Abstract
Introduction
Marek’s disease (MD), a herpesvirus-induced lymphoma of chickens is a unique natural model of CD30-overexpressing (CD30hi) lymphoma. We have previously proposed that the CD30hi neoplastically transformed CD4+ T cells in MD lymphomas have a phenotype antagonistic to cell mediated immunity. Here were test the hypothesis that the CD30hi neoplastically transformed MD lymphoma cells have a phenotype more closely resembling T-helper (Th)-2 or regulatory T (T-reg) cells.
Materials and methods
We separated ex vivo-derived CD30hi, from the CD30lo/− (non-transformed), MD lymphoma cells and then quantified the relative amounts of mRNA and proteins for cytokines and other genes that define CD4+ Th-1, Th-2 or T-reg phenotypes.
Results and discussion
Gene Ontology-based modeling of our data shows that the CD30hi MD lymphoma cells having a phenotype more similar to T-reg. Sequences that could be bound by the MD virus putative oncoprotein Meq in each of these genes’ promoters suggests that the MD herpesvirus may play a direct role in maintaining this T-reg-like phenotype.
Keywords: Regulatory T cell, Herpesvirus, Gene Ontology, Systems biology, Animal model
Introduction
Marek’s disease (MD), a lymphomatous disease of chickens caused by the MD α-herpesvirus (MDV) is also a unique natural animal model for classical Hodgkin’s, and non-Hodgkin’s, human lymphomas. MD neoplastically transformed cells over-express tumor necrosis factor receptor superfamily member (TNFSFR) 8 [the “Hodgkin’s disease antigen” (CD30)] [1]. Like human CD30hi lymphomas [2, 3], the MD CD30hi cells are rare [4] and surrounded by activated non-transformed lymphocytes. MDV latently infects CD30hi MD lymphoma cells [5]. MDV’s putative oncogenes are not acutely transforming in vitro [6–8], and survival and growth of MD CD30hi cells depends on the local lymphoma environment [4]. MD lymphoma growth occurs despite specific immune responses to virus and host proteins [9]. Here we test our hypotheses that the CD30hi MD lymphoma cells have a phenotype most resembling T-helper (Th)-2 [1, 4] or T-regulatory (T-reg) cells [10]; either of which could antagonize cytotoxic lymphocyte immunity and support tumorigenesis.
Th cells can be distinguished from T-reg cells based on gene expression. Th-1 cells express CD4 and produce high levels of interferon-gamma (IFN-γ) and interleukin (IL)-2, Th-2 cells (also CD4+) produce IL-4, -5, -10, and -13 [11]. In contrast, T-reg cells express CD4, major histocompatibility complex (MHC) class II (except in mouse), IFN-γ [12, 13], transforming growth factor beta (TGFβ), IL-10, the transcription factor forkhead box (FOX) P3, IL-2 receptor α chain (CD25), high levels of cytotoxic T-lymphocyte associated molecule-4 (CTLA-4) [14–17], G protein-coupled receptor (GPR)-83 [18], and decreased SMAD-7 [19, 20]. Furthermore, T-reg function depends on CD30 expression and signaling [21–23]. The neoplastically transformed cells in MD lymphomas express the highest levels of MHC class II, CD25 and CD30 [4]. Furthermore, the MDV oncoprotein “meq” transactivates the CD30 promoter [1].
Materials and methods
Chickens and MDV
Lymphomas were produced in outbred SPF, MD-maternal antibody-free white leghorn chickens (Charles River Laboratories, SPAFAS Avian Products and Services, Wilmington, MA, USA), infected (11 days old) with MDV (GA/22, passage 18, 500 pfu, from the Avian Disease and Oncology Laboratory, East Lansing, MI, USA), housed in Petersime units in isolated rooms at Mississippi State University College of Veterinary Medicine (ad libitum food and water).
Lymphoma cell sorting
Lymphomas were removed from ten chickens (kidney, sciatic nerves, testis, bursa, spleen, mesentery, lung and liver) and immediately placed into ice cold phosphate buffered saline (PBS). The CD30hi were separated from the CD30lo/−, lymphoma cells (Fig. 1a inset) by magnetic activated cell sorting and the CD30hi and CD30lo/− purity measured by flow cytometry (FACSCalibur, Becton Dickinson Biosciences) exactly as described [24].
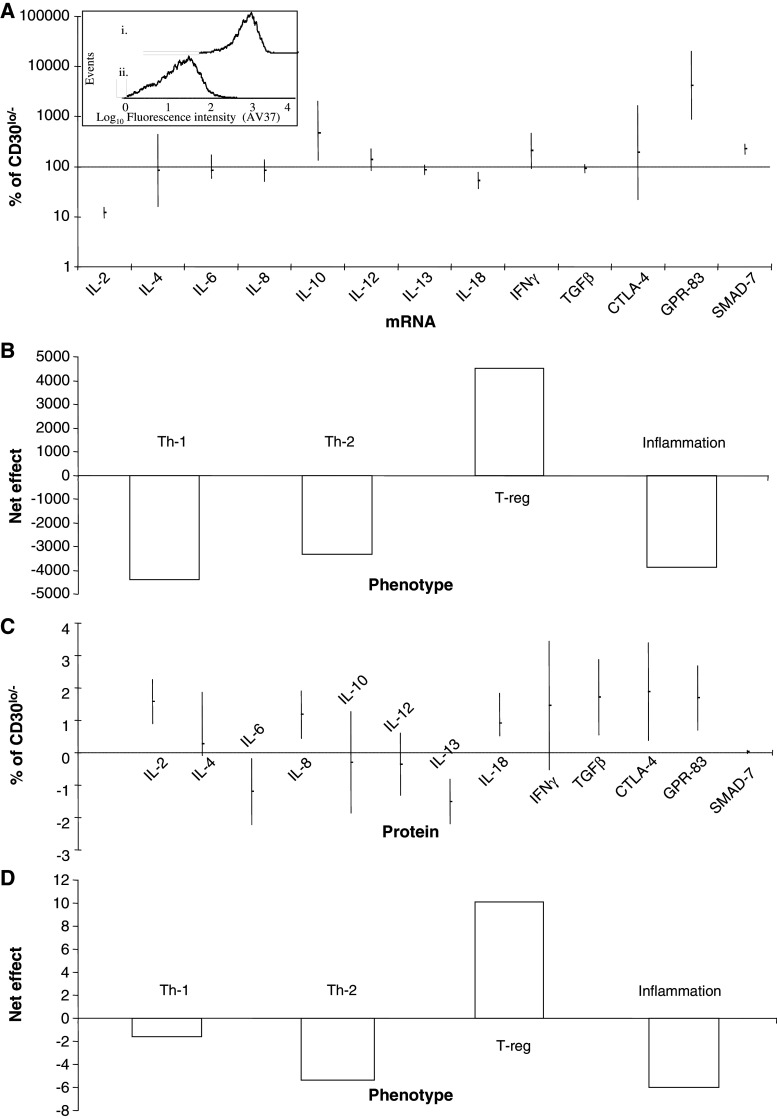
Fig. 1.
