Abstract
The BCR/ABL p210 fusion protein has long been considered an ideal target antigen for the development of immunotherapeutic strategies in chronic myeloid leukaemia (CML) due to its central role in malignant transformation and to its unique novel amino acid sequence solely expressed in leukaemia cells. However, the feasibility to expand BCR-ABL-specific T cells remains still controversial. Using BCR/ABL peptide/MHC tetramers, significantly higher frequencies of tetramer positive cells were detected in the peripheral blood of HLA-A*0301 (mean 0.38%) and HLA-B*0801 (mean 0.28%) CML patients than in healthy donors (P = 0.0025 and 0.0026, respectively). However, following stimulation with autologous peptide-pulsed DCs, BCR/ABL-specific T cells were only expanded from some healthy donors, suggesting that CML patients may have a specific immune deficit with respect to the BCR/ABL antigen.
Keywords: CML, BCR/ABL, HLA/tetramers, Antigen-specific T cells
Introduction
Chronic myeloid leukaemia (CML) is a clonal stem cell malignancy characterised by an acquired genetic abnormality, the Philadelphia chromosome that results in the formation of the chimeric and constitutively active BCR-ABL tyrosine kinase. The BCR-ABL fusion protein exhibits selective expression in Philadelphia chromosome positive leukaemic cells, expression, which is essential and sufficient for the development of CML. The fusion protein results from the reciprocal translocation t(9;22)(q34;q11), which transcribed into one of the most common chimeric bcr/abl mRNA (b3a2), and translated into BCR/ABL protein (p210BCR-ABL) [12, 28]. In addition, the protein fusion creates a codon split, which produces a new amino acid, lysine, in the b3a2 BCR-ABL protein; it is therefore considered as a truly tumour-specific antigen.
A large number of epitope peptides derived from the BCR/ABL b3a2 breakpoint protein have been demonstrated to bind with high or intermediate affinity to a wide range of both HLA-class I and HLA-class II molecules [3, 15, 18, 22, 29]. Six b3a2-derived peptides were shown to bind to HLA-A*0201, -A*0301, -A*1101 and -B*0801 molecules [3, 8, 34]. Interestingly some of these HLA types, HLA-A*0301 and/or HLA-B*0801, have been associated with a reduced incidence of CML [26]. From these observations, a hypothesis was developed as to whether the potential processing and presentation of BCR/ABL b3a2-derived antigens on HLA-A*0301 and HLA-B*0801 results in the generation of a specific immune response, which in turn protects individuals bearing these HLA types from developing CML.
In addition, BCR/ABL b3a2-derived peptides have been demonstrated to elicit reactive T cells in vitro that recognize peptide-pulsed target cells in an HLA-class I and HLA-class II manner [5, 19–21, 34]. In these studies, the BCR/ABL-specific responses were obtained from a limited number of donors who were mainly healthy individuals. The HLA restriction [21] and the efficiency of these specific T cells to lyse BCR/ABL b3a2 positive tumour cells, however, were not clearly demonstrated in all of these culture systems [19, 34]. These observations have subsequently questioned the natural processing and presentation of BCR/ABL-derived antigens on the tumour cell surface. Indirect evidence of intracellular processing and presentation of these antigens has been provided by the generation of BCR/ABL b3a2-specific CTLs following in vitro stimulation with peptide-unpulsed Ph positive DCs [10, 18, 32, 33]. Our group have demonstrated and confirmed the natural processing and presentation of the previously described HLA-A*0301 b3a2 KQSSKALQR peptide on the cell surface of HLA-A*0301 transfected K562 leukaemic cell line and primary tumour cells derived from HLA-A*0301 CML patients [11]. Here, we report the results of our attempt to generate-specific BCR-ABL cytotoxic T cells from HLA-A*0301 and HLA-B*0801 CML patients, as well as from healthy donors.
Materials and methods
Healthy donors and CML patients recruitment
Peripheral blood samples were obtained from 28 CML patients (Table 1) and 15 healthy volunteers, selected for the expression of HLA-A*0301 and/or HLA-B*0801, with informed consent. PBMCs were separated using Ficoll-Hypaque density gradient centrifugation (Cedarlane® Laboratories Ltd, Canada), and were directly used for ex vivo tetramer staining and T cells expansion.
Table 1.
