Abstract
The discovery of tumor-associated antigens, which are either selectively or preferentially expressed by tumors, together with an improved insight in dendritic cell biology illustrating their key function in the immune system, have provided a rationale to initiate dendritic cell-based cancer immunotherapy trials. Nevertheless, dendritic cell vaccination is in an early stage, as methods for preparing tumor antigen presenting dendritic cells and improving their immunostimulatory function are continuously being optimized. In addition, recent improvements in immunomonitoring have emphasized the need for careful design of this part of the trials. Still, valuable proofs-of-principle have been obtained, which favor the use of dendritic cells in subsequent, more standardized clinical trials. Here, we review the recent developments in clinical DC generation, antigen loading methods and immunomonitoring approaches for DC-based trials.
Keywords: Clinical applicability, Dendritic cell, Cancer immunotherapy
Introduction
The introduction, over a century ago, of the concept of “immune surveillance” led to the quest for ways to initiate de novo and enhance existing immune responses against tumors, thereby aiming at the specific eradication of cancer cells, whilst leaving normal tissues untouched [129]. This concept was initially supported by the detection of spontaneously developing tumor infiltrating lymphocytes (TIL) with the capacity to kill malignant cells in a HLA-restricted fashion and was later on substantiated by the discovery of tumor-associated antigens (TAA) against which anti-tumor immune responses can be directed [67, 128]. However, malignant tumor cells develop mechanisms to escape elimination by these immune responses and possess mechanisms to tolerize the immune system, leading to tumor establishment. Tumor cells that escape elimination can persist in equilibrium with the immune system until the balance between the immune response and the tumor tilts towards tumor growth due to the outgrowth of poorly immunogenic tumor cells (immunoediting) and suppression of the immune system [53, 205].
The field of cancer immunotherapy has grown very rapidly in the past few decades. In order to initiate an immune response, induce memory and break immunological tolerance against the tumor, dendritic cells (DC) have emerged as what appears to be the ideal cellular tools [13, 140, 158]. Since tumor cells can express a whole array of TAA, the ideal anti-cancer vaccine may consist of DC loaded with TAA expressed by the tumor of that particular patient [23, 119]. In recent years, strategies have been developed for the large-scale generation of DC, yielding sufficient numbers of cells for use in clinical trials. Meanwhile, many different protocols have been designed to load antigens onto DC. Together, these findings made it possible to start clinical studies with antigen-loaded DC in cancer patients. This review will focus on recent advances made in the procedures to generate large numbers of clinical-grade DC from various types of progenitor cells with a special focus on differentially isolated monocyte-derived DC and will discuss the problems associated with DC generated from cancer patients. After a brief description of the currently used strategies to load DC with antigens (Ag) and the possible methods for monitoring the induced immune response, a short overview will be given of DC-based clinical trials that have been carried out in cancer patients so far and their outcomes. Finally, we will focus on the problems arising in these first trials and point out new insights which should be taken into account to improve DC vaccinations in the future. Figure 1 gives an overview of DC-based immunotherapy and highlights the aspects discussed in this review which could have significant impact on the efficacy of DC-based immunotherapy.
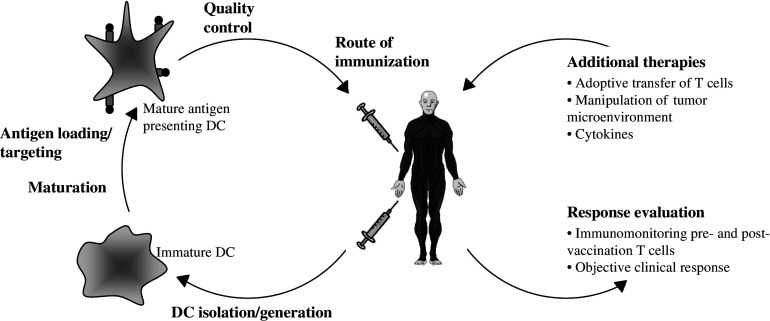
Fig. 1.
Advances in DC-based immunotherapy. DC-based therapy has been shown to be very promising but several variables still need to be optimized. Further research is needed to determine the optimal procedure for DC isolation and/or generation, the most efficient maturation stimulus to activate the DC and the optimal method to load DC with antigens. The DC preparation needs to be extensively controlled before administration to the patient and the optimal route of immunization still needs to be defined. An important issue is also the standardization of techniques used for monitoring of the vaccine-induced immune response and the use of objective clinical endpoints. Furthermore, DC-based immunotherapy could benefit from combination with additional therapies
Dendritic cell characteristics
The properties of DC that make them unique APC have been the subject of recent reviews and will only be briefly discussed here [12, 169].
The DC population is highly heterogeneous but their life cycle can be roughly divided in two stages: the immature and mature stage [12, 169]. In the immature stage, DC capture Ag through various mechanisms: macropinocytosis, receptor-mediated endocytosis and phagocytosis. After uptake, iDC start to process Ags into peptides for subsequent presentation to T cells as mDC. The phenotype of iDC is characterized by a low expression of MHC molecules, co-stimulatory molecules, adhesion molecules and DC markers. In contrast, they express a large amount of inflammatory chemokine receptors, allowing them to extravasate into inflamed tissues [12, 148]. The encounter of a so-called “danger signal” initiates maturation, whereby DC become highly motile, veiled cells and lose their ability for Ag uptake by down-regulating endocytic and phagocytic receptors. mDC optimize Ag processing through the up-regulation of components of the Ag-processing machinery and acquire the capacity to present antigens to and stimulate T cells by up-regulation of MHC molecules, adhesion/co-stimulatory molecules (CD40, CD54, CD58, CD80, CD83, CD86) and the DC marker DC-LAMP. Whereas most of these markers are already present at low levels on iDC, CD83 is absent on iDC and hence, CD83 expression allows discrimination between iDC and mDC. However, recently it has been shown that CD83 can be absent on monocyte-derived mDC generated in IL-3 and IFNβ and matured with poly(I:C), which nevertheless show a mature phenotype based on expression of other maturation markers. Maturation also induces acquisition of chemokine receptors such as CCR7 on the DC surface, which enable trafficking to lymphoid organs in response to chemokines secreted by stromal cells in the lymph nodes. mDC, in turn, secrete large amounts of chemokines to attract various cell types of the immune system and, depending on the maturation stimulus, they secrete particular cytokines to skew the immune response in a specific direction [12, 104, 141, 169].
Effective T-cell priming requires three consecutive signals between DC and T cells. DC can activate both CD4+ T cells and CD8+ T cells through Ag presentation via MHC class II and MHC class I, respectively (signal 1). Signal 2 consists of the interaction between CD80 and CD86 on the DC and CD28 on the T-cell surface. In the absence of co-stimulation, T cells recognizing the Ag presented by the DC are tolerized. Although the effector cells in tumor immunology consist mainly of TAA specific CTL, it has been well documented that CD4+ T-cell help is required for efficient priming of memory CD8+ T-cell responses (reviewed in [20]). For this to occur, DC need to be licensed by activated CD4+ T cells through reciprocal interactions between CD40 on the DC and CD40L on the activated CD4+ T cells, leading to IL-12 production by the DC (signal 3) [12, 73, 163]. The IL-12 produced by DC polarizes the T-cell response towards a Th1 profile, which is believed to be preferential for cancer immunotherapy.
The DC population residing in the human body is roughly divided into two groups: CD11c+ CD123lo myeloid DC and CD11c- CD123hi plasmacytoid DC (pDC). Plasmacytoid DC are important mediators of innate antiviral immune responses and are the main producers of IFNα in the body (hence they are also called natural interferon producing cells, NIPC). pDC are located in blood and lymphoid organs. Myeloid DC reside in the blood and lymphoid organs as well as in the dermis and the interstitial tissue of most organs. A special subtype of myeloid DC, the Langerhans cells, reside in the epidermis and mucosal tissues [43, 148]. Since most research in the field of DC vaccination is focused on monocyte-derived myeloid DC, this review will refer to this cell type, unless specifically mentioned.
Generation of clinical grade dendritic cells
Critical parameters for DC vaccines
When chosing a generation protocol for DC intended for vaccination purposes, several critical parameters must be considered.
A first parameter which is important for the generation of DC vaccines is the number of DC that need to be isolated/generated. Since it is believed that repeated immunizations are beneficial and most protocols use around 107 DC per injection, it is necessary to obtain large numbers of DC (∼108 DC per preparation) which can be frozen in aliquots for repeated vaccinations. DC viability should be >75%.
Contaminating cells in the DC preparation could affect the efficacy of DC vaccination. Since the type of contaminating cells present in the DC preparation can vary between protocols and in order to minimize the effect of the contaminants, it is necessary to aim for the highest possible DC purity. Based on data from the literature, we propose to aim for DC purities of >75% [60].
As discussed later in this review, the maturation state of DC is critical for their effectiveness. It is therefore recommended to use mDC for vaccination or to combine the use of iDC with the in vivo administration of maturation stimuli. For each DC isolation/generation protocol, it should be assessed whether the resulting DC are immature or mature and, if immature, investigated whether they can be induced to mature with activation stimuli.
DC isolation and generation
DC can be isolated directly ex vivo from the blood of patients, either through positive selection using DC-specific markers or by depletion of contaminating cells or by a combination of both. Myeloid-derived DC and pDC can be distinguished through the differential expression of CD11c/BDCA-1 and CD123/BDCA-2/BDCA-4 on these DC types [62, 78, 103, 144, 179]. A first approach to enrich blood DC entails the use of sequential density centrifugation of apheresis PBMC followed by a culture period of 24 h. Administration of Flt3-L significantly increases the number of DC obtained and the isolated DC show a mature phenotype. However, reagents for density gradient centrifugation are not GMP-grade and it is difficult to carry out this approach in a closed system [62]. Another method for the isolation of blood DC involves depletion of lineage marker positive cells, which can be followed by positive selection using DC-specific markers. The isolated DC are immature and comprise both myeloid DC and pDC. Maturation is achieved by in vitro culture [144, 179]. Recently, Fearnley et al. described a method for the isolation of high-potency blood DC by using the CMRF-44 antigen. Using this method, only mature DC are isolated. The DC preparation contains both myeloid DC and pDC. However, yield and purity of the isolated DC are highly variable [4, 56, 97]. DC can also be enriched by exploiting membrane expression of DC markers (e.g. CD1c, BDCA-4,...) for magnetic or flow cytometric enrichment. It has to be mentioned that only the pDC-specific antibodies are truly DC-specific, whereas other DC markers are also expressed in low amounts on other cell types, which need to be depleted. Myeloid DC and pDC can be isolated separately or together and the DC show an immature phenotype but can be matured with appropriate stimuli [78]. Nevertheless, despite the fact that it has now been shown that Flt3-L can be used to mobilize blood DC, yields are generally too low to obtain sufficient numbers of DC for vaccination [62, 103]. On the other hand, Lopez et al. recently described the isolation of blood DC from apheresis products in sufficient numbers for immunotherapy [4, 97] and Campbell et al. [31] described the isolation of large numbers of blood DC using the CliniMACS system. It remains a matter of debate, though, if it will become feasible to use DC isolated from the blood of cancer patients for vaccine preparations because some reports have been published showing that significantly reduced DC numbers were found in peripheral blood of cancer patients compared to healthy donors [156].
A second source for DC generation is the proliferating CD34+ progenitor cell. These CD34+ cells can be isolated from blood or bone marrow by positive selection through magnetic separation. In order to obtain higher yields of CD34+ cells, these cells can be mobilized into the blood via G-CSF administration alone or by a combination of stem cell-mobilizing chemotherapy (e.g. cyclophosphamide), G-CSF and/or IL-3 [120, 150]. However, caution has to be taken regarding the use of G-CSF for stem cell mobilization, since recent reports indicate that G-CSF can skew immune responses towards the Th2 phenotype and can induce/recruit Treg, which may both be undesirable for cancer immunotherapy [63, 86]. After isolation, CD34+ precursors can be differentiated into DC by the addition of different cytokine mixtures. Most frequently, CD34+ cells are cultured with GM-CSF and TNFα. After a 2 week culture period, iDC are obtained which mature asynchronously during the next 2 weeks, resulting in a mixture of iDC and mDC. A small proportion of the cells display the characteristics of Langerhans cells. IL-4, Flt3-L or c-kit ligand can be added to enhance DC yield. Purities are variable and rather low, with the contaminating cells being mainly granulocytes [98, 99, 162, 174]. Another protocol consists of a 2 week culture in the presence of GM-CSF and IL-4, resulting in the generation of iDC, followed by a 7 day period of maturation using CD40L or TNFα [38]. CD34+ cells can also be differentiated into iDC using GM-CSF and IL-13 during 2 weeks [98].
The most commonly used cell type for DC generation is the peripheral blood monocyte. Monocytes can be easily collected via buffy coat preparations or leukapheresis and can be enriched in various ways. The easiest and most cost-effective way for DC generation is through adherence of monocytes to plastic which has been developed for use in closed systems. However, the variability in DC purity of this approach remains an important shortcoming [17, 57, 108, 135, 167, 172, 183, 189]. Highly purified monocytes can also be obtained by positive immunomagnetic selection of CD14+ cells, but the reagents required are expensive, which limits their clinical use [10, 52, 57, 66, 108]. Furthermore, positive selection of monocytes raises concerns about the use of xenogeneic antibodies and possible activation/alteration of the monocytes [25, 54]. Although it has never been investigated whether monocyte activation has a negative effect on DC generation, this can be circumvented by a negative magnetic enrichment, but this method yields highly variable monocyte and DC purities [57, 108, 135, 172, 197]. Another technique to enrich monocytes involves the use of density gradient centrifugation, but this technique is difficult to integrate in a closed system and the available reagents are not GMP-grade [34, 93]. Recently, elutriation has been described as a means to isolate highly pure monocytes. This technique is based on a counter-flow centrifugation to physically separate cells depending on their size and density. The development of the Elutra™ device by Gambro.BCT facilitated the use of this approach in a closed system. Elutriation has been described to be fast and very cost-effective. Important parameters to consider when using the Elutra™ system are: (1) the dimensions of the elutriation chamber and the resulting requirement for high numbers of input cells (minimum 1 × 109 monocytes and minimum 5 × 109 PBMC); (2) due to the design of the system where cells are separated on the basis of cell size, there is no discrimination between monocytes and granulocytes leading to granulocytes being the major contaminants of the purified monocyte preparation; and (3) red blood cell (RBC) levels in the apheresis product should be kept low because RBC interfere with the purification process [2, 18, 66, 149, 167, 178, 197] (Dr. H. Vrielink, Sanquin Blood Bank, Amsterdam, The Netherlands: personal communication). Although significant differences exist in culture characteristics and study endpoints (iDC or mDC, evaluation of purity/yield) used by different groups, we attempted to compare the characteristics of the different available systems for monocyte enrichment and DC culture. An overview of the methods used for monocyte isolation, along with the resulting purities and DC yields is given in Table 1. In view of possible differences in phenotype and function of DC, some groups have compared DC generated from monocytes that were obtained using different isolation techniques. Again, results from different groups cannot easily be compared, due to differences in medium, cytokines and culture vessels used for DC generation. Table 2 gives an overview of the studies that have compared DC from differentially isolated monocytes at phenotypical and functional level. Taken together, these data indicate that the method of monocyte isolation has no major implications on DC phenotype and function.
Table 1.
Characteristics of the different available closed systems for monocyte enrichment and subsequent DC generation
| Adherence | Positive selection | Negative selection | Elutriation | |
|---|---|---|---|---|
| Cost | Low | High | High | Low |
| Monocyte puritya | ±60% | 91–99% | 8–75% | 55–90% |
| Main monocyte contaminantsb | B, NK cells | / |
NK cells Granulocytes |
Granulocytes (especially neutrophils) |
| Monocyte recovery | ND | 27–100% | 43–97% | 53.3–88.2% |
| Closed culture recipient |
Cell FactoriesTM Gas-permeable bags |
Gas-permeable bags | Gas-permeable bags | Gas-permeable bags |
| Monocyte activation | Possible | Possible | No | No |
| DC purityc | 34–98% | 59.5–98% | 31–86% | 62–98% |
| DC yield (PBMC) | 2.7–20% | 1–2% | 4.8–13% | 5–12% |
| DC yield (monocytes) | 12–68% | 4–41% | 16–95.1% | 20–100% |
| References | [17, 57, 72, 108, 135, 167, 172, 183, 189] | [10, 52, 57, 66, 108] | [57, 108, 135, 172, 197] |
[2, 18, 66, 149, 167, 178, 197] Dr. H. Vrielink (personal communication) |
a Monocyte purity was assessed by flow cytometry using the monocytic marker CD14
b Contaminants of monocyte preparations were assessed by flow cytometry using lineage-specific antibodies
c DC purity was evaluated by FSC/SSC characteristics or by CD83 positivity
Table 2.