Difference in cytokine and cell antigen mRNA (a) and protein (c) expression (measured by duplex real-time reverse transcriptase PCR and proteomics as described in M and M) of CD30hi, relative to CD30lo/−, MD lymphoma cells. The amount of CD30lo/− mRNA expression is set to 100% and protein expression is set to 0. The CD30hi, were separated from the CD30lo/−, lymphoma cells by magnetic activated cell sorting. Inset: the mean purity (±SEM) was 95.7 ± 3.3 and 93.9 ± 4.9%, for the (1) CD30hi and (2) CD30lo/− cells, respectively. GO-based hypothesis-driven quantitative modeling as described in M and M for the mRNA (b) and protein (d) shows that the CD30hi cells have a T-reg phenotype
RNA isolation and real-time PCR
We isolated RNA from three batches of 106 CD30hi and CD30lo/− cells using TRI reagent (Molecular Research Center, Inc.) and treated each with RNase-free DNAse 1 (Promega Corporation) exactly as described [24]. RNA concentrations were quantified (GeneSpec I spectrophotometer; MiraiBio, Alameda, CA, USA) and all RNA samples were adjusted to within a tenfold concentration of each other using RNase-free water. mRNA expression was measured for cytokines and other genes (shown in Fig. 1) to define CD4+ T cell phenotypes. We used a duplex real-time reverse transcriptase PCR (drtRT-PCR), with 28 S rRNA standard, exactly as described [25]: Platinum Quantitative RT-PCR ThermoScript One-Step System (Invitrogen, Carlsbad, CA, USA), 10 μM of each primer, 1 μM probe, an iCycler iQ Real-Time PCR Detection System [Bio-Rad Laboratories, Inc., Hercules, CA, USA; 50°C, 30 s; 95°C, 5 min + 45 × (95°C, 15 s; 60°C, 60 s)]. Most primer and probe sequences (Table 1) are previously published [26, 27], the novel primer/probe sets were designed using Beacon Designer (PREMIER Biosoft International, Palo Alto, CA, USA). All amplicons cross intron–exon boundaries. Each drtRT-PCR experiment, done in triplicate on 96-well plates, included no-template controls and a standard curve (10–1 – 10–6 total RNA made by mixing a 10 μl aliquot from all samples). All PCRs were normalized using the standard curves. The 28 S rRNA-specific mean threshold cycle (C t) value for all target genes was calculated and used to normalize across PCR plates and between samples.
Table 1.
PCR probes and primers (fluorophore)
| RNA target | Probe/primer | Sequence |
|---|---|---|
| 28 S | Probe | 5′-(HEX)-AGGACCGCTACGGACCTCCACCA-(TAMRA)-3′ |
| F | 5′-GGCGAAGCCAGAGGAAACT-3′ | |
| R | 5′-GACGACCGATTTGCACGTC-3′ | |
| IL-2 | Probe | 5′-(FAM)-ACTGAGACCCAGGAGTGCACCCAGC-(TAMRA)-3′ |
| F | 5′-TTGGAAAATATCAAGAACAAGATTCATC-3′ | |
| R | 5′-TCCCAGGTAACACTGCAGAGTTT-3′ | |
| IL-4 | Probe | 5′-(FAM)-AGCAGCACCTCCCTCAAGGCACC-(TAMRA)-3′ |
| F | 5′-AACATGCGTCAGCTCCTGAAT-3′ | |
| R | 5′-TCTGCTAGGAACTTCTCCATTGAA-3′ | |
| IL-6 | Probe | 5′-(FAM)-AGGAGAAATGCCTGACGAAGCTCTCCA-(TAMRA)-3′ |
| F | 5′-GCTCGCCGGCTTCGA-3′ | |
| R | 5′-GGTAGGTCTGAAAGGCGAACAG-3′ | |
| IL-8 | Probe | 5′-(FAM)-TCTTTACCAGCGTCCTACCTTGCGACA-(TAMRA)-3′ |
| F | 5′-GCCCTCCTCCTGGTTTCAG-3′ | |
| R | 5′-TGGCACCGCAGCTCATT-3′ | |
| IL-10 | Probe | 5′-(FAM)-CGACGATGCGGCGCTGTCA-(TAMRA)-3′ |
| F | 5′-CATGCTGCTGGGCCTGAA-3′ | |
| R | 5′-CGTCTCCTTGATCTGCTTGATG-3′ | |
| IL-12β | Probe | 5′-(FAM)-CTGAAAAGCTATAAAGAGCCAAGCAAGACGTTCT-(TAMRA)-3′ |
| F | 5′-TGGGCAAATGATACGGTCAA-3′ | |
| R | 5′-CAGAGTAGTTCTTTGCCTCACATTTT-3′ | |
| IL-13a | Probe | 5′-(FAM)-CTGCCACAGTGCTGGACAACATGACCG-(TAMRA)-3′ |
| F | 5′-CAAGGATCGGAAGCTGTCAGAG-3′ | |
| R | 5′-GGCGGGCAGTTCGTCATG-3′ | |
| IL-18 | Probe | 5′-(FAM)-CCGCGCCTTCAGCAGGGATG-(TAMRA)-3′ |
| F | 5′-AGGTGAAATCTGGCAGTGGAAT-3′ | |
| R | 5′-ACCTGGACGCTGAATGCAA-3′ | |
| CTLA-4a | Probe | 5′-(FAM)-TTGTCTTCTCTGAATCGCTTTGCCCACG-(TAMRA)-3′ |
| F | 5′-CAGCATCATCATCTCAGCCATTG-3′ | |
| R | 5′-GCATTTTCACATAGACCCCAGTAG-3′ | |
| GPR83a | Probe | 5′-(FAM)-TCCGCCACCAGCCTGTTCATCGTCA-(TAMRA)-3′ |
| F | 5′-CGTCATCATCAAGAGCAAACGC-3′ | |
| R | 5′-ACAAAACGAGCCAGTGTAAAAGG-3′ | |
| IFN-γ | Probe | 5′-(FAM)-TGGCCAAGCTCCCGATGAACGA-(TAMRA)-3′ |
| F | 5′- GTGAAGAAGGTGAAAGATATCATGGA-3′ | |
| R | 5′-GCTTTGCGCTGGATTCTCA-3′ | |
| SMAD7a | Probe | 5′-(FAM)-TCCCAGTAAGCCACCACGCACCAGT-(TAMRA)-3′ |
| F | 5′-GCTCTCAGATTCTCAAGTTATTCAGG-3′ | |
| R | 5′-CCGACCCACACGCATCTTC-3′ | |
| TGFβ | Probe | 5′-(FAM)-ACCCAAAGGTTATATGGCCAACTTCTGCAT-(TAMRA)-3′ |
| F | 5′-AGGATCTGCAGTGGAAGTGGAT-3′ | |
| R | 5′-CCCCGGGTTGTGTGTTGGT-3′ |
F forward, R reverse
aDesigned during this work
Proteomics
