Abstract
Circulating T lymphocytes enter a tissue if they express appropriate chemokine receptors and adhesion molecules to engage ligands presented at this site. To aid rational development of T cell-based therapies for Hodgkin’s lymphoma (HL), we have assessed the expression and function of homing receptors on tumour-infiltrating T cells in HL and compared them with T cells from unaffected lymph nodes and colorectal cancer tissue. Chemokine receptors CXCR3, CXCR4 and CCR7 were expressed on a large proportion of T cells within HL tissue and mediated chemotaxis to purified chemokine. The corresponding ligands (CXCL10, CXCL12, CCL21) were expressed on the malignant cells and/or vascular endothelium. Adhesion molecules including CD62L were widely expressed on HL-derived T cells and their corresponding ligands were detected on vessels within the tumour. This homing phenotype was distinct from T cells isolated from colorectal cancer, but matched closely the phenotype of T cells from unaffected lymph nodes. Thus, T cell recruitment to HL resembles entry of naïve/central memory T cells into normal lymph nodes. This has important implications for current approaches to treat HL using T cells activated and expanded in vitro that lack CCR7 and CD62L expression.
Keywords: Chemokines, Hodgkin’s lymphoma, Tumour Immunity, T cell homing
Introduction
Eliciting or boosting T cell responses to tumour-associated antigens through vaccination or infusion of in vitro expanded T cells can mediate objective clinical responses in cancer patients [3, 25, 29]. In particular, infusing Epstein Barr Virus (EBV)-specific T cells appears to be effective treatment for EBV-positive post-transplant lymphoproliferative disease [6, 10, 16, 17]. Consequently, there is considerable interest in developing a T cell-based therapy for other EBV positive tumours including Hodgkin’s lymphoma (HL) where 30–40% of cases carry the virus in the malignant Hodgkin Reed-Sternberg (H-RS) cells [4]. The first attempt to treat relapsed HL with infusions of EBV-specific T cell lines reported 2/11 patients experiencing a complete response [1]. T cells might also be used to treat EBV-negative HL since MAGE A4, a known target for cytotoxic T cells, is selectively expressed in H-RS cells [2] and screening a panel of randomly selected EBV-negative HL cases from the UK, 16/42 (38%) expressed MAGE A4 (P.M. and S.P.L., unpublished data).
A prerequisite for T cell-based therapies is that effector cells must be capable of entering the tumour tissue. However, the mechanisms that direct such T cells into tumours are poorly understood. Within HL, the bulk of the tumour comprises a reactive infiltrate of non-malignant CD4 + and CD8 + T lymphocytes along with eosinophils, macrophages, neutrophils and plasma cells [7]; the malignant H-RS cells are <2% of the tumour mass. Although the T cell infiltrate may represent a specific immune response against the malignant cells, there is as yet no evidence for this. Nevertheless, the presence of T cells within HL allows characterisation of the homing molecules that directed their recruitment.
Before a T cell can enter tissue it must first recognise and bind to molecules expressed by endothelial cells lining the vessels in that tissue. The initial step in this process involves transient, tethering interactions (classically mediated by selectins) that induce lymphocytes to roll along the vascular endothelium. The T cell can then interact with chemokines presented by the endothelial glycocalyx [18]. Upon binding to specific G-protein coupled receptors chemokines promote the arrest of rolling lymphocytes by activating stable integrin-mediated adhesion to ligands expressed on the endothelium. The cell can then undergo transendothelial migration into tissue, a process regulated by chemokines and adhesion molecules expressed at the endothelial cell-cell junction. A complex chemokine network then directs the localisation and retention of the T cell within that tissue. Thus lymphocytes will only be recruited to a tissue if they express the appropriate receptors to allow them to respond to adhesion molecules and chemokines present at this site.
HL generally presents within lymph nodes which are structures designed to recruit lymphocytes from blood facilitating interaction with antigen-presenting cells. The regulation of cellular trafficking to lymph nodes has been well characterised [27]. However, the pathogenesis of HL involves disruption of the lymph node architecture and dysregulation of cytokine production. This suggests that lymphocyte recruitment to HL may not recapitulate that observed within unaffected nodes. Therefore, to aid the development of effective T cell-based therapies for HL, we sought to determine the mechanisms whereby T cells are recruited to this tumour.
Methods
Tumour infiltrating and peripheral blood lymphocytes
Tumour biopsy samples were obtained from 15 newly diagnosed HL patients (see Table 1). Fresh biopsy material was rinsed with RPMI 1640 (Life Technologies) to remove traces of blood, then disaggregated using a scalpel or Medimachine (Dako, UK). Tumour infiltrating lymphocytes (TIL) were isolated by density gradient centrifugation on lymphoprep (Robbins Scientific, UK) either before or after cryopreservation. In the same way, infiltrating cells were prepared from tumour biopsies from five newly diagnosed colorectal cancer (CRC) patients and from five non-involved lymph nodes with no evidence of hyperplasia (isolated from three CRC patients and two liver donors). Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation on lymphoprep of blood from five additional HL patients and five age/sex-matched healthy donors. See Table 1 for further details on all these donors. Ethical approval was granted by the South Birmingham Research Ethics Committee, UK, and by the Scottish Multi-Centre Research Ethics Committee.
Table 1.
