these mice recovered to the average weight of control tumor-bearing mice within 2 weeks (data not shown).
Discussion
It has long been known that TNFα is a candidate for cancer biological therapy. However, its use clinically is limited due to its well-described systemic toxicity [20]. We have previously demonstrated that TM-TNFα could kill not only S-TNFα-sensitive tumor cells, but also S-TNFα-resistant tumor cells [21], indicating that TM-TNFα has potentially a wider anti-tumor spectrum than S-TNFα. It is clear that TM-TNFα exerts its actions by cell-cell contact since bioactive TM-TNFα is expressed as a transmembrane molecule on the cell surface of many cell types. The expression of TM-TNFα within a tumor may therefore exert antitumor activity without systemic side effects. Therefore, in the present study, the antitumor effects between TM-TNFα and S-TNFα in vitro and in vivo were compared and the possible mechanisms underlying their tumoricidal effect were explored.
To compare the effects of TM-TNFα and S-TNFα on tumor regression in vivo, we retrovirally inserted into H22 tumor cells cDNA encoding 17 kDa solely secretable, 26 kDa uncleavable transmembrane and wild type TNFα separately. We showed that the H22/wt-TNFα expressed both secretory and transmembrane TNFα forms and thus, both the fixed- and the supernatants of cultured-H22/wt-TNFα cells were highly cytotoxic against the TNF-sensitive target L929. The H22/TNFΔ1-12 cells expressed only cell surface, uncleavable TNF-α, and thus only the fixed cells (but not the cell supernatants), were cytotoxic towards the L929 cells. Since IL-2 is a typical secretory protein, the replacement of the TNFα signal peptide with the signal peptide of IL-2 leads to the synthesis of TNFα as a secretory protein without expression of 26 kDa pro-TNFα molecules. This was confirmed in the present study by our data, which indicated that H22/TNF-IL-2SIG cells could secrete large amounts of 17 kDa TNFα that was cytotoxic towards L929 cells. In these series of experiments, neither the cell surface TNFα nor cytotoxicity of fixed H22/TNF-IL-2SIG cells was detectable, although it is possible that secreted TNFα could have bound to TNF receptors on the cell surface. A potential explanation for the failure of receptor associated TNFα to be detectable may be due to the down-regulation or endocytosis of TNF receptors induced by binding to the ligand [22]. These results indicate that the three transductants that we employed in our experiments could produce biologically active, solely secretable form of TNFα, uncleavable transmembrane form of TNFα or wild-type TNFα (containing both the transmembrane form and the cleaved secretory form).
Our in vivo experiments demonstrated that the tumorigenicity of H22 tumor cells transfected by TNFα and its mutants, or mixed with parental H22 cells, was significantly reduced which manifested as a delay of 5 days for the tumor development and distinct reduction in tumor size. This indicates that both TM-TNFα and S-TNFα are able to reduce tumor mass. Similar results were also observed in the mice injected with retroviral vectors containing sequences encoding wt-TNFα, TNFΔ1-12 or TNF-IL-2SIG, into the tumor on day +3 after the H22 tumor cell challenge (data not shown). Importantly, the recent data from Marr et al. [12] support our experimental results showing that TM-TNFα could inhibit tumor growth in vivo just as S-TNFα did.
Our data show that the antitumor effects of both transmembrane and secretory TNFα were highly dependent on the E/T ratio in vitro and in vivo. However, the dependence of both forms of TNF-α in vivo was variable, namely the antitumor activity of TM-TNFα expressing tumor cells was more dependent on the E/T ratio than that of S-TNFα modified tumor cells. This conclusion is supported by the fact that the tumor growth inhibitory rate of H22/TNFΔ1-12 was significantly decreased (from 68% at E/T of 5:1 to 14% at 1:5). The inhibitory rate of H22/TNF-IL-2SIG was much less diminished at similar E/T ratios (from 72% at 5:1 to 48% at 1:5). The different dependences of TM-TNFα versus S-TNFα on E/T ratios seemed partially due to their mechanism of action. TM-TNFα acts by cell-cell contact, and is thus highly dependent on the E/T ratio. However, S-TNFα, like other proinflammatory cytokines, exerts its effects by autocrine, paracrine and endocrine mechansims and is therefore less dependent on E/T ratios, especially in the in vivo scenario.