CML patients’ characteristics
| Patients | HLA typesa | Transcript typeb | Treatmentc | Clinical responsesd | Tetramer frequencye | Expansionf |
|---|---|---|---|---|---|---|
| 1 | A3+ | b3a2 | IM | PR | 0.56 | Negative |
| 2 | A3+ | b3a2 | Auto HSCT | PR | 0.43 | N/P |
| 3 | A3+ | b3a2 | IM | PR | 0.36 | N/P |
| 4 | A3+ | b3a2 | Auto HSCT/IM | PR | 0.41 | Negative |
| 5 | A3+ | b3a2 | Allo HSCT | CR | 0.65 | Positive |
| 6 | A3+ | b3a2 | Allo HSCT | CR | 0.63 | Positive |
| 7 | A3+ | b3a2 | Allo HSCT | CR | 0.69 | Positive |
| 8 | A3+ | b3a2 | IM | PR | 0.51 | Negative |
| 9 | A3+ | b3a2 | Allo HSCT/DLI/IM | CR | 0.7 | Positive |
| 10 | A3+ | b3a2 | Allo HSCT/IM | NR | 0.22 | N/P |
| 11 | A3+ ; B8+ | b3a2 | IM | NR | 0.16/0.15 | N/P |
| 12 | A3+ ; B8+ | b3a2 | IM | PR | 0.37/0.23 | Negative/negative |
| 13 | A3+ ; B8+ | b3a2 | Auto HSCT/IM | PR | 0.16/0.26 | N/P |
| 14 | A3+ ; B8+ | b3a2 | Auto HSCT/IM | PR | 0.42/0.34 | Negative/negative |
| 15 | A3+ ; B8+ | b3a2 | Auto HSCT/IM | CR | 0.65/0.3 | Positive/negative |
| 16 | B8+ | b3a2 | IM | PR | 0.12 | Negative |
| 17 | B8+ | b3a2 | Allo HSCT/DLI | CR | 0.7 | Positive |
| 18 | B8+ | b3a2 | Auto HSCT/IM | PR | 0.62 | Positive |
| 19 | B8+ | b3a2 | Allo HSCT | PR | 0.17 | Positive |
| 20 | B8+ | b3a2 | Allo HSCT | PR | 0.29 | Positive |
| 21 | B8+ | b3a2 | Allo HSCT | NR | 0.11 | Negative |
| 22 | B8+ | b3a2 | IM | PR | 0.38 | Negative |
| 23 | A3+ | b3a2 | At diagnosis | CP | 0.064 | N/P |
| 24 | A3+ | b3a2 | At diagnosis | CP | 0.10 | N/P |
| 25 | A3+ | b3a2 | At diagnosis | CP | 0.21 | Negative |
| 26 | A3+ ; B8+ | b3a2 | At diagnosis | CP | 0.12/0.19 | N/P |
| 27 | A3+ ; B8+ | b3a2 | At diagnosis | CP | 0.23/0.05 | N/P |
| 28 | A3+ ; B8+ | b2a2 | At diagnosis | CP | 0.087/0.05 | N/P |
CML patients were selected for their HLA types, including 28 HLA-A*0301 and/or HLA-B*0801 patients and for their b3a2 bcr/abl transcript types. For control purposes, one b2a2+ CML patient (patients 28) was also included. The HLA-class I types, bcr/abl transcript types, treatments received and clinical responses at the time of blood harvest are detailed for each patient
aThe HLA types were obtained from the hospital centres or from the Histocompatibility Laboratories, Anthony Nolan Research Institute, London
bTranscript type: most of the bcr/abl transcript types were obtained from the hospital centres, the others were screened in house
cPatients’ treatments included: IM imatinib mesylate drug, Auto HSCT or Allo HSCT autologous or allogeneic haematopoietic stem cell transplantation, DLI donor lymphocytes infusion. Some samples were obtained from leucopheresis harvested at diagnosis. Patients that were treated with immunosuppressive drugs to prevent or treat GvHD were excluded from the study
dClinical responses: PR partial clinical response where patients demonstrated a complete cytogenetic response but bcr/abl transcripts were still detected at the molecular level, CR complete clinical response, including cytogenetic and molecular remission, CP chronic phase
eThe numbers of HLA-A*0301 and/or HLA-B*0801 BCR/ABL tetramer positive cells detected ex vivo from CML patients are expressed as percentage of CD3+CD8+ T cells
fThe ability of tetramer positive cells to expand upon specific peptide-pulsed DCs stimulation was assessed from some patients. For this, patient blood samples were drawn after autologous or allogeneic transplantation and during the course of imatinib treatment, when large blood sample were available. Positive expansion was considered when frequency of tetramer positive cells detected after stimulation was at least two times greater than the frequency detected in the control stimulation culture (unpulsed-DCs). Positive expansions observed in five HLA-A*0301 and in four HLA-B*0801 CML patients are represented in Fig. 2 (patients 5–7, 9, 15, 17–20). The HLA-A*0301 patient 15 also expressed the HLA-B*0801, however, no expansion in the context of HLA-B*0801 was obtained. N/P not performed due to the limited number of PBMCs from these patients
Synthetic peptides
BCR/ABL b3a2 17-mer (ATGFKQSSKALQRPVAS), HLA-A*0301-associated 9-mer (KQSSKALQR) and HLA-B*0801-associated 9-mer (GFKQSSKAL) peptides, as well as the HLA-A*0201 associated CMV peptide (NLVPMVATV) and the WT1 peptide (RMFPNAPYL) were synthesized on an ABI synthesiser using Fmoc chemistry and purified by high performance liquid chromatography by Alta Bioscience (The University of Birmingham, UK). The peptides were dissolved in DMSO at 10 mg/ml.
HLA/peptide complex synthesis
The HLA-A*0301/KQSSKALQR and the HLA-B*0801/GFKQSSKAL tetramers were produced as described previously [1]. In brief, the extracellular portion of the HLA coding DNA was amplified by polymerase chain reaction (PCR) and cloned into the pET-3d vector (Novagen), modified to contain a C-terminal peptide tag substrate for Bir A-dependent biotinylation. The HLA heavy chains were expressed in BL21(DE3)pLysS Escherichia coli as insoluble inclusion bodies. The β2-microglobulin was produced in a similar fashion. Both molecules were solubilised in 8 M urea and added with the appropriate peptide in a dilution refolding buffer under denaturing conditions [400 mM arginine (Sigma), 100 mM Tris, 5 mM reduced glutathione (Sigma), 0.5 mM oxidised glutathione (Sigma), 2 mM EDTA (GibcoBRL), pH8]. The refolded molecules were then purified by Fast Protein Liquid Chromatography (FPLC) gel filtration on a Superdex 75 column (Pharmacia, UK). Biotinylation was performed overnight using bacterially expressed Bir A enzyme, with comparable efficiency to commercially available Bir A (Avidity, Denver, CO, USA). Monomeric biotinylated complexes were further purified by gel filtration followed by anion exchange chromatography. Tetrameric molecules were then formed by the addition of phycoerythrin (PE)-labelled streptavidin at a 4:1 molar ratio.
