Abstract
We previously isolated the novel heteropolysaccharide maitake Z-fraction (MZF) from the maitake mushroom (Grifola frondosa), and demonstrated that MZF significantly inhibited tumor growth by inducing cell-mediated immunity. In this study, we demonstrated that MZF upregulated the expression of CD80, CD86, CD83, and MHC II on bone marrow-derived dendritic cells (DCs) and significantly increased interleukin-12 (IL-12) and tumor necrosis factor-alpha production by DCs in a dose-dependent manner. MZF-treated DCs significantly stimulated both allogeneic and antigen-specific syngenic T cell responses and enhanced antigen-specific interferon-gamma (IFN-γ) production by syngenic CD4+ T cells; however, MZF-treated DCs did not affect IL-4 production. Furthermore, the enhancement of IFN-γ production in CD4+ T cells, which was induced by MZF-treated DCs, was completely inhibited by the addition of an anti-IL-12 antibody. These results indicate that MZF induced DC maturation and antigen-specific Th1 response by enhancing DC-produced IL-12. We also demonstrated that DCs pulsed with colon-26 tumor lysate in the presence of MZF induced both therapeutic and preventive effects on colon-26 tumor development in BALB/c mice. These results suggest that MZF could be a potential effective adjuvant to enhance immunotherapy using DC-based vaccination.
Keywords: Dendritic cells, Vaccines, Grifola frondosa, BRM, IL-12
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs) that show an extraordinary capability to stimulate naïve T cells. Activated DCs are able to present antigens to T-helper (Th) cells and cytotoxic T lymphocytes (CTLs) and activate natural killer (NK) cells. DCs play important roles in both innate and adaptive immune responses [1]. Numerous DC-based vaccination studies aim to prevent tumor relapses and extend patient survival [2]. Immature DCs express low levels of costimulatory molecules and the major histocompatibility complex (MHC) class II antigen, and are functionally specialized in capturing and processing exogenous antigens in peripheral tissues. On encountering pathogens, pathogen-associated molecular patterns (PAMPs) [e.g., toll-like receptor (TLR) ligands] and inflammatory cytokines [e.g., tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β] induce DC maturation [3, 4]. After maturation, the DCs begin to migrate to the lymphoid organs; they also express elevated levels of MHC II and costimulatory molecules, and show high efficiency in stimulating T cells, accompanied by downregulated antigen uptake and processing mechanisms [5]. DC subsets show functional heterogeneity and may be immunogenic or tolerogenic depending on their maturation stage. In the presence of inflammatory cytokines or PAMPs, DCs become mature and efficiently stimulate the immune system; however, in the absence of such signals, they retain an immature phenotype that induces immune tolerance. Therefore, appropriate DC maturation is important for the augmentation of cancer immunity [6, 7].
Many studies have reported the immunomodulatory effects of polysaccharides isolated from mushrooms, fungi, yeast, algae, lichens, and plants. β-Glucan and mannan—components of the fungal cell wall—are recognized by several pattern-recognition receptors (PRRs), including TLRs, Dectin-1, complement receptor 3 (CR3), mannose receptor, and scavenger receptors on the surfaces of APCs such as macrophages and DCs, and function to activate these cells [8, 9]. Dectin-1 is the major receptor for β-1,3-glucans and induces the production of cytokines and chemokines, respiratory burst, and phagocytosis [10, 11]. Curdlan, a β-1,3-glucan, stimulates DCs via the Dectin-1/Syk pathway, resulting in CTL response induction [12]. In addition, some fungal polysaccharides have been reported to significantly induce DC maturation in vitro [13–16].
The maitake mushroom (Grifola frondosa) has become a very popular, edible mushroom in Japan since its fruiting body can be artificially produced. The immunological effects of a G. frondosa extract have already been demonstrated in human clinical trials [17]. Maitake D-fraction (MD-fraction), a β-(1,3)(1,6)-glucan extracted from G. frondosa, induces anti-tumor activity by activating the host immune system and enhancing hematopoiesis [18–20]. Recently, we isolated a novel polysaccharide, maitake Z-fraction (MZF), from G. frondosa, and demonstrated that MZF significantly inhibited tumor growth in BALB/c mice inoculated with colon-26 cancer cells. MZF also induced a Th1 response by increasing the percentage of IFN-γ-producing cells in both splenic CD4+ and CD8+ T cells and increasing the cytotoxic activity in NK cells and CTLs. MZF significantly induced the proliferation of resident peritoneal macrophages as did polysaccharides derived from other natural products [21]. This fact suggests that MZF initially acts on APCs such as macrophages and DCs. However, the effect of MZF on DC maturation and function is unclear. In the present study, we investigated whether MZF enhances the maturation and T cell priming ability of DCs in vitro. Furthermore, we examined the preventive and therapeutic effect of the transfer of MZF-stimulated DCs on the growth and survival rate of colon-26 tumors.
Materials and methods
Isolation of polysaccharide MZF from G. frondosa
The MZF was isolated from a boiling water extract of the fruiting bodies of G. frondosa provided by Yukiguni Maitake Co. (Minami-Uonuma City, Japan). A crude polysaccharide fraction was obtained by precipitation with ethanol from the aqueous extract and applied on a diethylaminoethyl (DEAE)-cellulofine anion exchange column (Seikagaku Biobusiness Co., Ltd., Tokyo, Japan) pre-equilibrated with 5 mM Tris–HCl (pH 8.0); the unabsorbed fraction was eluted with 5 mM Tris–HCl (pH 8.0). The supernatant was collected by the addition of ethanol to a concentration of 50%, and the precipitate was then collected by the addition of ethanol to a concentration of 80%. The precipitate was dissolved in sterilized water and loaded onto a gel filtration column of Sepharose CL-6B (GE Healthcare, Piscataway, NJ, USA). Two fractions were separated completely by gel filtration, and the first fraction was collected as MZF. Lipopolysaccharide (LPS) contamination was tested using an Endospecy ES-24S set (Seikagaku Biobusiness Co., Ltd.). The concentration of the LPS in MZF was <0.05 EU/mg of sugar. The concentrations of carbohydrate and protein were determined by the anthrone method and a BCA protein assay kit (Pierce, Rockford, IL, USA), respectively.
Mice
Female BALB/c mice (H-2d) and C3H/HeN mice (H-2k) were purchased from CLEA Japan Inc. (Higashimaya, Japan), and 6-week-old mice were used in the study. Animal care and processing were performed in accordance with the guidelines for proper conduct of animal experiments by the Science Council of Japan and approved by the animal care committee at this institution.
