Abstract
Nucleolin is multifunctional protein mainly present in nucleoli but also detected in cytoplasm and plasma membranes. Extranuclear nucleolin differs from the nuclear form by its glycosylation. Studies on expression of nucleolin in breast cancer suggest a possible association to the metastatic cascade. In the present study, Vicia villosa lectin (VVL) precipitation followed by subsequent polyacrylamide gel electrophoresis and mass spectrometry analysis demonstrates nucleolin as a VVL-positive glycoprotein expressed in melanoma. The presence of VVL-positive nucleolin in the melanoma cell membrane and cytoplasm was confirmed by confocal microscopy. Using bioinformatic peptide prediction programs, nucleolin was shown to contain multiple possible MHC class-I binding peptides in its sequence which makes nucleolin an interesting melanoma marker and target for immunodiagnostic and possibly therapeutic purposes.
Keywords: Nucleolin, Melanoma, Tn antigen
Introduction
Malignant transformation is associated with abnormal glycosylation resulting in synthesis of altered N- or O-glycans. Changes of cell surface carbohydrate have a profound influence on the cells’ behaviour and have been associated with metastasis formation [11, 24]. One of the most frequently described cancer-related changes in the pattern of glycosylation is the premature termination of biosynthesis resulting in the expression of uncompleted forms of O-linked glycans. Two such carbohydrate antigens are the Thomsen-Friedenreich (T) antigen (Galβ1-3GalNAcα1-O-Ser/Thr) and its immediate precursor, the Tn antigen (GalNAcα1-O-Ser/Thr). These antigens can be detected by antibodies or lectins. In normal tissues these antigens are often present in “cryptic” forms [6] but expressed in carcinomas of several organs. The expression of T and Tn antigens has been found to correlate with tumour aggressiveness [33]. Kanitakis et al. [20] have shown the accumulation of the Tn antigen in tumours metastasizing to the skin. The predominant Tn versus T antigen expression might be helpful in order to aid the differentiation of primary cutaneous melanoma from metastatic lesions. Thies et al. [35] analysed the association between lectin binding and metastasis in cutaneous malignant melanoma in 100 patients. The results clearly showed that GlcNAc/GalNAc residues, recognized by Helix pomatia agglutinin (HPA), are linked to metastasis in malignant melanoma. The close association between Tn and sialyl-Tn antigens and neoplastic transformation prompted some investigators to use such antigens for active immunotherapy [31].
In this study, we focus on the identification of Tn-antigen carrying proteins in primary and metastatic melanoma cell lines. We used Vicia villosa agglutinin (VVL) which specifically binds GalNAcα1-O-Ser/Thr for isolation of Tn-antigen bearing glycoproteins and nanoLC-MS/MS technique for identification of these proteins. We confirmed the mass spectrometry results using lectin precipitation followed by Western blotting. Using indirect immunofluorescence staining and laser scanning confocal microscopy, we showed nucleolin to be present in nuclei, cytoplasm and on the cell surface of human melanoma cells. In contrast to nuclear nucleolin, the surface-expressed and cytoplasmic nucleolin exhibited Tn antigen as shown by simultaneous immunofluorescence staining of nucleolin and VVL-positive glycoproteins in confocal microscopy.
Materials and methods
Materials
Biotinylated, FITC-conjugated and agarose-bound lectin VVL, as well as Vectashield HardsetTM mounting medium with DAPI were purchased from Vector (USA). Anti-mouse IgG/AP, foetal calf serum and Immunoprecipitation Kit (Protein G) were purchased from Boehringer (Mannheim, Germany). Trypsin was from Promega (USA). Immobilon-P transfer membrane and rabbit anti-mouse IgG/AP were obtained from Millipore and Chemicon (USA), respectively. RPMI 1640 medium, Protein Assay Kit, Brilliant Blue G Colloidal, GalNAc, ExtrAvidin/AP and normal goat serum were obtained from Sigma (St. Louis, MO). Mouse anti-human nucleolin mAbs, clone 3G4B2 were from Upstate (USA) and mouse anti-human nucleolin mAbs MS-3 were from Santa Cruz Biotechnology (USA). Alexa Fluor Cy3-conjugated goat anti-mouse mAbs were from Invitrogen (USA). All other chemicals were of the highest purity and were purchased from Sigma (St. Louis, MO).
Cell lines
Human cutaneous primary melanoma cell lines: WM35, WM115 and WM793 were obtained from The ESTDAB Melanoma Cell Bank (Tübingen). Metastatic melanoma cell lines: KNUD, Ma-Mel-04, Ma-Mel-8b, Ma-Mel-16, Ma-Mel-12, Ma-Mel-27 were obtained from Prof. D. Schadendorf, and FM55M2, WM1205Lu, WM39 were from The ESTDAB Melanoma Cell Bank (Tübingen).
Cell culture condition and cell extract preparation
All cell lines were maintained in RPMI 1640 medium with GlutaMAX-I, supplemented with 10% foetal calf serum, 100 units/ml penicillin and 100 μg/ml streptomycin. Cells were grown in monolayers at 95% air/5% CO2 atmosphere at 37°C in a humidified incubator. Cell extract proteins were prepared as described previously [22]. The protein concentrations were determined using Protein Assay Kit.
Isolation and mass spectrometry analysis of VVL-positive glycoproteins
Precipitation with VVL-agarose
The cleared cell extracts (600 μg) were incubated overnight at 4°C with 50 μl of VVL-agarose in 10 mM HEPES, 0.15 M NaCl, pH 7.5 on a circular rotator (Omega, Poland). Afterwards, the precipitates were washed three times with above buffer, and one time with PBS with subsequent centrifugations (10,000 g, 1 min, 4°C). Glycoproteins bound to VVL-agarose were released by boiling (100°C, 10 min) in sample buffer: 50 mM Tris, 5% mercaptoethanol, 2% SDS, 10% glycerol, 1 mM EDTA, pH 6.8 and then supernatants were collected.
SDS-PAGE and lectin blotting
Material obtained after lectin precipitation was divided into three nonequal parts: 80% was used for glycoprotein detection (Brilliant Blue G Colloidal staining, CBB) and remaining material (2 × 10%) for on-blot lectin probe, and then electrophoresed on 8% SDS-polyacrylamide gel according to Laemmli [23]. One part of gel was stained with CBB, and the second one was electrotransfered on PVDF membrane and probed with VVL-biotin lectin (1:125 dilution) pre-blocked with 0.2 M GalNAc for 2 h at RT. After washing, the peaces of membrane were incubated with ExtrAvidin/AP (1:4000 dilution) for 1 h at RT. The conjugated alkaline phosphatase was detected by NBT/X-phosphate staining.
