Abstract
Purpose
Vγ9Vδ2 (γδ) T lymphocytes, a critical peripheral blood lymphocyte subset, are directly cytotoxic against many solid and hematologic tumor types. Vγ9Vδ2 T lymphocytes can be selectively expanded in vivo with BrHPP (IPH1101) and IL-2. The present phase I trial was conducted with the aim of determining the maximum-tolerated dose (MTD) and safety of IPH1101 combined with a low dose of IL-2 in patients with solid tumors.
Experimental design
A 1-h intravenous infusion of IPH11 was administered alone at cycle 1, combined with a low dose of SC IL-2 (1 MIU/M2 d1 to d7) in the subsequent cycles (day 1 every 3 weeks). The dose of IPH1101 was escalated from 200 to 1,800 mg/m2.
Results
As much as 28 patients with solid tumors underwent a total of 109 treatment cycles. Pharmacodynamics data demonstrate that γδ T lymphocyte amplification in humans requires the co-administration of IL-2 and is dependent on IPH 1101 dose. Dose-limiting toxicity occurred in two patients at a dose of 1,800 mg/m2: one grade 3 fever (1 patient) and one grade 3 hypotension (1 patient) suggesting cytokine release syndrome immediately following the first infusion. At lower doses the treatment was well tolerated; the most frequent adverse events were mild fever, chills and abdominal pain, without exacerbation in the IL-2 combined cycles.
Conclusion
IPH1101 in combination with SC low-dose IL-2 is safe, well tolerated and induces a potent γδ T lymphocyte expansion in patients. Its clinical activity will be evaluated in phase II clinical trials.
Keywords: Bromohydrin pyrophosphate, Vγ9Vδ2 T lymphocyte, Phase I trial, Solid tumors
Introduction
An expansive body of literature in the field has documented that γδ T cells, which represent 1–10% of human peripheral T cells, kill solid and hematologic tumors originating from virtually any organ type. Over more than 15 years of research, in vitro γδ T cell cytotoxic activity has been reported against tumors of virtually all cellular origins. Among all these, the most remarkable data are based on two observations of particular relevance: (1) γδ T cells are able to kill autologous tumors; this has been formally established for mRCC [1, 2], melanoma [3], hepatocarcinoma [4], ovarian carcinoma [5], colorectal cancer [6], gastrointestinal carcinomas [7], primary glioblastoma [8] and, more importantly, for colon cancer stem cells [9]; and (2) some tumors present “spontaneous” infiltration by γδ T cells illustrating that, in vivo, there might exist a specific signal recognized by γδ T cells [2, 3, 6, 10–12]. Altogether, these observations emphasize the potential importance of γδ T cells, as innate immunity effectors, in the specific recognition, growth control and elimination of human tumors.
In contrast with their αβ couterparts, γδ T cells recognize and respond to non-peptidic natural and synthetic small molecular weight phosphorylated compounds (phosphoantigens), through a non-MHC-restricted mechanism. One such synthetic compound, easily synthesized and active at nanomolar concentrations similarly to natural phosphoantigens, is bromohydrin pyrophosphate (BrHPP, IPH1101, previously Phosphostim®) [13]. This pyrophosphate monoester was selected for clinical development on the basis of preclinical data in which this compound stimulates the γ9δ2 T cell subset, which has known anti-tumor activity. For example, in an autologous setting, in a study of ten metastatic renal cell carcinoma (mRCC) patients, γ9δ2 T cells expanded by BrHPP exhibited specific lytic activity against primary tumor cell lines but not against primary normal cell lines [12]. BrHPP induces both an expansion of the γ9δ2 compartment with the generation of large numbers of cytotoxic effectors, as well as an indirect pro-inflammatory and regulatory effect that orients the immune response toward a TH1 cellular response (tumor necrosis factor (TNF)-α and interferon (IFN)-γ). These cytokines can in turn either directly inhibit tumor cell growth or potentiate the anti-tumor activity of other lymphoid effectors. The expansion of the γ9δ2 cell population is dependent on the presence of IL-2.
There is a significant potential for the beneficial use of highly potent compounds stimulating the γ9δ2 T cell subset, such as pamidronate or BrHPP, in cancer immunotherapy. In a pilot clinical study set up to test the effects of a co-treatment of pamidronate, a bisphophonate, and IL-2 on 11 patients with non-Hodgkin’s lymphoma (NHL) and 8 patients with multiple myeloma (MM), it was established that pamidronate and IL-2 co-treatment induces specific γδ cell amplification. The clinical response observed in the patients (stabilization or partial response) was linked to γδ T cell proliferation in vivo [14]. A second study conducted in hormone refractory prostate cancer patients compared zoledronic acid alone or in combination with low-dose IL-2 as inducers of γδ T cells. In the combination arm, five out of nine patients showed appreciable increases in blood γδ T cells that correlated with favorable clinical outcome. At 9 months after therapy, an inverse correlation between serum PSA levels and blood effectors γδ T cells was observed [15].
BrHPP is active at nanomolar concentrations similarly to natural phosphoantigens [13] and is more potent than aminobisphophonates, such as pamidronate. In monkeys, BrHPP, particularly when combined with a low dose of IL-2, was shown to induce a strong activation and amplification of γ9δ2 T cells accompanied by the production of considerable amounts of cytokines, with no associated toxicity [16]. The present phase I clinical study was aimed to determine the maximum-tolerated dose of IPH1101 combined with a low dose of IL-2 in patients with solid tumors. The safety profile of IPH1101 was evaluated alone or in combination with repeated injection of a low dose of IL-2, sufficient to induce activation of γ9δ2 T cells. An additional objective was to examine the pharmacodynamic profile of IPH1101 alone and in combination with a low dose of IL-2.
