Abstract
Despite the immunogenicity of glioblastoma multiforme (GBM), immune-mediated eradication of these tumors remains deficient. Regulatory T cells (Tregs) in the blood and within the tumor microenvironment of GBM patients are known to contribute to their dismal immune responses. Here, we determined which chemokine secreted by gliomas can preferentially induce Treg recruitment and migration. In the malignant human glioma cell lines D-54, U-87, U-251, and LN-229, the chemokines CCL22 and CCL2 were detected by intracellular cytokine analysis. Furthermore, tumor cells from eight patients with GBM had a similar chemokine expression profile. However, only CCL2 was detected by enzyme-linked immunosorbent assay, indicating that CCL2 may be the principal chemokine for Treg migration in GBM patients. Interestingly, the Tregs from GBM patients had significantly higher expression levels of the CCL2 receptor CCR4 than did Tregs from healthy controls. Glioma supernatants and the recombinant human chemokines CCL2 and CCL22 induced Treg migration and were blocked by antibodies to the chemokine receptors. Production of CCL2 by glioma cells could also be mitigated by the chemotherapeutic agents temozolomide and carmustine [3-bis (2-chloroethyl)-1-nitrosourea]. Our results indicate that gliomas augment immunosuppression by selective chemokine-mediated recruitment of Tregs into the tumor microenvironment and that modulating this interaction with chemotherapy could facilitate the development of novel immunotherapeutics to malignant gliomas.
Keywords: Gliomas, Regulatory T cells, Chemokines, Tumor immunity
Introduction
Malignant gliomas express tumor-associated and tumor-specific antigens, making them detectable to immune responses [14]. However, there is a distinct lack of immune-mediated tumor eradication in glioma patients and most attempts at tumor immunotherapy in the clinic have been met with little success to date [22]. Many factors work in concert to inhibit anti-glioma immunity, including immunosuppressive cytokines [10, 23, 30] and impaired APC function [2, 11]. Recently, the role of Tregs in mediating this tolerance was demonstrated in glioma patients [7]. We have previously shown the preferential deposition of CD4+CD25+FoxP3+ Tregs within the glioma microenvironment [11] rather than in the peripheral blood, suggesting the presence of chemotactic factors.
Chemokines are small secreted peptides that play a role in the migration and homing of lymphocyte subpopulations, especially Tregs. Individual T-cell subsets express a specific set of chemokine receptors that respond to specific chemokines. In ovarian and prostate cancers, the chemokine CCL22 has been shown to induce the migration of Tregs into the tumors [5, 17]. These Tregs are CD4+CD25+FoxP3+ and express the CCL22 receptor CCR4. The CCR4 receptors also bind another chemokine CCL2, which is also produced by glioma cells [6] and has been shown to induce T-cell infiltration across the blood-brain barrier into the parenchyma [24, 29, 31]. CCL2 binds a second receptor, CCR2, which is expressed on a small percentage of Treg subsets [28].
To determine which chemokines may influence the migration of Tregs into the glioma microenvironment we evaluated chemokine production across a panel of glioma cell lines and directly from resected gliomas. We found that the chemokine CCL2 played a critical role in Treg migration via its receptors CCR2 and CCR4 in glioma patients. Furthermore, CCL2 production was directly suppressed by the chemotherapeutic agents temozolomide and carmustine [3-bis (2-chloroethyl)-1-nitrosourea] (BCNU), indicating the potential to overcome the negative influences of the tumor microenvironment in GBM patients by developing novel immunotherapeutics for malignant glioma.
Materials and methods
Human tumor and blood specimens
Low-grade and high-grade brain tumor tissues were obtained post surgical resection and were graded pathologically as anaplastic astrocytomas (AA, n = 4) and glioblastoma multiforme (GBM, n = 8), respectively, according to the World Health Organization’s classification system by a neuropathologist. Each patient provided written informed consent for tumor tissues as well as having blood drawn during surgical resection, and this study was conducted under protocol #LAB03-0687, which was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center. Blood from healthy individuals (as control subjects) was obtained from the Gulf Coast Blood Center (Houston, TX, USA).
Human glioma cell lines
Human brain tumor cell lines D-54, U-251, LN-229, and U-87 were purchased from the American Type Culture Collection (Manassas, VA, USA) and cultured in modified Eagle’s medium (MEM) (D-54 and U-251), Dulbecco’s MEM/F-12 (LN-229), and MEM + nonessential amino acid salts (U-87). To all media, 10% fetal bovine serum and 1% penicillin-streptomycin were added.
Chemicals
Temozolomide (Schering-Plough, Kenilworth, NJ, USA) was diluted in dimethyl sulfoxide to 20 mM and was then further diluted to 1 and 5 μM in the media appropriate to the respective tumor cell lines. The IC50 of U-87 cells treated with temozolomide for 24 h is 150 μM [27]. BCNU was purchased from Bristol Laboratories (Princeton, NJ, USA), stored at 4°C, and then diluted according to the manufacturer’s instructions: 3.3 mg/ml in 10% ethanol and further in cell-appropriate media to 0.06, and 0.6 μg/ml. The IC50 of U-87 cells treated with BCNU is 12 μg/ml (56 nM).