The antitumor effects of the three transductants in vivo appeared to be more effective because the inhibitory rates of tumor growth by both H22/TNFΔ1-12 and H22/wtTNF at E/T of 1:1 was about 78% of the rate at E/T of 5:1 in vivo, whereas the cytotoxic effect of both transductants at E/T of 1:1 was about only 39% of their effects at E/T 5:1 in vitro. Furthermore, even at an E/T of 1:5, the inhibitory rate of H22/TNF-IL-2SIG was still 66.7% of the rate at E/T of 5:1 in vivo, while the cytotoxic activity of H22/TNF-IL-2SIG was only 22.5% of the activity at E/T of 5:1 in vitro. These data suggest that both cell surface-expressed TM-TNFα and secretory TNFα may suppress tumor growth in vivo via other mechanisms besides cytotoxicity. In particular, the mechanism involved in the suppression of tumor growth by tumor secreting S-TNFα in vivo seems more likely to be activation of antitumor responses rather than direct killing, because only 9% of the S-TNFα mediated cytotoxicity was left at E/T of 1:5 in vitro, while its tumor growth inhibitory rate was still 48% at the same E/T ratio in vivo.
Our in vivo studies suggest different mechanisms by which TM-TNFα and S-TNFα inhibit growth of cancer cells. Immunocytochemistry examination showed that S-TNFα secreted from H22/TNF-IL-2SIG containing tumors induced increased number of lymphocytes infiltrated including CD4+ and CD8+ T cells into the local tumor tissue as compared to control tumors. The lymphocytes infiltrated in the control tumors were mainly CD8+ T cells, while in TNF-IL-2SIG modified tumors CD4+ T cells were attracted more than CD8+ T cells. Matory et al. [23] demonstrated that both CD8+ and CD4+ T cells were required for the regression of S-TNFα transduced tumors in vivo, because the depletion of CD8+ or CD4+ T cells caused a reversal of TNFα induced inhibitory effects. By contrast, increased infiltration of lymphocytes into TNFΔ1-12 modified tumors was not observed.
The similarity of the E/T ratio dependence of TM-TNFα activity between in vitro and in vivo suggests that the cytotoxicity of TM-TNFα may be preferable to triggering antitumor immunity for its modified tumor regression in vivo. This is supported by the evidence of a compromised growth of TM-TNFα expressing tumor in both immunosuppressed and severe combined immunodeficient mice [24]. The phenomenon that the action of TM-TNFα in vivo was more effective than that of in vitro, indicate that TM-TNFα also augmented other tumoricidal mechanism in vivo. This hypothesis was proved by our results that the tumor expressing uncleavable TM-TNFα was able to stimulate expression of Fas that mediate tumor cell apoptosis. We did not observe that human S-TNFα derived from the tumor could induce Fas expression in murine cells, although previous studies showed that S-TNFα was able to up-regulate Fas expression in many kinds of cells and render these cells sensitive to Fas ligand- or Fas mAb-induced apoptosis [25, 26]. The reason for this difference is that tumor secreting human TNFα can only interact with mouse TNFR1 but not with mouse TNFR2 [27], and is thus unable to induce murine cells to express Fas [28]. A previous study by Grell et al. [29] in human cells has demonstrated that stimulation of endogenous TM-TNFα induced cell death via an autotropic or paratropic manner by binding to TNFR1. Addition of soluble Fas/APO-1-Fc had no effect on this TM-TNFα-mediated cell death, indicating that the interaction of Fas/FasL is not involved in TM-TNFα mediated cytotoxicity [29]. Different from this observation, our results from study in vivo showed that human TM-TNFα was able to stimulate Fas expression in murine cells, implying the involvement of Fas/FasL in TM-TNFα –induced cell death. The discrepancy between Grell’s observation and ours is possibly due to studies in different systems (human versus mouse and in vitro versus in vivo).