Patients and methods
Patient selection
Patients were candidates for the study if they had solid tumors for which effective standard therapy was not available or if they had progressive disease after conventional therapeutic modalities for advanced or metastatic disease. The interval between completion of the last chemotherapy and/or radiotherapy and enrollment in the trial had to be at least 4 weeks. Recruited patients were between 18 and 70 years old, with World Health Organization (WHO) performance status (PS) score of 0–1 and an estimated life expectancy of at least 12 weeks. The biological criteria for eligibility were defined by laboratory tests of adequate hematological function: white blood cell (WBC) count ≥ 4.0 × 109 cells, neutrophils ≥ 2.0 × 109, platelets ≥ 100 × 109, hemoglobin ≥ 9 g/dl; and biochemistry: aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤ 2.5 × the upper normal limits (UNL), serum bilirubin ≤ UNL and serum creatinine ≤ 1.5 × UNL. For AST and ALT, ≤ 5 × UNL was acceptable in case of liver metastases. At least one measurable or evaluable lesion outside of a previous radiation field was required. Measurable lesions were identified as those with a diameter ≥20 mm on computed tomography (CT) scan. In addition, patients were selected according to the ability of their peripheral blood mononuclear cells (PBMC) to respond to BrHPP ex vivo: this standardized “BrHPP sensitivity test” quantified patients’ γδ T cells proliferative response to the drug in a short-term culture (8 days). Results of in vitro amplification of cells by BrHPP is expressed as (A)% of γδ T cells at the end of the culture and (B) total amplification rate of γδ T cells (i.e., ratio of the absolute count of γδ cells at day 8 on the absolute count at day 0). Only patients whose γδ T cells (A) reached at least 20% in the culture or (B) showed at least eightfold expansion were included in the trial.
Patients were excluded if they had clinical evidence of brain or leptomeningeal metastasis, uncontrolled hypercalcemia, known active infections including known HIV positivity or unstable cardiovascular conditions (heart failure, uncontrolled angina, uncontrolled hypertension, myocardial infarction within 6 months). The QTc interval duration was required to be inferior to 430 ms for men and 450 ms for women. Concurrent treatment with other experimental drugs or participation in another clinical trial within the 30-day period prior to study entry was not permitted.
Additional exclusion criteria were prior treatment with lymphocyte therapy, prior treatment with immunotherapy during the last 6 weeks, prior treatment with biphosphonates during the last 4 weeks, concomitant treatment with anticancer drugs or corticotherapy, active immune disease, previous high-dose chemotherapy bone marrow rescue and prior irradiation of >25% of the bone marrow reserve.
The study was approved by the Independent Ethics Committee of Nantes, France, and was conducted in two centers; all patients gave written informed consent before participating in the study.
Treatment plan
This was a two-center (Centre René Gauducheau, Nantes, France; Hôpital Saint-Louis, Paris, France), open-label, phase I study conducted in patients with advanced solid tumors.
Bromohydrin pyrophosphate (IPH1101) was supplied by INNATE Pharma (Marseille, France) as a white lyophilized powder (200 mg/vial) and was to be reconstituted immediately prior to use with 2 ml of water for injections to make a 100 mg/ml solution. For administration, the appropriate dose of BrHPP (IPH1101) was diluted in a volume of 100 ml of Ringer’s lactate and infused over 1 h via a central venous line.
Each patient was to receive infusions of IPH1101 at 21-day intervals until disease progression. During the first cycle, the patients received IPH1101 without IL-2 co-administration. During the subsequent cycles, the patients received subcutaneous injections of IL-2 (1 × 106 IU/m2/day) 10 min after starting IPH1101 perfusion on days 1–7.
The dose-escalation strategy involved five main dose levels (200, 600, 1,200, 1,800 and 2,400 mg/m2) to be tested successively in ascending order, subject to tolerability. At least three patients were to be treated at each dose level. Toxicity was assessed using the National Cancer Institute of Canada Common Toxicity Criteria version 3.0. DLT was defined as any one of the following: nadir neutrophils <0.5 × 109/l lasting 7 days or <0.1 × 109/l lasting 3 days; thrombocytopenia <25 × 109/l or thrombocytopenia with bleeding or requiring platelet transfusion; febrile neutropenia, defined as absolute neutrophil count <0.5 × 109/l and fever (three measured <38°C in 24 h or one >38.5°C); and/or any grade 3/4 major organ toxicity except alopecia or non-premedicated nausea/vomiting. The maximum-tolerated dose (MTD) of IPH1101 was defined as the highest-validated dose, i.e., with DLT in no more than one out of three, or two out of six patients. There was no intra-patient dose escalation.
Pretreatment and follow-up examinations
On entering into the study, patients were evaluated with a complete medical history and physical examination including measurement of vital signs (temperature, pulse rate and blood pressure), electrocardiogram (ECG), complete blood count (CBC), serum electrolytes, and hepatic and renal function. During the treatment period, laboratory tests were performed at each weekly visit.
Efficacy assessments
Tumor response (according to the RECIST criteria) to study treatment was assessed on the basis of CT scans examinations. These radiological examinations were performed at inclusion and for every three cycles.
BrHPP sensitivity test in vitro
Whole blood (20 mL) was taken pre-dose and PBMC were prepared after Ficoll-Paque™ PLUS (Amersham Pharmacia Biotech) density centrifugation. PBMC were cultured in RPMI1640 supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate (all from Invitrogen Life Sciences, Cergy-Pontoise, France), 10% irradiated fetal bovine serum (Fetal Clone I, HyClone/Thermo Fisher Scientific, Brebières, France), 3 μM BrHPP and 300 IU/mL rhIL-2 (Proleukin®, Chiron, 22 × 106 IU per vial) starting at 1 × 106 cells/mL. rhIL-2 was renewed on day 5 of the culture. On day 8, cells were counted for final density determination on a Guava® PCA apparatus (Guava Technologies Inc, Hayward, CA, USA) and then harvested by centrifugation and washed in RPMI before multi-color flow cytometry analysis of their percentage of γδ T cells (see below).
