Abstract
Topoisomerase II alpha (Top2α) is an attractive candidate to be used as a tumor antigen for cancer immunotherapy, because it is abundantly expressed in various tumors and serves as a target for a number of chemotherapeutic agents. In this study, we demonstrated the immunogenicity of Top2α, using dendritic cells (DC) electroporated with RNA encoding the Top2α C-terminus (Top2αCRNA/DC). Top2αCRNA/DC were able to demonstrate in vitro stimulation of T cells from mice that were previously vaccinated with Top2α-expressing tumor lysate-pulsed DC. Vaccination with Top2αCRNA/DC induced Top2α-specific T cell responses in vivo as well as antitumor effects in various murine tumor models including MC-38, B16F10, and GL26. DC pulsed with p1327 (DSDEDFSGL), defined as an epitope presented by H-2Kb, also induced Top2α-specific immune responses and antitumor effects. Based on these data, Top2α is suggested to be a universal target for cancer immunotherapy.
Keywords: Topoisomerase II alpha, RNA electroporation, Dendritic cell, Vaccine, Tumor antigen
Introduction
For successful cancer immunotherapy, it is essential to identify tumor antigens that can be recognized by cytotoxic T lymphocytes (CTL). Over the past two decades, many tumor antigens have been identified using various strategies and methods, such as T cell epitope cloning, serological identification of antigens by recombinant expression cloning (SEREX), DNA microarray analysis, and bioinformatics [13, 23, 26]. However, the number of tumor antigens that are available for immunotherapy is still limited.
Topoisomerase II alpha (Top2α) is an important DNA-binding enzyme that makes transient breaks in one segment of double-strand DNA and places an intact duplex into the broken DNA before resealing the break, thus altering DNA topology [27]. Top2α is preferentially expressed in proliferating cells [2, 33] and is over-expressed in a wide range of tumors [3, 28, 32, 35]. The copy number of the Top2α gene is also amplified in various tumors [16, 19]. Recently, several studies showed that Top2α could be used as a prognostic biomarker in tumors including prostate cancer and hepatocellular carcinoma [17, 34]. Top2α is a target of chemotherapeutic agents, such as etoposide and doxorubicin [11]. It has been reported that Top2α was recognized by serum antibodies in ovarian cancer patients, using the SEREX method [29].
It has been demonstrated that dendritic cells (DC) transfected with RNA encoding human telomerase reverse transcriptase (hTERT) are effective in inducing CTL responses and antitumor effects in human and murine systems [22]. Primary CTL responses can also be stimulated in vitro by human monocyte-derived DC transfected with RNA corresponding to carcinoembryonic antigen (CEA) or human papillomavirus E6 protein [7, 21]. Furthermore, DC transfected with RNA by electroporation have more effectively induced both MHC class I- and class II-mediated immune responses [20, 30]. These reports suggest that the use of DC electroporated with RNA is an efficient strategy for the identification of novel tumor antigens.
Topoisomerase II has two isoforms, Top2α and Top2β, which exhibit a similar structure and function. Top2α is essential for cell proliferation, whereas Top2β is constitutively present in most cells [1, 14, 18]. Among the three domains of these molecules, the N-terminal and central region have 81% identity between the two isoforms, whereas the C-terminal region shows low homology, bearing only 32% identical amino acid residues. Therefore, the C-terminal region could be a more specific target for Top2α. In the present study, we demonstrated Top2α-specific immune responses and antitumor effects in murine tumor models by vaccination with Top2α C-terminal RNA-electroporated DC (Top2αCRNA/DC).
Materials and methods
Animals and tumor cell culture
Female C57BL/6 mice (H-2b, 6–8 weeks old) were purchased from Orient Bio (Kapung, Gyeonggido, Korea). The mice were maintained and treated according to the guidelines of the Local Council for Animal Care.
GL26 (H-2b; glioma), MC-38 and MC-38-cea2 (H-2b; colon cancer), B16F10 (H-2b; melanoma) and CT26 (H-2d; colon cancer) were cultured in complete Dulbecco’s Modified Eagle Medium (DMEM; Cambrex, Walkerville, MD, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, Grand Island, NY, USA), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. EL4 (H-2b; lymphoma) and YAC-1 (H-2a; lymphoma) were cultured in complete RPMI-1640 (Cambrex) supplemented with 10% heat-inactivated fetal bovine serum, 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. GL26 was kindly provided by Dr. John S. Yu (Cedars Sinai Medical Center, Los Angeles, CA, USA). MC-38-cea2 expressing human CEA was kindly provided by Dr. J. Schlom (Division of Tumor Immunology and Biology, NIH, Bethesda, MD, USA). MC-38, EL4, B16F10, CT26, and YAC-1 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA).
Cloning of the Top2α C-terminal region
The C-terminal region of Top2α was used in this study and consisted of amino acid residues 1263–1528 (Top2αC). Reverse-transcribed mRNA for mouse Top2αC isolated from GL26 served as a template for polymerase chain reaction (PCR) amplification. PCR conditions were as follows: 2 min at 94°C; followed by 30 cycles of 15 s at 94°C, 30 s at 60°C, and 1 min at 72°C; ending with 10 min at 72°C. The respective primers for Top2αC were 5′-CTGAGGATGGTGCAGAAGAAAGAGCCGGT-3′ (forward primer) and 5′-TGCCTCAGAAGAGGTCGTCATCGTCATCGAA-3′ (reverse primer). The PCR product was inserted into the pcDNA3.1 TOPO vector (Invitrogen, Grand island, NY, USA). The cloned gene was confirmed by nucleotide sequencing.
Western blotting
Mouse tissue extracts were prepared by first mincing the tissue with a scalpel to yield small fragments. Tissue fragments were then suspended in RIPA buffer containing 1 mM DTT, 1 mM phenylmethanesulfonyl fluoride, 10 μg/ml aporotinin, and 5 μg/ml leupeptin. The suspension was then homogenized using the Precellys24 lyser (Bertin Technologies, Cedex, France). Tumor cells were lysed in the same buffer as above. Protein concentration was determined using a bicinchoninic acid assay (Pierce, Rockford, IL, USA). Total protein (50 μg) was separated on an 8% polyacrylamide gel, blotted onto nitrocellulose membranes (Schleicher & Schuell, Dassel, Germany), and incubated with an anti-Top2α antibody (Epitomics, Burlingame, CA, USA). Bands were visualized by enhanced chemiluminescence (ECL) staining (Amersham Pharmacia, Freiburg, Germany).
Generation of bone marrow-derived DC
Generation of DC from mouse bone marrow was performed as described previously [12]. Briefly, the bone marrow was flushed from the tibias and femurs of C57BL/6 mice, and the red cells were lysed with hypotonic buffer (9.84 g/l NH4Cl, 1 g/l KHCO3, and 0.1 mM EDTA). The cells were washed twice in serum-free RPMI-1640 medium and cultured to 5 × 106 cells/well in complete RPMI-1640 containing 20 ng/ml murine granulocyte–macrophage colony-stimulating factor (GM-CSF, R&D Systems, Minneapolis, MN, USA) and 20 ng/ml recombinant murine interleukin-4 (IL-4, R&D Systems). After 48 h, non-adherent cells were gently removed, and complete medium containing GM-CSF and IL-4 was replenished. On day 6 of culture, non-adherent and loosely adherent cells were collected as the DC and used for RNA electroporation.
