Abstract
Cell based therapies for acute myeloid leukaemia (AML) have made significant progress in the last decade benefiting the prognosis and survival of patients with this aggressive form of leukaemia. Due to advances in haematopoietic stem cell transplantation (HSCT) and particularly the advent of reduced intensity conditioning (RIC), the scope of transplantation has now extended to those patients previously ineligible due to age and health restrictions and has been associated with a decrease in transplant related mortality. The apparent graft versus leukaemia (GvL) effect observed following HSCT demonstrates the potential of the immune system to target and eradicate AML cells. Building on previously published pre-clinical studies by ourselves and others, we are now initiating a Phase I clinical study in which lentiviral vectors are used to genetically modify AML cells to express B7.1 (CD80) and IL-2. By combining allogeneic HSCT with immunisation, using the autologous AML cells expressing B7.1 and IL-2, we hope to stimulate immune eradication of residual AML cells in poor prognosis patients that have achieved donor chimerism. In this report we describe the background to cell therapy based approaches for AML, and discuss difficulties associated with the deployment of a chronically stimulated, hence exhausted/depleted immune system to eradicate tumour cells that have already escaped immune surveillance.
Keywords: Acute myeloid leukaemia, Whole cell vaccine, B7.1, IL-2, Immunotherapy, Lentivirus
Introduction
Advances in the field of tumour immunobiology have improved our understanding of the role of the immune system in preventing tumour development. We now understand that tumour antigens can be recognised by the immune system and that natural immune responses do occur against tumour cells. These responses, involving both the innate and adaptive arms of the immune system, can lead to the elimination of tumour cells. This occurrence is best exemplified by the increased rate of tumour development in immuno-compromised individuals [1]. Where the immune system can mount a response against tumour cells, then the development of antigen-specific CTL activities and the generation of memory T cells and protective immunity can be detected. However, tumour cells are able to escape immune surveillance. Immunological responses against tumour cells are likely to be most effective against the most immunogenic of the tumour cell population, and least effective against the escape mutants that have evolved various means of camouflage or resistance to immune mediated toxicity. This is a continuously evolving process and the most vulnerable of the tumour cells are likely to be the most efficiently eradicated, leaving an evolving population of tumour cells with increasingly more effective means of combating immune mediated eradication. This phenomenon, named “immune-editing” of tumour cells by Robert Schreiber, has important implications for the development of immune therapy strategies that may have any realistic chance of therapeutic benefit (see Schreiber et al. [2, 3] for recent reviews).
Tumour/immune interactions also affects the immune system. The continued survival of the tumour and chronic immune stimulation could result in the eventual exhaustion and even clonal deletion of the most stimulated components of the immune system (e.g. the corresponding CTLs). In addition, T cell encounters with tumour antigens in the absence of adequate co-stimulatory and helper functions could result in anergy and tolerance to these antigens [4, 5]. These tumour mediated influences on the immune system, the “tumour editing” of the immune system, has important implications for the development of tumour immune therapy. For instance, the strongest tumour antigens may not provide the best vaccination targets, as the corresponding T cells may be very effectively exhausted and/or clonally deleted. In contrast, some of the weaker tumour antigens, which may have failed to spontaneously induce an effective immunological response, may be able to draw on a reserve of unstimulated cells which may then be effectively stimulated in response to vaccination in the presence of the appropriate adjuvants and helper functions.
Acute myeloid leukaemia as a target for immune therapy
Acute myeloid leukaemia (AML) is one of the most aggressive forms of haematological malignancies affecting both the young and the old. This disease has a particularly poor prognosis (11% survival at 5 years post-diagnosis) in patients over 55 years of age, due to these patients’ difficulties in tolerating intensive chemotherapy or pre-transplant conditioning, and the likely absence of suitable transplant donors. Despite recent advances in chemotherapy and HSCT, particularly the advent of RIC, the survival rates amongst AML patients remain poor. It is for these patients that immunisation with AML cells expressing immune stimulatory factors may offer a new adjuvant therapy option.
A wealth of evidence exists in the literature to show that patients can generate immune responses against antigens expressed by their own tumour cells. AML is no exception and the presence of pre-treatment autoantigens and spontaneous T cell responses against tumour antigens have been demonstrated. Examples of some of the autoantigens identified in AML include RHAMM [6], MPP11 [7], SSX2IP and the cancer-testis antigen PASD1 [8]. WT1 is detectable in many forms of cancer (lung, breast, ovarian) and has been shown to be expressed at high levels in haematopoietic malignancies and to play a role in the pathogenesis of several leukaemias including the development of AML [9]. A number of studies have demonstrated the presence of antibodies against WT1 in AML patients [10–12]. The first evidence of spontaneous T cell responses against defined antigens in AML patients came from a study by Scheibenbogen et al. [13] when they described the presence of granzyme B positive CD8+ T cell responses against proteinase 3 and WT1. More recently the presence of RHAMM peptide-specific effector T cells in the peripheral blood of patients with AML was shown using HLA-A2/R3 tetramers in a population of CCR7-CD45RA+ cells. Spontaneously occurring T cell immune responses against BCL-2 have also been documented in patients with AML [14]. A number of other AML associated antigens ranging from mutated oncogenes to products of chromosomal translocations have been identified that can potentially act as tumour antigens.
The identification of AML associated antigens provides opportunities to generate tumour specific cytotoxic T cells. However this potential remains unrealised, not least because of technological difficulties in the expansion of functionally cytotoxic T cells. In addition, a fundamental hurdle in cancer immune therapy remains in the rejection of an immune edited tumour (thus selected for loss of susceptibility to immune rejection) by a tumour edited immune system, already depleted of its most potent effector cells by chronic stimulation mediated exhaustion and clonal deletion.