We isolated protein from three batches of 107 CD30hi and CD30lo/− cells by differential detergent fractionation (DDF) exactly as described to produce four fractions. Each DDF fraction predominantly contains proteins from different cellular locations (which directly relate to GO cell components) [28]. For each DDF, these were analyzed by two-dimensional liquid chromatography electrospray ionization tandem mass spectrometry (2D LC ESI MS2) exactly as described [10] with one exception. Because we were searching only for the specific proteins described in Fig. 1, we first identified the predicted masses of tryptic peptides between 6 and 30 amino acids in length that could be derived from these peptides using the ExPASy PeptideCutter tool [29] and then did tandem mass spectrometry only on precursor ions with these masses (±1.5 Da, i.e., within the accuracy of the LCQ dex Xp plus mass spectrometer, ThermoElectron Corp., San Jose, CA, USA). We used decoy database searching exactly as described [10, 30] to calculate the probability that a tandem mass spectrometry match occurred by chance and, from these, the probability of the protein identification occurring by chance exactly as described [31, 32] (Table 2). We used isotope-free quantitative analysis based on spectral counting combined with the increased specificity given by including the quantitative aspects of the Sequest cross correlation (XCorr) [33].
Table 2.
Proteins and probability (P) that the protein identification could have occurred by chance (based on decoy database searching as described in M and M)
| Protein | P |
|---|---|
| gi|3087785|emb|CAA12025.1| interleukin-2 | 2.0e–67 |
| gi|27803086|emb|CAC15566.2| interleukin-6 | 3.4e–63 |
| gi|1729918|sp|P09531|TGFB1_CHICK Transforming growth factor β-1 | 7.0e–52 |
| gi|47087195|ref|NP_998736.1| interleukin 12B | 1.7e–48 |
| gi|8919963|emb|CAB96214.1| interleukin-18 | 2.0e–47 |
| gi|54792251|emb|CAF18428.1| interleukin-13 | 4.9e–43 |
| gi|50745619|ref|XP_426254.1| Predicted: similar to G protein-coupled receptor 83 | 2.8e–36 |
| gi|90968221|emb|CAJ86460.1| cytotoxic T-lymphocyte-associated protein 4 | 4.9e–23 |
| gi|51173886|emb|CAF18432.1| interleukin-10 | 4.9e–21 |
| gi|119318|sp|P08317|IL8_CHICK Interleukin-8 | 8.2e–21 |
| gi|1708496|sp|P49708|IFNG_CHICK Interferon gamma | 4.9e–13 |
| gi|54792249|emb|CAF18427.1| interleukin-4 | 4.9e–13 |
| gi|50774673|ref|XP_427238.1| Predicted: similar to SMAD7 | 7.0e–04 |
Statistical analysis
Differences are always presented from the perspective of the neoplastically transformed CD30hi cells relative to the CD30lo/− cells. We express the results for the fold-difference in mRNA and the proteomics data for each gene as the mean percentage difference and mean difference ± 95% confidence interval (CI), respectively. The mean difference was used for the proteomics data because, on some occasions, the denominator (i.e., always the CD30lo/− value) was 0. Thus, for a given gene, if CD30hi cell mRNA expression was not different from the CD30lo/− cell mRNA expression then 100% would be included in the 95% CI Similarly, for the protein data, if the CD30hi cell protein expression was not different from the CD30lo/− cell mRNA expression then 0 would be included in the 95% CI.
GO-based quantitative modeling
Our GO-based modeling was based on specific hypotheses, framed in GO biological process (GOBP) terms, defining the phenotype of CD4+ T cells and inflammation. To derive quantitative data, we combined GO-annotation with our quantitative mRNA or protein expression data using the computaional tools at AgBase [34]. We had to overcome the limitation of the GO that most literature for any species is not yet curated and so functional annotations from this literature are not yet present in the GO databases. Although the GOBP terms exist for gene products controlling T-reg development and regulation (GO:0045066, GO:0045590, GO:0045591 and GO:00455890), and there is a literature on the genes involved, the literature and GO databases are unconnected [10, 34]. Thus, we first annotated the chicken genes, using orthology to the human and mouse genes, to GO:0045066, GO:0045590, GO:0045591 and GO:00455890. To retrieve existing GO annotations, and add futher computational- and literature-based annotations, the GO annotation processes we use are decribed elsewhere [35, 36]. The computational tool GOmodeler [35], first scores the effects of each gene product, a process as either “pro” (+1), “anti” (–1), “no effect” (0) or “no data” (blank cell) (Table 3). Then the quantitative drtRT-PCR or proteomics data are used to calculate a quantitative effect for each gene (i.e., to give a quantitative value in each cell). Finally, net effects are calculated and both the mRNA (Fig. 1b) and the protein data (Fig. 1d).