Details of donors analysed for T cell homing phenotype
| Sex | Age | Disease stage | HL subtype | EBV status of tumour | |
|---|---|---|---|---|---|
| HL TILs | |||||
| HL1 | F | 16 | nk | Nodular sclerosis | Negative |
| HL2 | F | 17 | 4a | Nodular sclerosis | Negative |
| HL3 | F | 19 | nk | Mixed cellularity | Negative |
| HL4 | F | 20 | 2a | Nodular sclerosis | Negative |
| HL5 | F | 33 | nk | Nodular sclerosis | Negative |
| HL6 | F | 40 | 2 | Nodular sclerosis | Negative |
| HL7 | F | 46 | nk | Nodular sclerosis | Negative |
| HL8 | F | 76 | 3a | Nodular sclerosis | Positive |
| HL9 | M | 8 | 3b | Mixed cellularity | Positive |
| HL10 | M | 14 | nk | Nodular sclerosis | Negative |
| HL11 | M | 27 | 2b | Nodular sclerosis | nk |
| HL12 | M | 29 | 3a | Nodular sclerosis | Negative |
| HL13 | M | 30 | 4b | Nodular sclerosis | Negative |
| HL14 | M | 37 | 1b | Mixed cellularity | Negative |
| HL15 | M | 51 | 2a | Nodular sclerosis | Positive |
| Uninvolved LNs | |||||
| LN1 (Liver donor) | F | 31 | n/a | n/a | n/a |
| LN2 (CRC patient) | F | 97 | n/a | n/a | n/a |
| LN3 (Liver donor) | M | 47 | n/a | n/a | n/a |
| LN4 (CRC patient) | M | 68 | n/a | n/a | n/a |
| LN5 (CRC patient) | M | 72 | n/a | n/a | n/a |
| HL PBMCs | |||||
| HL PBMC1 | F | 28 | 2a | Mixed cellularity | Negative |
| HL PBMC2 | F | 35 | 3b | Nodular sclerosis | Negative |
| HL PBMC3 | F | 52 | 1b | Nodular sclerosis | Negative |
| HL PBMC4 | M | 27 | 2a | Nodular sclerosis | Negative |
| HL PBMC5 | M | 32 | 2a | Nodular sclerosis | Negative |
| Healthy PBMCs | |||||
| Healthy PBMC1 | F | 27 | n/a | n/a | n/a |
| Healthy PBMC2 | F | 38 | n/a | n/a | n/a |
| Healthy PBMC3 | F | 51 | n/a | n/a | n/a |
| Healthy PBMC4 | M | 28 | n/a | n/a | n/a |
| Healthy PBMC5 | M | 33 | n/a | n/a | n/a |
| CRC TILs | |||||
| CRC1 | F | 76 | Dukes C | n/a | n/a |
| CRC2 | M | 53 | Dukes C | n/a | n/a |
| CRC3 | M | 67 | Dukes C | n/a | n/a |
| CRC4 | M | 70 | Dukes C | n/a | n/a |
| CRC5 | M | 80 | Dukes B | n/a | n/a |
nk not known; n/a not applicable
Tissue samples
Formaldehyde-fixed, paraffin-embedded tissue from nine HL cases (four nodular sclerosing, NS, five mixed cellularity, MC) were obtained from University Hospital, Birmingham, UK. Three cases were EBV positive (all MC) and the remainder were EBV negative. A further eight snap frozen HL tissues (six NS, two MC) were obtained from Birmingham Heartlands Hospital, UK. One MC case was EBV positive, three NS cases were EBV negative and four were not tested.
Flow cytometry
Mononuclear cells derived from tissue or blood samples were incubated with pretitred concentrations of chemokine and adhesion receptor-specific antibodies, or isotype-matched controls, diluted in buffer (PBS + 2% v/v FCS). Cells were washed in buffer and stained with biotinylated anti-mouse or anti-rat IgG, then with streptavidin Alexa 488 (Cambridge Biosciences, UK). Cells were washed and incubated with normal mouse serum (Dako, UK) on ice for 20 min. Cells were then stained with saturating concentrations of anti-CD3 (PE-Cy5-conjugated) and anti-CD4 (phycoerythrin-conjugated) antibodies (Coulter, UK). All incubations with antibodies were conducted on ice for 30 min. After washing, cells were analysed on an Epics XL Flow Cytometer (Beckman Coulter) and data analysed with WinMDI 2.8 (J. Trotter, Scripps Institute, San Diego).
Chemokine receptor-specific antibodies used were as follows: CCR1 (clone 53504.111), CCR2 (48607.121), CCR3 (61828.111), CCR6 (53103.111), CXCR4 (44716.111), CXCR5 (51505.111), CXCR6 (56811.111) (R&D systems, UK); CCR4 (1G1), CCR5 (2D7), CCR7 (3D12), CXCR3 (1C6) (Pharmingen, UK). Adhesion receptor-specific antibodies used were as follows: LFA-1 (HI111), PSGL-1 (KPL-1), CLA (HECA-452), L-selectin (DREG-56) (Pharmingen) and VLA-4 (P4G9) (Abcam).
Immunohistochemistry
Formalin-fixed tissue
Paraffin-embedded sections (5 μm) were mounted onto vectorbond-coated slides (Vector Laboratories, Peterborough, UK), deparaffinised in xylene, cleared in 100% ethanol and washed briefly in tap water. Endogenous peroxidase was blocked with 3% hydrogen peroxide in methanol for 10 min. Antigen retrieval was achieved by boiling slides for 30 min in a microwave (800 W) in 10 mM citrate buffer (pH 5.8). Sections were rehydrated in PBS and incubated with anti-chemokine or anti-adhesion receptor antibodies overnight at 4°C in PBS. Non-specific staining was assessed in all cases using isotype-matched antibody controls (Dako). After washing in PBS, bound antibody specific for CXCL12 (79018, 10 μg/ml), ICAM-1 (BBA17, 5 μg/ml), VCAM-1 (BBA19, 1/800) (R&D systems) and PNAd (MECA-79, 5 μg/ml) (Pharmingen), was detected using an ABC immunoperoxidase method (Universal vectastain Elite ABC kit, Vector Laboratories). Antibodies specific for CXCL10 (AF-266-NA, 5 μg/ml), CXCL13 (AF801, 1 μg/ml) and CCL21 (AF366, 1 μg/ml) (R&D systems) were detected using peroxidase-conjugated rabbit anti-goat immunoglobulin followed by peroxidase-conjugated goat anti-rabbit immunoglobulin (Dako). In all cases, bound antibody was visualised with the 3,3 diaminobenzidine-based detection reaction (Sigma). Images were recorded digitally using brightfield microscopy and a Nikon Coolpix camera.
Frozen tissue
Sections (5 μm) were fixed in acetone for 10 min, rehydrated with PBS and incubated overnight at 4°C with anti-CXCR3 (1C6, 20 μg/ml) (Pharmingen), anti-CXCR5 (51505.111, 10 μg/ml) (R&D systems), anti-VAP-1 (1B2, 5 μg/ml) (gift from Drs. M. Salmi and S. Jalkanen, Turku University, Finland) or isotype-matched antibody controls (Dako). Bound antibody was detected using biotinylated goat anti-mouse IgG1 or IgG2b followed by streptavidin Alexa 594 (Cambridge Biosciences). Sections stained for CXCR3 and CXCR5 were subsequently blocked with PBS + 20% (v/v) mouse serum (Dako), then incubated at room temperature for 45 min with anti-CD30 FITC (Ber-H2, 1/50) and anti-CD3 (A0452, 1/50) (Dako). Anti-CD30 and anti-CD3 reagents were visualised with anti-fluorescein Alexa 488 and anti-rabbit Alexa 350, respectively (Cambridge Biosciences). Sections stained for VAP-1 were blocked with PBS + 20% (v/v) mouse serum and incubated at room temperature for 45 min with anti-CD31 FITC (B-B38, 1/50). Images were recorded using confocal microscopy (Zeiss LSM510).