Generation of bone marrow-derived DCs
Bone marrow (BM)-derived DCs were generated as described previously [22]. Briefly, BM cells were flushed out from the femurs and tibias of BALB/c mice with serum-free RPMI-1640 media through a 2.5-ml syringe. Red blood cells were lysed using 0.83% NH4Cl solution, and the BM cells (1 × 106 cells/ml) were cultured in RPMI-1640 medium containing 10% heat-inactivated FBS, l-glutamine (0.03 mg/ml), penicillin (100 unit/ml), and streptomycin (100 μg/ml) supplemented with 20 ng/ml GM-CSF and 10 ng/ml IL-4 (Pepro Tech, Rocky Hill, NJ, USA). The culture medium containing cytokine was replaced at days 3 and 5. At day 6 of culture, nonadherent cells and loosely adherent cells were harvested. More than 85% of the cells expressing CD11c were used in further experiments.
Cytokine assay
Supernatants were collected for the determination of cytokine production after 24 h of stimulation of DCs (1 × 106 cells/ml) in 96-well plates with or without polymyxin B (30 μg/ml). TNF-α and IL-12 levels were determined by sandwich enzyme-linked immunosorbent assay (ELISA) as previously described [20]. The following monoclonal antibodies were used for ELISA: monoclonal anti-mouse IL-12 (clone C17.8) (Biolegend, CA, USA), biotinylated anti-mouse IL-12 (Pepro Tech), monoclonal anti-mouse TNF-α (clone TN3-19.12) (Santa Cruz Biotechnology, Santa Cruz, CA, USA), biotinylated anti-mouse TNF-α (R&D Systems, Minneapolis, MN, USA), anti-mouse IFN-γ (clone RMMG-1, PBL Biomedical Laboratories, NJ, USA), biotinylated anti-mouse IFN-γ (Pepro Tech), anti-mouse IL-4 (clone 30340.11), and biotinylated anti-mouse IL-4 (Genzyme-Techne, Minneapolis, MN, USA). LPS (Escherichia coli serotype 0111: B4) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Flow cytometric analysis
The DCs were incubated with fluorescein isothiocyanate (FITC) or R-phycoerythrin (PE)-conjugated anti-CD11c (clone HL3) and FITC anti-I-A/I-E (clone 2G9), PE anti-CD86 (clone GL1), PE anti-CD80 (clone 16-10A1), and PE anti-CD83 (clone Michel-19) for 20 min at 4°C. Appropriate isotype control was used for each antibody. All antibodies were purchased from BD Biosciences (San Jose, CA, USA). Stained cells were analyzed with a FACSCalibur™ (BD Biosciences).
Generation of fluorescent MZF
The MZF was labeled with fluorescein using 5-[(4,6-dichlorotriazin-2-yl) amino]-fluorescein hydrochloride (DTAF, Sigma-Aldrich). Briefly, 20 mg of MZF was diluted to 2 mg/ml with borate buffer (pH 10.8) and mixed with 20 mg of DTAF dissolved in DMSO and heated for 3 h at 70°C. The DTAF-labeled MZF (Fl-MZF) was then precipitated with ethanol. The pellet was dissolved in 200 mM NaOH, and the process of precipitation followed by solubilization was repeated several times until a clear supernatant free of unbound DTAF was obtained. After final precipitation, Fl-MZF was diluted in sterile water, and the pH was adjusted to 7.2.
Fluorescent MZF-binding assay
Ice-cold PBS containing 3% FBS with or without inhibitors [Laminarin: β-(1,6) (1,3)-glucan; Mannan: α-1,6-mannan; Dextran: α-(1,3) (1,6)-glucan, 1 mg/ml] or antibodies [anti-Dectin-1 Ab (2A11: Hycult Biotechnology B.V., Uden, The Netherlands), isotype Ab (rat IgG2bκ: Biolegend Inc.), 20 μg/ml] were added to DCs (2 × 105 cells/tube) and incubated on ice for 1 h before the addition of Fl-MZF (400 μg/ml). Cells were incubated on ice for 1 h, washed, and fixed with 1% paraformaldehyde. Fl-MZF+ DCs were identified by flow cytometry.
Mixed lymphocyte reaction induced by DCs
Responder T cells used for allogeneic T cell reaction were isolated with a MACS CD4+ T cell isolation kit (Miltenyi Biotec, Auburn, CA, USA) from whole spleen cells of C3H/HeN mice. Unstimulated or MZF-stimulated DCs were treated with 50 μg/ml mitomycin C (Kyowa Co. Ltd., Tokyo, Japan) for 1 h and cultured with allogeneic T cells at a density of 1 × 105 cells/well in U-bottom 96-well microtiter plates in 5% CO2 at 37°C for 72 h. Cell proliferation was measured with WST-8 reagent by using Cell Count Reagent SF (Nacalai Tesque Inc., Kyoto, Japan) according to the manufacturer’s instructions.
Antigen presentation assay
The keyhole limpet hemocyanin (KLH, CARBIOCHEM, Cambridge, MA, USA) was absorbed to an aluminum hydroxide adjuvant (LSL Co. Ltd., Tokyo, Japan), and 100 μg of KLH was injected into the footpads of BALB/c mice as previously described [23]. Seven days after the injection, draining axial, popliteal, and inguinal lymph nodes were collected from the mice, and CD4+ T cells were isolated by using a MACS CD4+ T cell isolation kit as responder T cells [24]. DCs were cultured for 24 h with MZF (0–400 μg/ml) in the presence or absence of KLH (100 μg/ml). DCs were washed and co-cultured with KLH-primed or unprimed T cells at a density of 1 × 105 cells/well in U-bottom 96-well microtiter plates in 5% CO2 at 37°C for 72 h. Cell proliferation was measured with the WST-8 reagent. Levels of IL-12, IFN-γ, and IL-4 in the culture supernatants were then measured by ELISA.
The KLH-pulsed, MZF-treated DCs were cultured with KLH-primed CD4+ T cells in the presence of anti-IL-12 Ab (C17.8) or isotype Ab (JES3-19F1) for 72 h. IFN-γ levels in the culture supernatants were then measured by ELISA.
Tumor antigen pulsing of DCs
Tumor cell lysates were prepared by four freeze–thaw cycles of colon-26 cells (1 × 107 cells/ml in PBS) as previous described [25]. Cellular debris was removed by centrifugation, and the lysate solution was passed through a 0.2-μm membrane filter and stored at −80°C. DCs were incubated with tumor lysates at a ratio of 3:1 tumor cell equivalents: DCs in the presence or absence of MZF (400 μg/ml) or LPS (100 ng/ml) overnight at 37°C in 5% CO2. The DCs were harvested by gentle pipetting and washed twice in PBS. The DCs were resuspended in PBS at specific vaccine concentrations for use in further studies.