Pharmacodynamics
The biological effect of IPH1101 in solid tumor patients was monitored throughout the trial and studied on peripheral blood cells. Whole blood for pharmacodynamic analyses was taken on day 1 and at several time points between days 1 and 21 for cycles 1 and 2. As much as 6 ml of blood was removed pre-dose and at days 6, 8 and 12. The volume of blood withdrawn for pharmacodynamic determinations from each patient did not exceed 24 ml for cycles 1 and 2, and 12 ml for each subsequent cycle. During the cycles 3, 4, 5 and 6, additional PD analyses were performed. At each mentioned cycles, 6 ml of blood was removed pre-dose and at day 6.
The biological effect study included taking whole blood sample for follow-up of γδ T cell amplification and for identification of other blood lymphocyte populations by multi-color flow cytometry (FacsCalibur, Becton–Dickinson Biosciences (BD), Le Pont de Claix, France) with the various combinations of commercially available anti-human receptors antibodies, used according to the manufacturers’ instructions. γδ T cells were defined as Vdelta2+/CD3+ cells (anti-Vdelta2, IMMU389 clone from Immunotech-Beckman-Coulter (IBC), Marseilles, France and anti-CD3, UCHT1 clone from BD); NK cells were CD3-/CD56+/CD16+ cells (anti-CD56, B159 clone and anti-CD16, 3G8 clone from BD); B cells were CD19+ cells (anti-CD19, 4G7 clone from BD) and conventional T cells were, respectively, CD3+/CD4+ (anti-CD4, SK3 clone from BD) and CD3+/CD8+ (anti-CD8, SK1 clone from BD) cells.
Results
Patient sample
Between October 2003 and August 2005, 48 patients were screened to participate in the study: 35 patients at the Nantes center and 13 at the Paris center. Of these patients, 20 were not included in the trial. The reason for non-inclusion was mainly deterioration of performance status (7 patients). Other reasons were the absence of lymphocytes response in vitro in the BrHPP expansion test (5 patients), abnormal liver functions (3 patients), abnormal ECG (3 patients), lymphopenia (1 patient) and age >70 years old (1 patient). The treated population ultimately included 28 patients receiving at least one treatment dose. Their demographic data are shown in Table 1. Because IPH1101 stimulates the immune system by activating the γ9δ2 subset, enrollment of patients with tumors regarded as relatively sensitive to immune intervention was favored: 18 of the 28 total patients suffered from a metastatic renal cell carcinoma.
Table 1.
Demographic data
| Demographic data | |
|---|---|
| Sex | |
| Male | 19 |
| Female | 9 |
| WHO | |
| 0 | 22 |
| 1 | 6 |
| Age (years) | |
| Median | 56 |
| Range | 44–71 |
| Primary cancer | |
| Renal cell carcinoma | 18 |
| Colon cancer | 3 |
| Esophagus carcinoma | 3 |
| Gastric cancer | 1 |
| Ovarian cancer | 1 |
| Breast cancer | 2 |
| Previous cancer therapy | |
| Surgery | 25 |
| Radiotherapy | 8 |
| Chemotherapy | 17 |
| 1 line | 12 |
| 2 lines | 2 |
| ≥3 lines | 3 |
| Hormonotherapy | 6 |
| Immunotherapy | 13 |
| IL2 and interferon | 8 |
| Interferon | 3 |
| IL2 | 1 |
| Monoclonal antibody | 1 |
| Organ involved | |
| Lung | 18 |
| Liver | 9 |
| Lymph nodes | 7 |
| Bone | 1 |
| Other | 10 |
Study progress
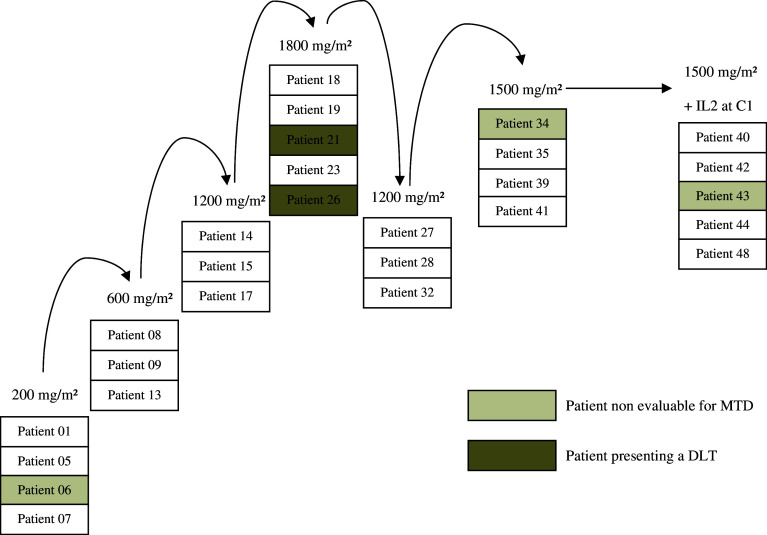
A total of 109 cycles were administered at five different dose levels (Fig. 1). At the first dose level (IPH1101, 200 mg/m2), four patients were treated but one was not evaluable for DLT because he did not receive the total dose of IL2 (5 days of IL2 instead of 7). Then, six patients were enrolled at the two subsequent dose levels, 600 mg/m2 and 1,200 mg/m2. At the 1,800 mg/m2 level, of the five patients enrolled, two experienced a DLT. This was therefore designated as MTD. Three additional patients were then included at the 1,200 mg/m2 dose level and, on confirmation of the absence of DLT, the intermediate dose level of 1,500 mg/m2 was tested. Of the four patients treated at 1,500 mg/m2, three were evaluable for MTD (one patient received 4 h of IPH1101 infusion instead of 1 h) and no DLT was recorded. Finally, an additional cohort of six patients was treated at the 1,500 mg/m2 dose level and received IL2 from cycle 1 as per protocol amendment. The assessment of DLT was then based on the first two cycles of treatment for 20 patients.