Sample preparation for mass spectrometry analysis
Individual protein bands corresponding to the VVL staining patterns were excised from the gel, chopped into cubes (ca. 1 × 1 mm), rinsed with water and transferred into siliconized centrifuge tubes. CBB stain was removed with 100 mM NH4HCO3 and an equal volume of acetonitrile was added after 10–15 min. Then, gel pieces were dehydrated with acetonitrile and re-swollen in 12 ng/μl trypsin in 100 mM NH4HCO3 in an ice bucket for 45 min. The supernatant which was not absorbed by gel particles was removed, and gel pieces were immersed in 50 mM NH4HCO3 and incubated overnight at 37°C. After completion of digestion, the supernatant was transferred into another tube, followed by addition of 50 mM NH4HCO3, and after 10–15 min an equal volume of acetonitrile was pipetted. The samples were incubated under shaking at 37°C for 30 min. Extraction of peptides was repeated twice with 5% formic acid (v/v) in acetonitrile, and combined extracts evaporated to dryness in a vacuum centrifuge (SpeedVac, Savant).
NanoLC-MS/MS investigation
Dried samples were prepared for LC-MS/MS by dissolving in 7 μl 0.1% trifluoroacetic acid. The LC-MS/MS analysis, used to separate the digests, was performed with the Ultimate LC microchromatography system (LC Packings/Dionex, Amsterdam, The Netherlands). The separation was made on an LC Packings capillary column filled with the PepMap reversed-phase material (15 cm long, 75 μm ID, C18, 2–3 μm bead size and 100 Å pore size). The gradient was formed using 0.1% HCOOH in 98:2 (v/v) water/acetonitrile solution (solvent A) and 0.1% HCOOH in 20:80 (v/v) water/acetonitrile solution (solvent B), and it was delivered at flow rate of 300 nl/min. The system was controlled by Chromeleon software (Dionex). A gradient was produced from 2 to 45% B in 30 min and up to 90% B at 60 min. The chromatographic system was coupled directly to the Esquire 3000 quadrupole ion-trap mass spectrometer (Bruker Saxonia, Leipzig, Germany) using home-made “black-dust” nanoelectrospray emitter. The instrument operated in positive-ion mode. During analysis, most intense peaks in the range 400–1,900 m/z were automatically fragmented by means of data-dependent fragmentation.
Bioinformatic analysis
Search parameters were set as follows: taxonomy: human, modification: carbamidomethyl (fixed), up to 1 missed cleavage, peptide charges +1, +2, and +3, mass tolerance 1.6 Da for precursor mass, and 0.5 Da for fragment mass. The acquired spectra were analysed using Bruker Data Analysis software and were interpreted using Mascot search engine against Swiss-Prot/TrEMBL sequence database.
Confirmation of mass spectrometry results
Lectin precipitation and immunobloting
Lectin precipitation was performed as above using 300 μg of cell extracts and 30 μl of VVL-agarose. Fifty percent of lectin precipitate was used for on-blot immunostaining of nucleolin and 2 × 25% for on-blot lectin probe, and then electrophoresed and electrotransfered, in parallel, with 15 μg of total protein from cell extract. Immunodetection of nucleolin on PVDF membrane was performed using mouse anti-human nucleolin mAb (clone 3G4B2, 1:6,000 dilution) in 50 mM Tris, 0.15 M NaCl, pH 7.5 with 0.1% Tween and 1% BSA for 2 h at RT. After washing, the membranes were incubated with rabbit anti-mouse IgG/AP (1:4,000 dilution) in 50 mM Tris, 0.15 M NaCl, pH 7.5 with 0.1% Tween and 1% BSA for 1 h at RT. The conjugated alkaline phosphatase was detected by NBT/X-phosphate staining. The lectin probe was done as described above.
Immunofluorescence and confocal microscopy
Cells were plated in glass slides and grown in four-well plates (Nunc, Germany). Growing medium was changed for fresh medium, and after 5 h cells were fixed with (i) 3% paraformaldehyd (PFA, for nucleolin cellular staining), 10 min, RT or (ii) 2% PFA, 10 min, RT, followed by permeabilisation with 0.1% Triton X-100 (for nucleolin nucleolar staining), 1 min, RT. After blocking with 10 normal goat serum (NGS), 2% BSA in PBS for 30 min at RT cells were incubated with mouse anti- human nucleolin IgG (MS-3) diluted 1:100 in 2% BSA/PBS for overnight at RT, and then with Cy3-conjugated goat anti-mouse IgG diluted 1:300 in 2% BSA/PBS for 2 h at RT.
For nucleolin cell surface staining (after 5 h of culture in fresh medium), primary antibodies (MS-3) diluted 8:200 in 10% NGS, 2% BSA/PBS containing 25 mM NaN3 were added to wells and incubated with the cells for 1 h at 37°C. Cells were then washed three times with PBS containing 25 mM NaN3 and fixed with 2% PFA for 10 min at RT. Secondary antibodies (Cy3-conjugated goat anti- mouse IgG) were diluted 1:300 in 1% BSA/PBS, and incubated with the cells for 2 h at RT.
For co-localization of nucleolin and VVL lectin, the experiments mentioned above were repeated using simultaneous staining with mAb MS-3 and VVL-FITC lectin (dilution 1:100), and then incubation with Cy3-conjugated goat anti-mouse IgG was performed as described above. Cells were mounted with Vectashield HardsetTM mounting medium with DAPI and analysed in confocal microscope (Zeiss LSM 510 Meta).
Results
Nucleolin is identified as one of the proteins bearing Tn antigen in human melanoma
Combination of lectin precipitation with subsequent polyacrylamide gel electrophoresis and mass spectrometry analysis demonstrates nucleolin as a VVL-positive glycoprotein in all analysed melanoma cell lines (An outline of the general protocol is shown in Fig. 1). Primarily in CBB-stained proteins resolved in SDS-PAGE from analysed cell extracts at least nine protein bands per cell line were found which were captured by VVL-agarose. The typical pattern of VVL-captured, then SDS-PAGE resolved and CBB-stained proteins from primary and metastatic melanoma cells were shown in Fig. 2 in lanes 2 and 7, respectively. CBB-stained protein bands corresponding to one of the most intense VVL-stained bands were excised from the gel and subjected to nanoLC-MS/MS analysis (the encircled bands in Fig. 2 lines 2 and 7). These bands were VVL-positive judging by on-blot lectin specificity test (Fig. 2 lines 4 and 9). Peptide mass fingerprint analysis in the data bank NCBI with the Mascot program identified these proteins as nucleolin. The matched peptides covered between 9 and 43% (64–305 amino acids) of the protein sequence. The detailed results from mass spectra are summarized in Fig. 3. To validate the mass spectrometry results, we used Western blot analysis to detect the presence of nucleolin in the VVL-agarose precipitates (Fig. 4). In all analysed cell lines, the molecular weight of Tn antigen-bearing nucleolin ranged between 102 and 108 kDa. The lower molecular weight range, 92–97 kDa, was also identified as nucleolin fragments suggesting that it may be a proteolytic product of nucleolin (Fig. 4). The partial proteolysis of nucleolin has been well documented in the literature [4].