Immunotherapy for AML
One possible approach to “re-activating” the immunogenicity of tumour cells is to turn them into effective antigen presenting cells (APCs). AML blasts are of the same developmental lineage as APC and therefore express a range of molecules required for the direct presentation of antigens to T-cells. Important amongst these are the expression of both MHC class I and class II for the activation of CD8+ and CD4+ cells, appropriate adhesion molecules and some co-stimulatory signals. However, AML blasts invariably lack the expression of a key co-stimulatory factor—B7.1 (also known as CD80) [15]. Several promising approaches have been developed for the immunotherapy of AML, including the modification of autologous AML blasts to act as a whole cell vaccine. This type of cellular therapy has included the in vitro differentiation of AML cells to a “leukaemia derived-dendritic cell” phenotype (LD-DC). Although LD-DCs have been demonstrated to stimulate autologous T-cell activation [16–19], efficient LD-DC generation has been variable in different studies, and LD-DCs may not be able to stimulate CTL activity in autologous T-cells [20, 21]. Alternatively, myeloid leukaemia cells have been genetically modified in order to counteract their inherent immune suppressive activity and to achieve T-cell stimulation [22–24]. Several studies in solid tumour models have now shown that the combined expression of B7.1 and stimulatory cytokines such as IL-2, IL-7 and GM-CSF, make tumour cells potent stimulators of both allogeneic and autologous T-cells. Where examined, the responding T-cells appear to have MHC restricted and antigen specific CTL activity [25–31].
In a recent clinical study, AML patients with chemotherapy induced cytopenia show reduced in vitro T cell responsiveness compared to normal subjects. However, this can be restored to normal levels by B7.1 co-stimulation [32], making it a highly promising molecule for immunogene therapy of AML.
T cell stimulation by B7.1/IL-2 transduced AML cells
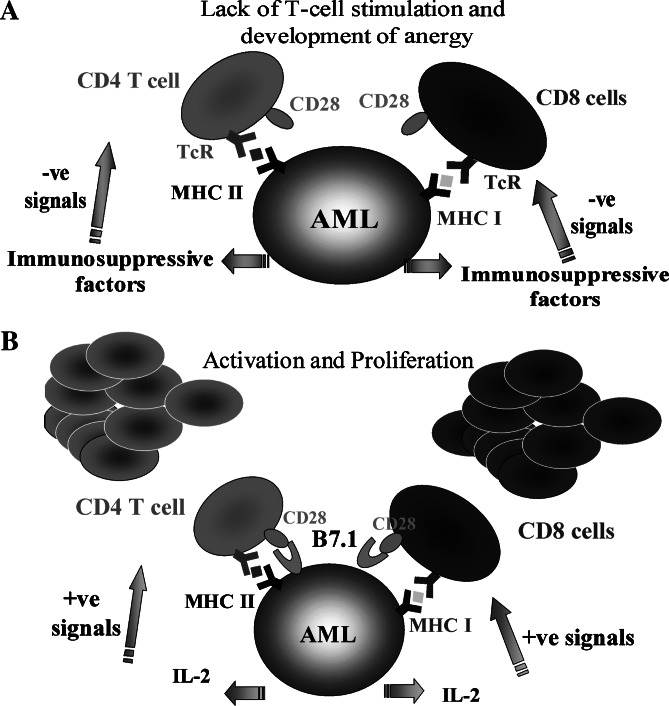
The absence of co-stimulation and the secretion of immunosuppressive factors are ways in which AML cells evade immune attack [33, 34]. However, it may be possible to overcome both effects by genetically modifying AML cells to express a potent co-stimulatory molecule such as B7.1, and to also provide additional helper functions by the expression of a pro-inflammatory cytokine such as IL-2. As a single agent IL-2 has been shown to reverse anergy both in vitro and in vivo [35] (Fig. 1). We and others have previously shown that B7.1/IL-2 expressing tumour cells can stimulate T-cell [23, 36–41] and NK cell [42, 43] responses against unmodified tumour cells, resulting in the immune mediated rejection of tumours and a prolonged survival in vaccinated animals ([44–46], see [47] for review). In human in vitro studies we have investigated the immunotherapeutic potential of primary AML blasts transduced with B7.1 and IL-2 [22, 48, 49]. When compared with single B7.1 or IL-2 gene modification or unmodified controls, B7.1/IL-2 transduction provided the best T-cell stimulation in allogeneic (threefold increase over unmodified control), autologous (7.4-fold) and chimeric settings (34.7-fold). The T-cell stimulation could be partially blocked by anti-B7.1 and anti-MHC class I antibodies [49]. This T-cell stimulation coincided with the expansion of activated CD8+ cells and increased secretion of Th1 cytokines (IFN-γ, TNF-α and IL-2). Importantly, this was observed with T cells from post-transplanted patients who had achieved donor chimerism. While this is encouraging, unless CTL activity against primary human AML cells can be demonstrated, hopes for successful immunotherapy of this disease must remain cautious. There are few reports of immunotherapy strategies successfully initiating CTL activity against AML cells in vitro [18, 50]. The absence of CTL activity could be due to the inherent resistance of primary human AML cells to cell mediated lysis. We have previously shown that AML cells secrete an inhibitory factor that is capable of limiting the secretion of Th1 cytokines by T cells and lowering T cell proliferative responses [33, 34]. Alternatively it could be that PBLs obtained from AML patients, although capable of some degree of proliferation and cytokine release, may lack effective end stage lytic function. However, our current studies using B7.1/IL-2 modified AML cells suggest that, at least in vitro, autologous cytolytic T cells can be generated against AML cells (Hardwick et al., manuscript in preparation). Therefore, the combined expression of B7.1/IL-2 by genetically modified AML cells could enable the stimulation of an effective lytic response.
Fig. 1.