Table 3.
GOmodeler table after scoring the regulatory effects of each gene product examined on Th-1, Th-2, T-reg cell differentiation and inflammation
| Gene product | Th-1 | Th-2 | T-reg | Inflammation |
|---|---|---|---|---|
| IL-2 | 1 | 1 | –1 | |
| IL-4 | –1 | 1 | 1 | |
| IL-6 | 1 | –1 | 1 | |
| IL-8 | 1 | 1 | ||
| IL-10 | –1 | 1 | 1 | 0 |
| IL-12 | 1 | –1 | ||
| IL-13 | –1 | 1 | ||
| IL-18 | 1 | 1 | 1 | 1 |
| IFNγ | 1 | –1 | 1 | 1 |
| TGFβ | –1 | 0 | 1 | –1 |
| CTLA-4 | –1 | –1 | 1 | –1 |
| GPR-83 | –1 | –1 | 1 | –1 |
| SMAD-7 | 1 | 1 | –1 | 1 |
Pro +1, Anti –1 and No effect 0. When no data is present in the published literature the cell is left blank. Annotation followed the GO Consortium guidelines (http://www.geneontology.org/GO.annotation.shtml)
The numbers of putative Meq binding sites in promoters
There is more Meq in the CD30hi, than the CD30lo/−, MD lymphoma cells [1] and Meq activates or represses gene transcription [1, 6, 37–39]. It is possible that differences in amounts of mRNA between CD30hi and CD30lo/− MD lymphoma cells may be caused by Meq directly. We identified the genomic location of each cytokine (by BLASTN searches against the chicken genome sequence) and extended each sequence by 2,500 bp 5′ of the annotated ORF start. These sequences were then searched using Alibaba2 and MatInspector (core and matrix similarity values of 1.0 and ≥0.9, respectively), for activator protein-1 (AP-1), MERE I and II sequences [39] that could potentially bind Meq, exactly as described [1].
Results
Cell sorting and differential mRNA and protein expression
The CD30hi and CD30lo/− purity was 95.7 ± 3.3 and 93.9 ± 4.9% (mean ± SEM), respectively (Fig. 1a inset). For statistical confidence that CD30hi lymphoma cells expressed a different amount of mRNA or protein compared to the CD30lo/− cells (at α = 0.05), then 100% or 0 cannot be included in the 95% CI of the mean percentage difference and mean difference for mRNA and protein, respectively. The CD30hi lymphoma cells express less IL-2 and IL-18 mRNA, but more IL-10, GPR-83 and SMAD-7 mRNA, than CD30lo/− lymphoma cells (Fig. 1a). CD30hi lymphoma cells express less IL-6 and IL-13 proteins, but more IL-2, IL-8, IL-18, TGFβ, CTLA-4 and GPR-83 proteins, than CD30lo/− lymphoma cells (Fig. 1a). Because we used the DDF method to isolate the proteins, we can identify the cellular component that the given protein was primarily isolated from [28], these are shown in the Table.
GO-based quantitative modeling
Even though there is no 100% agreement between differentially expressed mRNAs and proteins, GO-based model is pro T-reg, anti Th-1, -2 and inflammatory for both mRNA (Fig. 1b) and protein (Fig. 1d).
Meq binding sites in promoters of mRNAs examined
The numbers of putative Meq binding sites in promoters with relative rank order of mRNA expression for each cytokine. The predicted numbers of putative Meq binding AP1 and MERE I and II binding sequences are shown in Table 4.
Table 4.
Change in mRNA expression and numbers of AP1, MERE I and II in promoters of each gene
| Δ mRNA (%) | AP1 | MERE I/II | Sum | |
|---|---|---|---|---|
| IL-2 | 10 | 18 | 5 | 23 |
| IL-4 | 0 | 12 | 5 | 17 |
| IL-6 | 0 | 11 | 4 | 15 |
| IL-8 | 0 | 21 | 7 | 28 |
| IL-10 | 524 | 23 | 11 | 34 |
| IL-12 | 0 | 9 | 4 | 13 |
| IL-13 | 0 | 13 | 7 | 20 |
| IL-18 | 53 | 13 | 3 | 16 |
| CTLA-4 | 0 | 7 | 3 | 10 |
| GPR-83 | 4,154 | 8 | 5 | 13 |
| IFNγ | 0 | 18 | 4 | 22 |
| SMAD-7 | 224 | 6 | 3 | 9 |
| TGFβ | 0 | 5 | 1 | 6 |
Discussion
The suggestion that the MDV Meq protein’s presence in cells is necessary, but not alone sufficient, for lymphomagenesis, is clear from the range of genetic resistance and susceptibility of chickens to MD, i.e., whether a given MDV causes gross lymphomas depends on the chicken genotype. Furthermore, although MD resistance is mediated to some degree at the level of virus load [27, 40], we have demonstrated that MDV lymphomagenesis is a continuum and lymphomagenesis is also mediated at the level of numbers of transformed cells [4] and lesion development [41]. Here we did not examine the factors that may mediate genetic MD resistance and susceptibility. Instead we defined one aspect of the MD lymphoma immune environment and how the neoplastically transformed cells differ from the reactive cells in MD lymphomas. This is important because the outcome of any oncogenic herpesvirus infection depends not only on intracellular host virus gene interactions but also on interactions between the neoplastically transformed cells and the immune system. We show for the first time a herpesvirus transformed cell that has a phenotype most closely resembling T-reg.