Electroporation of RNA into DC
The Top2αC/pcDNA3.1 TOPO plasmid was linearized with Sma I and in vitro transcription (IVT) was performed with T7 RNA polymerase according to the manufacturer’s instructions (Ambion mMESSAGE mMACHINE T7 Ultra kit, Austin, TX, USA). The production of IVT RNA was confirmed by agarose gel electrophoresis, and the RNA concentration was measured spectrophotometrically. RNA samples were stored in small aliquots at −70°C.
After 6 days in culture, DC were resuspended in Opti-MEM medium at a concentration of 2.5 × 107 cells/ml. The cell suspension (200 μl) was placed in a 2-mm cuvette, and 20 μg of RNA was added. The cell suspension was then placed in the electroporator (ElectroSquarePorator, ECM 830, BTX, San Diego, CA, USA), and a 300 V current was delivered for 500 μs. The cells were immediately removed from the cuvette and placed in complete medium containing GM-CSF, IL-4, and LPS (1 μg/ml: Sigma, Saint Louis, MO, USA) to allow the DC to fully mature for 24 h. The electroporation transfection efficiency was determined with GFP RNA by FACS analysis.
Production of tumor lysate
In brief, MC-38, MC-38-cea2, or B16F10 cells were resuspended at a density of 1 × 107 cells/ml in PBS. The cell suspension was frozen in liquid nitrogen, and then thawed in a 37°C water bath. The freeze/thaw cycle was repeated four times in rapid succession. The larger particles were removed by centrifugation at 600 rpm for 10 min. The supernatant was passed through a 0.2 μm filter and an aliquot was stored at −70°C. The protein concentration of the lysate was determined using a bicinchoninic acid assay (Pierce). DC were incubated with the MC-38 tumor lysate (100 μg/ml) for 16–18 h to pulse the MC-38 tumor lysate.
Isolation of CD4+ and CD8+ T cells
To determine the immune responses of CD4+ and CD8+ T cells, splenocytes were incubated with magnetic beads conjugated to CD4+ or CD8+ specific monoclonal antibodies (magnetic antibody cell sorter (MACS); Miltenyi Biotec, Bergisch Gladbach, Germany) for 15 min at 4°C. Following incubation, the cells were washed with PBS containing 2 mM EDTA and processed through a MACS magnetic separation column. The purity of each T cell population after sorting was >90%, as determined by FACS analysis.
Enzyme-linked immunospot assay
An enzyme-linked immunospot (ELISPOT) kit purchased from BD Bioscience (Qume Drive, San Jose, CA, USA), was used according to the manufacturer’s instructions. Briefly, splenocytes (5 × 104 cells/well) were seeded into a 96-well plate coated with the anti-mouse IFN-γ antibody. Top2αCRNA/DC, MC-38TL/DC, SuvRNA/DC, and DC were added as target cells. The plates were incubated for 20 h at 37°C. The cells were then removed, and the plate was washed three times with PBS containing 0.05% Tween-20 PBS. Next, biotinylated anti-mouse IFN-γ antibody was added to the wells, and the plates were then incubated for 2 h at room temperature. After washing three times, streptavidin-horseradish peroxidase was added to each well, followed by incubation for 1 h at room temperature. After washing, a chromogenic substrate [3-amino-9-ethyl carbazole (AEC)] was added to each well, the reaction was quenched with distilled water, and the plates were inverted and allowed to dry overnight in the dark. The number of spots corresponding to the IFN-γ-secreting cells was determined using an automatic AID-ELISPOT-Reader (Strassberg, Germany).
Cytotoxicity assay