Strategies for AML-immune activation. a Immune evasion by AML. In the absence of co-stimulation, T cells develop anergy towards AML. This is augmented by the secretion of immunosuppressive factors, which further inhibits immune responses against AML. b Genetically modified AML cells for immune activation. B7.1 (CD80) and IL-2 gene transduction provides the essential co-stimulatory signal and IL-2 for induction of anti-leukaemia immunological responses
An efficient gene transfer protocol for AML blasts to co-express B7.1 and IL-2
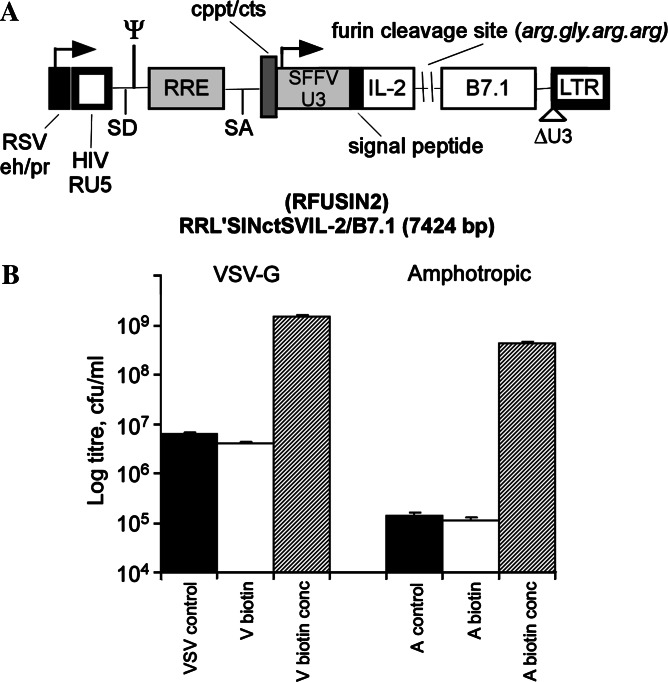
Until recently, expression of transgenes in human AML blasts have been limited to the use of retroviral [22, 51] and adenoviral vectors [51]. These transductions had low efficiencies due to the failure of primary AML blasts to cycle in culture, limiting transduction efficiencies and variable coxackie adenovirus receptor expression [52, 53], respectively. In addition, the use of vectors based on herpes simplex virus and adeno-associated virus give highly variable transduction efficiencies (our own unpublished observations), all of which has seriously hampered the development of immune gene therapy for AML. Ex vivo gene transfer presents many practical advantages over in vivo gene delivery in that isolating and targeting of the specific tissue type or organ is relatively easy. In contrast to classical retroviral vectors, lentiviral vectors readily transduce mitotic as well as a number of post-mitotic targets, owing to the HIV matrix protein and the accessory protein Vpr [54, 55]. Although lentiviral vectors have mainly been used for the long-term transduction of non-dividing cells, it has proved to be highly effective, in comparison to other commonly used vectors, in the transduction of primary AML blasts [23, 24, 56–58]. This is mainly due to the ability to pseudotype lentiviral vectors with VSV-G envelope protein for ubiquitous cell entry, the stability of VSV-G also allows centrifugal vector concentration and the capacity of lentiviral vectors to infect non-dividing cells is advantageous for the transduction of primary cells ex vivo. It is possible to remove viral genes from the vector and to provide the required products in trans in the vector producing cells, allowing the assessment of the therapeutic potential of the transgenes concerned (e.g. B7.1 and IL-2) without the risk of subverting immunological responses against viral proteins at the expense of the tumour-associated antigens. To this end, we have constructed second- and third-generation self-inactivating lentiviral vectors incorporating cppt/cts, a wpre enhancer element and a myeloid enhanced promoter—the U3 region of spleen focus forming virus [49, 59]. Transduction of primary AML blasts of multiple FAB subtypes can be achieved with an efficiency of 40–100% following a single round of infection at an MOI of 10.
To produce viral vectors at high titres, we have developed a highly efficient virus concentration strategy by capturing retroviral particles using conjugated paramagnetic microparticles (PMP) [60]. This has now been further developed for lentiviral vectors pseudotyped with both the VSV-G and amphotropic envelope [49, 61]. The result is a 380-fold increase in titre for VSV-G and 3,600-fold for amphotropic envelope, providing titres in the order of 109 cfu/ml (Fig. 2b exemplifies a typical PMP-mediated concentration). In addition, these PMP-concentrated lentiviral vectors displayed an increased infectivity of primary AML blasts. Compared with conventional ultracentrifugation based concentration strategies, we find a higher infection rate as a function of viral capsid protein p24 concentration, demonstrating an improvement in transduction efficiency.
Fig. 2.
Third generation self-inactivating lentiviral vector. a Backbone encoding B7.1 (CD80) and IL-2 cDNAs in a monocistronic expression cassette driven by a myeloid efficient promoter derived from spleen focus forming virus (SFFV). The IL-2/B7.1 fusion protein is separated by a recognition sequence for the endoprotease–furin, which cleaves to generate the biologically active constituents of soluble IL-2 and transmembrane B7.1. b Magnetic concentration following conjugation with paramagnetic microparticles (PMP). Titres presented as cfu/ml following infection and selection of infected leukaemic K562 cells in a soft agar cloning assay. VSV or A control unconcentrated, V or A biotin unconjugated control titre, V or A biotin conc PMP mediated concentrated samples (methodology described by Chan et al. [49])
To facilitate the efficient co-transduction of both B7.1 and IL-2, we have developed a “fusagene” strategy by which multiple gene products are expressed as a fusion protein from a single cistron [48]. The fusion protein contains recognition sequences for the golgi located, ubiquitous endoprotease and furin. Furin cleavage results in the generation of biologically active constituents from the fusion protein. Thus soluble IL-2 is released extracellularly whilst transmembrane B7.1 remains anchored at the cell surface. This strategy avoids the problems associated with transduction of AML blasts with two single gene vectors, or promoter interference when using two separate promoters. A well established alternative strategy is the use of internal ribosome entry sites (IRES). However, the instability of vectors with more than one IRES restricts the suitability of IRES vectors to express multiple genes.
Allogeneic HSCT and RIC
A lethargic immune system, exhausted by “tumour editing” of the immunological response and faced with the outgrowth of “immune edited” tumour cells, is unlikely to be able to mount a therapeutically effective immune response. However, haematopoietic stem cell transplantation (HSCT) mediates installation of an “unedited” immune system, and reduction of the mass of the tumour target (e.g. by chemotherapy mediated induction of albeit a transitory remission), may provide an opportunity for the donor derived immune system to eradicate the residual tumour.
The single most effective therapy for intermediate and poor risk AML is allogeneic HSCT. The therapeutic GvL effect that follows HSCT and donor leukocyte infusions (DLI) demonstrates the pivotal role that the immune system can play in eradicating leukaemia cells. This is best demonstrated by the reduced risk of relapse in patients receiving allogeneic HSCT, compared to autologous HSCT, showing both the ability of the immune system to recognise AML cells, and the susceptibility of AML cells to be eradicated by allogeneic T-cells. The involvement of T-cells in this response is further shown by the increased risk of relapse by T-cell depletion of the transplanted HSCT and by the efficacy of DLI.
