Abstract
Recent reports revealed that dendritic cell (DC)–natural killer (NK) cell interaction plays an important role in tumor immunity, but few DC vaccine studies have attempted to evaluate the non-specific, yet potentially clinically relevant, NK response to immunization. In this study, we first analyzed in vitro activation of NK cells by DCs similar to those used in clinical trials. Subsequently, NK cell responses were analyzed in a phase I clinical trial of a vaccine consisting of autologous DCs loaded with a fowlpox vector encoding CEA. The data were compared with the clinical outcome of the patients. DC enhances NK activity in vitro, partly by sustaining NK cell survival and by enhancing the expression of NK-activating receptors, including NKp46 and NKG2D. Among nine patients in our clinical trial, NK cytolytic activity increased in four (range 2.5–5 times greater lytic activity) including three who had increased NK cell frequency, was stable in two and decreased in three. NKp46 and NKG2D expression showed a good correlation with the patients’ NK activity. When patients were grouped by clinical activity (stable disease/no evidence of disease (stable/NE, n=5) vs progressive disease (N=4) at 3 months), the majority in the stable/NE group had increases in NK activity (P=0.016). Anti-CEA T cell response was enhanced in all the nine patients analyzed, but was not significantly different between the two groups (P=0.14). Thus, NK responses following DC vaccination may correlate more closely with clinical outcome than do T cell responses. Monitoring of NK response during vaccine studies should be routinely performed.
Keywords: Natural killer cell, Dendritic cell, Vaccine, Clinical trial
Introduction
Most cancer vaccines in development are intended to function by activating tumor antigen-specific T cells. Therefore, the goal of animal and human studies with these vaccines has been to demonstrate the induction of CD8+ and sometimes CD4+ antigen-specific T cells. Because dendritic cells (DCs) are the most important cells for processing and presenting antigen to T cells, a promising strategy for immunotherapy has been to utilize vaccines based on DC loaded with tumor antigen. Recently, we completed a study in which patients with advanced carcinoembryonic antigen (CEA) expressing malignancies were immunized with autologous, monocyte-derived DC modified to express the tumor antigen CEA and a triad of costimulatory molecules by infection with the fowlpox vector rF-CEA(6D)-TRICOM [1]. As expected, we found that the number of CEA-specific CD8+ and CD4+ T cells at the height of the immune response tended to correlate with the ability to maintain tumor stability. Nonetheless, there were patients with clinical stability and fairly low levels of CEA-specific T cell response. This suggested that there may be other mechanisms for immune response to the tumor, such as natural killer (NK) mediated tumor destruction.
Natural killer cells, members of the innate immune system, are important for the immunological control of infection and tumors due to their capacity for cellular cytotoxicity and production of cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor (TNF) [2]. Unstimulated NK cells are generally thought of as resting, but strong functional activity is induced in vivo by exposure to pathogen-produced molecules such as double-stranded RNA or CpG oligonucleotides, as well as tumors [3]. There is increasing evidence that activation of NK cells in vivo may be due to interactions with DCs [4–8]. In particular, co-culture of NK cells with DC enhances NK-mediated cytolytic activity and IFN-γ production.
Dendritic cell–NK cell interactions appear to be important for tumor rejection. For example, injected DCs substantially augment NK cell-mediated protection from challenge with tumor cells [4, 9]. In glioma models, Parajuli [10] demonstrated that both CTLs and NK-like T cells (CD3(+)CD56(+)) are expanded and stimulated by mature, tumor-pulsed DCs. NK cells also reciprocally provide activating stimuli to DCs. NK cells can cause immature DCs to undergo maturation with the result that they produce interleukin 12 [6, 7] and upregulate costimulatory molecules such as CD86. Furthermore, T cell responses are further influenced by IFN-γ produced by NK cells, which promotes antigen processing and presentation to T cells and T helper type 1 cell polarization [8]. Finally and surprisingly, control of DC, especially immature DC, may also be achieved by NK cell-mediated lysis of DCs [11, 12].
We wished to determine if NK cells were responsive to DC-mediated activation and whether this activation was more potent with DCs modified to express high levels of costimulatory molecules. Based on this data, we then evaluated whether NK cells might be responsive to vaccinations using DCs modified with fowlpox vectors encoding CEA and a triad of costimulatory molecules in our phase I clinical trial. Therefore, utilizing specimens available from the phase I clinical trial, we evaluated NK cell number, phenotype and function in the peripheral blood before and after immunization with the DC vaccine. We correlated the in vitro results with clinical outcomes and observed that there was an association between NK activity and clinical outcome in this phase I study. Based on these results, we conclude that DCs can sustain NK cell survival and upregulate NK activation receptors. By these mechanisms, DC-based vaccination seems to enhance innate immunity, and benefits to improve anti-tumor immune response.
Materials and methods
Patients and protocols
Participants were provided signed informed consent approved by the Duke University Medical Center Institutional Review Board before enrollment. This clinical trial was performed under FDA approved Investigational New Drug Exemption (IND). The participants were required to have a metastatic cancer expressing CEA as defined by immunohistochemical analysis or elevated CEA in peripheral blood. Detailed inclusion criteria were described in our previous report [1]. Of the 14 patients enrolled in the trial, blood samples from nine were available for this study. Five of them had colorectal cancer, three had lung cancer and the other had urachal adenocarcinoma. Most of these patients had failed at least two prior chemotherapeutic regimens. Vaccinations of the patients were done by the following method. Seven day cultured monocyte-derived DCs were infected with the recombinant fowlpox virus (rF-CEA(6D)-TRICOM) containing genes for human CEA and the three costimulatory molecules B7.1, ICAM-1 and LFA-3 at moi5 for at least 30 min before administration. rF-CEA(6D)-TRICOM was manufactured by Therion Biologics Corporation (Cambridge, MA) and supplied by the Cancer Treatment Evaluation Program (NCI). Patients received one or two cycles of the DC vaccines. A cycle consisted of the leukapheresis, generation of DCs, loading the DCs with antigen and administration of the fresh DCs followed by three, triweekly immunizations with previously cryopreserved DC. Those who had progressed on imaging studies did not receive the second cycle of vaccinations. Clinical activity was assessed by applying the RECIST criteria to CT or MRI scans obtained before and after each cycle of immunization.