Intracellular flow cytometric determination of chemokines
When the cells reached confluence, they were washed in phosphate-buffered saline (PBS) and trypsinized. Freshly resected tumors were processed immediately after surgery by being passed through a 70-μm pore size sieve and diluted with flow staining buffer (2% fetal calf serum-PBS). Samples were then passed again through a 70-μm filter to remove additional tumor debris. Aliquots of approximately 1 × 106 cells were plated in each well of a 96-well V-bottom plate and permeabilized with BD Cytofix/Cytoperm (BD Biosciences, San Diego, CA, USA) for 20 min at 4°C. After being washed in PermWash (BD Biosciences), the cells were stained intracellularly with fluorescein isothiocyanate (FITC)- or phycoerythrin-labeled antibodies against CXCL9, CCL3, CCL4, CCL2, and CCL22 (R&D Systems, Minneapolis, MN, USA) for 30 min at 4°C. The cells were then washed twice in PermWash and resuspended in 1% paraformaldehyde until analyzed by fluorescence-activated cell sorter (FACS) (FACSCalibur, BD Biosciences, San Jose, CA, USA) and CellQuest software (BD Biosciences, San Jose, CA).
Glioma supernatant chemokine measurement by ELISA
Supernatants of freshly resected glioma tissue and the human glioma cell lines U-87 and U-251 were measured for the concentrations of CCL2 and CCL22 by using ELISA kits according to the manufacturer’s instructions (R&D Systems). Glioma tissue surgically resected from GBM patients was processed as described above. The supernatants from tissue or cell lines were collected after 5 days in culture and stored at −20°C. They were then thawed and added in duplicate to appropriate precoated plates and incubated for 2 h at room temperature (CCL2) or 4°C (CCL22). After the wells were washed, horseradish peroxidase-conjugated detection antibody was added and plates were incubated for 1 h at room temperature for CCL2 and 2 h at 4°C for CCL22. The substrate used for color development was tetramethylbenzidine. We also assayed for CCL22 concentration by coating the 96-well ELISA plate (Corning, Corning, NY, USA) with antihuman CCL22 capture antibody (clone 57226, R&D Systems) overnight at 4°C. After blocking with 1% bovine serum albumin in PBS at room temperature for 1 h, standards and supernatant specimens were added to the plate and incubated 2 h at room temperature. Biotinylated chicken anti-human CCL22 detection antibody (R&D Systems) was then added to each well and incubated for another 2 h at room temperature. Finally, streptavidin-horseradish peroxidase solution (R&D Systems) was added to each well, and tetramethylbenzidine was used for color development. The optical density was measured at 450 nm with a microplate reader (Spectra Max 190; Molecular Devices, Sunnyvale, CA, USA), and chemokine concentrations were quantitated with the SoftMax Pro software (Molecular Devices). The detection limits for CCL2 and CCL22 were <5 and 62.5 pg/ml, respectively.
Flow cytometric analysis of CCR2 and CCR4 expression on peripheral FoxP3+ Tregs
Peripheral blood mononuclear cells (PBMCs) were isolated from newly diagnosed GBM patients (n = 6) and healthy individuals (n = 4) with Histopaque (Sigma-Aldrich, St Louis, MO, USA) density gradient centrifugation. The PBMCs were stained with PerCP-Cy5.5 conjugated mouse anti-human CD4, phycoerythrin-conjugated mouse anti-human CD25 (Pharmingen, San Diego, CA, USA), and allophycocyanin-conjugated mouse anti-human CCR2 or CCR4 (R&D Systems) for 20 min at 4°C and washed twice in flow staining buffer (2% fetal calf serum-PBS). Intracellular staining of those cells was performed by using FITC conjugated anti-human FoxP3 from eBioscience (San Diego, CA, USA) according to its staining protocol. Finally, the cells were washed twice in permeabilization buffer and resuspended in 1% paraformaldehyde until analyzed by FACSCalibur and CellQuest software (BD Biosciences). Isotype controls with corresponding fluorescence conjugation were used for each antibody in addition to surface stain controls to set appropriate quadrant boundaries for absolute-positive populations. Approximately 0.5 × 106 live, gated events based on forward scatter and side scatter parameters were assessed.
Chemotaxis assay
A T-cell migration assay was performed in micro-transwells of a 5-μM pore size membrane (96-well Boyden chamber, NeuroProbe, Cabin John, MD, USA). PBMCs were isolated from the buffy coats of normal donors (n = 3) or GBM patients (n = 2) with Histopaque (Sigma-Aldrich) density-gradient centrifugation. We purified unlabeled CD4+ T cells from normal PBMCs by using magnetic beads (CD4+ T cell isolation kit II; Miltenyi Biotech, Auburn, CA, USA) and seeded them into the upper chamber at a density of 1 × 106 cells per well in complete RPMI-1640 medium. The purity of the separated CD4+ T-cell population was >96% as determined by flow cytometry with allophycocyanin-conjugated anti-human CD4 monoclonal antibody. Human recombinant CCL2 (0.5 μg/ml) or CCL22 (10 ng/ml) (R&D Systems) was added to the lower chamber in complete RPMI-1640 medium. Mouse anti human CCL2 antibody (5.0 μg/ml, R&D Systems) was applied to neutralize the exogenous recombinant CCL2. The control group contained complete medium only.