Chemotaxis assays
Cryopreserved cells were thawed, washed twice in RPMI 1640 and resuspended at 106 cells/ml in RPMI 1640 containing 0.5% (w/v) BSA (Sigma) (chemotaxis media). Cells (105) were added to the top chamber of a transwell filter (6.5 mm diameter, 3 μm pore size) (Appleton Woods, UK) and 600 μl of chemotaxis media containing chemokine (prewarmed to 37°C) added to the lower chamber. Transwells were incubated at 37°C with 5% CO2 for 4 h. A 400 μl sample of medium was removed from the lower chamber and migrated cells counted by flow cytometry. Background migration was assessed using chemotaxis media alone as a stimulus. Chemotactic index values were calculated by dividing the number of cells migrating in response to specific chemokine by the number of cells migrating in medium alone. Recombinant human chemokines (R&D systems) were used at pretitred concentrations to achieve maximal migration of lymphocytes.
Results
Chemokine and adhesion receptor expression on T cells infiltrating HL
Flow cytometric analysis
To determine which homing receptors are important for the recruitment of T cells to HL we assessed expression of a wide range of chemokine receptors and adhesion molecules on TIL from HL. Using 3-colour flow cytometry, antibodies against homing markers were used together with anti-CD3 and anti-CD4 antibodies. CD3+ CD4− staining was used as a surrogate marker for CD8+ T cells. Figure 1 shows the mean percentage of cells within the CD3+, CD3+/CD4+ and CD3+/CD4− populations that expressed each of the homing receptors analysed. For comparison, homing receptor expression was analysed on T cells isolated from unaffected lymph nodes, circulating T cells from HL patients and age/sex-matched healthy donors, and T cells infiltrating a non-lymphoid tumour, colorectal carcinoma.
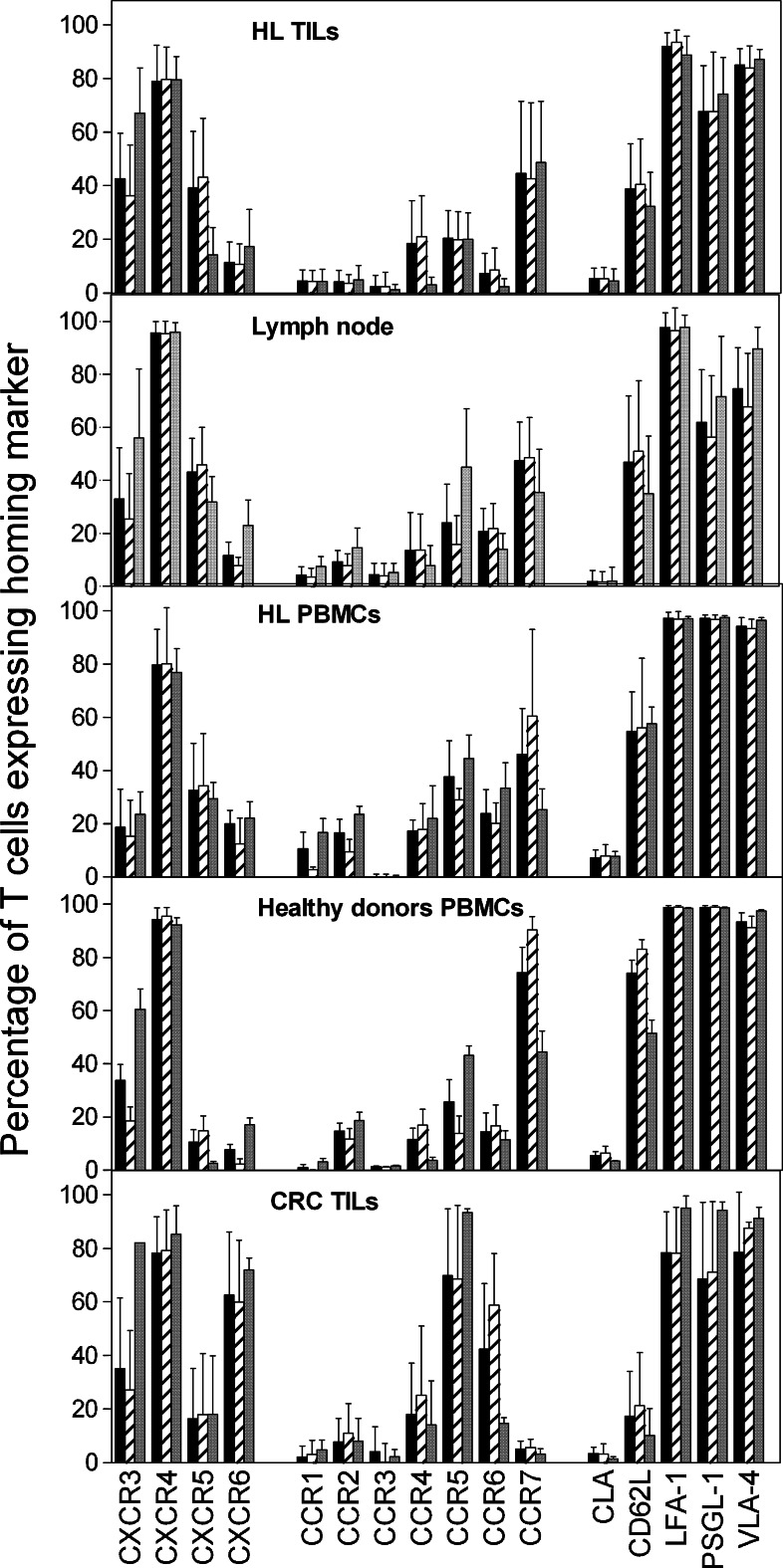
Fig. 1.