Flow cytometric analysis
PBMCs harvested from leukapheresis products were kept frozen in 10% DMSO+90% autologous plasma until use. The cells were analyzed on a FACSCaliber using the CellQuest software (Becton Dickinson, San Diego, CA). The monoclonal antibodies used were: APC-labeled CD56, FITC-labeled CD3, PerCP-labeled CD45 and the following PE-labeled antibodies: CD69, CD244 (2B4), NKG2A, NKG2C, NKG2D (PharMingen, San Diego, CA), NKp30, NKp44, NKp46 (Immunotech, Marseille, France). CD45+ cells were first gated by histograms and then lymphocytes were gated from these cells using forward scatter/side scatter dot plots. CD3-CD56+ NK cells were analyzed for their expression of those molecules mentioned above. The percentages of CD3-CD56+ NK cell and CD3+CD56- T cell were also analyzed.
51Cr release assay
Frozen stocked PBMCs were thawed and incubated overnight in RPMI1640 medium+10% human AB serum with or without IL-2 (1,000 IU/ml). In some cases, NK cells were isolated using NK cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions and were used as effector cells. The purity of CD3-CD56+ NK cell after magnetic beads isolation procedure was always more than 90%. K562 cells and Raji cells were labeled with 51Cr and used as target cells. Three to five thousand target cells were put into each well of 96-well U-bottomed plates and effector cells were added to make the E:T ratio appropriate (125:1, 25:1, 5:1, 1:1 for PBMCs, 10:1, 2:1 for NK cell). The cells were incubated for 4 h at 37°C, and 51Cr release was analyzed by TRILUX gamma counter (Wallac). More than 10% increase or decrease in cytotoxicity at 25:1 E:T ratio was evaluated as a significant change.
IFN-γ ELISPOT assay
Interferon-γ ELISPOT assay was performed using anti-human IFN-γ Mab (diaPharma Group, Inc., West Chester, OH) as reported previously [1]. Briefly, PBMCs were plated to 96-well plates in the presence of rF-CEA(6D)-TRICOM (moi 10) or rF-wild type (moi 10) as a control for any anti-fowlpox responses. The plates were incubated for 18–20 h at 37°C, 5% CO2, followed by incubation with anti-IFN-γ biotinylated Mab (diaPharma Group, Inc.) for 2 h and Vectastain ABC Peroxidase (Vector Labs, Inc., Burlingame, CA) for 1 h. Color was developed using 3-amino-9-ethyl-carbazole [AEC] (Sigma, St. Louis, MO). Spot numbers were estimated using the KS ELISPOT Automated Reader System with the KS ELISPOT 4.2 Software (Carl Zeiss, Inc., Thornwood, NY).
Cytokine analysis
The concentrations of IL-2, IL-4, IL-10, TNF-α and IFN-γ in the culture supernatant were measured with the Cytometric Bead Array (CBA) kit (BD Pharmingen), according to the manufacturer’s instructions, and analyzed on a FACSCaliber flow cytometer using BD CBA software (BD BioScience, San Diego, CA). The Interleukin-12 (IL-12) p70 concentration was measured by the enzyme-linked immunosorbant assay (Mabtech, Nacka, Sweden) according to the manufacturer’s instructions.
Effect of DCs on NK activity in vitro
The DCs were cultured from flask adherent cells as described above. After 7 days culture with GM-CSF and IL-4, cells were harvested from the flask. PBLs were prepared from PBMCs using the flask adherent technique, that is, eliminating flask adherent cells after 2 h of incubation at 37°C. CD56 MicroBeads (Miltenyi Biotec) was used to deplete NK cells from the PBLs and the DC population. One half of the DCs were infected with rF-CEA(6D)-TRICOM at moi 5 for 1 h, and further incubated overnight. To examine the effect of DCs on NK cell-activation status and NK killing activity, 5×106 of autologous PBLs or NK cell-depleted PBLs were cultured alone or with 5×105 of mock-infected DCs or rF-CEA(6D)-TRICOM-infected DCs at the ratio of 10:1 in 25-cm2 flask. After 3 or 7 days culture, the cells were harvested from the flasks and analyzed by flow cytometry. NK killing activity was analyzed using 7-day cultured cells by the standard 4 h 51Cr release assay.
Statistical analysis
Wilcoxon rank sum was used to compare the NK activities between patients with stable disease or minor response and those with progressive disease. Student’s t-test was used to compare expression of NK cell receptors between pre- and post-vaccination.
