Abstract
Our previous studies have described a rare type of antibody that spontaneously binds to itself, or homodimerizes. This self-binding, or autophilic antibody provides stronger protection against bacterial infection than a non-self-binding antibody with identical specificity and affinity, due to an increase of polymeric avidity. Furthermore, we have shown that a peptide derived from the self-binding domain of the autophilic T15 antibody can be crosslinked to the Fc carbohydrate of monoclonal antibodies specific for the B-cell receptor of B-cell tumors. These peptide-crosslinked antibodies can exert self-binding properties, leading to an increase in binding efficiency to the target cells as well as an increase in potential to induce apoptosis. Herein, we report a novel finding that crosslinking of the autophilic T15 peptide rescues a loss-of-function chimerized (ch) anti-GM2 antibody. The parental antibody demonstrates in vivo anti-tumor activity against melanoma xenografts. The T15 peptide-conjugated antibody shows the ability to bind to itself, as well as an increased binding to its antigen, ganglioside GM2. Moreover, the peptide-conjugated antibody also demonstrates an increased ability to bind to two GM2-positive tumor cell lines and notably important, restores its ability to induce apoptosis in two types of tumor cells. These results provide strong support for the clinical potential of the autophilic technology.
Keywords: Anti-GM2 antibody, Autophilic antibody, Self-binding, Ganglioside
Introduction
Monoclonal antibodies (mAbs) are known to be the first successful targeted therapy for cancer. In contrast to the non-specific nature of most chemotherapy, antibodies bind with high specificity to cell-surface antigens, resulting in targeted killing of malignant cells, relative sparing of normal tissues, and low toxicity. Antibody therapy has undergone substantial development since Ehrlich’s notion of a “magic bullet” in 1890. It was not until the 1970s, however, that mAbs became viable therapeutic tools with clinical studies showing them to be effective. As of late 2004, 26 antibody-based therapeutic agents have been approved in the European Union and the US. Some 500 such products are currently in development, ensuring that the number of approved antibody-based products will increase substantially over the coming years [1].
Accumulating evidence indicates that cellular function and phenotype are highly influenced by gangliosides, which are acidic glycosphingolipids that are characterized by the presence of at least one sialic acid linked to their unique oligosaccharide chain. They are present on the outer leaflet of plasma cell membranes of all types of tissues [2]. Gangliosides GD3, GD2, and GM2, which are the major gangliosides expressed on most human cancers of neuroectodermal and epithelial origin, have been focused on as effective targets for passive immunotherapy with mAbs [3]. A hybridoma anti-GM2 mAb, DMF10.167.4, was previously developed that specifically bound with high affinity to the respective antigen and exhibited potent immune effector functions [4]. While humanization of the anti-ganglioside antibody is expected to enhance its use for human cancer therapy, a partially humanized (chimerized) anti-GM2 mAb was developed which maintains its antigen binding ability, but weakened immune effector functions, by losing its ability to induce apoptosis of target cancerous cells (unpublished data).
In order to “restore” the immunological function of this chimeric antibody, we proposed to endow the self-binding feature into it. The self-binding property of antibodies was identified in mouse antibodies directed against phosphorylcholine that form soluble self-complexes without chemical bonds [5]. Subsequently, we described a peptide derived from the variable domain of the heavy chain that comprises regions of CDR2 and FR3 of the germline encoded S107/TEPC15 (T15) antibody, being able to inhibit the homodimerization [6]. Furthermore, our studies have also shown that after being crosslinked with this self-binding domain peptide (T15), mAbs specific for the cancerous B-cell receptor (BCR) established monomer-dimer equilibrium in solution. The peptide-conjugated antibodies also showed enhanced induction of apoptosis of target tumor cells [7, 8]. In our current study, we covalently attached the self-binding peptide to the chimeric anti-GM2 antibody as described previously [7, 8]. We were able to detect the self-binding of T15 ch anti-GM2 antibody while retaining GM2 antigen specificity, and also the ability to induce apoptosis in GM2-positive Jurkat and SBC-3 cells, which have high and intermediate expression levels of GM2, respectively.
Materials and methods
Cell lines and antibody
Human T-cell leukemia Jurkat and small cell lung carcinoma SBC-3 lines were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotic (penicillin, streptomycin and amphotericin). Human cervical carcinoma HeLa cells were grown in DMEM supplemented with 10% fetal bovine serum and antibiotic (penicillin, streptomycin, and amphotericin). Chimeric anti-GM2 antibody (ch-α-GM2, IgG1) was obtained from Corixa Corporation (Seattle, WA, USA). After chimerization, the resulting antibody lost its ability to induce apoptosis in ganglioside GM2 expressing target cells (unpublished data). Herceptin (an anti-HER2 antibody) was purchased from Genentech (San Francisco, CA, USA).