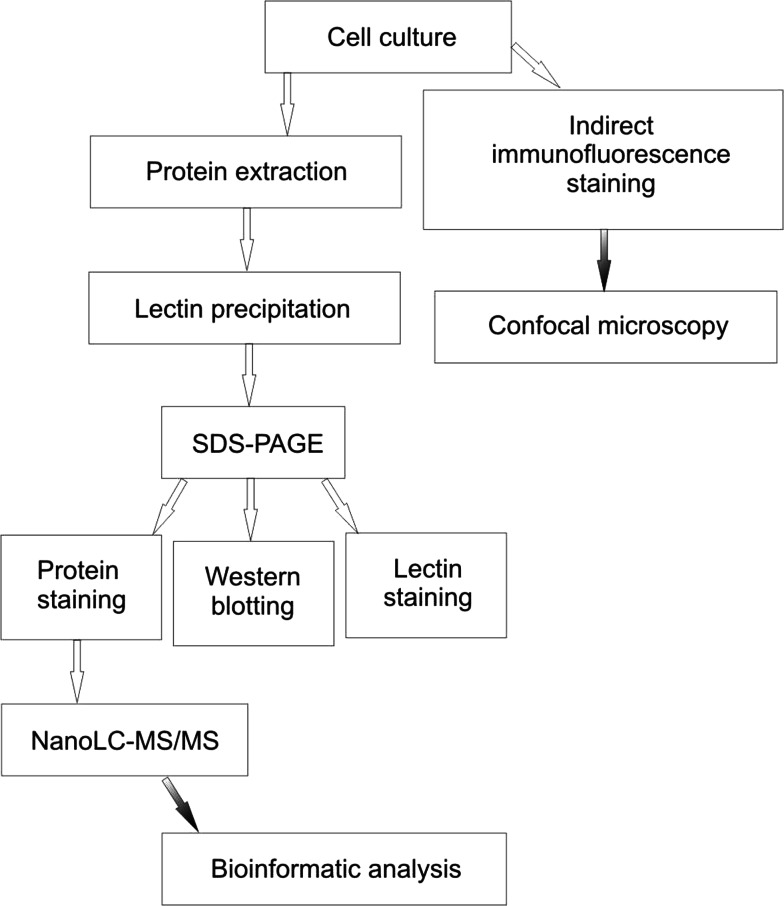
Fig. 1.
Overview of the technique for analysis of Tn antigen-bearing glycoproteins
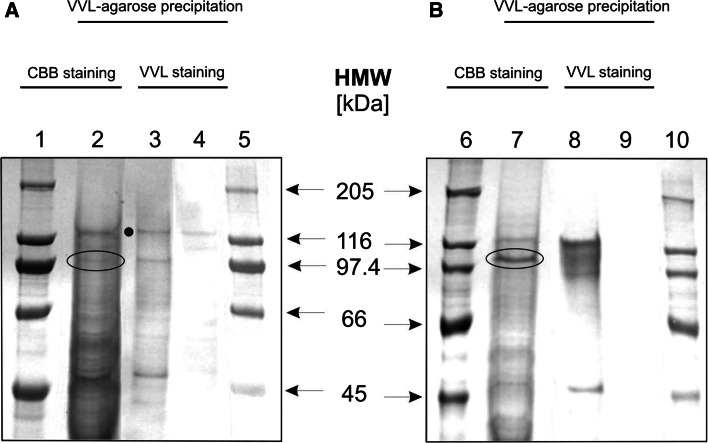
Fig. 2.
Lectin precipitation of Tn antigen-bearing glycoproteins in primary and metastatic melanoma. Extracts of WM793 (a) and Ma-Mel-27 (b) cellular proteins were precipitated using VVL-agarose, separated by SDS-PAGE and stained with CBB (lanes 2 and 7) or electrotransferred on PVDF membrane and probed with VVL (lanes 3 and 8) or VVL pre-blocked with GalNAc (lanes 4 and 9). In parallel, protein standards were resolved (lanes 1, 5, 6 and 10). Bands of interest (103 kDa for WM793 cell extract and 105 kDa for Ma-Mel-27 cell extract) were excised from the gel and subjected to mass spectrometry analysis (the encircled bands in lines 2 and 7). Note. One non-specific band was observed and marked with a black dot
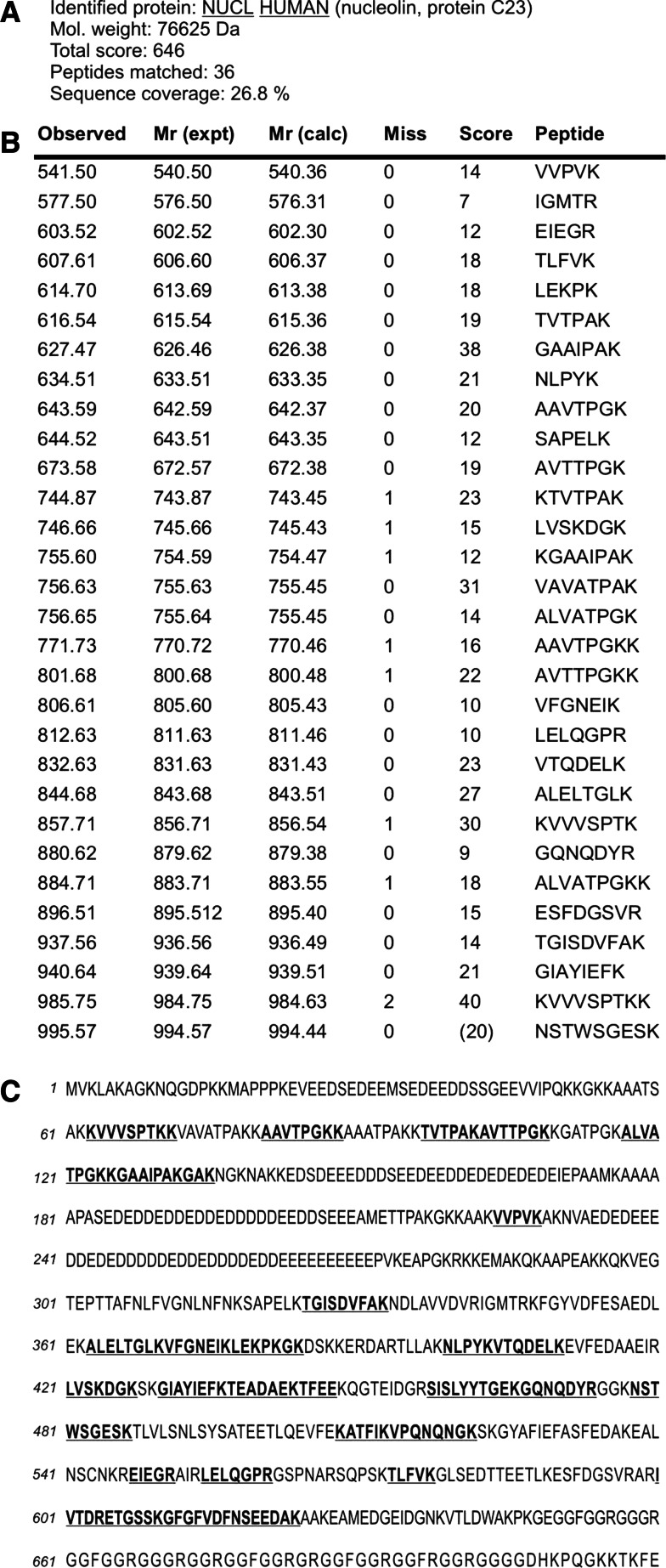
Fig. 3.