For CD4+ T cells blocked by anti-human CCR2 or CCR4 antibodies, 1 × 106 CD4+ T cells were preincubated with 5 μg/ml anti-human CCR2 or CCR4 antibody (clones 48607 and 205410, respectively; R&D Systems) for 20 min at 4°C. After the cells were washed and the supernatants removed, the CD4+ T cells were resuspended in complete RPMI-1640 medium and added to the upper chamber of their designated wells. All experimental groups were duplicated, and the chambers were incubated at 37°C in 5% CO2 for 2 h. Migrated T cells were collected from the bottom chamber and surface stained with allophycocyanin-labeled anti-CD4 and phycoerythrin-labeled anti-CD25 antibodies. They were then stained with fluorescein isothiocyanate-labeled anti-FoxP3 and analyzed with FACSCalibur and CellQuest software. The results were recorded as percentage increases in migrated FoxP3+ Tregs under various chemoattractant circumstances over the numbers of migrated FoxP3+ Tregs in the medium-only control group, plus or minus standard error. The experiment was reproduced three times with similar findings.
Measurement of CCL2 production from U-87 cells treated with BCNU and temozolomide
U-87 cells at 5 × 105 cells/well were seeded in a 12-well plate (BD Falcon, San Jose, CA, USA) in 10% fetal bovine serum /MEM-1 medium and cultured at 37°C in 5% CO2 for 4 days. On day 5, temozolomide (1 and 5 μM) or BCNU (0.28 or 2.8 nM) doses that are achievable within the central nervous system [8, 18] were added into each experimental group for two more days. Untreated U-87 cells were used as a control. Supernatants were then collected and stored at −20°C for ELISA detection of CCL2 production and chemotaxis assay as described above. U-87 cells were then trypsinized and washed with complete MEM-1 medium. We checked viabilities by using the trypan blue exclusion test with the Vi-Cell cell viability analyzer from Beckman Coulter (Fullerton, CA, USA).
Statistics
All p values were calculated with the Student’s t test, and P values <0.05 were considered statistically significant.
Results
Human gliomas secrete CCL2
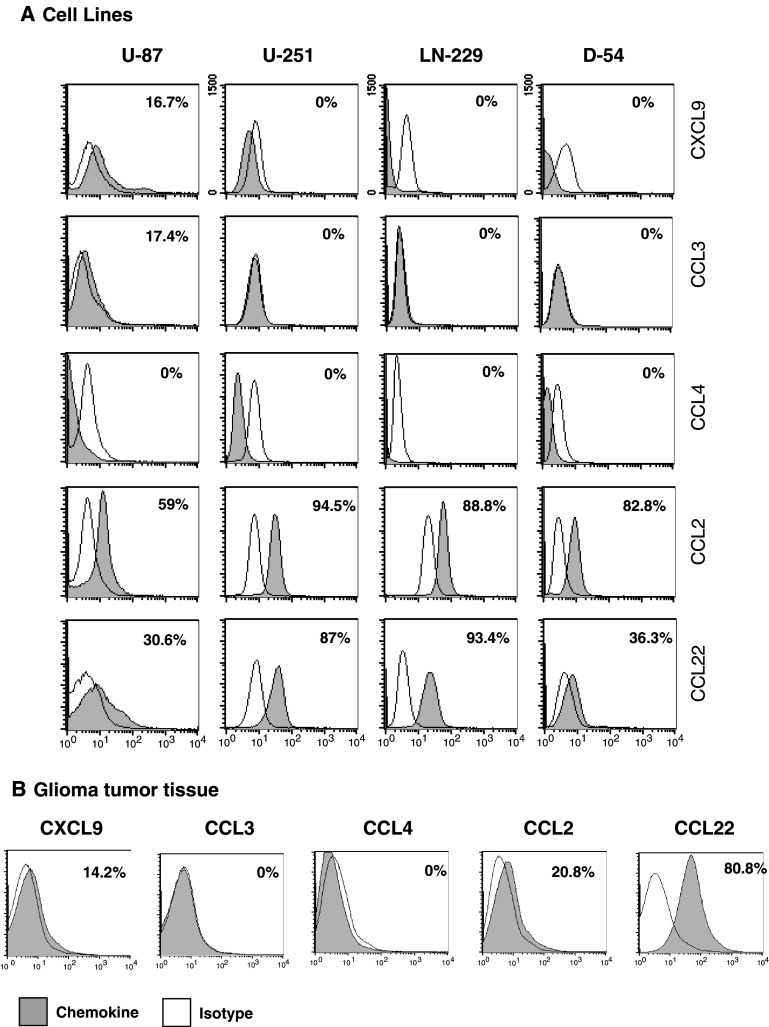
Because malignant gliomas contain substantially more Tregs than normal tissues, we wanted to determine which T-cell-attractant chemokines are produced by the glioma cells. In the malignant human glioma cell lines U-87, U-251, LN-229, and D-54, significant levels of CCL2 and CCL22 were found within intracellular compartment (Fig. 1a). Furthermore, CXCL9, another chemokine that attracts Tregs, was present but in smaller amounts than those of CCL22 and CCL2. Chemokines CCL3 and CCL4 were not produced in significant quantities.
Fig. 1.