Homing receptors expressed on tissue-infiltrating T lymphocytes. Three colour flow cytometry was used to determine the proportion of CD3+ (filled square), CD3+/CD4+ (striped square) and CD3+/CD4− (grey shaded square) T cells derived from HL tumour that express the chemokine and adhesion receptors indicated. The number of HL cases analysed for each chemokine receptor were as follows: CXCR3, CXCR4, CXCR6 and CCR7 (n = 11), CXCR5 (n = 10), CCR1, CCR2, CCR4, CCR5 (n = 8), CCR6 (n = 7) and CCR3 (n = 6). Expression of adhesion molecules was analysed on T cells from seven HL cases. Results were compared with T cells isolated from unaffected lymph nodes (n = 5), PBMCs from HL patients (n = 5) and age/sex-matched healthy donors (n = 5), and T cells isolated from colorectal carcinoma biopsies (n = 5). Data represent mean percentage of cells expressing a marker ± SD of the cases examined having corrected for non-specific staining (measured using isotype-matched antibody controls)
Chemokine receptors CXCR3, CXCR4, CXCR5 and CCR7 were expressed by at least one third of all T cells within HL. A smaller proportion (10–20%) expressed CXCR6, CCR4 and CCR5, whereas CCR1, CCR2, CCR3 and CCR6 were rarely detected on T cells within this tumour (Fig. 1). For most of these receptors expression was similar on both CD3+/CD4+ and CD3+/CD4− T cell subsets. However, correcting for multiple comparisons, CXCR3 expression was significantly greater in the CD3+/CD4− T cell subset (p = 0.002 Mann–Whitney test) whereas CXCR5 was significantly greater in the CD3+/CD4+ T cell subset (p = 0.003 Mann–Whitney test).
Adhesion molecules LFA-1, PSGL-1 and VLA-4 were detected on the majority of HL-derived T cells and at least one third of T cells within the tumour expressed CD62L. In contrast, the skin associated homing marker CLA was virtually absent (Fig. 1).
This expression pattern of chemokine receptors and adhesion molecules on HL TIL closely resembled that detected on T cells isolated from unaffected lymph nodes and PBMCs from HL patients or age/sex-matched healthy controls (Fig. 1). This was also true when the expression levels of each homing marker (measured as mean fluorescence intensity) were compared (data not shown). CXCR3 was expressed on a greater proportion of T cells in HL TILs compared with HL PBMCs (p = 0.04; Mann–Whitney test) suggesting selective recruitment of CXCR3+ T cells to the tumour site (Fig. 1). However, it should be noted that these were not matched samples and this difference was not statistically significant when corrected for multiple comparisons.
Although the homing phenotype of T cells in HL tissue was comparable with that of T cells from unaffected lymph nodes and PBMCs, it was quite distinct from that of T cells from CRC, a tumour of non-lymphoid tissue (Fig. 1). Thus, in CRC a significantly greater proportion of T cells expressed CXCR6, CCR5 and CCR6 (p < 0.05; Dunn’s multiple comparison test) whereas the classical lymph node homing markers CCR7 and CD62L were less common. The average age of the CRC patients (69 years) was significantly higher than the HL patients (31 years) but we consider it unlikely that this would account for the different T cell homing phenotypes observed since we are not aware of any evidence that the host’s age influences the homing phenotype of T cells. Indeed the homing phenotype of T cells from CRC tissue was also distinct from that of T cells in unaffected lymph nodes, yet the average age of the lymph node donors (63 years) was similar to that of the CRC patients.
Immunohistochemical analysis
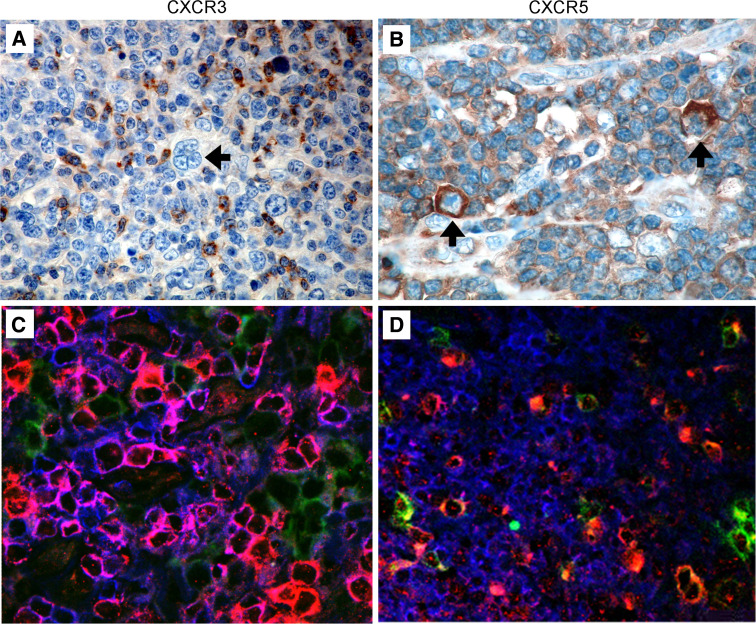
Where suitable antibody reagents were available, we used immunohistochemistry to explore the distribution within HL tumour tissue of chemokine receptors expressed on a significant proportion of infiltrating T cells. Using an anti-CXCR3 antibody showed that this receptor was widely distributed on cells throughout the tumour, including those in close proximity to H-RS cells, but was not expressed on the malignant cells. Figure 2a shows staining of one HL case that is representative of all nine cases studied. To determine whether the cells expressing CXCR3 were T cells, triple immunofluorescent staining was conducted using antibodies specific for CD3 (a marker for T cells), CD30 (a marker for H-RS cells) and CXCR3. The pattern of staining shown in Fig. 2c was again representative of all eight cases studied, and demonstrates that CXCR3 was expressed on T cells in close proximity to the malignant H-RS cells. Using CD31 as a marker for the vascular endothelium, CXCR3+ CD3+ T cells were also detected in close association with vessels in HL (data not shown). CXCR5 was expressed by a smaller proportion of T cells in HL but some of these were again intimately associated with H-RS cells (representative staining shown in Figs. 2b, d). Note that CXCR5 was also expressed by some H-RS cells.
Fig. 2.
