Abstract
Objective
Chronic inflammation and cancer development are associated with dysregulated immune responses and the presence of regulatory T cells (Treg). To study the role of Treg in tumor cell escape from immune surveillance, an in vitro model simulating the tumor microenvironment and promoting the induction and expansion of IL-10+ Treg type 1 (Tr1) was established.
Methods
An in vitro co-culture system (IVA) included an irradiated head and neck squamous cell carcinoma cell line, immature dendritic cells (iDC), CD4+CD25− T cells and cytokines, IL-2 (10 IU/ml), IL-10 (20 IU/ml), IL-15 (20 IU/ml) ± 1 nM rapamycin. Autologous iDC and CD4+CD25− T cells were obtained from the peripheral blood of 15 normal donors. Co-cultures were expanded for 10 days. Proliferating lymphocytes were phenotyped by multi-color flow cytometry. Their suppressor function was measured in CFSE inhibition assays ± neutralizing anti-IL-10 mAb and using transwell cultures. Culture supernatants were tested for IL-4, IL-10, TGF-β and IFN-γ in ELISA.
Results
In the IVA, low doses of IL-2, IL-10 and IL-15 promoted induction and expansion of CD3+CD4+CD25−IL2Rβ+IL2Rγ+FoxP3+CTLA-4+IL-10+ cells with suppressor activity (mean suppression ± SD = 58 ± 12%). These suppressor cells produced IL-10 (mean ± SD = 535 ± 12 pg/ml) and TGF-β (mean ± SD = 512 ± 38 pg/ml), but no IL-4 or IFN-γ. Suppressor function of co-cultures correlated with the percent of expanding IL-10+ Tr1 cells (r 2 = 0.9; P < 0.001). The addition of rapamycin enriched Tr1 cells in all co-cultures. Neutralizing anti-IL-10 mAb abolished suppressive activity. Suppression was cell-contact independent.
Conclusion
The tumor microenvironment promotes generation of Tr1 cells which have the phenotype distinct from that of CD4+CD25highFoxP3+ nTreg and mediate IL-10 dependent immune suppression in a cell-contact independent manner. Tr1 cells may play a critical role in cancer progression.
Keywords: Tr1 cells, Treg, HNSCC, Immune suppression, Tumor escape, Rapamycin
Introduction
Human tumors have evolved a variety of molecular mechanisms which induce dysfunction in immune cells, including functional suppression of effector T cells and are thus responsible for peripheral tolerance [26]. Autoimmunity and tumor immunity may be two sides of related immune phenomena and indeed can accompany one another [37]. The mechanisms of tumor escape are, at least in part, mediated by subsets of regulatory T cells (Treg), which may play a critical role in the induction of tolerance to self-antigens, including those expressed by tumors. It has been suggested that tumors overexpressing self-antigens induce tumor/self-specific Treg cells which interfere with effective anti-tumor responses [11]. The biologic importance of Treg in human cancers, including ovarian carcinoma [12], and lung cancer [38], has recently been documented, and extensive data obtained in mice support the Treg involvement in control of anti-tumor immunity [5].
To date, several distinct cell subsets with regulatory activity have been described including: (1) naturally occurring T regulatory cells (nTreg) characterized by the CD4+CD25highFoxP3high phenotype, (2) CD4+ T regulatory type 1 (Tr1) cells inducible under specific conditions from naive precursors and (3) Th3 cells, which are dependent on IL-4 for functional differentiation [18 ]. Tr1 cells were first described in 1997 [16] and were characterized and identified mainly by high levels of IL-10 and TGF-β production, with negligible IL-4 and IFN-γ secretion [9]. This T-cell subset regulates immune responses via the process referred to as infectious tolerance [32]. Previous data indicate that the generation of Tr1 cells depends on the environmental context in which a T cell encounters its cognate antigen (self or foreign), and since then several models have been reported to induce and expand Tr1 cells in vitro. These models included modified antigen-presenting cells (APC) and/or exposure to immunoregulatory cytokines.
Dendritic cells (DCs) are professional APCs specialized to initiate and drive T-cell immunity. Depending on their maturational state and their location, DCs perform different functions within the immune system. While CD83+ mature DCs induce Th1 inflammatory responses, immature DCs (iDC) participate in the generation of IL-10-producing Tr1 cells [22].
IL-10 plays a pivotal role in the immunosuppression of cancer [20]. It inhibits the stimulatory capacity of DCs through downregulation of MHC class II and the costimulatory molecules, CD80 and CD86, thus inhibiting DC maturation and polarizing DCs to tolerogenic rather than stimulatory activity [33]. iDCs drive the differentiation of IL-10-producing Tr1 cells, and not CD4+CD25+ Treg [20, 22]. Several groups reported the in vitro induction of IL-10-producing Tr1 cells through stimulation with anti-CD3 and anti-CD46 Abs [7], Vitamin D3 and dexamethasone [8], IL-10/IFN-anti- [21] or IL-4 [2]. The immunosuppressive drug, rapamycin, which has been reported to block intracellular signaling responses to T-cell growth factors, was used for selective survival of nTreg in humans [34] and, in the presence of IL-10, reported to induce Tr1 cells in mice [10].
IL-15 shares with IL-2 the ability to support the growth of various T-cell subsets and mediates costimulatory activity for proliferation. More recently, it was shown that IL-15 expands Tr1 cells in vitro [4]. IL-2, mainly derived from CD4+ T cells, supports the growth of T cells in vitro [27]. It also plays a role in the development of tolerance mediated by Treg in vivo [25]. Signaling through the heterotrimeric IL-2Ranti-βγc, expressed on Treg, prolongs their survival and is critical for Treg function in vivo [14].
In the tumor microenvironment, a crosstalk existing between tumor-derived soluble factors, TAA, APC and T lymphocytes promotes carcinogenesis and immune tolerance and thus plays a key role in downregulation of anti-tumor host immunity [31]. In this context, IL-10, IL-15 and IL-2 may be considered as a mix of cytokines necessary for Tr1 cell expansion. In order to investigate this hypothesis, we have established a Tr1 in vitro system simulating a tumor microenvironment and promoting development of Tr1 cells. In this system, it is possible to induce cells which express the Tr1-specific phenotype and mediate suppressor function by co-incubating naive T cells of normal donors with autologous iDCs in a mix of cytokines and in the presence of tumor cells.