Human glioma cells demonstrate intracellular production of T-cell-attractant chemokines CCL2 and CCL22. a Malignant human glioma cell lines U-87, U-251, LN-229, and D-54 and b freshly resected human glioma cells were permeabilized and stained with fluorescent-conjugated antibodies to the chemokines CCL2, CCL3, CCL4, CCL22, and CXCL9. The percentage of cells expressing each intracellular chemokine was assessed by flow cytometric analysis, and each histogram plot (grey) was overlaid with the plot of its respective isotype control (clear). These data are representative of eight separate experiments
Since perpetuated cell lines may not accurately reflect the in vivo characteristics of gliomas, freshly resected gliomas were disassociated and stained for intracellular chemokines. In GBM specimens (n = 8), most cells produced CCL22 (60.97 ± 17.12%) and, to a lesser degree, CCL2 (23.61 ± 4.72%) (Fig. 1b). In lower-grade tumors (n = 4), there was a trend of diminished production of CCL22 (data not shown).
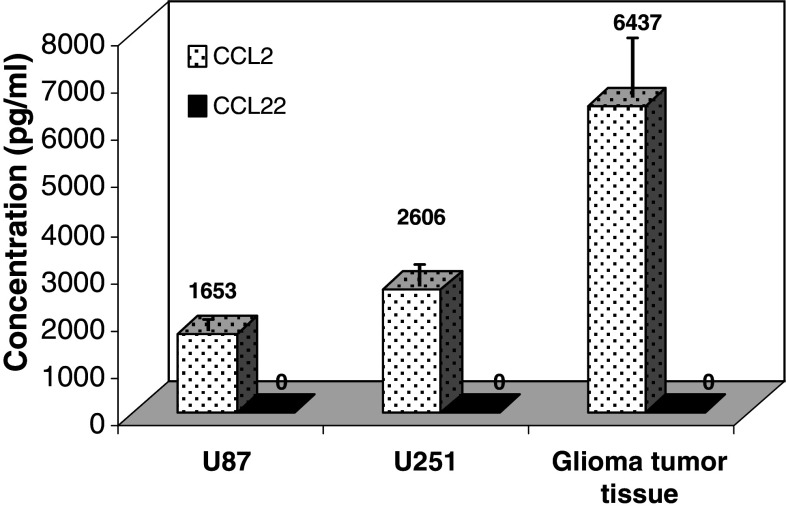
To confirm that CCL2 and CCL22 were secreted into the tumor microenvironment, the supernatants from resected human glioma tumor tissue and the glioma cell lines U-87 and U-251 were measured by ELISA. Both glioma cell lines produced large quantities of CCL2, but the tumor tissue produced even larger amounts (4072.16 − 7666.90 pg/ml, n = 3, P < 0.01) (Fig. 2). In contrast, we detected no CCL22 in the supernatants from the glioma tissue or either cell line despite having used two different ELISA assays, including the one utilized to detect CCL22 in other cancers [5, 17].
Fig. 2.
Human glioma cells and glioma tumor tissue secrete CCL2. Supernatants from glioma cell lines U-87, U-251 and freshly resected human glioma tissue analyzed by ELISA for CCL2 and CCL22. These data are representative of three separate experiments. Numbers on the graph denote the concentration of each chemokine produced by the respective cells
Increased percentage of peripheral FoxP3+ Tregs expressing CCR4 from GBM patients
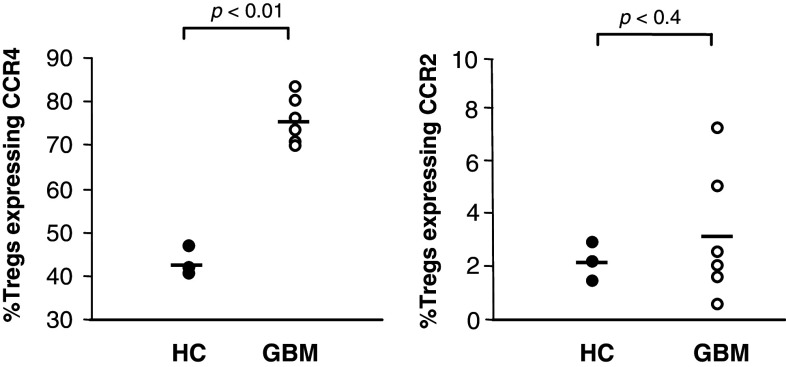
To characterize the percentage of FoxP3+ Tregs expressing the CCL2 and CCL22 receptors, we analyzed the peripheral blood of patients with GBM and of healthy controls. Flow cytometric analyses showed that the percentage of FoxP3+ Tregs expressing CCR4 were significantly higher in the GBM patients’ blood (73.92 ± 3.93%) than in those of healthy controls (42.67 ± 2.85%) (P < 0.01). In contrast, the percentage of FoxP3+ Tregs expressing CCR2 in the GBM patients’ blood (3.11 ± 2.74%) did not differ significantly from that in the healthy controls (2.09 ± 0.68%) (Fig. 3).
Fig. 3.