The standard 51Cr-release assay was performed as described previously [24]. Splenocytes were harvested from each mouse. The harvested cells were restimulated in vitro with 4% paraformaldehyde-fixed MC-38 cells for 5 days and used as effectors. MC-38, GL26, Top2αCRNA/DC, DC, YAC-1, and CT26 labeled with 100 mCi [51Cr]-sodium chromate/1 × 106 cells for 1 h at 37°C in 5% CO2 were used as target cells. 51Cr-labeled target cells were incubated with effectors for 4 h at 37°C. Supernatant from each well (100 μl) was harvested, and the radioactivity was counted with a gamma counter. Percentage of specific lysis was calculated as 100 × [(experimental release − spontaneous release)/(maximal release − spontaneous release)]. Spontaneous and maximal release was determined in the presence of medium and 2% triton-X100, respectively.
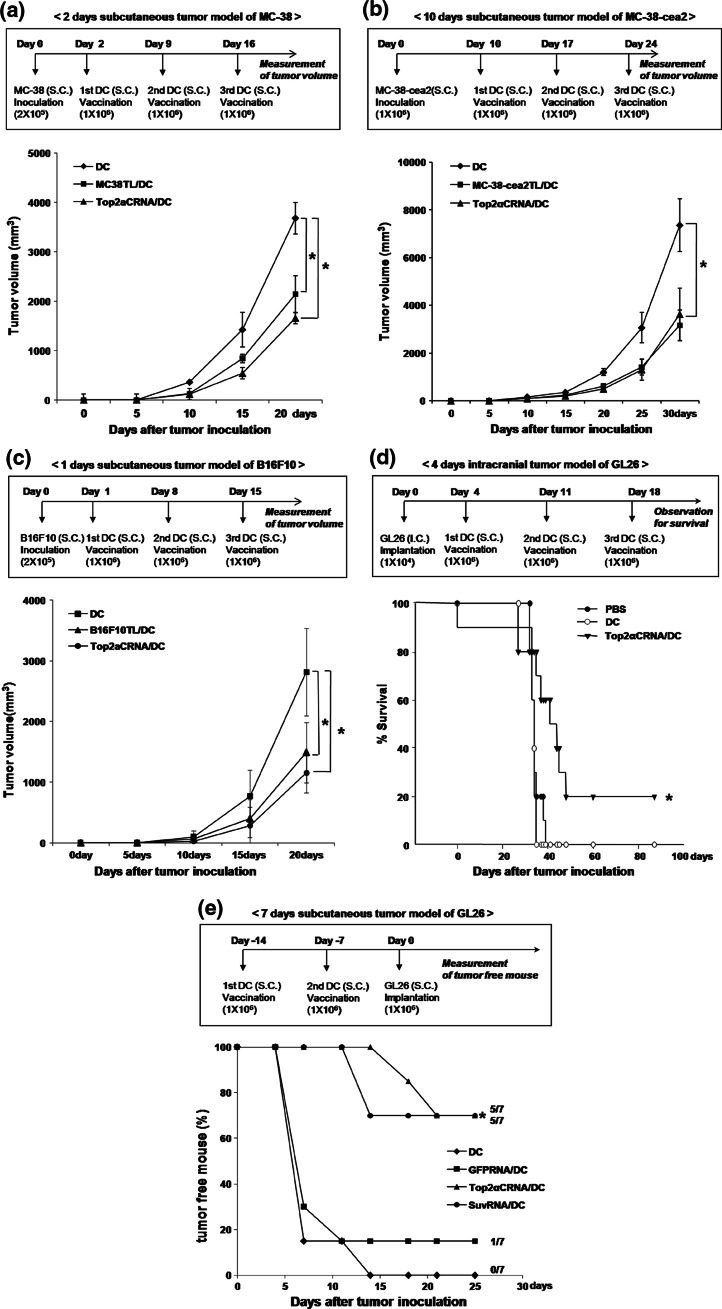
Tumor models and DC vaccination
For the MC-38 tumor model, C57BL/6 mice (6–8 weeks old) were injected subcutaneously with 2 × 105 MC-38 cells. On day 2 after MC-38 inoculation, the mice were subcutaneously vaccinated with 1 × 106 Top2αCRNA/DC, MC38TL/DC, p1327/DC, or DC once per week for 3 weeks. For the MC-38-cea2 tumor model, C57BL/6 mice (6–8 weeks old) were injected subcutaneously with 1 × 106 MC-38-cea2 cells. On day 10, 17, 24 after MC-38-cea2 inoculation, the mice were subcutaneously vaccinated with 1 × 106 Top2αCRNA/DC, MC-38-cea2TL/DC, or DC. For the B16F10 tumor model, C57BL/6 mice (6–8 weeks old) were injected subcutaneously with 2 × 105 MC-38 cells. On day 1 after B16F10 inoculation, the mice were subcutaneously vaccinated with 1 × 106 Top2αCRNA/DC, B16F10TL/DC, or DC once per week for 3 weeks. For the GL26 tumor model, C57BL/6 mice (6–8 weeks old) were injected subcutaneously with 1 × 106 GL26 cells. On day −14 and −7 before GL26 inoculation, the mice were subcutaneously vaccinated with 1 × 106 Top2αCRNA/DC, SuvRNA/DC, GFPRNA/DC, or DC. For the intracranial implantation of GL26 glioma cells, C57BL/6 mice (6–8 weeks old) were anesthetized with ketamine/xylazine. The head was shaved, and the skull was exposed. The animal was positioned into a stereotactic frame with small animal earbars. A burr hole was made using a Dremel drill approximately 2 mm lateral and 1 mm posterior to the intersection of the coronal and sagittal sutures (bregma). GL26 cells (1 × 104) were injected using a Hamilton syringe at a depth of 3 mm in a volume of 4 μl. Mice were subcutaneously vaccinated with 1 × 106 Top2αCRNA/DC or DC on days 4, 11, and 18 after GL26 implantation. DC that went through the transfection procedure without Top2αCRNA were used as a negative control. Reduction of tumor growth and survival was compared with the negative control.
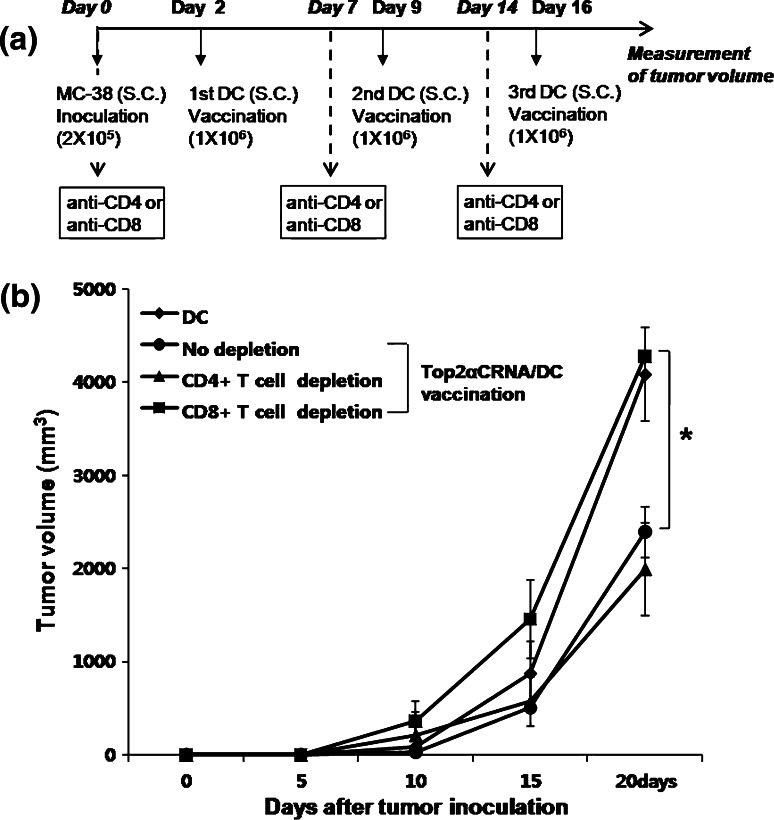
In vivo depletion of the T cell subset
To clarify the role of CD4+ and CD8+ T cells in the antitumor effect of the MC-38 tumor model, an anti-CD4 monoclonal antibody (GK1.5; eBioscience, San Diego, USA) and an anti-CD8 monoclonal antibody (2.43; a gift from Prof. Byoung S. Kwon at Ulsan University, Ulsan, Korea) were used for depletion of CD4+ and CD8+ T cell, respectively. C57BL/6 mice were injected intraperitoneally with 200 μg of purified anti-mouse CD4 or CD8 antibody or IgG at 0, 7, 14 days in the MC-38 tumor model and vaccinated at 2, 9, 16 days with Top2αCRNA/DC. CD4 and CD8 depletion (>95%) was verified during the treatment by FACS analysis using antibodies specific for CD4 and CD8. DC without tumor antigen were vaccinated as a control for normal tumor growth.
Peptide epitopes
The Top2αC sequence was analyzed for peptides that could potentially bind to H-2Kb, using a peptide motif scoring system (Bioinformatics and Molecular Analysis Section (BIMAS) of NIH, http://www-bismas.cit.nih.gov; SYFPEITHI, http://www.syfpeithi.de). Five peptides with high prediction scores in two programs were selected for further analysis (Table 1). Synthetic peptides were purified by high-performance liquid chromatography at a minimum purity of 90%. The CEA526 (526-533: EAQNTTYL) peptide was used as a negative control.
Table 1.