Materials and methods
Reagents and antibodies
Ficoll-Hypaque was purchased from GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA. Recombinant human IL-4 (rhIL-4) was purchased from Cellgenix, Antioch, IL,USA, and recombinant human granulocyte macrophage–colony-stimulating factor (rhGM-CSF) from Berlex, Bothell, WA, USA. All cell culture reagents including AIM V medium, DMEM medium, RPMI 1640 medium, phosphate-buffered saline (PBS), heat-inactivated fetal calf serum (ΔFCS), streptomycin, penicillin, recombinant Trypsin-like enzyme (TrypLE) and Trypan-blue dye were purchased from Gibco/Invitrogen, Grand Island, NY, USA. CD4+CD25+ regulatory T cell isolation kit, T cell activation kit, and the AutoMACS cell separator were purchased from Miltenyi Biotec, Auburn, CA, USA. Cytokines rhIL-2, rhIL-10 and rhIL-15 were purchased from Peprotech, Rocky Hill, NJ, USA. Bovine serum albumin (BSA), rapamycin, PMA, Ionomycin, saponin and Brefeldin A were from Sigma, St. Louise, MO, USA. Anti-CD3 mAb was obtained from ATCC, Rockville, MD, USA. The following mAbs for flow cytometry analysis were purchased from Beckman Coulter, Miami, FL, USA: anti-CD28 mAb, anti-CD3-ECD, anti-CD4-PC5, anti-CD25-FITC and -PE, anti-CD122-FITC, anti-CD132-PE, anti-CD152-PE and isotype controls IgG1, IgG2a and IgG2b. Anti-IL-10-PE and its isotype control PE rat IgG2a,κ was purchased from BD Pharmingen, San Jose, CA, USA; anti-FoxP3-FITC and anti-IL-4-FITC from eBioscience, San Diego, CA, USA. Carboxyfluoresceine diacetate succinimidylester (CFSE) was purchased from Molecular Probes/Invitrogen, Carlsbad, CA, USA. anti-GITR-FITC, rhIL-10 mAb, and EIA kits were purchased from R&D, Minneapolis, MN, USA. NO-detection kit was obtained from Assay Designs, Ann Arbor, MI, USA and the Luminex cytokine reagents from Biosource/Invitrogen, Grand Island, NY, USA. Antibodies for HLA-phenotyping, MA2.1, W6.32, and BB7.2, were produced from hybridomas obtained from ATCC, Manassas, VA by Dr. DeLeo, UPCI, Pittsburgh, PA, USA. GAM-IgG-FITC and GAH IgG were obtained from Caltag/Invitrogen, Carlsbad, CA, USA.
Cell culture
All hematopoetic cells were cultured in a complete medium consisting of AIM V supplemented with 10% (v/v) ΔFCS, 100 IU/ml penicillin, 100 μg/ml streptomycin and l-glutamine (2 mmol/l). Tumor cell lines were maintained in DMEM medium supplemented with 10% ΔFCS, 100 IU/ml penicillin, 100 μg/ml streptomycin and l-glutamine (2 mmol/l) at 37°C in an atmosphere of 5% CO2 in air. RPMI 1640 medium was used for washing lymphocytes.
Tumor cell lines
PCI-1, PCI-6A and PCI-13 are HLA-A2+ human squamous cell carcinomas of the head and neck (HNSCC) cell lines established from a primary tumor and maintained in our laboratory at the University of Pittsburgh, as previously described [17]. All tumor cell lines were routinely tested and confirmed to be mycoplasma free.
Generation of immature dendritic cells (iDC) and isolation of CD4+CD25− T cells
Peripheral blood mononuclear cells (PBMCs) obtained from normal donors were tested for the HLA-A2 phenotype by flow cytometry, and only HLA-A2+ PBMC were used in this study. PBMC were isolated by centrifugation over Ficoll-Hypaque gradients. CD14+ monocytes were isolated via plastic adherence for 1 h at 37°C in an atmosphere of 5% CO2 in air, and the non-adherent PBMCs were collected as a T-lymphocyte fraction. Monocytes were cultured in a serum-free AIM V medium supplemented with 10 ng/ml rhIL-4 and 103 IU/ml rhGM-CSF at 37°C in an atmosphere of 5% CO2 in air for 7 days. Cells were harvested using TrypLE solution. Lymphocytes were washed twice with RPMI 1640 medium, counted and immediately used for magnetic cell separation (MACS). Briefly, CD4+CD25− T cells were purified by negative selection of CD4+ T cells followed by positive depletion of CD25+ T cells using the Regulatory T cell Isolation Kit on an AutoMACS system according to the manufacturers’ instructions.
In vitro assay (IVA) for Tr1 generation
MACS-isolated CD4+CD25− T cells (106) from PBMC of normal donors were co-incubated with 105 iDC and 105 tumor cells (previously irradiated with 15,000 rad to inhibit proliferation) in a complete medium (1 ml) in duplicate wells of 24-well plates for 10 days at 37°C in an atmosphere of 5% CO2 in air. IL-2 (10 IU/ml), IL-10 (20 IU/ml), IL-15 (20 IU/ml) and—where specified—rapamycin (1 nM) were added on day 0, 3 and 6. On day 9, culture medium was replaced by fresh medium, containing anti-CD3 mAb (1 μg/ml) and Brefeldin A (1 μg/ml). On day 10, lymphocytes and culture medium were separately harvested.
IVA for generation of reference cells
Human nTreg and activated CD4+ T cells were used for comparison with Tr1 cells. nTreg cells were cultured as previously described [34]. Briefly, MACS-isolated CD4+CD25+ T cells (106) from PBMC of normal donors were cultured with anti-CD3/anti-CD28 mAb-coated magnetic beads (T-cell expansion kit) in presence of 1,000 IU/ml IL-2 and rapamycin (1 nM). Activated CD4+ T cells were expanded starting with MACS-isolated CD4+CD25− T cells (106) from PBMC of normal donors by culture in the presence of 1,000 IU/ml IL-2. These two types of reference cells were cultured for 10 days in a complete medium, in wells of 24-well plates at 37°C in an atmosphere of 5% CO2 in air. Cells and supernatants were harvested on day 10 and used for phenotypic, functional and cytokine analyses.
Phenotypic characterization
Cells harvested from IVA or reference cultures (at least 200,000 cells/tube) were washed twice in staining buffer, consisting of PBS, containing 0.1% (w/v) BSA and 0.1% (w/v) NaN3. Cells were stained for flow cytometry as previously described [30]. Briefly, for surface staining, cells were incubated with the optimal dilution of each Ab for 25 min at 4°C in the dark, washed twice with staining buffer and finally fixed by resuspending them in 2% (v/v) paraformaldehyde (PFA) in PBS. The following Abs were used for surface staining: anti-CD3-ECD (TCR), anti-CD4-PC5 (Th1), anti-CD25-FITC and -PE (IL2Ranti-), anti-CD122-FITC (IL2Rβ), anti-CD132-PE (IL2Rγ). For intracellular staining, surface staining for T-cell markers was followed by two washes with staining buffer and by subsequent cell fixation in 2% (v/v) PFA for 10 min at RT in the dark. After another wash with the same buffer, PBMC were permeabilized by incubating them in saponin (0.1% v/v in PBS) for 30 min at 37°C in an atmosphere of 5% CO2 in air. Next, conjugated Abs were added to the cells. After 25 min incubation at 4°C in the dark, cells were washed again with 0.1% saponin, followed by another wash with staining buffer and fixation with 2% PFA in PBS. The following Abs were used for intracellular staining: anti-FoxP3-FITC, anti-CD152-PE (CTLA-4), anti-IL-10-PE (IL-10) and anti-IL-4-FITC (IL-4). CD4+CD25− T cells were stimulated with PMA (1 ng/ml) and ionomycin (1 μM) for 6 h in the presence of Brefeldin A (1 μg/ml) to serve as a positive control for intracellular staining. The cells were stored at 4°C in the dark until acquisition on the flow cytometer. The appropriate isotype controls were used in all experiments. All mAbs used were pre-titred on fresh PBMC to determine their optimal working dilutions.