Preventive tumor challenge experiments
The BALB/c mice were treated with three subcutaneous (s.c.) injections (a week apart) of 2 × 105 DCs prepared as follows: unpulsed DC, colon-26 cell lysate-pulsed DC, colon-26 cell lysate and MZF-pulsed DC, or colon-26 cell lysate and LPS-pulsed DC. A week after the final DC injection, colon-26 cells (1 × 105 cells/mouse) were s.c. inoculated. The tumor volume was calculated using the formula: tumor volume (cm3) = longest diameter × shortest diameter2/2.
Therapeutic implanted tumor experiments
Colon-26 carcinoma cells (1 × 105 cells/mouse) were s.c. inoculated into BALB/c mice on day 0. On days 3, 10, and 17, the mice were treated by s.c. injections of 2 × 105 DCs, which were prepared as follows: unpulsed DC, colon-26 cell lysate-pulsed DC, colon-26 cell lysate and MZF-pulsed DC, or colon-26 cell lysate and LPS-pulsed DC.
Statistical analysis
Values are presented as mean ± standard error (SE). Student’s t test was used to analyze the significance. Tumor volumes of different groups were analyzed using a nonparametric two-tailed test (Mann–Whitney test) for unpaired samples. Kaplan–Meier survival curves were compared using the log-rank test. p value of <0.05 was considered to be significant.
Results
MZF induces DC maturation and function in vitro
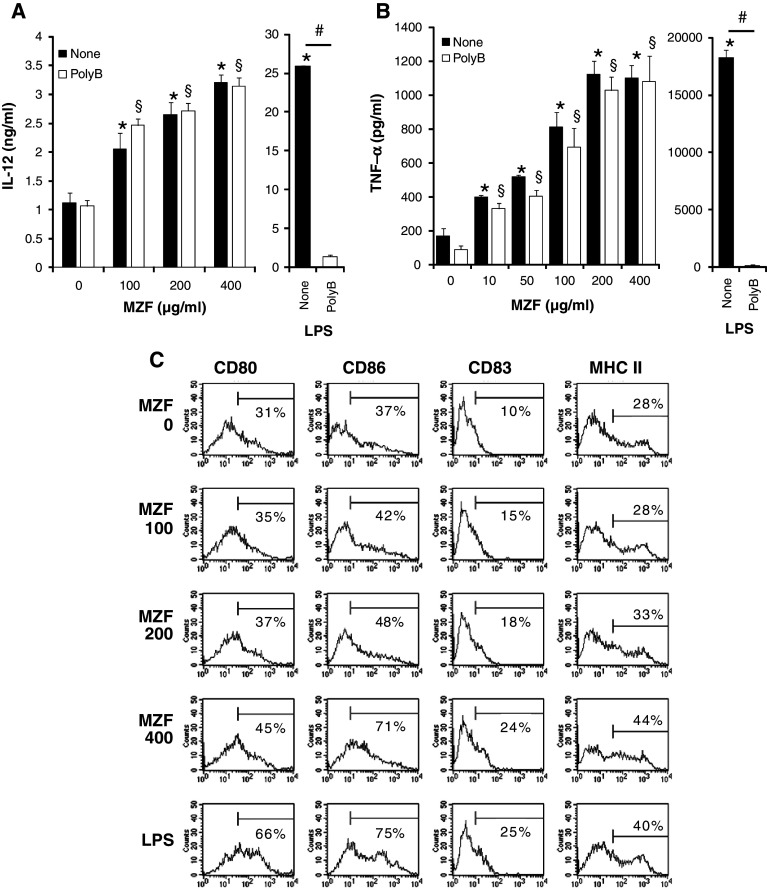
We first examined whether MZF could induce IL-12 and TNF-α production by BM-derived DCs in vitro. As shown in Fig. 1a, b, MZF significantly increased IL-12 and TNF-α production by DCs in a dose-dependent manner. MZF contained less than 0.05 EU/mg LPS as assessed by the Limulus test. To further exclude the possibility of endotoxin contamination in MZF-mediated production of IL-12 and TNF-α, we conducted additional experiments using polymyxin B. As expected, LPS-induced cytokine production was abrogated in the presence of polymyxin B, but MZF-induced cytokine production was not affected by polymyxin B (Fig. 1a, b). These results indicated that MZF-induced IL-12 and TNF-α production was not due to endotoxin contamination.
Fig. 1.
MZF induces IL-12 and TNF-α production and expression of DC maturation markers. a, b DCs (1 × 106 cells/ml) were cultured in RPMI-1640 medium containing 10% FBS and MZF (0–400 μg/ml) or LPS (100 ng/ml) with or without polymyxin B (poly B) for 24 h. TNF-α and IL-12 in the culture supernatants were measured by ELISA. The data shown are representative of three experiments. Values are expressed as mean ± SE (n = 5). *p < 0.05 versus control without poly B. § p < 0.05 versus control with poly B. # p < 0.05, poly B-treated group versus non-treated group. c DCs (1 × 106 cells/ml) were stimulated with MZF (0–400 μg/ml) or LPS (100 ng/ml) for 24 h. Expression of CD80, CD86, CD83, and MHC II (I-Ad) on CD11c+ cells was determined by flow cytometry and demonstrated by representative histogram profiles. Numbers reflect the percentage of positive cells
Mature DCs express high levels of maturation markers such as MHC molecules, CD80, CD83, and CD86. To evaluate whether MZF influences DC phenotypic maturation, DCs were cultured with MZF or LPS (positive control) for 24 h, and then analyzed for the expression of the DC maturation markers. As shown in Fig. 1c and Table 1, MZF upregulated the expression of CD80, CD83, CD86, and MHC II on CD11c+ cells in a dose-dependent manner. LPS, which induces DC activation and maturation, also enhanced the expression of these markers as expected.
Table 1.