Effect of monocyte purification on DC phenotype and function
| Reference | Monocyte selection | DC phenotype | DC function |
|---|---|---|---|
| Suen et al. [172] | Negative selection vs. adherence | Similar CD1a, CD80, CD83, CD86 |
Similar uptake FITC-dextran DC from negative selection slightly better in allo-MLR |
| Pullarkat et al. [135] | Negative selection vs. adherence |
Similar CD11c, CD40, CD44, CD58, CD80, CD83, CD86, HLA-DR DC from negative selection higher CD1a expression Similar phenotype stability |
Comparable allogeneic and peptide-specific (IMP-1, gp100) proliferative T-cell responses and gp100 specific cytotoxic T-cell responses |
| Berger et al. [18] | Elutriation vs. adherence |
Comparable CD1a, CD14, CD25, CD83, CD86, HLA-DR Equal phenotype stability |
DC from elutriated monocytes were better in allo-MLR |
| Felzmann et al. [57] | Adherence vs. positive selection vs. negative selection | No differences in CD1a, CD14, CD45, CD80, CD83, CD86, HLA-ABC, HLA-DR | No difference in IL-12 secretion or allo-stimulatory capacity |
| Wong et al. [197] | Elutriation vs. negative selection |
Equal CD1a, CD14, CD80, CD83, CD86, HLA-DR DC from elutriation higher contamination with CD3+ cells |
No difference in allo-MLR or TT/influenza recall response |
| Garlie et al. [66] | Elutriation vs. positive selection |
Similar CD11c, CD83, CD86, HLA-ABC HLA-DR higher on DC from elutriated monocytes |
Similar FITC-dextran uptake and induction of allogeneic MLR responses |
| Meyer-Wentrup et al. [108] | Adherence vs. positive selection vs. negative selection | DC from positive or negative selection slightly more mature than adherence-DC (CD83, CD86, HLA-DR, CD14) | No difference in allo-MLR |
Classically, immature, “myeloid-type” DC are generated from enriched monocytes by a 5–7 day culture in the presence of GM-CSF and IL-4, which can afterwards be matured using different stimuli. IL-4 can be replaced by IL-13, which induces the same type of DC. Recently, several groups have published optimized protocols to reduce this culture period to 48 h without affecting the phenotypical or functional properties of the resulting DC [46, 121, 133, 198]. This shorter culture period has several advantages such as reduced labor, cost and time. Since it has been proposed that DC generated in the presence of IL-4 display several functional alterations, a search for other differentiation cocktails has been carried out [180]. DC develop quickly in a model of trans-endothelial trafficking, which has been suggested to be operating in vivo [138]. In the presence of GM-CSF and type I IFN monocytes also develop quickly into DC, which might therefore be more physiological. However, although several authors have reported the efficient generation of highly stimulatory DC using GM-CSF and type I IFN in a short culture period, some contradictory results have been published [45, 113, 153, 154, 182]. Recently it was discovered that DC can also be differentiated from monocytes in the presence of type I IFN and IL-3, where substitution of GM-CSF by IL-3 rescues the cells from apoptosis [24, 29, 142]. Another pathway of monocyte differentiation into DC has been described where incubation of monocytes in the presence of GM-CSF and IL-15 results in differentiation of Langerhans-like cells [111].
Comparison of different DC types
Freshly isolated DC or DC generated from different precursors are not equivalent, which raises the question of which DC type is optimal for the induction of an anti-tumor response. Many comparisons between these different DC subtypes have been published, which we briefly summarize hereafter. Again, these studies are difficult to compare due to differences in media and cytokines used.
Several groups have compared freshly isolated blood DC and monocyte-derived DC. These DC types are phenotypically and functionally distinct. At the phenotypical level, blood DC seem to be more activated, because blood DC require a short period of culture to mature, whereas the maturation of monocyte-derived DC is variable and largely dependent on the stimulus. However, compared to monocyte-derived DC, blood DC are relatively poor cytokine producers, are reported to be less active in endocytosis and lack DC-SIGN expression. Both DC types are equal stimulators in allo-MLR, but blood DC seem to be better initiators of Ag-specific T-cell responses and have a higher Th1 polarization capacity [79, 84, 126].
Many groups have reported comparisons between CD34- and monocyte-derived DC. In general, yields of CD34-derived DC, as calculated from the starting number of PBMC, appeared to be somewhat higher. Depending on the cytokine combinations used to generate DC, iDC or mDC were obtained, but both CD34- and monocyte-derived DC showed a comparable morphology and phenotype. However, when CD34- or monocyte-derived DC displaying comparable phenotypes were used for the stimulation of T cells, the induction of Ag-specific T-cell responses was enhanced when using CD34-derived DC [11, 37, 174].
The group of Dauer et al. performed an extensive comparison between the classical monocyte-derived DC generated in the presence of GM-CSF and IL-4 during 5–7 days and DC generated from monocytes in a reduced period of 48 h. They show that both DC populations have a comparable phenotype, IL-12 secretion and Ag uptake, processing and presenting capacity. Both DC types migrate equally well towards the CCR7 ligand 6CKine/CCL21. However, yield and purity of the fast DC population are higher compared to the classical monocyte-derived DC. Furthermore, fast DC induced similar numbers of Ag-specific T cells, but with higher lytic capacity compared to T cells induced by classical monocyte-derived DC [46, 121, 198].
Many reports have been published comparing the classical monocyte-derived DC (GM-CSF + IL-4) with DC generated in the presence of GM-CSF/IL-3 and type I interferon. In general, DC differentiation seems to occur more rapidly in the presence of type I IFN and these DC acquire a semi-mature phenotype and a high migratory capacity at day 3 of culture without any other maturation stimulus, in contrast to IL-4 DC which need exogenous maturation stimuli to mature. The cytokine secretion pattern is largely different between type I IFN DC and IL-4 DC, with the major difference being a decreased IL-12p70 secretion and an increased IFNα secretion by type I IFN DC as compared to IL-4 DC. However, type I IFN DC are short-lived because a considerable percentage of the cells undergo apoptosis by day 5 of culture, which can be counteracted by adding IL-3 [24, 29, 49, 113, 142, 154, 182]. The comparison of T-cell stimulation by these two DC types has been troubled by the fact that immature type I IFN DC already display a semi-mature phenotype. Consequently, when immature type I IFN DC and immature IL-4 DC were compared, type I IFN DC were more potent at inducing Ag-specific T-cell responses than DC differentiated with GM-CSF and IL-4 [131, 154]. However, the capacity to induce Ag-specific T-cell responses was comparable between immature type I IFN DC and mature IL-4 DC [182]. When both DC types were matured, conflicting results have been published, showing either an equal capacity of both DC types to induce Ag-specific T-cell responses [142] or an enhanced capacity of type I IFN DC [24, 113]. Type I IFN DC have been shown to polarize CD4+ T-cell responses either only towards Th1 [154] or to both Th1 and Th2 [29, 49]. In contrast, Dauer et al. [45] reported that DC fail to develop in the presence of type I IFN and that type I IFN even disables DC precursors. It should be mentioned however that these authors compared both DC derived in the presence of IL-4 or type I IFN at day 6 of culture, a time point at which type I IFN DC already undergo apoptosis. McRae et al. [106] described that when type I IFN is added to cultures of monocytes together with IL-4 and GM-CSF, TNFα-mediated maturation is impaired, resulting in a decreased T-cell stimulatory capacity and reduced IL-12 secretion. This contrasts with findings described by Radvanyi et al. [136], who observed that addition of type I IFN to GM-CSF/IL-4 cultures of monocytes accelerated DC generation and maturation. Furthermore, Tamir et al. reported that the environment in which type I IFN is present plays a central role in determining its effects on DC function and Lehner et al. showed that adding both type I IFN and bacterial stimuli to GM-CSF and IL-4 containing DC cultures induces apoptosis in monocyte-derived DC [92, 176].
Differentiation of monocytes with GM-CSF and IL-15 was decribed by Mohamadzadeh et al. and compared to the classical DC generation in the presence of GM-CSF and IL-4. The authors reported that both DC types share: (1) the basic DC phenotype; (2) the FITC-dextran uptake capacity; (3) maturation capacity upon incubation with various stimuli; and (4) the capacity to stimulate allogeneic and Ag-specific T cells. As opposed to GM-CSF/IL-4 DC however, DC generated in the presence of IL-15 express several Langerhans cell markers: E-Cadherin, Langerin and CCR6. As a consequence, IL-15 DC migrate towards the CCR6 ligand MIP-3α/CCL20 [111].
Importance of maturation state
The induction of a successful anti-tumor immune response requires the use of immuno-stimulatory mDC, because of their enhanced capacity to induce Ag-specific T-cell responses and because iDC tend to induce tolerance. Furthermore, iDC have been shown to expand regulatory T cells (Treg), although it has been described that DC matured with certain stimuli can also induce Treg expansion and even provoke de novo Treg generation from CD4+CD25− effector T cells [14, 48, 105]. In addition, mDC have been shown to be resistant to immunosuppressive factors produced by tumors and are phenotypically and functionally stable in the absence of cytokines [145, 168]. Maturation can be achieved by a wide array of different stimuli: monocyte-conditioned medium (MCM), different pro-inflammatory cytokine cocktails, TLR ligands,... [1, 32, 39, 90, 109, 122, 152, 166]. These stimuli are schematically represented in Fig 2. Currently, the most widely used stimulus is a cocktail containing IL-1β, IL-6, TNFα and PGE2 but extensive controversy exists about which stimulus might be optimal. The different stimuli used to induce maturation give rise to subtle differences in the DC maturation stage. These differences can be observed at the phenotypical level (expression of maturation markers CD25, CD40, CD83, CCR7,...), the functional level (allo-MLR, Ag-specific T-cell induction...) and at the level of cytokine/chemokine secretion (IL-6, IL-10, IL-12p70, IFNα...). Furthermore, the DC phenotype, functional characteristics and cytokine/chemokine secretion pattern can also be influenced by the culture medium used to generate the DC [54, 181]. The concept arising now is that DC do not need to have a completely mature phenotype, but need to secrete cytokines/chemokines which polarize T-cell responses and express chemokine receptors for efficient migration to lymphoid organs.
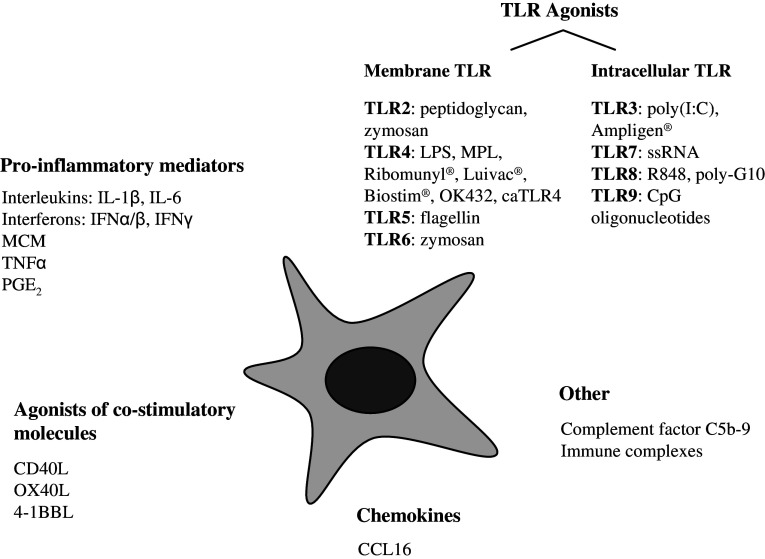
Fig. 2.
Schematic representation of the currently available maturation stimuli. To date, several stimuli have been identified which promote DC maturation. The extent to which DC maturation is affected varies considerably between individual stimuli. In addition, it has been shown that distinct combinations of maturation stimuli can act synergistically to promote DC maturation and Th1 polarizing capacity of DC. Ongoing research will possibly identify optimal combinations of stimuli for DC maturation. Although clinical-grade bacterial immunomodulators (Ribomunyl®, Luivac®, Biostim®, OK432) are classified in this figure as TLR4 agonists, the nature of these stimuli suggests that they possibly trigger a combination of distinct TLR. Agonists of co-stimulatory molecules can be delivered by agonistic Ab, soluble ligands (if available) and transfection with ligands. MCM monocyte-conditioned medium, MPL monophosphoryl lipid A, caTLR4 constitutively active TLR4
Recent insights indicate that phenotypic DC maturation is not a distinguishing feature of immunogenic versus tolerogenic DC, since tolerance can be induced by phenotypically immature, semi-mature and fully mature DC. In this regard, semi-mature DC, which express high amounts of MHC and co-stimulatory molecules and produce high amounts of IL-10 but only trace amounts of IL-12, have been implicated in the conversion of naïve T cells into Treg [151]. Recently, IDO expressing mDC were shown to be tolerogenic and could be further identified by CD123 and CCR6 expression. These authors suggest that IDO expression by mDC might be determined by the prevailing regulatory influences during maturation [116]. This has led to the concept of defining DC not only by their maturation status but also based on effector function [141]. However, the precise DC characteristics leading to Th1, Th2, Th17, Treg or cytotoxic T-cell development are not yet completely identified. It is conceivable that small differences in environmental stimuli drive DC to acquire different effector functions, which could be characterized by subtle differences in the expression of various known molecules or DC longevity. In this view, distinct DC subsets can each acquire characteristics of immunogenic and tolerogenic DC depending on the stimuli they receive.
Although it is difficult to define precise characteristics for DC preparations, we can point out some parameters that can be considered important. First of all, mDC can be characterized by a high expression of MHC and co-stimulatory molecules and the expression of CD83, which has recently been shown not only to be a marker of phenotypical maturation, but also a modulator of the immune response [3]. Next, expression of CCR7 on DC displays their capacity to migrate to the lymphoid organs. In order to get licensed by CD4+ T cells for the induction of a memory CD8+ T-cell response, DC should express the CD40 molecule for signalling through CD40L, which, in turn, leads to IL-12p70 production. Cytokine secretion is another means by which DC can modulate T-cell responses. IL-12p70 and IFNα can induce a Th1 response, whereas IL-6 can rescue effector T cells from the suppressive effect of Treg cells. In contrast, expression of IDO and secretion of IL-10 can lead to the induction of Treg and is therefore undesirable.
Clinical-grade DC
DC for use in cancer immunotherapy should be produced according to good manufacturing practise (GMP) guidelines. This implies that the procedure of DC generation is validated and that protocols are available for each step in the DC generation process. Finally, a quality control system must be developed to examine the quality of every final DC preparation. In order to avoid infections, DC cultures should be performed in closed recipients or gas-permeable culture bags with sterile connections, using GMP-grade reagents and culture media (AIM-V, X-VIVO-15, X-VIVO-20) either serum-free or supplemented with autologous heat-inactivated plasma to avoid exposure of DC to xenoantigens [65, 145]. Recently, several groups have developed closed culture systems for DC generation. In view of repeated immunizations, it is desirable to generate a large amount of DC from one single leukapheresis, which can then be frozen and thawed before each injection [59, 94]. The generated DC vaccines should be subjected to an extensive quality control procedure before use. Since our understanding of DC biology and T-cell activation is continuously evolving based on ongoing research, a standardized set of specific parameters for DC quality which have to be fulfilled can not be defined. Instead, a minimal set of release criteria have to be fulfilled and additional parameters, which are thought to be important, can be analyzed to gain information for later comparison. Microbiological tests have to be performed in order to demonstrate the lack of bacterial, fungal or mycoplasma contamination and absence of endotoxins in the DC preparation. The viability of the final DC preparation before injection should be at least 75%. The DC preparation should display the typical morphologic features of DC with maximally 25% of contaminating cells. Minimal phenotyping should be performed to ensure that the DC are MHC class I and class II positive and express the co-stimulatory molecules CD80 and CD86 to some extent. In addition to these minimal criteria, we recommend the examination of additional parameters which give more information about the phenotypical and functional characteristics of the DC vaccine. Complete phenotyping provides additional information about the activation status of the DC (CCR7, CD1a, CD11c, CD40, CD80, CD83, CD86, CD123, HLA-ABC, HLA-DR) and the type of contaminating cells (CD3, CD14, CD16, CD19, CD56). However, it should be mentioned that different culture media can give rise to phenotypic differences. DC with a fully mature phenotype maintain their morphological and phenotypical characteristics after cytokine withdrawal, which can be examined using the washout test. The T-cell stimulatory capacity of the DC can be tested using an allo-MLR (non Ag-specific) or by an Ag presentation assay (Ag specific). Another characteristic of DC that can influence the induced T-cell response is their cytokine secretion pattern (IL-6, IL-10, IL-12p70, IFNα,...). Functionally, fully mature DC can also be characterized by their capacity to migrate towards the CCR7 ligand CCL19 [60].