Flow cytometry
Flow cytometry was performed using a FACScan flow cytometer (Beckman Coulter) equipped with Expo32 software (Beckman Coulter). The acquisition and analysis gates were initially restricted to the small lymphocyte gate as determined by the characteristic forward and side scatter properties of lymphocytes and collecting data on 105 cells. Further, analysis gates were restricted to the CD3+CD4+ T-cell subset. Data were analyzed using Coulter EXPO 32vl.2 analysis software.
CFSE-based suppression assay
Responder CD4+CD25− T cells (R) were MACS-isolated from PBMC, stained with 1.5 μM CFSE as previously described [34] and cultured in a complete AIM V medium in the presence of IL-2 (50 IU/ml) and anti-CD28 mAb (1 μg/ml) in wells of 96-well plates (5 × 105/well) coated with anti-CD3 mAb (1 μg/ml). T cells obtained from 10 day cultures in the presence of tumor, iDCs, and cytokines (IVA) or from reference cultures were plated at different ratios of T cells to autologous R (i.e., 1:1, 1:5, 1:10), and cultures were incubated for 5 days at 37°C in an atmosphere of 5% CO2 in air. Flow cytometry analysis was performed to evaluate suppression of proliferation of CFSE-labeled R as previously described [34]. All CFSE data were analyzed using the ModFit LT for Win32 software provided by Verity Software House, Inc., Topsham, ME, USA. The program quantifies the proliferation of R by evaluating the number of cell divisions. The percentages of suppression were calculated based on the proliferation index (PI) of R alone compared with the PI of cultures containing R + T effector cells. The PI is the sum of the cells in all generations divided by the computed number of original parental cells theoretically present at the start of the experiment. It is a measure of the increase in cell number in the culture during the course of an experiment.
To test the role of IL-10 in Tr1-mediated suppression, we added neutralizing anti-IL-10 mAb or the corresponding GAH-IgG to CFSE-based suppression assays. Following initial titration experiments, the anti-IL-10 mAb was added to the assays at the final concentration of 2 μg/ml. Cells were cultured and analyzed as described above.
In additional CFSE assays, responder CD4+CD25− T cells (R) were plated at 106 cells/well in wells of 24-well plates (Corning, Acton, MA, USA), coated with anti-CD3 mAb (1 μg/ml) equipped with 6.5 mm transwell inserts (pore size: 0.4 μm). Cells were cultured in complete AIM V medium in the presence of IL-2 (50 IU/ml) and anti-CD28 mAb (1 μg/ml) for 5 days at 37°C in an atmosphere of 5% CO2 in air. T cells obtained from 10 day IVA or from reference cultures were either plated in the bottom wells containing R or placed in the transwell insert at the 1:1 ratio. After the harvest, suppression of proliferation of CFSE-labeled R was analyzed using ModFit software as described above.
Tr1 cell expansion
For evaluation of Tr1 cell expansion levels, naïve T cells from normal donors were plated in duplicate wells and co-cultured with autologous iDCs and tumor cells as described above. T cells were harvested after 2, 4, 6, 8 and 10 days, washed in medium, counted in a 0.4% (v/v) trypan blue dye, and phenotyped by four-color flow cytometry. A part of the harvested cells was used for CFSE assays, and their suppressive function was evaluated after 5 days of co-incubation with CFSE-labeled autologous CD4+CD25− T responder cells.
ELISA
On day 9 of IVA co-cultures, supernatants (SN) were replaced by fresh complete medium (supplemented AIM V) containing anti-CD3 mAb (1 μg/ml) and Brefeldin A (1 μg/ml), but no exogenous cytokines. After 24 h SN was collected and stored frozen. SN in cultures of tumor cell lines were likewise replaced by fresh culture medium (supplemented DMEM), which was collected after 48 h and stored frozen. CD4+CD25− T cells were stimulated with PMA (1 ng/ml) and ionomycin (1 μM) in the presence of Brefeldin A (1 μg/ml), for 6 h and their SN served as controls. Levels of IL-4, IL-10, IL-12 and IFN-γ were determined by capture enzyme-linked immunosorbend assay (ELISA). Levels of TGF-β1 in acidified SN was also determined by ELISA. Nitric oxide was evaluated using the NO-detection EIA. Levels of IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IFN-γ, TNF-anti-, and GM-CSF were evaluated by Luminex technology, using a Human Cytokine 10-plex Ab bead kit. All assays have been performed according to manufacturers’ instructions.
Statistical analysis
All statistical analyses were performed using the Student’s t test, and P values < 0.05 were considered significant.
Results
Components of the model for Tr1 cell growth
The objective of this study was to establish growth conditions for a subset of CD4+ T cells, which produce IL-10 and suppress proliferation of autologous T cells. We aimed at simulating a tumor microenvironment in order to promote outgrowth of Tr1 cells [26]. Components selected for achieving their outgrowth included naive CD4+CD25− T cells obtained from PBMC of HLA-A2+ normal donors, cytokines (IL-2, IL-10, IL-15), autologous iDC to serve as antigen cross-presenting cells and irradiated human HLA-A2+ tumor cells as a source of antigen and tumor-derived soluble factors. All components were plated in duplicate wells of 24-well plates and cultured for up to 10 days.