Results
NK cell activation by DC in vitro
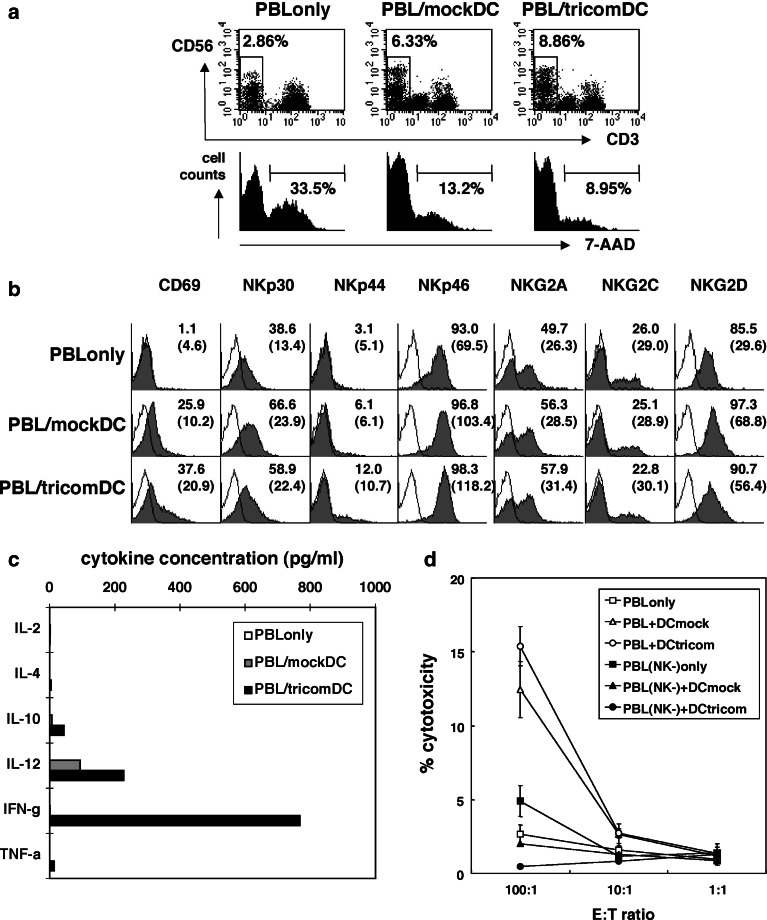
We wished to determine if NK cells and their functional activity were modified by the DC platform used in our clinical vaccination study. Therefore, we cultured PBL with DC alone (mockDC) or DC modified with a recombinant fowlpox encoding a triad of costimulatory molecules (tricomDC). We demonstrated that after 3 days of stimulation, the percentage of CD56+CD3- NK cells was greater when PBL were exposed to DC, and was preserved to a greater degree with tricomDC (Fig. 1a). As shown in the histograms, 33.5% of the NK cells in the PBL culture alone were dead (7-AAD positive), whereas lesser percentages of NK cells were dead in the co-culture with DCs (PBL/mock DC, 13.2%; PBL/tricomDC, 8.95%). Thus, DCs in the culture helped the survival of NK cells in the culture condition without IL-2.
Fig. 1.
NK cell activation by DC–NK cell interaction in vitro. DCs were cultured from flask adherent cells in AIM V media containing rh-GM-CSF and rh-IL-4 for 7 days. CD56 MicroBeads (Miltenyi Biotec) was used to deplete NK cells from PBLs and DC population. One half of the DCs were infected with rF-CEA(6D)-TRICOM at moi 5 and incubated overnight. Then, PBLs or NK-depleted PBLs were incubated alone or with mock-infected (mockDC) or rF-CEA(6D)-TRICOM-infected DCs (tricomDC). a, b After a 3-day culture, the cells were harvested and analyzed for NK cell percentages, NK cell viability (a) and receptor expression on NK cells (b). Cells were stained with anti-CD3-FITC, anti-CD56-APC, 7-AAD and PE-labeled antibodies for the following antigens: CD69, CD161, CD244 (2B4), NKp30, NKp44, NKp46, NKG2A, NKG2C, and NKG2D. a Percentages of CD56+CD3- NK cells are shown in each dot plot. In each histogram, percentages of dead cells (7-AAD positive) in total NK cells are shown. b Percentages of receptor positive cells are shown in each histogram and mean fluorescence intensities are shown in parentheses. c, d After a1-week culture, the cells were harvested for the following assays. Representative data of two experiments with similar results are shown. c Culture supernatants were collected and their cytokine concentrations were analyzed by Cytometric Beads Array assay and IL-12 ELISA. d Cells were used as effector cells in standard 4 h 51Cr release assay. K562 cells were used as target cells. Open symbols PBLs cultured alone or with DCs. Filled symbols NK-depleted PBLs cultured alone or with DCs. Square symbols PBLs cultured without DC addition. Triangle symbols PBLs cultured with mock-infected DCs (mockDC). Circle symbols PBLs cultured with fp-TRICOM-infected DCs (tricomDC)
These NK cells cultured with DCs demonstrated increased activation as determined by an increased expression of CD69 (Fig. 1b). Furthermore, there were subtle differences in NK receptor phenotype with a slightly greater expression of NKp46 and NKG2D, both activating receptors (Fig. 1b). The NK cell cultures exposed to DCs generated substantially more IL-12, but only the cultures with DC modified to hyperexpress the costimulatory molecules (tricomDC) produced IFN-γ (Fig. 1c). The cytolytic activity of these cultures was greater when exposed to DC with a trend for greater cytolytic activity for cultures stimulated with tricomDC (Fig. 1d). Removing the NK cells from the culture before starting co-culture abolished the cytolytic activity. These data taken together demonstrate that DCs, particularly those hyperexpressing costimulatory molecules as used in our clinical trial, activate NK cells.