Increased expression percentage of CCR4 on FoxP3+ Tregs. PBMCs from GBM patients (n = 6) and healthy controls (HC, n = 4) were isolated, and the percentages of CD4+CD25+FoxP3+ T cells expressing CCR4 and CCR2 were measured by flow cytometry
CCL2 and CCL22 induce the migration of CD4+CD25+FoxP3+ Tregs
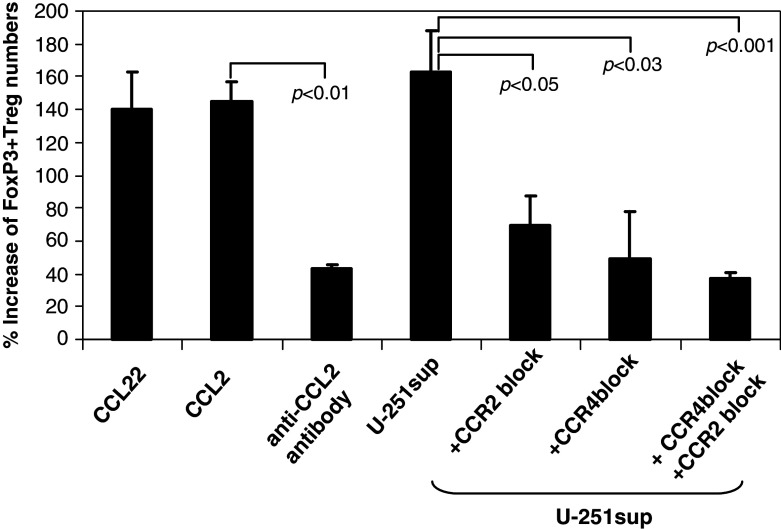
We used purified CD4+ T cells from PBMCs of healthy control subjects (n = 3) to determine whether CCL2 from the supernatants of glioma cell lines can recruit FoxP3+ Tregs. The directional migration of CD4+CD25+FoxP3+ Tregs toward recombinant CCL22 (positive control), CCL2, and U-251 supernatant was determined using a standard Boyden chamber system. Normal peripheral CD4+CD25+FoxP3+ Tregs exhibited increased migration in the presence of human recombinant CCL2 (145% increase of Treg numbers). This is equivalent to the increase seen in response to recombinant CCL22 (140% increase of Treg numbers), the chemokine previously described as primarily responsible for Treg recruitment to solid tumors [5]. The supernatant from U-251 also induced the migration of Tregs (163% increase of Treg numbers compared to that of control group), and this could be abrogated by using blocking antibody to CCR2 or CCR4 (Fig. 4). Similar data were obtained from Tregs isolated from patients with GBM. However, because of limitations in obtaining the requisite quantities of blood (approximately 150 ml) from intraoperative patients, these data were insufficiently powered (data not shown).
Fig. 4.
CCL2 induces the migration of CD4+CD25+FoxP3+ Tregs. CD4+ T cells were isolated from PBMCs of healthy donors (n = 3). Cells that migrated toward the chemokines in the supernatant (sup) were stained with fluorescent-conjugated antibodies to CD4, CD25, FoxP3. The percentage increase in numbers of Tregs over that of the control group (CD4+ T cells assayed with medium alone) was analyzed by flow cytometry. Bars standard error
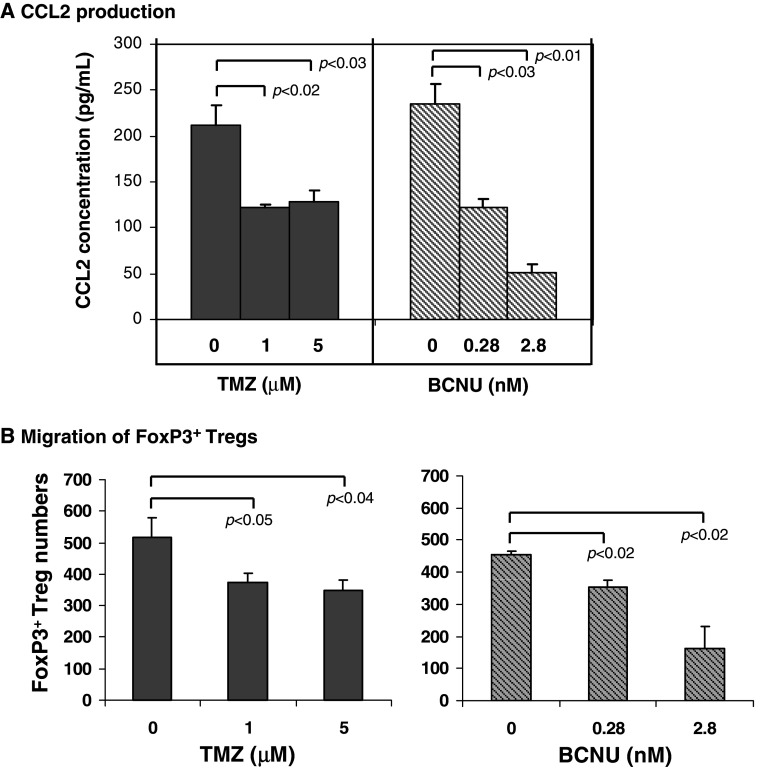
Temozolomide and BCNU inhibit U-87 cells’ production of CCL2 and migration of FoxP3+ Tregs
To determine whether chemotherapeutic agents could inhibit CCL2 production by gliomas, we treated U-87 cells with temozolomide or BCNU, both of which are currently used in GBM treatment. The ELISA results showed that CCL2 concentrations in the supernatants from U-87 cells treated with 1 and 5 μM temozolomide significantly decreased from 211.64 pg/ml (untreated control group) to 122.56 pg/ml (P < 0.02) and 128.45 pg/ml (P < 0.03), respectively (Fig. 5a). In addition, CCL2 concentrations in the supernatants from U-87 cells treated with 0.28 and 2.8 nM BCNU also significantly decreased from 234.57 pg/ml (untreated control group) to 122.16 pg/ml (P < 0.03) and 51 pg/ml (P < 0.01), respectively (Fig. 5a), demonstrating a dose-dependent relationship. The viability of U-87 cells treated with either chemotherapeutic agent was unaffected (>90% viability by trypan blue exclusion, data not shown), indicating that these chemotherapeutic agents exert immune modulatory properties below the threshold of glioma cytotoxicity.