Reduced intensity conditioning prior to allogeneic HSCT has considerably reduced transplant related mortality, thus extending the eligibility criteria to AML patients that were previously at too great a risk of transplant related toxicity. The additional inclusion of anti-CD52 (CAMPATH-1) in the transplant conditioning regimen has allowed efficient in vivo purging of donor T cells, with the consequent reduction in graft versus host disease (GvHD). We have recently reported the results of allografting following conditioning with fludarabine, busulphan and CAMPATH-1H in 62 patients with high risk myelodysplastic syndrome (MDS) and AML ([24] with matched sibling donors and 32 volunteer unrelated donors, VUD) [62]. The median age for sibling recipients was 56 (41–70) and for VUD recipients 52 (22–65) with a median follow up of 524 days (93–1,392) and 420 days (53–1,495), respectively. The non-relapse mortality at day 100, 200 and 360 days were 0, 5 and 5%, respectively for siblings; and 11, 17 and 21% for VUD. The overall survival at one year was in excess of 70% for both groups with a disease free survival of 61 and 59%. In addition, 86% of the recipients achieved full donor chimerism. The cumulative incidence of grade 3/4 GvHD at day 100 for VUD recipients was 9% and for sibling recipients 0%, indicating the effectiveness of CAMPATH-1 in reducing GvHD. The results were particularly encouraging in low risk MDS patients and AML patients who were in complete morphological and/or cytogenetic remission at the time of transplantation. In contrast, poor prognosis AML patients (i.e. poor risk cytogenetics at diagnosis, only partial remission or progressive leukaemia at the time of transplant) have a high risk of relapse, despite repeated DLIs, with median survival from transplantations for those with relapsed/progressive leukaemia at 68 days and those with impartial remission at 162 days. For these patients there are currently few other treatment options.
Recomposition of an anti-tumour immune response
Whilst immune editing of the tumour takes place through a strong anti-tumour response against the most immunogenic tumour antigens, the immune system also becomes simultaneously “edited” through exhaustion [63, 64]. By combining the essence of surgery, chemotherapy, HSC transplantation and immunotherapy, a window of opportunity may be created to stimulate an un-edited, or at least less edited immune system, to combat a reduced tumour mass which is more likely to be susceptible to eradiation (e.g. reduced expression of immune suppressive factors, numerically reduced chance of generating escape mutants, etc). An example of this strategy is outlined in our Phase I clinical study which combines RIC and HSCT with immune gene therapy of AML.
A Phase-I clinical study for the induction of GvL effect in poor prognosis AML
We have recently obtained Gene Therapy Advisory Committee (GTAC) approval to undertake a Phase-I study involving the immunisation of poor prognosis AML patients who have had an allogeneic HSCT, and have achieved partial or complete remission and clear evidence of donor chimerism, with their own leukaemic cells that are transduced by a self-inactivating lentiviral vector encoding B7.1 and IL-2 (Fig. 2a). This trial, which builds on the established therapeutic efficacy of allogeneic HSCT and DLI, will have a number of important and relatively unique features:
The tumour cell vaccine naturally express both MHC class I and II, as well as a large number of adhesion and co-stimulatory molecules that are normally expressed by the professional APCs—but importantly lack innate B7.1 expression.
The genetically modified AML cells will express the missing B7.1 in combination with IL-2. They may therefore represent effective APCs that could stimulate both helper and effector T cell functions.
The vaccinated patients will have recently received an allogeneic HSCT and DLI, providing a relatively intact, donor derived, immune system and reduced numbers of tolerised, anergised and regulatory T cells.
The vaccinated patients will be in early relapse, therefore, the leukaemic cell burden will be low, thus reducing the chances of “blunting” an otherwise effective immunological response, or the risk of generating escape mutants.
IL-2 a double edge sword!
The clinical application of IL-2 mediated therapies carry a number of potential side effects. The first is the well established systemic toxicity of high dose IL-2 (vascular leak syndrome). In particular, the higher relative toxicity of IL-2 in man, compared to mice, has prevented the clinical administration of IL-2 at doses that are high enough for maximal therapeutic efficacy. Therefore, many of the most promising anti-tumour effects of IL-2 in murine studies have not been reproducible in clinical trials. The second major consideration is the ability of IL-2 to stimulate the expansion and/or activity of regulatory T cells (TReg: CD4+/CD25Hi/FoxP3 +). IL-2 therapy can therefore impede anti-tumour immunity by activating the immune suppressive effects of TReg [65, 66]. Indeed, recent studies have shown that elimination of TReg cells (with the aid of a recombinant IL-2 diphteria toxin conjugate—Ontak) substantially enhances anti-tumour immunity in renal cell carcinoma patients that are subsequently vaccinated with tumour RNA transfected dendritic cells [67]. The IL-2 toxicity, and in particular its effect on regulatory T cells and thus the potential abrogation of anti-tumour immunity, are both concentration dependent. The systemic dose of IL-2 at which both the vascular leak syndrome and increased numbers of TRegs are detectable is in the order of about 700,000 IU (1 IU equals approx. 0.4 ng) per Kg weight, administered as bolus injections up to three times a day for several days [66]. However the in vitro concentration of IL-2 at which the expansion/activation of tumour reactive/specific T cells become clearly visible is at least a 103–104 fold lower than this (i.e. 1–20 ng/ml). Furthermore, in murine tumour models the therapeutic effect of vaccination with IL-2 secreting tumour cells are visible at IL-2 doses that are 103–104 fold lower than the levels used in systemic IL-2 administration in the treatment of renal cell carcinoma. The highest dose of administration with B7.1/IL-2 expressing AML cells in our trial will be 108 lethally irradiated cells per dose injected at three weekly intervals for up to a maximum of six doses. Therefore the systemic IL-2 levels in these patients are likely to be at least a 1,000 fold lower than the levels associated with either in vivo toxicity or stimulation of TRegs; although the minimum IL-2 dose at which TReg stimulation is achieved has not yet been established in clinical trials [66]. Of course the local concentration of IL-2 in the vicinity of the B7.1/IL-2 expressing AML cells is likely to be substantially higher, thus resulting not only in the effective stimulation of cytotoxic T cells, but also the possible expansion/activation of the immune suppressive TReg.
In the light of these considerations, the risk of either IL-2 toxicity or the suppression of anti-leukaemic immunity due to its activation of TReg remain potential risks associated with vaccination using IL-2 expressing tumour cells. Therefore, the detailed analysis of both toxicity, and TReg proliferation/activity remain amongst the central objectives of our B7.1/IL-2 vaccination studies. Furthermore, it remains entirely conceivable that elimination of TReg will enhance the anti-leukaemic effect of HSCT and donor leukocyte infusion, either with or without concomitant vaccination with B7.1/IL-2 expressing AML cells.