Peptide conjugation to ch-GM2-antibody
Both the T15 peptide (ASRNKANDYTTEYSASVKGRFIVSR), a VH-derived peptide from a self-binding antibody-T15 [6], and a scrambled peptide, which was randomly generated using the T15 amino acid sequence, were synthesized by Genemed Synthesis (South San Francisco, CA, USA). The scrambled peptide was used as a control. Antibodies were dialyzed against PBS (pH 6.0), then 1/10 volume of 200 μM NaIO4 was added and incubated at 4°C for 30 min in the dark. The reaction was stopped by adding glycerol to a final concentration of 30 μM, and the samples were dialyzed at 4°C for 30 min against PBS (pH 6.0). Fifty times molecular excess of T15 or scrambled peptide was added to the antibodies and incubated at 37°C for 1 h. The T15 peptide is covalently linked to the antibody through any amino terminal or the epsilon amino group of the lysine. L-Lysine was added and incubated at 37°C for 30 min to block any remaining reactive aldehyde group. After this blocking step, the antibody-conjugates were dialyzed against PBS (pH 7.2) at 4°C overnight, and stored at 4°C until use.
Direct antigen binding ELISA
The GM2 ganglioside was dissolved in methanol, and 0.5 μg was coated per well in a 96-well polystyrene plate (Costar, Cambridge, MA, USA) and allowed to dry overnight. The wells were blocked with 1% BSA for 2 h at room temperature and 500 ng of anti-GM2 or anti-HER2 antibody, diluted in 1% BSA, were added in the first well and serially diluted in 1:1 ratio. After incubation for 1 h, the wells were washed five times with washing solution and HRP-conjugated goat anti-human IgG (Sigma, St Louis, MO, USA) was added at a 1:1000 dilution and incubated for 1.5 h. After washing three times, the bound antibodies were visualized using a substrate o-phenylenediamine and ELISA plate was read at OD 492 using a Microplate Reader (Fisher Scientific, Pittsburgh, PA, USA).
Antigen binding ELISA with biotinylated antibodies
Anti-GM2 antibody was chemically biotinylated with 15 M excess of NHS-biotin according to manufacturer’s protocol (Pierce, Rockford, IL, USA). Subsequent to biotinylation, anti-GM2 was conjugated to T15 peptide, as described previously.
GM2 ganglioside was dissolved in methanol, and 0.5 μg was coated per well in a 96-well polystyrene plate and allowed to dry overnight. Biotinylated antibody diluted in 1% BSA was added at 2 μg/200 μl to the first well, and serially diluted in 1:1 ratio. After incubation for 1 h, the wells were washed five times with washing solution. 100 microliter of avidin-HRP (Sigma) at a 1:1000 dilution was added and incubated for 1.5 h. After washing three times, the bound antibodies were visualized using a substrate o-phenylenediamine and ELISA plate was read at OD 492 using a Microplate Reader.
Specific antigen binding ELISA
Gangliosides GM1, GM2, and GM3 were dissolved in methanol and 0.5 μg was coated in a 96-well polystyrene plate, and dried overnight. The wells were blocked with 1% BSA for 2 h at room temperature, followed by the addition of 400 ng of T15 peptide-conjugated anti-GM2 antibodies (anti-GM2-T15) to the first well and then serially diluted in 1:1 ratio. After incubation for 1 h, the wells were washed five times with washing solution and HRP-conjugated goat anti-human IgG was added and incubated for 1.5 h. After washing three times, the bound antibodies were visualized using a substrate o-phenylenediamine and assayed as described previously.
Antibody self-binding ELISA
Four microgram per milliliter of anti-GM2-T15 was used to coat a 96-well polyvinyl chloride plate (Fisher Scientific). After blocking with 3% BSA, 2 μg of NHS-biotinylated [7] anti-GM2 or anti-GM2-T15 was added into first well and serially diluted in 1:1 ratio. The antibodies were then incubated for 2 h at room temperature. After washing three times, avidin-HRP was added at a 1:1,000 dilution and incubated for 1 h. The bound antibodies were visualized by adding a substrate o-phenylenediamine and assayed as described previously.
Cell surface binding detected by FACS
The 2 × 105 per well of Jurkat or HeLa cells were seeded in a 6-well plate and incubated overnight. Cells were then collected and washed twice with P/B/G/A buffer (0.5% BSA, 5% goat serum in PBS [pH 7.4]). The cells were then resuspended in 100 μl P/B/G/A buffer containing 5 μg/ml anti-GM2 antibodies on ice for 30 min. After washing with P/B/G/A buffer, FITC-conjugated anti-Human IgG (Sigma, 1:1,000 dilution in 100 μl P/B/G/A) was added and incubated on ice for another 30 min. After washing with P/B/G/A buffer, cells were resuspended in 400 μl P/B/G/A buffer containing 10 μg/ml propidium iodide as viability probe, and analyzed by flow cytometry (Cellquest FACS Instrument, BD Bioscience, Mountain View, CA, USA). The index as defined fluorescence increase percentage of cells was determined by gating positive fluorescence of cells with or without antibody treatment. The increase in fluorescence was calculated according to the formula: (% gated cells with antibody)/(% gated cells without antibody) × 100.