Fig. 5.
a The chemotherapeutic agents temozolomide and BCNU inhibit CCL2 production by gliomas. U-87 glioma cells were treated with temozolomide (1 and 5 μM) or BCNU (0.28 and 2.8 nM). Untreated U-87 cells were used as a control. After 48 h supernatants were collected and assayed for CCL2 production (pg/ml) by ELISA. This data was replicated twice with similar statistically significant results. b Migrated FoxP3+ Treg numbers from healthy individuals induced by supernatants from U-87 glioma cells treated with temozolomide or BCNU. Bars standard deviation
We then performed the chemotaxis assay with the supernatants from U-87 cells treated with temozolomide or BCNU to investigate whether the inhibition of CCL2 production by chemotherapeutic drugs could help to suppress the migratory effect of glioma cells’ supernatant. The migrated FoxP3+ Treg numbers decreased from 517 ± 63 (untreated control group) to 376 ± 26 (1 μM TMZ, P < 0.05) and 350 ± 31(5 μM TMZ, P < 0.04), respectively, in the temozolomide treated group. In addition, significant decreases of migrated FoxP3+ Treg numbers (both P < 0.02) from 456 ± 9 (control group) to 352 ± 23 (0.28 nM BCNU) and 163 ± 67 (2.8 nM BCNU) were also observed in BCNU group (Fig. 5b).
Discussion
In contrast to Curiel et al. [5], who demonstrated that Tregs migrated to solid tumors through an interaction between the receptor CCR4 and its ligand CCL22, we show that CCL2 mediates this function within malignant glioma patients. CCL2 was first reported to be present in cerebrospinal fluid of patients with malignant glioma and is considered a surrogate marker of subarachnoid dissemination of tumor cells [13]. CCL2 is produced by human astrocytomas and glioblastomas in vivo and in vitro [6] and has been shown to attract Tregs to the brain in neuro-inflammatory conditions such as experimental autoimmune encephalomyelitis [16]. In this study, we showed that human gliomas secrete CCL2 and that Treg migration is blocked by anti-CCR2 or anti-CCR4. Cumulatively, these findings indicate that the recruitment of Tregs into the tumor microenvironment is not exclusively secondary to the production of CCL22 but that other chemokines, such as CCL2, can also mediate this role.
We found that intracellular staining of not only glioma cell lines but also of freshly resected GBM tumor tissue demonstrated constitutive expression of CCL2 and CCL22. However, although the high concentrations of secreted CCL2 were confirmed by ELISA, CCL22 was not detected in the same batch of supernatants by ELISA. This phenomenon can be explained by the existence of dipeptidyl peptidase IV on the surfaces of glioma cells [25]. Processing of mature CCL22 protein (CCL22 1-69) by the peptidase generates two types of truncated CCL22, which are missing the first two (CCL22 3-69) or four amino acids (CCL22 5-69), making them undetectable by CCL22 ELISA antibodies [26]. However, intracellular CCL22 antibody can detect unprocessed CCL22 proteins before they are secreted through the cell surfaces. More importantly, truncated CCL22 cannot interact with CCR4 and is therefore incapable of attracting Tregs [20]. This supports the probability that not all types of cancers rely solely on CCL22 for Treg intratumoral recruitment. In gliomas, for example, CCL2 appears to be the major chemokine attracting Tregs into the tumor microenvironment. Glioma-infiltrating microglia could be a source of CCL22, but whether they secrete functional forms of this chemokine is unclear. We were unable to detect CCL22 by ELISA in the supernatants from human glioma tissue, and that tissue would also contain microglia capable of elaborating chemokines. In addition, macrophages have been shown to express dipeptidyl peptidase IV [15], suggesting that this enzyme is also present on microglia, thereby resulting in truncated and inactive CCL22 secretion.
The importance of Treg-attractant chemokine production by cancers is compounded by the higher expression of the CCR4 receptor on FoxP3+ Tregs isolated from patients with GBM than on those from healthy donors. Although CCL2 primarily binds the receptor CCR2, it is nonetheless promiscuous and can also bind CCR4 [24]. Since CCL22, the dominant ligand for CCR4, appears to be truncated and nonfunctional in gliomas, our studies suggest that in GBM patients, CCL2 is primarily responsible for attracting CCR4+ Tregs to the tumor microenvironment.