NK cell frequency in the peripheral blood of patients
We wished to analyze the NK cell frequency, phenotype and function in our DC vaccine clinical trial. Among nine patients analyzed, four patients received two cycles of vaccination. CD3-CD56+ NK cell frequency was analyzed by flow cytometric analysis (Table 1). NK cell frequency increased by more than 5% in the lymphocytes of four patients (TRI-07, -21, -22, -38) and slightly in one (TRI-37) after vaccinations. Likewise, two patients (TRI-26 and TRI-40) had stable (changes less than 1%) NK cell proportion and the two others (TRI-06 and TRI-32) showed a slight decrease. Absolute numbers of NK cells in the peripheral blood were calculated based on the NK cell frequency in the lymphocytes analyzed by flow cytometry and the absolute lymphocyte numbers obtained by the automated cell counter, and are shown in Table 1. In five patients (TRI-07, -21, -22, -37, -38), NK cell number increased during vaccination, two (TRI-26 and TRI-40) had no significant change and two others (TRI-06 and TRI-32) showed a decrease. Changes in the absolute NK cell number showed a similar trend to the changes in NK cell frequencies in the PBMCs.
Table 1.
White blood cell counts and NK cell numbers in patients
| Pt-number | Time (before/after vaccination) | WBC (×103/ml) | Lymphocyte (×103/ml) | NK cell in lymphocyte (%) | NK cell (×103/ml) |
|---|---|---|---|---|---|
| TRI-06 | Before | 6,900 | 1,380 | 8.81 | 122 |
| After first | 6,700 | 1,005 | 7.62 | 77 | |
| TRI-07 | Before | 4,600 | 860 | 32.11 | 276 |
| After first | 6,700 | 864 | 39.99 | 346 | |
| TRI-21 | Before | 4,000 | 688 | 12.46 | 86 |
| After first | 6,000 | 978 | 15.00 | 147 | |
| After second | 5,200 | 842 | 18.36 | 155 | |
| TRI-22 | Before | 4,100 | 1,415 | 10.20 | 144 |
| After first | 4,600 | 1,306 | 6.17 | 81 | |
| After second | 4,800 | 955 | 19.84 | 190 | |
| TRI-26 | Before | 3,900 | 901 | 19.91 | 179 |
| After first | 4,100 | 951 | 12.55 | 119 | |
| After second | 4,800 | 806 | 20.89 | 168 | |
| TRI-32 | Before | 6,300 | 1,273 | 9.90 | 126 |
| After first | 7,700 | 924 | 8.45 | 78 | |
| TRI-37 | Before | 6,200 | 1,364 | 10.31 | 141 |
| After first | 6,000 | 1,848 | 11.87 | 219 | |
| TRI-38 | Before | 8,700 | 2,862 | 5.57 | 159 |
| After first | 5,900 | 1,929 | 10.85 | 209 | |
| TRI-40 | Before | 3,900 | 1,162 | 13.91 | 162 |
| After first | 3,900 | 1,127 | 13.25 | 149 | |
| After second | 3,900 | 1,104 | 14.75 | 163 |
NK cell percentages in lymphocytes were analyzed using flow cytometry by gating CD3-CD56+ NK cell population in CD45+ FSClow SSClow lymphocytes population. Absolute NK cell numbers were calculated based on lymphocytes number in peripheral blood and NK cell percentages in the lymphocyte population
NK cytolytic activity of patients treated with rF-CEA(6D)-TRICOM-infected DCs
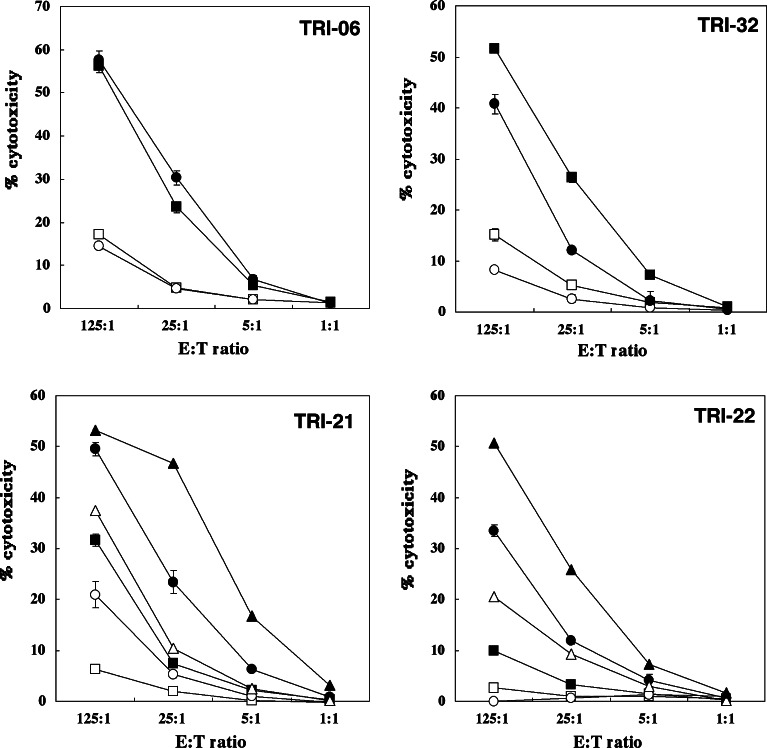
Among the five patients who finished one cycle of vaccination, three patients (TRI-07, -32, -37) showed decreased NK activity after treatment, while one patient (TRI-06) showed no change and the other (TRI-38) showed a moderate increase (Fig. 2, Table 2). Among the four patients who finished two cycles, three (TRI-21, -22, -40) showed a gradually enhanced NK activity (Fig. 2). The other one patient (TRI-26) showed a slight decrease after one cycle treatment and had recovered NK activity at the end of the second cycle (estimated as “no change”). Cytotoxicity against Raji cells was always significantly lower than that against K562 target cells. In summary, NK cytolytic activity increased in four patients (range 2.5–5 times greater lytic activity), was stable in two and decreased in three. Five patients showed good correlation between NK killing activity and absolute NK cell number in the peripheral blood, whereas two patients had the opposite trend.