Analyzing T cells by phenotype analysis alone is not trivial because, with the exception of the T and the B cell receptors, proteins are not definitive markers of cell phenotype per se. CD25, though considered a phenotypic marker of human, mouse and rat T-reg cells, is expressed by non-suppressive, activated CD4+ T cells [42]. In addition to the antigens that we used to define T cell phenotype, tumor necrosis factor receptor superfamily members (TNFRSF)-4 (a.k.a. CD134 and OX40), TNFRSF9 (a.k.a. CD137 and 4-1BB) and TNFRSF18 (a.k.a. GITR) are also suggested to be markers of T-reg phenotype and could provide additional information. However, all are non-specific as T-reg markers [43–47]. TNFRSF-4 has also recently been demonstrated to negatively regulate T-reg development [48] and enhances the numbers of tumor antigen-reactive CD4 T cells [49]. Regardless, the GO annotation for all three is very poor and thus these genes would not significantly contribute to our GO modeling. In contrast, FOXP3 is considered a “master gene required for the development and function of T-reg” [50]. We would have liked to use FOXP3 in this work but no FOXP3 ortholog has (yet) been identified in the chicken and we were unable to identify any ESTs with high-enough sequence identity in the EST databases to identify an ortholog. However, although FOXP3 is currently considered as one of the most specific markers for naturally occurring CD4(+)CD25(+) T-regulatory cells, expression of FOXP3 is a normal consequence of CD4(+) T cell activation and is not an exclusive marker of human T-reg [51, 52]. FOXP3 expression occurs after in vitro stimulation of human CD4(+)CD25(–) cells and although FOXP3 expression is strongly associated with hyporesponsiveness of activated T cells, it is not directly correlated with T-reg suppressive capabilities and in humans, expression of endogenous FOXP3 is not sufficient to induce regulatory T cell activity or to identify T-reg cells [52]. The lack of FOXP3 in the chicken was the reason that we used GPR-83 and SMAD7. GPR-83 is up-regulated in human CD4(+)CD25(+) T-regulatory cells, is directly linked to FOXP3 expression in human cells and in mouse it is “critically involved in the peripheral conversion of FOXP3-negative to FOXP3-expressing regulatory T cells in vivo” [18]. SMAD7 negatively regulates FOXP3 and thus T-reg cannot have high SMAD7 expression [19].
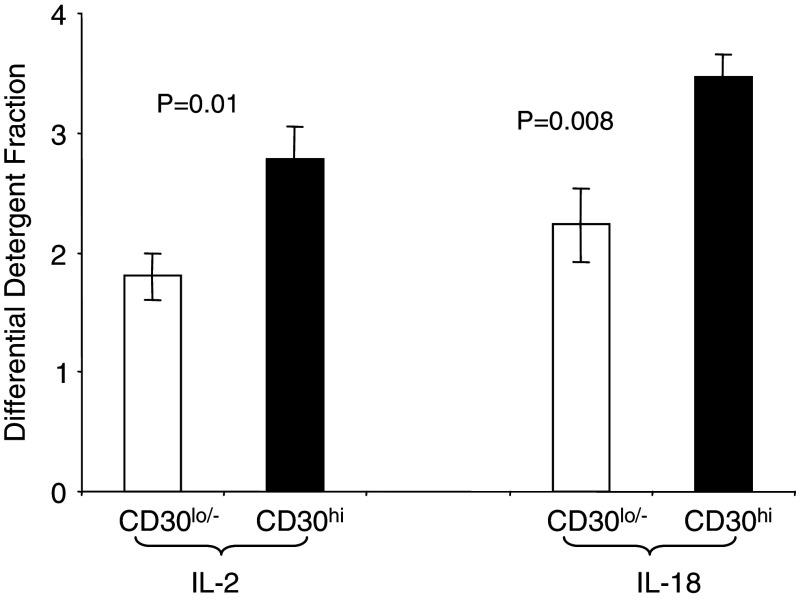
Apart from surface phenotype, T cells are described functionally and because of this, we used cytokine production profile to define T-reg in addition to cellular antigens. Generally, the cytokine profiles that are used to distinguish (the various subtypes of) T-reg cells from T effector cells are increased levels of IL-10, TGFβ and IFNγ, and decreased IL-2. Although our modeling overall suggests that the CD30hi MD lymphoma cells are more similar to a T-reg, then Th1 or Th2 cells, our data are not completely consistent with this classical T-reg phenotype at a gene-by-gene level. This is perhaps not surprising given that these CD30hi MD lymphoma cells are not physiological T-reg cells, but are virus transformed CD4+ T cells. Our protein and mRNA data also do not correlate perfectly but this is expected to some degree because there is a generally low correlation between amounts of an mRNA and amounts of protein in a cell [53, 54]. IL-2 and IL-18 are of most concern, as these have the greatest conflict and decreased IL-2 expression is one critical determinant of a T-reg phenotype. However, because each DDF fraction contains proteins predominantly from different cellular locations [28], we can quantitatively define the differences in cellular distribution of protein between the CD30lo/− and CD30hi MD lymphoma cells (Fig. 2). In the CD30hi MD lymphoma cells both IL-2 and IL-18 are preferentially distributed in the differential detergent fractions that represent the least superficial areas of the cell [28]. This suggests that IL-18 is retained in the cell in its pre-pro and/or pro-protein isoforms, which fits with the known physiology of IL-18 secretion. IL-2 may be actively or passively retained in the cell; regardless, the IL-2 DDF profile in the CD30hi MD lymphoma cells suggests that IL-2 secretion will be lower in the CD30hi MD lymphoma cells and this is consistent with a T-reg phenotype and with low IL-2 mRNA expression.
Fig. 2.
Difference in IL-2 and IL-18 protein distribution in CD30lo/− and CD30hi lymphoma cells mean (±SEM). Both IL-2 and IL-18 protein are preferentially distributed in the differential detergent fractions representing the least superficial areas of the cell and the less soluble proteins
Although we have circumstantial evidence suggesting that the MD lymphoma environment is antagonistic to T cell mediated immunity [1, 4, 9, 41], after cytokine expression the next logical functional evidence to define a T-reg-like phenotype would be to demonstrate T-reg-associated suppressor activity in an in vitro assay [e.g. contact-dependent CD30hi MD lymphoma cell inhibition of proliferation and cytokine secretion induced by TCR cross-linking of CD4(+)CD25(–) responder T cells]. However, such experiments are challenging in MD. The CD30hi MD lymphoma cells are absolutely dependent on the lymphoma environment for their survival [4]. Even when MD cell lines can be produced from lymphomas, such cell lines are highly in vitro-adapted and may not be good functional models of the MD lymphoma cells in vivo [4, 9]. Finally, there is persistent MDV reactivation with concomitant cell death, in both ex vivo CD30hi MD lymphoma cells and MD cell lines.