Dendritic cells in cancer patients
Some authors have reported alterations in number and phenotype of peripheral blood DC as well as functional defects in freshly isolated DC from the blood of cancer patients compared to healthy donors. These defects appear to be more severe in more advanced stages of disease and are induced by factors secreted by tumor cells (IL-6, IL-10, TGF-β, VEGF,...) [200]. This has been described for patients with AML [112], CLL [124], multiple myeloma [139], colorectal cancer [50] and breast, head and neck and lung cancer [5]. This raises the question whether DC generated from precursors obtained from cancer patients will also display differences compared to DC from normal donors. Although several authors have reported no phenotypical and functional differences between DC derived from precursors from cancer patients compared to healthy donors [10, 88, 201, 203], recently some concerns have been raised regarding the generation of DC from cancer patients. Orsini et al. [125] describe that monocyte-derived DC from CLL patients with active disease display an abnormal phenotype and functional defects, whereas monocyte-derived DC from CLL patients in remission show no differences compared to DC from normal donors, indicating that patients with minimal residual disease (MRD) after conventional treatment are the most suitable candidates for DC immunotherapy. Schütt et al. [159] report the efficient generation of monocyte-derived DC in multiple myeloma patients, but found that their phenotype can be altered depending on the treatment the patients received previously. Pedersen et al. [132] show that monocyte-derived DC from breast cancer patients are more activated but less sensitive to maturation signals compared to healthy donor-derived DC. However, these DC can mount Ag-specific T-cell responses in vitro. Makino et al. show intrinsic abnormalities of monocytes and a defect of DC differentiation in adult T-cell leukaemia (ATL) patients. The authors provide evidence that these alterations arise because of infection with the human T lymphotropic virus type I (HTLV-1), which is responsible for the induction of ATL [102]. Furthermore, factors in the patient’s autologous serum can influence DC generation from precursors in cancer patients, but this could be easily circumvented by using serum-free culture conditions [139]. Clearly, DC generation in cancer patients can be altered, but a lot of reports have been published where DC from cancer patients are able to induce Ag-specific immune responses. It is therefore recommended to carefully test DC generated from all cancer patients before using them in immunotherapy trials.
Antigen loading
The ideal target for cancer immunotherapy would be a TAA which is exclusively expressed in tumor cells and not in normal tissues, to avoid potential induction of auto-immunity. A prerequisite for a broad therapeutic potential in several cancers is the wide expression of the TAA on different tumor types. In addition, the TAA should be important for tumor growth and survival, so down-regulation is impossible [71, 202]. Most TAA are self-derived proteins and thus poorly immunogenic. Nevertheless, DC loaded with these Ags can be used to initiate Ag-specific T-cell responses. In recent years a large number of strategies have been developed to deliver Ags to DC, using defined epitopes, specific TAA or whole tumor cell material and employing both viral and non-viral techniques.
Peptide/protein approaches
The most commonly used protocol for loading Ags onto DC is pulsing with synthetic peptides. Advantages of this technique are the ease of manufacturing GMP-grade peptides, obviation of the need for tumor tissue and simplification of immunomonitoring. Important drawbacks of this technique are MHC restriction, the need for identification of TAA epitopes, low affinity binding of self-derived peptides and lack of CD4+ T-cell help (since only a limited number of CD4 epitopes are known) [194]. The need for MHC typing and peptide identification can be circumvented by using acid-eluted peptides from autologous tumor cells, but in general the large amounts of tumor material needed for this procedure are not available. Moreover, tumor cells also present shared self Ags, which could give rise to unwanted autoimmune responses [51]. In order to increase the binding affinity of self-derived peptides, peptide analogues (so-called heteroclitic peptides) can be generated by modifying the anchor residues which mediate binding to MHC molecules [130]. However, it was recently described that vaccination with these heteroclitic peptides results in poor recognition of endogenous peptides and less efficient tumor cell killing [170]. CD4+ T-cell help can be obtained by addition of a xenoantigen such as Keyhole Limpet Hemocyanin (KLH). Along with the discovery of more CD4 epitopes, the use of long peptides comprising both CTL and CD4 epitopes has been developed to generate both Ag-specific CD4+ and CD8+ T cells for optimal anti-tumor immune responses [204, 206]. In order to avoid tumor escape by TAA down-regulation, a mixture of different TAA peptides could be loaded onto DC. However, this could lead to epitope competition which, in turn, can be easily avoided by loading the different peptides on different DC batches [127]. In addition, the opposite phenomenon termed “epitope spreading” where vaccination with a single TAA epitope results in the induction of T-cell responses directed against other, non-related TAA, has also been reported [100].
In order to address some of these issues, purified whole TAA proteins have been used for loading DC [193]. This method has the advantage of being independent of the knowledge of the MHC haplotype of each patient and of prior identification of defined TAA-derived peptide epitopes. Furthermore, multiple immunogenic epitopes can be processed by DC in the context of both MHC class I and class II, resulting in both CD4+ and CD8+ T-cell responses. However, proteins are only efficiently taken up by iDC; protein loading should therefore occur in an immature state, after which maturation has to be induced. In addition, because of the combined use of standard and immunoproteasomes by mDC, some epitopes could be less efficiently processed and presented to T cells. Proteins can also be delivered as immune complexes, enhancing the efficiency of MHC class I presentation. Antigens can be conjugated to IgG mAb for uptake by Fc receptors or to Ab targeting endocytic receptors (mannose receptor, DEC-205, DC-SIGN). The latter receptors are more DC restricted and can thus be used for in vivo DC targeting. Furthermore, loading DC with immune complexes has been shown to bypass the need for CD4 licensing of DC [137, 160, 175].
Genetic approaches
Problems related to whole protein loaded DC (such as the intensive process of protein purification) can be overcome by gene-based delivery of TAA into DC. Advantages are the ease of cloning genetic constructs and the possibility to include sequences for improving Ag presentation in both MHC class I and class II. Furthermore, different TAA can be simultaneously delivered to DC, thereby broadening the T-cell repertoire that can be activated. DNA and mRNA can be delivered as naked strands, but transfection efficiencies are enhanced by lipid-mediated transfection or electroporation. Transfection of DC with DNA has not met with great success and important concerns can be raised regarding safety, because DNA can integrate into the host genome [190, 191]. mRNA delivery to DC proved to be more effective and safe and is surrounded by significantly less safety issues, because mRNA is only transiently expressed in the cells and does not integrate into the genome. Recently, several groups have developed mRNA electroporation strategies, resulting in very high transfection efficiencies of DC [69, 188, 190]. This technique has also been applied to amplified whole tumor mRNA. Using microscopic amounts of tumor tissue, total tumor RNA is amplified by a PCR-based protocol. Thus, this approach can even be applied when TAA are not defined and only limited amounts of tumor material are available, giving the opportunity to induce a broad patient specific immune response against both known and unknown TAA [16, 118].
Viral gene delivery systems are very efficient strategies to introduce genetic material into various cell types, including DC. We will only give a brief description of currently used viral vector systems for DC transduction, a more comprehensive overview has been given by Breckpot et al. [26]. As with non-viral gene delivery methods, several TAA can be combined in one viral vector and target sequences to obtain Ag presentation in both MHC class I and class II can be incorporated. A large variety of viral vectors have been developed and optimized for high-efficiency transduction of DC: adenovirus, adeno-associated virus, herpes simplex virus, vaccinia/pox virus, retrovirus and lentivirus. Depending on the viral system used, great variability exists in the size of genetic material that can be incorporated, the stability of transgene expression, the capability to transduce both dividing and non-dividing cells and the viral titres that can be obtained. Furthermore, large scale production of viral vectors needs further research. In addition, major concerns are raised regarding possible effects of viral transduction on DC phenotype/function, immunogenicity of the virus, insertional mutagenesis and biosafety; issues which have to be resolved. Nevertheless, some viral vectors are already being used in cancer immunotherapy.
Whole tumor approaches
Methods that take advantage of the complete protein content of the tumor cell, thereby broadening the induced immune response and avoiding tumor escape, have been developed, including loading DC with tumor lysate, engulfment of necrotic/apoptotic cells by DC and fusion of DC with tumor cells. Tumor lysates contain the whole protein content of lysed tumor cells, which can be loaded on iDC in the same way as purified proteins. Using this approach, induction of Ag-specific CD4+ and CD8+ T cells can be achieved, but a relatively high amount of tumor cells is required [21]. Induction of necrosis and apoptosis in tumor cells can be achieved by mechanical/thermal lysis and UV irradiation, respectively. However, it is not easy to induce pure necrotic or apoptotic cell populations and it is still a matter of debate which type is needed for induction of immunity. The general concept arising now is that necrotic cells induce immunity, whereas apoptotic cells induce tolerance, because of the lack of DC activation signals. However, although this is an important issue in vivo, it can be circumvented by exposing ex vivo generated DC loaded with apoptotic cells to additional maturation stimuli to ensure full maturation of the DC [58, 77, 155]. Furthermore, using these techniques, it is essential that every tumor cell is rendered necrotic/apoptotic, because residual viable tumor cells in the DC preparation could theoretically lead to metastatic spread in patients. The requirement for relatively large amounts of tumor material is another important drawback. Another attractive approach consists of fusion of DC with tumor cells, which generates hybrids expressing the DC characteristics of Ag processing and presentation together with the unaltered antigenic spectrum of the tumor cell [87, 147, 185]. Fusion can be obtained by using either chemical fusogens or electrofusion. However, until now, simple and reliable protocols to generate a highly efficient DC-tumor cell fusion are not available and caution has to be taken regarding the safety of this technique. As for loading DC with tumor lysates and necrotic/apoptotic cells, this technique also requires the availability of a large number of viable tumor cells. An important issue to take into account when using whole tumor cell-derived material is the risk of inducing autoimmunity. Furthermore, evaluation of the immune response becomes more complex. Another concern relevant to the use of whole tumor cell-derived material (tumor mRNA, tumor-DC fusions and possibly also tumor lysates and necrotic/apoptotic tumor cells) is the risk of transferring immunosuppressive factors from the tumor cells to the DC, which would generate deficient DC.
A recently developed approach consists of the use of DC derived exosomes. Exosomes are 50–90 nm vesicles originating from multivesicular endosomes and contain Ag presenting molecules, adhesion and co-stimulatory molecules, i.e., the necessary machinery required to generate potent immune responses. Exosomes need to be transferred to mDC to promote T-cell activation leading to tumor eradication. Exosomes pulsed with tumor peptides can successfully prime Ag-specific CTL responses [7, 36, 55].
Monitoring the immune response and clinical outcome
Reproducible monitoring of the immunologic outcome of DC vaccination could facilitate the interpretation of study results. The establishment of reliable, reproducible and quantitative assays to evaluate vaccine-induced immune responses should be regarded as of critical importance [41, 85]. Immunomonitoring methods depend on the vaccine design: strategies employing defined epitopes, defined TAA, or undefined Ags need different approaches. Another issue complicating immunomonitoring of the vaccine-induced response is the phenomenon of epitope spreading: even when immunizing with a single peptide, the immune response can be broadened to other epitopes [30, 143, 192]. Thus, when measuring the immune response after vaccination, one has to discriminate between anti-vaccine and anti-tumor T cells. During the course of tumor progression, a spontaneous anti-tumor T-cell response develops, but these anti-tumor T cells become inactivated due to tumor-induced immunosuppression. Upon vaccination, anti-vaccine T cells are induced and migrate to the tumor. In some cases, these anti-vaccine T cells are able to overcome immunosuppression, thereby destroying local tumor cells and activating both pre-existing and new anti-tumor T cells which can eliminate the bulk of the tumor cells. This concept, introduced by Boon et al., implies that successful vaccination does not depend on the number of the induced anti-vaccine T cells, but rather on the production of an anti-vaccine T-cell clone which is able to migrate to the tumor and resist local immunosuppressive mechanisms [22, 100]. Thus, besides quantification of the number of anti-vaccine T cells, it is also important to determine their qualitative aspect. In principle, the elicited anti-vaccine T cells should have the ability to migrate to the tumor site, produce cytokines, proliferate after Ag re-exposure and mediate tumor cell lysis. Therefore, both T cells at the tumor site and in the circulation should be analyzed if possible, as the immune response monitored in the blood does not always reflect the situation in the tumor [8, 91]. In addition, tumor-specific T cells should be analyzed by combining different methods in order to get a complete picture of the induced T-cell functional profile. Furthermore, if possible, the tumor site should also be screened for Treg, because intra-tumoral accumulation of Treg is associated with poor prognosis and it has been described that certain DC vaccination modalities can induce/expand Treg [14, 42]. Up to now, a big discrepancy has been observed between induced immune responses and clinical outcome of the patients, which is probably related to the breadth and quality of the induced T-cell response, resulting in the frequent observation of induction of anti-vaccine immune responses in the absence of an objective clinical response. Thus, either the induced anti-vaccine T cells in patients lacking a clinical response are not capable of destroying the tumor, or the tumor-induced immunosuppressive mechanisms between patients with and without a clinical response are of a different magnitude. The methods for immunomonitoring described hereafter are also schematically represented in Fig 3.
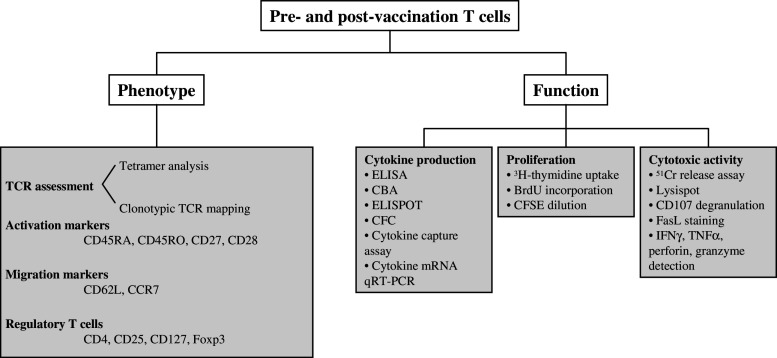
Fig. 3.
Schematic overview of immunomonitoring methods to characterize the induced T-cell response. Several assays have been developed to determine phenotypical and functional characteristics of T cells. The combined use of these assays provides information about the breadth and the quality of the induced immune response
Target cells and time schedule for immunomonitoring
When immunizing with DC, it is important to evaluate immune responses with cellular targets other than DC, since this could result in significant background responses. When using peptide-pulsed DC, other target cells expressing the relevant HLA type pulsed with either an irrelevant peptide or the immunizing peptide can be used (e.g. T2 cells for HLA-A2 restricted peptides). However, with all other approaches for Ag loading of DC, this method is not applicable, since the immune response can be directed against various epitopes (both MHC class I and class II restricted). In this case, it is recommended to use autologous APC as targets (e.g. EBV transformed B cells or PHA blasts), since these cells express all the relevant MHC molecules. These autologous APC can then be loaded with the Ag(s) used for vaccination using one of the approaches described for loading DC with Ag. However, if possible, it would be optimal to use a distinct approach as the one used for Ag loading of DC for vaccination to avoid background. Perhaps the best target to use, at least for MHC class I responses, is the autologous tumor or cell lines derived thereoff, which is, however, not always available.