TopαC-derived peptides predicted for binding to MHC class I molecules (H-2Kb)
| Peptide name | Peptide position | Peptide sequence | SYFPEITHI score |
|---|---|---|---|
| pl327 | 1327–1355 | DSDEDFSGL | 16 |
| pl339 | 1339–1447 | DEDEDFLPL | 13 |
| pl470 | 1470–1478 | SDSDFERAI | 13 |
| pl490 | 1490–1498 | EEQDFPVDL | 11 |
| p1509 | 1509–1518 | RARKPIKYI | 14 |
These peptides were searched using BIMAS (http://www-bimas.dt.nih.gov/cgi-bin/molbio) and SYFPEITHI (http://www.sytpeithi.de) software
Enzyme-linked immunosorbent assay (ELISA)
Splenocytes (2 × 105 cells/200 μl) from mice vaccinated with Top2αCRNA/DC were incubated with peptide 1 μg/ml in round-bottomed 96-well plate. After 3 days, the plates were centrifuged and 100 μl of supernatant was removed from each well. The level of IFN-γ production was determined by ELISA (eBioscience).
Statistical analysis
The results are shown as the mean ± SD or SE. Statistical analysis was performed using a Student’s t test, with the exception of the survival data. Survival data were analyzed using the Kaplan–Meier test, and the difference between the groups was compared using a log-rank test. A P value <0.05 was considered to reflect a statistically significant difference.
Results
Expression of Top2α in murine tumor cell lines and normal murine tissues
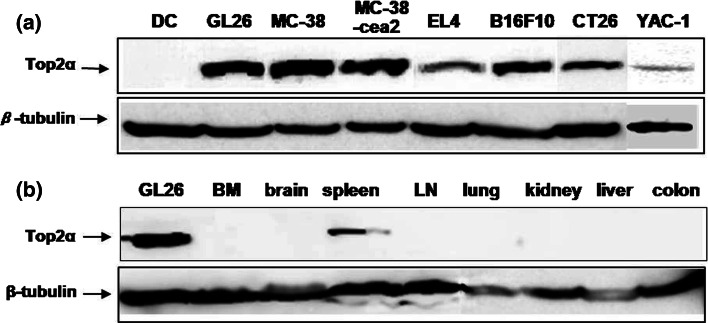
We evaluated the expression of Top2α in murine tumor cell lines by Western blot analysis using an anti-Top2α antibody. Figure 1a shows that the Top2α protein was expressed in various murine tumor cell lines, including MC-38 and GL26.
Fig. 1.
Expression of Top2α in murine tumor cell lines and normal murine tissues by Western blot analysis. Western blotting was performed using an anti-Top2α antibody against protein from cultured tumor cell lines, including GL26 (glioma), MC-38, MC-38-cea2 and CT26 (colon cancer), EL4 (leukemia), B16F10 (melanoma), and YAC-1(lymphoma) (a) and tissues of normal mice (b). BM bone marrow, LN lymph node. The data are representative of three independent experiments
Top2α expression in normal murine tissues was examined in bone marrow, brain, spleen, lymph node, lung, kidney, liver, and colon. As shown in Fig. 1b, the spleen expressed Top2α, but other tissues including the brain and colon, did not.
Detection of T cell responses specific for Top2α and survivin
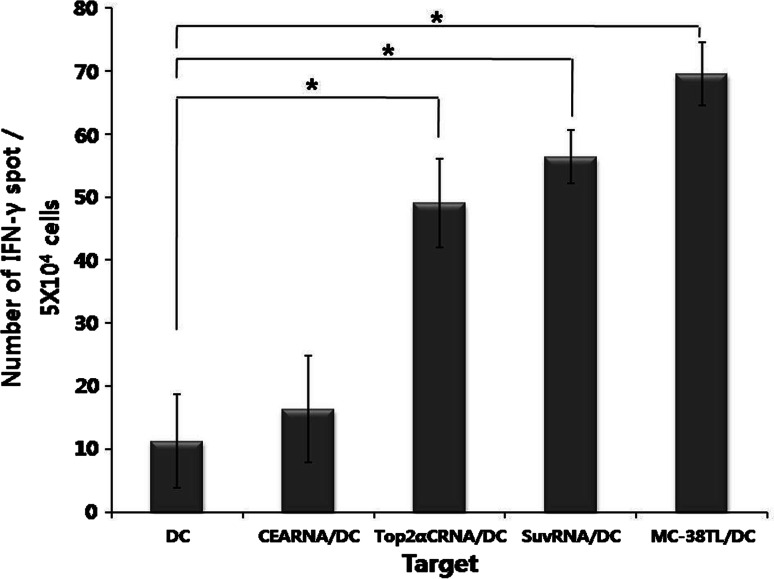
To investigate whether mice vaccinated with MC-38 tumor lysate-pulsed DC (MC-38TL/DC) induce an immune response against Top2α, we determined the number of IFN-γ-secreting cells by ELISPOT assay. The C-terminal region of Top2α was used in this study, and consisted of amino acid residues 1263–1528. Because MC-38 has been shown to express several tumor antigens, including survivin and Top2α (data not shown), C57BL/6 mice were vaccinated with MC-38TL/DC. One week after the second vaccination, splenocytes were harvested and activated with various target cells. The number of IFN-γ-secreting cells stimulated by Top2αCRNA/DC was significantly higher than that stimulated by unpulsed DC. A high number of IFN-γ-secreting cells was also detected in splenocytes stimulated by MC-38TL/DC and DC transfected with RNA encoding survivin (SuvRNA/DC) (Fig. 2). DC pulsed with CEA, an irrelevant tumor antigen, and unpulsed DC were used as Top2α-negative cells.
Fig. 2.
Detection of T cell responses specific for Top2α and survivin in mice vaccinated with DC pulsed with MC-38 tumor lysate. Top2α-specific immune responses were measured with the ELISPOT assay using DC, CEARNA/DC, Top2αCRNA/DC, survivinRNA/DC (SuvRNA/DC), or MC-38 tumor lysate/DC (MC38TL/DC) as the target cells. Results are given as the mean number of IFN-γ spots per 5 × 104 splenocytes from individually tested mice ±SE. The statistical significance was evaluated with a t test of the pooled results of two experiments. *P < 0.05 (DC vs. each group)
These results indicate that immune responses to several tumor antigens were induced in mice vaccinated with MC-38TL/DC, and that Top2αCRNA/DC were capable of presenting Top2α epitopes.