Virus survival critically depends on immune evasion and virus activation of T-reg is a known immune evasion strategy, e.g. herpes simplex virus [55] and the tumorigenic Epstein-Barr [56], hepatitis C [57] and murine leukemia [58] viruses. Tumor growth also depends on immune evasion. The T-reg-like phenotype of the CD30hi MD cells could help to explain the lack of CD8+ T cells in developing MD lesions in susceptible, compared with resistant, chicken genotypes [41]. In support, the T-reg-like response induced by persistent infection of mice by Friend retrovirus causes the loss of tumor transplant rejection ability [59].
Anti-tumor immunity not targeting virus- or mutated host-antigens is fundamentally “autoimmunity”. T-reg are essential for controlling autoimmunity [60, 61] and CD30 signaling very early in disease pathogenesis is critical to T-reg function [23]. Such CD30 signaling is likely in MD [4]. T-reg requires activation with specific antigens to develop suppressive activity but subsequent immune suppression is antigen- and MHC-independent [62]. This property is consistent with a hypothesized mechanism of CD4+: CD4+ T cell co-antigen presentation via MHC class II in MD lymphomas [4].
MDV appears to be similar to other transforming viruses that have usurped T-reg to perturb tumor immunity. Meq is a transcriptional regulator of the CD30 gene [1], and meq transcriptionally regulates the expression of many other mRNAs [37]. Because the CD30hi lymphoma cells have the most meq protein [1], we speculated that the differences in cytokine mRNA expression may be due to greater numbers of meq binding sequences in the promoters of these genes. However, there was a low correlation coefficient (–0.11) between the numbers of predicted AP1, MERE I and II binding sites, and the difference in mRNA expression. Despite a poor overall correlation, T cell lymphomas generally have AP-1 activation [63, 64] and one of the target genes is IL-2 and IL-2 is a critical T cell proliferation regulator [65–67]. Notably our ex vivo data do agree with previous in vitro data that Meq (presumably homodimers) represses IL-2 [38]. TGFβ, which has the lowest number of predicted AP1, MERE I and II binding sites, is particularly interesting because previous in vitro work has demonstrated that Meq increases TGFβ expression [37]. Though we would have predicted differential expression of TGFβ mRNA, we identified higher expression by the CD30hi cells at the protein level only. Regardless, and although the mechanistic details need further experimental clarification, our work suggests that the MD herpesvirus may play a direct role in maintaining a T-reg-like phenotype via the meq protein.
Acknowledgments
This work was supported by a USDA NRI 2004-35204-14829. We acknowledge Tibor Pechan for running the mass spectrometer and the extremely valuable input of two anonymous reviewers.
Footnotes
L. A. Shack and J. J. Buza equally contributed to this work.
References
- 1.Burgess SC, Young JR, Baaten BJG, Hunt L, Ross LNJ, Parcells MS, Kumar PM, Lee LF, Davison TF. Marek’s disease is a natural model for lymphomas over-expressing Hodgkin’s disease antigen (CD30) Proc Natl Acad Sci USA. 2004;101:13879–13884. doi: 10.1073/pnas.0305789101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heine B, Hummel M, Demel G, Stein H. Hodgkin and Reed-Sternberg cells of classical Hodgkin’s disease overexpress the telomerase RNA template (hTR) J Pathol. 1999;188:139–145. doi: 10.1002/(SICI)1096-9896(199906)188:2<139::AID-PATH344>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Maggio E, van den Berg A, Diepstra A, Kluiver J, Visser L, Poppema S. Chemokines, cytokines and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Oncol. 2002;1(Suppl 13):52–56. doi: 10.1093/annonc/13.s1.52. [DOI] [PubMed] [Google Scholar]
- 4.Burgess SC, Davison TF. Identification of the neoplastically transformed cells in Marek’s disease herpesvirus-induced lymphomas: recognition by the monoclonal antibody AV37. J Virol. 2002;76:7276–7292. doi: 10.1128/JVI.76.14.7276-7292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan RW, Xie Q, Cantello JL, Miles AM, Bernberg EL, Kent J, Anderson A. Marek’s disease virus latency. Curr Top Microbiol Immunol. 2001;255:223–243. [PubMed] [Google Scholar]
- 6.Kung HJ, Nair V (2004) Marek’s disease virus oncogenecity: molecular mechanisms. In: Davison TF, Venugopal K (eds) Marek’ s disease: an evolving problem. Academic Press, London
- 7.Osterrieder N, Kamil JP, Schumacher D, Tischer BK, Trapp S. Marek’s disease virus: from miasma to model. Nat Rev Microbiol. 2006;4:283–294. doi: 10.1038/nrmicro1382. [DOI] [PubMed] [Google Scholar]
- 8.Trapp S, Parcells MS, Kamil JP, Schumacher D, Tischer BK, Kumar PM, Nair VK, Osterrieder N. A virus-encoded telomerase RNA promotes malignant T cell lymphomagenesis. J Exp Med. 2006;203:1307–1317. doi: 10.1084/jem.20052240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess SC, Venugopal KN. ChapterVII: anti-tumor immune responses after infection with the Marek’s disease and avian leukosis oncogenic viruses of poultry. In: Mathew T, editor. Advances in medical and veterinary virology, immunology and epidemiology. Modern concepts of immunology in veterinary medicine: poultry immunology. West Orange: Thajema; 2002. pp. 236–291. [Google Scholar]
- 10.Buza JJ, Burgess SC. Modeling the proteome of a Marek’s disease transformed cell line: a natural animal model for CD30 over-expressing lymphomas. Proteomics. 2007;7:1316–1326. doi: 10.1002/pmic.200600946. [DOI] [PubMed] [Google Scholar]
- 11.O’Garra A, Arai N. The molecular basis of T helper 1 and T helper 2 cell differentiation. Trends Cell Biol. 2000;10:542–550. doi: 10.1016/S0962-8924(00)01856-0. [DOI] [PubMed] [Google Scholar]