Effect of MZF on the expression of DC maturation markers
| Maturation marker | MZF concentration (μg/ml) | LPS | |||
|---|---|---|---|---|---|
| 0 | 100 | 200 | 400 | ||
| CD80 | |||||
| Percentage of positive cells | 31.96 ± 0.58 | 34.91 ± 0.62* | 36.34 ± 0.36* | 44.35 ± 0.38* | 66.33 ± 0.39* |
| MFI | 74.68 ± 2.08 | 70.28 ± 2.20 | 75.04 ± 1.91 | 102.88 ± 2.97* | 165.98 ± 4.43* |
| CD86 | |||||
| Percentage of positive cells | 35.20 ± 2.94 | 42.49 ± 0.35 | 47.16 ± 1.14* | 61.29 ± 8.47* | 72.97 ± 0.78* |
| MFI | 55.56 ± 6.81 | 88.70 ± 4.04* | 92.01 ± 2.03* | 109.76 ± 2.26* | 186.50 ± 2.25* |
| CD83 | |||||
| Percentage of positive cells | 10.13 ± 0.38 | 13.06 ± 1.13 | 17.94 ± 0.43* | 24.69 ± 0.59* | 24.83 ± 0.49* |
| MFI | 4.98 ± 0.08 | 5.99 ± 0.25* | 6.43 ± 0.20* | 8.72 ± 0.54* | 9.50 ± 0.59* |
| MHC class II | |||||
| Percentage of positive cells | 28.25 ± 0.43 | 29.06 ± 0.71 | 32.69 ± 0.69* | 43.95 ± 0.24* | 40.17 ± 0.22* |
| MFI | 131.59 ± 2.85 | 143.05 ± 2.90* | 144.75 ± 3.17* | 179.11 ± 1.97* | 182.85 ± 3.97* |
DCs (1 × 106 cells/ml) were stimulated with MZF (0–400 μg/ml) or LPS (100 ng/ml) for 24 h. The values are percentages of DCs (CD11c+ cells) expressing indicated markers and the mean fluorescence intensity (MFI). Values are expressed as mean ± SE (n = 3)
* p < 0.05 versus control
Recognition of MZF by DCs requires β-glucan and mannan recognition
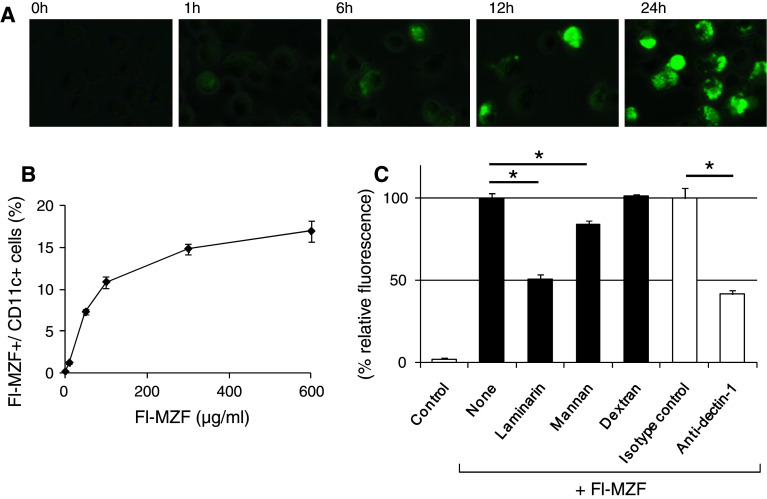
We next investigated whether fluorescence-labeled MZF directly binds to and is internalized by DCs. DCs were incubated with Fl-MZF at 37°C. Cells were collected at various time periods and then washed, fixed, and observed by fluorescence microscopy. As shown in Fig. 2a, fluorescence microscopy demonstrated an association of Fl-MZF with DCs after 1-h incubation. Fl-MZF was then internalized by DCs in a time-dependent manner at 37°C. In addition, we performed binding experiments with Fl-MZF on DCs on ice. The percentage of Fl-MZF+ DCs increased at higher FI-MZF concentrations, and binding saturation was reached at 300 μg/ml (Fig. 2b). We already demonstrated that MZF is a heteropolysaccharide consisting of → 6)-α-d-Galp-(1 → (36.2%), → 3)-α-l-Fucp-(1 → (14.5%), → 6)-α-d-Manp-(1 → (9.4%), → 3)-β-d-Glcp-(1 → (10.1%), α-d-Manp-(1 → (23.2%), and → 3,6)-β-d-Glcp-(1 → (6.5%) [21]. Therefore, we investigated whether DC recognition of Fl-MZF was competitively inhibited by laminarin (β1,6-branched β1,3-glucan), mannan (α1,6-mannan), or dextran (α1,3-branched α1,6-glucan). Although binding of Fl-MZF to DCs was significantly blocked by laminarin (49.3%) and mannan (15.8%), it was not affected by dextran (Fig. 2c). Consistent with this observation, binding was blocked by a Dectin-1-neutralizing antibody (58.7%), suggesting that DCs recognize the β-1,3- and 1,3,6-linked glucose contained in MZF via cell surface Dectin-1, a β-glucan receptor (Fig. 2c). These findings indicate that the recognition of β-glucan and α-mannan is involved in the binding of Fl-MZF to DCs.
Fig. 2.
Binding of MZF by DCs requires β-glucan and mannan recognition. a Internalization of Fl-MZF by DCs. DCs were cultured in RPMI-1640 medium containing 10% FBS with Fl-MZF (400 μg/ml) at 37°C. Cells were collected at various time periods and then washed, fixed, and observed by fluorescence microscopy. b Binding of MZF by DCs. DCs (2 × 105 cells/tube) were incubated on ice for 1 h in PBS containing 3% FBS and Fl-MZF at various concentrations. After incubation, the cells were washed and fixed. Fl-MZF+ DCs were identified by flow cytometry. c Laminarin, mannan, and anti-Dectin-1 Ab inhibited the DC recognition of Fl-MZF. Ice-cold PBS containing 3% FBS with or without inhibitors (laminarin, mannan and dextran; 1 mg/ml), anti-Dectin-1 Ab (2A11), or isotype Ab (rat IgG2bκ) was added to the DCs (2 × 105 cells/tube) and incubated on ice for 1 h before the addition of Fl-MZF (400 μg/ml). Cells were incubated on ice for 1 h, washed, and fixed. Fl-MZF+ DCs were identified by flow cytometry. The data shown have been normalized to the percentages of Fl-MZF+ CD11c+ cells of uninhibited control. Values are expressed as mean ± SE, n = 3; *p < 0.05
MZF enhances DC allostimulatory activity
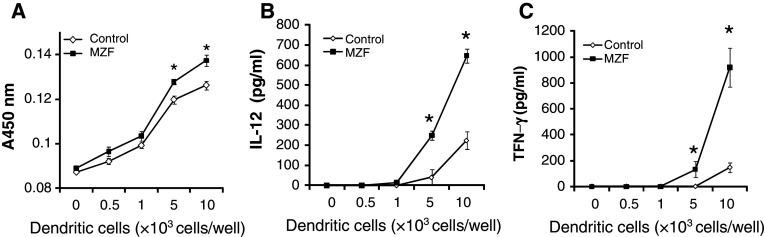
The MZF increased the expression of DC maturation markers involved in the presentation of antigens to T cells. Therefore, we investigated the ability of MZF-treated DC to stimulate T cell proliferation and cytokine production in an allogeneic mixed lymphocyte reaction (MLR). CD4+ T cells were collected from C3H/HeN mice (I-Ak) and cultured with MZF-treated or untreated DCs from BALB/c mice (I-Ad) for 72 h. As shown in Fig. 3a, MZF-treated DCs slightly but significantly induced proliferation of allogeneic CD4+ T cells. In addition, IL-12 and IFN-γ levels were more significantly increased by MZF-treated DCs than by untreated DCs at 104 DCs/well (Fig. 3b, c). These results suggest that MZF-treated DCs have the ability to prime and activate allogeneic T cells.
Fig. 3.