Regarding the time schedule at which samples should be taken for immunomonitoring, comparisons of pre- and post-vaccination samples are probably most informative, and should preferably take place concomitantly with the evaluation of clinical parameters. When the DC vaccine is repeatedly administered, several samples for monitoring can also be taken during the course of vaccination. If the patient is subjected to a follow-up period (either with or without any further treatment), it is advisable to take samples for monitoring at later time points as well to address the induction of a sustained/memory response.
T-cell receptor assessment
A strategy to enumerate the percentage of T cells recognizing a certain epitope in the context of a defined MHC molecule is by using tetramers. Tetramers are soluble complexes of recombinant MHC molecules folded in the presence of antigenic epitopes. MHC class I tetramers are relatively easy to produce, whereas MHC class II tetramer production is more challenging. With these reagents, T cells recognizing specific antigenic epitopes can be enumerated, but it does not give information about their functional capacity. However, tetramers can only be used when the patient’s HLA haplotype is known and screening can only be done for known TAA epitopes. Tetramer staining can be combined with other techniques, described hereafter, in order to obtain information about the phenotypical and functional profile of the T cells [19, 196]. The group of Coulie et al. developed a tetramer-based mixed lymphocyte peptide culture (MLPC) approach to carefully estimate the frequency of peptide-specific CTL. In this assay, PBMC are re-stimulated twice with peptide and cytokines in limiting dilution conditions. After this culture period the separate cultures are stained with tetramers and the CTL precursor frequency is deduced from the proportion of positive wells. Cells from the positive cultures are subcloned and the growing subclones are then tested for specificity with tetramers and analyzed further with a variety of techniques to determine their functional characteristics [70, 83]. Recently, a qRT-PCR-based method has been developed for clonotypic TCR mapping by which vaccine-reactive T-cell clones can be identified and enumerated [82, 157].
Analysis of T-cell phenotype
Analyzing the T-cell phenotype might be valuable to gather information about the activation status of Ag-specific T cells. Discrimination of naïve and activated/memory CTL solely on the basis of differential CD45RA/CD45RO expression has proven to be unreliable, since CD45RA is also expressed at high levels on stable resting memory CD8+ T cells which did not encounter their cognate Ag for a long period of time. Further characterization can be achieved using markers such as CD27 and CD28 and the lymphocyte migration markers CD62L and CCR7. Table 3 shows the expression profile of these molecules on CD8+ T cells in the naïve stage and during the course of activation. By combining tetramer staining with these cellular markers, the activation status and homing potential of the antigen-specific CD8+ T cells can be assessed [6, 33, 134, 165]. Treg can be detected through the combined staining of CD4, CD25, CD127 and Foxp3, together with several other non-distinctive markers like CTLA-4 and GITR [95, 161].
Table 3.
CD8+ T-cell characterization according to expression of activation markers and chemokine receptors
| CCR7 | CD62L | CD45RO | CD45RA | CD27 | CD28 | |
|---|---|---|---|---|---|---|
| Naïve T cells | + | + | − | + | + | + |
| TCM, central memory T cells | + | + | + | − | + | + |
| TEM, effector memory T cells | − | − | + | − | − | − |
| TEMRA, stable resting Ag-experienced T cells | − | − | − | + | − | − |
Central memory T cells home to the lymph nodes, whereas effector memory T cells home to the tissues
Measurement of cytokine production
An important parameter of CTL effector function is cytokine secretion. Depending on the stimulus, CTL can produce a variety of cytokines including IL-2, IL-4, IL-10, IFNγ and TNFα. Over the years, several assays have been developed to measure cytokine production after Ag-specific stimulation. Bulk assays such as ELISA and cytometric bead array (CBA) measure the total amount of cytokines secreted by a whole cell population and do not provide information about the percentage of cells producing these cytokines [177, 195]. Furthermore, the detection limit of ELISA is rather high. Newer methods were then developed at the single-cell level: ELISPOT, cytokine flow cytometry (CFC) and cytokine capture assays. Using these assays, the percentage of CD8+ T cells secreting a specific cytokine can be enumerated. In ELISPOT, cytokine secreting cells are visible as single spots on a nitrocellulose membrane and the frequency can be calculated from the number of cells plated [110, 196]. CFC measures the intracellular cytokine content of individual cells and offers the subsequent advantage of combined evaluation of T-cell phenotype [173, 196]. The above-mentioned assays do not provide the opportunity to specifically isolate cytokine secreting cells. Therefore, cytokine capture assays were developed: this assay uses bispecific antibodies that bind the cell surface and capture specific cytokines directly after their release by the cell. These cytokine secreting cells can then be isolated and either used in other assays or cloned [28]. Recently, a sensitive functional assay to directly measure CTL anti-tumor activity by qRT-PCR of cytokine mRNA was developed [81].
Proliferative capacity
Another characteristic feature of effector T cells is their capacity to proliferate upon Ag recognition in order to expand to sufficient numbers. The standard assay used to measure proliferation consists of the uptake of 3H-labelled thymidine by proliferating cells and subsequent measurement of radioactive signals. This assay, however, does not provide information on the percentage of proliferating cells, nor on their phenotype. In order to obviate the need for using radioactivity, another assay was developed where the incorporation of 5-bromo-2′-deoxyuridine (BrdU) is measured by ELISA. BrdU is a pyrimidine analogue and is incorporated instead of thymidine into DNA of proliferating cells [107]. In addition, BrdU can be coupled to fluorochromes for FACS analysis. Dilution of carboxyfluorescein diacetate succimidyl ester (CFSE) is another FACS-based technique to measure cell division. CFSE fluorescence is halved upon every division and can be measured by FACS. Advantages of this technique are the possibility to enumerate proliferating cells at the single-cell level, which can be simultaneously evaluated for their expression of other activation markers. Furthermore, the ability to manipulate the fluorescence intensity of a stained population allows for the differential labelling of two or more cell populations and the technique can be used both in vitro and in vivo [101].
Cytotoxic activity
CTL-mediated cytotoxicity involves three distinct pathways: (1) indirect killing through release of cytokines IFNγ and TNFα; (2) induction of apoptosis through Fas–FasL interactions using FasL expressed by the CTL; (3) direct killing by secretion of perforin and granzymes into the intercellular space [6]. A variety of methods have been developed to measure either total cytotoxicity or one of the aforementioned aspects in particular. The golden standard method is the 51Cr release assay, where total lysis of target cells by CTL is measured. An important drawback however is that no information is obtained about the actual percentage of lytic cells. Consequently, the Lysispot assay was introduced where lytic CTL are visualized as single spots from which the precise percentage of lytic cells can be calculated [164]. Recently, the CD107 assay was shown to be a very attractive method to calculate the percentage of lytic CTL. As a marker of degranulation, CD107a/b is transiently expressed on the plasma membrane, and lytic cells are stained by adding anti-CD107a/b mAbs during culture. An extra advantage of this technique is that it can be combined with tetramer staining and membrane or intracellular FACS staining for activation markers and cytokines, respectively [19]. Other techniques providing information about specific aspects of CTL-mediated cytotoxicity comprise membrane staining of FasL and intracellular staining of IFNγ, TNFα, perforin and granzymes.
Delayed type hypersensitivity reaction (DTH test)
The DTH test is a method to assess the anti-tumor immune response initiated by a vaccine in vivo. The Ag(s) used for vaccination are injected intradermally into the patient, which attract immune cells to the sensitization site, leading to induration and erythema. The extent of induration/erythema is then a measure for the strength of the immune response, but this assay is not reliable unless the immune cells invading the sensitization site are phenotypically and functionally characterized, providing detailed information about the in vivo immune response against the immunogen(s) [47].
Clinical response
In cancer immunotherapy, as with any cancer therapy, the desired outcome of any treatment is tumor control (either by prolonging the tumor free interval following resection of all disease, tumor stabilisation or regression). The most important appraisal for the success of cancer immunotherapy therefore remains the evaluation of objective clinical responses. At present most clinical trials with DC vaccines have been conducted in pretreated advanced-stage patients, very often with a large tumor volume. In such patients objective clinical endpoints would be easy to assess but have been rarely observed. Moreover, DC vaccination might not be able to induce tumor regression, but might result in slowing the rate of progression. In addition, these patients often have a compromised immune system, which makes them not the ideal patient population to test DC-based therapy which depends on an effective immune system for activity. DC vaccines can also show a delayed onset of activity, based on the time required to initiate an immune response. Therefore, patients could show early tumor progression before eventual tumor regression, which is another factor complicating the evaluation of the clinical response. Furthermore, the tumor can also be controlled by the immune system without complete tumor eradication, in which case only prolonged survival or time-to-progression are good criteria for evaluating the clinical response. In less advanced patients with no or clinically not-evaluable disease or patients with minimal residual disease (MRD) after debulking by other approaches, clinical estimation of the effect of immunotherapy would only be possible by analysis of the time to progression/recurrence and demonstration of a prolonged overall survival of the patients [76]. In some specific types of cancer, tumor markers in the blood could serve as a surrogate (e.g. PSA, idiotype protein, CEA,...). However, it is important to determine objective criteria (e.g. the recently proposed Response Evaluation Criteria in Solid Tumors, RECIST criteria) to evaluate the clinical benefit, as the use of non-standard criteria can lead to over-optimistic interpretation of the results [146].
Overview of clinical trials
A substantial number of clinical trials using dendritic cells has been carried out over the last decade. A recently updated list of published trials has been made available on the Internet by the group of Dr. D. Hart (http://www.mmri.mater.org.au/). Overall, tumor-specific immune responses have been frequently observed in patients vaccinated with DC, but durable clinical responses were exceedingly rare. In general, results from clinical trials published by different groups are difficult to compare because of a variety of reasons: (1) the variability in the type and activation status of the DC used (blood, CD34- or monocyte-derived DC, iDC versus mDC, cytokines used for DC generation,...); (2) the variation in Ag loading methods; (3) the use of different immunomonitoring methods; (4) the use of non-objective clinical criteria, resulting in an over-optimistic representation of clinical outcome; (5) overall study design (number of DC injected, route and number of vaccinations,...). Nevertheless, certain conclusions can be drawn from these trials. An important overall observation that can be made is that DC vaccination is safe, as no or only mild and self-limiting adverse effects have been reported in a small number of patients. We will now discuss in more depth some trials which highlight aspects that could be important for future design of clinical trials using DC.
Several trials have assessed the influence of the maturation status of the DC on clinical and immunological responses. Jonuleit et al. compared GM-CSF/IL-4 monocyte-derived iDC or mDC matured using a cocktail of IL-1β, TNFα, IL-6 and PGE2 loaded with distinct peptides and recall Ags for vaccination of melanoma patients. Peptide-specific CTL and recall Ag-specific CD4+ T-cell responses were enhanced when mDC were used for vaccination, indicating that mDC are superior to iDC for use in cancer vaccination [80]. A study by de Vries et al. reported vaccination of melanoma patients with either GM-CSF/IL-4 monocyte-derived iDC or mDC matured with MCM pulsed with peptides, followed by vaccination with peptides alone in combination with KLH. KLH-specific cellular and humoral responses were enhanced when using mDC. DTH responses were only observed in patients receiving mDC and T cells isolated from DTH sites showed peptide specificity. No clinical responses were observed in patients vaccinated with iDC (n = 8), whereas in patients receiving mDC (n = 9), four had disease progression, three had stable disease, one showed a mixed response and one showed a partial response [48]. A recent report by Yamanaka et al. used GM-CSF/IL-4 monocyte-derived iDC or mDC matured with OK-432 (a lyophilized mixture of Streptococcus pyogenes and benzylpenicillin) pulsed with tumor lysate and KLH for vaccination of glioma patients. A higher percentage of patients vaccinated with mDC developed a tumor-specific DTH reaction and tumor-specific CD8+ T cells in blood, compared to patients receiving iDC. In the group of patients vaccinated with iDC alone (n = 17), six had stable disease, nine progressive disease and two showed a mixed response. In the cohort of patients treated with mDC or mDC combined with iDC (n = 7), there was one partial response, one mixed response, four patients had stable disease and one progressive disease. Altogether, results obtained in these trials clearly indicate that mDC are required for optimal induction of tumor-specific immune responses in cancer patients [48, 80, 199]. However, recent evidence indicates that iDC can still be used for vaccination, when combined with in vivo maturation approaches [117].
Another variable possibly affecting the effectiveness of DC vaccination is the route of vaccine administration. Fong et al. used protein-pulsed DC isolated from blood for vaccination of metastatic prostate cancer patients by intravenous (i.v.), intradermal (i.d.) or intralymphatic (i.l.) route. All patients developed Ag-specific T-cell proliferative responses, regardless of the immunization route. TNFα secretion was only observed after i.v. vaccination, whereas IFNγ was only detected after i.d. and i.l. vaccination. None of the patients developed an IL-4 response. Ag-specific Abs were predominantly detected in i.v. treated patients. In conclusion, i.d. and i.l. DC vaccination leads to induction of Th1 immunity, whereas i.v. vaccination leads predominantly to a humoral response [61]. The group of Bedrosian reported a study in melanoma patients where monocyte-derived mDC were administered i.v., intranodally (i.n.) or i.d. Tetramer-positive CD8+ T cells were induced/enhanced in the majority of patients but IFNγ production by these T cells was only seen in 6/7 i.n. treated patients and 2/6 i.d. treated patients. In the i.v. treated group 4/8 patients had stable disease and 4/8 had progressive disease. In the i.n. group, 2/8 patients showed a minor response, 2/8 remained stable and 4/8 progressed. In the i.d. treated group, 1/10 patients had a minor response, 3/10 had stable disease and 6/10 progressed. These results point to the intranodal route as the preferred mode of DC injection [15]. Kyte et al. describe the treatment of melanoma patients with monocyte-derived mDC transfected with autologous tumor RNA using either i.d. and i.n. injections. Tumor-specific T-cell proliferation was observed in 6/10 i.d. immunized patients and 4/12 i.n. immunized patients, whereas tumor-specific DTH responses developed in 6/10 i.d. treated patients and 2/12 i.n. treated patients. Clinically, in the i.d. treated group (n = 10), two patients had no evidence of disease, one remained stable and seven progressed. In the i.n. treated group (n = 12) 1 patient was stable and 11 progressed, which indicates that, in this study, i.d. administration of DC was more efficient [89]. Recently, Trakatelli et al. reported immunization of patients with IFNβ/IL-3 monocyte-derived mDC pulsed with peptides via the combined subcutaneous (s.c.), i.d. and i.n. routes. DC migration was only observed after i.d. injection, not after s.c. injection. Peptide-specific CTL were detected in 3/8 patients and these patients also showed DC migration. Regarding clinical outcome, 3/8 patients had no evidence of disease, 1/8 remained stable and 4/8 progressed [184]. Butterfield et al. compared i.v. and i.d. administration of peptide-pulsed iDC in melanoma patients. Both routes of immunization resulted in development of peptide-specific T cells in the same percentage of patients, but higher levels of IFNγ were secreted by these T cells in the i.d. group. Determinant spreading occurred in one i.d. treated patient and not in the i.v. group and this was correlated with the induction of a durable complete response [30]. Altogether, these results indicate that i.v. injection primarily induces humoral immune responses, whereas i.d. and i.n./i.l. injection mediates induction of Th1 immunity. Intradermal DC administration induces DC migration, as opposed to s.c. injection. Since i.n. administration is rather complicated and because i.v. injection is less effective at inducing Th1 responses, results so far indicate that i.d. administration of DC is probably preferable for inducing anti-tumor immunity and clinical responses [15, 30, 61, 89, 184]. However, these results are very preliminary, since only a small number of patients have been treated in these studies.
As already mentioned, epitope spreading occurring after vaccination might be an important factor to counteract tumor escape and elicit durable clinical responses. Brossart et al. vaccinated seven breast and three ovarian cancer patients with peptide-pulsed monocyte-derived mDC. Upon vaccination two patients developed disease stabilization whereas the others progressed. Ag-specific T-cell responses developed in five patients, with two of them showing evidence for epitope spreading. These same patients also showed a period of disease stabilization, suggesting that epitope spreading could be correlated with clinical response [27]. The group of Trefzer vaccinated melanoma patients with irradiated fusions of allogeneic mDC and autologous tumor cells. Upon vaccination 1/17 patients developed a complete response, 1/17 developed a mixed response, 6/17 patients remained stable and 9/17 patients progressed. Tumor-specific CD8+ T-cell responses were mounted in 11 patients, 3 of which showed epitope spreading. However, in all patients analyzed (n = 6) immune evasion was detected, as tumor cells were found to lose either TAA expression or molecules of the Ag presenting machinery, or both [185, 186]. These data indicate that the phenomenon of epitope spreading is often correlated with a positive clinical outcome and could thus be a predictive factor of vaccination efficiency [27, 185, 186]. Although these data are promising, more patients have to be treated in order to be able to draw definitive conclusions.