Apoptosis detected by annexin V staining
The 2×105 per well of Jurkat, SBC-3 or HeLa cells were seeded in a 6-well plate. After 6 h, the cells were incubated with 20 μg/ml of human IgG, anti-GM2 or anti-GM2-T15 or scramble T15-conjugated anti-GM2 (anti-GM2-sT15) antibodies for 20 h. After the incubation, a small portion (50 μl) of Jurkat cells was assayed for cell viability. The remaining cells were harvested and washed with cold PBS (pH 7.4). Cells were then resuspended in 100 μl of 1× annexin binding buffer containing annexin V (Molecular Probes, Eugene, OR, USA) and prepared according to manufacture’s instructions. After incubation for 15 min at room temperature, the cells were diluted with 400 μl of 1× annexin binding, and analyzed by flow cytometry (Cellquest FACS instrument, BD Bioscience, Mountain View, CA, USA).
Cell viability assay
The small portion of the Jurkat cell samples saved from the previous step was used for viability assay. Ten-microliter aliquots from the cell suspension were taken to determine viability using trypan blue exclusion assay.
Statistical analysis
Statistical analysis was performed using one-way ANOVA followed by Newman–Keuls post-test. Data are reported as means ± SD.
Results
Self-binding peptide enhanced antibody binding to its specific ganglioside
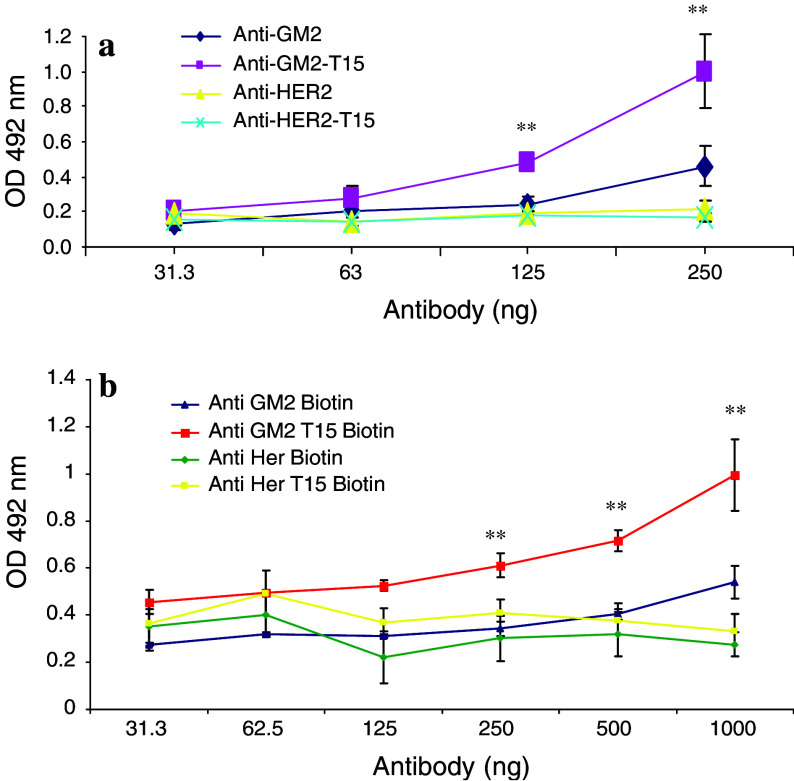
Following antibody-peptide conjugation, the binding capacity of the T15-conjugated ch-α-GM2 antibody (anti-GM2-T15) was determined using a direct antigen binding ELISA assay. As seen in Fig. 1a, both ch-α-GM2 antibody (anti-GM2) and anti-ch-GM2-T15 antibody (anti-GM2-T5 antibody) showed a dose-dependent increase in binding to ganglioside GM2. Anti-GM2-T15 showed a significantly higher binding capacity compared with anti-GM2 at almost all the doses tested, confirming that by attaching the self-binding peptide, the antigen binding capacity of the ch-α-GM2 antibody has been increased at the given antibody concentrations. To demonstrate that the autophilic antibody has increased potency in detecting binding to target antigen compared to native antibody a direct antigen binding assay was performed. The control for this increase is the use of the naked anti-GM2, which shows less binding than anti-GM2-T15 (see Fig. 1a). Furthermore, to demonstrate that autophilic antibody with different specificity (anti-HER2-T15) does not bind to the GM2 target. The increased potency is achieved by conjugating the autophilic peptide. This control shows that the conjugation of the autophilic peptide does not change the specificity (Fig. 1a).
Fig. 1.
a Direct antigen binding to ganglioside GM2 of anti-GM2 and anti-GM2-T15 detected by ELISA assay. GM2 ganglioside was coated onto the ELISA plate. An anti-HER2 antibody (Herceptin) was applied as non-specific control. Three independent experiments have been performed to determine the means and standard deviations. b GM2 ganglioside antigen was coated onto plates, antibody was added and serially diluted. An avidin-HRP conjugate was used to rule out Fc effects. Double asterisk, significant difference compared with anti-GM2 group, p < 0.01
In addition, a biotin-avidin system was used to show that the increased signal is due to the increased number of autophilic antibodies binding to the target and not due to the increased Fc affinity of the secondary antibody. The autophilic anti-GM2 and anti-HER2 were biotinylated. As shown in Fig. 1b, the anti-HER2 and autophilic anti-HER2 (anti-HER2-T15) did not bind to GM2 ganglioside. The autophilic anti-GM2 showed a significant increase in binding to GM2 ganglioside compared with naked anti-GM2, confirming the increased binding with the secondary antibody seen in Fig. 1a.
