Abstract
There are good arguments for suggesting that two seminal papers published 50 years ago can be taken as the beginning of modern tumour immunology. These papers by R. Baldwin, “Immunity to transplanted tumour: the effect of tumour extracts on the growth of homologous tumours in rats” and “Immunity to methylcholanthrene-induced tumours in inbred rats following atrophy and regression of the implanted tumours” (Br J Cancer 9:646–51 and 652–657, 1955) showed that once tumours are established, they and their products can be recognised by the adaptive immune system and rejected. However, the tumour normally co-evolves with immunity, like a parasite, rather than being suddenly introduced as in these, and many other, experimental models. Dynamics of this co-evolution are illustrated by findings that inflammation enhances tumorigenicity, yet is important to enable T cells to respond properly to tumour antigen and exert anti-tumour effects. The important thing is to maintain the balance between effective anti-tumour immunity and tumour escape and/or stimulatory mechanisms. Tumours almost always co-exist with immune defence systems over extended periods and interact chronically with T cells. The effect of this is potentially similar to other situations of chronic antigenic stress, particularly lifelong persistent virus infection, most strikingly, CMV infection. The questions briefly explored in this symposium paper are what happens when T lymphocyte clones are chronically stimulated by antigen which is not or cannot be eliminated? What are the similarities and differences between chronic antigenic stimulation by tumour antigen versus CMV antigen? What can we learn in one system which may illuminate the other?
Keywords: Population Doubling, Renal Cell Cancer Patient, Chronic Antigenic Stimulation, Cell Clonal Expansion, Dysfunctional Cell
What happens when T lymphocyte clones are chronically stimulated by antigen which cannot be eliminated?
We have sought to answer this question using three approaches: (1) by examining T cells from cancer patients; (2) by examining T cells from cancer-free elderly people; and (3) by modelling T cell clonal expansion under conditions of chronic antigenic stimulation in vitro. We will start with the last first.
The T cell clonal expansion model
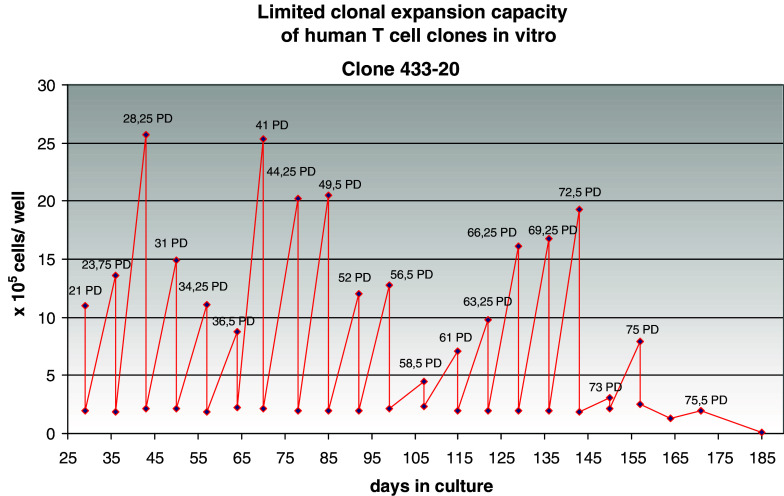
Human T cells can be cloned and maintained in long-term tissue culture by providing them with a source of growth factors and intermittent antigenic or mitogenic stimulation via their T cell receptors (TCR). Like other normal somatic cells, such clones possess finite lifespans, ranging in each experiment up to an average maximum of around 50–80 population doublings (PD) (reviewed in Ref. [1]). An example of the growth curve of such a clone is shown in Fig. 1. The cells were subcultured weekly, when they were restimulated via the TCR and given fresh medium and growth factor. The growth curves indicate a fairly constant, rigorous clonal expansion, as would be required for prolonged T cell responses to pathogens and cancer. However, population increase eventually slows and reverses, resulting in the demise of the clone. This is caused by an increasing fraction of apoptotic cells at this time, representing clonal deletion by apoptosis. Our attempts to prevent this using anti-apoptotic cytokines, caspase inhibitors or a variety of other approaches have all failed. Thus far, only transfection of the catalytic component of telomerase has been reported to (sometimes) overcome this process of clonal deletion [2, 3]. There is good reason to believe that a similar process occurs in vivo, where it has been dubbed “clonal exhaustion” in mice [4, 5]. In humans, it has been shown that the resolution of acute infectious mononucleosis is accompanied by marked expansion of several EBV-specific clones (estimated equivalent to ca. 30 PD), followed by retention of only a proportion of these for extended periods and a loss of others [6]. Therefore, we feel that the in vitro clonal T cell model is an appropriate tool for exploring the behaviour of T cells under chronic antigenic stress, such as occurring during anti-tumour responses. We can use this model to explore the correlates of the clonal exhaustion process and possibly as a test bed for interventions aimed at preventing or reversing this in patients.