Results of the mass spectrometry analysis of in-gel tryptic digest of 105 kDa protein band isolated from total lysate of Ma-Mel-27 cells using VVL-agarose. The encircled band shown in Fig. 2 lane 7 was excised from polyacrylamide gel and digested with trypsin, and the tryptic peptides were sequenced. The acquired spectra were analysed using Bruker Data Analysis software and interpreted using Mascot search engine against Swiss-Prot/TrEMBL sequence database. Peptides determined by sequencing were found to correspond to a high degree of certainty to human nucleolin (a). The table columns contain: Observed, experimental m/z value; Mr(expt), experimental m/z transformed to a relative molecular mass; Mr(calc), relative molecular mass calculated from the matched peptide sequence; Miss, number of missed cleavage sites; Score, ions score; Peptide, sequence of the matched peptide in 1-letter code (b). Localization of the identified peptides within the nucleolin sequence. The primary structure of human nucleolin is shown in the single-letter amino acid code sequence. Matched tryptic peptides are in bold and underlined (c). Note. Matched peptides equally cover the nucleolin sequence
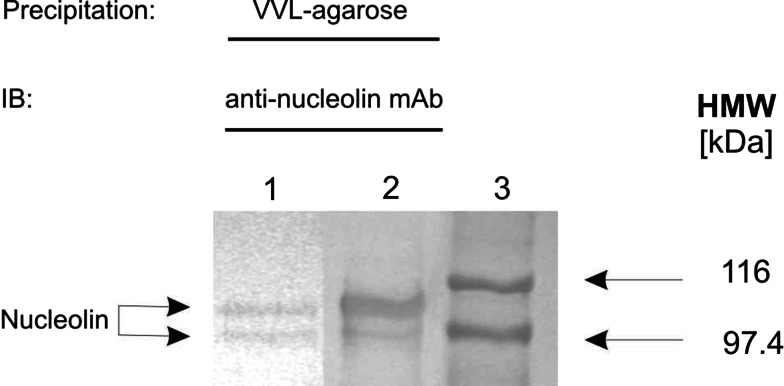
Fig. 4.
Confirmation of mass spectrometry results. Extracts of WM793 (lane 1) and Ma-Mel-27 (lane 2) cellular proteins were precipitated using VVL-agarose, separated by SDS-PAGE and blotted on PVDF membrane. In parallel, protein standards were resolved (lane 3). Immunodetection of nucleolin was performed using anti-human nucleolin mAb, clone 3G4B2
Nucleolin is present on the surface of melanoma cells
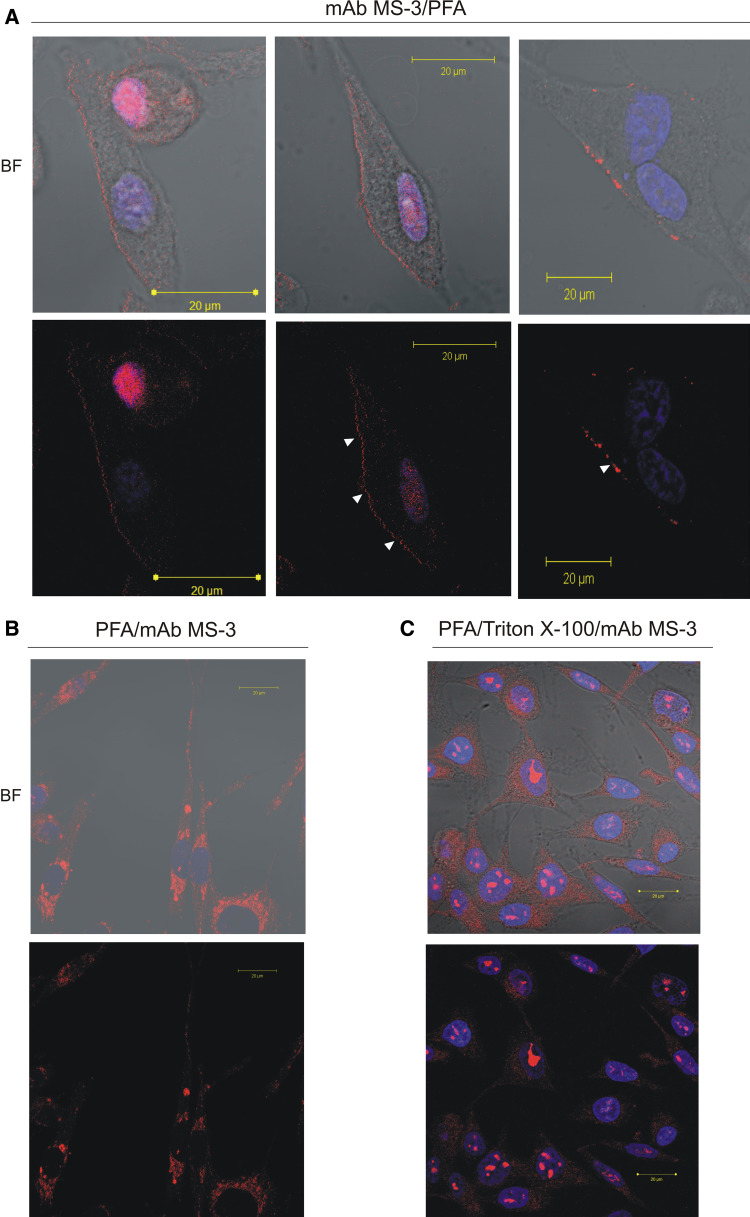
Originally nucleolin was reported as exclusively localized within the nucleus [9]; however, more recent studies have shown that nucleolin is also present on the surface of a variety of cells [12, 17, 19, 25, 29] including endothelial cells [5, 18]. To address whether nucleolin is also present on the surface of human melanoma cells, we carried out immunofluorescence analysis of Ma-Mel-27 cells. This cell line was grown in fresh medium for 5 h before immunostaining as it was reported to promote the cell surface localisation of nucleolin in other cell types [17]. Live, non-permeabilised cells and fixed, semi-permeabilised cells and permeabilised cells were stained with antinucleolin antibodies. To abolish antibody internalization into viable cells during cell surface nucleolin staining sodium azide was used as metabolic inhibitor [7]. Anti-nucleolin staining of the live non-permeabilised cells showed delicate punctate patches on the outside of the cells (Fig. 5a) suggesting a clustering of nucleolin mediated by the antibodies in the living melanoma cells. The semi-permeabilised cells (fixation with 3% paraformaldehyde (PFA)) showed moderate level of nucleolin staining within the cytoplasm (Fig. 5b). Since PFA was used for partial permeabilisation, nucleolar localisation of nucleolin was not expected. The completely permeabilised cells (fixation with 2% PFA and 0.1% Triton X-100) showed intracellular nucleolin as intense stained fine dot-like structures in nucleoli and moderate staining of cytoplasmic nucleolin (Fig. 5c). It should be pointed out that the detection of cytoplasmic nucleolin in permeabilised cells required scanning at an elevated intensity that gives a highly saturated signal in the nucleolus.