Antibody self-binding behavior was demonstrated by ELISA assay
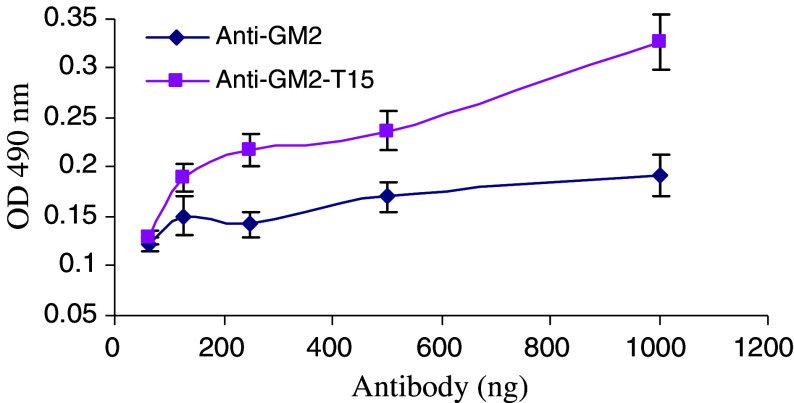
Next, we investigated by ELISA whether the increase in binding to ganglioside GM2 shown by anti-GM2-T15 was due to a new self-binding feature. As seen in Fig. 2, the anti-GM2-T15 demonstrated a greater dose-dependent increase in binding to itself, whereas non-peptide conjugated anti-GM2 antibody did not show significant binding. These data demonstrate that the anti-GM2-T15 can bind to itself or homodimerize through the Fc-conjugated, autophilic peptide moiety.
Fig. 2.
Self-binding activity of anti-GM2-T15 detected by ELISA assay. Anti-GM2-T15 was coated onto the ELISA plate. Three independent experiments have been performed to determine the means and SD. Double asterisk, significant difference compared with anti-GM2 group, p < 0.01
T15 conjugation did not change the specificity of the ch-α-GM2 antibody
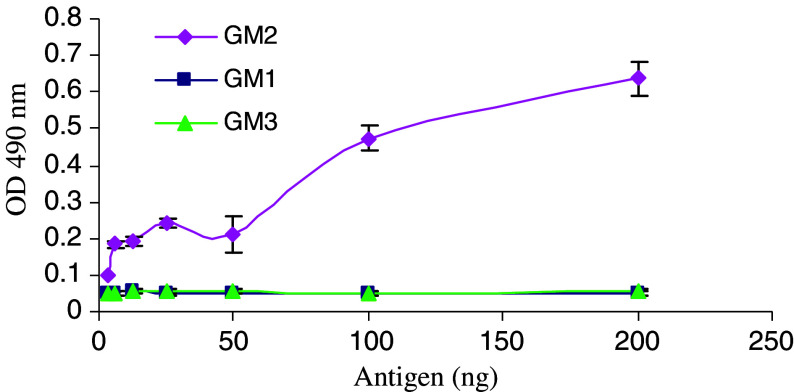
To assess whether conjugation of the T15 peptide might alter the cognate binding specificity of the antibody, a direct antigen binding ELISA assay was performed. As shown in Fig. 3, the anti-GM2-T15 demonstrated a specific, almost dose-dependent increase in binding to ganglioside GM2, whereas no binding above background levels to gangliosides GM1 or GM3 was detected. These results confirm that addition of the self-binding T15 peptide does not alter nor reduce the specificity of the ch-α-GM2 antibody.
Fig. 3.