The group of Su et al. investigated whether targeting of the Ag for presentation in both MHC class I and II could improve anti-tumor immune responses. DC were electroporated with either hTERT mRNA or LAMP-hTERT mRNA. After injection, 9/11 hTERT immunized patients and 9/9 LAMP-hTERT immunized patients developed pronounced inflammatory responses at the injection site. Immune reactions were more pronounced in the LAMP-hTERT group. hTERT-specific CD8+ T cells were detected in the blood of 8/9 hTERT immunized patients and 9/9 LAMP-hTERT immunized patients. hTERT-specific CD4+ T cells were detected in 6/9 hTERT vaccinated patients, compared to 9/9 LAMP-hTERT vaccinated patients. Furthermore, CD4+ T-cell responses induced in LAMP-hTERT vaccinated patients were of higher magnitude compared to responses induced by hTERT vaccination. CTL from LAMP-hTERT immunized subjects showed higher lytic activity against hTERT-expressing targets than CTL from the hTERT group. In addition, whereas CTL from hTERT immunized patients developed mainly into effector memory T cells, CTL from LAMP-hTERT vaccinated patients developed into central memory T cells. hTERT-specific CD4+ T-cell proliferation was only observed in LAMP-hTERT vaccinated patients. Four out of nine patients in the LAMP-hTERT group had circulating tumor cells, all of which were transiently reduced during and after vaccination. Six out of nine patients in the hTERT group had circulating tumor cells, which were transiently cleared in 5/6 subjects. Altogether, these data suggest that the use of LAMP targeting can induce more pronounced anti-tumor immune responses with an improved T-cell memory [171]. These results thus seem promising and are prompting us to investigate this issue in larger cohorts of patients to draw further conclusions.
A key obstacle hindering the induction of successful anti-tumor immune responses by DC vaccination is the presence of suppressive mediators. One of the factors that has clearly been implicated in suppression of tumor specific immune responses are Treg. These cells are able to suppress Ag-specific effector T cells and were found in elevated numbers in the peripheral blood of cancer patients compared to healthy volunteers. Furthermore, large numbers of Treg were found intratumorally. Dannull et al. investigated whether elimination of Treg using denileukin diftitox/ONTAK (recombinant IL-2 diphteria toxin conjugate) could enhance the efficacy of tumor RNA-transfected mDC vaccines. In this respect, we have to mention that although Dannull et al. observed a reduction of Treg numbers using this regimen, this result could not be obtained by Attia and coworkers [9]. CD8+ T-cell responses were increased 2.7-fold in patients receiving only DC and 7.9-fold in patients receiving ONTAK and DC. CD4+ T-cell responses were increased 2-fold in patients immunized with DC alone, compared to 7.2-fold in patients vaccinated with ONTAK and DC. The results of this study indicate that combination of Treg depletion by ONTAK and DC vaccination might lead to improved anti-tumor immune responses [44]. The group of Höltl et al. investigated whether co-treatment with cyclophosphamide could enhance the efficacy of vaccination with allogeneic monocyte-derived mDC pulsed with tumor lysate and KLH. Although the dose and administration schedule of cyclophosphamide used here do not mediate Treg depletion [68], cyclophosphamide could down-regulate Treg activity. KLH-specific proliferative responses were only observed when DC vaccination was combined with cyclophosphamide, whereas tumor-specific responses could not be detected in any group. In the group receiving DC alone (n = 11), two patients remained stable and the others progressed. In the group receiving combined cyclophosphamide and DC treatment (n = 7), two patients developed a mixed response, one remained stable and four progressed. These results suggest that combining DC vaccination with cyclophosphamide administration could be an effective means to counteract the suppressive effect of Treg on anti-tumor responses [75]. Although the number of treated patients was rather low in these studies, results indicate that either Treg depletion or inactivation combined with DC vaccination might lead to improved anti-tumor immune responses [44, 75].
Very few data are available on the role of tumor volume on the outcome of DC vaccination therapy. It has been postulated that in patients with large tumor burden active suppressive mechanisms of the tumor prevent the induction of effective anti-tumor immune responses. A study reported by O’Rourke and colleagues used the measurement of the S-100B protein in the blood of stage IV melanoma patients as a means to estimate tumor burden. They show that patients with low S-100B concentration (<0.36 μg/ml plasma) before vaccination show a significantly better survival upon DC vaccination compared to patients with high S-100B levels before vaccination. Furthermore, patients with initially low S-100B levels and concomitantly low bulk disease were shown to have significantly better objective clinical response rates. Thus, S-100B levels in melanoma patients could function as a measure for the tumor burden and could be predictive for the outcome of therapy. The authors postulate that a large tumor burden prevents the induction of an anti-tumor response by DC vaccination and suggest that surgical debulking before DC vaccination could improve the outcome of DC vaccination [123]. Another study reported by Tuettenberg et al. compared results of a study in stage II melanoma patients with MRD at high risk of relapse with previous results of a study in stage IV melanoma patients with large tumors. They point to several important differences: (1) the strength of DTH responses obtained in stage II patients was significantly higher and longer-lived compared to stage IV patients; (2) although tumor-specific CD8+ T-cell responses were observed in both stage II and IV patients, the expansion of these tumor-specific CD8+ T cells was much higher in stage II patients; (3) vaccine-induced IFNγ producing effector CD8+ T cells were observed in a larger proportion of stage II patients compared to stage IV patients. Altogether, vaccination-induced expansion and differentiation of Ag-specific CD8+ T cells was more prominent in stage II patients [187]. Although preliminary, these results suggest that a large volume of tumor indeed has a negative effect on the outcome of DC vaccination. Therefore, surgical debulking before vaccination to obtain a state of MRD could be advantageous for subsequent DC vaccination.
Although many issues still need to be resolved, the following conclusions can be drawn from the clinical trials carried out with DC so far. First, the present view is that mDC need to be used for vaccination. Maturation stimuli can be provided either in vitro or in vivo. However, considerable controversy still exists about the selection of optimal maturation stimuli. Second, concerning the immunization route, the few studies that compared different routes point to i.d. administration as the preferred method. However, combining different routes of immunization might also be beneficial because depending on the location of the tumor a different injection route might be required [115]. Third, a whole variety of methods has been used to load DC with Ags. Immunization with a single antigenic epitope could lead to the emergence of Ag-loss variants, although it is now clear that T cells directed towards the immunizing epitope can lead to secondary activation of T cells recognizing other TAA. Furthermore, the emergence of anti-tumor T cells or epitope spreading could be predictive for a clinical response. However, the use of approaches employing different TAA could select for the most immunogenic responses and, when a genetic approach is used, the TAA can be targeted for presentation in MHC class II, thereby providing CD4+ T-cell help. Little is known about the optimal number of vaccinations, but it is generally believed that repeated injections are beneficial. However, there is little or no information on the optimal interval between vaccinations [60]. Finally, a number of studies combine DC therapy with other agents. KLH has been frequently used to provide non-specific CD4+ T-cell help, but more recently other stimuli have been used in conjunction with DC vaccination to either activate DC in vivo (e.g. TLR ligands), activate the immune system (e.g. cytokines) or attenuate tumor-induced immunosuppression (e.g. cyclophosphamide, ONTAK). However, more research is needed to identify possible advantageous combinatorial therapies.
Concerning the design of clinical studies using DC, minimal criteria have to be fulfilled in order to be able to compare different studies and eventually come to a standardized protocol [60]. The method of vaccine preparation must be carefully described and it must be indicated if DC vaccination is combined with other treatments. The studies should provide precise information on the maturation stimulus used and the quality of the injected DC. Next, there has to be a careful description of the route of DC administration, the number of DC injected, the vaccination schedule and the time points at which samples are taken for monitoring. Lastly, a careful description of clinical outcome of all treated patients together with the results of immunological monitoring before and after treatment needs to be provided.
Conclusions and future perspectives
Promising results have been obtained with DC vaccination in mouse models, with induction of both anti-tumor immune responses and tumor regression. Initial clinical trials in humans highlight the potential of DC for the induction of tumor-specific immune responses. Nevertheless, durable clinical responses have been rarely achieved and often a correlation between the induction of tumor-specific immunity and clinical outcome could not be observed. Several factors might contribute to this discrepancy. First of all, patients treated up to now generally suffered from advanced-stage disease, were heavily pre-treated and had a compromised immune system, which has considerable impact on the efficacy of DC vaccination. Furthermore, most patients have excessive, highly vascularized tumor burden, which may be difficult to reject by T cells induced by DC vaccination. We should now move to vaccination of less-advanced patients or even patients with MRD, which might be the ideal treatment population for DC vaccination. Another important variable which complicates comparison of clinical trials is the use of different DC subsets for vaccination. New insights in DC biology are continuously generated which makes it difficult to standardize the DC population to be used in cancer trials. Although DC vaccination proved to be safe in the short term in these first trials, concerns are raised regarding the long-term effect since it has been described that some DC subsets can induce/expand Treg. Preferably, DC generated from distinct progenitors and activated using different stimuli should be compared in small clinical trials in order to define optimal DC preparations and standardize vaccination protocols. Maybe a DC vaccine consisting of several DC types could be developed in order to try to activate all components of the immune system. The same holds true for the Ag type to be used for DC loading. Although epitope spreading has been described when using only one defined epitope for vaccination, optimal loading strategies should use a broad spectrum of potential TAA, in order to avoid tumor escape. Next, immunomonitoring methods used should preferably be standardized in order to be able to compare immunological outcomes for different trials. A broad spectrum of methods should be used to not only enumerate tumor-specific effectors, but also to characterize their activation status and functional properties. Furthermore, in order to fully characterize the immune response, it is important to analyze both T cells in the circulation and at the tumor site, since important phenotypical and functional differences were found, which might in part explain the lack of clinical efficacy of DC vaccination. One important contributing factor is probably the existence of a suppressive tumor microenvironment. Several mechanisms cooperate to establish this tumor-induced immunosuppression. Tumor cells display an over-consumption of glucose, leading to glucose deprivation which could inhibit effector T-cell functions. B7-H1/PD-L1 expression by human tumors leads to inhibition of T-cell responses through interaction with PD-1 on activated T cells. Tumors show an elevated expression of COX-2, leading to secretion of PGE2 which inhibits T-cell effector functions. Indoleamine 2,3-dioxygenase (IDO) is frequently expressed and activated in tumor cells, which leads to tryptophan deprivation and production of toxic metabolites, causing cell-cycle arrest and T-cell death. Myeloid suppressor cells (MSC) are present in various tumors and express Arginase I and the inducible nitric oxide synthetase (iNOS), which lead to arginine deprivation and the production of toxic O and NO radicals within the tumor. Soluble immunosuppressive mediators (TGF-β, VEGF, IL-10, IL-23) are present within the tumor microenvironment and interfere with effective T-cell function. Finally, Treg are recruited and/or induced in tumors and exert direct immunosuppressive effects on effector T cells. Therefore, it will probably be advantageous to combine DC vaccination with agents tackling these immunosuppressive mechanisms. Among these agents are blocking Abs (PD-1/B7-H1, TGF-β, VEGF, IL-10, IL-23), enzyme inhibitors (IDO, Arginase I, iNOS) and strategies to eliminate or inhibit Treg (low-dose chemotherapy, ONTAK, CTLA-4 blockade, TLR8 stimulation) [35, 40, 64, 74, 96, 114]. Thus, in a first stage it is necessary to gather more information about the variety of escape strategies used by tumors. In the next step, we can identify which mechanism(s) is used by the tumor of individual patients in order to select for each patient the most appropriate approach to counteract tumor escape.
It has become clear that, in order to eradicate large tumors, a combination of vaccination approaches targeting several aspects of the immune system is necessary: (1) adoptive transfer of tumor-specific T cells to directly attack the tumor; (2) TAA-expressing DC to induce tumor-specific effector and eventually memory T cells; (3) interference with the suppressive tumor microenvironment; and (4) administration of immune-activating cytokines (GM-CSF, IFNα, IL-2, IL-15, TNFα...).
In conclusion, DC vaccination has proved to be very safe and can induce tumor-specific immune responses. However, objective clinical responses so far have been scarce. Further optimization of several parameters is needed, including defining the preferred DC type and the optimal Ag loading technique, which will eventually all lead to a standardized DC vaccine. Efforts should also focus on the combination of DC vaccination with other therapies, to further enhance clinical efficacy.
Acknowledgment
This work was supported by the Belgian Program on Interuniversity Poles of Attraction, by a grant of the Belgian Foundation against Cancer, by an Integrated Project and Network of Excellence sponsored by the EU and by a grant of the Fund for Scientific Research (FWO-V). ST was supported by a grant from the ‘Vlaamse Liga tegen Kanker’. JLA, KB and AB are post-doctoral fellows of the Fund for Scientific Research Flanders, Belgium (FWO Vlaanderen).