Generation of monocyte-derived dendritic cells
A minimum of 10 × 106 cells per well (in 12-well plate) to up to 30 × 106 cells per well (in 6-well plate) were incubated in complete X-VIVO 10 medium (10% AB serum, 1 U/ml penicillin, 1 μg/ml streptomycin, BioWhittaker) and left for 4–12 h at 37°C. The non-adherent cells were removed by gently resuspending and washing the wells with medium and cryopreserved until required. The adherent cells were incubated at 37°C in 2 ml of complete X-VIVO 10 medium supplemented with 200 ng/ml GMCSF (R&D) and 100 ng/ml IL-4 (R&D). On day 2 and day 4, 2 ml of medium supplemented with 400 ng/ml GMCSF and 200 ng/ml IL-4 was added and exchanged. On day 6, DCs were matured with the addition of TNFα (10 ng/ml, R&D), poly I:C (12.5 μg/ml, Sigma) and sCD40L (1 μg/ml, PeproTech Inc., Peterborough, UK). Antigenic peptides that were required to be up-taken and processed by monocyte-derived dendritic cells (mDCs) (i.e. 17-mers antigenic peptides) were added at 20 μg/ml on the same day of maturation. Short 9-mers peptides were pulsed, also at 20 μg/ml on day 8, prior to their use as APC. Maturation of mDCs was confirmed by cell surface up-regulation of co-stimulatory molecules CD80, CD83 (B7.1) and CD86 (B7.2).
Stimulation of BCR-ABL-specific T cells
Lymphocytes were plated at 2 × 106 cells/ml in 24-well plate and were primed with autologous irradiated monocyte-derived DCs (ratio of T cells:DC of 10:1) on day 1 and day 10. A third stimulation was performed with autologous irradiated PBMCs (ratio of T cells:APC of 5:1) on day 17. The APCs were pulsed with 20 μg/ml antigenic peptide for 3 h at 37°C, washed, irradiated at 30 Gy and resuspended in 0.5 ml complete medium plus 10 ng/ml IL-7. Three days after stimulation, half of the medium was exchanged with fresh medium supplemented with IL-2 (10–50 UI/ml, R&D) and IL-15 (10 ng/ml, R&D).
Flow cytometry/HLA tetramer analysis
PBMCs (1 × 106) were incubated for 30 min at 37°C with 2 μg HLA/peptide tetramer in staining medium (PBS with 10% heated inactivated FCS and 0.1% sodium azide). Cells were washed and incubated for 15 min at 4°C with CD3-FITC and CD8-PerCP (BD Pharmingen, San Diego, USA). Cells were fixed with 1% w/v of paraformaldehyde in PBS and acquired within 24 h on FACSCalibur flow cytometer (BD) using CellQuest™ software version 3.3 (BD); the analysis was performed using the FlowJo software (Tree Star).
Statistical analysis
The statistical analysis of the data was performed using Prism® 4 Software (GraphPad, San Diego, USA). P ≤ 0.05 was considered statistically significant.
Results
BCR/ABL-specific T cells were detected in HLA-A*0301 and HLA-B*0801 CML patients
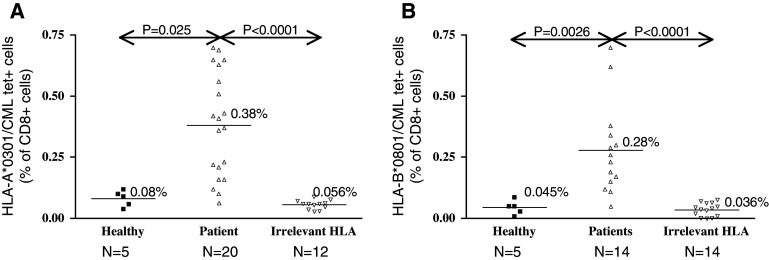
In accordance with prior observations [6, 7, 11, 31], BCR/ABL tetramer positive cells were detected in the peripheral blood of CML patients. The frequencies of HLA-A*0301 and HLA-B*0801 BCR/ABL-specific T cells detected in the peripheral blood of our CML patient cohort, as well as in healthy individuals and control CML patients lacking expression of the HLA-A*0301 or HLA-B*0801 alleles are represented in Fig. 1a and b, respectively. None or very low levels of tetramer positive cells were detected in patients bearing irrelevant HLA types (Fig. 1) or in patients bearing the b2a2 transcript type (not shown).
Fig. 1.
Frequencies of ex vivo HLA-A*0301 and HLA-B*0801 BCR/ABL-specific T cells. The frequencies of HLA-A*0301/KQSSKALQR (a) and HLA-B*0801/GFKQSSKAL (b) specific CD8+ T cells detected in the peripheral blood of CML patients, as well as in healthy individuals and CML patients bearing irrelevant HLA types (non-HLA-A*0301 and/or non-HLA-B*0801) are represented. Tetramer positive cells are expressed as a percentage of CD3+CD8+ T cells. Bars indicate the mean values for each category. The frequencies of BCR/ABL-specific T cells circulating in the peripheral blood of CML patients are in both the HLA-A*0301 and the HLA-B*0801 group significantly higher than in healthy individuals (P = 0.0025 and 0.0026, respectively, Mann–Whitney U test) or in patients bearing irrelevant HLA types (P < 0.0001, Mann–Whitney U test). N represents the number of samples
The levels of BCR/ABL-specific CD8+ T cells detected in CML patients were very heterogeneous. Higher frequencies were found in the context of HLA-A*0301 than in HLA-B*0801 positive CML patients (mean of 0.38 vs. 0.28%, respectively, Fig. 1). Additionally, CML patients bearing both HLA-A*0301 and HLA-B*0801 molecules have circulating BCR/ABL-specific T cells recognizing both HLA/peptide tetramers with reproducibly higher frequencies in the context of HLA-A*0301 molecule than HLA-B*0801 molecule. In contrast to CML patients, the basal frequency of BCR/ABL-specific T cells detected in the peripheral blood of healthy individuals was negligible. The frequency of BCR/ABL-specific T cells detected in all CML patients was significantly higher than in healthy individuals with both HLA-A*0301 tetramers and HLA-B*0801 tetramers (P = 0.0025 and 0.0026, Mann–Whitney U test, Fig. 1). The majority of the CML patients included in this study were demonstrating at least a partial clinical response following auto HSCT, allo HSCT and/or Imatinib mesylate treatment (Table 1). It was not possible to correlate the frequency of BCR/ABL-specific T cells detected in the peripheral blood of patients with their respective treatment and subsequent clinical responses, as the clustering of patients assessed in each category resulted in a too small number. However, higher frequencies of BCR/ABL-specific T cells were detected in patients experiencing at least a complete cytogenetic response following treatment than in patients remaining in active chronic phase of the disease (≤0.21%, Table 1). Thus, these data tend to suggest that the large tumour burden in CML patients may affect the generation of BCR/ABL-specific T cell responses. Recently, the presence of BCR/ABL tetramer positive cells in CML patients was associated with a lower tumour burden, suggesting that BCR/ABL-specific T cells may participate in disease control [7].