Fig. 1.
Growth curve of a CD4 T cell clone originating from an 85-year-old donor. Cells were counted every week and subcultured at a standard concentration in fresh medium with new irradiated feeder cells. The estimated number of population doublings (PD) is given at each time point
Correlates of clonal expansion under chronic antigenic stress
We have established that the majority of T cell clones (TCC) maintained in long-term culture show the expected age-associated reduction in the length of telomeric repeats capping the chromosomes and guarding against chromosomal instability [7]. This may be because, although T cells express native telomerase on activation, they rapidly become “desensitised” on repetitive stimulation and no longer upregulate this enzyme [8]. We believe this, in turn, is because although the TCR remains expressed at the proper level and at least in CD4 cells seems to remain fully functional, the level of expression of important costimulatory molecules, first and foremost CD28, is decreased [9]. This is associated with changed patterns of cytokine secretion because of the altered balance of stimulatory and costimulatory signals. A striking finding in many TCC is that they show not only decreased capacity to secrete IL-2, but also an enhanced capacity to produce IL-10 [9]. This may be at least partly responsible for our earlier observations that late- but not early-passage TCC are able to suppress T cell proliferation in mixed lymphocyte cultures [10, 11]. This in turn perhaps implies that some descriptions of “peripheral T regulatory” cells in the literature are in fact referring to such anergic suppressive cells. It should also be borne in mind that there are many situations involving chronic antigenic stimulation in vivo where a similar loss of CD28 expression has been observed, in association with viral disease, parasitic disease, autoimmune disease or cancer, as well as “normal” ageing, and that these have been associated with increased “suppressor cells” in the past.
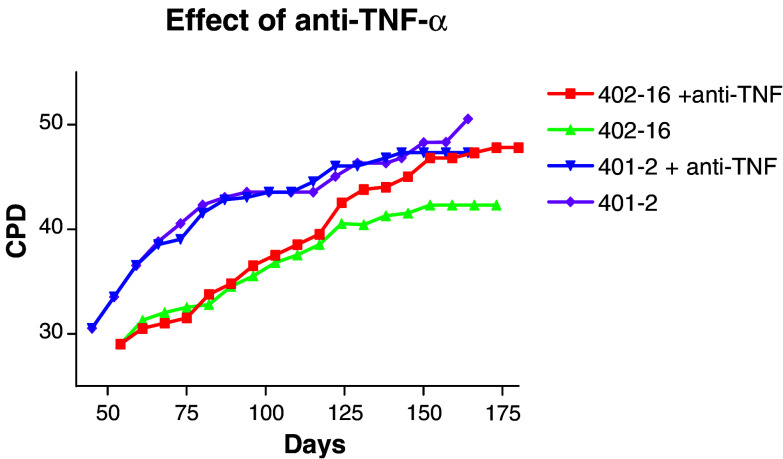
Our dissection of the mechanisms responsible for gradual loss of CD28 expression on the surface of TCC as they progress through their finite lifespan indicates that it has much to do with the level of TNF-α that they secrete in an autocrine fashion. Blockade of TNF-α can result in CD28 upregulation, re-acquisition of proliferative capacity and lifespan extension. An example is given in Fig. 2, which shows growth curves of clone 402-16 (a typical clone which continued to secrete autocrine TNF-α throughout its lifespan) and clone 401-2 (a rarer type for unknown reasons spontaneously losing the ability to secrete TNF-α at ca. 35 PD). Both clones were cultured in the presence or absence of a saturating concentration of neutralising TNF-α antiserum from 35 PD (added weekly at each subculture). The antiserum had no effect on the clone which no longer secreted TNF-α, but after a short lag phase of about 5 PD, a beneficial effect of neutralising this cytokine begins to be noticeable for the clone continuing to secrete TNF-α (Fig. 2). However, even when autocrine TNF-α is blocked, TCC still have finite lifespans. We believe that this is related to the level of oxidative stress manifested as DNA damage and to compromised DNA repair mechanisms in these cells [12]. Again, however, although anti-oxidants may extend clonal lifespan, this remains finite. There must, therefore, still be factors of which we are unaware, which ensure that T cell proliferation is always limited, possibly itself an anti-cancer mechanism. This work has been carried out on CD4 TCC. The other half of the story, CD8 cells, has not yet been told and awaits future study. In the elderly, however, it is the CD8 cells which have been most intensely scrutinised; in this case, it is the CD4 cells which await further study.