To determine optimal experimental conditions for outgrowth of Tr1 cells, culture conditions were varied until consistent results were obtained that met the a priori criteria we established for the Tr1 cell phenotype and function. Expression of IL-10 in the majority of outgrowing cells and IL-10 levels in culture supernatant, suppressor function as well as the outgrowing phenotype were systematically tested during culture. The optimal ratio of CD4+CD25− T cells, iDCs and tumor cells was found to be 10:1:1. Cytokine concentrations that best supported Tr1 outgrowth were: IL-2 at 10 IU/ml, IL-10 at 20 IU/ml and IL-15 at 20 IU/ml. This cytokine cocktail is referred to as “Tr1 cytokines” from now on. In the presence of Tr1 cytokines, MACS-separated HLA-A2+ CD4+CD25− T cells (non-adherent), cultured in the presence of autologous iDCs (adherent) and irradiated HLA-A2+ tumor cells (adherent), proliferated vigorously, as confirmed by viable cell counts of the non-adherent fraction. Culture conditions which gave the highest cell number of CD4+CD25− cells on day 10 were thus established (Fig. 1a). As the CD4+ T cells expanded, their ability to express IL-10 and mediate suppression of proliferating autologous CD4+CD25− T cells in CFSE assays increased (Fig. 1b), whereas activated effector cells used as controls did not proliferate or suppress. The data in Fig. 1c show that the presence of tumor was necessary for enrichment of IL-10+ T cells in the co-cultures. In the absence of tumor cells, both the fold expansion and IL-10 expression in CD4+CD25− T cells were significantly (P < 0.01) decreased (Fig. 1a, c.3). We performed the Tr1 IVA with three different HNSCC cell lines. Comparable phenotypic and functional results for outgrowing cells were obtained with all three tumor cell lines. In the absence of iDC, both the fold expansion and the percent of IL-10+ cells were also decreased (Fig. 1a, c.4). It is important to note that in reference cultures, as represented by activated CD4+CD25− T cells expanding in the presence of 1,000 IU/ml of IL-2 (Fig. 1c.5) or nTreg expanding in the presence of rapamycin (R1) (Fig. 1c.6), IL-10+ CD4+ T cells were absent. Results obtained from experiments performed with PBMC of 15 different HLA-A2+ normal donors under the conditions, which were selected as optimal for in vitro generation of Tr1 cells, were consistently reproducible.
Fig. 1.

Components of the model for outgrowth of Tr1 cells. a Numbers of proliferating CD4+CD25− T cells co-cultured with tumor cells, iDC and cytokines used in different combinations. In this HLA-A2-restricted model, T cells and iDCs are autologous. Tumor is an HNSCC cell line, PCI-13. Cells were co-cultured for 10 days. Growth was determined after harvest by viable cell counts of the non-adherent fraction. The data are from three experiments performed with cells of different HLA-A2+ normal donors. b Kinetics of CD4+IL-10+ cell growth in culture and the development of their suppressor activity, analyzed by flow cytometry and in CFSE suppression assays, respectively. The data are mean values ± SD from five experiments performed with cells of two normal donors (solid line). Activated T cells were used as controls (broken line). c Phenotypic analysis of MACS-separated CD4+CD25− T cells (black bar) co-cultured in the IVA under different conditions. c.1 A profile of CD4+CD25− T cells prior to the IVA; c.2 phenotype of CD4+CD25− T cells proliferating under the conditions established and optimized for Tr1 cell expansion. c.3 In absence of tumor cells, the outgrowth of IL-10+CD4+ T cells was significantly decreased (P < 0.01); c.4 outgrowth of Tr1 cells in absence of iDCs; c.5 activated CD4+CD25− T cells (white bar) cultured in presence of IL-2 (1,000 IU/ml) for 10 days were used as reference culture; All data are mean % ± SD of CD4+CD25− cells expressing a given marker were obtained with cells of 15 normal donors. c.6 nTreg generated from MACS-separated CD4+CD25+ T cells (stripped bar) and cultured in the presence of IL-2 (1,000 IU/ml), rapamycin (1 nM), anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (1 μg/ml) for 10 days, were used as reference. The data obtained with nTreg reference cultures are mean % ± SD of positive cells from experiments with cells of five normal donors
Phenotype of Tr1 cells
Under the conditions established for Tr1 cell IVA, proliferating lymphocytes obtained from normal donors had the following phenotype: CD3+CD4+CD25−CD122+CD132+FoxP3+ CTLA-4+IL-10+IL-4− (Figs. 1c.2, 2a). Cultures of activated T cells served as reference cultures (Fig. 2a). Importantly, the generated CD3+CD4+ T cells were CD25− (IL2Rα-), although most expressed CD122 (IL2Rβ+) and CD132 (IL2Rγ+). In contrast, CD25 expression was strongly up-regulated on CD4+CD25− T cells expanding in presence of 1,000 IU/ml IL-2. Most cells in experimental cultures were FoxP3+ and expressed IL-10 (Fig. 2b). They were negative for intracellular IL-4 expression. The phenotype of the lymphocytes which outgrow in IVA is significantly distinct (P = 0.01) from that of activated CD4+ T cells (Fig. 1c.5) and of nTreg (Fig. 1c.6). Percentages of Tr1 cells expressing CTLA-4 or GITR (data not shown), the markers usually associated with the nTreg phenotype profile [29], were not higher than those in reference cultures (Fig. 1c.5).
Fig. 2.
Phenotypic characteristics of in vitro expanded Tr1 cells. a The phenotype of CD3+CD4+ lymphocytes proliferating under conditions established for the Tr1 IVA (black bar). Activated T cells, cultured in presence of IL-2 (1,000 IU/ml) for 10 days served as reference cultures (white bar). Cells were stained with mAb and evaluated by multi-color flow cytometry with gates set on CD3+CD4+. The data are mean % of positive cells ± SD from experiments with cells of 15 normal donors. Asterisks indicate significant differences (*P < 0.05 and **P < 0.01) between % positive cells in the IVA versus reference cultures; b flow cytometry histograms showing relative levels of expression of the selected markers on lymphocytes cultured in the Tr1 IVA for 10 days. Gates were set on CD3+CD4+ cells. The data obtained with T cells of one donor are representative for 15 different normal donors tested
Effects of rapamycin on Tr1 cell expansion
The immunosuppressive drug rapamycin (sirolimus) has been used for selective survival and expansion of nTreg cells in humans [34] and Tr1 cells in mice [10]. Therefore, rapamycin was added to the IVA culture system to study its effects on expansion of Tr1 cells. The cells with Tr1 phenotype outgrew to a higher extent in the presence of rapamycin, with CD122+ and IL-10+ T cells representing close to 100% of cultured cells (Fig. 3). Consistently, no cells expressing CD25 surface marker were observed in these cultures.
Fig. 3.