BCR/ABL-specific T cells could only be expanded from healthy donors
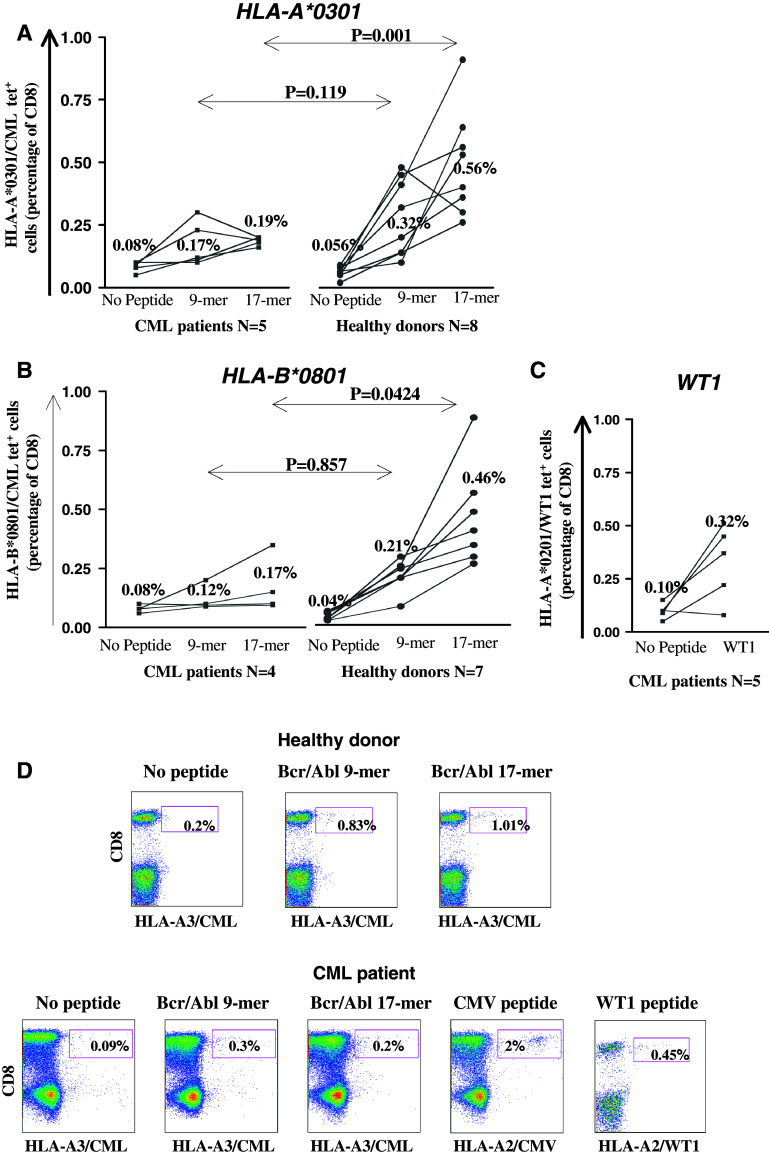
The stimulation of CML patient lymphocytes with the 9-mer and 17-mer BCR/ABL peptide-pulsed mDCs induced a small increase of tetramer positive T cells in the context of both HLA-A*0301 and HLA-B*0801 (from mean of 0.08 to 0.19% and 0.08 to 0.17%, respectively, Fig. 2a, b) and were not found to be statistically significant compared to the frequencies of BCR/ABL-specific T cells detected in cultures without antigen stimulation (No peptide, P > 0.05, Wilcoxon signed ranked test). The majority of the patients assessed here were at least in complete cytogenetic remission (8 out of 9 patients, Table 1). The highest frequencies of tetramer positive T cells were detected in patients receiving an allo HSCT and experiencing complete cytogenetic and molecular responses compared to patients treated with auto HSCT and/or Imatinib mesylate. This was observed both in the context of HLA-A*0301 and HLA-B*0801 (patients 5, 6 and 17, Table 1). These tetramer positive T cells may be donor-derived and therefore more capable of generating an anti-leukaemic response. However, the numbers of BCR/ABL-specific T cells detected from all the CML patient cultures remained low, with or without peptide stimulations (<0.2%). Thus, despite our effort BCR/ABL-specific T cells could not be generated and/or expanded from these CML patients.
Fig. 2.