Our finding that the uncleavable TM-TNFα modified tumors were wrapped with a sharply demarcating capsule has led us to infer that TM-TNFα may play an important role in the inhibition of tumor metastasis. Much interest has currently been taken concerning the role of altered CD44 isoform expression during tumor progression and metastasis [30]. It is generally accepted that CD44s are expressed mainly on cells of normal tissues and non-metastatic tumors, while CD44v are present in many kinds of metastatic tumors [31]. It was found by us that the expression of CD44v3 was inhibited in TNFΔ1-12 modified tumors, denoting possibly that TM-TNFα may be involved in the suppression of tumor metastasis. In contrast, TNF-IL-2SIG modified tumors which were shown to be devoid of demarcating capsules manifested erosion and necrosis, suggesting that S-TNFα may promote tumor metastasis. Recently MacNeil and colleagues demonstrated that stimulation with S-TNFα significantly enhanced melanoma cell migration at 24 h as well as tumor invasion mediated by fibronectin [32], which seems to be consistent with the results of our study.
In conclusion, the results of the present study demonstrate that both TM-TNFα and S-TNFα derived from TNFα- and its mutants-modified tumors have cytotoxic and inhibitory effects on tumor cells, but the antitumor activity of TM-TNFα is more dependent on E/T ratio as compared to S-TNFα. The antitumor mechanisms of both forms of human TNF-α in tumor bearing mouse model may be different. S-TNFα appears to be able to recruit lymphocytes in the tumor to augment the activity of immune effector cells, while TM-TNFα seems to be capable of induction of Fas expression to promote tumor cell apoptosis via the Fas/FasL pathway. Furthermore, TM-TNFα might have an ability to suppress tumor metastasis by inhibiting CD44v3 expression. As the side effects of S-TNFα, but not TM-TNFα, namely a transient weight loss and a possible boost of tumor metastasis, were observed in our experiments. Therefore, use of a non-secreted form of TNF-α may greatly limit the side effects and remain antitumor activity for tumor gene therapy.
Acknowledgments
The authors wish to thank Mary Reiche for careful review of the manuscript and Professor Xuetao Cao for his generous gift of the PA317 and H22 cell lines. This project was supported by the National Natural Science Foundation of China (30200257), Key Project of Science and Technology from the Education Ministry of China (02136), the Hi-tech Research and Development Program of China (2001AA215431) and National Key Basic Research Program of China from the Ministry of Science and Technology of the People’s Republic of China (2001CB510008), and The Leukemia and Lymphoma Society of the United States of America (6249-05, to M.Z.).
References
- 1.Kriegler M, Perez C, DeFay K, Albert I, Lu SD. A novel form of TNF/cachectin is a cell surface cytotoxic transmembrane protein: ramifications for the complex physiology of TNF. Cell. 1988;53:45. doi: 10.1016/0092-8674(88)90486-2. [DOI] [PubMed] [Google Scholar]
- 2.Li X, Fan H. Loss of ectodomain shedding due to mutations in the metalloprotease and cysteine-rich/disintegrin domains of the tumor necrosis factor-alpha converting enzyme (TACE) J Biol Chem. 2004;279:27365. doi: 10.1074/jbc.M401690200. [DOI] [PubMed] [Google Scholar]
- 3.Haas E, Grell M, Wajant H, Scheurich P. Continuous autotropic signaling by membrane-expressed tumor necrosis factor. J Biol Chem. 1999;274:18107. doi: 10.1074/jbc.274.25.18107. [DOI] [PubMed] [Google Scholar]