Fig. 1.
Study population and escalating dose. A total of 28 patients were treated in this study, 22 in the dose-escalation phase up to a dose level of 1,800 mg/m2. An amendment was introduced at the end of the first part of the escalating dose to have IL2 administered from cycle 1 at a dose level of 1,500 mg/m2
Toxicity and dose-limiting toxicity
All 28 patients were evaluable for toxicity, which is summarized in Tables 2 and 3. No grade 4 toxicity was observed at any dose levels. Flu-like symptoms were the main toxicities in evaluations after cycles 1 and 2. For most of these symptoms, their incidence increased as a function of the IPH1101 dose. Grade 1 and 2 adverse events included: fever (26 patients), chills (15 patients), fatigue (11 patients), headache (11 patients), abdominal pain (10 patients), diarrhea (9 patients), nausea (15 patients), vomiting (14 patients), hypotension (8 patients) and hypertension (1 patient). As discussed, the assessment of DLT was based on the first two cycles of treatment for 20 patients. The remaining patients, treated at 1,500 mg/m2, received IL-2 from cycle 1 as per protocol amendment and did not form part of this assessment. Of the five patients included in the highest dose level of IPH1101, 1,800 mg/m2, two experienced a DLT: one had a grade 3 fever associated with grade 3 hypertension and the other had a grade 3 hypotension, both at the first cycle (without IL-2 co-administration). The first patient was withdrawn from the study, and the second was subsequently treated at the 1,200 mg/m2 dose level for another two cycles. The first patient, 71 years old, had an mRCC. At cycle 1, 10 min after the end of IPH1101 infusion, he presented with grade 3 fever, grade 3 arterial hypertension, grade 2 chills, grade 2 confusional syndrome and grade 2 junctional tachycardia. All symptoms returned to a grade 0 or 1 within 2 days. Nevertheless, the patient was withdrawn from the study. The second patient, 65 years old, had a metastatic breast cancer. At cycle 1, 4 h after the infusion, she reported grade 1 chills, grade 1 vomiting, grade 2 headache, grade 1 dorsal pain and grade 1 asthenia. The blood pressure was low (71/41 mmHg) and required fluid completion and dopamine support (grade 3). All symptoms resolved within 24 h. The patient was then treated at dose 1,200 mg/m2 for two more cycles without any serious problems. However, the MTD was considered to have been met. When IPH1101, 1,500 mg/m2, was given in combination with IL-2 for cycle 1–6 patients, no grade 3 and 4 drug-related adverse events were reported (Table 3).
Table 2.
Clinical adverse events: worst drug-related toxicity (NCI/CTC) adverse events by patient and by cycle
| Dose level of Phosphotim® | 200 (mg/m2) | 600 (mg/m2) | 1,200 (mg/m2) | 1,500 (mg/m2) | 1,800 (mg/m2) | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients/cycles | 4 | 18 | 3 | 10 | 6 | 26 | 10 | 39 | 5 | 16 | 28 | 109 |
| Constitutional | ||||||||||||
| Fatigue | ||||||||||||
| Grade | ||||||||||||
| 1 | 1 | 1 | – | – | – | – | 4 | 5 | 3 | 4 | 8 | 10 |
| 2 | 1 | 1 | – | – | 1 | 2 | – | – | 1 | 1 | 3 | 4 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Fever | ||||||||||||
| Grade | ||||||||||||
| 1 | 2 | 3 | 3 | 5 | 3 | 5 | 8 | 9 | 3 | 5 | 19 | 26 |
| 2 | – | – | 1 | 1 | – | – | 6 | 10 | – | – | 7 | 11 |
| 3 | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 1 |
| Chills | ||||||||||||
| Grade | ||||||||||||
| 1 | 2 | 2 | – | – | 5 | 8 | 3 | 8 | 3 | 5 | 13 | 23 |
| 2 | – | – | – | – | – | – | 1 | 1 | 1 | 1 | 2 | 2 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Gastrointestinal | ||||||||||||
| Abdominal pain | ||||||||||||
| Grade | ||||||||||||
| 1 | 1 | 3 | – | – | 2 | 2 | 3 | 5 | 1 | 1 | 7 | 11 |
| 2 | 1 | 1 | 1 | 1 | – | – | – | – | 1 | 1 | 3 | 3 |
| 3 | – | – | 1 | 1 | – | – | – | – | – | – | – | – |
| Diarrhea | ||||||||||||
| Grade | ||||||||||||
| 1 | 1 | 1 | – | – | 2 | 3 | 2 | 3 | 1 | 1 | 6 | 8 |
| 2 | 1 | 1 | – | – | – | – | 2 | 2 | – | – | 3 | 3 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Nausea | ||||||||||||
| Grade | ||||||||||||
| 1 | 1 | 3 | 1 | 3 | 2 | 4 | 2 | 2 | 4 | 11 | 10 | 23 |
| 2 | – | – | – | – | 1 | 1 | 1 | 2 | 2 | 2 | 4 | 5 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Vomiting | ||||||||||||
| Grade | ||||||||||||
| 1 | – | – | 1 | 1 | 2 | 4 | 3 | 3 | 4 | 8 | 10 | 16 |
| 2 | – | – | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 1 | 5 | 7 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
| Cardiovascular | ||||||||||||
| Hypotension | ||||||||||||
| Grade | ||||||||||||
| 1 | – | – | – | – | 1 | 2 | 1 | 1 | 2 | 2 | 4 | 5 |
| 2 | – | – | – | – | 1 | 1 | 2 | 3 | 1 | 1 | 4 | 5 |
| 3 | – | – | – | – | – | – | – | – | 1 | 3 | 1 | 3 |
| Hypertension | ||||||||||||
| Grade | ||||||||||||
| 1 | – | – | – | – | – | – | 1 | 1 | – | – | 1 | 1 |
| 2 | – | – | – | – | – | – | – | – | – | – | – | – |
| 3 | – | – | – | – | – | – | – | – | 1 | 1 | 1 | 1 |
| Other | ||||||||||||
| Headache | ||||||||||||
| Grade | ||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 3 | 4 | 7 | 2 | 2 | 9 | 14 |
| 2 | – | – | – | – | – | – | 1 | 3 | 1 | 3 | 2 | 6 |
| 3 | – | – | – | – | – | – | – | – | – | – | – | – |
No grade 4 toxicity occurred
Table 3.