BCR/ABL-specific CD8+ T cell responses generated with peptide-pulsed autologous mDCs. The generation of BCR/ABL-specific T cells using monocytes-derived DCs as APCs was assessed from HLA-A*0301 (a) and HLA-B*0801 (b) CML patients (left) and healthy donors (right). In parallel, WT1-specific T cells were primed from HLA-A*0201 CML patients (c). Lymphocytes were stimulated with autologous irradiated DCs pulsed with no peptide (No Peptide) or BCR/ABL-derived 9-mer and 17-mer peptides. Activated T cells were harvested 3 days after the last stimulation (day 21) and stained with the appropriate HLA/CML tetramer. BCR/ABL-specific T cells are expressed as a percentage of CD3+CD8+ T cells, with the bar mean values shown for each condition. The P values differences between CML patients and healthy individuals were calculated with the Mann–Whitney U test. d Representative tetramer staining dot plots from an HLA-A*0201/HLA-A*0301 healthy donor and CML patient are shown
In contrast to CML patients, the stimulation of PBMCs derived from healthy donors induced a statistically significant expansion of BCR/ABL-specific T cells. The frequencies of BCR/ABL-specific CD8+ T cells detected in healthy donors after stimulation with the 9-mer BCR/ABL peptides increased significantly compared to the frequencies detected in the cultures without antigen stimulation (No peptide). This was observed both in the context of HLA-A*0301 (from mean of 0.056 to 0.32%, P = 0.0078 Wilcoxon signed ranked test, Fig. 2a) and HLA-B*0801 (from mean of 0.04 to 0.21%, P = 0.0156, Fig. 2b). The frequencies of BCR/ABL-specific T cells generated after stimulation with the 17-mer BCR/ABL peptide were also found to be statistically significant compared to the negative control (mean of 0.56%, P = 0.0039 for HLA-A*0301 and mean of 0.46%, P = 0.0125 for HLA-B*0801, Wilcoxon signed ranked test). The degree of the BCR/ABL-specific CD8+ T cell responses generated was affected by the length of the BCR/ABL peptides used as a source of antigen to stimulate these responses. The frequencies of tetramer positive T cells detected in healthy donors after stimulation with the 17-mer peptide were significantly higher when compared to the 9-mer peptide (P = 0.0391 for HLA-A*0301 and P = 0.0156 for HLA-B*0801, Wilcoxon signed ranked test). This longer 17-mer BCR/ABL peptide may contain other HLA-class I and/or HLA-class II epitopes that may enhance the activation of HLA-A*0301 and HLA-B*0801 associated BCR/ABL-specific T cells.
Although the frequencies of BCR/ABL tetramer positive cells detected ex vivo were significantly higher in CML patients, higher frequencies of antigen-specific T cells were generated from both HLA-A*0301 and HLA-B*0801 healthy donors than from patients (mean of 0.21–0.56 vs. 0.12–0.19%, respectively, Fig. 2). The numbers of tetramer positive T cells detected following stimulation with the BCR/ABL-derived 17-mer peptide were significantly higher from healthy donors than from CML patients in the context of both HLA-A*0301 and of HLA-B*0801 molecules (P = 0.001 and 0.0424, respectively, Mann–Whitney U test). As no HLA-A*0301 and HLA-B*0801 defined antigens and tetramer tools were available at the time of the study to control each patient expansion, the well-defined viral CMV and tumour associated antigen WT1-restricted peptides to HLA-A*0201 were used as positive controls whenever possible (when patients also expressed the HLA-A*0201 allele). HLA-A*0201 positive CML patients were capable of generating CMV-specific T cell responses following the same protocol as shown for a patient in Fig. 2d. In addition it was possible to expand, from some patients, WT1-specific T cells (Fig. 2c, d). Therefore, the lack of BCR/ABL-specific responses is unlikely to be due to the inefficiency of CML patient DCs to prime an immune response, and CML patients are fully capable of generating an antigen-specific T cell response, at least in the context of a viral antigen. Thus, our data suggest that CML patients may have a specific immune deficit with respect to the BCR/ABL antigen.
Furthermore, the BCR/ABL peptide presented by HLA-A*0301 was demonstrated to induce a greater expansion of antigen-specific T cells than the HLA-B*0801 associated peptide. The frequencies of BCR/ABL-specific T cells detected after stimulation with the BCR/ABL 9-mer peptide in both HLA-A*0301 CML patients and healthy donors were higher than those detected in HLA-B*0801 donors (mean of 0.17 and 0.32% vs. 0.12 and 0.21%, respectively, Fig. 2a, b, 9-mer). The expansion of these BCR/ABL-specific T cells after peptide stimulation was also statistically more significant in the context of the HLA-A*0301 molecule than of the HLA-B*0801 molecule compared to the control (No peptide stimulation). This was observed only for healthy donors (P = 0.0078 vs. 0.0156, Wilcoxon signed ranked test). The expansion of these cells was not significantly different to the controls for CML patients, though there were a trend towards a greater expansion in the context of HLA-A*0301 than HLA-B*0801 molecule (P = 0.1250 vs. 0.250). It has recently been described that the proteosomal digestion of BCR/ABL fusion peptide could lead to the generation of the HLA-A*0301 associated KQSSKALQR peptide but not the HLA-B*0801 associated GFKQSSKAL [17, 25].
Discussion
The BCR/ABL p210 fusion protein has long been considered an ideal target antigen for the development of immunotherapeutic strategies in CML due to its central role in malignant transformation and to its unique novel amino acid sequence solely expressed in leukaemia cells. In fact, the BCR/ABL breakpoint protein represents a rare truly tumour-specific antigen. The data presented here illustrate the problems associated with generating BCR/ABL-specific CD8+ T cell responses in vitro for the development of immunotherapeutic treatment of CML patients.