Expression of CXCR3 and CXCR5 in HL tissue. CXCR3 and CXCR5 chemokine receptor expression was examined by immunohistochemical staining of formalin-fixed HL biopsies (n = 9). a CXCR3+ lymphoid cells were detected in the vicinity of cells having H-RS morphology. b CXCR5 was expressed on a minor population of lymphoid cells as well as H-RS cells. H-RS cells are indicated with an arrow. CXCR3 (c) and CXCR5 (d) expression was further investigated with triple immunofluorescent staining of snap frozen HL biopsies (n = 8) for CD3 (blue), CD30 (green) and CXCR3 or CXCR5 (red). Individual images were merged to provide a composite with CXCR3+ or CXCR5+ T cells staining pink
Chemokine and adhesion molecule expression in HL
Chemokines
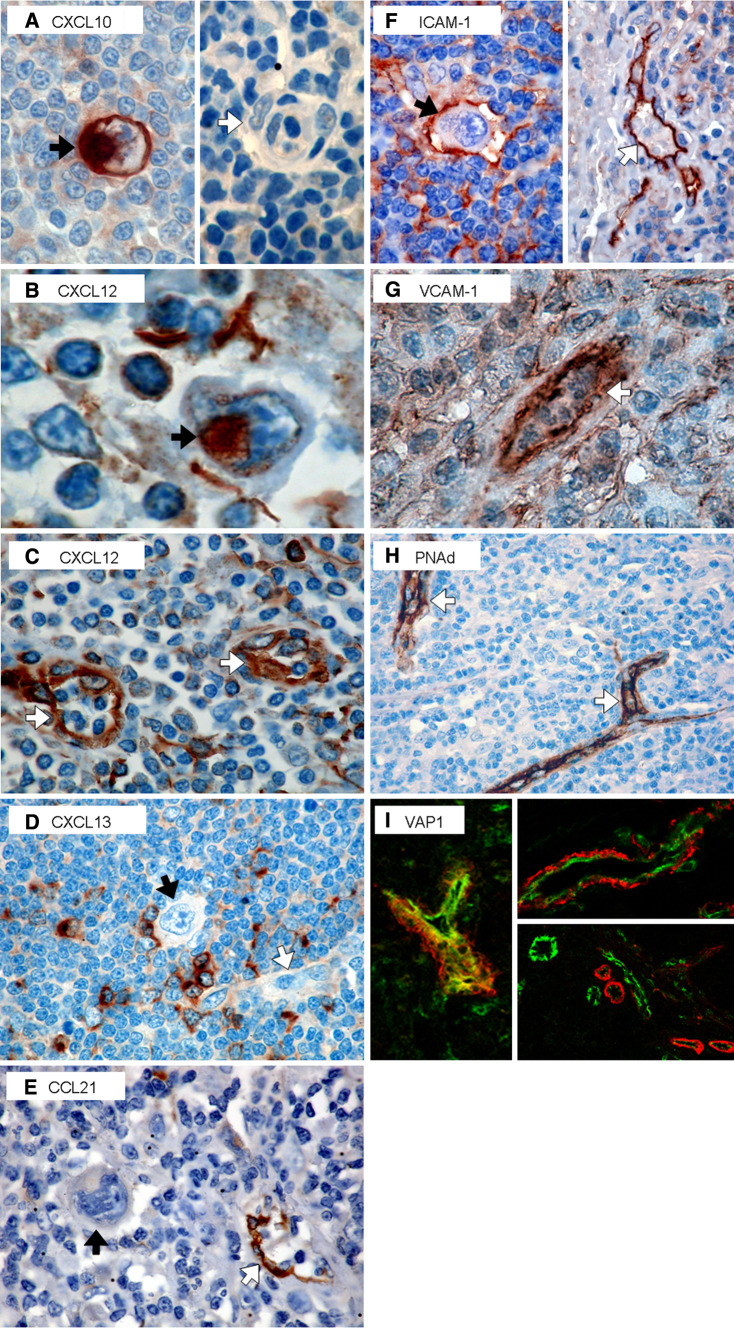
Having identified chemokine receptors expressed by a significant proportion of T cells in HL tissue, we sought to determine whether the corresponding ligands were also expressed in the tumour. Using immunohistochemistry, formalin-fixed sections from nine HL biopsies were stained using antibodies specific for CXCL10, CXCL12, CXCL13 and CCL21. Figure 3 shows staining for each of these chemokines. CXCL10, a ligand for CXCR3, was expressed in 40–100% of H-RS cells but only in EBV positive cases (which were all MC subtype). However, this chemokine was absent from the vascular endothelium (Figure 3a). CXCL12 is the sole ligand for the receptor CXCR4 and in all cases was detected on H-RS cells (Fig. 3b) as well as the vascular endothelium [including high endothelial venules (HEVs)], macrophages and some lymphocytes (Fig. 3c). CXCL13, the sole ligand for CXCR5, was not expressed by H-RS cells but was detected on variable numbers of cells in the reactive infiltrate and often on cells located close to H-RS cells (Fig. 3d). CCL21, one of two ligands for CCR7, was also absent on H-RS cells but was detected on the majority of small vessels (including HEVs) with a membranous luminal localisation (Fig. 3e).
Fig. 3.
Chemokine and adhesion molecule expression in HL. HL cases (n = 9) were examined by immunohistochemistry for expression of the following chemokines and adhesion molecules: CXCL10 (a), CXCL12 (b, c), CXCL13 (d), CCL21 (e), ICAM-1 (f), VCAM-1 (g), and PNAd (h). H-RS cells and vessels are indicated with black and white arrows, respectively. VAP-1 expression on 8 HL biopsies was examined by immunofluorescence (i). Colocalisation of VAP-1 (red) with the vascular endothelium was investigated by co-staining with CD31 (green)
Adhesion molecules
We then investigated the expression of ICAM-1, VCAM-1, and PNAd, selected counter-receptors for adhesion molecules expressed on HL-derived T cells. Expression of the orphan receptor VAP-1 (AOC3) was also studied as it is important in T cell trafficking to lymph nodes and chronic inflammatory sites [20, 23]. ICAM-1 was expressed by the majority of H-RS cells in all cases examined, as well as a proportion of vessels and reactive lymphocytes (Fig. 3f). VCAM-1 was detected on vessels as well as reactive cells including those with DC morphology and occasional lymphocytes (Fig. 3g). In contrast, VCAM-1 was absent from H-RS cells (data not shown). The expression of PNAd was restricted to the vascular endothelium (Fig. 3h). VAP-1 expression was examined by immunofluorescence on frozen sections from eight HL cases and demonstrated expression on the vascular endothelium including HEVs (using CD31 as a marker) (Fig. 3i). Note that VAP-1 expression did not always colocalise with CD31. Rather CD31 expression was luminal whereas VAP-1 expression was often subendothelial (this pattern of expression was also observed in tonsil tissue; data not shown). Furthermore, whereas VAP-1 was restricted to CD31+ vessels not all CD31+ vessels expressed VAP-1 (Fig. 3i). Triple staining for VAP-1, CD3 and CD30 (as a marker for H-RS cells) showed that VAP-1 was not expressed on the malignant cells or on T cells in HL (data not shown).