Clinical adverse events in the extended cohort (6 patients) treated with Phosphotim®, 1,500 mg/m2 and IL2 at cycle 1
| Grade 1 | Grade 2 | All grades | |
|---|---|---|---|
| Asthenia | 1 | – | 1 |
| Abdominal pain | 1 | – | 1 |
| Diarrhea | 1 | – | 1 |
| Headache | 3 | – | 3 |
| Hypotension | 1 | – | 1 |
| Fever | 3 | 1 | 4 |
| Vomiting | 1 | 1 | 2 |
Clinical activity
A total of 28 patients were treated in the study and available for the assessment of tumor response. Tumor assessments were performed at baseline and responses were evaluated every three cycles using the RECIST criteria. In this phase I trial, no patients achieved an objective response; 12 patients had stable disease and 16 had progressive disease after three cycles.
Pharmacodynamics
To establish the pharmacodynamics of IPH1101 in solid tumor patients, targeted γδ T lymphocytes were followed in blood samples taken pre-dose and on three occasions following the first two infusions of IPH1101 (day 5 or 6, day 7 or 8 and day 11, 12 or 13), and pre-dose and day 6 after the subsequent infusions of IPH1101. A total of 24 patients were evaluable for pharmacodynamic analysis, with the following numbers per dose group: 200 mg/m2, N = 4; 600 mg/m2, N = 3; 1,200 mg/m2, N = 6; 1,500 mg/m2, N = 8; 1,800 mg/m2, N = 3. In the 1,500 mg/m2 dose group, three patients received IPH1101 alone at the first cycle, then IPH1101 and IL-2 at subsequent cycles, whereas five supplementary patients received IPH1101 combined with IL-2 from the first cycle of treatment onward.
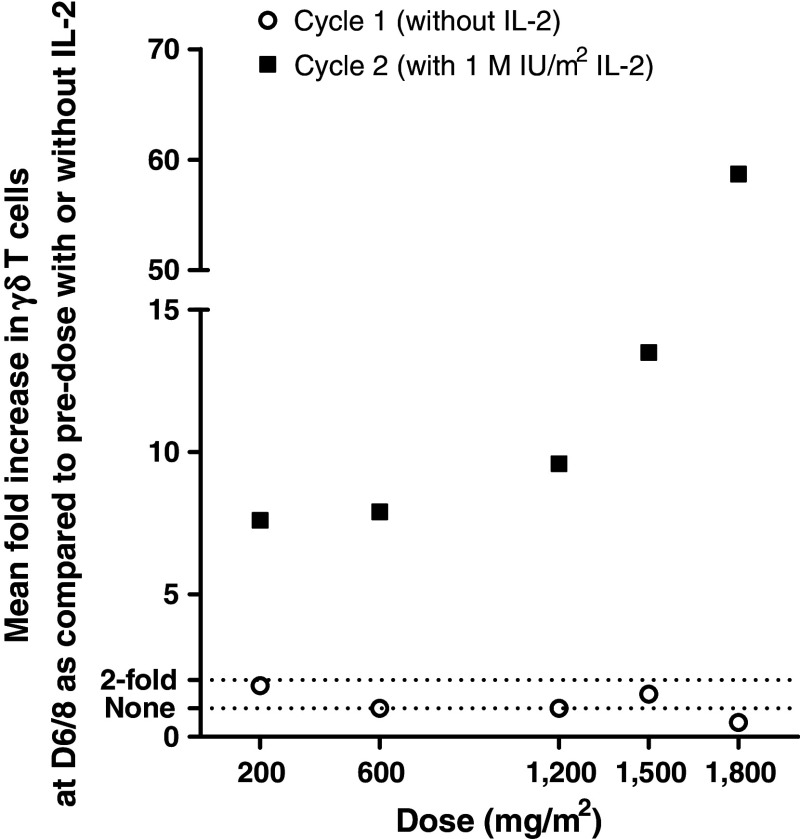
No significant amplification of γδ T cells was observed at the first cycle of IPH1101 infusion, which was in the absence of IL-2. The overall kinetics of γδ T cell amplification in vivo is similar in all patients, with a peak around days 6–8 and a progressive decrease onward. Amplification was calculated as the ratio of maximal absolute counts of Vδ2+ cells (in number of cells/μL blood) at cycle n/absolute counts of Vδ2+ cells at cycle n, day 1 (pre-dose) (in number of cells/μL blood). Mean amplifications by dose level for the five cohorts receiving IL-2 from cycle 2 are presented in Fig. 2 and are IPH1101 dose dependent. Interestingly, even at the lowest level of IPH1101 tested (200 mg/m2 dose group), significant γδ T cell amplification could already be observed in all three patients (mean 7.6-fold) and this effect peaked in the 1,800 mg/m2 dose group, with a mean amplification of 58.7-fold (Fig. 2). In four patients at the highest dose levels (1,200, 1,500 or 1,800 mg/m2), at the peak of the expansion, γδ T cells accounted for more than 20% of all circulating T cells (which represented more than 2,000 γδ T cells per μL blood for the best pharmacodynamic responses).
Fig. 2.