Antigen binding specificity of anti-GM2-T15 detected by ELISA. Three types of ganglioside (GM1, GM2, and GM3) were coated onto the ELISA plate. Three independent experiments have been performed to determine the means and SD
Anti-GM2-T15 showed enhancement of cell surface binding
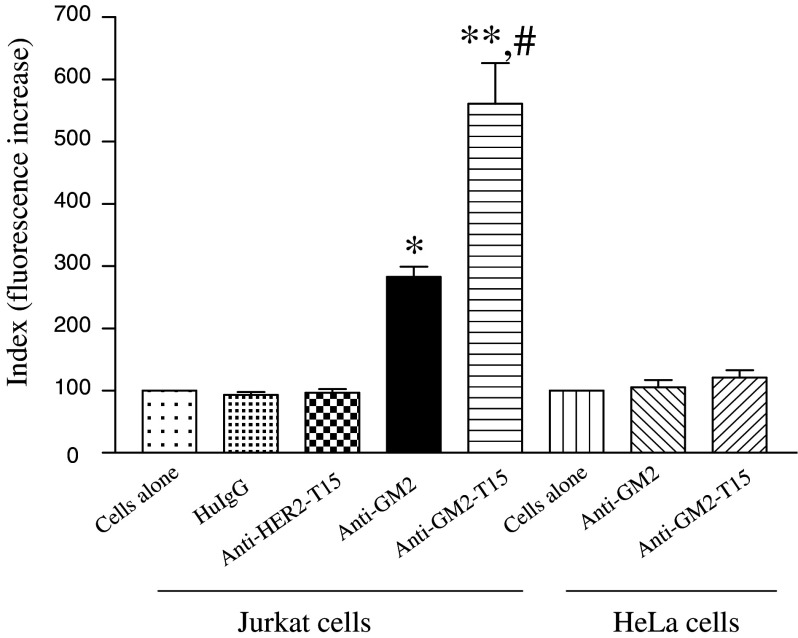
The human T-cell leukemic cell line Jurkat is known to express high level of ganglioside GM2 [9], whereas HeLa cells express undetectable level of GM2 [10]. The ability of anti-GM2-T15 to bind to native ganglioside GM2 expressed on the surface of Jurkat cells was compared to that of anti-GM2 by flow cytometry. As shown in Fig. 4, anti-GM2 demonstrated a GM2 specific binding signal 3-fold greater than background levels, whereas the binding demonstrated by the anti-GM2-T15 was 2-fold higher than that of anti-GM2. These results suggest that the enhanced binding demonstrated by the peptide-conjugated antibody is due to homodimerization, which generates a lattice formation on the surface of the tumor cells.
Fig. 4.
Comparison of cell surface binding using FACS analysis with Jurkat (GM2 positive) and HeLa cells (GM2 negative). Calculation of florescence increase is described in the Materials and Methods section. Single asterisk, significant difference compared with cells-alone group, p < 0.05. Double asterisk, significant difference compared with cells-alone group, p < 0.01. Hash, significant difference compared with anti-GM2 group, p < 0.05
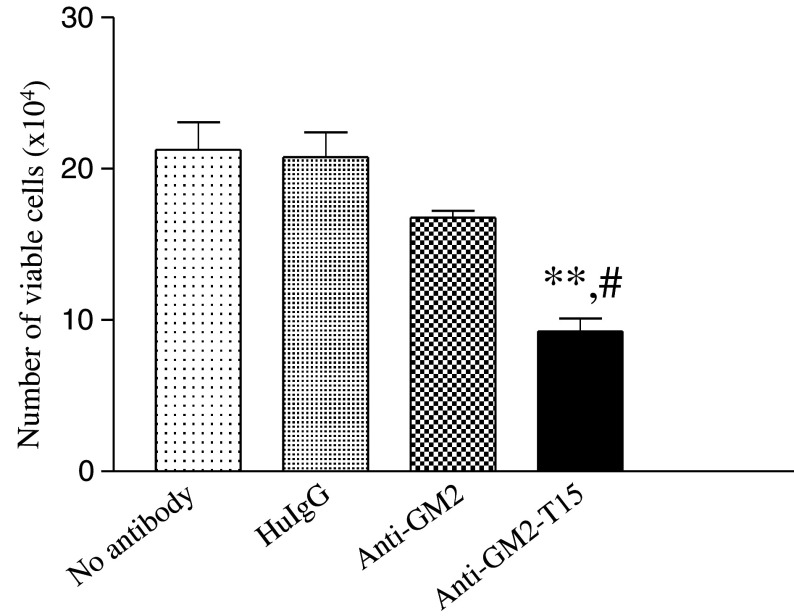
Anti-GM2-T15 inhibited tumor cell growth
Antibodies binding to the BCR have been shown to induce crosslinking of the BCR, leading to greater inhibition of cell proliferation [11] and producing a death signal [12, 13]. Furthermore, chemically dimerized antibodies directed against a B-cell tumor induce hyper-crosslinking of the BCR followed by inhibition of cell division and induction of apoptosis of the tumor cells [14, 15]. To determine whether anti-GM2-T15 induced a similar anti-proliferative effect corresponding to a reduced number of cells, cell viability was measured as described in material and methods. As summarized in Fig. 5, human IgG antibody treatment had no effect on cell growth or viability, whereas there was a slight reduction with the anti-GM2 antibody, which was judged not to be statistically different. However, anti-GM2-T15 demonstrated a significant inhibition of Jurkat cell growth, as cell numbers were reduced greater than 2-fold compared to naked anti-GM2 treated sample.
Fig. 5.