Our findings on the expression of chemokine receptors, chemokine ligands and adhesion molecules in HL tissue sections are summarised in Table 2.
Table 2.
The expression of homing markers in HL tissue (summarising data from this study)
| Molecule investigated | Expression pattern in HL tissue | ||
|---|---|---|---|
| HR-S cells | Reactive cellular infiltrate | Tumour vasculature | |
| Chemokine receptors | |||
| CXCR3 | − | ++ (including T cells) | − |
| CCR5 | + | + (including T cells) | − |
| Chemokines | |||
| CXCL10 | ++ (EBV + cases only) | − | − |
| CXCL12 | ++ | + | ++ |
| CXCL13 | − | + | − |
| CCL21 | − | − | ++ |
| Adhesion molecules | |||
| ICAM-1 | ++ | ++ | ++ |
| VCAM-1 | − | ++ | ++ |
| PNAd | − | − | ++ |
| VAP1 | − | − | ++ |
− not detected, + detectable but on <20% of cells, ++ detected on 20–100% of cells
Chemokine receptor function on T cells derived from HL
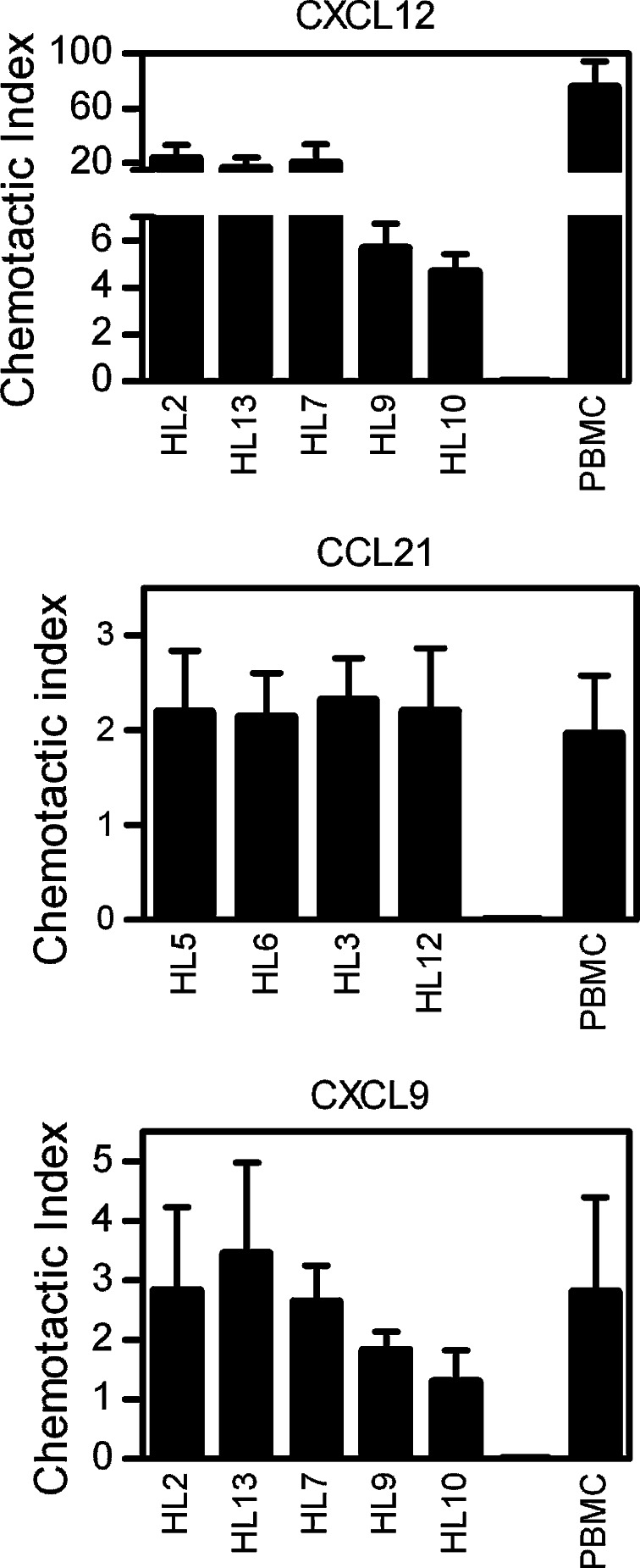
To test the function of chemokine receptors detected on HL-derived T cells, HL TIL were tested for their ability to migrate in response to purified chemokine (Fig. 4). Migration to the CXCR4 ligand CXCL12 elicited the strongest response but migration was also stimulated by ligands for the CXCR3 and CCR7 receptors (CXCL9 and CCL21, respectively) at levels comparable to those seen with PBMCs from a healthy donor. The migrating cells were identified as T lymphocytes by immunofluorescent staining for CD3.
Fig. 4.
Chemotaxis of HL TIL to purified chemokine. Isolated TIL from HL biopsies were tested for chemotaxis to recombinant human CXCL12 (200 ng/ml), CCL21 (500 ng/ml) and CXCL9 (500 ng/ml). All HL cases were NS subtype except HL3 and HL9 which were MC. Cryopreserved PBMCs derived from a healthy donor were thawed and used as positive controls. Results are shown as chemotactic index values calculated by dividing the number of cells migrating in response to specific chemokine by the number of cells migrating in medium alone (spontaneous migration). The mean value for spontaneous migration was 384 cells. Data represent the mean of triplicate tests ± SD
Discussion
The molecular mechanisms of T cell homing to lymph nodes, gut and skin have been extensively studied and are well-described. T cells also infiltrate many solid tumours but it is not clear whether recruitment to tumours recapitulates the tissue-specific environment or whether more generalised inflammatory signals are involved. Defining the molecular mechanisms of T cell recruitment to tumours is particularly important for the rational design of T cell-based therapies for cancer where efficacy will be determined in part by the ability of lymphocytes to access and be retained within the target tumour.
The present study aimed to define these mechanisms in the context of HL by studying the homing phenotype of T cells naturally recruited to this tumour. Previous studies have shown that CCL17 and CCL22 are expressed by H-RS cells and may recruit CCR4+ T cells (possibly of a Th2 or regulatory T cell phenotype) into HL tissue [9, 26]. However, our data suggest that cells recruited through this pathway represent a minor population of the T cell infiltrate (Fig. 1). Clearly therefore, other pathways must be involved in recruiting the majority of infiltrating T cells to HL.