Allostimulatory activity of MZF-treated DCs stimulates allogeneic T cells. Unstimulated or MZF-stimulated DCs were treated with 50 μg/ml mitomycin C for 1 h and cultured with allogeneic C3H/HeN CD4+ T cells at a density of 1 × 105 cells/well in U-bottom 96-well microtiter plates in 5% CO2 at 37°C for 72 h. a Cell proliferation was measured with WST-8 reagent. b, c IL-12 and IFN-γ levels in supernatants were analyzed by ELISA. Data (n = 5) represent the mean ± SE of two separate experiments. *p < 0.01 versus control
MZF increases DC antigen-presenting function
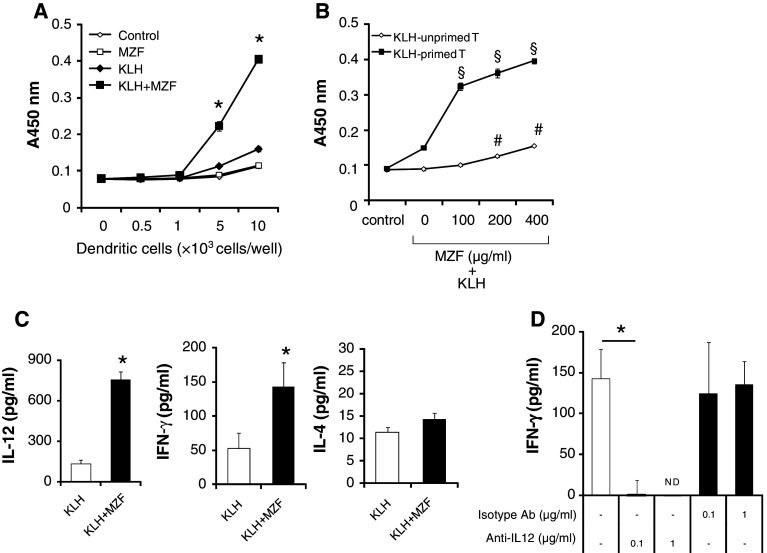
We next investigated whether MZF enhances the antigen-presenting function of DCs. KLH (100 μg in alum) was injected into the footpads of BALB/c mice. Seven days later, CD4+ T cells were isolated with the MACS system as responder T cells from draining lymph node cells and cultured with MZF + KLH-DCs from BALB/c mice for 72 h. As a control, KLH-DCs or DCs were cultured with KLH-T cells. As shown in Fig. 4a, the proliferation of KLH-T cells was more significantly induced by MZF + KLH-DCs than by all the other groups. On the other hand, there was no significant difference between the MZF-DCs and DCs that were unpulsed with KLH (Fig. 4a). The proliferation of KLH-T cells was increased in a MZF dose-dependent manner (Fig. 4b). The proliferation of KLH-unprimed T cells was slightly increased by MZF + KLH-DCs (Fig. 4b). These results suggest that MZF induces Ag-specific T cell proliferation.
Fig. 4.
MZF increases DC Ag-presenting activity. a DCs were stimulated with MZF (400 μg/ml) for 24 h in the presence or absence of KLH (100 μg/ml). DCs were washed and cultured with KLH-primed T cells (1 × 105 cells/well) in U-bottom 96-well microtiter plates for 72 h. Cell proliferation was measured with WST-8 reagent. *p < 0.05 versus all other groups. b DCs were stimulated with MZF at various concentrations for 24 h in the presence or absence of KLH. DCs were washed and cultured with KLH-primed or unprimed T cells (1 × 105 cells/well) for 72 h. § p < 0.05 versus KLH-DCs (MZF 0 μg/ml) co-cultured with KLH-T cells. # p < 0.05 versus KLH-DCs (MZF 0 μg/ml) co-cultured with unprimed T cells. c Levels of IL-12, IFN-γ, and IL-4 in the culture supernatants (DCs/T cells ratio, 0.1:1) were then measured by ELISA. d KLH + MZF-DCs were cultured with KLH-T cells (DCs/T cells ratio, 0.1:1) in the presence of anti-IL-12 Ab (C17.8) or isotype Ab (JES3-19F1) for 72 h. IFN-γ levels in the culture supernatants were then measured by ELISA. *p < 0.05 versus KLH-DCs co-cultured with KLH-T cells. Data (n = 4) represent the mean ± SE of two separate experiments. ND not detected
To investigate whether the Th1 or Th2 phenotype is induced by MZF + KLH-DCs, cytokine levels in the culture supernatants were determined by ELISA. IL-12 production was more significantly induced by MZF + KLH-DCs (104 cells/well) cultured with KLH-T cells (105 cells/well) than by KLH-DCs (Fig. 4c). Enhancement of IL-12 production might induce the Th1 phenotype. We therefore investigated the level of IFN-γ, a Th1 cytokine, in the culture supernatants. As shown in Fig. 4c, MZF + KLH-DCs induced IFN-γ production from KLH-T cells. On the other hand, the production of IL-4, a Th2 cytokine, from KLH-T cells was not significantly induced by MZF (Fig. 4c).
IL-12 induces IFN-γ production by T cells, drives the development of Th1 cells, and promotes cell-mediated immunity. As shown in Fig. 4d, MZF + KLH-DC-induced IFN-γ production was strongly reduced in co-culture with the IL-12 neutralizing antibody. In contrast, the isotype antibody did not affect MZF + KLH-DC-induced IFN-γ production (Fig. 4d). This indicates that IL-12 production by MZF + KLH-DCs is a significant factor in MZF + KLH-DC-induced IFN-γ production by T cells.