The frequencies of BCR/ABL-specific T cells detected in the peripheral blood of CML patients were significantly higher than those detected in healthy donors. In our cohort of CML patients, higher frequencies of tetramer positive T cells were detected in patients who were at least in partial clinical remission compared to those remaining in active phase of the disease. In the latter patients, the large tumour burden may have affected their ability to generate antigen-specific T cell responses in vivo. The standard protocol for the generation of immune responses utilises DCs, as these cells are capable of both priming naïve T cells and boosting memory T cell responses. In this study, DCs were not sufficiently efficient to stimulate and expand BCR/ABL-specific T cells from CML patients. However, CMV and WT1-specific T cell responses were successfully generated in vitro from these patients following identical stimulation protocols. It is possible that the large tumour burden and the defective leukaemia DC functions described in CML patients [13] may have induced BCR/ABL-specific T cell exhaustion, tolerance and/or anergy in vivo, thus rendering their expansion highly difficult in vitro. Additionally, given the observation that HLA-A*0301 and HLA-B*0801 alleles appear to be protective against developing CML [26], it may be considered therefore that individuals who bear these HLA types yet still develop CML and have high tumour burden, may belong to one of the worst groups from whom to stimulate BCR/ABL-specific T cell responses in vitro. No higher frequencies of CD4+CD25+ T cells were detected in the peripheral blood of CML patients compared to healthy donors (data not shown), suggesting that Tregs were not involved. To understand the mechanism leading to the observed BCR/ABL-specific T cells deficiency in CML patients, it would be important to better characterize these BCR/ABL-specific T cells in terms of their central memory or terminally differentiated phenotype, their possible T cell exhaustion phenotype and their capacity (or not) to produce IFNγ in response to BCR/ABL peptide stimulation. However, due to the restricted blood supplies, to the low frequencies of BCR/ABL-specific T cells (as compared to viral antigen), and because we wanted to generate DCs to expand those BCR/ABL-specific T cells, we were unfortunately unable to assess those characteristics.
In contrast to CML patients, the stimulation of PBMCs derived from healthy donors induced a statistically significant expansion of BCR/ABL-specific T cells. However, the levels of these responses remained low. The use of the longer 17-mer BCR/ABL peptide to stimulate antigen-specific T cell responses was demonstrated to induce a higher frequency of tetramer positive T cell expansion when compared to the use of the 9-mer BCR/ABL peptides. Thus, this longer peptide may contain other HLA-class I and/or HLA-class II epitope, which enhanced the stimulation and expansion of HLA-A*0301 and HLA-B*0801 restricted BCR/ABL-specific CD8+ T cell responses. Moreover this longer peptide has been demonstrated to efficiently generate HLA-class II restricted BCR/ABL-specific T cell responses in healthy donors [2, 5, 21, 33].
The data presented here also showed that higher expansions of BCR/ABL-specific T cells were achieved in the context of HLA-A*0301 in contrast to HLA-B*0801 molecule. The proteosomal digestion of BCR/ABL fusion peptide so far, has been demonstrated for the generation of the HLA-A*0301 associated KQSSKALQR peptide but not for the HLA-B*0801 associated GFKQSSKAL [17, 25]. Thus, it is not yet known whether the GFKQSSKAL BCR/ABL-derived peptide is physiologically presented in the context of HLA-B*0801.
In conclusion, though our stimulatory condition were able to generate responses to viral antigens, it was not possible to generate BCR/ABL-specific T cells from CML patients. BCR/ABL-specific T cells could neither be detected nor induced from a CML patient responding to donor lymphocytes infusion [25]. Additionally, using DCs electroporated with RNA harbouring the chimeric bcr-abl transcript, Brossart [16] could elicit CTLs capable of recognizing certain CML-derived antigens but not the BCR/ABL fusion peptides. Vaccinations with BCR/ABL-derived peptides induced mainly CD4+ T cell responses in patients [4, 9, 24, 27]. Thus, it appears that the HLA-class I restricted BCR/ABL fusion peptides are not immunodominant antigens. Recently though, the modification of an amino acid at the HLA-binding anchor residues in BCR/ABL peptide sequences showed that CTL could be elicited against the altered peptide ligands and that they produce greater cytotoxicity without loss of original sequence specificity [23]. However, these CTLs failed to specifically lyse fresh CML cells. Peptides derived from the whole BCR/ABL protein, including other bcr/abl transcript types appear to induce stronger immune responses [8, 14, 17, 30] and may therefore represent more appropriate CML-associated antigens for immunotherapeutic approaches.
Acknowledgments
This work was supported by the Kay Kendall Leukaemia Fund and the European Union study No: 503319 “Allostem”.
Abbreviations
- APC
Antigen presenting cells
- CML
Chronic myelogenous leukaemia
- CMV
Cytomegalovirus
- DC
Dendritic cells
- HLA
Human leukocyte antigen
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cells
Footnotes
Professor A. I. Dodi passed away recently.
This paper is an original contribution from the meeting which took place 28–29 May 2008 in Nottingham, UK, celebrating the contribution of Prof. A. I. “Tony” Dodi (29 January 2008) to the EU project “Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumour immunology (ENACT)”.