Fig. 2.
Changes of NK activity in patients vaccinated with rF-CEA(6D)-TRICOM-infected DCs. Frozen stocked PBMCs were thawed the day before the assay, and were incubated overnight in RPMI1640 medium+10% human AB serum with or without IL-2 (1,000 IU/ml). K562 cells labeled with 51Cr were used as target cells. Raji cells were used as negative controls. Three to five thousand K562 cells were put into each well of 96-well U-bottom plates, and PBMCs were added to make appropriate effector:target ratios. After 4 h of incubation, 51Cr release was analyzed by gamma counter. TRI-06 and TRI-32 underwent one cycle of vaccination, whereas TRI-21 and TRI-22 had two cycles. Square before vaccination, circle after first cycle of vaccination, triangle after second cycle of vaccination, filled symbol with IL-2, open symbol without IL-2
Table 2.
Changes of NK activity and clinical outcome
| Pt | Treatment cycle | T cell response (baseline) | NK activity | Clinical outcome |
|---|---|---|---|---|
| TRI-06 | 1 | 13 (2.7) | No change | Progressive |
| TRI-07 | 1 | 69 (10.2) | Decreased | Progressive |
| TRI-21 | 2 | 228.5 (1.2) | Increased | Stable/NE |
| TRI-22 | 2 | 26.3 (0.3) | Increased | Stable/NE |
| TRI-26 | 2 | 169.7 (0.2) | No change | Stable/NE |
| TRI-32 | 1 | 53 (2.5) | Decreased | Progressive |
| TRI-37 | 1 | 57 (2) | Decreased | Progressive |
| TRI-38 | 1 | 72 (0.7) | Increased | Stable/NE |
| TRI-40 | 2 | 91.5 (1) | Increased | Stable/NE |
NK activity was examined by 4 h 51Cr release assay against NK-sensitive K562 cells. T cell response shows maximum response for fp-TRICOM-CEA transduced DCs in ELISPOT assay (IFN-γ+ cells in 100,000 PBMCs) after first vaccination. The values before vaccinations are shown in parentheses. Change of NK activity was estimated at 25:1 effector to target ratio, and less than 10% increase or decrease was regarded as no change. Clinical outcome was assessed by applying the RECIST criteria to CT or MRI scans obtained before and after each cycle of immunization. Progressive progressive disease, stable/NE stable disease or no evidence of disease
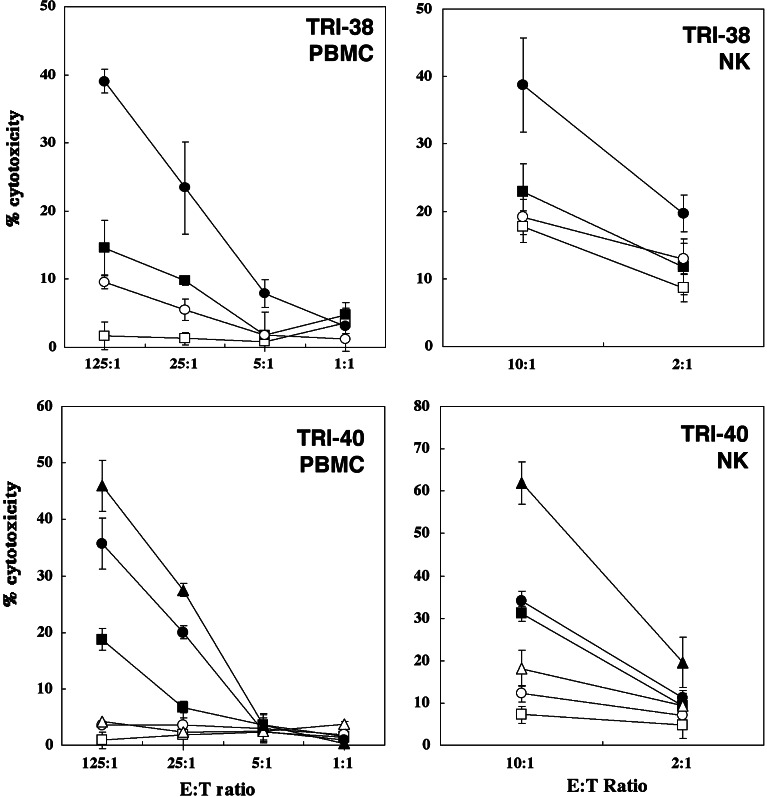
From three patients’ PBMCs (TRI-37, -38, -40), NK cells were negatively isolated using NK Cell Isolation Kit and NK killing activity of each time point was compared with those of the PBMCs. As expected, PBMCs and NK cells showed a similar trend with regard to the change of NK cytolytic activity. In two patients (TRI-38 and TRI-40), NK cell showed gradual increase in killing activity just like the PBMCs did (Fig. 3), and the other patient (TRI-37) had decreased activity compared to the pre-vaccination status for both the PBMCs and the isolated NK cells. Thus, we believe that the analysis of NK activity in PBMCs reflects not only NK cell numbers in the peripheral blood at each time point but also indicates, at least in some cases, the activation status of individual NK cells.
Fig. 3.