References
- 1.Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R) Vaccine. 2003;21:787–790. doi: 10.1016/S0264-410X(02)00599-6. [DOI] [PubMed] [Google Scholar]
- 2.Adamson L, Palmborg A, Svensson A, Lundqvist A, Hansson M, Kiessling R, Masucci G, Mellstedt H, Pisa P. Development of a technology platform for large-scale clinical grade production of DC. Cytotherapy. 2004;6:363–371. doi: 10.1080/14653240410004934. [DOI] [PubMed] [Google Scholar]
- 3.Aerts-Toegaert C, Heirman C, Tuyaerts S, Corthals J, Aerts JL, Bonehill A, Thielemans K, Breckpot K. CD83 expression on dendritic cells and T cells: Correlation with effective immune responses. Eur J Immunol. 2007;37:686–695. doi: 10.1002/eji.200636535. [DOI] [PubMed] [Google Scholar]
- 4.Alejandro Lopez J, Crosbie G, Kelly C, McGee AM, Williams K, Vuckovic S, Schuyler R, Rodwell R, Wright SJ, Taylor K, Hart DN. Monitoring and isolation of blood dendritic cells from apheresis products in healthy individuals: a platform for cancer immunotherapy. J Immunol Methods. 2002;267:199–212. doi: 10.1016/S0022-1759(02)00185-0. [DOI] [PubMed] [Google Scholar]
- 5.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 6.Andersen MH, Schrama D, Thor Straten P, Becker JC. Cytotoxic T cells. J Invest Dermatol. 2006;126:32–41. doi: 10.1038/sj.jid.5700001. [DOI] [PubMed] [Google Scholar]
- 7.Andre F, Chaput N, Schartz NE, Flament C, Aubert N, Bernard J, Lemonnier F, Raposo G, Escudier B, Hsu DH, Tursz T, Amigorena S, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J Immunol. 2004;172:2126–2136. doi: 10.4049/jimmunol.172.4.2126. [DOI] [PubMed] [Google Scholar]
- 8.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC, Romero P, Leyvraz S, Rufer N, Speiser DE. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol. 2006;177:1670–1678. doi: 10.4049/jimmunol.177.3.1670. [DOI] [PubMed] [Google Scholar]
- 9.Attia P, Powell DJ, Jr, Maker AV, Kreitman RJ, Pastan I, Rosenberg SA. Selective elimination of human regulatory T lymphocytes in vitro with the recombinant immunotoxin LMB-2. J Immunother. 2006;29:208–214. doi: 10.1097/01.cji.0000187959.45803.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babatz J, Rollig C, Oelschlagel U, Zhao S, Ehninger G, Schmitz M, Bornhauser M. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: a phase I study. J Hematother Stem Cell Res. 2003;12:515–523. doi: 10.1089/152581603322448222. [DOI] [PubMed] [Google Scholar]
- 11.Bai L, Feuerer M, Beckhove P, Umansky V, Schirrmacher V. Generation of dendritic cells from human bone marrow mononuclear cells: advantages for clinical application in comparison to peripheral blood monocyte derived cells. Int J Oncol. 2002;20:247–253. [PubMed] [Google Scholar]
- 12.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 13.Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5:296–306. doi: 10.1038/nri1592. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee D, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar K. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after DC injection of cytokine matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedrosian I, Mick R, Xu S, Nisenbaum H, Faries M, Zhang P, Cohen PA, Koski G, Czerniecki BJ. Intranodal administration of peptide-pulsed mature dendritic cell vaccines results in superior CD8+ T-cell function in melanoma patients. J Clin Oncol. 2003;21:3826–3835. doi: 10.1200/JCO.2003.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Bergant M, Meden L, Repnik U, Sojar V, Stanisavljevic D, Jeras M. Preparation of native and amplified tumour RNA for dendritic cell transfection and generation of in vitro anti-tumour CTL responses. Immunobiology. 2006;211:179–189. doi: 10.1016/j.imbio.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G, Schuler-Thurner B. Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods. 2002;268:131–140. doi: 10.1016/S0022-1759(02)00189-8. [DOI] [PubMed] [Google Scholar]
- 18.Berger TG, Strasser E, Smith R, Carste C, Schuler-Thurner B, Kaempgen E, Schuler G. Efficient elutriation of monocytes within a closed system (Elutra) for clinical-scale generation of dendritic cells. J Immunol Methods. 2005;298:61–72. doi: 10.1016/j.jim.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/S0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 20.Bevan MJ. Helping the CD8(+) T-cell response. Nat Rev Immunol. 2004;4:595–602. doi: 10.1038/nri1413. [DOI] [PubMed] [Google Scholar]
- 21.Bohnenkamp HR, Coleman J, Burchell JM, Taylor-Papadimitriou J, Noll T. Breast carcinoma cell lysate-pulsed dendritic cells cross-prime MUC1-specific CD8+ T cells identified by peptide-MHC-class-I tetramers. Cell Immunol. 2004;231:112–125. doi: 10.1016/j.cellimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 22.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 23.Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breckpot K, Corthals J, Bonehill A, Michiels A, Tuyaerts S, Aerts C, Heirman C, Thielemans K. Dendritic cells differentiated in the presence of IFN-{beta} and IL-3 are potent inducers of an antigen-specific CD8+ T cell response. J Leukoc Biol. 2005;78:898–908. doi: 10.1189/jlb.0105052. [DOI] [PubMed] [Google Scholar]
- 25.Breckpot K, Corthals J, Heirman C, Bonehill A, Michiels A, Tuyaerts S, De Greef C, Thielemans K. Activation of monocytes via the CD14 receptor leads to the enhanced lentiviral transduction of immature dendritic cells. Hum Gene Ther. 2004;15:562–573. doi: 10.1089/104303404323142015. [DOI] [PubMed] [Google Scholar]
- 26.Breckpot K, Heirman C, Neyns B, Thielemans K. Exploiting dendritic cells for cancer immunotherapy: genetic modification of dendritic cells. J Gene Med. 2004;6:1175–1188. doi: 10.1002/jgm.615. [DOI] [PubMed] [Google Scholar]
- 27.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 28.Brosterhus H, Brings S, Leyendeckers H, Manz RA, Miltenyi S, Radbruch A, Assenmacher M, Schmitz J. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol. 1999;29:4053–4059. doi: 10.1002/(SICI)1521-4141(199912)29:12<4053::AID-IMMU4053>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 29.Buelens C, Bartholome EJ, Amraoui Z, Boutriaux M, Salmon I, Thielemans K, Willems F, Goldman M. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–998. doi: 10.1182/blood.V99.3.993. [DOI] [PubMed] [Google Scholar]
- 30.Butterfield LH, Ribas A, Dissette VB, Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH, Mukherji B, Cochran AJ, Glaspy JA, Economou JS. Determinant spreading associated with clinical response in dendritic cell-based immunotherapy for malignant melanoma. Clin Cancer Res. 2003;9:998–1008. [PubMed] [Google Scholar]
- 31.Campbell JD, Piechaczek C, Winkels G, Schwamborn E, Micheli D, Hennemann S, Schmitz J. Isolation and generation of clinical-grade dendritic cells using the CliniMACS system. Methods Mol Med. 2005;109:55–70. doi: 10.1385/1-59259-862-5:055. [DOI] [PubMed] [Google Scholar]
- 32.Cappello P, Fraone T, Barberis L, Costa C, Hirsch E, Elia AR, Caorsi C, Musso T, Novelli F, Giovarelli M. CC-chemokine ligand 16 induces a novel maturation program in human immature monocyte-derived dendritic cells. J Immunol. 2006;177:6143–6151. doi: 10.4049/jimmunol.177.9.6143. [DOI] [PubMed] [Google Scholar]
- 33.Carrasco J, Godelaine D, Van Pel A, Boon T, van der Bruggen P. CD45RA on human CD8 T cells is sensitive to the time elapsed since the last antigenic stimulation. Blood. 2006;108:2897–2905. doi: 10.1182/blood-2005-11-007237. [DOI] [PubMed] [Google Scholar]
- 34.Celluzzi CM, Welbon C. Dendritic cell culture: a simple closed culture system using ficoll, monocytes, and a table-top centrifuge. J Hematother Stem Cell Res. 2003;12:575–585. doi: 10.1089/152581603322448286. [DOI] [PubMed] [Google Scholar]
- 35.Cham CM, Gajewski TF. Metabolic mechanisms of tumor resistance to T cell effector function. Immunol Res. 2005;31:107–118. doi: 10.1385/IR:31:2:107. [DOI] [PubMed] [Google Scholar]
- 36.Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 37.Chen B, Stiff P, Sloan G, Kash J, Manjunath R, Pathasarathy M, Oldenburg D, Foreman KE, Nickoloff BJ. Replicative response, immunophenotype, and functional activity of monocyte-derived versus CD34(+)-derived dendritic cells following exposure to various expansion and maturational stimuli. Clin Immunol. 2001;98:280–292. doi: 10.1006/clim.2000.4968. [DOI] [PubMed] [Google Scholar]
- 38.Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–252. doi: 10.1007/BF02255855. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Yang C, Jin N, Xie Z, Tang Y, Fei L, Jia Z, Wu Y. Terminal complement complex C5b-9-treated human monocyte-derived dendritic cells undergo maturation and induce Th1 polarization. Eur J Immunol. 2007;37:167–176. doi: 10.1002/eji.200636285. [DOI] [PubMed] [Google Scholar]
- 40.Coulie PG, Connerotte T. Human tumor-specific T lymphocytes: does function matter more than number? Curr Opin Immunol. 2005;17:320–325. doi: 10.1016/j.coi.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Coulie PG, van der Bruggen P. T-cell responses of vaccinated cancer patients. Curr Opin Immunol. 2003;15:131–137. doi: 10.1016/S0952-7915(03)00009-8. [DOI] [PubMed] [Google Scholar]
- 42.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 43.Dakic A, Wu L. Hemopoietic precursors and development of dendritic cell populations. Leuk Lymphoma. 2003;44:1469–1475. doi: 10.1080/1042819031000083370. [DOI] [PubMed] [Google Scholar]
- 44.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dauer M, Pohl K, Obermaier B, Meskendahl T, Robe J, Schnurr M, Endres S, Eigler A. Interferon-alpha disables dendritic cell precursors: dendritic cells derived from interferon-alpha-treated monocytes are defective in maturation and T-cell stimulation. Immunology. 2003;110:38–47. doi: 10.1046/j.1365-2567.2003.01702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dauer M, Schad K, Herten J, Junkmann J, Bauer C, Kiefl R, Endres S, Eigler A. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J Immunol Methods. 2005;302:145–155. doi: 10.1016/j.jim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 47.de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol. 2005;23:5779–5787. doi: 10.1200/JCO.2005.06.478. [DOI] [PubMed] [Google Scholar]
- 48.de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, Figdor CG, Punt CJ. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res. 2003;9:5091–5100. [PubMed] [Google Scholar]
- 49.Della Bella S, Nicola S, Riva A, Biasin M, Clerici M, Villa ML. Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J Leukoc Biol. 2004;75:106–116. doi: 10.1189/jlb.0403154. [DOI] [PubMed] [Google Scholar]
- 50.Della Porta M, Danova M, Rigolin GM, Brugnatelli S, Rovati B, Tronconi C, Fraulini C, Russo Rossi A, Riccardi A, Castoldi G. Dendritic cells and vascular endothelial growth factor in colorectal cancer: correlations with clinicobiological findings. Oncology. 2005;68:276–284. doi: 10.1159/000086784. [DOI] [PubMed] [Google Scholar]
- 51.Delluc S, Tourneur L, Fradelizi D, Rubio MT, Marchiol-Fournigault C, Chiocchia G, Buzyn A. DC-based vaccine loaded with acid-eluted peptides in acute myeloid leukemia: the importance of choosing the best elution method. Cancer Immunol Immunother. 2007;56:1–12. doi: 10.1007/s00262-006-0170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dietz AB, Padley DJ, Butler GW, Maas ML, Greiner CW, Gastineau DA, Vuk-Pavlovic S. Clinical-grade manufacturing of DC from CD14+ precursors: experience from phase I clinical trials in CML and malignant melanoma. Cytotherapy. 2004;6:563–570. doi: 10.1080/14653240410005357. [DOI] [PubMed] [Google Scholar]
- 53.Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol. 2006;6:836–848. doi: 10.1038/nri1961. [DOI] [PubMed] [Google Scholar]
- 54.Elkord E, Williams PE, Kynaston H, Rowbottom AW. Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells. Immunology. 2005;114:204–212. doi: 10.1111/j.1365-2567.2004.02076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of the first phase I clinical trial. J Transl Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fearnley DB, McLellan AD, Mannering SI, Hock BD, Hart DN. Isolation of human blood dendritic cells using the CMRF-44 monoclonal antibody: implications for studies on antigen-presenting cell function and immunotherapy. Blood. 1997;89:3708–3716. [PubMed] [Google Scholar]
- 57.Felzmann T, Witt V, Wimmer D, Ressmann G, Wagner D, Paul P, Huttner K, Fritsch G. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy. 2003;5:391–398. doi: 10.1080/14653240310003053. [DOI] [PubMed] [Google Scholar]
- 58.Ferlazzo G, Semino C, Spaggiari GM, Meta M, Mingari MC, Melioli G. Dendritic cells efficiently cross-prime HLA class I-restricted cytolytic T lymphocytes when pulsed with both apoptotic and necrotic cells but not with soluble cell-derived lysates. Int Immunol. 2000;12:1741–1747. doi: 10.1093/intimm/12.12.1741. [DOI] [PubMed] [Google Scholar]
- 59.Feuerstein B, Berger TG, Maczek C, Roder C, Schreiner D, Hirsch U, Haendle I, Leisgang W, Glaser A, Kuss O, Diepgen TL, Schuler G, Schuler-Thurner B. A method for the production of cryopreserved aliquots of antigen-preloaded, mature dendritic cells ready for clinical use. J Immunol Methods. 2000;245:15–29. doi: 10.1016/S0022-1759(00)00269-6. [DOI] [PubMed] [Google Scholar]
- 60.Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 61.Fong L, Brockstedt D, Benike C, Wu L, Engleman EG. Dendritic cells injected via different routes induce immunity in cancer patients. J Immunol. 2001;166:4254–4259. doi: 10.4049/jimmunol.166.6.4254. [DOI] [PubMed] [Google Scholar]
- 62.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher GA, Davis MM, Engleman EG. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franzke A. The role of G-CSF in adaptive immunity. Cytokine Growth Factor Rev. 2006;17:235–244. doi: 10.1016/j.cytogfr.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother. 2006;29:233–240. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 65.Garderet L, Cao H, Salamero J, Verge V, Tisserand E, Scholl S, Gorin NC, Lopez M. In vitro production of dendritic cells from human blood monocytes for therapeutic use. J Hematother Stem Cell Res. 2001;10:553–567. doi: 10.1089/15258160152509163. [DOI] [PubMed] [Google Scholar]
- 66.Garlie N, Timler A. Dendritic cell generation from cryopreserved monocytes enriched using the Elutra versus the CliniMACS Cell Separation Systems. J Immunother. 2005;28:613–613. doi: 10.1097/01.cji.0000190949.33127.9a. [DOI] [Google Scholar]
- 67.Gervois N, Heuze F, Diez E, Jotereau F. Selective expansion of a specific anti-tumor CD8+ cytotoxic T lymphocyte clone in the bulk culture of tumor-infiltrating lymphocytes from a melanoma patient: cytotoxic activity and T cell receptor gene rearrangements. Eur J Immunol. 1990;20:825–831. doi: 10.1002/eji.1830200417. [DOI] [PubMed] [Google Scholar]
- 68.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4(+)CD25 (+) regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56:641–648. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gilboa E, Vieweg J. Cancer immunotherapy with mRNA-transfected dendritic cells. Immunol Rev. 2004;199:251–263. doi: 10.1111/j.0105-2896.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 70.Godelaine D, Carrasco J, Lucas S, Karanikas V, Schuler-Thurner B, Coulie PG, Schuler G, Boon T, Van Pel A. Polyclonal CTL responses observed in melanoma patients vaccinated with dendritic cells pulsed with a MAGE-3.A1 peptide. J Immunol. 2003;171:4893–4897. doi: 10.4049/jimmunol.171.9.4893. [DOI] [PubMed] [Google Scholar]
- 71.Gordan JD, Vonderheide RH. Universal tumor antigens as targets for immunotherapy. Cytotherapy. 2002;4:317–327. doi: 10.1080/146532402760271091. [DOI] [PubMed] [Google Scholar]
- 72.Guardino AE, Rajapaksa R, Ong KH, Sheehan K, Levy R. Production of myeloid dendritic cells (DC) pulsed with tumor-specific idiotype protein for vaccination of patients with multiple myeloma. Cytotherapy. 2006;8:277–289. doi: 10.1080/14653240600735701. [DOI] [PubMed] [Google Scholar]