Fig. 2.
Two CD4 clones were cultured as in Fig. 1. CPD cumulative PD. Clone 402-16 is a typical clone which continued to secrete autocrine TNF-α throughout its lifespan, whereas 401-2 is a rare clone which spontaneously lost the ability to secrete TNF-α at ca. 35 PD. Both clones were cultured in the presence or absence of a saturating concentration of neutralising TNF-α antiserum from 35 PD (added weekly at each subculture)
Chronic antigenic stimulation in the elderly
Over a decade ago it was noted that peripheral blood of elderly people commonly contained clonal expansions of CD8 cells, suggested to be a non-malignant T cell equivalent of the “benign gammopathy” of B cells [13]. However, it was later found that such clonal expansions, and the quantitative level thereof, were in fact associated with CMV infection and not age per se [14]. The apparent association with age was believed to have been due to the increasing rate of infection with age and the increasing duration of infection of those already carrying this persistent virus. By means of HLA/CMV peptide multimers used to identify CD8 cells carrying receptors for an immunodominant epitope of the HCMV pp65 protein (NLVPMVATV, binding HLA-A2), we have established the frequencies, surface marker phenotypes and some of the functional characteristics of these cells [15]. They are typically a mixture of CD45RA+ and CD45RA-negative cells in both young and old, but the proportion of cells expressing CD28 is very low in the elderly (<10% on average) and relatively high (>75% on average) in the young. A high proportion of CMV-specific CD8 cells in the young is still included in the phenotypically naïve subpopulation (CCR7+CD45RA+), but in the elderly most have a more differentiated phenotype commonly associated with effector memory cells (CCR7-negative, CD45RA-low) or effector cells (CCR7-negative, CD45RA+). Differences between young and old donors' CMV-specific cells may be due to the more extended periods of persistent infection in the latter, but the rapidity with which these phenotypic changes occur on infection (perhaps only 6 weeks) probably makes this unlikely [16]. Thus, in congenital fetal CMV infection, already less CD8 cells express CD28, indicating a rapid shift from naïve phenotype to effector memory and effector phenotype [17].
We have studied the frequencies of CMV-specific cells, as detected by tetramers, in several different young and old populations. In longitudinal studies carried out in Sweden, we were able to distinguish an “immune risk phenotype” predictive of 2 and 4-year mortality, which was associated with CMV seropositivity and CD8 CMV specificity [18]. Moreover, clonotypic analysis of the clonal composition of these CMV-specific cells indicates that not only are the numbers of CMV-specific cells increased, but also multiple different clones are expanded in the healthy elderly, more than in the young, indicating the overriding importance of CMV immunosurveillance. Strikingly, however, in the elderly assigned to the at-risk group, a reduction in the anti-CMV clonal repertoire is seen, which is predictive of incipient mortality (S. Reker et al., submitted for publication). Nonetheless, despite repertoire contraction, again the actual number of CMV receptor-bearing cells increases. So what is the nature of these cells?