Fig. 5.
Detection of membrane-associated and intracellular nucleolin in melanoma cells by confocal immunofluorescence laser microscopy. For the detection of the cell-surface-clustered nucleolin (a), Ma-Mel-27 cells cultured in fresh medium for 5 h were further incubated for 1 h at 37°C in the presence of mAb MS-3 diluted 1:25 in 10% NGS, 2% BSA/PBS containing 25 mM NaN3 before PFA fixation. For staining of semi-permeabilised (b) or permeabilised cells (c) Ma-Mel-27 cells (after 5 h of culture in fresh medium) were fixed with 3% PFA or 2% PFA/Triton before incubation with mAb MS-3 in 1% BSA/PBS (overnight, RT) diluted 1:100, respectively. The bound anti-nucleolin antibody was revealed by Cy3-labeled goat anti-mouse antibodies (red). Nuclei were counterstained with DAPI (blue). BF; bright field. Note. In PFA/Triton-fixed cells, nucleolin was detected primarily as fine dot-like structures in the nuclei and also as spots in the cytoplasm (c). In cells preincubated with anti-nucleolin mAb (living unpermabilised cells) red patches indicate cell surface nucleolin (arrows in a) (color figure online)
Surface-expressed and cytoplasmic nucleolin are carrying Tn antigen
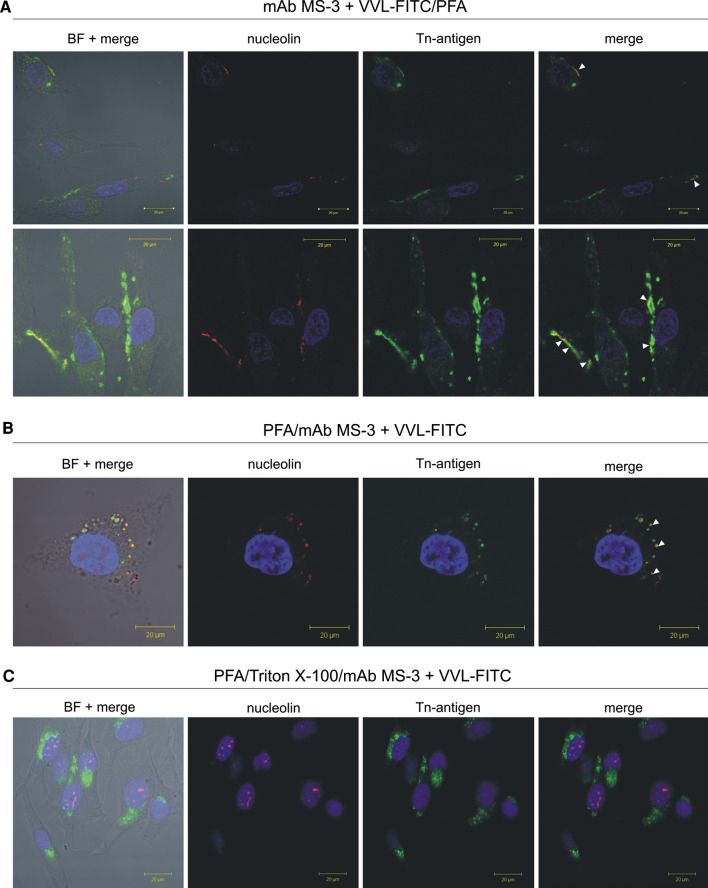
The laser scanning confocal microscopy studies for simultaneous immunostaining of nucleolin and VVL-binding glycoproteins showed overlapping of cell surface-localised as well as cytoplasmic nucleolin and VVL lectin. This overlap was observed as yellow punctate regions (arrows in Fig. 6).
Fig. 6.
Cell surface and cytoplasmic nucleolin are carrying Tn antigen. Living unpermeabilised melanoma Ma-Mel-27 cells (after 5 h of culture in fresh medium) were simultaneously incubated with anti-nucleolin mAb MS-3 (dilution 1:25) and with FITC-labeled VVL (green, dilution 1:100) in 10% NGS, 2% BSA/PBS containing 25 mM NaN3 before PFA fixation (a). For staining of semi-permeabilised (b) or permeabilised cells (c) Ma-Mel-27 cells (after 5 h of culture in fresh medium) were fixed with 3% PFA or 2% PFA/Triton before simultaneous incubation with mAb MS-3 (dilution 1:100) and with FITC-labeled VVL (green, dilution 1:100) in 1% BSA/PBS (overnight, RT), respectively. The bound anti-nucleolin antibody was revealed by Cy3-labeled goat anti-mouse antibodies (red). Nuclei were counterstained with DAPI (blue). Arrows indicate colocalization (yellow) of VVL with nucleolin present on the cell surface. BF; bright field (color figure online)
The prediction of MHC class-I binding sites in nucleolin sequence (http://www.imtech.res.in/raghava/propred1/page2.html)
ProPred1 allows the prediction of MHC binding peptides in nucleolin sequence yielding 47 potential MHC class-I binders to various alleles. ProPred1 also allows the prediction of the standard proteasome and immunoproteasome cleavage sites in antigenic sequence and filtering the MHC binder with cleavage sites at C terminus. Only the MHC class-I present on analysed cell lines were taken into consideration. The potential epitopes identified using ProPred1 searching, as yet not validated experimentally, are shown in Table 1.