Phenotypic characteristics of Tr1 cells expanded in presence of rapamycin. a The phenotype of CD3+CD4+ lymphocytes proliferating in the presence of 1 nM rapamycin and under the conditions established for the Tr1 cell IVA (black bar). Activated T cells, cultured in presence of IL-2 (1,000 IU/ml) for 10 days served as reference cultures (white bar). b Flow cytometry dot blots showing relative expression of selected markers on lymphocytes cultured in the Tr1 IVA and in the presence of rapamycin for 10 days. Gates were set on CD3+CD4+ cells. The data are representative for cells of one donor out of 15 tested
Functional properties of Tr1 cells
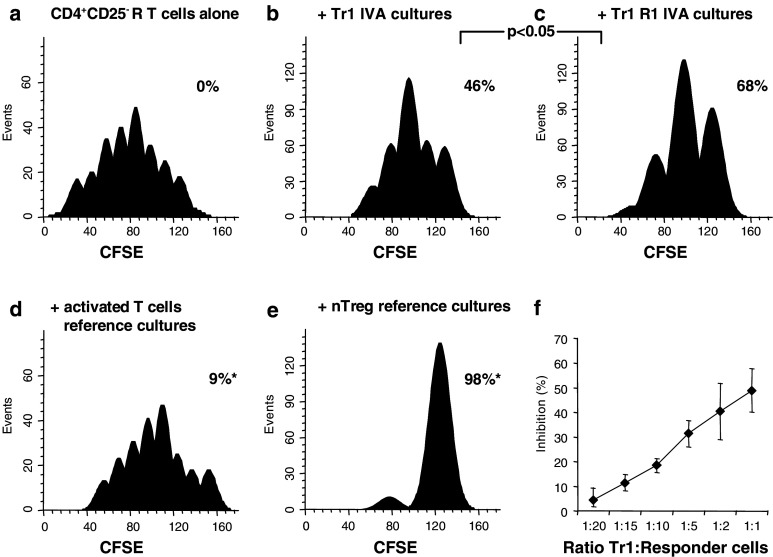
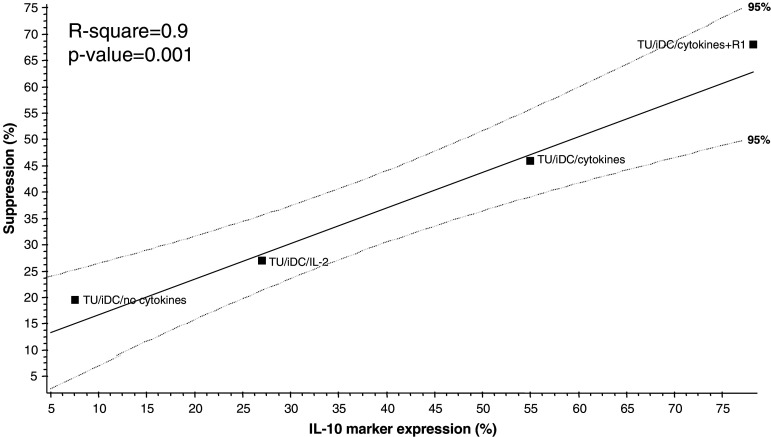
The ability to mediate suppression is one of the major characteristics of regulatory T cells. In the next series of experiments, lymphocytes from the IVA and reference cultures were co-incubated with autologous CD4+CD25− T responder (R) cells labeled with CFSE (1.5 μM) and activated with anti-CD3 and anti-CD28 mAbs. As shown in Fig. 4a, CFSE-labeled (R) cells proliferated well under these conditions. Lymphocytes from the Tr1 IVA co-incubated with autologous CFSE-labeled CD4+CD25− T cells suppressed proliferation of the CFSE-labeled (R) cells (Fig. 4b). A significantly enhanced suppression (P < 0.05) was observed, when Tr1 cells generated in the presence of rapamycin were added to CFSE-labeled (R) cells (Fig. 4c). In these cultures suppression of (R) by Tr1 cells increased linearly with increasing numbers of Tr1 cells (Fig. 4f) Activated T cells from reference cultures showed significantly lower (P < 0.0001) suppressor activity (Fig. 4d) than Tr1 cells. However, nTreg cells from reference cultures mediated potent suppression (Fig. 4e), as recently reported [34]. To establish a correlation between suppressor function of T cells generated in the co-cultures and their IL-10 expression, a linear regression model was used. It demonstrated a significant direct correlation (r 2 = 0.9) between increased percentages of cells expressing IL-10 and their ability to suppress proliferation of autologous CD4+CD25− cells, under various culture conditions applied in the model (Fig. 5).
Fig. 4.
Suppression of proliferation by Tr1 cells ± rapamycin. a Freshly MACS-purified CD4+CD25− responder (R) T cells were labeled with CFSE, stimulated with plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (1 μg/ml) mAb and cultured in the presence of 50 IU/ml IL-2 for 5 days. Cell proliferation of CFSE-labeled R cells were evaluated by flow cytometry and subsequent analysis using the ModFit software as described in Materials and methods. The histograms show CFSE intensity (X axis) and the number of events (Y axis). The acquisition gate was restricted to lymphocytes, as determined by characteristic forward and side scatter properties of these cells. Further, analysis gates were restricted to the CD3+CD4+ T-cell and CD4+CFSE+ T-cell subsets; b, c lymphocytes from the Tr1 IVA performed in the absence or presence of rapamycin (R1) were added at the start of the culture (ratio 1:1) and co-incubated with autologous CFSE-labeled R cells; d, e lymphocytes from the reference cultures (activated T cells or nTreg) were added at the start of the culture (ratio 1:1) and co-incubated with autologous CFSE-labeled R cells. The percentages of proliferation inhibition of CFSE labeled R cells alone or after additions of Tr1 cells are indicated in each panel. The results of one representative experiment out of 15 performed with cells of different donors are shown. f A representative experiment shows linearity of suppression depending on the number of Tr1 cells in co-cultures
Fig. 5.