Induction of Top2α-specific immune responses by Top2αCRNA/DC vaccination
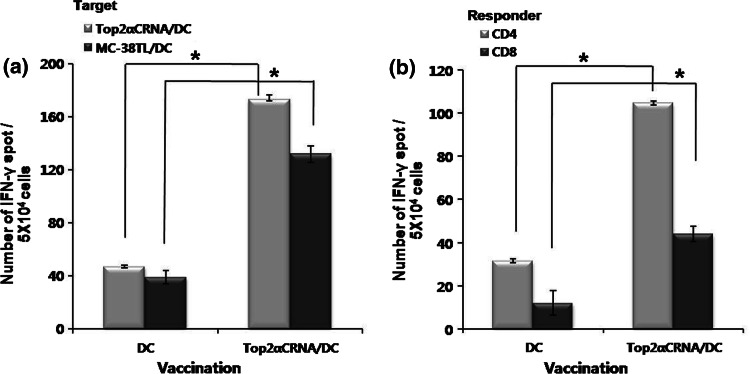
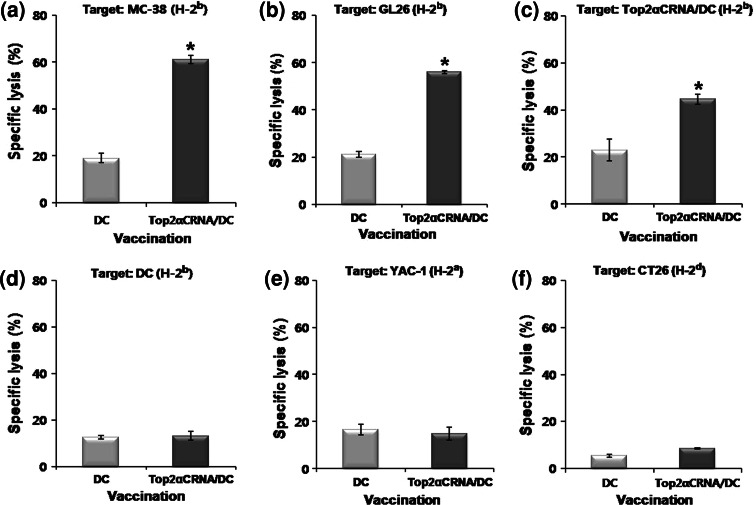
To examine the immune responses induced by Top2αCRNA/DC in vivo, the IFN γ-secreting T cells specific for Top2α were quantified by ELISPOT assay after the third vaccination with Top2αCRNA/DC. When stimulated by Top2αCRNA/DC, the frequencies of IFN γ-secreting cells in splenocytes, CD4+, and CD8+ T cells were significantly higher in mice vaccinated with Top2αCRNA/DC than those vaccinated with DC (Fig. 3). The frequency of Top2αC-specific CD4+ T cells was higher than that of CD8+ T cells. When stimulated by DC pulsed with MC-38 tumor lysate, a tumor cell line that expresses Top2α endogenously, high frequencies of IFN γ-secreting cells in splenocytes were also observed (Fig. 3a). The cytotoxicity of Top2αC-reactive CTL was assessed on several target cells. T cells from C57BL/6 mice vaccinated with Top2αCRNA/DC showed killing activity against MC-38 (H-2b), GL26 (H-2b), and Top2αCRNA/DC (H-2b), as Top2α-expressing cells with a E/T ratio of 40, but there was very low lysis against unpulsed DC, as Top2α-non-expressing cells (Fig. 4a–d). Targeting YAC-1 (H-2a) showed low lysis, indicating that the killing activity against target cells was not affected by NK cells (Fig. 4e). CT26, Top2α-expressing cells with a H-2d haplotype, were not able to be lysed because the Top2α-specific T cells from C57BL/6 mice were restricted to H-2b MHC class I molecules (Fig. 4f). These results demonstrate that Top2α-specific immune responses were successfully induced in vivo by vaccination with Top2αCRNA/DC.
Fig. 3.
Induction of Top2α-specific immune responses by Top2αCRNA/DC vaccination. a IFN-γ-secreting T cells specific for Top2α in splenocytes from mice vaccinated with Top2αCRNA/DC. Splenocytes stimulated with Top2αCRNA/DC and MC-38TL/DC as Top2α-expressing cells. IFN-γ-secreting T cells were measured by ELISPOT assay. b IFN-γ-secreting T cells specific for Top2α in CD4+ or CD8+ T cells sorted from splenocytes of mice vaccinated with Top2αCRNA/DC. CD4+ and CD8+ T cells were stimulated by Top2αCRNA/DC. Results are given as the mean number of IFN-γ spots per 5 × 104 cells from individually tested mice ±SD. The statistical significance was evaluated using a t test. *P < 0.05 (DC vs. Top2αCRNA/DC). The data are representative of three independent experiments
Fig. 4.
Cytotoxic T cell activity against Top2α-expressing cells. Cytotoxic activity was assessed with the 51Cr release assay against several target cells [Top2α-expressing target: MC-38 (a), GL26 (b), Top2αCRNA/DC (c), CT26 (f); Top2α-non-expressing target: unpulsed DC (d); NK cell-sensitive target: YAC-1 (e)] at a 40:1 E/T ratio. The percentage of specific lysis was calculated from triplicate wells. The results are given as mean ± SD. The statistical significance was evaluated using a t test. *P < 0.05 (DC vs. Top2αCRNA/DC). The data are representative of three independent experiments
Antitumor effect by Top2αCRNA/DC vaccination in murine tumor models
We investigated the antitumor effect of Top2αCRNA/DC in a MC-38, MC-38-cea2, and B16F10 subcutaneous tumor models and an established GL26 intracranial and subcutaneous tumor model because Top2α was expressed in several different tumor cell lines including MC-38, MC-38-cea2, B16F10, and GL26. Mice vaccinated with Top2αCRNA/DC in MC-38, MC-38-cea2, and B16F10 tumor models showed significant inhibition of tumor growth during progression through the chosen end point of these experiments, compared with mice vaccinated with DC (P < 0.05, Fig. 5a–c). After 4 days inoculation of GL26, mice vaccinated with Top2αCRNA/DC showed significantly longer survival compared with mice vaccinated with DC (P < 0.05), with 20% of mice vaccinated with Top2αCRNA/DC surviving beyond 85 days after tumor implantation (Fig. 5d). In the GL26 subcutaneous tumor model, the generation of tumors was inhibited in five of seven mice by vaccination with Top2αCRNA/DC, whereas the generation of tumors was not inhibited by vaccination with DC (Fig. 5e).
Fig. 5.
Antitumor effects of Top2αCRNA/DC vaccination in murine tumor models. a Tumor growth of mice vaccinated with Top2αCRNA/DC in the MC-38 subcutaneous tumor model. The time schedule for the experiment is shown in the upper panel. The results are given as mean ± SD. The statistical significance was evaluated using a t test. *P < 0.05. The data are representative of three independent experiments, consisting of seven mice per group. b Tumor growth of mice vaccinated with Top2αCRNA/DC in the MC-38-cea2 subcutaneous tumor model. The time schedule for the experiment is shown in the upper panel. The results are given as mean ± SD. The statistical significance was evaluated using a t test. *P < 0.05. Each group consisted of ten mice. c Tumor growth of mice vaccinated with Top2αCRNA/DC in the B16F10 subcutaneous tumor model. The time schedule for the experiment is shown in the upper panel. The results are given as mean ± SD. The statistical significance was evaluated using a t test. *P < 0.05. Each group consisted of eight mice. d Survival by Top2αCRNA/DC in the GL26 intracranial tumor model. The time schedule for the experiment is shown in the upper panel. Survival data were analyzed using the Kaplan–Meier method. The difference between the groups was compared using a log-rank test. *P < 0.05 (DC vs. Top2αCRNA/DC). Each group consisted of five mice. e Tumor free mice after vaccination with Top2αCRNA/DC in the GL26 subcutaneous tumor model. The time schedule for the experiment is shown in the upper panel. The statistical significance was evaluated using a t test. *P < 0.05 (DC vs. Top2αCRNA/DC). Each group consisted of seven mice
Depletion of CD4+ and CD8+ T cells in the MC-38 tumor model
To investigate which type of T cells are involved in the antitumor effect in the MC-38 tumor model vaccinated with Top2aCRNA/DC, we treated with anti-CD4 and anti-CD8 antibody 0, 7, and 14 days after MC-38 inoculation to remove CD4+ and CD8+ T cells, respectively. When CD8+ T cells were depleted, the inhibition of tumor growth by Top2αCRNA/DC vaccination almost completely disappeared (Fig. 6). However, depletion of CD4+ T cells did not affect the antitumor effect of vaccination with Top2αCRNA/DC. These results suggest that the CD8+ T cells are the population responsible for the inhibition of tumor growth in vivo by Top2αCRNA/DC vaccination.