Mean amplifications of γδ-T cells at cycle 1 (without IL-2) and 2 (first cycle with IL-2) of treatment with increasing doses of IPH1101
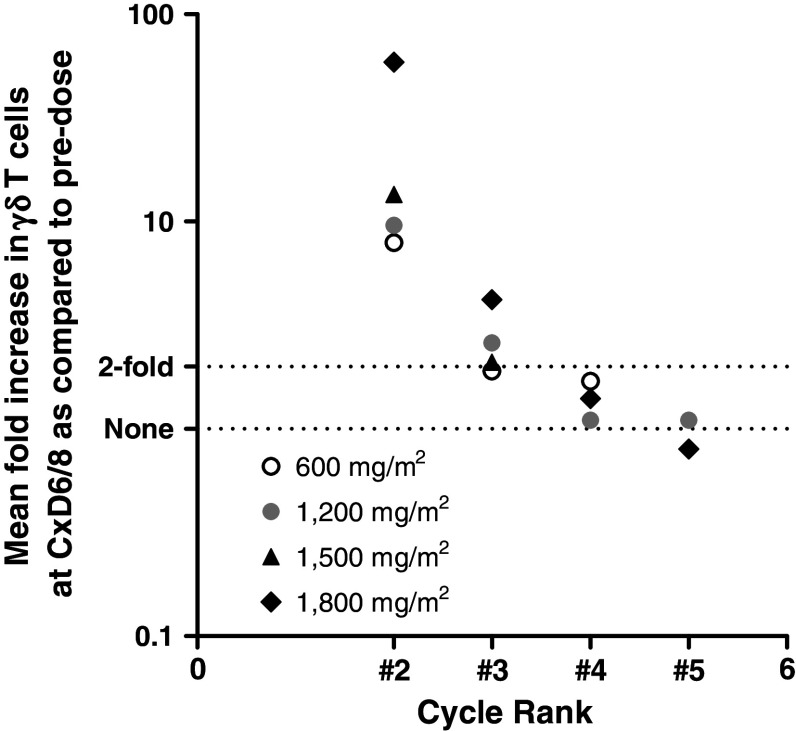
Specific γδ T cell amplifications were found in response to IPH1101/IL-2 decrease with successive administrations (every 3 weeks). This tachyphylaxy is observed whatever IPH1101 dose, at each administration rank (Fig. 3). It ultimately results in an absence of significant amplification at the fourth infusion of IPH1101 (namely, the third in combination with IL-2), whatever dose is administered.
Fig. 3.
Mean amplifications of γδ-T cells at cycles 2–5 of IPH1101 administrations (with low-dose IL-2) for dose groups 600–1,800 mg/m2
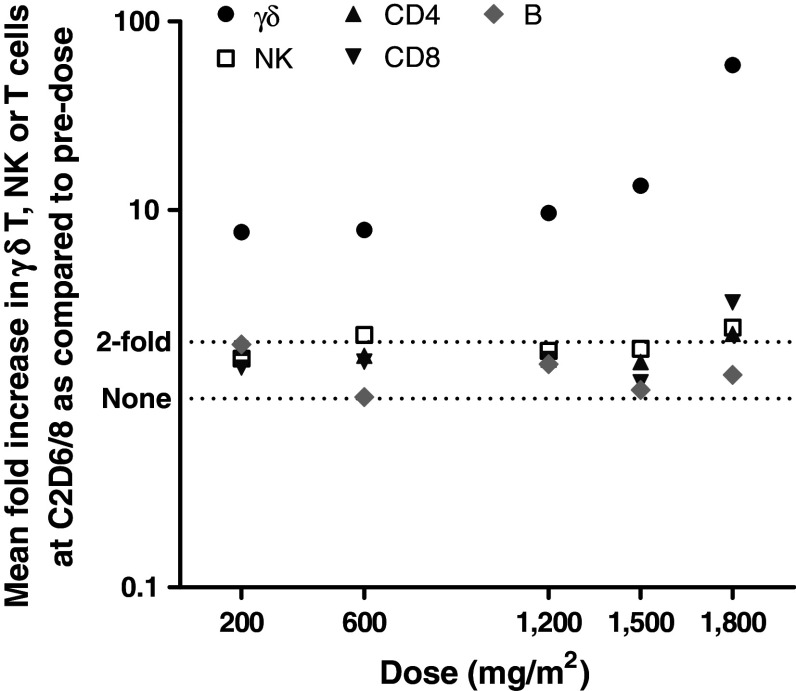
The effect of the combination of IPH1101 and IL-2 on the other blood populations of lymphocytes has also been monitored (namely B, NK, CD4 and CD8 cells). Their amplification is calculated as the maximal ratio of absolute counts at cycle n/absolute counts at cycle n day 1 (pre-dose) (Table 4). NK cells, CD3+ CD4+, CD8+ and B cells increased only in very low proportions (if any), irrespective of the IPH1101 dose (and most probably due to unspecific stimulation by IL-2). While increasing IPH1101 dose (combined with low-dose IL-2), only γδ T cell amplification improved, demonstrating the specificity of IPH1101 on these target cells (Fig. 4).
Table 4.
NK, T, CD4, CD8, B and γδ T cells mean maximal amplification
| Dose level of IPH1101 (mg/m2) | 200 | 600 | 1,200 | 1,500 | 1,500 + IL-2 from first cycle | 1,800 |
|---|---|---|---|---|---|---|
| N | 4 | 3 | 6 | 3 | 5 | 3 |
| NK cells (CD3-CD16+ CD56+) | 1.6 | 2.2 | 1.8 | 1.8 | 2.5 | 2.4 |
| T lymphocytes (CD3+) | 1.7 | 1.7 | 1.6 | 1.5 | 1.5 | 2.2 |
| CD4+ T lymphocytes | 1.9 | 1.7 | 1.6 | 1.6 | 1.4 | 2.2 |
| CD8+ T lymphocytes | 1.5 | 1.6 | 1.6 | 1.2 | 1.4 | 3.2 |
| B lymphocytes (CD19+) | 1.9 | 1.0 | 1.5 | 1.1 | 1.1 | 1.3 |
| γδ T lymphocytes | 7.6 | 7.9 | 9.6 | 13.5 | 15.5 | 58.7 |
Fig. 4.