Correlation between IL-10 expression by Tr1 cells and suppressor activity Correlation between suppressor function of Tr1 cells generated in the IVA model and their IL-10 expression was established using a linear regression model. Median percentages of IL-10+CD4+ T cells cultured under various conditions were plotted against their corresponding suppressor activity, determined as % suppression of proliferation of CFSE-labeled autologous CD4+CD25− responder T cells. Medians were calculated from up to 15 unique experiments
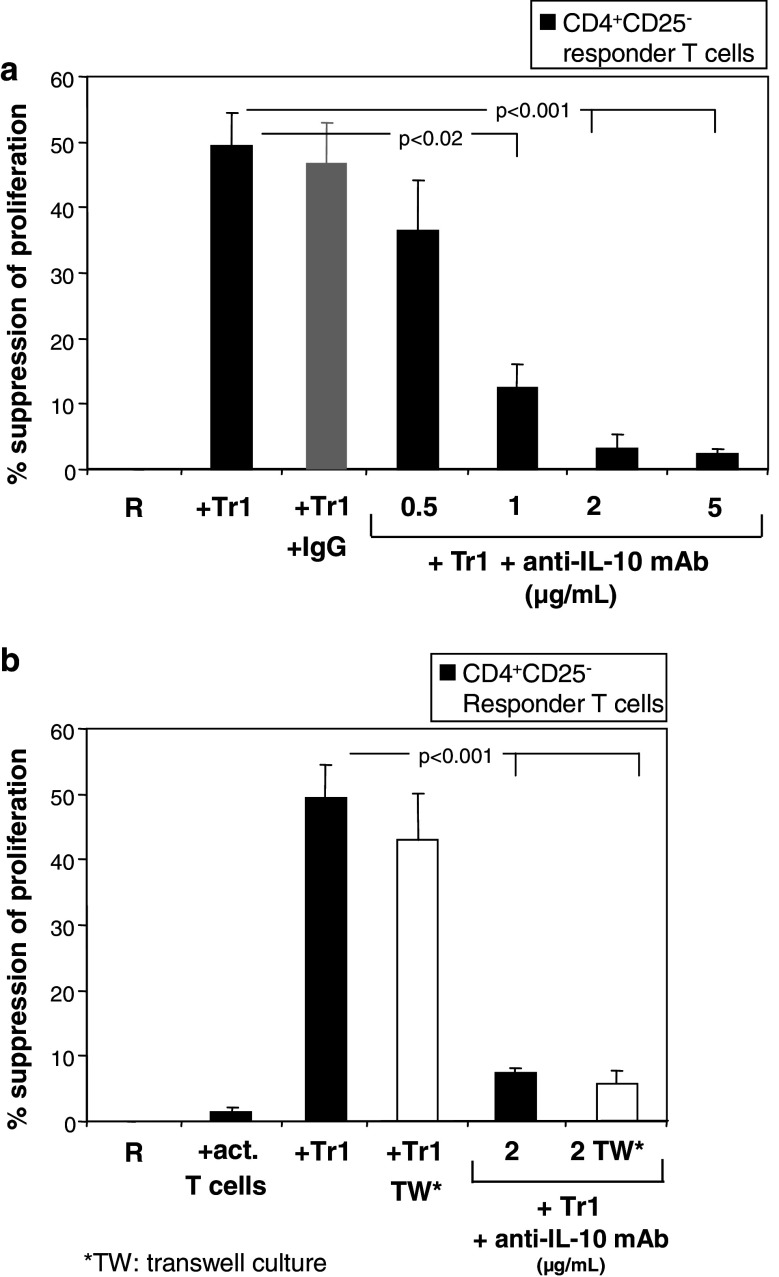
Neutralizing anti-IL-10 mAb was added to the co-cultures to evaluate the impact of IL-10 expressed and presumably secreted by Tr1 cells on their suppressor activity. As shown in Fig. 6a anti-IL-10 mAb neutralized suppression of proliferation of naïve CD4+CD25− T cells in response to immobilized anti-CD3 mAb and soluble anti-CD28 mAb in a dose-dependent manner. Furthermore, we co-cultured R cells with Tr1 cells separated by transwell inserts (Fig. 6b). Suppression levels of R proliferation remained unchanged when R and Tr1 cells were separated by the transwell insert, indicating that suppression was mediated by a soluble factor independent on cell-to-cell contact. Upon the addition of neutralizing anti-IL-10 mAb (2 μg/ml) to these cultures suppressor activity was inhibited in the presence or absence of the transwell inserts.
Fig. 6.
Tr1 cells mediate suppression by IL-10 secretion. a Tr1 cells generated in IVA were tested for their ability to suppress anti-CD3/CD28 mAbs-induced proliferation of autologous CD4+CD25− T cells in the presence or absence of neutralizing anti-IL-10 mAb (2 μg/ml) (black bar) or control (GAH-IgG: gray bar); b Tr1 cells separated from (R) cells in a transwell system inhibited proliferation of the (R) cells at the levels observed in unseparated co-cultures. Upon the addition of anti-IL-10 mAb to theses cultures almost completely abolished Tr1−mediated suppression (P < 0.001). The results are mean % of suppression ± SD obtained in three experiments with cells of three different normal donors
Supernatants of Tr1 cells
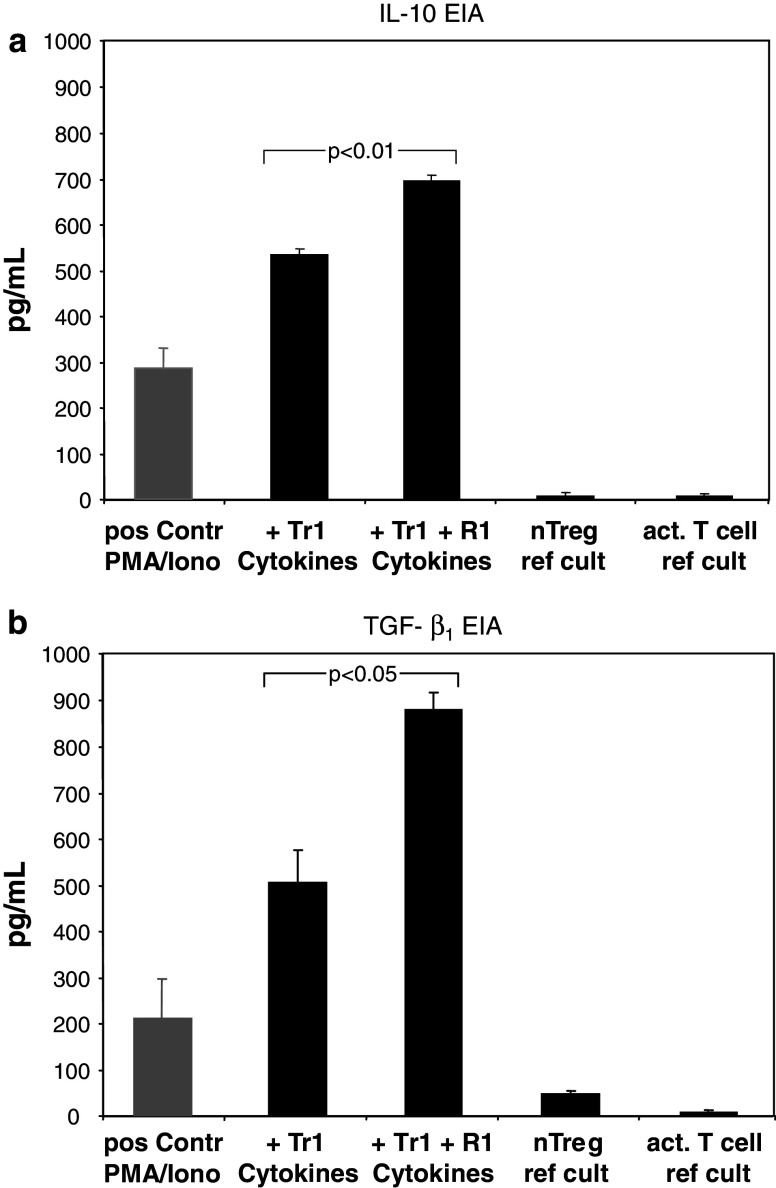
SN from the IVA were collected on day 10 of co-culture to determine levels of the following cytokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10 IL-12, TGF-β1, IFN-γ, TNF-anti-, GM-CSF and NO. The IVA cultures supplemented with Tr1 cytokines for 10 days contained high levels of endogenous IL-10 and TGF-β1, but were negative or contained only very low levels of all the other cytokines tested and, most notably, were negative for IL-2, IL-4 and IFN-γ. The presence of rapamycin significantly enhanced production of IL-10 and TGF-β1 (Fig. 7a, b). SN from reference cultures were negative for IL-10 and TGF-β1 and contained low to intermediate levels of some of the other cytokines tested (e.g., IL-2, IL-4, IFN-γ; data not shown).