Concluding remarks
The success of haematopoietic stem cell transplantation (HSCT) and donor leukocyte infusions (DLI) in the rejection of acute myeloid leukaemia, demonstrate the efficacy of the donor immune system in recognising and eradicating AML blasts. The success of this established form of immune therapy against AML, provides grounds for optimism to be able to enhance the efficacy of immune mediated rejection of AML, and in particular eradication of the minimal residual disease that is the cause of subsequent relapse in the poor prognosis patients. Given the fact that the leukaemia and the immune system are likely to have sculpted each other in ways dictated by their continued evolution in the presence of each other, effective stimulation of immune therapy requires both the reduction of the tumour mass, and elimination of immune tolerance. The former is frequently achievable by chemotherapy, albeit for a transitory period in the poor prognosis AML, and the latter can be achieved, at least in part by HSCT and DLI. Pre-clinical studies showing that the B7.1/IL-2 expressing AML cells can stimulate in vitro cytolytic activity against the unmodified AML cells suggest the potential possibility of vaccination mediated enhancement of immune mediated rejection of residual AML by a donor immune system that is not exhausted by the process of tumour editing. Potential hazards with such vaccinations include the stimulation of graft versus host disease without an even greater stimulation of leukaemia rejection, IL-2 associated toxicities, and possibly even TReg mediated abrogation of HSCT and DLI associated immunity against AML. Given the established role of immune therapy in AML (HSCT and DLI), and the availability of clinically feasible strategies for the elimination of TReg, the clinical effect of TReg elimination in the treatment of AML, warrants direct evaluation.
Acknowledgements
L.C. is funded by Elimination of Leukaemia Fund. N.R.H., J.G.-L. and B.G. are funded by Leukaemia Research Fund. The tumour immune therapy programme in this department is funded by grants from the UK Department of Health, Elimination of Leukaemia Fund, Leukaemia Research Fund, Rose Trees Trust, John and Holly Burton Myeloma Research Programme, Biotechnology & Biological Sciences Research Council (BBSRC), and the Engineering and Physical Sciences Research Council (EPSRC).
Abbreviations
- HSCT
Haematopoietic stem cell transplant
- AML
Acute myeloid leukaemia
- GvL
Graft versus leukaemia
- DLI
Donor leukocyte infusion
- VUD
Volunteer unrelated donor
- GvHD
Graft versus host disease
- MDS
Myelodysplastic syndrome
- RIC
Reduced intensity conditioning
- APC
Antigen presenting cell
- mHA
Minor histocompatibility antigens
- LD-DC
Leukaemia derived-dendritic cell
- CTL
Cytotoxic T lymphocytes
Footnotes
This article is a symposium paper from the “Robert Baldwin Symposium: 50 years of Cancer Immunotherapy”, held in Nottingham, Great Britain, on 30th June 2005.
References
- 1.Laimer M, Lanschuetzer CM, Hintner H. Interaction between the immune system and tumor cells: cutaneous disorders as a consequence of autoimmunity and immunosuppression. Ann N Y Acad Sci. 2004;1028:375–379. doi: 10.1196/annals.1322.043. [DOI] [PubMed] [Google Scholar]
- 2.Dunn GP, Ikeda H, Bruce AT, Koebel C, Uppaluri R, Bui J, Chan R, Diamond M, White JM, Sheehan KC, Schreiber RD. Interferon-gamma and cancer immunoediting. Immunol Res. 2005;32:231–246. doi: 10.1385/IR:32:1-3:231. [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RD. Cancer vaccines 2004 opening address: the molecular and cellular basis of cancer immunosurveillance and immunoediting. Cancer Immun. 2005;5(Suppl 1):1. [PubMed] [Google Scholar]
- 4.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 5.Carreno BM, Carter LL, Collins M. Therapeutic opportunities in the B7/CD28 family of ligands and receptors. Curr Opin Pharmacol. 2005;5:424–430. doi: 10.1016/j.coph.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Greiner J, Ringhoffer M, Taniguchi M, Schmitt A, Kirchner D, Krahn G, Heilmann V, Gschwend J, Bergmann L, Dohner H, Schmitt M. Receptor for hyaluronan acid-mediated motility (RHAMM) is a new immunogenic leukemia-associated antigen in acute and chronic myeloid leukemia. Exp Hematol. 2002;30:1029–1035. doi: 10.1016/S0301-472X(02)00874-3. [DOI] [PubMed] [Google Scholar]
- 7.Greiner J, Ringhoffer M, Taniguchi M, Hauser T, Schmitt A, Dohner H, Schmitt M. Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer. 2003;106:224–231. doi: 10.1002/ijc.11200. [DOI] [PubMed] [Google Scholar]
- 8.Guinn BA, Bland EA, Lodi U, Liggins AP, Tobal K, Petters S, Wells JW, Banham AH, Mufti GJ. Humoral detection of leukaemia-associated antigens in presentation acute myeloid leukaemia. Biochem Biophys Res Commun. 2005;335:1293–1304. doi: 10.1016/j.bbrc.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann L, Miething C, Maurer U, Brieger J, Karakas T, Weidmann E, Hoelzer D. High levels of Wilms’ tumor gene (wt1) mRNA in acute myeloid leukemias are associated with a worse long-term outcome. Blood. 1997;90:1217–1225. [PubMed] [Google Scholar]
- 10.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96:1480–1489. [PubMed] [Google Scholar]
- 11.Elisseeva OA, Oka Y, Tsuboi A, Ogata K, Wu F, Kim EH, Soma T, Tamaki H, Kawakami M, Oji Y, Hosen N, Kubota T, Nakagawa M, Yamagami T, Hiraoka A, Tsukaguchi M, Udaka K, Ogawa H, Kishimoto T, Nomura T, Sugiyama H. Humoral immune responses against Wilms tumor gene WT1 product in patients with hematopoietic malignancies. Blood. 2002;99:3272–3279. doi: 10.1182/blood.V99.9.3272. [DOI] [PubMed] [Google Scholar]