Transfer of MZF-treated DCs could achieve preventive effect on tumor challenge
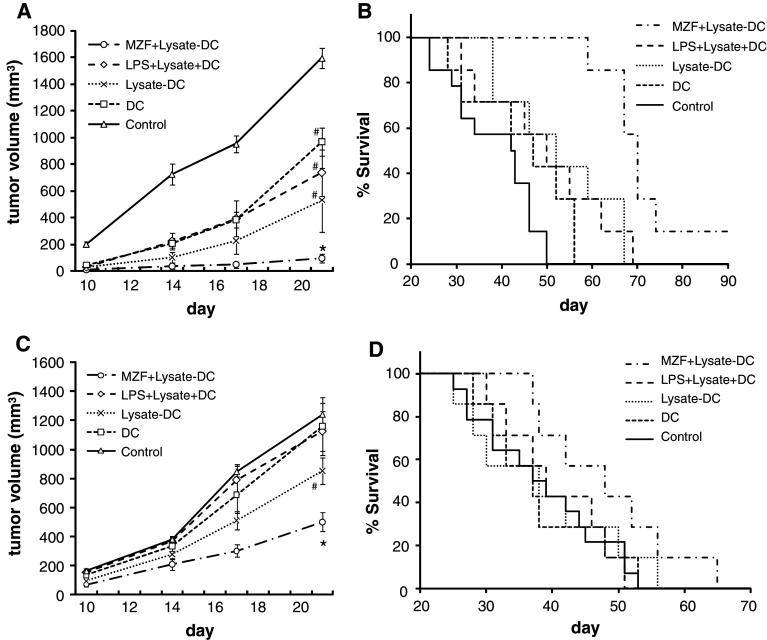
In BALB/c mice inoculated with colon-26 cancer cells, tumor growth was more significantly inhibited (47.6%) by MZF (8 mg/kg/day) than by the control group [21]. In this study, we demonstrated that MZF induced DC maturation and facilitated T cell priming. Therefore, we inoculated mice s.c. with MZF-treated tumor cell lysate-pulsed DCs to investigate the preventive effect on tumor challenge. BALB/c mice were given three s.c. injections (a week apart) of saline, unpulsed DCs, colon-26 lysate-pulsed DCs (Lysate-DCs), LPS-treated colon-26 lysate pulsed DCs (LPS + Lysate-DCs), or MZF-treated colon-26 lysate pulsed DCs (MZF + Lysate-DCs) 1 week before tumor inoculation. As shown in Fig. 5a, tumor growth was more dramatically inhibited (94.0%) by the transfer of MZF + Lysate-DCs than by all the other groups (vs. control, p = 0.0003; vs. DCs, p = 0.0017; vs. Lysate-DCs, p = 0.0350; vs. LPS + Lysate-DCs, p = 0.0181) on day 21 after tumor inoculation. The data also show that the survival of mice was more significantly prolonged by the transfer of MZF + Lysate-DCs than by all the other groups (vs. control, p < 0.0001; vs. DCs, p = 0.0002; vs. Lysate-DCs, p = 0.006; vs. LPS + Lysate-DCs, p = 0.0038; Fig. 5b). These results show that the transfer of MZF + Lysate-DCs before tumor inoculation could achieve a preventive effect on tumor challenge. Interestingly, LPS + Lysate-DCs were less capable of a preventive effect on tumor challenge than MZF + Lysate-DCs although LPS induced higher amounts of IL-12 and upregulated the expression of maturation markers on DCs.
Fig. 5.
Preventive and therapeutic effect of MZF in a subcutaneous colon-26 tumor model. a, b Preventive effect of MZF. BALB/c mice were treated with three s.c. injections (a week apart) of 2 × 105 DCs prepared as follows: DC, Lysate-DC, LPS + Lysate-DC, or MZF + Lysate-DC. A week after the final DC injection, colon-26 cells (1 × 105 cells/mouse) were s.c. inoculated. The tumor growth (a) and survival rates (b) were monitored (control, n = 14; other groups, n = 7). c, d Therapeutic effect of MZF. Colon-26 cells (1 × 105 cells/mouse) were s.c. inoculated into BALB/c mice on day 0. On days 3, 10, and 17, mice were treated by s.c. injections of 2 × 105 DCs. The tumor growth (c) and survival rates (d) were monitored (control, n = 14; other groups, n = 7). a, c The Mann–Whitney U test was used to analyze the statistical significance of tumor growth on day 21. *p < 0.05 versus all other groups; # p < 0.05 versus control. b, d Survival rates are represented using Kaplan–Meier curves
Transfer of MZF-treated DCs could achieve a therapeutic effect on tumor challenge
Since the preventive effect on colon-26 tumor challenge by MZF + Lysate-DCs was observed, we further examined whether MZF + Lysate-DCs inhibited tumor growth, even though DCs were transferred after tumor inoculation. Colon-26 cells were s.c. inoculated into BALB/c mice on day 0. On days 3, 10, and 17, mice were treated by s.c. injections of saline, DCs, Lysate-DCs, LPS + Lysate-DCs, or MZF + Lysate-DCs. As shown in Fig. 5c, tumor growth was more significantly inhibited by the transfer of MZF + Lysate-DCs (59.6%) than by all the other groups (vs. control, p = 0.0003; vs. DCs, p = 0.0253; vs. Lysate-DCs, p = 0.0127; vs. LPS + Lysate-DCs, p = 0.0127). However, there was no significant difference in survival rates between the MZF + Lysate-DCs groups and the other groups (vs. control, p = 0.0598; vs. DCs, p = 0.0897; vs. Lysate-DCs, p = 0.2081; vs. LPS + Lysate-DCs, p = 0.0916; Fig. 5d).
Discussion
In this study, we demonstrated that the polysaccharide MZF isolated from G. frondosa induces maturation of BM-derived DCs in vitro. MZF upregulated the expression of maturation markers such as CD80, CD86, CD83, and MHC II on DC surfaces (Fig. 1c; Table 1). MZF significantly and dose dependently increased IL-12 and TNF-α production by DCs (Fig. 1a, b). Furthermore, MZF-treated DCs significantly stimulated both allogeneic and antigen-specific syngenic T cell responses (Figs. 3, 4). These results indicate that MZF induces maturation of the DC phenotype and stimulates T cell proliferation.
The interactions of DCs with T cells are important to regulate the immune system. Immature DCs expressing low levels of co-stimulatory molecules (CD80, CD86) and MHC molecules do not have the capability to stimulate T cells [1]. They capture and process exogenous antigens in peripheral tissues, thereby triggering DC maturation in the presence of PAMPs and inflammatory cytokines. Matured DCs express high levels of co-stimulatory and MHC molecules and migrate to the lymphoid organs where they stimulate naïve T cells. MHC molecules presenting antigen peptides and co-stimulatory molecules provide several signals that determine the fate of naïve T cells [5]. Signal 1 is provided by the T cell receptor-recognizing antigen peptides bound to MHC molecules. Signal 1 alone induces T cell inactivation, T cell deletion, or inducible regulatory T cell generation. Signal 2, a co-stimulatory signal, induces T cell responses resulting in cell clonal expansion and differentiation into effector/memory cells when combined with Signal 1. Signal 2 promotes strong antigen-specific immunity by binding CD80/86 on DCs to its receptor, CD28, on T cells. However, Signal 2 can also induce tolerogenic signals through interaction between CD80/86 on DCs and cytotoxic T-lymphocyte antigen 4 (CTLA4) expressed on regulatory T cells. DCs also deliver Signal 3, which determines the differentiation of Th1 cells, Th2 cells, and CTLs. IL-12, a Signal 3 molecule, induces the differentiation of Th1 cells and CTLs [6, 26]. The Notch ligand leads to the development of Th2 cells as Signal 3 [27]. Controlling these multiple interactions between DCs and T cells helps induce efficient anti-tumor immunity.