Characteristics and functions of CMV-specific CD8 cells in the young and old
A major finding is that many of the CMV-specific cells in the elderly appear to be anergic [19]. In other words, they can be stimulated by certain mitogens, but not by cognate antigens, to secrete cytokines such as IFN-γ. As is commonly the case with anergic CD8 cells, and in contrast to CD4 cells, they appear also to be relatively resistant to apoptosis. The increased numbers of CMV-specific cells in the elderly are mostly dysfunctional cells of this type. They may exert suppressive activity by competing with functional cells for antigen presented by APC, by secreting suppressive cytokines (e.g. they do secrete IL-10) or by absorbing stimulatory cytokines like IL-2. They are characterised by containing a large fraction of cells bearing inhibitory NK receptors, particularly CD85j (ILT-2) (S. Koch, unpublished results). However, CMV-specific cells from young donors express similar levels of markers such as CD85j, except for one: KLRG-1. This negative receptor, when present on T cells, was thought to mark a cell population unable to undergo any further cell division [20]. Moreover, we have found that CD8+ KLRG-1+ T cells are relatively resistant to apoptosis induced even by powerful agents like staurosporine (Table 1). They may thus accumulate at the expense of functionally intact cells. Again, however, the KLRG-1+ population is not homogeneous, and such cells are also present in the young, albeit generally at lower levels than in the elderly. KLRG-1+ cells can be subdivided based on the expression of another T cell “senescence” marker, CD57. It may be the KLRG-1, CD57 double-positive cells which represent the real dysfunctional population of non-proliferative cells, whereas the KLRG-1+ CD57-negative population can still be stimulated to divide [21].
Table 1.
KLRG-1-positivity confers resistance to apoptosis
| KLRG-1-negative (%) | KLRG-1-positive (%) | P | |
|---|---|---|---|
| Spontaneous | 8 | 4 | <0.05 |
| Induced | 20 | 5 | <0.005 |
Peripheral blood mononuclear cells were cultured in medium alone or with 100 ng/ml staurosporine for 24 h. Annexin V staining was assessed by flow cytometry in KLRG-1-positive and negative cells from several different late middle-aged donors (>60 years) and average apoptosis levels are given
Is the situation similar in cancer patients?
As discussed above, in the elderly, chronic antigenic stimulation due to long-term exposure to antigens derived from persistent viral infection, notably CMV, first causes an increase in the number of different TCC responding to the virus as well as an increase in the number of cells contained within each clone. Ageing is accompanied by an increase in both these parameters. However, in the terminal phase of life, the anti-CMV repertoire shrinks, but the number of CMV-specific cells continues to increase. However, the majority of these are dysfunctional, and it is their accumulation which we believe is detrimental to the host. Does something similar happen in cancer patients exposed long term to tumour antigens? This would imply that immune responses to the tumour are initially amplified, both in terms of the numbers of different clones expanded and the numbers of these clonally expanded cells, but that, over time, the number of different anti-tumour clones would decrease as would the number of functional cells, but the overall number of tumour antigen-specific cells would be maintained or increase. However, these would be predicted to be dysfunctional.