Inhibition of Jurkat cell growth. The 2 × 105 Jurkat cells were incubated with antibodies for 20 h. Three independent experiments have been performed to determine the means and SD. HuIgG, human IgG. Double asterisk, significant difference compared with no-antibody group, p < 0.01. Hash, significant difference compared with anti-GM2 group, p < 0.05
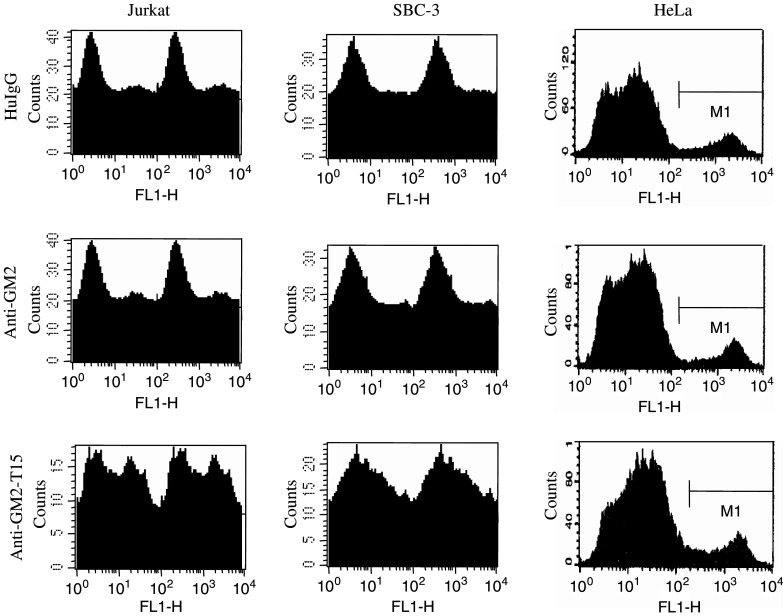
Anti-GM2-T15 could induce apoptosis of target cells
In order to determine whether the anti-tumor effect of the T15-conjugated antibodies directed against cell surface expressed gangliosides might be due to the induction of apoptosis, we treated GM2-positive Jurkat and SBC-3 cells with anti-GM2-T15 and various controls. We also included HeLa cells expressing only transient, low levels of GM2 ganglioside as negative control cell line. Apoptosis was then analyzed by measuring annexin V staining by FACS. The results are summarized in Table 1 and an example of representative FACS results shown in Fig. 6. Treatment of Jurkat/SBC-3 cells with the anti-GM2 or the anti-GM2 conjugated scrambled T15 peptide (anti-GM2-sT15) did not induce apoptosis as compared with control human IgG treatment. However, Jurkat/SBC-3 cells treated with the anti-GM2-T15 conjugated underwent a significant amount of apoptosis, nearly 4-fold higher than that induced by the non-conjugated antibody or the scrambled T15-conjugated antibody. Furthermore, we tested T15-conjugated anti-GM2 antibody on HeLa cells, and a slight, but non-significant increase in apoptosis was observed in HeLa cells. These results confirm that we could restore the apoptosis-inducing ability of the chimeric anti-GM2 without altering its specificity by endowing the self-binding feature.
Table 1.
Apoptosis analysis using Annexin V staining
| Treatment | Samples | ||
|---|---|---|---|
| Jurkat | SBC-3 | HeLa | |
| HuIgG | 9.90 ± 1.6 | 8.80 ± 2.8 | 17.4 ± 4.0 |
| Anti-GM2 | 10.0 ± 1.9 | 7.50 ± 2.0 | 16.3 ± 3.8 |
| Anti-GM2-sT15 | 10.3 ± 0.1 | 6.50 ± 0.2 | 18.3 ± 4.5 |
| Anti-GM2-T15 | 41.3 ± 4.8a | 25.0 ± 8.1a | 25.7 ± 14.0 |
| Staurosporine | 99.0 ± 0.1a | 77.7 ± 5.5a | 54.2 ± 12.0a,b |
Data were summarized from three sets of experiments, the percentage of Annexin V positive cells was presented. HuIgG, human IgG; staurosporine (1 nmol/l) was used as an inducer of apoptosis
a P < 0.01 when compared with HuIgG group
bHeLa cells were treated with actinomycin D (1 μg/ml)
Fig. 6.
A set of representative FACS results of annexin V staining. Twenty microgram of antibodies was incubated with cells overnight before being collected and stained for annexin V. HuIgG, human IgG
Discussion
Advances in our understanding of the molecular mechanisms underlying the development and progression of diseases have resulted in the discovery of new therapeutic interventions that target specific molecular abnormalities. As “magic bullets”, antibodies have been developed to target cell-surface antigens with expression restricted to disease status. Currently, antibodies are in use for many clinical applications and now comprise the second largest category of medicines in clinical development after vaccine [16]. A critical limitation of the first animal-derived mAbs was the induction of a human anti-mouse immunoglobulin immune response in the majority of patients [17]. This resulted in rapid clearance of the antibody and reduced tumor targeting with subsequent dosing. To overcome this defect, strategies aimed to decrease the immunogenicity of the antibodies, ranging from chimeric antibodies with a combination of human constant regions with rodent variable regions to fully reshaped antibodies where the variable regions are also humanized, were developed. However, the potency of these modified antibodies has become a major obstacle of this approach. A monoclonal hamster anti-GM2 antibody (DMF10.167.4, IgG, kappa of unknown subclass) was previously developed which specifically bound to the respective antigen with high affinity and showed potent immune effector functions [4]. Since complete or partial humanization (chimerization) of this anti-ganglioside antibody is required for human cancer therapy, a chimeric (ch) -anti-GM2 mAb (IgG1, kappa), was later developed. Ch-anti-GM2 mAb maintained its ganglioside GM2 antigen binding but weakened its immune effector functions to induce apoptosis of target cancerous cells. This specific case illustrates a common dilemma in antibody humanization.