- 73.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–667. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 74.Hayakawa S. No cancer in cancers: evolutionary trade-off between successful viviparity and tumor escape from the adaptive immune system. Med Hypotheses. 2006;66:888–897. doi: 10.1016/j.mehy.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 75.Holtl L, Ramoner R, Zelle-Rieser C, Gander H, Putz T, Papesh C, Nussbaumer W, Falkensammer C, Bartsch G, Thurnher M. Allogeneic dendritic cell vaccination against metastatic renal cell carcinoma with or without cyclophosphamide. Cancer Immunol Immunother. 2005;54:663–670. doi: 10.1007/s00262-004-0629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hoos A, Parmiani G, Hege K, Sznol M, Loibner H, Eggermont A, Urba W, Blumenstein B, Sacks N, Keilholz U, Nichol G. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 77.Jarnjak-Jankovic S, Pettersen RD, Saeboe-Larssen S, Wesenberg F, Gaudernack G. Evaluation of dendritic cells loaded with apoptotic cancer cells or expressing tumour mRNA as potential cancer vaccines against leukemia. BMC Cancer. 2005;5:20. doi: 10.1186/1471-2407-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::AID-IMMU3388>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 79.Jefford M, Schnurr M, Toy T, Masterman KA, Shin A, Beecroft T, Tai TY, Shortman K, Shackleton M, Davis ID, Parente P, Luft T, Chen W, Cebon J, Maraskovsky E. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 80.Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, Enk AH. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer. 2001;93:243–251. doi: 10.1002/ijc.1323. [DOI] [PubMed] [Google Scholar]
- 81.Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol. 1999;163:6867–6875. [PubMed] [Google Scholar]
- 82.Kammula US, Marincola FM, Rosenberg SA. Real-time quantitative polymerase chain reaction assessment of immune reactivity in melanoma patients after tumor peptide vaccination. J Natl Cancer Inst. 2000;92:1336–1344. doi: 10.1093/jnci/92.16.1336. [DOI] [PubMed] [Google Scholar]
- 83.Karanikas V, Lurquin C, Colau D, van Baren N, De Smet C, Lethe B, Connerotte T, Corbiere V, Demoitie MA, Lienard D, Dreno B, Velu T, Boon T, Coulie PG. Monoclonal anti-MAGE-3 CTL responses in melanoma patients displaying tumor regression after vaccination with a recombinant canarypox virus. J Immunol. 2003;171:4898–4904. doi: 10.4049/jimmunol.171.9.4898. [DOI] [PubMed] [Google Scholar]
- 84.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC-dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–1519. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 85.Keilholz U, Martus P, Scheibenbogen C. Immune monitoring of T-cell responses in cancer vaccine development. Clin Cancer Res. 2006;12:2346s–2352s. doi: 10.1158/1078-0432.CCR-05-2540. [DOI] [PubMed] [Google Scholar]
- 86.Klangsinsirikul P, Russell NH. Peripheral blood stem cell harvests from G-CSF-stimulated donors contain a skewed Th2 CD4 phenotype and a predominance of type 2 dendritic cells. Exp Hematol. 2002;30:495–501. doi: 10.1016/S0301-472X(02)00785-3. [DOI] [PubMed] [Google Scholar]
- 87.Koido S, Nikrui N, Ohana M, Xia J, Tanaka Y, Liu C, Durfee JK, Lerner A, Gong J. Assessment of fusion cells from patient-derived ovarian carcinoma cells and dendritic cells as a vaccine for clinical use. Gynecol Oncol. 2005;99:462–471. doi: 10.1016/j.ygyno.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 88.Kufner S, Zitzelsberger H, Kroell T, Pelka-Fleischer R, Salem A, de Valle F, Schweiger C, Nuessler V, Schmid C, Kolb HJ, Schmetzer HM. Leukemia-derived dendritic cells can be generated from blood or bone marrow cells from patients with acute myeloid leukaemia: a methodological approach under serum-free culture conditions. Scand J Immunol. 2005;62:86–98. doi: 10.1111/j.1365-3083.2005.01630.x. [DOI] [PubMed] [Google Scholar]
- 89.Kyte JA, Mu L, Aamdal S, Kvalheim G, Dueland S, Hauser M, Gullestad HP, Ryder T, Lislerud K, Hammerstad H, Gaudernack G. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13:905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 90.Lee AW, Truong T, Bickham K, Fonteneau JF, Larsson M, Da Silva I, Somersan S, Thomas EK, Bhardwaj N. A clinical grade cocktail of cytokines and PGE2 results in uniform maturation of human monocyte-derived dendritic cells: implications for immunotherapy. Vaccine. 2002;20(suppl 4):A8–A22. doi: 10.1016/S0264-410X(02)00382-1. [DOI] [PubMed] [Google Scholar]
- 91.Lee KH, Panelli MC, Kim CJ, Riker AI, Bettinotti MP, Roden MM, Fetsch P, Abati A, Rosenberg SA, Marincola FM. Functional dissociation between local and systemic immune response during anti-melanoma peptide vaccination. J Immunol. 1998;161:4183–4194. [PubMed] [Google Scholar]
- 92.Lehner M, Felzmann T, Clodi K, Holter W. Type I interferons in combination with bacterial stimuli induce apoptosis of monocyte-derived dendritic cells. Blood. 2001;98:736–742. doi: 10.1182/blood.V98.3.736. [DOI] [PubMed] [Google Scholar]
- 93.Lehner M, Holter W. Endotoxin-free purification of monocytes for dendritic cell generation via discontinuous density gradient centrifugation based on diluted Ficoll-Paque Plus. Int Arch Allergy Immunol. 2002;128:73–76. doi: 10.1159/000058006. [DOI] [PubMed] [Google Scholar]
- 94.Lewalle P, Rouas R, Lehmann F, Martiat P. Freezing of dendritic cells, generated from cryopreserved leukaphereses, does not influence their ability to induce antigen-specific immune responses or functionally react to maturation stimuli. J Immunol Methods. 2000;240:69–78. doi: 10.1016/S0022-1759(00)00173-3. [DOI] [PubMed] [Google Scholar]
- 95.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Improving antitumor immune responses by circumventing immunoregulatory cells and mechanisms. Clin Cancer Res. 2006;12:4794–4803. doi: 10.1158/1078-0432.CCR-06-0944. [DOI] [PubMed] [Google Scholar]
- 97.Lopez JA, Bioley G, Turtle CJ, Pinzon-Charry A, Ho CS, Vuckovic S, Crosbie G, Gilleece M, Jackson DC, Munster D, Hart DN. Single step enrichment of blood dendritic cells by positive immunoselection. J Immunol Methods. 2003;274:47–61. doi: 10.1016/S0022-1759(02)00429-5. [DOI] [PubMed] [Google Scholar]
- 98.Lopez M, Amorim L, Gane P, Cristoph A, Bardinet D, Abina AM, Minty A, Bernard J. IL-13 induces CD34+ cells isolated from G-CSF mobilized blood to differentiate in vitro into potent antigen presenting cells. J Immunol Methods. 1997;208:117–129. doi: 10.1016/S0022-1759(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 99.Luft T, Pang KC, Thomas E, Bradley CJ, Savoia H, Trapani J, Cebon J. A serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489–500. [PubMed] [Google Scholar]
- 100.Lurquin C, Lethe B, De Plaen E, Corbiere V, Theate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lyons AB. Divided we stand: tracking cell proliferation with carboxyfluorescein diacetate succinimidyl ester. Immunol Cell Biol. 1999;77:509–515. doi: 10.1046/j.1440-1711.1999.00864.x. [DOI] [PubMed] [Google Scholar]
- 102.Makino M, Wakamatsu S, Shimokubo S, Arima N, Baba M. Production of functionally deficient dendritic cells from HTLV-I-infected monocytes: implications for the dendritic cell defect in adult T cell leukemia. Virology. 2000;274:140–148. doi: 10.1006/viro.2000.0445. [DOI] [PubMed] [Google Scholar]
- 103.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski CR, Hoek J, Caron D, Lebsack ME, McKenna HJ. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 104.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 105.McIlroy D, Gregoire M. Optimizing dendritic cell-based anticancer immunotherapy: maturation state does have clinical impact. Cancer Immunol Immunother. 2003;52:583–591. doi: 10.1007/s00262-003-0414-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McRae BL, Nagai T, Semnani RT, van Seventer JM, van Seventer GA. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14(+) precursors. Blood. 2000;96:210–217. [PubMed] [Google Scholar]
- 107.Messele T, Roos MT, Hamann D, Koot M, Fontanet AL, Miedema F, Schellekens PT, Rinke de Wit TF. Nonradioactive techniques for measurement of in vitro T-cell proliferation: alternatives to the [(3)H]thymidine incorporation assay. Clin Diagn Lab Immunol. 2000;7:687–692. doi: 10.1128/CDLI.7.4.687-692.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Meyer-Wentrup F, Burdach S. Efficacy of dendritic cell generation for clinical use: recovery and purity of monocytes and mature dendritic cells after immunomagnetic sorting or adherence selection of CD14+ starting populations. J Hematother Stem Cell Res. 2003;12:289–299. doi: 10.1089/152581603322023025. [DOI] [PubMed] [Google Scholar]
- 109.Michiels A, Breckpot K, Corthals J, Tuyaerts S, Bonehill A, Heirman C, Thielemans K, Aerts JL. Induction of antigen-specific CD8+ cytotoxic T cells by dendritic cells co-electroporated with a dsRNA analogue and tumor antigen mRNA. Gene Ther. 2006;13:1027–1036. doi: 10.1038/sj.gt.3302750. [DOI] [PubMed] [Google Scholar]
- 110.Miyahira Y, Murata K, Rodriguez D, Rodriguez JR, Esteban M, Rodrigues MM, Zavala F. Quantification of antigen specific CD8+ T cells using an ELISPOT assay. J Immunol Methods. 1995;181:45–54. doi: 10.1016/0022-1759(94)00327-S. [DOI] [PubMed] [Google Scholar]
- 111.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohty M, Jarrossay D, Lafage-Pochitaloff M, Zandotti C, Briere F, de Lamballeri XN, Isnardon D, Sainty D, Olive D, Gaugler B. Circulating blood dendritic cells from myeloid leukemia patients display quantitative and cytogenetic abnormalities as well as functional impairment. Blood. 2001;98:3750–3756. doi: 10.1182/blood.V98.13.3750. [DOI] [PubMed] [Google Scholar]
- 113.Mohty M, Vialle-Castellano A, Nunes JA, Isnardon D, Olive D, Gaugler B. IFN-alpha skews monocyte differentiation into Toll-like receptor 7-expressing dendritic cells with potent functional activities. J Immunol. 2003;171:3385–3393. doi: 10.4049/jimmunol.171.7.3385. [DOI] [PubMed] [Google Scholar]
- 114.Muller AJ, Scherle PA. Targeting the mechanisms of tumoral immune tolerance with small-molecule inhibitors. Nat Rev Cancer. 2006;6:613–625. doi: 10.1038/nrc1929. [DOI] [PubMed] [Google Scholar]
- 115.Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Munn DH, Sharma MD, Lee JR, Jhaver KG, Johnson TS, Keskin DB, Marshall B, Chandler P, Antonia SJ, Burgess R, Slingluff CL, Jr, Mellor AL. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297:1867–1870. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 117.Nair S, McLaughlin C, Weizer A, Su Z, Boczkowski D, Dannull J, Vieweg J, Gilboa E. Injection of immature dendritic cells into adjuvant-treated skin obviates the need for ex vivo maturation. J Immunol. 2003;171:6275–6282. doi: 10.4049/jimmunol.171.11.6275. [DOI] [PubMed] [Google Scholar]
- 118.Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–549. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187–207. doi: 10.1007/s00262-004-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nowrousian MR, Waschke S, Bojko P, Welt A, Schuett P, Ebeling P, Flasshove M, Moritz T, Schuette J, Seeber S. Impact of chemotherapy regimen and hematopoietic growth factor on mobilization and collection of peripheral blood stem cells in cancer patients. Ann Oncol. 2003;14(suppl 1):i29–i36. doi: 10.1093/annonc/mdg706. [DOI] [PubMed] [Google Scholar]
- 121.Obermaier B, Dauer M, Herten J, Schad K, Endres S, Eigler A. Development of a new protocol for 2-day generation of mature dendritic cells from human monocytes. Biol Proced Online. 2003;5:197–203. doi: 10.1251/bpo62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.O’Neill DW, Adams S, Bhardwaj N. Manipulating dendritic cell biology for the active immunotherapy of cancer. Blood. 2004;104:2235–2246. doi: 10.1182/blood-2003-12-4392. [DOI] [PubMed] [Google Scholar]
- 123.O’Rourke MG, Johnson M, Lanagan C, See J, Yang J, Bell JR, Slater GJ, Kerr BM, Crowe B, Purdie DM, Elliott SL, Ellem KA, Schmidt CW. Durable complete clinical responses in a phase I/II trial using an autologous melanoma cell/dendritic cell vaccine. Cancer Immunol Immunother. 2003;52:387–395. doi: 10.1007/s00262-003-0375-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Orsini E, Guarini A, Chiaretti S, Mauro FR, Foa R. The circulating dendritic cell compartment in patients with chronic lymphocytic leukemia is severely defective and unable to stimulate an effective T-cell response. Cancer Res. 2003;63:4497–4506. [PubMed] [Google Scholar]
- 125.Orsini E, Pasquale A, Maggio R, Calabrese E, Mauro FR, Giammartini E, Guarini A, Foa R. Phenotypic and functional characterization of monocyte-derived dendritic cells in chronic lymphocytic leukaemia patients: influence of neoplastic CD19 cells in vivo and in vitro. Br J Haematol. 2004;125:720–728. doi: 10.1111/j.1365-2141.2004.04971.x. [DOI] [PubMed] [Google Scholar]
- 126.Osugi Y, Vuckovic S, Hart DN. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858–2866. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 127.Palmowski MJ, Choi EM, Hermans IF, Gilbert SC, Chen JL, Gileadi U, Salio M, Van Pel A, Man S, Bonin E, Liljestrom P, Dunbar PR, Cerundolo V. Competition between CTL narrows the immune response induced by prime-boost vaccination protocols. J Immunol. 2002;168:4391–4398. doi: 10.4049/jimmunol.168.9.4391. [DOI] [PubMed] [Google Scholar]
- 128.Pandolfino MC, Viret C, Gervois N, Guilloux Y, Davodeau F, Diez E, Jotereau F. Specificity, T cell receptor diversity and activation requirements of CD4+ and CD8+ clones derived from human melanoma-infiltrating lymphocytes. Eur J Immunol. 1992;22:1795–1802. doi: 10.1002/eji.1830220719. [DOI] [PubMed] [Google Scholar]
- 129.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–839. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 130.Parkhurst MR, Salgaller ML, Southwood S, Robbins PF, Sette A, Rosenberg SA, Kawakami Y. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 131.Parlato S, Santini SM, Lapenta C, Di Pucchio T, Logozzi M, Spada M, Giammarioli AM, Malorni W, Fais S, Belardelli F. Expression of CCR-7, MIP-3beta, and Th-1 chemokines in type I IFN-induced monocyte-derived dendritic cells: importance for the rapid acquisition of potent migratory and functional activities. Blood. 2001;98:3022–3029. doi: 10.1182/blood.V98.10.3022. [DOI] [PubMed] [Google Scholar]
- 132.Pedersen AE, Thorn M, Gad M, Walter MR, Johnsen HE, Gaarsdal E, Nikolajsen K, Buus S, Claesson MH, Svane IM. Phenotypic and functional characterization of clinical grade dendritic cells generated from patients with advanced breast cancer for therapeutic vaccination. Scand J Immunol. 2005;61:147–156. doi: 10.1111/j.0300-9475.2005.01531.x. [DOI] [PubMed] [Google Scholar]
- 133.Ponsaerts P, Van den Bosch G, Cools N, Van Driessche A, Nijs G, Lenjou M, Lardon F, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN, Van Tendeloo VF. Messenger RNA electroporation of human monocytes, followed by rapid in vitro differentiation, leads to highly stimulatory antigen-loaded mature dendritic cells. J Immunol. 2002;169:1669–1675. doi: 10.4049/jimmunol.169.4.1669. [DOI] [PubMed] [Google Scholar]
- 134.Powell DJ, Jr, Rosenberg SA. Phenotypic and functional maturation of tumor antigen-reactive CD8+ T lymphocytes in patients undergoing multiple course peptide vaccination. J Immunother. 2004;27:36–47. doi: 10.1097/00002371-200401000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pullarkat V, Lau R, Lee SM, Bender JG, Weber JS. Large-scale monocyte enrichment coupled with a closed culture system for the generation of human dendritic cells. J Immunol Methods. 2002;267:173–183. doi: 10.1016/S0022-1759(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 136.Radvanyi LG, Banerjee A, Weir M, Messner H. Low levels of interferon-alpha induce CD86 (B7.2) expression and accelerates dendritic cell maturation from human peripheral blood mononuclear cells. Scand J Immunol. 1999;50:499–509. doi: 10.1046/j.1365-3083.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- 137.Ramakrishna V, Treml JF, Vitale L, Connolly JE, O’Neill T, Smith PA, Jones CL, He LZ, Goldstein J, Wallace PK, Keler T, Endres MJ. Mannose receptor targeting of tumor antigen pmel17 to human dendritic cells directs anti-melanoma T cell responses via multiple HLA molecules. J Immunol. 2004;172:2845–2852. doi: 10.4049/jimmunol.172.5.2845. [DOI] [PubMed] [Google Scholar]