At present, this scenario in cancer patients remains hypothetical, but amenable to investigation. However, there are some scattered data which are consistent with this notion, although it must be borne in mind that it is necessary to consider different cancers separately. It is known that there are differences in the way that more or less immunogenic tumours interact with the immune system and some data on the effects of age on this in animal models. Thus, early data suggested that when tumours are highly immunogenic, young immune-intact animals survive longer than old immunosenescent ones; but in contrast, old animals do better when the tumours are less immunogenic [22–24]. It is also known that tumour-infiltrating lymphocytes (TIL) are of diverse clonotypes and that TIL and peripheral blood CD8 cells represent the same pool. However, we are aware of no studies that have followed clonotypes and their potential changes over time in cancer patients in vivo. One study did examine what happened during in vitro expansion of TIL and found that many clonotypes were lost on culture [25]. This is consistent with the idea that such clones rapidly reached the end of their proliferative lifespan due to extensive in vivo stimulation, as seen in EBV and CMV infection in the elderly.
We have studied phenotypes and functions of certain CD8 T cells found in renal cell cancer (RCC) patients in this context. This represents a cancer generally considered to be more rather than less immunogenic based on clinical experience of response to immunotherapy, and therefore predicted to be more sensitive to immunosenescence of the host. Using HLA tetramers, we have sought tumour antigen-specific T cells in the peripheral blood and in TIL in these patients. In rare RCC patients, we observed a striking peripheral expansion—up to 9% of the CD8 repertoire—of T cells specific for a peptide derived from the protein cytokeratin 18 (CK18) (C. Gouttefangeas, unpublished results). This peptide had originally been identified by elution and mass spectrometric sequencing methods as an HLA ligand in fresh renal cell tumours. Such CK18-specific CD8 cells were also detected in healthy donors, albeit at a frequency not exceeding 0.6%. Interestingly, CK18-specific cells were similar in several respects to the CMV-specific cells present in the elderly. First, they have a highly differentiated phenotype (CCR7-negative, CD45RA+, CD28-negative and co-express various inhibitory NK receptors including KLRG-1). Second, they represent oligoclonal expansions of CD8 cells, as shown by TCR Vbeta and TCR excision circle analysis. Third, they show no response to peptide-specific stimulation in terms of cytokine secretion and killing [26] and are in this respect anergic. We have also meticulously sought other CD8 populations specific for more than 10 described or candidate epitopes derived from RCC-associated antigens, but have never detected any T cell expansions similar to the CK18-specific cells in the blood of RCC patients (H. Griesemann, unpublished results). We believe that CK18-specific cells have been expanded by chronic antigenic stimulation, are also present in the TIL population of RCC and are anergic. Why they appear in certain individuals, either healthy or cancer patients, and what function they may have remains to be explored.
Concluding remarks
In this symposium paper, we argue that T cell immunity to persistent antigens follows a similar course in cancer patients and the elderly. Initially clonally heterogeneous responses maintain immunosurveillance, but chronic antigenic stress due to persistence of the source of antigen causes T cell clonal exhaustion. This first manifests as oligoclonal accumulations of dysfunctional cells followed by a reduction in the number of different clones present (repertoire shrinkage) due to clonal deletion but no reduction in the actual number of dysfunctional cells present. Thus, attempts by the immune system to maintain responses against persistent antigen result in accumulations of dysfunctional cells which reduce the diversity of the T cell repertoire for other antigens, but still eventually result in loss of receptor diversity also for the persistent antigen driving this phenomenon. The eventual result is immunodeficiency both for novel antigenic challenges and the persistent antigen itself. Hence, for this reason, both generalised and specific immunodeficiency contribute to morbidity and mortality in cancer patients and the elderly. In this respect, what we have learned in the elderly and from in vitro clonal T cell expansion models may be useful in understanding and manipulating T cell responses to tumours in cancer patients.
Acknowledgements
The work mentioned here was most recently supported by the Deutsche Forschungsgemeinschaft through SFB 685 “Immunotherapy: from molecular basis to clinical application” and the EU through projects 6FP 503306 “European Network for the Identification and Validation of Antigens and Biomarkers in Cancer and their Application in Tumour Immunology, ENACT”; QLRT-2001-00668 “Outcome and impact of specific treatment in European research on melanoma” and QLK6-CT-2002-02283 “T cells in ageing, T-CIA”.