CCL2 production by glioma cells was effectively suppressed by non-cytotoxic concentrations of the chemotherapeutic agents temozolomide and BCNU. Clinical studies have shown that 50 μM peak plasma levels of temozolomide are achieved after systemic administration, however, the sustained plasma concentrations range from 0.1 to 10 μM [19]. Based on the vascular permeability of gliomas, the dose ranges utilized in our experiments are projected to be achieved in vivo. The greatest CNS penetration of Gliadel was reported as 24 ± 4 μM in the CSF [18] and 9.3 nM/gm within brain tissue [9]. Systemic administration of BCNU results in CNS concentration of approximately 11.7 nM; we have again employed concentrations well within this achievable in vivo range [9]. The efficacy of some of these compounds in current practice could be contributed to their immune modulatory effects. We speculate that the mechanism underlying these effects could be attributed to interference of intracellular signaling pathways that regulate chemokine gene transcription, as has been previously described [1].
Marginal efficacy and severe toxic effects have limited the use of BCNU as an effective antiglioma agent; however, this does not preclude its use as an immune modulatory drug. As a means of overcoming systemic toxicity, BCNU is now delivered via the Gliadel wafer (MGI Pharma, Bloomington, MN, USA). In a previous study [21], marked therapeutic efficacy and synergy were observed after the concomitant administration of IL-2 immunotherapeutic agents with Gliadel. We propose that one of the underlying mechanisms facilitating this synergy between immunotherapy and chemotherapy could be the ability of BCNU and/or temozolomide to inhibit CCL2 production and prevent Treg recruitment to the glioma microenvironment. At the doses utilized in this manuscript, which can be achieved within the central nervous system, chemotherapy induced T-cell death was not observed based on trypan blue exclusion (AB Heimberger, unpublished data). It is possible that these agents may adversely affect production of chemokines that regulate the influx of antitumor effector CD8+ T cells. However, this effect is likely not significant, as glioma production of CD8-attractant chemokines is very low [12] and migration of antigen-experienced T cells to gliomas is largely mediated by tissue-specific homing molecules [3, 4].
Chemotherapy and immunotherapy have previously been used as independent forms of treatment, and conventional wisdom has been that the two are antagonistic forms of therapy. This idea was due, in part, to the fact that many chemotherapeutic agents induce lymphopenia. However, this does not preclude the use of chemotherapeutic agents administered at appropriate times with immunotherapy or at lower doses to minimize lymphopenia. The depletion or inhibition of certain immune effector cells, such as Tregs, may be a highly desirable outcome of chemotherapy, yielding greater immunotherapeutic efficacy, or it may promote a desirable cytokine profile for adequate tumor control. Understanding the mechanisms involved in the delicate balance of Treg migration and recruitment is key to limiting the immunosuppressive function of these cells. Combination therapies that inhibit CCL2 and/or CCL22 secretion and block the inhibitory effect of CCR4+ Tregs on antitumor immune responses could be effective treatment modalities for patients with malignant gliomas.
Acknowledgments
We are grateful to Lamonne Crutcher for assistance in obtaining patient specimens. This work was supported by recruitment start-up funds for the Department of Neurosurgery, The University of Texas M. D. Anderson Cancer Center; by the National Brain Tumor Foundation Translational Grant; by The Rose Foundation; and by National Institutes of Health grant RO1 CA120813-01A1.
Abbreviations
- APC
Antigen presenting cell
- BCNU
Carmustine [3-bis (2-chloroethyl)-1-nitrosourea]
- ELISA
Enzyme-linked immunosorbent assay
- FACS
Fluorescence-activated cell sorter
- FITC
Fluorescein isothiocyanate
- GBM
Glioblastoma multiforme
- IL
Interleukin
- MEM
Minimum essential medium
- PBMC
Peripheral blood mononuclear cell
- PBS
Phosphate-buffered saline
- Treg
Regulatory T cell
Footnotes
Justin T. Jordan and Wei Sun are contributed equally to this work.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00262-007-0403-3
References
- 1.Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, de Braud F, Jimeno J, D’Incalci M. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- 3.Calzascia T, Di Berardino-Besson W, Wilmotte R, Masson F, de Tribolet N, Dietrich PY, Walker PR. Cutting edge: cross-presentation as a mechanism for efficient recruitment of tumor-specific CTL to the brain. J Immunol. 2003;171:2187–2191. doi: 10.4049/jimmunol.171.5.2187. [DOI] [PubMed] [Google Scholar]
- 4.Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, Aurrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 6.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 7.Fecci PE, Mitchell DA, Whitesides JF, Xie W, Friedman AH, Archer GE, Herndon JE, 2nd, Bigner DD, Dranoff G, Sampson JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 8.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41:403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Hassenbusch SJ, Anderson JH, Colvin OM. Predicted and actual BCNU concentrations in normal rabbit brain during intraarterial and intravenous infusions. J Neurooncol. 1996;30:7–18. doi: 10.1007/BF00177438. [DOI] [PubMed] [Google Scholar]