Th1 response-induced activation of Th1 cells, CTLs, and NK cells is significantly efficient for cancer immunotherapies with cancer vaccines. IL-12 plays a key role in Th1 cell differentiation by DC stimulation. Mature DCs together with IL-12 they produce induce IFN-γ-producing Th1 cells and tumor-specific CTLs and augment the cytotoxic activity of tumor-specific CTLs and non-specific NK cells [1, 28–30]. We demonstrated that MZF-treated DCs enhanced antigen-specific IFN-γ production by syngenic CD4+ T cells. In contrast, it did not affect IL-4 production by CD4+ T cells (Fig. 4c). Furthermore, the enhancement of IFN-γ production in CD4+ T cells induced by MZF-treated DCs was completely inhibited by the addition of the anti IL-12 antibody (Fig. 4d). These results indicate that MZF induces the maturation of Th1 cell-inducing DCs, and that IL-12 produced by MZF-treated DCs is essential for the enhancement of IFN-γ production by CD4+ T cells.
The DCs pulsed with synthetic tumor peptides elicit antigen-specific immunity. Systemic or intradermal injection of DCs pulsed with specific tumor-associated antigens (TAAs) has been clinically shown to elicit an immune response [25, 31, 32]. In general, DCs generated ex vivo are pulsed with TAAs and re-injected into patients to stimulate Th1 cell- and CTL-mediated anti-tumor immunity [2, 7]. In the present study, DCs generated from BM cells in the presence of GM-CSF and IL-4 were co-stimulated with freeze–thaw cell lysates of colon-26 cells and LPS or MZF, and they were subsequently injected s.c. into syngenic BALB/c mice. MZF + Lysate-DCs significantly inhibited colon-26 tumor growth both preventively and therapeutically to a greater extent than unpulsed DCs, Lysate-DCs, and LPS + Lysate-DCs (Fig. 5a, c). On the other hand, LPS + Lysate-DCs had a minimal effect on tumor growth although LPS induced high amounts of IL-12 and upregulated the expression of maturation markers on DCs (Fig. 1). This may be due to the fact that DCs become “exhausted” by excessive stimulation with LPS and become incapable of secreting IL-12 when they encounter T cells in the body [7, 29]. These results indicate the possibility that MZF-treated DCs are more appropriate for cancer therapy than unpulsed or LPS-treated DCs. MZF + Lysate-DCs induced survival prolongation in the preventive model; however, it did not significantly improve survival in the therapeutic model (Fig. 5b, d). Although administration of MZF + Lysate-DCs inhibited colon-26 tumor growth, it did not significantly prolong survival owing to the very rapid growth rate of the colon-26 tumor in the therapeutic model. Optimization of the number of DCs, administration schedule, and administration route are required to improve the effect of this DC vaccine therapy. Some fungal polysaccharides have been reported to induce DC maturation in vitro [13–16]; however, there have been few in vivo studies to confirm the antitumor effect of mature DCs induced by fungal polysaccharides. Our study has provided important findings about the pharmacological effect of fungal polysaccharide-treated DC vaccination in vivo.
This study also demonstrated the possible mechanism of the effects of fungal polysaccharide on DC stimulation in vitro. Internalization of Fl-MZF by DCs was observed using a fluorescence microscope after incubation at 37°C in a time-dependent manner (Fig. 2a). MZF is a heteropolysaccharide consisting of → 6)-α-d-Galp-(1 →, → 3)-α-l-Fucp-(1 →, → 6)-α-d-Manp-(1 →, → 3)-β-d-Glcp-(1 →, α-d-Manp-(1 →, and → 3,6)-β-d-Glcp-(1 → [21]. DCs express PRRs such as TLRs, mannose receptor, dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin 2 (DC-SIGN), complement receptors, scavenger receptors, and Dectin-1, all of which recognize various microbial PAMPs [10]. Zymosan extracted from Saccharomyces cerevisiae is a stimulatory polysaccharide that contains β-glucans and other components such as mannans, mannoproteins, and chitin. Zymosan has been reported to be recognized by DCs through TLR2 and Dectin-1 [33]. As shown in Fig. 2c, the binding of Fl-MZF to DCs was significantly inhibited by laminarin (49.3%) and mannan (15.8%), indicating that the recognition of β-glucan and α-mannan is partially involved in the binding Fl-MZF to DCs. The binding of Fl-MZF to DCs was also inhibited by a Dectin-1-neutralizing antibody (58.7%). These results suggest that β-glucan contained in MZF is recognized by Dectin-1 on DCs, and that recognition by Dectin-1 might contribute to MZF-induced DC stimulation. Because Dectin-1 not only regulates gene expression but can also function as a phagocytic receptor [34], the Dectin-1-mediated endocytotic pathway may partially mediate internalization of MZF. However, the detailed recognition mechanism of MZF by DCs remains unclear because MZF is a heteropolysaccharide. More studies at the molecular level are needed to define the recognition mechanism of MZF by DCs.
In conclusion, the polysaccharide MZF isolated from G. frondosa induced DC maturation and antigen-specific Th1 response through the enhancement of IL-12 production by DCs. Furthermore, we demonstrated that the injection of MZF + Lysate-DCs induced both therapeutic and preventive effects on tumor development. These results suggest that MZF could be a possible effective adjuvant to enhance immunotherapy using DC vaccination. The immunological effects of a G. frondosa extract have already been demonstrated in human clinical trials without dose-limiting toxicity, and MZF contained in the extract has been reported to induce an anti-tumor effect without side effects in mice [17, 21]. The possibility that MZF could enhance the effect of DC vaccination may provide new insights for clinical studies.