Changes of NK activity in PBMCs and purified NK cells. Frozen stocked PBMCs were thawed the day before the assay, and were incubated with or without IL-2 (1,000 IU/ml). NK cells were purified using NK Cell Isolation Kit after overnight incubation. K562 cells labeled with 51Cr were used as target cells. Isolated NK cells were put to 96-well plates to make 10:1 and 2:1 effector:target ratios. Square before vaccination, circle after first cycle of vaccination, triangle after second cycle of vaccination, filled symbol with IL-2, open symbol without IL-2
NK cell receptor expression on NK cells
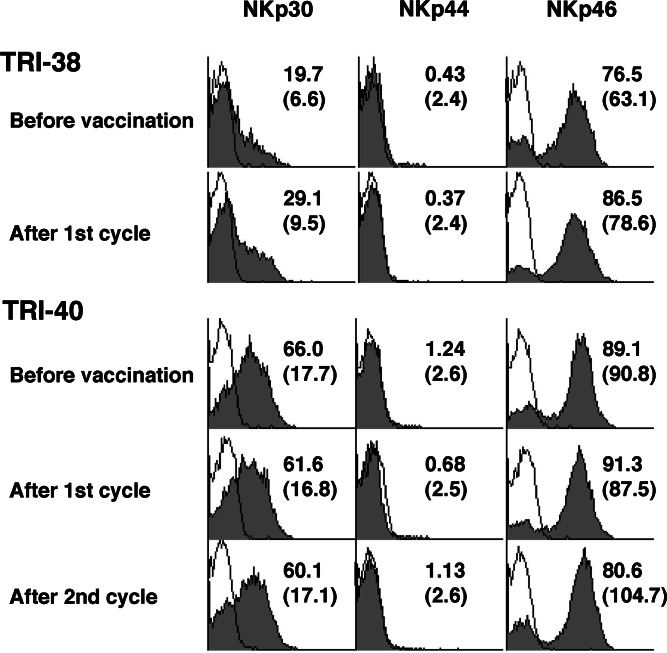
For nine patients, we analyzed CD69, 2B4(CD244), NKG2A, NKG2C and NKG2D expression on CD3-CD56+ NK cells (Fig. 4), and for four patients among them, we also analyzed the expression of natural cytotoxicity receptors, NKp30, NKp44 and NKp46 (Fig. 5). Expression of NKG2A, one of the inhibitory NK cell receptors, was enhanced in five patients (TRI-21, -22, -37, -38, -40) and decreased in two (TRI-06 and TRI-26). Expression of NKG2C, one of the activating NK cell receptors, was stable in most cases except in one patient (TRI-37). NKG2D, another c-type lectin receptor, was enhanced in expression in three (TRI-32, -38, -40), and two of them had enhanced NK killing activity. Four patients, however, had stable NKG2D expression, while two (TRI-06 and TRI-37) had lower NKG2D expression, among them one (TRI-37) had decreased NK activity. There were no significant changes in 2B4 expression. NKp30 and NKp46 showed enhanced expression in two (TRI-37 and TRI-38) of the four patients, another patient (TRI-40) showed mildly increased MFI (90.8→104.7) after two cycles of treatment and two of those three patients who showed stronger NKp46 expression had enhanced NK activity after vaccination. The other patient (TRI-32) showed lower expression of NKp46 after vaccination, which coincided with the change of NK activity. The other natural cytotoxicity receptor, NKp44, did not show any significant changes in expression. Thus, NKG2D and NKp46 expression of NK cell seemed to have a good correlation with NK activity in this study.
Fig. 4.
Expression of natural killing receptors on NK cells in patients vaccinated with rF-CEA(6D)-TRICOM-infected DCs. Frozen stocked PBMCs were thawed the day before the staining. Cells were stained with FITC-labeled anti-CD3, PerCP-labeled anti-CD45, APC-labeled anti-CD56 and PE-labeled anti-NKp30, 44 or 46. CD45+ FSClow SSClow CD3-CD56+ NK cells were analyzed for their expression of natural killing receptors. Two representative cases, which had increased NK activities after vaccination, are shown. Filled histograms show the NK receptor staining and solid lines show isotype controls. The numbers indicated in histogram show percentage of positive cells and the numbers in parentheses show mean fluorescence intensity
Fig. 5.
Expression of NK-activating receptors and inhibitory receptors on NK cells. Frozen stocked PBMCs were thawed the day before the staining. Cells were stained with FITC-labeled anti-CD3, PerCP-labeled anti-CD45, APC-labeled anti-CD56 and PE-labeled anti-CD69, 2B4 (CD244), NKG2A, NKG2C or NKG2D. CD45+ FSClow SSClow CD3-CD56+ NK cells were analyzed for their expression of these receptors. Two representative cases are shown, TRI-32: decreased NK activity, TRI-40: increased NK activity after vaccination. Filled histograms show the NK receptor staining and solid lines show isotype controls. The numbers indicated in the histogram show the percentage of positive cells and the numbers in parentheses show mean fluorescence intensity
Correlation between NK activity and clinical outcome
When the patients were grouped by clinical activity (stable disease/no evidence of disease (n=5) vs progressive disease (N=4) at 3 months), we observed that the majority (four of five cases) of those with stable disease/no evidence of disease had increases in their NK activity (chi square, P=0.0163) and the other one case showed no significant change (Table 2). All the patients who had increased NK activity after vaccination showed better prognosis. On the contrary, the majority (three of four cases) of those patients with clinically progressive disease had decreased NK activity after their vaccinations. All three patients who showed a decrease in NK activity had progressive disease. However, based on the results of ELISPOT assay, all the patients included in this analysis showed enhanced T cell response after vaccination (more than 10 IFN-γ positive spots per 100,000 PBMCs). Although it was not significant, stable/NE patients tended to have higher numbers of IFN-γ+ spots than patients with the progressive disease (118±80.8 vs 48.0±24.3, respectively, P=0.14). Thus, importantly, the NK results predicted more closely with the clinical outcome than did the T cell responses (chi square, P=NS).