- 138.Randolph GJ, Beaulieu S, Lebecque S, Steinman RM, Muller WA. Differentiation of monocytes into dendritic cells in a model of transendothelial trafficking. Science. 1998;282:480–483. doi: 10.1126/science.282.5388.480. [DOI] [PubMed] [Google Scholar]
- 139.Ratta M, Fagnoni F, Curti A, Vescovini R, Sansoni P, Oliviero B, Fogli M, Ferri E, Della Cuna GR, Tura S, Baccarani M, Lemoli RM. Dendritic cells are functionally defective in multiple myeloma: the role of interleukin-6. Blood. 2002;100:230–237. doi: 10.1182/blood.V100.1.230. [DOI] [PubMed] [Google Scholar]
- 140.Reichardt VL, Brossart P, Kanz L. Dendritic cells in vaccination therapies of human malignant disease. Blood Rev. 2004;18:235–243. doi: 10.1016/j.blre.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 141.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 142.Renneson J, Salio M, Mazouz N, Goldman M, Marchant A, Cerundolo V. Mature dendritic cells differentiated in the presence of interferon-beta and interleukin-3 prime functional antigen-specific CD8 T cells. Clin Exp Immunol. 2005;139:468–475. doi: 10.1111/j.1365-2249.2005.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ribas A, Glaspy JA, Lee Y, Dissette VB, Seja E, Vu HT, Tchekmedyian NS, Oseguera D, Comin-Anduix B, Wargo JA, Amarnani SN, McBride WH, Economou JS, Butterfield LH. Role of dendritic cell phenotype, determinant spreading, and negative costimulatory blockade in dendritic cell-based melanoma immunotherapy. J Immunother. 2004;27:354–367. doi: 10.1097/00002371-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 144.Robinson SP, Patterson S, English N, Davies D, Knight SC, Reid CD. Human peripheral blood contains two distinct lineages of dendritic cells. Eur J Immunol. 1999;29:2769–2778. doi: 10.1002/(SICI)1521-4141(199909)29:09<2769::AID-IMMU2769>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 145.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 146.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rosenblatt J, Kufe D, Avigan D. Dendritic cell fusion vaccines for cancer immunotherapy. Expert Opin Biol Ther. 2005;5:703–715. doi: 10.1517/14712598.5.5.703. [DOI] [PubMed] [Google Scholar]
- 148.Rossi M, Young JW. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- 149.Rouard H, Leon A, De Reys S, Taylor L, Logan J, Marquet J, Jouault H, Loper K, Maison P, Delfau-Larue MH, Beaujean F, Farcet JP, Noga SJ. A closed and single-use system for monocyte enrichment: potential for dendritic cell generation for clinical applications. Transfusion. 2003;43:481–487. doi: 10.1046/j.1537-2995.2003.00353.x. [DOI] [PubMed] [Google Scholar]
- 150.Russell NH, McQuaker G, Stainer C, Byrne JL, Haynes AP. Stem cell mobilisation in lymphoproliferative diseases. Bone Marrow Transplant. 1998;22:935–940. doi: 10.1038/sj.bmt.1701477. [DOI] [PubMed] [Google Scholar]
- 151.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 152.Sakakibara M, Kanto T, Inoue M, Kaimori A, Yakushijin T, Miyatake H, Itose I, Miyazaki M, Kuzushita N, Hiramatsu N, Takehara T, Kasahara A, Hayashi N. Quick generation of fully mature dendritic cells from monocytes with OK432, low-dose prostanoid, and interferon-alpha as potent immune enhancers. J Immunother. 2006;29:67–77. doi: 10.1097/01.cji.0000183093.77687.46. [DOI] [PubMed] [Google Scholar]
- 153.Santini SM, Di Pucchio T, Lapenta C, Parlato S, Logozzi M, Belardelli F. A new type I IFN-mediated pathway for the rapid differentiation of monocytes into highly active dendritic cells. Stem Cells. 2003;21:357–362. doi: 10.1634/stemcells.21-3-357. [DOI] [PubMed] [Google Scholar]
- 154.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Savary CA, Grazziutti ML, Melichar B, Przepiorka D, Freedman RS, Cowart RE, Cohen DM, Anaissie EJ, Woodside DG, McIntyre BW, Pierson DL, Pellis NR, Rex JH. Multidimensional flow-cytometric analysis of dendritic cells in peripheral blood of normal donors and cancer patients. Cancer Immunol Immunother. 1998;45:234–240. doi: 10.1007/s002620050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schrama D, Fuchs E, Brocker EB, Thor Straten P, Becker JC. Identical T-cell receptor transcripts in multiple melanoma metastases. Cancer Res. 2002;62:5664–5667. [PubMed] [Google Scholar]
- 158.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15:138–147. doi: 10.1016/S0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 159.Schutt P, Buttkereit U, Brandhorst D, Lindemann M, Schmiedl S, Grosse-Wilde H, Seeber S, Nowrousian MR, Opalka B, Moritz T. In vitro dendritic cell generation and lymphocyte subsets in myeloma patients: influence of thalidomide and high-dose chemotherapy treatment. Cancer Immunol Immunother. 2005;54:506–512. doi: 10.1007/s00262-004-0633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schuurhuis DH, van Montfoort N, Ioan-Facsinay A, Jiawan R, Camps M, Nouta J, Melief CJ, Verbeek JS, Ossendorp F. Immune complex-loaded dendritic cells are superior to soluble immune complexes as antitumor vaccine. J Immunol. 2006;176:4573–4580. doi: 10.4049/jimmunol.176.8.4573. [DOI] [PubMed] [Google Scholar]
- 161.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Siena S, Di Nicola M, Bregni M, Mortarini R, Anichini A, Lombardi L, Ravagnani F, Parmiani G, Gianni AM. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–1471. [PubMed] [Google Scholar]
- 163.Smith CM, Wilson NS, Waithman J, Villadangos JA, Carbone FR, Heath WR, Belz GT. Cognate CD4(+) T cell licensing of dendritic cells in CD8(+) T cell immunity. Nat Immunol. 2004;5:1143–1148. doi: 10.1038/ni1129. [DOI] [PubMed] [Google Scholar]
- 164.Snyder JE, Bowers WJ, Livingstone AM, Lee FE, Federoff HJ, Mosmann TR. Measuring the frequency of mouse and human cytotoxic T cells by the Lysispot assay: independent regulation of cytokine secretion and short-term killing. Nat Med. 2003;9:231–235. doi: 10.1038/nm821. [DOI] [PubMed] [Google Scholar]
- 165.Speiser DE, Lienard D, Pittet MJ, Batard P, Rimoldi D, Guillaume P, Cerottini JC, Romero P. In vivo activation of melanoma-specific CD8(+) T cells by endogenous tumor antigen and peptide vaccines. A comparison to virus-specific T cells. Eur J Immunol. 2002;32:731–741. doi: 10.1002/1521-4141(200203)32:3<731::AID-IMMU731>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 166.Spisek R, Brazova J, Rozkova D, Zapletalova K, Sediva A, Bartunkova J. Maturation of dendritic cells by bacterial immunomodulators. Vaccine. 2004;22:2761–2768. doi: 10.1016/j.vaccine.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 167.Spisek R, Bretaudeau L, Barbieux I, Meflah K, Gregoire M. Standardized generation of fully mature p70 IL-12 secreting monocyte-derived dendritic cells for clinical use. Cancer Immunol Immunother. 2001;50:417–427. doi: 10.1007/s002620100215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 169.Steinman RM. Some interfaces of dendritic cell biology. APMIS. 2003;111:675–697. doi: 10.1034/j.1600-0463.2003.11107802.x. [DOI] [PubMed] [Google Scholar]
- 170.Stuge TB, Holmes SP, Saharan S, Tuettenberg A, Roederer M, Weber JS, Lee PP. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 2004;1:e28. doi: 10.1371/journal.pmed.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 172.Suen Y, Lee SM, Aono F, Hou S, Loudovaris M, Ofstein G, Bender JG. Comparison of monocyte enrichment by immuno-magnetic depletion or adherence for the clinical-scale generation of DC. Cytotherapy. 2001;3:365–375. doi: 10.1080/146532401753277184. [DOI] [PubMed] [Google Scholar]
- 173.Suni MA, Picker LJ, Maino VC. Detection of antigen-specific T cell cytokine expression in whole blood by flow cytometry. J Immunol Methods. 1998;212:89–98. doi: 10.1016/S0022-1759(98)00004-0. [DOI] [PubMed] [Google Scholar]
- 174.Syme R, Bajwa R, Robertson L, Stewart D, Gluck S. Comparison of CD34 and monocyte-derived dendritic cells from mobilized peripheral blood from cancer patients. Stem Cells. 2005;23:74–81. doi: 10.1634/stemcells.2004-0070. [DOI] [PubMed] [Google Scholar]
- 175.Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, Faas SJ, Punt CJ, Torensma R, Adema GJ, Figdor CG. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–1285. doi: 10.1182/blood-2005-01-0318. [DOI] [PubMed] [Google Scholar]
- 176.Tamir A, Jordan WJ, Ritter M, Habib N, Lechler RI, Foster GR, Lombardi G. Interferon-alpha2a is sufficient for promoting dendritic cell immunogenicity. Clin Exp Immunol. 2005;142:471–480. doi: 10.1111/j.1365-2249.2005.02933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Tassignon J, Burny W, Dahmani S, Zhou L, Stordeur P, Byl B, De Groote D. Monitoring of cellular responses after vaccination against tetanus toxoid: comparison of the measurement of IFN-gamma production by ELISA, ELISPOT, flow cytometry and real-time PCR. J Immunol Methods. 2005;305:188–198. doi: 10.1016/j.jim.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 178.Ten Brinke A, Karsten ML, Dieker MC, Zwaginga JJ, Vrielink H, Marieke van Ham S. Generation of dendritic cells for immunotherapy is minimally impaired by granulocytes in the monocyte preparation. Immunobiology. 2006;211:633–640. doi: 10.1016/j.imbio.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 179.Thomas R, Lipsky PE. Human peripheral blood dendritic cell subsets. Isolation and characterization of precursor and mature antigen-presenting cells. J Immunol. 1994;153:4016–4028. [PubMed] [Google Scholar]
- 180.Thurnher M, Zelle-Rieser C, Ramoner R, Bartsch G, Holtl L. The disabled dendritic cell. FASEB J. 2001;15:1054–1061. doi: 10.1096/fj.00-0508hyp. [DOI] [PubMed] [Google Scholar]
- 181.Tkachenko N, Wojas K, Tabarkiewicz J, Rolinski J. Generation of dendritic cells from human peripheral blood monocytes-comparison of different culture media. Folia Histochem Cytobiol. 2005;43:25–30. [PubMed] [Google Scholar]
- 182.Tosi D, Valenti R, Cova A, Sovena G, Huber V, Pilla L, Arienti F, Belardelli F, Parmiani G, Rivoltini L. Role of cross-talk between IFN-alpha-induced monocyte-derived dendritic cells and NK cells in priming CD8+ T cell responses against human tumor antigens. J Immunol. 2004;172:5363–5370. doi: 10.4049/jimmunol.172.9.5363. [DOI] [PubMed] [Google Scholar]
- 183.Toungouz M, Quinet C, Thille E, Fourez S, Pradier O, Delville JP, Velu T, Lambermont M. Generation of immature autologous clinical grade dendritic cells for vaccination of cancer patients. Cytotherapy. 1999;1:447–453. doi: 10.1080/0032472031000141304. [DOI] [PubMed] [Google Scholar]
- 184.Trakatelli M, Toungouz M, Blocklet D, Dodoo Y, Gordower L, Laporte M, Vereecken P, Sales F, Mortier L, Mazouz N, Lambermont M, Goldman S, Coulie P, Goldman M, Velu T. A new dendritic cell vaccine generated with interleukin-3 and interferon-beta induces CD8+ T cell responses against NA17-A2 tumor peptide in melanoma patients. Cancer Immunol Immunother. 2006;55:469–474. doi: 10.1007/s00262-005-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sharav T, Sparbier K, Sterry W, Walden P. Tumour-dendritic hybrid cell vaccination for the treatment of patients with malignant melanoma: immunological effects and clinical results. Vaccine. 2005;23:2367–2373. doi: 10.1016/j.vaccine.2005.01.081. [DOI] [PubMed] [Google Scholar]
- 186.Trefzer U, Herberth G, Wohlan K, Milling A, Thiemann M, Sherev T, Sparbier K, Sterry W, Walden P. Vaccination with hybrids of tumor and dendritic cells induces tumor-specific T-cell and clinical responses in melanoma stage III and IV patients. Int J Cancer. 2004;110:730–740. doi: 10.1002/ijc.20191. [DOI] [PubMed] [Google Scholar]
- 187.Tuettenberg A, Becker C, Huter E, Knop J, Enk AH, Jonuleit H. Induction of strong and persistent MelanA/MART-1-specific immune responses by adjuvant dendritic cell-based vaccination of stage II melanoma patients. Int J Cancer. 2006;118:2617–2627. doi: 10.1002/ijc.21679. [DOI] [PubMed] [Google Scholar]
- 188.Tuyaerts S, Michiels A, Corthals J, Bonehill A, Heirman C, de Greef C, Noppe SM, Thielemans K. Induction of Influenza Matrix Protein 1 and MelanA-specific T lymphocytes in vitro using mRNA-electroporated dendritic cells. Cancer Gene Ther. 2003;10:696–706. doi: 10.1038/sj.cgt.7700622. [DOI] [PubMed] [Google Scholar]
- 189.Tuyaerts S, Noppe SM, Corthals J, Breckpot K, Heirman C, De Greef C, Van Riet I, Thielemans K. Generation of large numbers of dendritic cells in a closed system using Cell Factories. J Immunol Methods. 2002;264:135–151. doi: 10.1016/S0022-1759(02)00099-6. [DOI] [PubMed] [Google Scholar]
- 190.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 191.Van Tendeloo VF, Snoeck HW, Lardon F, Vanham GL, Nijs G, Lenjou M, Hendriks L, Van Broeckhoven C, Moulijn A, Rodrigus I, Verdonk P, Van Bockstaele DR, Berneman ZN. Nonviral transfection of distinct types of human dendritic cells: high-efficiency gene transfer by electroporation into hematopoietic progenitor- but not monocyte-derived dendritic cells. Gene Ther. 1998;5:700–707. doi: 10.1038/sj.gt.3300626. [DOI] [PubMed] [Google Scholar]
- 192.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 193.Vari F, Hart DN. Loading DCs with Ag. Cytotherapy. 2004;6:111–121. doi: 10.1080/14653240410005230. [DOI] [PubMed] [Google Scholar]
- 194.Velders MP, Markiewicz MA, Eiben GL, Kast WM. CD4+ T cell matters in tumor immunity. Int Rev Immunol. 2003;22:113–140. doi: 10.1080/08830180305220. [DOI] [PubMed] [Google Scholar]
- 195.Westermann J, Lessen A, Schlimper C, Baskaynak G, le Coutre P, Dorken B, Pezzutto A. Simultaneous cytokine analysis by cytometric bead array for the detection of leukaemia-reactive T cells in patients with chronic myeloid leukaemia. Br J Haematol. 2006;132:32–35. doi: 10.1111/j.1365-2141.2005.05844.x. [DOI] [PubMed] [Google Scholar]
- 196.Whiteside TL, Zhao Y, Tsukishiro T, Elder EM, Gooding W, Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res. 2003;9:641–649. [PubMed] [Google Scholar]
- 197.Wong EC, Lee SM, Hines K, Lee J, Carter CS, Kopp W, Bender J, Read EJ. Development of a closed-system process for clinical-scale generation of DCs: evaluation of two monocyte-enrichment methods and two culture containers. Cytotherapy. 2002;4:65–76. doi: 10.1080/146532402317251545. [DOI] [PubMed] [Google Scholar]
- 198.Xu S, Koski GK, Faries M, Bedrosian I, Mick R, Maeurer M, Cheever MA, Cohen PA, Czerniecki BJ. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J Immunol. 2003;171:2251–2261. doi: 10.4049/jimmunol.171.5.2251. [DOI] [PubMed] [Google Scholar]
- 199.Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11:4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 200.Yang L, Carbone DP. Tumor-host immune interactions and dendritic cell dysfunction. Adv Cancer Res. 2004;92:13–27. doi: 10.1016/S0065-230X(04)92002-7. [DOI] [PubMed] [Google Scholar]
- 201.Yannelli JR, Sturgill J, Foody T, Hirschowitz E. The large scale generation of dendritic cells for the immunization of patients with non-small cell lung cancer (NSCLC) Lung Cancer. 2005;47:337–350. doi: 10.1016/j.lungcan.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 202.Yannelli JR, Wroblewski JM. On the road to a tumor cell vaccine: 20 years of cellular immunotherapy. Vaccine. 2004;23:97–113. doi: 10.1016/j.vaccine.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 203.Yoshida S, Yamamoto K, Tanaka R. Generation of dendritic cells from the ventricular fluid in patients with meningeal carcinomatosis. J Neuroimmunol. 2003;140:172–176. doi: 10.1016/S0165-5728(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 204.Zeng G, Li Y, El-Gamil M, Sidney J, Sette A, Wang RF, Rosenberg SA, Robbins PF. Generation of NY-ESO-1-specific CD4+ and CD8+ T cells by a single peptide with dual MHC class I and class II specificities: a new strategy for vaccine design. Cancer Res. 2002;62:3630–3635. [PMC free article] [PubMed] [Google Scholar]
- 205.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 206.Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJ. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169:350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]