- 4.Peck R, Brockhaus M, Frey JR. Cell surface tumor necrosis factor (TNF) accounts for monocyte- and lymphocyte-mediated killing of TNF-resistant target cells. Cell Immunol. 1989;122:1. doi: 10.1016/0008-8749(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 5.Mueller C, Corazza N, Trachsel-Loseth S, Eugster HP, Buhler-Jungo M, Brunner T, Imboden MA. Noncleavable transmembrane mouse tumor necrosis factor-alpha (TNFalpha) mediates effects distinct from those of wild-type TNFalpha in vitro and in vivo. J Biol Chem. 1999;274:38112. doi: 10.1074/jbc.274.53.38112. [DOI] [PubMed] [Google Scholar]
- 6.Aversa G, Punnonen J, de Vries JE. The 26-kD transmembrane form of tumor necrosis factor alpha on activated CD4+ T cell clones provides a costimulatory signal for human B cell activation. J Exp Med. 1993;177:1575. doi: 10.1084/jem.177.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkland TP, Sypek JP, Wyler DJ. Soluble TNF and membrane TNF expressed on CD4+ T lymphocytes differ in their ability to activate macrophage antileishmanial defense. J Leukoc Biol. 1992;51:296. doi: 10.1002/jlb.51.3.296. [DOI] [PubMed] [Google Scholar]
- 8.Baxevanis CN, Voutsas IF, Tsitsilonis OE, Tsiatas ML, Gritzapis AD, Papamichail M. Compromised anti-tumor responses in tumor necrosis factor-alpha knockout mice. Eur J Immunol. 2000;30:1957. doi: 10.1002/1521-4141(200007)30:7<1957::AID-IMMU1957>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Williamson BD, Carswell EA, Rubin BY, Prendergast JS, Old LJ. Human tumor necrosis factor produced by human B-cell lines: synergistic cytotoxic interaction with human interferon. Proc Natl Acad Sci USA. 1983;80:5397. doi: 10.1073/pnas.80.17.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichtner I, Lemm M, Becker M, Tanneberger S. Determination of antineoplastic activity and toxicity of tumor necrosis factor (TNF) in animal experiments. Correlation to clinical findings. Neoplasma. 1990;37:301. [PubMed] [Google Scholar]
- 11.Karp SE, Hwu P, Farber A, Restifo NP, Kriegler M, Mule JJ, Rosenberg SA. In vivo activity of tumor necrosis factor (TNF) mutants. Secretory but not membrane-bound TNF mediates the regression of retrovirally transduced murine tumor. J Immunol. 1992;149:2076. [PMC free article] [PubMed] [Google Scholar]
- 12.Marr RA, Addison CL, Snider D, Muller WJ, Gauldie J, Graham FL. Tumour immunotherapy using an adenoviral vector expressing a membrane-bound mutant of murine TNF alpha. Gene Ther. 1997;4:1181. doi: 10.1038/sj.gt.3300528. [DOI] [PubMed] [Google Scholar]
- 13.Hay H, CJ (1989) Studies on the specificity of the L929 cell bioassay for the measurement of tumour necrosis factor. J Clin Lab Immunol 29:151 [PubMed]
- 14.Bosselman RA, Hsu RY, Bruszewski J, Hu S, Martin F, Nicolson M. Replication-defective chimeric helper proviruses and factors affecting generation of competent virus: expression of Moloney murine leukemia virus structural genes via the metallothionein promoter. Mol Cell Biol. 1987;7:1797. doi: 10.1128/mcb.7.5.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji YB, Gao SY, Cheng WP. Effect of Haimiding on the functioning of red cell membrane of FC and H22 tumor-bearing mice. World J Gastroenterol. 2005;11:823. doi: 10.3748/wjg.v11.i6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez C, Albert I, DeFay K, Zachariades N, Gooding L, Kriegler M. A nonsecretable cell surface mutant of tumor necrosis factor (TNF) kills by cell-to-cell contact. Cell. 1990;63:251. doi: 10.1016/0092-8674(90)90158-B. [DOI] [PubMed] [Google Scholar]
- 17.Decoster E, Vanhaesebroeck B, Vandenabeele P, Grooten J, Fiers W. Generation and biological characterization of membrane-bound, uncleavable murine tumor necrosis factor. J Biol Chem. 1995;270:18473. doi: 10.1074/jbc.270.31.18473. [DOI] [PubMed] [Google Scholar]
- 18.Renz H, Gong JH, Schmidt A, Nain M, Gemsa D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J Immunol. 1988;141:2388. [PubMed] [Google Scholar]