Comparative mean maximal amplifications of γδ-T cells and NK, CD4+, CD8+ and B cells in each IPH1101 dose group (at cycle 2)
Discussion
This study is the first clinical trial conducted in humans with IPH1101, the lead drug candidate (phosphorylated compound) from a family of molecules obtained by chemical synthesis as analogs of a naturally occurring substance derived from mycobacterium that specifically activates Vγ9Vδ2 T cell subset. In this phase I study, the schedule of IPH1101 evaluated consisted of 1-h i.v. infusions every 3 weeks in combination with s.c. low doses of IL-2 from days 1 to 7. In the first cycle, IPH1101 was administered alone, without IL-2, with the aim of evaluating the tolerability profile of this new compound. We have previously reported a phase I study using a γ9δ2 cell therapy product named Innacell γδ™. Innacell γδ™ was manufactured in vitro from an autologous peripheral blood mononuclear cell (PBMC) preparation, by a single stimulation with BrHPP (IPH 11001) followed by a 2-week period of culture and expansion with IL-2 [17]. The data collected from this study indicated that repeated infusions of Innacell γδ™, either alone or with co-administration of IL-2, was well tolerated up to the dose of 4 × 109 cells. However, the maximum-tolerated dose could not be determined though, due to discontinuation of the study because of the relative complexity of cell therapy. With IPH1101, the three-first-dose levels (200, 600 and 1,200 mg/m2) were well tolerated without DLT. At the dose level of 1,800 mg/m2, two patients experienced grade 3 toxicities: fever and arterial hypertension in the first case; arterial hypotension in the second case requiring fluid completion and dopamine support. Severe adverse events were observed only after the administration of IPH1101 alone (first cycle) and were not enhanced by co-treatment with IL-2. The intermediate dose, 1,500 mg/m2, was the highest well-tolerated dose and has been considered as the recommended dose for further clinical development. At this level, four patients were treated and three were evaluable for DLT. Six additional patients were treated with IPH1101 at 1,500 mg/m2 in an extended cohort where IL-2 was administered from cycle 1. Safety assessment of IPH1101 showed classical tolerance signs of activation cytokine release syndrome (fever, nausea, vomiting, chills, abdominal pain, asthenia, headache, diarrhea and hypotension) for most of the patients in the hour following the infusion. With respect to efficacy, disease stabilization according to RECIST criteria was observed in 12 patients, although it must be kept in mind that this population of patients presented advanced disease.
This dose-ranging clinical trial provided the first opportunity to establish the pharmacodynamic profile of a specific γδ T cell agonist in vivo in humans. As demonstrated in non-clinical animal models [16, 18], amplification of γδ T cells on IPH1101 treatment in vivo requires the co-administration of low doses of IL-2. These non-human primate studies had also predicted the kinetics of γδ T cell proliferation in human blood (with a peak around days 6–8), the relationship between IPH1101 dose and γδ T cell amplification, as well as the progressively decreased ability to respond to subsequent administrations of the drug [16] that were in this phase I study. The progressive decrease in γδ amplification in blood following successive administrations was also observed in clinical trials using aminobisphosphonates and low-dose IL-2 [14]. The major limitation of these studies is the monitoring of γδ T cells only in blood and not of tissue/tumor resident ones that might behave differently. The observation of this decreased activation of γδ in vivo questions the interest of using ex vivo γδ T cell therapy rather than attempting to stimulate in vivo γδ T cells with systemic drugs: in a cell therapy setting [17], virtually as many γδ T cells as desired could be injected several times. However, it is still not known whether these ex vivo expanded cells would be maintained long enough in the body and would retain efficient effector properties, owing to their terminal differentiation in in vitro cultures. We lack convenient animal models of cancer to address this highly relevant question and, ultimately, only a comparative trial in human can tell which is the more efficient of the two approaches.
Today, we are leading a set of phase 2 clinical trials with IPH1101, combined with other drugs (e.g., the therapeutic antibody rituximab) and various doses of IL-2, and have already obtained encouraging data in terms of improved pharmacological response in vivo. In particular, we have been able to demonstrate that IPH1101-dependent γδ T cell amplification in vivo is also proportional to IL-2 dose in the non-human primate (H. Sicard, unpublished observation): this prompted us to test higher doses of IL-2 in phase II clinical trials to further improve IPH1101 pharmacodynamics in patients [19]. In the present phase I trial, at the highest doses of IPH101 tested, a few patients’ γδ T cells reached transiently more than 20% of all their blood T cells, without any significant changes in the numbers of the other potential effector lymphocytes such as CD8+ or NK cells. This is a more radical change in blood lymphocytes repertoire than that observed after adoptive transfer of γδ T cells [17] or even as compared to that achieved using pamidronate and low-dose IL-2 to activate γδ T cells in hemato-oncology patients [14]. The relationship between pharmacodynamic response to IPH1101 and clinical efficacy is currently being assessed in several phase II clinical trials in oncology (metastatic renal carcinoma, chronic myeloid leukemia and follicular lymphoma) and infectious disease (chronic hepatitis C viral infection).