Fig. 7.
IL-10 and TGF-β1 levels in culture supernatants. Supernatants (SN) from different IVA cultures were collected on day 10 as described in Materials and methods and tested for cytokine levels by EIA; a IL-10 was not detectable in SN from reference cultures. SN from the Tr1 IVA ± rapamycin (R1) were positive for IL-10; b TGF-β1 was undetectable/low in acidified SN from T cells obtained from IL-2 activated or nTreg reference cultures. SN from the Tr1 IVA ± R1 contained high levels of TGF-β1
The tumor cell lines cultured alone (PCI-1, PCI-6A and PCI-13) were also tested for the production of cytokines, but were negative for IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, GM-CSF, INF-γ, NO, TNF-anti- and TGF-β1 (data not shown).
Discussion
Naive CD4+ T cells from peripheral blood of normal donors can be differentiated in vitro into Th1 or Th2 cells [2]. In addition, they may also contain Tr1 cells. Similar to Th1 and Th2, the differentiation of Tr1 cells is regulated by cytokines, although little is known about conditions required for Tr1 cell generation or their phenotypic and functional characteristics. In order to induce and expand Tr1 cells, an in vitro model was established. Isolated CD4+CD25− T cells from HLA-A2+ normal donors plus autologous iDCs and HLA-A2-matched tumor cells were co-cultured in the presence of a cytokine combination consisting of low doses of IL-2, IL-10 and IL-15. Under optimal conditions expansion of the cell subset with a unique phenotype (CD3+CD4+CD25−IL2Rβ+IL2Rγ+FoxP3+CTLA-4+IL-10+) and strong suppressor function was observed in this model. This T cell subset significantly and consistently inhibited proliferation of autologous responder CD4+CD25− T cells (P < 0.01). This suppression was mediated by IL-10 secreted by Tr1 cells and independent on cell-to-cell contact. Thus, the cells generated in these conditions had attributes previously described for Tr1 cells [28].
Characteristics identifying Tr1 cells are based on the cytokine profile associated with suppressive functions [9]. These cells secrete IL-10 and TGF-β or IL-5, but no Th1- or Th2-type cytokines. They mediate suppression in vitro and in vivo [3, 16]. IL-10 secretion has been proposed as the hallmark of Tr1 cells [9]. IL-10 is an anti-inflammatory cytokine, which inhibits cytokine production and proliferation of CD4+ T cells or T-cell clones. It also had downregulatory effects on APC functions [13]. In the IVA, IL-10-producing CD4+ T cells were induced as confirmed by flow cytometry, but it is possible that IL-10 also originated from iDCs or tumor cells present in culture, as recently reported [40]. No cells expressing intracellular IL-4 were detected in IVA cultures, and soluble IL-4, IL-2 or INF-γ were not detected in the supernatants, suggesting that this IVA system induced a Tr1 response rather than Th1 or Th2 responses [16]. Moreover, these supernatants contained high levels of TGF-β1, supporting the role of TGF-β1 in T-cell immunoregulation, as reported in the current literature [39].
Regulatory activity manifested as the ability to suppress T-cell proliferation is another major characteristic of Tr1 cells [9]. The lymphocytes generated in the IVA system suppressed autologous T-cell proliferation upon polyclonal activation. Importantly, we observed a direct correlation between the percentage of IL-10-expressing T cells and suppressor function mediated by Tr1 cells. Furthermore, suppressor activity of Tr1 cells was completely inhibited in the presence of neutralizing anti-IL-10 mAb. This is in agreement with other studies, which consider IL-10 as the key cytokine utilized by Tr1 cells to mediate suppression [23]. Transwell suppression assays confirmed a cell-contact independent activity of Tr1 cells, as also indicated earlier [28].
Controversy exists regarding the phenotypic characteristics of Tr1 cells. While nTregs are identified by a defined marker expression profile [6], including constitutively expressed CD25, FoxP3, a transcription factor associated with suppressor function [29], CTLA-4, CD62L and CD45RO, all expressed with a high density, the Tr1 subset remains poorly defined. Reports describing the Tr1 phenotype are inconsistent, perhaps because of different experimental conditions used for culture [2, 20, 22, 23, 33]. In our study, the Tr1 cellular marker profile differed significantly from that of nTreg, in support of the current view that these regulatory T cells represent distinct lymphocyte subsets rather than the same subset at different states of activation [28]. The Tr1 phenotypic profile was also significantly distinct from that of activated T cells. Besides moderate upregulation of common activation markers like CTLA-4 and GITR (not shown), the T cells outgrowing in the IVA cultures were consistently highly positive for IL2Rβ and IL2Rγ, as also reported by others [4]. In contrast, the IL2Rα- was largely absent on the surface of these cells. The absence of CD25 expression upon antigen or polyclonal stimulation in Tr1 cell populations may be the major discriminating feature between Tr1 cells and nTreg. It suggests that these two regulatory cell subsets are differentially responsive to IL-2. The absence of CD25 with upregulation of CD122 and CD132 could be critical for Tr1 differentiation and function, as the β (CD122) and γ (CD132) receptor chains are essential for ligand internalization, signal transduction and T-cell development as well as differentiation [35]. The upregulated expression of the IL2Rβ and -γ chains on Tr1 cells suggests that in addition to IL-2, these cells are able to respond to other growth factors, i.e., IL-10 (via the γc-chain), IL-15 (via β- and γc-chains [15]), and furthermore IL-7 (via the γc-chain) [24]. Moreover, knock-out of the IL2Rβ chain in mice resulted in severe autoimmunity [36] as mutations in the human IL2Rγ chain led to severe combined immunodeficiency [19], suggesting an important role for the IL2R subunits β and γ for signaling in Tr1 cell regulation of responses to self.
To test effects of the immunosuppressive drug, rapamycin, on selective expansion of regulatory T-cells, as was previously shown with Tr1 cells in mice [10] or CD4+CD25+ nTreg in humans [34], we compared the fold expansion, phenotype and functions of Tr1 cells in IVA cultures ± rapamycin. Our data showed significant outgrowth of Tr1 cells, when cytokines, IL-2, IL-10 and IL-15, were combined with 1 nM rapamycin. Rapamycin has been reported to block intracellular signaling in response to T-cell growth factors, e.g. IL-2, which is required for the progression of activated T cells from the G1 to S phase [1], but molecular mechanisms by which it selectively promotes expansion of regulatory T cells are yet to be defined. We hypothesize that rapamycin inhibits proliferation by induction of apoptosis activated CD4+ T cells and thereby enables Tr1 cells to outgrow and thus selectively survive in the IVA [34].