- 12.Wu F, Oka Y, Tsuboi A, Elisseeva OA, Ogata K, Nakajima H, Fujiki F, Masuda T, Murakami M, Yoshihara S, Ikegame K, Hosen N, Kawakami M, Nakagawa M, Kubota T, Soma T, Yamagami T, Tsukaguchi M, Ogawa H, Oji Y, Hamaoka T, Kawase I, Sugiyama H. Th1-biased humoral immune responses against Wilms tumor gene WT1 product in the patients with hematopoietic malignancies. Leukemia. 2005;19:268–274. doi: 10.1038/sj.leu.2403539. [DOI] [PubMed] [Google Scholar]
- 13.Scheibenbogen C, Letsch A, Thiel E, Schmittel A, Mailaender V, Baerwolf S, Nagorsen D, Keilholz U. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 14.Andersen MH, Svane IM, Kvistborg P, Nielsen OJ, Balslev E, Reker S, Becker JC, Straten PT. Immunogenicity of Bcl-2 in patients with cancer. Blood. 2005;105:728–734. doi: 10.1182/blood-2004-07-2548. [DOI] [PubMed] [Google Scholar]
- 15.Whiteway A, Corbett T, Anderson R, Macdonald I, Prentice HG. Expression of co-stimulatory molecules on acute myeloid leukaemia blasts may effect duration of first remission. Br J Haematol. 2003;120:442–451. doi: 10.1046/j.1365-2141.2003.04085.x. [DOI] [PubMed] [Google Scholar]
- 16.Boyer MW, Waller EK, Bray RA, Unangst T, Johnson TS, Phillips C, Jurickova I, Winton EF, Yeager AM. Cytokine upregulation of the antigen presenting function of acute myeloid leukemia cells. Leukemia. 2000;14:412–418. doi: 10.1038/sj.leu.2401685. [DOI] [PubMed] [Google Scholar]
- 17.Charbonnier A, Gaugler B, Sainty D, Lafage-Pochitaloff M, Olive D. Human acute myeloblastic leukemia cells differentiate in vitro into mature dendritic cells and induce the differentiation of cytotoxic T cells against autologous leukemias. Eur J Immunol. 1999;29:2567–2578. doi: 10.1002/(SICI)1521-4141(199908)29:08<2567::AID-IMMU2567>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Harrison BD, Adams JA, Briggs M, Brereton ML, Yin JA. Stimulation of autologous proliferative and cytotoxic T-cell responses by “leukemic dendritic cells” derived from blast cells in acute myeloid leukemia. Blood. 2001;97:2764–2771. doi: 10.1182/blood.V97.9.2764. [DOI] [PubMed] [Google Scholar]
- 19.Roddie PH, Horton Y, Turner ML. Primary acute myeloid leukaemia blasts resistant to cytokine-induced differentiation to dendritic-like leukaemia cells can be forced to differentiate by the addition of bryostatin-1. Leukemia. 2002;16:84–93. doi: 10.1038/sj.leu.2402335. [DOI] [PubMed] [Google Scholar]
- 20.Cignetti A, Vallario A, Roato I, Circosta P, Allione B, Casorzo L, Ghia P, Caligaris-Cappio F. Leukemia-derived immature dendritic cells differentiate into functionally competent mature dendritic cells that efficiently stimulate T cell responses. J Immunol. 2004;173:2855–2865. doi: 10.4049/jimmunol.173.4.2855. [DOI] [PubMed] [Google Scholar]
- 21.Kufner S, Fleischer RP, Kroell T, Schmid C, Zitzelsberger H, Salih H, Valle FD, Treder W, Schmetzer HM (2005) Serum-free generation and quantification of functionally active leukemia-derived DC is possible from malignant blasts in acute myeloid leukemia and myelodysplastic syndromes. Cancer Immunol Immunother. DOI 10.1007/s00262–004–0657-y [DOI] [PMC free article] [PubMed]
- 22.Hirst WJ, Buggins A, Darling D, Gaken J, Farzaneh F, Mufti GJ. Enhanced immune costimulatory activity of primary acute myeloid leukaemia blasts after retrovirus-mediated gene transfer of B7.1. Gene Ther. 1997;4:691–699. doi: 10.1038/sj.gt.3300437. [DOI] [PubMed] [Google Scholar]
- 23.Stripecke R, Cardoso AA, Pepper KA, Skelton DC, Yu XJ, Mascarenhas L, Weinberg KI, Nadler LM, Kohn DB. Lentiviral vectors for efficient delivery of CD80 and granulocyte-macrophage-colony-stimulating factor in human acute lymphoblastic leukemia and acute myeloid leukemia cells to induce antileukemic immune responses. Blood. 2000;96:1317–1326. [PubMed] [Google Scholar]
- 24.Koya RC, Kasahara N, Pullarkat V, Levine AM, Stripecke R. Transduction of acute myeloid leukemia cells with third generation self-inactivating lentiviral vectors expressing CD80 and GM-CSF: effects on proliferation, differentiation, and stimulation of allogeneic and autologous anti-leukemia immune responses. Leukemia. 2002;16:1645–1654. doi: 10.1038/sj.leu.2402582. [DOI] [PubMed] [Google Scholar]
- 25.Bain C, Merrouche Y, Puisieux I, Duc A, Colombo MP, Favrot M. B7.1 gene transduction of human renal-cell-carcinoma cell lines restores the proliferative response and cytotoxic function of allogeneic T cells. Int J Cancer. 1996;67:769–776. doi: 10.1002/(SICI)1097-0215(19960917)67:6<769::AID-IJC4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 26.Fenton RG, Turcovski-Corrales SM, Taub DD. Induction of melanoma antigen-specific cytotoxic T lymphocytes in vitro by stimulation with B7-expressing human melanoma cell lines. J Immunother. 1998;21:95–108. doi: 10.1097/00002371-199803000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Kim SJ, Sadelain M, Lee JS, Seong RH, Yun YS, Jang YJ, Chung HY. Adoptive-transfer therapy of tumors with the tumor-specific primary cytotoxic T cells induced in vitro with the B7.1-transduced MCA205 cell line. Cancer Immunol Immunother. 1999;47:257–264. doi: 10.1007/s002620050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyazono Y, Kamogawa Y, Ryo K, Furukawa T, Mitsuhashi M, Yamauchi K, Kameoka T, Hayashi N. Effect of B7.1-transfected human colon cancer cells on the induction of autologous tumour-specific cytotoxic T cells. J Gastroenterol Hepatol. 1999;14:997–1003. doi: 10.1046/j.1440-1746.1999.01990.x. [DOI] [PubMed] [Google Scholar]