Table 1.
ProPred-1 prediction of MHC class-I binding sites in nucleolin
| First position of binder | Sequence | ALLELE: HLA | Cell line |
|---|---|---|---|
| 168 | EIEPAAMKA | A1 | KNUD |
| 617 | NSEEDAKAA | ||
| 481 | SGESKTLVL | ||
| 95 | KTVTPAKAV | A2 | Ma-Mel-12 |
| 522 | KGYAFIEFA | Ma-Mel-04 | |
| 300 | TEPTTAFNL | ||
| 223 | VVPVKAKNV | ||
| 223 | VVPVKAKNV | A*0201 | WM115 |
| 63 | VVVSPTKKV | ||
| 522 | KGYAFIEFA | ||
| 95 | KTVTPAKAV | ||
| 493 | SYSATEETL | A24 | Ma-Mel-27 |
| 314 | NFNKSAPEL | Ma-Mel-8b | |
| 355 | SAEDLEKAL | ||
| 109 | KGATPGKAL | ||
| 391 | ARTLLAKNL | B14 | Ma-Mel-8b |
| 318 | SAPELKTGI | B*3501 | Ma-Mel-8b |
| 170 | EPAAMKAAA | Ma-Mel-12 | |
| 109 | KGATPGKAL | ||
| 75 | TPAKKAAVT | ||
| 632 | GEIDGNKVT | B*4403 | WM115 |
| 281 | KEMAKQKAA | KNUD | |
| 300 | TEPTTAFNL | ||
| 169 | IEPAAMKAA | ||
| 281 | KEMAKQKAA | B61 | Ma-Mel-27 |
| 297 | VEGTEPTTA | ||
| 169 | IEPAAMKAA | ||
| 632 | GEIDGNKVT | ||
| 407 | ELKEVFEDA | B62 | Ma-Mel-27 |
| 109 | KGATPGKAL | B7 | Ma-Mel-04 |
| Ma-Mel-12 | |||
| 216 | KGKKAAKVV | B*0702 | Ma-Mel-04 |
| 281 | KEMAKQKAA | Ma-Mel-12 | |
| 122 | GKKGAAIPA | ||
| 95 | KTVTPAKAV | ||
| 357 | EDLEKALEL | Cw*0301 | Ma-Mel-12 |
| 351 | VDFESAEDL | ||
| 493 | SYSATEETL | Cw*0401 | Ma-Mel-12 |
| 314 | NFNKSAPEL | ||
| 479 | TWSGESKTL | ||
| 391 | ARTLLAKNL | Cw*0602 | WM115 |
| 355 | SAEDLEKAL | Ma-Mel-12 |
N potential N-glycosylation sites, T potential O-glycosylation sites
The alleles of MHC class-I present on analysed cell lines: KNUD, Ma-Mel-04, Ma-Mel-8b, Ma-Mel-12, Ma-Mel-16, Ma-Mel-27 and WM115 were taken into consideration. All peptides having score greater than threshold score at 4% were considered as predicted binders for selected MHC alleles
Discussion
Nucleolin (C23) is an abundant, ubiquitously expressed protein that is found in nucleoli, nucleoplasma, cytoplasm and on the cell surface. Nucleolin has been described as a major nuclear protein having an apparent molecular mass of 100–110 kDa in SDS-PAGE and a calculated molecular mass of 76 kDa as predicted by the amino acid sequence. This difference in molecular masses is most likely due to the post-translational modification. As a result of intracellular trafficking associated with its numerous functions, nucleolin is very promising molecule [34]. Various post-translational modifications that might regulate its function and trafficking have been described [34]. Recently, Carpentier et al. [3] have demonstrated that part of extranuclear nucleolin, identified as a 113-kDa isoform, was N- and O-glycosylated. Two N-glycosylated sites were defined at position N317 and N492. In addition, five potential O-glycosylation sites were predicted: T84, T92, T2105, T106 and T113. Using lectins, Carpantier et al. [3] assumed that nucleolin from Jurkat cells had two sialyl-T antigens. These preliminary results indicated that the glycosylated nucleolin isoform was not present in the nucleus. It seems to be located at the surface of the cell and in the cytosol suggesting that glycosylation influence the fate of the molecule. On the other hand, Salazar et al. [30] found that only membrane-bound nucleolin in U937 cells was enzymatically glycosylated while the cytosolic form of the protein was free from carbohydrates. Our results obtained from confocal microscopy are in agreement with these observations. In addition, Aldi et al. [1] have recently demonstrated the presence of fucosyl-containing nuclear and extranuclear nucleolin in cultured bovine endothelial cells (CVEC) and cultured human malignant A431 cells. Those results are not in contradiction as fucosylation of melanoma glycoproteins was not studied here and therefore one cannot exclude that nucleolin from melanoma cells contains fucosyl residues. In the present study, performed on melanoma cell lines derived from primary and metastatic lesions, we have shown the presence of Tn antigen on nucleolin by binding to VVL. Moreover, we observed T antigen-bearing nucleolin in some of those cell lines, too (data not shown) and in human uveal melanoma mel-202 cells [16]. The synthesis of nucleolin is positively correlated with increased rates of cell division; therefore, nucleolin levels are highest in tumours or other rapidly dividing cells [34]. The presence of antigens on cancer cell surface had led to studies directed towards the development of anticancer vaccines. Also some specific carbohydrate structures have been identified as promising tumour markers [28].