The model we established mimics the tumor microenvironment, in which interactions of TAA, infiltrating leukocytes and tumor- or stromal cell-derived soluble factors favor the induction of Tr1 cells with suppressive activity from naive CD4+ T-cell precursors. These regulatory T cells subsequently impair antitumor immune responses, contribute to tumor progression and may mediate systemic immunosuppression. Upon validation, such an in vitro Tr1 model is likely to be useful to better define the role of the tumor microenvironment in orchestrating cellular and molecular mechanisms which control immune responses. Improved understanding of these mechanisms is essential for future control of Tr1 cell expansion in cancer and their contributions to tumor escape.
Acknowledgments
Research described in this article was supported in part by Philip Morris USA, Inc. and Philip Morris International (SL, RZ and TLW) and by the NIH grant PO-1 DE12321 (TLW).
References
- 1.Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- 2.Assenmacher M, Lohning M, Scheffold A, Richter A, Miltenyi S, Schmitz J, Radbruch A. Commitment of individual Th1-like lymphocytes to expression of IFN-gamma versus IL-4 and IL-10: selective induction of IL-10 by sequential stimulation of naive Th cells with IL-12 and IL-4. J Immunol. 1998;161:2825–2832. [PubMed] [Google Scholar]
- 3.Bacchetta R, Bigler M, Touraine JL, Parkman R, Tovo PA, Abrams J, de Waal Malefyt R, de Vries JE, Roncarolo MG. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J Exp Med. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacchetta R, Sartirana C, Levings MK, Bordignon C, Narula S, Roncarolo MG. Growth and expansion of human T regulatory type 1 cells are independent from TCR activation but require exogenous cytokines. Eur J Immunol. 2002;32:2237–2245. doi: 10.1002/1521-4141(200208)32:8<2237::AID-IMMU2237>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J Exp Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baecher-Allan C, Anderson DE. Regulatory cells and human cancer. Semin Cancer Biol. 2006;16:98–105. doi: 10.1016/j.semcancer.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Barchet W, Price JD, Cella M, Colonna M, MacMillan SK, Cobb JP, Thompson PA, Murphy KM, Atkinson JP, Kemper C. Complement-induced regulatory T cells suppress T-cell responses but allow for dendritic-cell maturation. Blood. 2006;107:1497–1504. doi: 10.1182/blood-2005-07-2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrat FJ, Cua DJ, Boonstra A, Richards DF, Crain C, Savelkoul HF, de Waal-Malefyt R, Coffman RL, Hawrylowicz CM, O’Garra A. In vitro generation of interleukin 10-producing regulatory CD4 (+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Battaglia M, Gregori S, Bacchetta R, Roncarolo MG. Tr1 cells: from discovery to their clinical application. Semin Immunol. 2006;18:120–127. doi: 10.1016/j.smim.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Battaglia M, Stabilini A, Draghici E, Gregori S, Mocchetti C, Bonifacio E, Roncarolo MG. Rapamycin and interleukin-10 treatment induces T regulatory type 1 cells that mediate antigen-specific transplantation tolerance. Diabetes. 2006;55:40–49. doi: 10.2337/diabetes.55.01.06.db05-0613. [DOI] [PubMed] [Google Scholar]
- 11.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 12.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 13.de Waal Malefyt R, Haanen J, Spits H, Roncarolo MG, te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, de Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991;174:915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giri JG, Anderson DM, Kumaki S, Park LS, Grabstein KH, Cosman D. IL-15, a novel T cell growth factor that shares activities and receptor components with IL-2. J Leukoc Biol. 1995;57:763–766. doi: 10.1002/jlb.57.5.763. [DOI] [PubMed] [Google Scholar]
- 16.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 17.Heo DS, Snyderman C, Gollin SM, Pan S, Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB, Whiteside TL. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–5175. [PubMed] [Google Scholar]
- 18.Inobe J, Slavin AJ, Komagata Y, Chen Y, Liu L, Weiner HL. IL-4 is a differentiation factor for transforming growth factor-beta secreting Th3 cells and oral administration of IL-4 enhances oral tolerance in experimental allergic encephalomyelitis. Eur J Immunol. 1998;28:2780–2790. doi: 10.1002/(SICI)1521-4141(199809)28:09<2780::AID-IMMU2780>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 20.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4 (+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levings MK, Sangregorio R, Galbiati F, Squadrone S, de Waal Malefyt R, Roncarolo MG. IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J Immunol. 2001;166:5530–5539. doi: 10.4049/jimmunol.166.9.5530. [DOI] [PubMed] [Google Scholar]
- 22.Levings MK, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo MG. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 23.Levings MK, Roncarolo MG. Phenotypic and functional differences between human CD4+CD25+ and type 1 regulatory T cells. Curr Top Microbiol Immunol. 2005;293:303–326. doi: 10.1007/3-540-27702-1_14. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 26.Mocellin S, Marincola FM, Young HA. Interleukin-10 and the immune response against cancer: a counterpoint. J Leukoc Biol. 2005;78:1043–1051. doi: 10.1189/jlb.0705358. [DOI] [PubMed] [Google Scholar]
- 27.Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976;193:1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- 28.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 29.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3CD25CD4 natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer. 2005;92:913–920. doi: 10.1038/sj.bjc.6602407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shevach EM. Fatal attraction: tumors beckon regulatory T cells. Nat Med. 2004;10:900–901. doi: 10.1038/nm0904-900. [DOI] [PubMed] [Google Scholar]
- 32.Stassen M, Schmitt E, Jonuleit H. Human CD(4+)CD(25+) regulatory T cells and infectious tolerance. Transplantation. 2004;77:S23–S25. doi: 10.1097/00007890-200401151-00009. [DOI] [PubMed] [Google Scholar]
- 33.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–4780. [PubMed] [Google Scholar]
- 34.Strauss L, Whiteside TL, Knights A, Bergmann C, Knuth A, Zippelius A. Selective in vitro survival of naturally occuring human CD4+CD25+FOXP3+ regulatory T cells with rapamycin. J Immunol. 2007;178(1):320–329. doi: 10.4049/jimmunol.178.1.320. [DOI] [PubMed] [Google Scholar]
- 35.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJ, Ohashi PS, Griesser H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 37.Whiteside TL. Immune suppression in cancer: effects on immune cells, mechanisms and future therapeutic intervention. Semin Cancer Biol. 2006;16:3–15. doi: 10.1016/j.semcancer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Woo EY, Chu CS, Goletz TJ, Schlienger K, Yeh H, Coukos G, Rubin SC, Kaiser LR, June CH. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–4772. [PubMed] [Google Scholar]
- 39.You S, Thieblemont N, Alyanakian MA, Bach JF, Chatenoud L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol Rev. 2006;212:185–202. doi: 10.1111/j.0105-2896.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 40.Zhang M, Tang H, Guo Z, An H, Zhu X, Song W, Guo J, Huang X, Chen T, Wang J, Cao X. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]