- 29.Schendel DJ, Frankenberger B, Jantzer P, Cayeux S, Nobetaner E, Willimsky G, Maget B, Pohla H, Blankenstein T. Expression of B7.1 (CD80) in a renal cell carcinoma line allows expansion of tumor-associated cytotoxic T lymphocytes in the presence of an alloresponse. Gene Ther. 2000;7:2007–2014. doi: 10.1038/sj.gt.3301349. [DOI] [PubMed] [Google Scholar]
- 30.Wang YC, Zhu L, McHugh R, Graham SD, Jr, Hillyer CD, Dillehay D, Sell KW, Selvaraj P. Induction of autologous tumor-specific cytotoxic T-lymphocyte activity against a human renal carcinoma cell line by B7–1 (CD8O) costimulation. J Immunother Emphasis Tumor Immunol. 1996;19:1–8. doi: 10.1097/00002371-199601000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Yang S, Darrow TL, Seigler HF. Generation of primary tumor-specific cytotoxic T lymphocytes from autologous and human lymphocyte antigen class I-matched allogeneic peripheral blood lymphocytes by B7 gene-modified melanoma cells. Cancer Res. 1997;57:1561–1568. [PubMed] [Google Scholar]
- 32.Wendelbo O, Nesthus I, Sjo M, Paulsen K, Ernst P, Bruserud O. Functional characterization of T lymphocytes derived from patients with acute myelogenous leukemia and chemotherapy-induced leukopenia. Cancer Immunol Immunother. 2004;53:740–747. doi: 10.1007/s00262-004-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buggins AG, Lea N, Gaken J, Darling D, Farzaneh F, Mufti GJ, Hirst WJ. Effect of costimulation and the microenvironment on antigen presentation by leukemic cells. Blood. 1999;94:3479–3490. [PubMed] [Google Scholar]
- 34.Buggins AG, Milojkovic D, Arno MJ, Lea NC, Mufti GJ, Thomas NS, Hirst WJ. Microenvironment produced by acute myeloid leukemia cells prevents T cell activation and proliferation by inhibition of NF-kappaB, c-Myc, and pRb pathways. J Immunol. 2001;167:6021–6030. doi: 10.4049/jimmunol.167.10.6021. [DOI] [PubMed] [Google Scholar]
- 35.Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- 36.Gaken J, Darling D, Hollingsworth S, Hirst W, Peakman M, Kuiper M, Humphries S, Towner P, Mufti GJ, Farzaneh F. Synergy between B7.1 and IL-2 gene modification in the induction of tumour rejection. Cancer Gene Therapy. 1994;1:212. [Google Scholar]
- 37.Salvadori S, Gansbacher B, Wernick I, Tirelli S, Zier K. B7–1 amplifies the response to interleukin-2-secreting tumor vaccines in vivo, but fails to induce a response by naive cells in vitro. Hum Gene Ther. 1995;6:1299–1306. doi: 10.1089/hum.1995.6.10-1299. [DOI] [PubMed] [Google Scholar]
- 38.Gaken JA, Hollingsworth SJ, Hirst WJ, Buggins AG, Galea-Lauri J, Peakman M, Kuiper M, Patel P, Towner P, Patel PM, Collins MK, Mufti GJ, Farzaneh F, Darling DC. Irradiated NC adenocarcinoma cells transduced with both B7.1 and interleukin-2 induce CD4+-mediated rejection of established tumors. Hum Gene Ther. 1997;8:477–488. doi: 10.1089/hum.1997.8.4-477. [DOI] [PubMed] [Google Scholar]
- 39.Bubenik J, Rosser P, Bubenikova D, Simova J, Indrova M, Sloncova E. Tumour vaccines expressing IL-2, CD80, and IL-2 plus CD80 gene. Intl J Oncol. 1997;11:1213–1219. doi: 10.3892/ijo.11.6.1213. [DOI] [PubMed] [Google Scholar]
- 40.Emtage PC, Wan Y, Bramson JL, Graham FL, Gauldie J. A double recombinant adenovirus expressing the costimulatory molecule B7-1 (murine) and human IL-2 induces complete tumor regression in a murine breast adenocarcinoma model. J Immunol. 1998;160:2531–2538. [PubMed] [Google Scholar]
- 41.Cayeux S, Richter G, Becker C, Beck C, Aicher A, Pezzutto A, Dorken B, Blankenstein T. Lack of correlation between rejection of tumor cells co-expressing interleukin-2 and B7.1 and vaccine efficiency. Eur J Immunol. 1997;27:1657–1662. doi: 10.1002/eji.1830270710. [DOI] [PubMed] [Google Scholar]
- 42.Galea-Lauri J, Darling D, Gan SU, Krivochtchapov L, Kuiper M, Gaken J, Souberbielle B, Farzaneh F. Expression of a variant of CD28 on a subpopulation of human NK cells: implications for B7-mediated stimulation of NK cells. J Immunol. 1999;163:62–70. [PubMed] [Google Scholar]
- 43.Gao JX, Liu X, Wen J, Caligiuri MA, Stroynowski I, Zheng P, Liu Y. Two-signal requirement for activation and effector function of natural killer cell response to allogeneic tumor cells. Blood. 2003;102:4456–4463. doi: 10.1182/blood-2003-07-2480. [DOI] [PubMed] [Google Scholar]
- 44.Barnard AL, Farzaneh F, Gaken J, Darling D. Local versus systemic interleukin-2: tumor formation by wild-type and B7-1-positive murine melanoma cells. Cancer Gene Ther. 2000;7:207–214. doi: 10.1038/sj.cgt.7700087. [DOI] [PubMed] [Google Scholar]
- 45.Ge NL, Ye SL, Zheng N, Sun RX, Liu YK, Tang ZY. Prevention of hepatocellular carcinoma in mice by IL-2 and B7-1 genes co-transfected liver cancer cell vaccines. World J Gastroenterol. 2003;9:2182–2185. doi: 10.3748/wjg.v9.i10.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larchian WA, Horiguchi Y, Nair SK, Fair WR, Heston WD, Gilboa E. Effectiveness of combined interleukin 2 and B7.1 vaccination strategy is dependent on the sequence and order: a liposome-mediated gene therapy treatment for bladder cancer. Clin Cancer Res. 2000;6:2913–2920. [PubMed] [Google Scholar]
- 47.Kochling J, Konig-Merediz SA, Stripecke R, Buchwald D, Korte A, Von Einsiedel HG, Sack F, Henze G, Seeger K, Wittig B, Schmidt M. Protection of mice against Philadelphia chromosome-positive acute lymphoblastic leukemia by cell-based vaccination using nonviral, minimalistic expression vectors and immunomodulatory oligonucleotides. Clin Cancer Res. 2003;9:3142–3149. [PubMed] [Google Scholar]