- 12.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65:2457–2464. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 13.Uraushihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, Nakamura T, Watanabe M. Regulation of murine inflammatory bowel disease by CD25+ and CD25– CD4+ glucocorticoid-induced TNF receptor family-related gene + regulatory T cells. J Immunol. 2003;171:708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- 14.Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896–904. doi: 10.4049/jimmunol.177.2.896. [DOI] [PubMed] [Google Scholar]
- 15.Kabelitz D, Wesch D, Oberg HH. Regulation of regulatory T cells: role of dendritic cells and toll-like receptors. Crit Rev Immunol. 2006;26:291–306. doi: 10.1615/critrevimmunol.v26.i4.10. [DOI] [PubMed] [Google Scholar]
- 16.Liu H, Leung BP. CD4 + CD25+ regulatory T cells in health and disease. Clin Exp Pharmacol Physiol. 2006;33:519–524. doi: 10.1111/j.1440-1681.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfoertner S, Jeron A, Probst-Kepper M, Guzman CA, Hansen W, Westendorf AM, Toepfer T, Schrader AJ, Franzke A, Buer J, Geffers R. Signatures of human regulatory T cells: an encounter with old friends and new players. Genome Biol. 2006;7:R54. doi: 10.1186/gb-2006-7-7-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansen W, Loser K, Westendorf AM, Bruder D, Pfoertner S, Siewert C, Huehn J, Beissert S, Buer J. G protein-coupled receptor 83 overexpression in naive CD4 + CD25– T cells leads to the induction of Foxp3+ regulatory T cells in vivo. J Immunol. 2006;177:209–215. doi: 10.4049/jimmunol.177.1.209. [DOI] [PubMed] [Google Scholar]
- 19.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4 + CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 20.Mizobuchi T, Yasufuku K, Zheng Y, Haque MA, Heidler KM, Woods K, Smith GN, ummings OWC, Jr, Fujisawa T, Blum JS, Wilkes DS. Differential expression of Smad7 transcripts identifies the CD4 + CD45RC high regulatory T cells that mediate type V collagen-induced tolerance to lung allografts. J Immunol. 2003;171:1140–1147. doi: 10.4049/jimmunol.171.3.1140. [DOI] [PubMed] [Google Scholar]
- 21.Dai Z, Li Q, Wang Y, Gao G, Diggs LS, Tellides G, Lakkis FG. CD4 + CD25+ regulatory T cells suppress allograft rejection mediated by memory CD8+ T cells via a CD30-dependent mechanism. J Clin Invest. 2004;113:310–317. doi: 10.1172/JCI19727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Kleer IM, Kamphuis SM, Rijkers GT, Scholtens L, Gordon G, De Jager W, Hafner R, van de Zee R, van Eden W, Kuis W, Prakken BJ. The spontaneous remission of juvenile idiopathic arthritis is characterized by CD30+ T cells directed to human heat-shock protein 60 capable of producing the regulatory cytokine interleukin-10. Arthritis Rheum. 2003;48:2001–2010. doi: 10.1002/art.11174. [DOI] [PubMed] [Google Scholar]
- 23.Zeiser Early CD30 signaling is critical for adoptively transferred CD4 + CD25+ regulatory T cells in prevention of acute graft-versus-host disease. Blood. 2007;109:2225–2233. doi: 10.1182/blood-2006-07-038455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burgess SC, Davison TF. A quantitative duplex PCR technique for measuring amounts of cell-associated Marek’s disease virus: differences in two populations of lymphoma cells. J Virol Methods. 1999;82:27–37. doi: 10.1016/S0166-0934(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 25.Levy AM, Burgess SC, Davidson I, Underwood G, Leitner G, Heller ED. Interferon-containing supernatants increase Marek’s disease herpesvirus genomes and gene transcription levels, but not virion replication in vitro. Viral Immunol. 2003;16:501–509. doi: 10.1089/088282403771926328. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser MG, Cheeseman JH, Kaiser P, Lamont SJ. Cytokine expression in chicken peripheral blood mononuclear cells after in vitro exposure to Salmonella enterica serovar enteritidis. Poult Sci. 2006;85:1907–1911. doi: 10.1093/ps/85.11.1907. [DOI] [PubMed] [Google Scholar]
- 27.Kaiser P, Underwood G, Davison F. Differential cytokine responses following Marek’s disease virus infection of chickens differing in resistance to Marek’s disease. J Virol. 2003;77:762–768. doi: 10.1128/JVI.77.1.762-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCarthy FM, Burgess SC, van den Berg BHJ, Koter MD, Pharr GT. Differential detergent fractionation for non-electrophoretic eukaryote cell proteomics. J Proteome Res. 2005;4:316–324. doi: 10.1021/pr049842d. [DOI] [PubMed] [Google Scholar]
- 29.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005) Protein identification and analysis tools on the ExPASy server. In: Walker JM (eds) The proteomics protocols handbook. Humana Press, Totowa
- 30.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 31.MacCoss MJ, Wu CC, Yates JR. Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal Chem. 2002;74:5593–5599. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- 32.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 33.Nanduri B, Lawrence ML, Vanguri S, Burgess SC. Proteomic analysis using an unfinished bacterial genome: the effects of sub-minimum inhibitory concentrations of antibiotics on Mannheimia haemolytica virulence factor expression. Proteomics. 2005;5:4852–4863. doi: 10.1002/pmic.200500112. [DOI] [PubMed] [Google Scholar]
- 34.McCarthy FM, Wang N, Magee GB, Nanduri B, Lawrence ML, Camon EB, Barrell DG, Hill DP, Dolan ME, Williams WP, Luthe DS, Bridges SM, Burgess SC. AgBase: a functional genomics resource for agriculture. BMC Genomics. 2006;7:229. doi: 10.1186/1471-2164-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarthy FM, Bridges SM, Burgess SC. Going from functional genomics to biological significance. Cytogenet Genome Res. 2007;117((1–4)):278–287. doi: 10.1159/000103189. [DOI] [PubMed] [Google Scholar]
- 36.McCarthy FM, Bridges SM, Wang N, Magee GB, Williams WP, Luthe DS, Burgess SC (2007) AgBase: a unified resource for functional genomics analysis in agriculture. Nucleic Acids Res. doi:10.1093/nar/gkl936 [DOI] [PMC free article] [PubMed]