In the present study we have analysed the expression pattern of chemokine receptors CXCR3-6 and CCR1-7, as well as adhesion molecules CLA, CD62L, LFA-1, PSGL-1 and VLA-4 on T cells derived from HL tissue and found they matched closely the phenotype of T cells from unaffected lymph nodes (comparing both the percentage of cells expressing the receptor and the levels of expression as measured by mean fluorescence intensity). Thus a high proportion of T cells in HL expressed the classical lymph node homing markers CCR7, CD62L and LFA-1 (Fig. 1). Furthermore the corresponding ligands (CCL21, PNAd, and ICAM-1) were all expressed on the tumour vasculature (Fig. 3). There was a slight increase in the proportion of CD3+ CD4− T cells in unaffected LNs that expressed chemokine receptors CCR5 (p = 0.03; Mann–Whitney test) and CCR6 (p = 0.01; Mann–Whitney test) but this was not significant when correcting for multiple comparisons. Thus despite disruption of the lymph node architecture and dysregulation of cytokine production seen in HL, T cell recruitment to this tumour closely resembled entry of naïve/central memory T cells into normal lymph nodes.
In contrast, T cells recruited to a non-lymphoid tumour CRC, displayed a quite distinct homing phenotype with few cells expressing CCR7 or CD62L, and a much larger proportion expressing chemokine receptors involved in non-lymphoid tissue infiltration such as CCR6 and CXCR6. These comparative data suggest that recruitment of T cells to tumours requires tissue-specific signals.
Studying eleven chemokine receptors, we identified four expressed on at least a third of all T cells within HL, namely CCR7, CXCR3, CXCR4 and CXCR5 (Fig. 1). CCR7 was expressed by half the T cells within HL and in chemotaxis assays was shown to be functional (Fig. 4). There are two known ligands for this receptor, CCL19 and CCL21. In situ hybridisation previously suggested that these chemokines are expressed by the reactive infiltrate but not H-RS cells [8]. Using immunohistochemical analysis of CCL21 expression we have confirmed these findings at the protein level but importantly have demonstrated that this chemokine is also strongly expressed on endothelial cells lining the vessels and thus may be involved in recruiting CCR7-positive cells from the circulation.
Our findings on the expression of the CXCR3 ligand CXCL10 are consistent with previous reports [11, 14, 24] which also detected a second ligand, CXCL9 on H-RS cells and on endothelial cells lining vessels within HL [11, 24]. This suggests that these ligands could be involved in activation-induced arrest of CXCR3+ T cells on the vascular endothelium. However, we did not detect CXCL10 expression on vessels (Fig. 3a) suggesting that this chemokine is involved in local migration to H-RS cells rather than recruitment of T cells from the circulation.
CXCR4 was expressed by the majority of T cells within HL and mediated potent chemotactic responses in vitro. Previous studies have demonstrated that transcripts encoding the corresponding ligand CXCL12 are expressed by the reactive cells but not by the H-RS cells [8], whereas at the protein level we detected CXCL12 on both. This difference may be explained by the observation that chemokines can be transcytosed from the cell producing them [12]. Importantly, we also detected CXCL12 on the vascular endothelium indicating that it may be involved in recruiting T cells directly from the circulation.
CXCR5 is expressed mainly on CD4+ T cells in HL, and although the corresponding ligand CXCL13 was not detected on the H-RS cells it was expressed by the surrounding infiltrate where it could provide an autocrine feedback loop. Interestingly, we also detected the CXCR5 receptor on H-RS cells. It has been suggested that infiltrating cells promote growth and/or survival of the neoplastic cells, possibly via CD40 signalling [22], and it is tempting to speculate that CXCL13 might also be important in this context.
Recent reports have demonstrated that HL patients possess elevated serum levels of chemokines CCL17 and CCL22 compared with healthy controls [13, 28]. CXCL9 and CXCL10 levels are not increased [13] but serum levels of CCL19, CCL21 and CXCL11-13 have yet to be determined. Elevated serum levels of some chemokines might interfere with their action at the endothelial surface by competing for receptor binding and thereby affect T cell recruitment to tumour tissue. However this is a stochastic process and other factors including binding to decoy receptors, enzymatic modifications and accessibility will impact on the biological outcome.
Adoptive T cell therapy for cancer generally involves selective expansion of T cells in vitro using protocols that favour the generation of cells with an effector/effector-memory phenotype lacking CCR7 or CD62L [1, 15]. Both these homing receptors are key mediators of T cell recruitment to lymph nodes and, based on the present study, likely to be of similar importance for entry into HL. Lymphocyte homing to lymph nodes can occur using other receptors such as CXCR4 but this process is inefficient [21]. Recently it has been shown that CD8+ T cells in mice home to reactive lymph nodes in a CXCR3-dependent but CCR7/CD62L-independent manner [5]. CXCR3 levels were slightly increased on HL TILs compared with unaffected lymph nodes however this difference did not reach statistical significance (p = 0.43; Mann–Whitney test). Therefore optimal delivery of T cells to HL tissue is likely to require generation of CCR7+CD62L+ effectors. In this regard it is interesting to note that 2–3 days after T cell receptor stimulation, effector T cells upregulate expression of several chemokine receptors including CCR7 [19]. Therefore, in future trials of adoptive T cell therapy it will be interesting to explore whether stimulating T cells prior to infusion improves their ability to access HL tumours.
Acknowledgment
We wish to thank June Freeland for assistance in collection of clinical material.
Footnotes
This work was supported by a Studentship from the University of Birmingham (EB Jones bequest) to LRM and a Cancer Research UK Senior Fellowship to SPL.