It would be advantageous if T cells could be directed to tumour-associated carbohydrate antigens. However, malignant transformation is frequently associated with the loss of function of MHC class I gene which were expressed in cell precursors [2]. The MHC class I down-regulation results in decreased sensitivity of the tumour cells to MHC class I- restricted CD8+ cytotoxic T lymphocytes (CTLs), the major component of the tumour rejection reaction. The mechanisms by which antigenic peptides bearing a glycosylation sites may be processed and presented by MHC class I is poorly understood. The development of strategies for identification tumour-associated antigens (TAAs) recognized by specific CTLs have let to the characterization of more than 60 such molecules [28]. Among them are also glycoproteins. Monzavi-Karbassi et al. [27] have demonstrated by lectin reactivity and crystallographic studies that MHC class I molecules can be present in the immune system as post-translationally modified cytosolic peptides carrying O-β-linked N-acetylglucosamine (GlcNAc). Crystal structure analysis of T-cell receptor binding to model glycopeptides has shown that T cells can recognize GlcNAc-linked glycopeptides bound by the MHC molecules [10, 32] suggesting that T cells can be targeted to presented carbohydrate antigens on tumour cells. Haurum et al. [13–15] have shown that peptide with O-linked GlcNAc can be transported by peptide specific transporter (TAP) into ER, bind to MHC class I and elicit glycopeptides-specific CTL response in mice. Finally, Kastrup et al. [21] provided evidence for the natural presentation by human MHC class I of glycopeptides carrying O-β-GlcNAc residues in vivo using lectin affinity chromatography. Presentation of O-β-GlcNAc modified or other post-translationally modified peptides by MHC class I molecules may have significant immunological implication, since the T-cell response could be modulated due the changes in glycosylation caused by malignant transformation. Moreover, the effect of glycosylation is greatly dependent on carbohydrate location with presented peptide sequence. Carbohydrate linked to other than anchor amino acid residues, i.e. located within or outside the MHC groove, was tolerated. In most cases, immunization of mice with glycosylated peptides is known to bind to MHC elicited the T cell clones which required both amino acid residues and sugar in the recognized glycopeptidic epitope [26]. The immmunodominant peptides of these proteins are considered as candidates for immunotherapy. It has been demonstrated that MHC binders having proteasome cleavage site at C-terminus are mostly responsible for the activation of CTLs. Therefore, we decided to analyse by PreProd1 program the theoretical ability of MHC class I molecules expressed on these particular cells to present the peptides predicted from nucleolin (Table 1). Among the nucleolin peptides identified as a potential MHC class I binder were peptides with potential O-glycosylation sites. One of them, i.e. KGATPGKAL peptide (amino acids positions 109–117), that could be presented by HLA alleles: A24, B*3501 and B7, is carrying an O-glycosylation site at the central position of its sequence.
The study clearly demonstrated that nucleolin is also present in the plasma membrane of melanoma cells. Therefore, it may become a potential target for not only T-cell but also antibody based therapy [8, 36] what apparently increase the interest in further study on its possible use as a target in cancer immunotherapy. The investigation extended to more melanoma cell lines and, if possible, tissue samples as well as experimental validation, in order to know which of theoretically predicted peptides have the highest potency to become T cell epitopes, should be continued in the future.
In summary, the present study showed that nucleolin was presented on the cell surface and in cytoplasm as Tn antigen-bearing glycoprotein in the analysed human melanoma cells. Moreover, it was possible to predict potential T-cell epitopes in nucleolin sequence as suitable vaccine candidates.
Acknowledgments
This research was supported by grants from: The European Network for the Identification and Validation of Antigens and Biomarkers in Cancer and their Application in Clinical Tumour Immunology (ENACT, 6FP of EU, LSHC-CT-2004-503306) and the Institute of Zoology, Jagiellonian University (K/ZDS/000782).
Footnotes
This paper is an original contribution from the meeting which took place on 28–and 29 May, 2008, in Nottingham, UK, celebrating the contribution of Professor I.A. “Tony” Dodi (+29.1.2008) to the EU project “Network for the identification and validation of antigens and biomarkers in cancer and their application in clinical tumour immunology (ENACT)”.
References
- 1.Aldi S, Della Giovampaola C, Focarelli R, Armini A, Ziche M, Finetti F, Rosati F. A fucose-containing O-glycoepitope on bovine and human nucleolin. Glycobiology. 2009;19:337–343. doi: 10.1093/glycob/cwn126. [DOI] [PubMed] [Google Scholar]
- 2.Bubenik J. MHC class I down-regulation, tumour escape from immune surveillance and design of therapeutic strategies. Folia Biol (Praha) 2005;51:1–2. [PubMed] [Google Scholar]
- 3.Carpentier M, Morelle W, Coddeville B, Pons A, Masson M, Mazurie J, Legrand D. Nucleolin undergoes partial N- and O-glycosylations in the extracellular cell compartment. Biochemistry. 2005;44:5804–5815. doi: 10.1021/bi047831s. [DOI] [PubMed] [Google Scholar]
- 4.Chen C, Chiang S, Yeh N. Increased stability of nucleolin in proliferating cells by inhibition of its self-cleaving activity. J Biol Chem. 1991;266:7754–7758. [PubMed] [Google Scholar]
- 5.Christian S, Pilch J, Akerman ME, Porkka K, Laakkonen P, Ruoslahti E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J Cell Biol. 2003;163:871–878. doi: 10.1083/jcb.200304132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dall’Olio F. Protein glycosylation in cancer biology: an overview. Clin Mol Pathol. 1996;49:126–135. doi: 10.1136/mp.49.3.M126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng J-S, Ballou B, Hofmeister JK. Internalization of anti-nucleolin antibody into viable HEp-2 cells. Mol Biol Rep. 1996;23:191–195. doi: 10.1007/BF00351168. [DOI] [PubMed] [Google Scholar]
- 8.Fonsatti E, Di Giacomo AM, Maio M. Optimizing complement-activating antibody-based cancer immunotherapy: a feasible strategy? J Trans Med. 2004;2:21. doi: 10.1186/1479-5876-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and function of nucleolin. J Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 10.Glithero A, Tormo J, Haurum JS, Arsequell G, Valencia G, Edwards J, Springer S, Townsend A, Pao YL, Wormald M, Dwek RA, Jones EY, Elliott T. Crystal structures of two H-2Db/glycopeptides complexes suggest a molecular basis for CTL cross-reactivity. Immunity. 1999;10:63–74. doi: 10.1016/S1074-7613(00)80007-2. [DOI] [PubMed] [Google Scholar]