- 10.Hu S, Chao CC, Ehrlich LC, Sheng WS, Sutton RL, Rockswold GL, Peterson PK. Inhibition of microglial cell RANTES production by IL-10 and TGF-beta. J Leukoc Biol. 1999;65:815–821. doi: 10.1002/jlb.65.6.815. [DOI] [PubMed] [Google Scholar]
- 11.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating anti-tumor immune responses. Neuro-oncol. 2006;8:261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura T, Takeshima H, Nomiyama N, Nishi T, Kino T, Kochi M, Kuratsu JI, Ushio Y. Expression of lymphocyte-specific chemokines in human malignant glioma: essential role of LARC in cellular immunity of malignant glioma. Int J Oncol. 2002;21:707–715. doi: 10.3892/ijo.21.4.707. [DOI] [PubMed] [Google Scholar]
- 13.Kuratsu J, Yoshizato K, Yoshimura T, Leonard EJ, Takeshima H, Ushio Y. Quantitative study of monocyte chemoattractant protein-1 (MCP-1) in cerebrospinal fluid and cyst fluid from patients with malignant glioma. J Natl Cancer Inst. 1993;85:1836–1839. doi: 10.1093/jnci/85.22.1836. [DOI] [PubMed] [Google Scholar]
- 14.Kurpad SN, Zhao XG, Wikstrand CJ, Batra SK, McLendon RE, Bigner DD. Tumor antigens in astrocytic gliomas [Review] GLIA. 1995;15:244–256. doi: 10.1002/glia.440150306. [DOI] [PubMed] [Google Scholar]
- 15.Laouar A, Wietzerbin J, Bauvois B. Divergent regulation of cell surface protease expression in HL-60 cells differentiated into macrophages with granulocyte macrophage colony stimulating factor or neutrophils with retinoic acid. Int Immunol. 1993;5:965–973. doi: 10.1093/intimm/5.8.965. [DOI] [PubMed] [Google Scholar]
- 16.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/S1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 17.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, Pisa P. CD4+CD25high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–7405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 18.Motl S, Zhuang Y, Waters CM, Stewart CF. Pharmacokinetic considerations in the treatment of CNS Tumours. Clin Pharmacokinet. 2006;45:871–903. doi: 10.2165/00003088-200645090-00002. [DOI] [PubMed] [Google Scholar]
- 19.Newlands ES, Stevens MF, Wedge SR, Wheelhouse RT, Brock C. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23:35–61. doi: 10.1016/S0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 20.Proost P, Struyf S, Schols D, Opdenakker G, Sozzani S, Allavena P, Mantovani A, Augustyns K, Bal G, Haemers A, Lambeir AM, Scharpe S, Van Damme J, De Meester I. Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Biol Chem. 1999;274:3988–3993. doi: 10.1074/jbc.274.7.3988. [DOI] [PubMed] [Google Scholar]
- 21.Rhines LD, Sampath P, DiMeco F, Lawson HC, Tyler BM, Hanes J, Olivi A, Brem H. Local immunotherapy with interleukin-2 delivered from biodegradable polymer microspheres combined with interstitial chemotherapy: a novel treatment for experimental malignant glioma. Neurosurgery. 2003;52:872–879. doi: 10.1227/01.NEU.0000053211.39087.D1. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roussel E, Gingras MC, Grimm EA, Bruner JM, Moser RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol. 1996;105:344–352. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166:996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- 25.Sedo A, Malik R, Vicar J, Simanek V, Ulrichova J. Quaternary benzo[c]phenanthridine alkaloids as inhibitors of dipeptidyl peptidase IV-like activity baring enzymes in human blood plasma and glioma cell lines. Physiol Res. 2003;52:367–372. [PubMed] [Google Scholar]
- 26.Struyf S, Proost P, Sozzani S, Mantovani A, Wuyts A, Declercq E, Schols D, Van Damme J. Enhanced anti-HIV-1 activity and altered chemotactic potency of NH2-terminally processed macrophage-derived chemoking (MDC) imply an addtional MDC receptor. J Immunol. 1998;161:2672–2675. [PubMed] [Google Scholar]
- 27.Su YB, Sohn S, Krown SE, Livingston PO, Wolchok JD, Quinn C, Williams L, Foster T, Sepkowitz KA, Chapman PB. Selective CD4+ lymphopenia in melanoma patients treated with temozolomide: a toxicity with therapeutic implications. J Clin Oncol. 2004;22:610–616. doi: 10.1200/JCO.2004.07.060. [DOI] [PubMed] [Google Scholar]
- 28.Szanya V, Ermann J, Taylor C, Holness C, Fathman CG. The subpopulation of CD4+CD25+ splenocytes that delays adoptive transfer of diabetes expresses L-selectin and high levels of CCR7. J Immunol. 2002;169:2461–2465. doi: 10.4049/jimmunol.169.5.2461. [DOI] [PubMed] [Google Scholar]
- 29.Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- 30.Vincent VA, Tilders FJ, Van Dam AM. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta. Glia. 1997;19:190–198. doi: 10.1002/(SICI)1098-1136(199703)19:3<190::AID-GLIA2>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang T, Somasundaram R, Berencsi K, Caputo L, Gimotty P, Rani P, Guerry D, Swoboda R, Herlyn D. Migration of cytotoxic T lymphocytes toward melanoma cells in three-dimensional organotypic culture is dependent on CCL2 and CCR4. Eur J Immunol. 2006;36:457–467. doi: 10.1002/eji.200526208. [DOI] [PubMed] [Google Scholar]