Acknowledgments
We thank Yukiguni Maitake Co., Ltd. for supplying maitake. We also thank F. Yamamoto for supporting our experiment.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Nencioni A, Brossart P. Cellular immunotherapy with dendritic cells in cancer: current status. Stem Cells. 2004;22:501–513. doi: 10.1634/stemcells.22-4-501. [DOI] [PubMed] [Google Scholar]
- 3.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–550. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama T, Hoshino K, Saito M, Yano T, Sasaki I, Yamazaki C, Akira S, Kaisho T. Immunoadjuvant effects of polyadenylic:polyuridylic acids through TLR3 and TLR7. Int Immunol. 2008;20:1–9. doi: 10.1093/intimm/dxm112. [DOI] [PubMed] [Google Scholar]
- 5.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 6.Curtsinger JM, Lins DC, Mescher MF. Signal 3 determines tolerance versus full activation of naive CD8 T cells: dissociating proliferation and development of effector function. J Exp Med. 2003;197:1141–1151. doi: 10.1084/jem.20021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ballestrero A, Boy D, Moran E, Cirmena G, Brossart P, Nencioni A. Immunotherapy with dendritic cells for cancer. Adv Drug Deliv Rev. 2008;60:173–183. doi: 10.1016/j.addr.2007.08.026. [DOI] [PubMed] [Google Scholar]
- 8.Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol Rev. 2007;219:75–87. doi: 10.1111/j.1600-065X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 9.Yan J, Vetvicka V, Xia Y, Coxon A, Carroll MC, Mayadas TN, Ross GD. Beta-glucan, a “specific” biologic response modifier that uses antibodies to target tumors for cytotoxic recognition by leukocyte complement receptor type 3 (CD11b/CD18) J Immunol. 1999;163:3045–3052. [PubMed] [Google Scholar]
- 10.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 11.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230:38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leibundgut-Landmann S, Osorio F, Brown GD, Reis e Sousa C. Stimulation of dendritic cells via the dectin-1/Syk pathway allows priming of cytotoxic T-cell responses. Blood. 2008;112:4971–4980. doi: 10.1182/blood-2008-05-158469. [DOI] [PubMed] [Google Scholar]
- 13.Sheng KC, Pouniotis DS, Wright MD, Tang CK, Lazoura E, Pietersz GA, Apostolopoulos V. Mannan derivatives induce phenotypic and functional maturation of mouse dendritic cells. Immunology. 2006;118:372–383. doi: 10.1111/j.1365-2567.2006.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren Z, Guo Z, Meydani SN, Wu D. White button mushroom enhances maturation of bone marrow-derived dendritic cells and their antigen presenting function in mice. J Nutr. 2008;138:544–550. doi: 10.1093/jn/138.3.544. [DOI] [PubMed] [Google Scholar]
- 15.Kim GY, Lee MY, Lee HJ, Moon DO, Lee CM, Jin CY, Choi YH, Jeong YK, Chung KT, Lee JY, et al. Effect of water-soluble proteoglycan isolated from Agaricus blazei on the maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2005;5:1523–1532. doi: 10.1016/j.intimp.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 16.Park SK, Kim GY, Lim JY, Kwak JY, Bae YS, Lee JD, Oh YH, Ahn SC, Park YM. Acidic polysaccharides isolated from Phellinus linteus induce phenotypic and functional maturation of murine dendritic cells. Biochem Biophys Res Commun. 2003;312:449–458. doi: 10.1016/j.bbrc.2003.10.136. [DOI] [PubMed] [Google Scholar]
- 17.Deng G, Lin H, Seidman A, Fornier M, D’Andrea G, Wesa K, Yeung S, Cunningham-Rundles S, Vickers AJ, Cassileth B. A phase I/II trial of a polysaccharide extract from Grifola frondosa (Maitake mushroom) in breast cancer patients: immunological effects. J Cancer Res Clin Oncol. 2009;135:1215–1221. doi: 10.1007/s00432-009-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nanba H, Hamaguchi A, Kuroda H. The chemical structure of an antitumor polysaccharide in fruit bodies of Grifola frondosa (maitake) Chem Pharm Bull. 1987;35:1162–1168. doi: 10.1248/cpb.35.1162. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Masuda Y, Yamasaki Y, Yokota Y, Nanba H. Maitake beta-glucan enhances granulopoiesis and mobilization of granulocytes by increasing G-CSF production and modulating CXCR4/SDF-1 expression. Int Immunopharmacol. 2009;9:1189–119615. doi: 10.1016/j.intimp.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Masuda Y, Inoue M, Miyata A, Mizuno S, Nanba H. Maitake beta-glucan enhances therapeutic effect and reduces myelosupression and nephrotoxicity of cisplatin in mice. Int Immunopharmacol. 2009;9:620–626. doi: 10.1016/j.intimp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Masuda Y, Matsumoto A, Toida T, Oikawa T, Ito K, Nanba H. Characterization and antitumor effect of a novel polysaccharide from Grifola frondosa . J Agric Food Chem. 2009;57:10143–10149. doi: 10.1021/jf9021338. [DOI] [PubMed] [Google Scholar]
- 22.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim E, Kim SH, Kim S, Cho D, Kim TS. AIMP1/p43 protein induces the maturation of bone marrow-derived dendritic cells with T helper type 1-polarizing ability. J Immunol. 2008;180:2894–2902. doi: 10.4049/jimmunol.180.5.2894. [DOI] [PubMed] [Google Scholar]
- 24.DeKruyff RH, Fang Y, Wolf SF, Umetsu DT. IL-12 inhibits IL-4 synthesis in keyhole limpet hemocyanin-primed CD4+ T cells through an effect on antigen-presenting cells. J Immunol. 1995;154:2578–2587. [PubMed] [Google Scholar]
- 25.Lambert LA, Gibson GR, Maloney M, Durell B, Noelle RJ, Barth RJ., Jr Intranodal immunization with tumor lysate-pulsed dendritic cells enhances protective antitumor immunity. Cancer Res. 2001;61:641–646. [PubMed] [Google Scholar]
- 26.Kalinski P, Hilkens CM, Wierenga EA, Kapsenberg ML. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today. 1999;20:561–567. doi: 10.1016/S0167-5699(99)01547-9. [DOI] [PubMed] [Google Scholar]
- 27.Amsen D, Blander JM, Lee GR, Tanigaki K, Honjo T, Flavell RA. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/S0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 28.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164:4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 29.Giermasz AS, Urban JA, Nakamura Y, Watchmaker P, Cumberland RL, Gooding W, Kalinski P. Type-1 polarized dendritic cells primed for high IL-12 production show enhanced activity as cancer vaccines. Cancer Immunol Immunother. 2009;58:1329–1336. doi: 10.1007/s00262-008-0648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 31.Gao FG, Wan da F, Gu JR. Ex vivo nicotine stimulation augments the efficacy of therapeutic bone marrow-derived dendritic cell vaccination. Clin Cancer Res. 2007;13:3706–3712. doi: 10.1158/1078-0432.CCR-07-0028. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Carmona MA, Lukacs-Kornek V, Timmerman A, Shabani S, Kornek M, Vogt A, Yildiz Y, Sievers E, Schmidt-Wolf IG, Caselmann WH, et al. CD40 ligand-expressing dendritic cells induce regression of hepatocellular carcinoma by activating innate and acquired immunity in vivo. Hepatology. 2008;48:157–168. doi: 10.1002/hep.22296. [DOI] [PubMed] [Google Scholar]
- 33.Rogers NC, Slack EC, Edwards AD, Nolte MA, Schulz O, Schweighoffer E, Williams DL, Gordon S, Tybulewicz VL, Brown GD, Reis e Sousa C. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–517. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 34.Herre J, Marshall AS, Caron E, Edwards AD, Williams DL, Schweighoffer E, Tybulewicz V, Reis e Sousa C, Gordon S, Brown GD. Dectin-1 uses novel mechanisms for yeast phagocytosis in macrophages. Blood. 2004;104:4038–4045. doi: 10.1182/blood-2004-03-1140. [DOI] [PubMed] [Google Scholar]