References
- 1.Bollard CM, Aguilar L, Straathof KC, Gahn B, Huls MH, Rousseau A, Sixbey J, Gresik MV, Carrum G, Hudson M, Dilloo D, Gee A, Brenner MK, Rooney CM, Heslop HE. Cytotoxic T lymphocyte therapy for Epstein–Barr virus + Hodgkin’s disease. J Exp Med. 2004;200:1623. doi: 10.1084/jem.20040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chambost H, Van Baren N, Brasseur F, Godelaine D, Xerri L, Landi SJ, Theate I, Plumas J, Spagnoli GC, Michel G, Coulie PG, Olive D. Expression of gene MAGE-A4 in Reed-Sternberg cells. Blood. 2000;95:3530. [PubMed] [Google Scholar]
- 3.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glaser SL, Lin RJ, Stewart SL, Ambinder RF, Jarrett RF, Brousset P, Pallesen G, Gulley ML, Khan G, OGrady J, Hummel M, Preciado MV, Knecht H, Chan JC, Claviez A. Epstein-Barr virus-associated Hodgkin’s disease: epidemiologic characteristics in international data. Int J Cancer. 1997;70:375. doi: 10.1002/(SICI)1097-0215(19970207)70:4<375::AID-IJC1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Guarda G, Hons M, Soriano SF, Huang AY, Polley R, Martin-Fontecha A, Stein JV, Germain RN, Lanzavecchia A, Sallusto F. L-selectin-negative CCR7-effector and memory CD8 + T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol. 2007;8:743. doi: 10.1038/ni1469. [DOI] [PubMed] [Google Scholar]
- 6.Haque T, Taylor C, Wilkie GM, Murad P, Amlot PL, Beath S, McKiernan PJ, Crawford DH. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation. 2001;72:1399. doi: 10.1097/00007890-200110270-00012. [DOI] [PubMed] [Google Scholar]
- 7.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JK, Cleary ML, Delsol G, Wolf-Peeters C, Falini B, Gatter KC. A revised European–American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994;84:1361. [PubMed] [Google Scholar]
- 8.Hopken UE, Foss HD, Meyer D, Hinz M, Leder K, Stein H, Lipp M. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood. 2002;99:1109. doi: 10.1182/blood.V99.4.1109. [DOI] [PubMed] [Google Scholar]
- 9.Ishida T, Ishii T, Inagaki A, Yano H, Komatsu H, Iida S, Inagaki H, Ueda R. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res. 2006;66:5716. doi: 10.1158/0008-5472.CAN-06-0261. [DOI] [PubMed] [Google Scholar]
- 10.Khanna R, Bell S, Sherritt M, Galbraith A, Burrows SR, Rafter L, Clarke B, Slaughter R, Falk MC, Douglass J, Williams T, Elliott SL, Moss DJ. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. 1999;96:10391. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggio EM, Van Den BA, Visser L, Diepstra A, Kluiver J, Emmens R, Poppema S. Common and differential chemokine expression patterns in rs cells of NLP, EBV positive and negative classical Hodgkin lymphomas. Int J Cancer. 2002;99:665. doi: 10.1002/ijc.10399. [DOI] [PubMed] [Google Scholar]
- 12.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell. 1997;91:385. doi: 10.1016/S0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 13.Niens M, Visser L, Nolte IM, van der SG, Diepstra A, Cordano P, Jarrett RF, Te Meerman GJ, Poppema S, Van Den BA (2008) Serum chemokine levels in Hodgkin lymphoma patients: highly increased levels of CCL17 and CCL22. Br J Haematol 140: 527 [DOI] [PubMed]
- 14.Ohshima K, Tutiya T, Yamaguchi T, Suzuki K, Suzumiya J, Kawasaki C, Haraoka S, Kikuchi M. Infiltration of Th1 and Th2 lymphocytes around Hodgkin and Reed-Sternberg (H&RS) cells in Hodgkin disease: relation with expression of CXC and CC chemokines on H&RS cells. Int J Cancer. 2002;98:567. doi: 10.1002/ijc.10218. [DOI] [PubMed] [Google Scholar]
- 15.Powell DJ, Jr, Dudley ME, Robbins PF, Rosenberg SA. Transition of late-stage effector T cells to CD27 + CD28 + tumor-reactive effector memory T cells in humans after adoptive cell transfer therapy. Blood. 2005;105:241. doi: 10.1182/blood-2004-06-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rooney CM, Smith CA, Ng CC, Loftin S, Li CF, Krance RA, Brenner MK, Heslop HE. Use of gene modified virus-specific T lymphocytes to control Epstein-Barr virus-related lymphoproliferation. Lancet. 1995;345:9. doi: 10.1016/S0140-6736(95)91150-2. [DOI] [PubMed] [Google Scholar]
- 17.Rooney CM, Smith CA, Ng CC, Loftin SK, Sixbey JW, Gan YJ, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549. [PubMed] [Google Scholar]
- 18.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Salmi M, Tohka S, Berg EL, Butcher EC, Jalkanen S. Vascular adhesion protein 1 (VAP-1) mediates lymphocyte subtype-specific, selectin-independent recognition of vascular endothelium in human lymph nodes. J Exp Med. 1997;186:589. doi: 10.1084/jem.186.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scimone ML, Felbinger TW, Mazo IB, Stein JV, von Andrian UH, Weninger W. CXCL12 mediates CCR7-independent homing of central memory cells, but not naive T cells, in peripheral lymph nodes. J Exp Med. 2004;199:1113. doi: 10.1084/jem.20031645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skinnider BF, Mak TW. The role of cytokines in classical Hodgkin lymphoma. Blood. 2002;99:4283. doi: 10.1182/blood-2002-01-0099. [DOI] [PubMed] [Google Scholar]
- 23.Stolen CM, Marttila-Ichihara F, Koskinen K, Yegutkin GG, Turja R, Bono P, Skurnik M, Hanninen A, Jalkanen S, Salmi M. Absence of the endothelial oxidase AOC3 leads to abnormal leukocyte traffic in vivo. Immunity. 2005;22:105. doi: 10.1016/j.immuni.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 24.TeruyaFeldstein J, Jaffe ES, Burd PR, Kingma DW, Setsuda JE, Tosato G. Differential chemokine expression in tissues involved by Hodgkin’s disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999;93:2463. [PubMed] [Google Scholar]
- 25.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with mage-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van den Berg A, Visser L, Poppema S. High expression of the CC Chemokine TARC in Reed-Sternberg cells: a possible explanation for the characteristic T-cell infiltrate in Hodgkin’s lymphoma. Am J Pathol. 1999;154:1685. doi: 10.1016/S0002-9440(10)65424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nat Rev Immunol. 2003;3:867. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- 28.Weihrauch MR, Manzke O, Beyer M, Haverkamp H, Diehl V, Bohlen H, Wolf J, Schultze JL. Elevated serum levels of CC thymus and activation-related chemokine (TARC) in primary Hodgkin’s disease: potential for a prognostic factor. Cancer Res. 2005;65:5516. doi: 10.1158/0008-5472.CAN-05-0100. [DOI] [PubMed] [Google Scholar]
- 29.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8 + T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]