References
- 1.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274(5284):94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 2.Bertazzoli C, Marchesi E, Passoni L, Barni R, Ravagnani F, Lombardo C, Corneo GM, Pioltelli P, Pogliani E, Gambacorti-Passerini C. Differential recognition of a BCR/ABL peptide by lymphocytes from normal donors and chronic myeloid leukemia patients. Clin Cancer Res. 2000;6(5):1931–1935. [PubMed] [Google Scholar]
- 3.Bocchia M, Wentworth PA, Southwood S, Sidney J, McGraw K, Scheinberg DA, Sette A. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood. 1995;85(10):2680–2684. [PubMed] [Google Scholar]
- 4.Bocchia M, Gentili S, Abruzzese E, Fanelli A, Iuliano F, Tabilio A, Amabile M, Forconi F, Gozzetti A, Raspadori D, Amadori S, Lauria F. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet. 2005;365(9460):657–662. doi: 10.1016/S0140-6736(05)17945-8. [DOI] [PubMed] [Google Scholar]
- 5.Bosch GJ, Joosten AM, Kessler JH, Melief CJ, Leeksma OC. Recognition of BCR-ABL positive leukemic blasts by human CD4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood. 1996;88(9):3522–3527. [PubMed] [Google Scholar]
- 6.Butt NM, Wang L, Abu-Eisha HM, Christmas SE, Clark RE. BCR-ABL-specific T cells can be detected in healthy donors and in chronic myeloid leukemia patients following allogeneic stem cell transplantation. Blood. 2004;103(8):3245. doi: 10.1182/blood-2003-11-4086. [DOI] [PubMed] [Google Scholar]
- 7.Butt NM, Rojas JM, Wang L, Christmas SE, Abu-Eisha HM, Clark RE. Circulating bcr-abl-specific CD8+ T cells in chronic myeloid leukemia patients and healthy subjects. Haematologica. 2005;90(10):1315–1323. [PubMed] [Google Scholar]
- 8.Buzyn A, Ostankovitch M, Zerbib A, Kemula M, Connan F, Varet B, Guillet JG, Choppin J. Peptides derived from the whole sequence of BCR-ABL bind to several class I molecules allowing specific induction of human cytotoxic T lymphocytes. Eur J Immunol. 1997;27(8):2066–2072. doi: 10.1002/eji.1830270834. [DOI] [PubMed] [Google Scholar]
- 9.Cathcart K, Pinilla-Ibarz J, Korontsvit T, Schwartz J, Zakhaleva V, Papadopoulos EB, Scheinberg DA. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103(3):1037–1042. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 10.Choudhury A, Gajewski JL, Liang JC, Popat U, Claxton DF, Kliche KO, Andreeff M, Champlin RE. Use of leukemic dendritic cells for the generation of antileukemic cellular cytotoxicity against Philadelphia chromosome-positive chronic myelogenous leukemia. Blood. 1997;89(4):1133–1142. [PubMed] [Google Scholar]
- 11.Clark RE, Dodi IA, Hill SC, Lill JR, Aubert G, Macintyre AR, Rojas J, Bourdon A, Bonner PL, Wang L, Christmas SE, Travers PJ, Creaser CS, Rees RC, Madrigal JA. Direct evidence that leukemic cells present HLA-associated immunogenic peptides derived from the BCR-ABL b3a2 fusion protein. Blood. 2001;98(10):2887–2893. doi: 10.1182/blood.V98.10.2887. [DOI] [PubMed] [Google Scholar]
- 12.Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96(10):3343–3356. [PubMed] [Google Scholar]
- 13.Dong R, Cwynarski K, Entwistle A, Marelli-Berg F, Dazzi F, Simpson E, Goldman JM, Melo JV, Lechler RI, Bellantuono I, Ridley A, Lombardi G. Dendritic cells from CML patients have altered actin organization, reduced antigen processing, and impaired migration. Blood. 2003;101(9):3560–3567. doi: 10.1182/blood-2002-06-1841. [DOI] [PubMed] [Google Scholar]
- 14.Gannage M, Abel M, Michallet AS, Delluc S, Lambert M, Giraudier S, Kratzer R, Niedermann G, Saveanu L, Guilhot F, Camoin L, Varet B, Buzyn A, Caillat-Zucman S. Ex vivo characterization of multiepitopic tumor-specific CD8 T cells in patients with chronic myeloid leukemia: implications for vaccine development and adoptive cellular immunotherapy. J Immunol. 2005;174(12):8210–8218. doi: 10.4049/jimmunol.174.12.8210. [DOI] [PubMed] [Google Scholar]
- 15.Greco G, Fruci D, Accapezzato D, Barnaba V, Nisini R, Alimena G, Montefusco E, Vigneti E, Butler R, Tanigaki N, Tosi R. Two brc-abl junction peptides bind HLA-A3 molecules and allow specific induction of human cytotoxic T lymphocytes. Leukemia. 1996;10(4):693–699. [PubMed] [Google Scholar]
- 16.Grunebach F, Mirakaj V, Muller MR, Brummendorf T, Brossart P. BCR-ABL is not an immunodominant antigen in chronic myelogenous leukemia. Cancer Res. 2006;66(11):5892–5900. doi: 10.1158/0008-5472.CAN-05-2868. [DOI] [PubMed] [Google Scholar]
- 17.Kessler JH, Bres-Vloemans SA, van Veelen PA, de Ru A, Huijbers IJ, Camps M, Mulder A, Offringa R, Drijfhout JW, Leeksma OC, Ossendorp F, Melief CJ. BCR-ABL fusion regions as a source of multiple leukemia-specific CD8+ T-cell epitopes. Leukemia. 2006;20(10):1738–1750. doi: 10.1038/sj.leu.2404354. [DOI] [PubMed] [Google Scholar]
- 18.Mannering SI, McKenzie JL, Fearnley DB, Hart DN. HLA-DR1-restricted bcr-abl (b3a2)-specific CD4+ T lymphocytes respond to dendritic cells pulsed with b3a2 peptide and antigen-presenting cells exposed to b3a2 containing cell lysates. Blood. 1997;90(1):290–297. [PubMed] [Google Scholar]