- 19.Hahnfeldt P, Panigrahy D, Folkman J, Hlatky L. Tumor Development under Angiogenic Signaling: a dynamical theory of tumor growth, treatment response, and postvascular dormancy. Cancer Res. 1999;59:4770. [PubMed] [Google Scholar]
- 20.Mocellin S, Rossi CR, Pilati P, Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16:35. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Shi W, Li Z, Gong F. Comparison of the cytocidal effect induced by transmembrane and secreted TNF-alpha. Chin J Microbiol Immunol. 1998;18:499. [Google Scholar]
- 22.Higuchi M, Aggarwal BB. TNF induces internalization of the p60 receptor and shedding of the p80 receptor. J Immunol. 1994;152:3550. [PubMed] [Google Scholar]
- 23.Matory YL, Dorfman DM, Wu L, Chen M, Goedegebuure P, Eberlein TJ. Treatment of established tumor is associated with ICAM-1 upregulation and reversed by CD8 depletion in a tumor necrosis factor-alpha gene transfected mouse mammary tumor. Pathobiology. 1999;67:186. doi: 10.1159/000028071. [DOI] [PubMed] [Google Scholar]
- 24.Nagy T, Glavinas H, Szincsak N, Hunyadi J, Janossy T, Duda E, Vizler C, Juhasz I. Tumor cells expressing membrane-bound tumor necrosis factor activate macrophages and have a compromised growth in immunosuppressed and immunodeficient mice. Cancer Lett. 2003;196:49. doi: 10.1016/S0304-3835(03)00181-2. [DOI] [PubMed] [Google Scholar]
- 25.Matsumura R, Umemiya K, Goto T, Nakazawa T, Ochiai K, Kagami M, Tomioka H, Tanabe E, Sugiyama T, Sueishi M. Interferon gamma and tumor necrosis factor alpha induce Fas expression and anti-Fas mediated apoptosis in a salivary ductal cell line. Clin Exp Rheumatol. 2000;18:311. [PubMed] [Google Scholar]
- 26.Spanaus KS, Schlapbach R, Fontana A. TNF-alpha and IFN-gamma render microglia sensitive to Fas ligand-induced apoptosis by induction of Fas expression and down-regulation of Bcl-2 and Bcl-xL. Eur J Immunol. 1998;28:4398. doi: 10.1002/(SICI)1521-4141(199812)28:12<4398::AID-IMMU4398>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 27.Lewis M, Tartaglia L, Lee A, Bennett G, Rice G, Wong G, Chen E, Goeddel D. Cloning and expression of cDNAs for two distinct murine tumor necrosis factor receptors demonstrate one receptor is species specific. PNAS. 1991;88:2830. doi: 10.1073/pnas.88.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quirk SM, Porter DA, Huber SC, Cowan RG. Potentiation of fas-mediated apoptosis of murine granulosa cells by interferon-{gamma}, tumor necrosis factor-{alpha}, and cycloheximide. Endocrinology. 1998;139:4860. doi: 10.1210/en.139.12.4860. [DOI] [PubMed] [Google Scholar]
- 29.Grell M, Zimmermann G, Gottfried E, Chen CM, Grunwald U, Huang DC, Wu Lee YH, Durkop H, Engelmann H, Scheurich P, Wajant H, Strasser A. Induction of cell death by tumour necrosis factor (TNF) receptor 2, CD40 and CD30: a role for TNF-R1 activation by endogenous membrane-anchored TNF. Embo J. 1999;18:3034. doi: 10.1093/emboj/18.11.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson DL, Speight PM, Watt FM. Altered expression of CD44 isoforms in squamous-cell carcinomas and cell lines derived from them. Int J Cancer. 1996;66:457. doi: 10.1002/(SICI)1097-0215(19960516)66:4<457::AID-IJC8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 31.Chuang CK, Liao SK. Differential expression of CD44 variant isoforms by cell lines and tissue specimens of transitional cell carcinomas. Anticancer Res. 2003;23:4635. [PubMed] [Google Scholar]
- 32.Katerinaki E, Evans GS, Lorigan PC, MacNeil S. TNF-alpha increases human melanoma cell invasion and migration in vitro: the role of proteolytic enzymes. Br J Cancer. 2003;89:1123. doi: 10.1038/sj.bjc.6601257. [DOI] [PMC free article] [PubMed] [Google Scholar]