The avidity increase due to covalent antibody polymerization can also be obtained through non-covalent inter-molecular homophilic interactions in mouse IgG3 class antibodies via the Fc–Fc mediated dimerization [18–21]; and Fab–Fab mediated self-binding which includes the monoclonal anti-ganglioside GD3 antibodies [22, 23]. One of us [5, 6] discovered a type of anti-phosphorylcholine antibodies exerting self-binding property. A peptide was identified derived from the variable domain of the heavy chain of the germline encoded S107/TEPC15 (T15) antibody, through which the antibody homodimerizes [6]. Homodimerization is not mediated by the Fc fragment since it could be demonstrated that F(ab’)2 of T15 formed self-complexing in solution [24].
Furthermore, endowment of self-binding feature to non-autophilic antibodies by attaching T15 peptide has previously been shown to increase the efficacy of the antibodies without affecting the non-target cells [7, 8]. We used this strategy to endow self-binding properties to the chimeric anti-GM2 mAb. The results are encouraging, as the self-binding antibody increases antigen binding both to insolubilized GM-2 antigen (Fig. 1) and cell surface antigen (Fig. 4), regaining the ability of the parental antibody (DMF-10.167.4) to induce apoptosis in GM2-positive human T-cell leukemia Jurkat cells and small cell lung carcinoma SBC-3 cells (Fig. 6 and Table 1). In addition to the increase in antibody avidity, the T15-conjugated antibody also shows an increase in affinity as suggested by BioCore analysis (data not shown). Moreover, the T15-conjugation does not change the specificity or cross-reactivity of the antibody as it shows no significant induction of apoptosis in GM2 HeLa cells, which is consistent with our previous finding on T15-conjugated anti-CD20 antibodies [7, 8]. This is corroborated by our latest finding that anti-HER2 (Herceptin)-T15 does not cross-react with T-cell leukemia Jurkat cells (Fig. 4). Anti-HER2-T15 showed enhanced binding to low Her2-receptor expressing breast cancer cells compared to native Herceptin (manuscript in preparation).
Chimeric and humanized antibodies have been proven non-toxic in patients, and can be given for a long time in chronic diseases. Since T15 idiotype is shared by human polyclonal anti-phosphorylcholine antibodies [25], representing a naturally occurring, evolutionary conserved idiotype expressed in mouse and man, a toxic effect in human would not be expected. However, further testing is needed and warrants a non-human primate (Macacc mulatta) model to evaluate the effect in vivo of T15-peptide linked chimeric or humanized antibodies.
In summary, our results demonstrate a novel approach: endowing self-binding to a functionally weakened chimeric anti-GM2 antibody by T15 peptide-conjugation leads to increased binding to specific antigen, and restores apoptosis-inducing activity in target tumor cells. We believe the increase in avidity of antibodies results in aggregation by cross-linking on tumor cell surface, and thereby lowers the threshold for initiation of cell signaling [26].
Acknowledgment
InNexus Biotechnology Inc. Vancouver, BC was funding this study. We thank Leslie Miller for the technical assistance in the InNexus/ImmPheron lab, and Jennifer Strange and Greg Bauman at University of Kentucky for their technical support on FACS analysis.
References
- 1.Walsh G (2004) Modern antibody-based therapeutics. BioPharm Int 1–5
- 2.Birkle S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression (Review) Biochimie. 2003;85:455–463. doi: 10.1016/S0300-9084(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 3.Nakamura K, Tanaka Y, Shitara K, Hanai N. Construction of humanized anti-ganglioside monoclonal antibodies with potent immune effector functions. . Cancer Immunol Immunother. 2001;50:275–284. doi: 10.1007/PL00006689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Retter MW, Johnson JC, Peckham DW, Bannink JE, Bangur CS, Dresser K, Cai F, Foy TM, Fanger NA, Fanger GR, Woda B, Rock KL. Characterization of a proapoptotic antiganglioside GM2 monoclonal antibody and evaluation of its therapeutic effect on melanoma and small cell lung carcinoma xenografts. Cancer Res. 2005;65:6425–6434. doi: 10.1158/0008-5472.CAN-05-0300. [DOI] [PubMed] [Google Scholar]