- 19.Nieda M, Nicol A, Kikuchi A, Kashiwase K, Taylor K, Suzuki K, Tadokoro K, Juji T. Dendritic cells stimulate the expansion of bcr-abl specific CD8+ T cells with cytotoxic activity against leukemic cells from patients with chronic myeloid leukemia. Blood. 1998;91(3):977–983. [PubMed] [Google Scholar]
- 20.Norbury LC, Clark RE, Christmas SE. b3a2 BCR-ABL fusion peptides as targets for cytotoxic T cells in chronic myeloid leukaemia. Br J Haematol. 2000;109(3):616–621. doi: 10.1046/j.1365-2141.2000.02090.x. [DOI] [PubMed] [Google Scholar]
- 21.Osman Y, Takahashi M, Zheng Z, Koike T, Toba K, Liu A, Furukawa T, Aoki S, Aizawa Y. Generation of bcr-abl specific cytotoxic T-lymphocytes by using dendritic cells pulsed with bcr-abl (b3a2) peptide: its applicability for donor leukocyte transfusions in marrow grafted CML patients. Leukemia. 1999;13(2):166–174. doi: 10.1038/sj.leu.2401311. [DOI] [PubMed] [Google Scholar]
- 22.Pawelec G, Max H, Halder T, Bruserud O, Merl A, da Silva P, Kalbacher H. BCR/ABL leukemia oncogene fusion peptides selectively bind to certain HLA-DR alleles and can be recognized by T cells found at low frequency in the repertoire of normal donors. Blood. 1996;88(6):2118–2124. [PubMed] [Google Scholar]
- 23.Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Roberts W, Scheinberg DA. Synthetic peptide analogs derived from bcr/abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematologica. 2005;90(10):1324–1332. [PubMed] [Google Scholar]
- 24.Pinilla-Ibarz J, Cathcart K, Korontsvit T, Soignet S, Bocchia M, Caggiano J, Lai L, Jimenez J, Kolitz J, Scheinberg DA. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95(5):1781–1787. [PubMed] [Google Scholar]
- 25.Posthuma EF, van Bergen CA, Kester MG, de Paus RA, van Veelen PA, de Ru AH, Drijfhout JW, Lurvink EG, Willemze R, Falkenburg JH. Proteosomal degradation of BCR/ABL protein can generate an HLA-A*0301-restricted peptide, but high-avidity T cells recognizing this leukemia-specific antigen were not demonstrated. Haematologica. 2004;89(9):1062–1071. [PubMed] [Google Scholar]
- 26.Posthuma EF, Falkenburg JH, Apperley JF, Gratwohl A, Roosnek E, Hertenstein B, Schipper RF, Schreuder GM, D’Amaro J, Oudshoorn M, van Biezen JH, Hermans J, Willemze R, Niederwieser D. HLA-B8 and HLA-A3 coexpressed with HLA-B8 are associated with a reduced risk of the development of chronic myeloid leukemia. The Chronic Leukemia Working Party of the EBMT. Blood. 1999;93(11):3863–3865. [PubMed] [Google Scholar]
- 27.Rojas JM, Knight K, Wang L, Clark RE. Clinical evaluation of BCR-ABL peptide immunisation in chronic myeloid leukaemia: results of the EPIC study. Leukemia. 2007;21(11):2287–2295. doi: 10.1038/sj.leu.2404858. [DOI] [PubMed] [Google Scholar]
- 28.Rowley JD. Letter: a new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 29.ten Bosch GJ, Toornvliet AC, Friede T, Melief CJ, Leeksma OC. Recognition of peptides corresponding to the joining region of p210BCR-ABL protein by human T cells. Leukemia. 1995;9(8):1344–1348. [PubMed] [Google Scholar]
- 30.Volpe G, Cignetti A, Panuzzo C, Kuka M, Vitaggio K, Brancaccio M, Perrone G, Rinaldi M, Prato G, Fava M, Geuna M, Pautasso M, Casnici C, Signori E, Tonon G, Tarone G, Marelli O, Fazio VM, Saglio G. Alternative BCR/ABL splice variants in Philadelphia chromosome-positive leukemias result in novel tumor-specific fusion proteins that may represent potential targets for immunotherapy approaches. Cancer Res. 2007;67(11):5300–5307. doi: 10.1158/0008-5472.CAN-06-3737. [DOI] [PubMed] [Google Scholar]
- 31.Westermann J, Schlimper C, Richter G, Mohm J, Dorken B, Pezzutto A. T cell recognition of bcr/abl in healthy donors and in patients with chronic myeloid leukaemia. Br J Haematol. 2004;125(2):213–216. doi: 10.1111/j.1365-2141.2004.04873.x. [DOI] [PubMed] [Google Scholar]
- 32.Yasukawa M, Ohminami H, Kojima K, Hato T, Hasegawa A, Takahashi T, Hirai H, Fujita S. HLA class II-restricted antigen presentation of endogenous bcr-abl fusion protein by chronic myelogenous leukemia-derived dendritic cells to CD4(+) T lymphocytes. Blood. 2001;98(5):1498–1505. doi: 10.1182/blood.V98.5.1498. [DOI] [PubMed] [Google Scholar]
- 33.Yasukawa M, Ohminami H, Kaneko S, Yakushijin Y, Nishimura Y, Inokuchi K, Miyakuni T, Nakao S, Kishi K, Kubonishi I, Dan K, Fujita S. CD4(+) cytotoxic T-cell clones specific for bcr-abl b3a2 fusion peptide augment colony formation by chronic myelogenous leukemia cells in a b3a2-specific and HLA-DR-restricted manner. Blood. 1998;92(9):3355–3361. [PubMed] [Google Scholar]
- 34.Yotnda P, Firat H, Garcia-Pons F, Garcia Z, Gourru G, Vernant JP, Lemonnier FA, Leblond V, Langlade-Demoyen P. Cytotoxic T cell response against the chimeric p210 BCR-ABL protein in patients with chronic myelogenous leukemia. J Clin Invest. 1998;101(10):2290–2296. doi: 10.1172/JCI488. [DOI] [PMC free article] [PubMed] [Google Scholar]