Discussion
We wished to study NK cell activation by DCs to determine if part of the clinical activity ascribed to DC-based vaccines might be related to enhanced NK cell function. We first demonstrated that DC, particularly those hyperexpressing the costimulatory molecules CD54, CD58 and CD80, activated NK cells in vitro and maintained their survival. Subsequently, we showed that changes in NK activity following DC vaccination correlated with clinical outcome. Previously, we had observed a correlation between the magnitude of the CEA-specific T cell response and clinical activity (stable disease and minor response) [1]. We now hypothesize that those patients who experienced clinical benefit did so because of T cell and/or NK cell activation.
Dendritic cells have long been recognized as highly versatile components of the immune system that can affect adaptive immunity at multiple levels. Interaction of DCs with T cells, B cells and NKT cells have been well described. Recent studies indicate that cell-to-cell contact between DC and NK cell increases NK cell cytolytic activity and IFN-γ secretion in mice [4]. Gerosa [13] reported that myeloid DC induced NK cell secretion of IFN-γ through a mechanism dependent on both IL-12 secretion and cell contact between NK cells and DC. Interestingly, Van den Broeke et al. [14] found in their murine tumor model that vaccination with antigen-unpulsed DC induced tumor immunity through the activation of NK cells. They showed increased NK activity and increased NK cell infiltration into the tumors in these mice, and, similar to our observations, demonstrated that activation of NK cells relied on the expression of costimulatory molecules on DCs. Similarly, Adam et al. [15] recently reported that antigen-unloaded DCs induced tumor rejection and tumor-specific long-term memory independent of CD4+ helper cell. NK activation following DC–NK cell interaction was necessary for the primary rejection and NKG2D–NKG2D ligand were involved in this process. In addition, the long-term protection against tumor was provided by cytolytic T cells activated by IL-12 and IFN-γ secreted in the course of the DC–NK cell interaction. Interestingly, we also observed increases in IL-12 and IFN-γ in our NK cell cultures. These findings suggest that DC–NK cell interaction may result in anti-tumor activity through enhanced NK cell killing or by enhanced activation of cytolytic T cells. Nieda et al. [16] recently reported a clinical trial of cancer immunotherapy using DCs pulsed with CD1d-specific ligand, α-galactosylceramide. They observed the activation of NK cells as well as NKT cells after DC administration, and found that NK cytolytic activity was enhanced in five out of 11 patients. Thus, DCs might be able to activate NK cells directly or indirectly through activation of other cell types, such as the NKT cells. We found the prolonged survival of NK cells when they were co-cultured with DCs (Fig. 1a), which is consistent with the previous report [17]. Direct secretion of cytokines, such as IL-12 and IL-15, by matured DCs can reciprocally promote NK cell survival and differentiation in vivo [18, 19]. This might be the mechanism for the increased NK cell number and enhanced NK activity in DC-vaccinated patients in our study.
Conversely, it is becoming clear that activated NK cells can reciprocally modify DC function. Kalinski [20] reported that activated NK cells induce maturation of DCs with a 100-fold enhanced ability to produce IL-12p70 in response to subsequent interaction with T helper cells. Our observations on increased IL-12 production in DC/PBL cultures are consistent with these reports. Thus, DC activation by NK cells may result in more efficient T cell stimulation. Moreover, recent study has demonstrated that NK cells by themselves can augment the proliferation of antigen-specific T cells through interactions between 2B4 (CD244) on NK cells and CD48 on T cells [21]. Thus, NK cells can be involved in adaptive immunity through T–NK cell interaction as well as DC–NK cell interaction.
The mechanism for the enhanced NK function and its correlation with clinical activity may be related to the enhanced expression of the stimulatory receptors. For example, engagement of NKG2D directly stimulates NK cells and induces natural killing activity [22–24]. In the present study, we demonstrated that NK–DC interaction enhanced NKG2D expression on NK cells in vitro, supporting the enhancement of NK killing activity. In the present clinical trial, NKG2D expression on NK cells were enhanced or stable in seven patients, and among them five patients had stable disease, whereas two patients who showed a downregulated expression of NKG2D after vaccination had progressive disease. Other stimulatory receptors include NKp30, NKp44 and NKp46 [25–27]. These receptors synergize in tumor cell recognition. It has been reported that NK cells recognize DCs as well as tumor cells through NKp30 and NKp46 [5, 28], although the ligands expressed by DCs are still unknown. Among the four patients analyzed in the present study, NKp30 expression was enhanced in two and NKp46 expression was stronger in three after vaccination. Our in vitro experiments also demonstrated the enhanced expression of these activating NK receptors on NK cells by co-culturing with DCs. Thus, DCs might be able to activate NK cells by enhancing the expression of activating NK receptors. Alternatively, upregulation of NKG2D and NKp46 expression could represent the selective expansion of NKG2D and NKp46 positive NK cell subsets upon DC vaccination. Since NKG2A expression was enhanced in some patients, this could represent rather the accumulation of terminally differentiated cytolytic CD56dimCD16+ NK cells, which is also suggested by the increased cytolytic activity (Figs. 2, 3).