Footnotes
This article is a symposium paper from the “Robert Baldwin Symposium: 50 years of Cancer Immunotherapy”, held in Nottingham, Great Britain, on 30 June 2005.
References
- 1.Pawelec G (2003) T cell immunosenescence. In: Kaul SC, Wadhwa R (eds) Aging of cells in and outside the body, chapter 6. Biology of aging and its modulation, vol 1, series editor Suresh Rattan. Kluwer, Dordrecht, pp 85–100
- 2.Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–4245. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 3.Migliaccio M, Amacker M, Just T, Reichenbach P, Valmori D, Cerottini JC, Romero P, Nabholz M. Ectopic human telomerase catalytic subunit expression maintains telomere length but is not sufficient for CD8+ T lymphocyte immortalization. J Immunol. 2000;165:4978–4984. doi: 10.4049/jimmunol.165.9.4978. [DOI] [PubMed] [Google Scholar]
- 4.Welsh RM, McNally JM. Immune deficiency, immune silencing, and clonal exhaustion of T cell responses during viral infections. Curr Opin Microbiol. 1999;2:382–387. doi: 10.1016/S1369-5274(99)80067-8. [DOI] [PubMed] [Google Scholar]
- 5.Kiessling R, Pawelec G, Welsh RM, Barry JD, Ferrone S. Have tumor cells learnt from microorganisms how to fool the immune system? Escape from immune surveillance of tumors and microorganisms: emerging mechanisms and shared strategies. Mol Med Today. 2000;6:344–346. doi: 10.1016/S1357-4310(00)01778-0. [DOI] [PubMed] [Google Scholar]
- 6.Soares MV, Maini MK, Beverley PC, Salmon M, Akbar AN. Regulation of apoptosis and replicative senescence in CD8+ T cells from patients with viral infections. Biochem Soc Trans. 2000;28:255–258. doi: 10.1042/bst0280255. [DOI] [PubMed] [Google Scholar]
- 7.Pawelec G, Mariani E, Solana R, Forsey R, Larbi A, Neri S, Dela Rosa O, Barnett Y, Tolson J, Fülöp T (2005) Human T cell clones in long-term culture as models for the impact of chronic antigenic stress in ageing. In: Conn M (ed) Handbook of models of human aging. Elsevier, Amsterdam (in press)
- 8.Pawelec G, Rehbein A, Hähnel K, Adibzadeh M, Wagner W, Engel A. In vitro senescence models for human T lymphocytes. Vaccine. 2000;18:1666–1674. doi: 10.1016/S0264-410X(99)00504-6. [DOI] [PubMed] [Google Scholar]
- 9.Pawelec G, Rehbein A, Haehnel K, Merl A, Adibzadeh M. Human T cell clones in long-term culture as a model of immunosenescence. Immunol Rev. 1997;160:31–42. doi: 10.1111/j.1600-065X.1997.tb01025.x. [DOI] [PubMed] [Google Scholar]
- 10.Pawelec G, Schneider EM, Wernet P. Acquisition of suppressive activity and natural killer-like cytotoxicity by human alloproliferative “helper” T cell clones. J Immunol. 1986;136:402–411. [PubMed] [Google Scholar]
- 11.Pawelec G, Fernandez N, Brocker T, Schneider EM, Festenstein H, Wernet P. DY determinants, possibly associated with novel class II molecules, stimulate autoreactive CD4+ T cells with suppressive activity. J Exp Med. 1988;167:243–261. doi: 10.1084/jem.167.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hyland P, Barnett C, Pawelec G, Barnett Y. Increased levels of oxidative DNA damage and alterations in the levels of the mitotic inhibitors p16INK4a/CDKN2a, p21WAF1/CIP1/SDI1, p27KIP1 leads to T cell replicative senescence in vitro. Mech Ageing Dev. 2001;122:1151–1167. doi: 10.1016/S0047-6374(01)00254-8. [DOI] [PubMed] [Google Scholar]