References
- 1.Choudhary A, Davodeau F, Moreau A, Peyrat MA, Bonneville M, Jotereau F. Selective lysis of autologous tumor cells by recurrent gamma delta tumor-infiltrating lymphocytes from renal carcinoma. J Immunol. 1995;154:3932–3940. [PubMed] [Google Scholar]
- 2.Viey E, Fromont G, Escudier B, et al. IPH1101-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–1347. doi: 10.4049/jimmunol.174.3.1338. [DOI] [PubMed] [Google Scholar]
- 3.Bachelez H, Flageul B, Degos L, Boumsell L, Bensussan A. TCR gamma delta bearing T lymphocytes infiltrating human primary cutaneous melanomas. J Invest Dermatol. 1992;98:369–374. doi: 10.1111/1523-1747.ep12499808. [DOI] [PubMed] [Google Scholar]
- 4.Bouet-Toussaint F, Cabillic F, Toutirais O, Le Gallo M, Thomas de la Pintière C, Daniel P, Genetet N, Meunier B, Dupont-Bierre E, Boudjema K, Catros V. Vgamma9Vdelta2 T cell-mediated recognition of human solid tumors Potential for immunotherapy of hepatocellular and colorectal carcinomas. Cancer Immunol Immunother. 2008;57:531–539. doi: 10.1007/s00262-007-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wrobel P, Shojaei H, Schittek B, Gieseler F, Wollenberg B, Kalthoff H, Kabelitz D, Wesch D. Lysis of a broad range of epithelial tumour cells by human gamma delta T cells: involvement of NKG2D ligands and T-cell receptor-versus NKG2D-dependent recognition. Scand J Immunol. 2007;66:320–328. doi: 10.1111/j.1365-3083.2007.01963.x. [DOI] [PubMed] [Google Scholar]
- 6.Corvaisier M, Moreau-Aubry A, Diez E, Bennouna J, Mosnier JF, Scotet E, Bonneville M, Jotereau F. V gamma 9V delta 2 T cell response to colon carcinoma cells. J Immunol. 2005;175:5481–5488. doi: 10.4049/jimmunol.175.8.5481. [DOI] [PubMed] [Google Scholar]
- 7.Murayama M, Tanaka Y, Yagi J, Uchiyama T, Ogawa K. Antitumor activity and some immunological properties of gammadelta T-cells from patients with gastrointestinal carcinomas. Anticancer Res. 2008;28:2921–2931. [PubMed] [Google Scholar]
- 8.Bryant NL, Suarez-Cuervo C, Gillespie GY, Markert JM, Nabors LB, Meleth S, Lopez RD, Lamb LS., Jr Characterization and immunotherapeutic potential of gammadelta T-cells in patients with glioblastoma. Neuro Oncol. 2009;11:3557–3567. doi: 10.1215/15228517-2008-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, Orlando V, La Mendola C, Gulotta G, Salerno A, Dieli F, Stassi G. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol. 2009;182:7287–7296. doi: 10.4049/jimmunol.0804288. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Niu H, He W, Ba D. Antitumor activity of expanded human tumor-infiltrating gammadelta T lymphocytes. Int Arch Allergy Immunol. 2001;125:256–263. doi: 10.1159/000053824. [DOI] [PubMed] [Google Scholar]
- 11.Kang N, Zhou J, Zhang T, Wang L, Lu F, Cui Y, Cui L, He W. Adoptive immunotherapy of lung cancer with immobilized anti-TCR gammadelta antibody-expanded human gammadelta T-cells in peripheral blood. Cancer Biol Ther. 2009;8:1540–1549. doi: 10.1158/1535-7163.MCT-08-0811. [DOI] [PubMed] [Google Scholar]
- 12.Viey E, Lucas C, Romagne F, Escudier B, Chouaib S, Caignard A. Chemokine receptors expression and migration potential of tumor-infiltrating and peripheral-expanded Vgamma9Vdelta2 T cells from renal cell carcinoma patients. J Immunother. 2008;31:313–323. doi: 10.1097/CJI.0b013e3181609988. [DOI] [PubMed] [Google Scholar]
- 13.Espinosa E, Belmant C, Pont F, Luciani B, Poupot R, Romagné F, Brailly H, Bonneville M, Fournié JJ. Chemical synthesis and biological activity of bromohydrin pyrophosphate, a potent stimulator of human gamma delta T cells. J Biol Chem. 2001;276:18337–18344. doi: 10.1074/jbc.M100495200. [DOI] [PubMed] [Google Scholar]
- 14.Wilhelm M, Kunzmann V, Eckstein S, Reimer P, Weissinger F, Ruediger T, Tony HP. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 15.Dieli F, Vermijlen D, Fulfaro F, Caccamo N, Meraviglia S, Cicero G, Roberts A, Buccheri S, D’Asaro M, Gebbia N, Salerno A, Eberl M, Hayday AC. Targeting human gammadelta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7457. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sicard H, Ingoure S, Luciani B, Serraz C, Fournié JJ, Bonneville M, Tiollier J, Romagné F. In vivo immunomanipulation of V gamma 9V delta 2 T cells with a synthetic phosphoantigen in a preclinical nonhuman primate model. J Immunol. 2005;175:5471–5480. doi: 10.4049/jimmunol.175.8.5471. [DOI] [PubMed] [Google Scholar]
- 17.Bennouna J, Bompas E, Neidhardt EM, Rolland F, Philip I, Galéa C, Salot S, Saiagh S, Audrain M, Rimbert M, Lafaye-de Micheaux S, Tiollier J, Négrier S. Phase-I study of Innacell gammadelta, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Casetti R, Perretta G, Taglioni A, Mattei M, Colizzi V, Dieli F, D’Offizi G, Malkovsky M, Poccia F. Drug-induced expansion and differentiation of V gamma 9V delta 2 T cells in vivo: the role of exogenous IL-2. J Immunol. 2005;175:1593–1598. doi: 10.4049/jimmunol.175.3.1593. [DOI] [PubMed] [Google Scholar]
- 19.Laurent G, Lafaye de Micheaux S, Solal-Celigny P, Soubeyran P, Delwail V, Ghesquieres H, Thieblemont C, Jourdan E, Beautier L, Audibert F, Squiban P, Sicard H, Rossi JF. Phase I/II study of IPH1101, γδ T cell agonist, combined with rituximab, in low grade follicular lymphoma patients. Blood. 2009;114:658–659. [Google Scholar]