- 37.Levy AM, Gilad O, Xia L, Izumiya Y, Choi J, Tsalenko A, Yakhini Z, Witter R, Lee L, Cardona CJ, Kung HJ. Marek’s disease virus Meq transforms chicken cells via the v-Jun transcriptional cascade: a converging transforming pathway for avian oncoviruses. Proc Natl Acad Sci USA. 2005;102:14831–14836. doi: 10.1073/pnas.0506849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levy AM, Izumiya Y, Brunovskis P, Xia L, Parcells MS, Reddy SM, Lee L, Chen HW, Kung HJ. Characterization of the chromosomal binding sites and dimerization partners of the viral oncoprotein Meq in Marek’s disease virus-transformed T cells. J Virol. 2003;77:12841–12851. doi: 10.1128/JVI.77.23.12841-12851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu JL, Kung HJ. Marek’s disease herpesvirus transforming protein MEQ: a c-Jun analogue with an alternative life style. Virus Genes. 2000;21:51–64. doi: 10.1023/A:1008132313289. [DOI] [PubMed] [Google Scholar]
- 40.Bumstead N, Sillibourne J, Rennie M, Ross N, Davison F. Quantification of Marek’s disease virus in chicken lymphocytes using the polymerase chain reaction with fluorescence detection. J Virol Methods. 1997;65:75–81. doi: 10.1016/S0166-0934(96)02172-6. [DOI] [PubMed] [Google Scholar]
- 41.Burgess SC, Basaran BH, Davison TF. Resistance to Marek’s disease herpesvirus-induced lymphoma is multiphasic and dependent on host genotype. Vet Pathol. 2001;38:129–142. doi: 10.1354/vp.38-2-129. [DOI] [PubMed] [Google Scholar]
- 42.Valitutti S, Muller S, Dessing M, Lanzavecchia A. Different responses are elicited in cytotoxic T lymphocytes by different levels of T cell receptor occupancy. J Exp Med. 1996;183:1917–1921. doi: 10.1084/jem.183.4.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Croft M. Co-stimulatory members of the TNFR family: keys to effective T cell immunity. Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 44.Kanamaru F, Youngnak P, Hashiguchi M, Nishioka T, Takahashi T, Sakaguchi S, Ishikawa I, Azuma M. Costimulation via glucocorticoid-induced TNF receptor in both conventional and CD25+ regulatory CD4+ T cells. J Immunol. 2004;172:7306–7314. doi: 10.4049/jimmunol.172.12.7306. [DOI] [PubMed] [Google Scholar]
- 45.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. doi: 10.1016/S1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 46.Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, Ayroldi E, Riccardi C. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- 47.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22:621–631. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 48.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25 + Foxp3+ T cells. J Immunol. 2007;179:1427–1430. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 49.Song A, Song J, Tang X, Croft M. Cooperation between CD4 and CD8 T cells for anti-tumor activity is enhanced by OX40 signals. Eur J Immunol. 2007;37:1224–1232. doi: 10.1002/eji.200636957. [DOI] [PubMed] [Google Scholar]
- 50.Long E, Wood KJ. Understanding FOXP3: progress towards achieving transplantation tolerance. Transplantation. 2007;84:459–461. doi: 10.1097/01.tp.0000275424.52998.ad. [DOI] [PubMed] [Google Scholar]
- 51.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 52.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–38. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 53.Beynon RJ. The dynamics of the proteome: strategies for measuring protein turnover on a proteome-wide scale. Brief Funct Genomic Proteomic. 2005;3:382–390. doi: 10.1093/bfgp/3.4.382. [DOI] [PubMed] [Google Scholar]
- 54.Goodlet D. Correlation of mRNA and protein expression. In: Lorkowski S, Cullen P, editors. Analysing gene expression: a handbook of methods: possibilities and pitfalls. Weinheim: Wiley-VCH; 2003. pp. 58–63. [Google Scholar]
- 55.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4 + CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall NA, Vickers MA, Barker RN. Regulatory T cells secreting IL-10 dominate the immune response to EBV latent membrane protein 1. J Immunol. 2003;170:6183–6189. doi: 10.4049/jimmunol.170.12.6183. [DOI] [PubMed] [Google Scholar]
- 57.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, Drapeau CM, Rocchi G, Bergamini A. Increased hepatitis C virus (HCV)-specific CD4 + CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–196. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beilharz MW, Sammels LM, Paun A, Shaw K, van Eeden P, Watson MW, Ashdown ML. Timed ablation of regulatory CD4+ T cells can prevent murine AIDS progression. J Immunol. 2004;172:4917–4925. doi: 10.4049/jimmunol.172.8.4917. [DOI] [PubMed] [Google Scholar]
- 59.Iwashiro M, Messer RJ, Peterson KE, Stromnes IM, Sugie T, Hasenkrug KJ. Immunosuppression by CD4+ regulatory T cells induced by chronic retroviral infection. Proc Natl Acad Sci USA. 2001;98:9226–9230. doi: 10.1073/pnas.151174198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 61.von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat Rev Immunol. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- 62.Thornton AM, Shevach EM. Suppressor effector function of CD4 + CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 63.Iwai K, Mori N, Oie M, Yamamoto N, Fujii M. Human T cell leukemia virus type 1 tax protein activates transcription through AP-1 site by inducing DNA binding activity in T cells. Virology. 2001;279:38–46. doi: 10.1006/viro.2000.0669. [DOI] [PubMed] [Google Scholar]
- 64.Mori N, Fujii M, Iwai K, Ikeda S, Yamasaki Y, Hata T, Yamada Y, Tanaka Y, Tomonaga M, Yamamoto N. Constitutive activation of transcription factor AP-1 in primary adult T cell leukemia cells. Blood. 2000;95:3915–3921. [PubMed] [Google Scholar]
- 65.Cantrell DA, Smith KA. The interleukin-2 T cell system: a new cell growth model. Science. 1984;224:1312–1316. doi: 10.1126/science.6427923. [DOI] [PubMed] [Google Scholar]
- 66.Merlo JJ, Tsygankov AY. Herpesvirus saimiri oncoproteins Tip and StpC synergistically stimulate NF-κB activity and interleukin-2 gene expression. Virology. 2001;279:325–338. doi: 10.1006/viro.2000.0714. [DOI] [PubMed] [Google Scholar]
- 67.Yamada Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J. 1987;6:2705–2709. doi: 10.1002/j.1460-2075.1987.tb02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]