- 11.Hakomori S. Glycosylation defining cancer malignancy: new wine in an old bottle. Proc Natl Acad Sci USA. 2002;99:10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harms G, Kraft R, Grelle G, Volz B, Dernedde J, Tauber R. Identification of nucleolin as a new L-selectin ligand. Biochem J. 2001;360:531–538. doi: 10.1042/0264-6021:3600531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haurum JS, Arsequell G, Lellouch AC, Wong SY, Dwek RA, McMichael AJ, Elliott T. Recognition of carbohydrate by major histocompatibility comlex class I-restricted, glycopeptides-specific cytotoxic T lymphocytes. J Exp Med. 1994;180:739–744. doi: 10.1084/jem.180.2.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haurum JS, Tan L, Arsequell G, Frodsham P, Lellouch AC, Moss PA, Dwek RA, McMichael AJ, Elliott T. Peptide anchor residue glycosylation: effect on class I major histocompatibility complex binding and cytotoxic T lymphocyte recognition. Eur J Immunol. 1995;25:3270–3276. doi: 10.1002/eji.1830251211. [DOI] [PubMed] [Google Scholar]
- 15.Haurum JS, Hoier IB, Arsequell G, Neisig A, Valencia G, Zeuthen J, Neefjes J, Elliott T. Presentation of cytosolic glycosylated peptides by human class I major histocompatibility complex molecules in vivo. J Exp Med. 1999;190:145–150. doi: 10.1084/jem.190.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoja-Łukowicz D, Lityńska A, Pocheć E, Przybyło M, Kremser E, Ciołczyk-Wierzbicka D, Laidler P. Identification of PNA-positive proteins in the primary uveal melanoma cell line by mass spectrometry. Acta Biol Cracov Ser Zool. 2006;48:39–47. [Google Scholar]
- 17.Hovanessian AG, Puvion-Dutilleul F, Nisole S, Svab J, Perret E, Deng JS, Krust B. The cell-surface-expressed nucleolin is associated with the actin cytoskeleton. Exp Cell Res. 2000;261:312–328. doi: 10.1006/excr.2000.5071. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Shi H, Zhou H, Song X, Yuan S, Luo Y. The angiogenic function of nucleolin is mediated by vascular endothelial growth factor and nonmuscle myosin. Blood. 2006;107:3564–3571. doi: 10.1182/blood-2005-07-2961. [DOI] [PubMed] [Google Scholar]
- 19.Joo EJ, ten Dam GB, van Kuppevelt TH, Toida T, Linhardt RJ, Kim YS. Nucleolin: acharan sulfate-binding protein on the surface of cancer cells. Glycobiology. 2005;15:1–9. doi: 10.1093/glycob/cwh132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanitakis J, Al-Rifai I, Faure M, Claudy A. Differential expression of cancer associated antigen T (Thomsen-Friedenrich) and Tn to the skin in primary and metastatic carcinomas. J Clin Pathol. 1998;51:588–592. doi: 10.1136/jcp.51.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kastrup IB, Stevanovic S, Arsequell G, Valencia G, Zeuthen J, Rammensee HG, Elliot T, Haurum JS. Lectin purified human class I MHC-derived peptides: evidence for presentation of glycopeptides in vivo. Tissue Antigens. 2000;56:129–135. doi: 10.1034/j.1399-0039.2000.560203.x. [DOI] [PubMed] [Google Scholar]
- 22.Laidler P, Lityńska A, Hoja-Łukowicz D, Łabędź M, Przybyło M, Ciołczyk-Wierzbicka D, Pocheć E, Trębacz E, Kremser E. Characterization of glycosylation and adherent properties of melanoma cell lines. Cancer Immunol Immunother. 2006;55:112–118. doi: 10.1007/s00262-005-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lau SK, Dennis JW. N-glycans in cancer progression. Glycobiology. 2008;18:750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 25.Legrand D, Vigie K, Said EA, Elass E, Masson M, Slomiany MC, Carpentier M, Briand JP, Mazurier J, Hovanessian AG. Surface nucleolin participates in both the binding and endocytosis of lactoferrin in target cells. Eur J Biochem. 2004;271:303–317. doi: 10.1046/j.1432-1033.2003.03929.x. [DOI] [PubMed] [Google Scholar]
- 26.Lisowska E. The role of glycosylation in protein antigenic properties. CMLS, Cell Mol Life Sci. 2002;59:445–455. doi: 10.1007/s00018-002-8437-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monzavi-Karbassi B, Luo P, Jousheghany F, Torres-Quinones M, Cunto-Amesty G, Artaud C, Kieber-Emmos T. A mimic of tumor rejection antigen-associated carbohydrates mediates an antitumor cellular response. Cancer Res. 2004;64:2162–2166. doi: 10.1158/0008-5472.CAN-03-1532. [DOI] [PubMed] [Google Scholar]
- 28.Renkvist N, Castelli C, Robbins PF, Parmanini G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3–15. doi: 10.1007/s002620000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Said EA, Courty J, Svab J, Delbe J, Krust B, Hovanessian AG. Pleiotrophin inhibits HIV infection by binding the cell surface-expressed nucleolin. FASEB J. 2005;272:4646–4659. doi: 10.1111/j.1742-4658.2005.04870.x. [DOI] [PubMed] [Google Scholar]
- 30.Salazar R, Brandt R, Kellermann J, Krantz S. Purification and characterization of a 200 kDa fructosyllysine-specific binding protein from cell membranes of U937 cells. Glycoconjugate J. 2000;17:713–716. doi: 10.1023/A:1011074705615. [DOI] [PubMed] [Google Scholar]
- 31.Singhal A, Fohn M, Hakomori S. Induction of α-N-acetylgalactosamine-O-serine/threonine (Tn) antigen-mediated cellular response for active immunotherapy in mice. Cancer Res. 1991;51:1406–1411. [PubMed] [Google Scholar]
- 32.Speir JA, Abde-Motal UM, Jondal M, Wilson IA. Crystal structure of an MHC class I presented glycopeptides that generates carbohydrate-specific CTL. Immunity. 1999;10:51–61. doi: 10.1016/S1074-7613(00)80006-0. [DOI] [PubMed] [Google Scholar]
- 33.Springer GF. Tn epitope (N-acetyl-D-galactosamineα-O-serine/threonine) density in primary breast carcinoma: a functional predictor of aggressiveness. Mol Immunol. 1989;26:1–5. doi: 10.1016/0161-5890(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava M, Pollard HB. Molecular dissection of nucleolin’s role in growth and cell proliferation: new insights. FASEB J. 1999;13:1911–1922. [PubMed] [Google Scholar]
- 35.Thies A, Moll I, Berger J, Schumacher U. Lectin binding to cutaneous malignant melanoma: HPA is associated with metastasis formation. Br J Cancer. 2001;84:819–823. doi: 10.1054/bjoc.2000.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiner LM, Dhodapkar MV, Ferrone S. Monoclonal antibodies for cancer immunotherapy. Lancet. 2009;373:1033–1040. doi: 10.1016/S0140-6736(09)60251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]