Fig. 6.
Depletion of CD4+ and CD8+ T cells in the MC-38 tumor model vaccinated with Top2aCRNA/DC. a The time schedule for the experiment is shown. b Treatment with the anti-CD4+ and anti-CD8+ antibodies was completed on days 0, 7, and 14 in the MC-38 tumor model vaccinated with Top2aCRNA/DC. DC without tumor antigen was vaccinated as a control for normal tumor growth. The results are given as mean ± SD. The statistical significance was evaluated using a t test. *P < 0.05 (no depletion vs. CD8+ T cell depletion). Each group consisted of eight mice
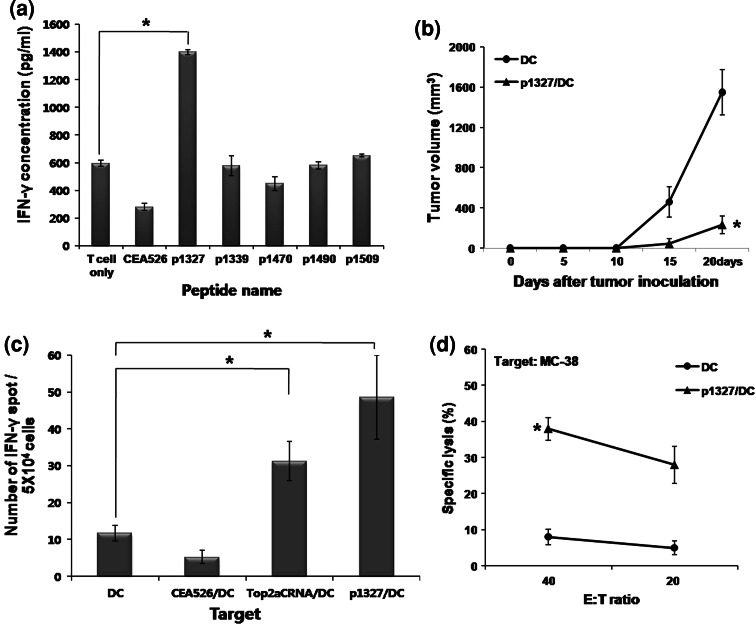
Peptide epitopes of Top2αC
Five Top2αC-derived peptides were selected that bind MHC class I molecules (H-2Kb) with a high score using BIMAS and SYFPEITHI program (Table 1). To identify the T cell epitopes in Top2αC, splenocytes from mice vaccinated with Top2αCRNA/DC were stimulated with Top2αC-derived peptides and production of IFN-γ was measured by ELISA. Splenocytes stimulated with p1327 (DSDEDFSGL) produced a high level of IFN-γ as compared to those stimulated with other peptides (Fig. 7a). Vaccination with DC pulsed with p1327 (p1327/DC) significantly inhibited the tumor growth of MC-38 as compared to mice vaccinated with unpulsed DC (Fig. 7b). To investigate the immune responses induced by p1327/DC in vivo, the IFN γ-secreting T cells specific for Top2α were quantified by ELISPOT assay after the third vaccination with p1327/DC. The frequencies of IFN γ-secreting cells were significantly higher when stimulated by Top2αCRNA/DC and p1327/DC than when stimulated by unpulsed DC or CEA526 peptide-pulsed DC (CEA526/DC) (Fig. 7c). Splenocytes from mice vaccinated with p1327/DC showed cytotoxic activity to MC-38 tumor cells (Fig. 7d). These results suggest that p1327 may be one of the T cell epitopes located in the Top2αC region.
Fig. 7.
Production of IFN-γ by the indicated peptides in mice vaccinated with Top2αCRNA/DC, and the antitumor effect of p1327 peptide-pulsed DC. a Production of IFN-γ by peptide epitopes derived from Top2αC. Five Top2αC-derived peptides were selected that bind MHC class I molecules (H-2Kb) with a high score using the BIMAS and SYFPEITHI programs. Splenocytes from mice vaccinated with Top2αCRNA/DC were incubated with individual peptides (1 μg/ml) for 3 days. The level of IFN-γ production was measured by ELISA. The statistical significance was evaluated using a t test. *P < 0.05 (T cell only vs. p1327). The data are representative of three independent experiments. b Tumor growth by p1327-pulsed DC (p1327/DC) in the MC-38 subcutaneous tumor model. Two days after MC-38 tumor cell inoculation, mice were vaccinated with p1327/DC once per week for 3 weeks, and tumor volume was measured every 5 days. The statistical significance was evaluated using a t test. *P < 0.05 (DC vs. p1327/DC). Each group consisted of seven mice. c Induction of Top2α-specific immune responses by p1327/DC vaccination. IFN-γ-secreting T cells specific for Top2α in splenocytes from mice vaccinated with p1327/DC were measured with the ELISPOT assay. Splenocytes stimulated with Top2αCRNA/DC and p1327/DC as positive targets, and DC and CEA526/DC as negative targets. The results are given as mean ± SE. The statistical significance was evaluated using a t test of the pooled results of two experiments. *P < 0.05 (DC vs. Top2αCRNA/DC or p1327/DC). d Cytotoxic activity specific for Top2α. Cytotoxic activity was assessed with the 51Cr release assay against MC-38 as Top2α-expressing target at a 40:1 and 20:1 E/T ratio. The statistical significance was evaluated using a t test. *P < 0.05 (DC vs. p1327/DC). The data are representative of two independent experiments
Discussion
The identification of antigens processed and presented by tumors of various histologic origins is an interesting research subject for the development of antitumor vaccines with broad applications. Over the past years, some tumor antigens with these characteristics have been described as “universal tumor antigens”, such as hTERT, survivin, and NY-ESO-1 [4, 6, 10, 31]. Over-expression of Top2α has been reported in various tumors including lymphoma, glioma and colon cancer [3, 28, 32, 35]. We also confirmed the expression of Top2α in the murine tumor cell lines used in this study (Fig. 1a). Top2α-specific T cells from mice vaccinated with Top2αCRNA/DC killed different types of tumors that expressed Top2α endogenously, including MC-38 (colon cancer) and GL26 (glioma), in vitro (Fig. 4a, b). As depicted in Fig. 5, Top2αCRNA/DC showed antitumor effects in terms of inhibition of tumor volume and prolonged survival in MC-38, B16F10, and GL26 murine tumor models. Splenocytes from mice vaccinated with MC-38TL/DC were stimulated by Top2αCRNA/DC as well as by SuvRNA/DC in vitro (Fig. 2). These results suggest that Top2α is a candidate for universal tumor antigens.