Owing to the ability of DCs to initiate immune responses, DC-based immunotherapy protocols are undergoing increasing clinical trial testing. The intermediate endpoint is typically the induction of tumor antigen-specific CD4+ or CD8+ T cell responses. However, NK cells, important effector cells in tumor surveillance, can be activated by autologous DCs, resulting in longer survival of NK cells, IFN-γ secretion and enhanced expression of activating NK receptors as shown in the present study. Although relevant mechanisms of NK activation require further clarification, understanding DC–NK cell interactions will render possible the development of more effective and specific immunotherapies that will elicit both NK cell and T cell responses and will provide long-term protective immunity. Since NK activity correlated well with the clinical outcome of the patients in this study, it might be also important to monitor NK activity to estimate the effect of vaccination and to find optimal treatments for individual patients.
References
- 1.Morse MA, Clay TM, Hobeika AC, Osada T, Khan S, Chui S, Niedzwiecki D, Panicali D, Schlom J, Lyerly HK. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 2.Yokoyama WM, Kim S, French AR. The dynamic life of natural killer cells. Annu Rev Immunol. 2004;22:405–429. doi: 10.1146/annurev.immunol.22.012703.104711. [DOI] [PubMed] [Google Scholar]
- 3.Glas R, Franksson L, Une C, Eloranta ML, Ohlen C, Orn A, Karre K. Recruitment and activation of natural killer (NK) cells in vivo determined by the target cell phenotype. An adaptive component of NK cell-mediated responses. J Exp Med. 2000;191:129–138. doi: 10.1084/jem.191.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 5.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raulet DH. Interplay of natural killer cells and their receptors with the adaptive immune response. Nat Immunol. 2004;5:996–1002. doi: 10.1038/ni1114. [DOI] [PubMed] [Google Scholar]
- 9.Kim A, Noh YW, Kim KD, Jang YS, Choe YK, Lim JS. Activated natural killer cell-mediated immunity is required for the inhibition of tumor metastasis by dendritic cell vaccination. Exp Mol Med. 2004;36:428–443. doi: 10.1038/emm.2004.55. [DOI] [PubMed] [Google Scholar]
- 10.Parajuli P, Mathupala S, Sloan AE. Systematic comparison of dendritic cell-based immunotherapeutic strategies for malignant gliomas: in vitro induction of cytolytic and natural killer-like T cells. Neurosurgery. 2004;55:1194–1204. doi: 10.1227/01.NEU.0000141082.20865.48. [DOI] [PubMed] [Google Scholar]
- 11.Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, Karre K, Zappacosta S. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 12.Wilson JL, Heffler LC, Charo J, Scheynius A, Bejarano MT, Ljunggren HG. Targeting of human dendritic cells by autologous NK cells. J Immunol. 1999;163:6365–6370. [PubMed] [Google Scholar]
- 13.Gerosa F, Gobbi A, Zorzi P, Burg S, Briere F, Carra G, Trinchieri G. The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions. J Immunol. 2005;174:727–734. doi: 10.4049/jimmunol.174.2.727. [DOI] [PubMed] [Google Scholar]
- 14.van den Broeke LT, Daschbach E, Thomas EK, Andringa G, Berzofsky JA. Dendritic cell-induced activation of adaptive and innate antitumor immunity. J Immunol. 2003;171:5842–5852. doi: 10.4049/jimmunol.171.11.5842. [DOI] [PubMed] [Google Scholar]
- 15.Adam C, King S, Allgeier T, Braumuller H, Luking C, Mysliwietz J, Kriegeskorte A, Busch DH, Rocken M, Mocikat R (2005) DC–NK cell cross-talk as a novel CD4+ T cell-independent pathway for antitumor CTL induction. Blood Mar 15 [Epub ahead of print] [DOI] [PubMed]
- 16.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, Abraham R, Juji T, Macfarlane DJ, Nicol AJ. Therapeutic activation of Va24+Vb11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–389. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 17.Valteau-Couanet D, Leboulaire C, Maincent K, Tournier M, Hartmann O, Benard J, Beaujean F, Boccaccio C, Zitvogel L, Angevin E. Dendritic cells for NK/LAK activation: rationale for multicellular immunotherapy in neuroblastoma patients. Blood. 2002;100:2554–2561. doi: 10.1182/blood.V100.7.2554. [DOI] [PubMed] [Google Scholar]
- 18.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.V97.1.14. [DOI] [PubMed] [Google Scholar]
- 19.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 20.Kalinski P, Giermasz A, Nakamura Y, Basse P, Storkus WJ, Kirkwood JM, Mailliard RB. Helper role of NK cells during the induction of anticancer responses by dendritic cells. Mol Immunol. 2005;42:535–539. doi: 10.1016/j.molimm.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 21.Assarsson E, Kambayashi T, Schatzle JD, Cramer SO, von Bonin A, Jensen PE, Ljunggren HG, Chambers BJ. NK cells stimulate proliferation of T and NK cells through 2B4/CD48 interactions. J Immunol. 2004;173:174–180. doi: 10.4049/jimmunol.173.1.174. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/S1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 23.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 24.Wu J, Song Y, Bakker AB, Bauer S, Spies T, Lanier LL, Phillips JH. An activating immunoreceptor complex formed by NKG2D and DAP10. Science. 1999;285:730–732. doi: 10.1126/science.285.5428.730. [DOI] [PubMed] [Google Scholar]
- 25.Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A. NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med. 1998;187:2065–2072. doi: 10.1084/jem.187.12.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spaggiari GM, Carosio R, Pende D, Marcenaro S, Rivera P, Zocchi MR, Moretta L, Poggi A. NK cell-mediated lysis of autologous antigen-presenting cells is triggered by the engagement of the phosphatidylinositol 3-kinase upon ligation of the natural cytotoxicity receptors NKp30 and NKp46. Eur J Immunol. 2001;31:1656–1665. doi: 10.1002/1521-4141(200106)31:6<1656::AID-IMMU1656>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]