- 13.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to “benign monoclonal gammopathy”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Looney RJ, Falsey A, Campbell D, Torres A, Kolassa J, Brower C, McCann R, Menegus M, McCormick K, Frampton M, Hall W, Abraham GN. Role of cytomegalovirus in the T cell changes seen in elderly individuals. Clin Immunol. 1999;90:213–219. doi: 10.1006/clim.1998.4638. [DOI] [PubMed] [Google Scholar]
- 15.Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A. Human immunosenescence. Is it infectious? Immunol Rev. 2005;205:257–268. doi: 10.1111/j.0105-2896.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 16.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8+ T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8+ T cell response. J Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 17.Elbou Ould MA, Luton D, Yadini M, Pedron B, Aujard Y, Jacqz-Aigrain E, Jacquemard F, Sterkers G. Cellular immune response of fetuses to cytomegalovirus. Pediatr Res. 2004;55:280–286. doi: 10.1203/01.PDR.0000104150.85437.FE. [DOI] [PubMed] [Google Scholar]
- 18.Wikby A, Ferguson F, Forsey R, Thompson J, Strindhall J, Löfgren S, Nilsson B-O, Ernerudh J, Pawelec G, Johansson B. An immune risk phenotype, cognitive impairment and survival in very late life: the impact of allostatic load in Swedish Octo- and Nonagenarian humans. J Gerontol B. 2005;60:556–565. doi: 10.1093/gerona/60.5.556. [DOI] [PubMed] [Google Scholar]
- 19.Ouyang Q, Wagner WM, Zheng W, Wikby A, Remarque EJ, Pawelec G. Dysfunctional CMV-specific CD8+ T cells accumulate in the elderly. Exp Gerontol. 2004;39:607–613. doi: 10.1016/j.exger.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Voehringer D, Koschella M, Pircher H. Lack of proliferative capacity of human effector and memory T cells expressing killer cell lectin-like receptor G1 (KLRG1) Blood. 2002;100:3698–3702. doi: 10.1182/blood-2002-02-0657. [DOI] [PubMed] [Google Scholar]
- 21.Ibegbu CC, Xu YX, Harris W, Maggio D, Miller JD, Kourtis AP. Expression of killer cell lectin-like receptor G1 on antigen-specific human CD8+ T lymphocytes during active, latent, and resolved infection and its relation with CD57. J Immunol. 2005;174:6088–6094. doi: 10.4049/jimmunol.174.10.6088. [DOI] [PubMed] [Google Scholar]
- 22.Kaesberg PR, Ershler WB. The change in tumor aggressiveness with age: lessons from experimental animals. Semin Oncol. 1989;16:28–33. [PubMed] [Google Scholar]
- 23.Provinciali M, Smorlesi A. Immunoprevention and immunotherapy of cancer in ageing. Cancer Immunol Immunother. 2005;54:93–106. doi: 10.1007/s00262-004-0539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Effros RB. Replicative senescence of CD8 T cells: potential effects on cancer immune surveillance and immunotherapy. Cancer Immunol Immunother. 2004;53:925–933. doi: 10.1007/s00262-004-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.thor Straten P, Kirkin AF, Siim E, Dahlstrom K, Drzewiecki KT, Seremet T, Zeuthen J, Becker JC, Guldberg P. Tumor infiltrating lymphocytes in melanoma comprise high numbers of T-cell clonotypes that are lost during in vitro culture. Clin Immunol. 2000;96:94–99. doi: 10.1006/clim.2000.4890. [DOI] [PubMed] [Google Scholar]
- 26.Walter S, Bioley G, Bühring H-J, Koch S, Wernet D, Zippelius A, Pawelec G, Romero P, Stevanović S, Rammensee H-G, Gouttefangeas C. High frequencies of functionally impaired cytokeratin 18-specific CD8+ T cells in healthy HLA-A2+ donors. Eur J Immunol. 2005;35:2876–2885. doi: 10.1002/eji.200526207. [DOI] [PubMed] [Google Scholar]