- 48.Gaken J, Jiang J, Daniel K, van Berkel E, Hughes C, Kuiper M, Darling D, Tavassoli M, Galea-Lauri J, Ford K, Kemeny M, Russell S, Farzaneh F. Fusagene vectors: a novel strategy for the expression of multiple genes from a single cistron. Gene Ther. 2000;7:1979–1985. doi: 10.1038/sj.gt.3301341. [DOI] [PubMed] [Google Scholar]
- 49.Chan L, Nesbeth D, Mackey T, Galea-Lauri J, Gaken J, Martin F, Collins M, Mufti G, Farzaneh F, Darling D. Conjugation of lentivirus to paramagnetic particles via nonviral proteins allows efficient concentration and infection of primary acute myeloid leukemia cells. J Virol. 2005;79:13190–13194. doi: 10.1128/JVI.79.20.13190-13194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bello-Fernandez C, Stasakova J, Renner A, Carballido-Perrig N, Koening M, Waclavicek M, Madjic O, Oehler L, Haas O, Carballido JM, Buschle M, Knapp W. Retrovirus-mediated IL-7 expression in leukemic dendritic cells generated from primary acute myelogenous leukemias enhances their functional properties. Blood. 2003;101:2184–2190. doi: 10.1182/blood-2002-02-0378. [DOI] [PubMed] [Google Scholar]
- 51.Roddie PH, Paterson T, Turner ML. Gene transfer to primary acute myeloid leukaemia blasts and myeloid leukaemia cell lines. Cytokines Cell Mol Ther. 2000;6:127–134. doi: 10.1080/mccm.6.3.127.134. [DOI] [PubMed] [Google Scholar]
- 52.Wattel E, Vanrumbeke M, Abina MA, Cambier N, Preudhomme C, Haddada H, Fenaux P. Differential efficacy of adenoviral mediated gene transfer into cells from hematological cell lines and fresh hematological malignancies. Leukemia. 1996;10:171–174. [PubMed] [Google Scholar]
- 53.Gonzalez R, Vereecque R, Wickham TJ, Vanrumbeke M, Kovesdi I, Bauters F, Fenaux P, Quesnel B. Increased gene transfer in acute myeloid leukemic cells by an adenovirus vector containing a modified fiber protein. Gene Ther. 1999;6:314–320. doi: 10.1038/sj.gt.3300836. [DOI] [PubMed] [Google Scholar]
- 54.Heinzinger NK, Bukinsky MI, Haggerty SA, Ragland AM, Kewalramani V, Lee MA, Gendelman HE, Ratner L, Stevenson M, Emerman M. The Vpr protein of human immunodeficiency virus type 1 influences nuclear localization of viral nucleic acids in nondividing host cells. Proc Natl Acad Sci USA. 1994;91:7311–7315. doi: 10.1073/pnas.91.15.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yao XJ, Subbramanian RA, Rougeau N, Boisvert F, Bergeron D, Cohen EA. Mutagenic analysis of human immunodeficiency virus type 1 Vpr: role of a predicted N-terminal alpha-helical structure in Vpr nuclear localization and virion incorporation. J Virol. 1995;69:7032–7044. doi: 10.1128/jvi.69.11.7032-7044.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bambacioni F, Casati C, Serafini M, Manganini M, Golay J, Introna M. Lentiviral vectors show dramatically increased efficiency of transduction of human leukemic cell lines. Haematologica. 2001;86:1095–1096. [PubMed] [Google Scholar]
- 57.Biagi E, Bambacioni F, Gaipa G, Casati C, Golay J, Biondi A, Introna M. Efficient lentiviral transduction of primary human acute myelogenous and lymphoblastic leukemia cells. Haematologica. 2001;86:13–16. [PubMed] [Google Scholar]
- 58.Stripecke R, Koya RC, Ta HQ, Kasahara N, Levine AM. The use of lentiviral vectors in gene therapy of leukemia: combinatorial gene delivery of immunomodulators into leukemia cells by state-of-the-art vectors. Blood Cells Mol Dis. 2003;31:28–37. doi: 10.1016/S1079-9796(03)00062-7. [DOI] [PubMed] [Google Scholar]
- 59.Chan L, Hardwick N, Darling D, Galea-Lauri J, Gaken J, Devereux S, Kemeny M, Mufti G, Farzaneh F. IL-2/B7.1 (CD80) fusagene transduction of AML blasts by a self-inactivating lentiviral vector stimulates T cell responses in vitro: a strategy to generate whole cell vaccines for AML. Mol Ther. 2005;11:120–131. doi: 10.1016/j.ymthe.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 60.Hughes C, Galea-Lauri J, Farzaneh F, Darling D. Streptavidin paramagnetic particles provide a choice of three affinity-based capture and magnetic concentration strategies for retroviral vectors. Mol Ther. 2001;3:623–630. doi: 10.1006/mthe.2001.0268. [DOI] [PubMed] [Google Scholar]
- 61.Nesbeth D, Williams S, Chan L, Brain T, Slater N, Farzaneh F, Darling D (2005) Metabolic biotinylation of lentiviral pseudotypes for scaleable paramagnetic microparticle dependent manipulation. Mol Ther [DOI] [PubMed]
- 62.Ho AY, Pagliuca A, Kenyon M, Parker JE, Mijovic A, Devereux S, Mufti GJ. Reduced-intensity allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome and acute myeloid leukemia with multilineage dysplasia using fludarabine, busulphan, and alemtuzumab (FBC) conditioning. Blood. 2004;104:1616–1623. doi: 10.1182/blood-2003-12-4207. [DOI] [PubMed] [Google Scholar]
- 63.Molldrem JJ, Lee PP, Kant S, Wieder E, Jiang W, Lu S, Wang C, Davis MM. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J Clin Invest. 2003;111:639–647. doi: 10.1172/JCI16398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Klenerman P, Cerundolo V, Dunbar PR. Tracking T cells with tetramers: new tales from new tools. Nat Rev Immunol. 2002;2:263–272. doi: 10.1038/nri777. [DOI] [PubMed] [Google Scholar]
- 65.Nelson BH. IL-2, regulatory T cells, and tolerance. J Immunol. 2004;172:3983–3988. doi: 10.4049/jimmunol.172.7.3983. [DOI] [PubMed] [Google Scholar]
- 66.Ahmadzadeh M, Rosenberg SA (2005) IL-2 administration increases CD4+CD25hiFoxp3+ regulatory T cells in cancer patients. Blood [DOI] [PMC free article] [PubMed]
- 67.Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, Zhang A, Dahm P, Chao N, Gilboa E, Vieweg J. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]