Top2α was also detected in normal spleen tissues although its level is lower in spleen than in the GL26 tumor cell line (Fig. 1b). Our immunoblotting showed a general agreement with Top2α expression in the spleen [8, 15]. Top2α is expressed in proliferating cells with levels varying throughout the cell cycle, and its expression is transient in normal cells [2, 33]. Expression of Top2α in normal spleen tissue may induce tolerance. However, we could effectively induce Top2α-specific T cell responses because the most potent antigen-presenting cells, DC, were used as a cellular adjuvant. Topoisomerase II has two distinct isoforms, designated Top2α and Top2β. The amino acid sequence of Top2α showed considerable homology with that of Top2β, except in the C-terminal domain [18]. Therefore, vaccination with full-length Top2α may induce autoimmune responses because Top2β is constantly present in most cells. In this study, we focused on the Top2α C-terminus, which shares low homology with Top2β.
DC transfected with RNA encoding whole tumor antigen have been known to effectively induce both CD4+ and CD8+ T cell responses [20, 30]. In this study, both CD4+ and CD8+ T cells were induced by this method (Fig. 3b). Different frequencies of CD4+ and CD8+ T cell responses may be associated with structural and functional differences that depend on tumor antigens. From our in vivo depletion model, CD8+ T cells were found to be effector cells of the antitumor effect of Top2αCRNA/DC vaccination (Fig. 6). However, the depletion of CD4+ T cells did not affect the antitumor effect of Top2αCRNA/DC vaccination. The combination of the two antibodies, agonistic anti-4-1BB and depleting anti-CD4, resulted in an enhanced production of efficient tumor-killing CTLs, facilitation of their infiltration, and production of a susceptible tumor microenvironment [9]. Elimination of CD4+ T cells in mice bearing an advanced sarcoma augments the antitumor action of interleukin-2 therapy by releasing CD8+ T cell-mediated immunity from T cell-mediated suppression [25]. Treating the spleen cells of the tumor-bearing mice with an anti-CD4 antibody completely eliminated their ability to block adoptive immunotherapy [5]. The immune system may contain various CD4+ cell-mediated regulatory mechanisms against CD8+ T cell-mediated antitumor immunity.
We selected five potential CTL epitopes from the Top2αC sequence using the BIMAS and SYFPEITHI programs. Only p1327 (DSDEDFSGL), which gave the highest score in the prediction program, successfully stimulated T cells from mice vaccinated with Top2αCRNA/DC. The antitumor effects resulting from vaccination with p1327/DC indicate that p1327 is a T cell epitope presented by tumor cells that could be used only within tumors with the same haplotype. To demonstrate the universality with other haplotypes, full-length Top2a should be used, rather than a peptide, in order to overcome MHC restriction. To define whether p1327 is immunogenic in other haplotypes, further studies should be performed using mice with different H-2 haplotypes. Additional control experiments, as well as studies in additional tumor models and in a more rigorous setting of established tumors are needed to confirm the efficacy of this peptide as a therapeutic reagent.
Conclusion
We provide the first evidence that Top2α could be used as a universal tumor antigen for cancer immunotherapy. Because 89% of the amino acid residues of Top2α were identical between mice and humans [1], Top2α may also be a potential target for immunotherapy in the human system. Further studies are needed to demonstrate the presence of specific T cells in cancer patients, the generation of CTL specific for Top2α in vitro, and the antitumor effect in xenogenic tumor models.
Acknowledgments
This study was supported by a grant from the Korea Research Foundation (Grant KRF 2006-005-J00602).
References
- 1.Adachi N, Miyaike M, Kato S, Kanamaru R, Koyama H, Kikuchi A. Cellular distribution of mammalian DNA topoisomerase II is determined by its catalytically dispensable C-terminal domain. Nucleic Acids Res. 1997;25:3135–3142. doi: 10.1093/nar/25.15.3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adachi N, Nomoto M, Kohno K, Koyama H. Cell-cycle regulation of the DNA topoisomerase II alpha promoter is mediated by proximal CCAAT boxes: possible involvement of acetylation. Gene. 2000;245:49–57. doi: 10.1016/S0378-1119(00)00040-8. [DOI] [PubMed] [Google Scholar]
- 3.Al-Kuraya K, Novotny H, Bavi P, Siraj AK, Uddin S, Ezzat A, Sanea NA, Al-Dayel F, Al-Mana H, Sheikh SS, Mirlacher M, Tapia C, Simon R, Sauter G, Terracciano L, Tornillo L. HER2, TOP2A, CCND1, EGFR and C-MYC oncogene amplification in colorectal cancer. J Clin Pathol. 2007;60:768–772. doi: 10.1136/jcp.2006.038281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 5.Awwad M, North RJ. Radiosensitive barrier to T-cell-mediated adoptive immunotherapy of established tumors. Cancer Res. 1990;50:2228–2233. [PubMed] [Google Scholar]
- 6.Baumgaertner P, Rufer N, Devevre E, Derre L, Rimoldi D, Geldhof C, Voelter V, Lienard D, Romero P, Speiser DE. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer Res. 2006;66:1912–1916. doi: 10.1158/0008-5472.CAN-05-3793. [DOI] [PubMed] [Google Scholar]
- 7.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Capranico G, Tinelli S, Austin CA, Fisher ML, Zunino F. Different patterns of gene expression of topoisomerase II isoforms in differentiated tissues during murine development. Biochim Biophys Acta. 1992;1132:43–48. doi: 10.1016/0167-4781(92)90050-a. [DOI] [PubMed] [Google Scholar]
- 9.Choi BK, Kim YH, Kang WJ, Lee SK, Kim KH, Shin SM, Yokoyama WM, Kim TY, Kwon BS. Mechanisms involved in synergistic anticancer immunity of anti-4-1BB and anti-CD4 therapy. Cancer Res. 2007;67:8891–8899. doi: 10.1158/0008-5472.CAN-07-1056. [DOI] [PubMed] [Google Scholar]
- 10.Gordan JD, Vonderheide RH. Universal tumor antigens as targets for immunotherapy. Cytotherapy. 2002;4:317–327. doi: 10.1080/146532402760271091. [DOI] [PubMed] [Google Scholar]