- 5.Kang CY, Kohler H. Immunoglobulin with complementary paratope and idiotope. J Exp Med. 1986;163:787–792. doi: 10.1084/jem.163.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kang CY, Brunck TK, Kieber-Emmons T, Blalock JE, Kohler H. Inhibition of self-binding antibodies (autobodies) by a VH-derived peptide. Science. 1988;240:1034–1036. doi: 10.1126/science.3368787. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Lou D, Burke J, Kohler H. Enhanced anti B-Cell tumor effects with anti-CD20 superantibody. . J Immunother. 2002;25:57–62. doi: 10.1097/00002371-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Y, Kohler H. Enhancing tumor targeting and apoptosis using non-covalent antibody homodimers. J Immunother. 2002;25:396–404. doi: 10.1097/00002371-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Hirabayashi Y, Matsumoto N, Kato H, Hidari K, Tsuchiya K, Matsumoto M, Hoshino H, Tozawa H, Miwa M. Aberrant expression of ganglioside and asialoglycosphingolipid antigens in adult T-cell leukemia cells. Jpn J Cancer Res. 1987;78:1112–1120. [PubMed] [Google Scholar]
- 10.Fernandes DM, Baird AM, Berg LJ, Rock KL. A monoclonal antibody reactive with a 40-kDa molecule on fetal thymocytes and tumor cells blocks proliferation and stimulates aggregation and apoptosis. J Immunol. 1999;163:1306–1314. [PubMed] [Google Scholar]
- 11.Ward RE, McNamara-Ward M, Webb CF, Altman D, Lim PK, Tucker PW, Kohler H. Regulation of an idiotype+ B cell lymphoma: effects of antigen and anti-idiotypic antibodies on proliferation and Ig secretion. J Immunol. 1988;141:340–345. [PubMed] [Google Scholar]
- 12.Hasbold J, Klaus GGB. Anti-immunoglobulin antibodies induce apoptosis in immature B cell lymphomas. Eur J Immunol. 1990;20:1685–1690. doi: 10.1002/eji.1830200810. [DOI] [PubMed] [Google Scholar]
- 13.Wallen-Ohman M, Lonnbro P, Schon A, Borrebaeck CAK. Antibody-induced apoptosis in a human leukemia cell line is energy dependent: thermochemical analysis of cellular metabolism. Cancer Lett. 1993;75:103–109. doi: 10.1016/0304-3835(93)90194-E. [DOI] [PubMed] [Google Scholar]
- 14.Ghetie MA, Picker LJ, Richardson JA, Tucker K, Uhr JW, Vitetta ES. Anti-CD19 inhibits the growth of human B-cell tumor lines in vitro and of Daudi cells in SCID mice by inducing cell cycle arrest. Blood. 1994;83:1329–1336. [PubMed] [Google Scholar]
- 15.Ghetie MA, Podar EM, Ilgen A, Gordon BE, Uhr JW, Vitetta ES. Homodimerization of tumor-reactive monoclonal antibodies markedly increases their ability to induce growth arrest or apoptosis of tumor cells. Proc Natl Acad Sci USA. 1997;94:7509–7514. doi: 10.1073/pnas.94.14.7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chester K, Pedley B, Tolner B, Violet J, Mayer A, Sharma S, Boxer G, Green A, Nagl S, Begent R. Engineering antibodies for clinical applications in cancer (Review) Tumour Biol. 2004;25:91–98. doi: 10.1159/000077727. [DOI] [PubMed] [Google Scholar]
- 17.Gorman SD, Clark MR. Humanisation of monoclonal antibodies for therapy (Review) Semin Immunol. 1990;2:457–466. [PubMed] [Google Scholar]
- 18.Grey HM, Hirst JW, Cohn M. A new mouse immunoglobulin: IgG3. J Exp Med. 1971;133:289–304. doi: 10.1084/jem.133.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehrlich PH, Moyle WR, Moustafa ZA, Canfield RE. Mixing two monoclonal antibodies yields enhanced affinity for antigen. J Immunol. 1982;128:2709–2713. [PubMed] [Google Scholar]
- 20.Greenspan NS, Monafo WJ, Davie JM. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol. 1987;138:285–292. [PubMed] [Google Scholar]
- 21.Greenspan NS, Dacek DA, Cooper LJ. Fc region-dependence of IgG3 anti-streptococcal group A carbohydrate antibody functional affinity. I. The effect of temperature. J Immunol. 1988;141:4276–4282. [PubMed] [Google Scholar]
- 22.Chapman PB, Yuasa H, Houghton AN. Homophilic binding of mouse monoclonal antibodies against GD3 ganglioside. J Immunol. 1990;145:891–898. [PubMed] [Google Scholar]
- 23.Yan X, Evans SV, Kaminki MJ, Gillies SD, Reisfeld RA, Houghton AN, Chapman PB. Characterization of an Ig VH idiotope that results in specific homophilic binding and increased avidity for antigen. J Immunol. 1996;157:1582–1588. [PubMed] [Google Scholar]
- 24.Kaveri SV, Halpern R, Kang CY, Kohler H. Self-binding antibodies (autobodies) form specific complexes in solution. J Immunol. 1990;145:2533–2538. [PubMed] [Google Scholar]
- 25.Halpern R, Kaveri SV, Kohler H. Human anti-phosphorylcholine antibodies share idiotopes and are self-binding. J Clin Invest. 1991;88:476–482. doi: 10.1172/JCI115328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Russ M, Morgan C, Muller S, Kohler H. Therapeutic applications of superantibodies. Drug Discov Today. 2005;10:1231–1236. doi: 10.1016/S1359-6446(05)03530-0. [DOI] [PubMed] [Google Scholar]