- 11.Hande KR. Clinical applications of anticancer drugs targeted to topoisomerase II. Biochim Biophys Acta. 1998;1400:173–184. doi: 10.1016/s0167-4781(98)00134-1. [DOI] [PubMed] [Google Scholar]
- 12.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ito M, Shichijo S, Tsuda N, Ochi M, Harashima N, Saito N, Itoh K. Molecular basis of T cell-mediated recognition of pancreatic cancer cells. Cancer Res. 2001;61:2038–2046. [PubMed] [Google Scholar]
- 14.Jenkins JR, Ayton P, Jones T, Davies SL, Simmons DL, Harris AL, Sheer D, Hickson ID. Isolation of cDNA clones encoding the beta isozyme of human DNA topoisomerase II and localisation of the gene to chromosome 3p24. Nucleic Acids Res. 1992;20:5587–5592. doi: 10.1093/nar/20.21.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juenke JM, Holden JA. The distribution of DNA topoisomerase II isoforms in differentiated adult mouse tissues. Biochim Biophys Acta. 1993;1216:191–196. doi: 10.1016/0167-4781(93)90144-3. [DOI] [PubMed] [Google Scholar]
- 16.Kanta SY, Yamane T, Dobashi Y, Mitsui F, Kono K, Ooi A. Topoisomerase II alpha gene amplification in gastric carcinomas: correlation with the HER2 gene. An immunohistochemical, immunoblotting, and multicolor fluorescence in situ hybridization study. Hum Pathol. 2006;37:1333–1343. doi: 10.1016/j.humpath.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Kosari F, Munz JM, Savci-Heijink CD, Spiro C, Klee EW, Kube DM, Tillmans L, Slezak J, Karnes RJ, Cheville JC, Vasmatzis G. Identification of prognostic biomarkers for prostate cancer. Clin Cancer Res. 2008;14:1734–1743. doi: 10.1158/1078-0432.CCR-07-1494. [DOI] [PubMed] [Google Scholar]
- 18.Linka RM, Porter AC, Volkov A, Mielke C, Boege F, Christensen MO. C-terminal regions of topoisomerase II alpha and II beta determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 2007;35:3810–3822. doi: 10.1093/nar/gkm102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mano MS, Awada A, Di Leo A, Durbecq V, Paesmans M, Cardoso F, Larsimont D, Piccart M. Rates of topoisomerase II-alpha and HER-2 gene amplification and expression in epithelial ovarian carcinoma. Gynecol Oncol. 2004;92:887–895. doi: 10.1016/j.ygyno.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Muller MR, Grunebach F, Nencioni A, Brossart P. Transfection of dendritic cells with RNA induces CD4- and CD8-mediated T cell immunity against breast carcinomas and reveals the immunodominance of presented T cell epitopes. J Immunol. 2003;170:5892–5896. doi: 10.4049/jimmunol.170.12.5892. [DOI] [PubMed] [Google Scholar]
- 21.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 22.Nair SK, Heiser A, Boczkowski D, Majumdar A, Naoe M, Lebkowski JS, Vieweg J, Gilboa E. Induction of cytotoxic T cell responses and tumor immunity against unrelated tumors using telomerase reverse transcriptase RNA transfected dendritic cells. Nat Med. 2000;6:1011–1017. doi: 10.1038/79519. [DOI] [PubMed] [Google Scholar]
- 23.Nakatsura T, Senju S, Ito M, Nishimura Y, Itoh K. Cellular and humoral immune responses to a human pancreatic cancer antigen, coactosin-like protein, originally defined by the SEREX method. Eur J Immunol. 2002;32:826–836. doi: 10.1002/1521-4141(200203)32:3<826::AID-IMMU826>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 24.Park MY, Kim HS, Woo SJ, Kim CH, Park JS, Sohn HJ, Kim HJ, Oh ST, Kim TG. Efficient antitumor immunity in a murine colorectal cancer model induced by CEA RNA-electroporated B cells. Eur J Immunol. 2008;38:2106–2117. doi: 10.1002/eji.200737960. [DOI] [PubMed] [Google Scholar]
- 25.Rakhmilevich AL, North RJ. Elimination of CD4+ T cells in mice bearing an advanced sarcoma augments the antitumor action of interleukin-2. Cancer Immunol Immunother. 1994;38:107–112. doi: 10.1007/BF01526205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanlan MJ, Simpson AJ, Old LJ. The cancer/testis genes: review, standardization, and commentary. Cancer Immun. 2004;4:1. [PubMed] [Google Scholar]
- 27.Schoeffler AJ, Berger JM. Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism. Biochem Soc Trans. 2005;33:1465–1470. doi: 10.1042/BST20051465. [DOI] [PubMed] [Google Scholar]
- 28.Schrader C, Meusers P, Brittinger G, Teymoortash A, Siebmann JU, Janssen D, Parwaresch R, Tiemann M. Topoisomerase IIalpha expression in mantle cell lymphoma: a marker of cell proliferation and a prognostic factor for clinical outcome. Leukemia. 2004;18:1200–1206. doi: 10.1038/sj.leu.2403387. [DOI] [PubMed] [Google Scholar]
- 29.Stone B, Schummer M, Paley PJ, Thompson L, Stewart J, Ford M, Crawford M, Urban N, O’Briant K, Nelson BH. Serologic analysis of ovarian tumor antigens reveals a bias toward antigens encoded on 17q. Int J Cancer. 2003;104:73–84. doi: 10.1002/ijc.10900. [DOI] [PubMed] [Google Scholar]
- 30.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 31.Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674–679. doi: 10.1038/sj.onc.1205074. [DOI] [PubMed] [Google Scholar]
- 32.Willman JH, Holden JA. Immunohistochemical staining for DNA topoisomerase II-alpha in benign, premalignant, and malignant lesions of the prostate. Prostate. 2000;42:280–286. doi: 10.1002/(SICI)1097-0045(20000301)42:4<280::AID-PROS5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 33.Woessner RD, Mattern MR, Mirabelli CK, Johnson RK, Drake FH. Proliferation- and cell cycle-dependent differences in expression of the 170 kilodalton and 180 kilodalton forms of topoisomerase II in NIH-3T3 cells. Cell Growth Differ. 1991;2:209–214. [PubMed] [Google Scholar]
- 34.Wong N, Yeo W, Wong WL, Wong NL, Chan KY, Mo FK, Koh J, Chan SL, Chan AT, Lai PB, Ching AK, Tong JH, Ng HK, Johnson PJ, To KF. TOP2A overexpression in hepatocellular carcinoma correlates with early age onset, shorter patients survival and chemoresistance. Int J Cancer. 2009;124:644–652. doi: 10.1002/ijc.23968. [DOI] [PubMed] [Google Scholar]
- 35.Zhao H, Yu H, Liu Y, Wang Y, Cai W. DNA topoisomerase II-alpha as a proliferation marker in human gliomas: correlation with PCNA expression and patient survival. Clin Neuropathol. 2008;27:83–90. doi: 10.5414/npp27083. [DOI] [PubMed] [Google Scholar]