Abstract
Th17 cells have been recently identified as a distinct Th cell lineage and found in an experimental animal model of cancer and in human cancers, but whether these cells promote tumor growth or regulate antitumor responses remains controversial. This review provides a summary of the current literature regarding interleukin (IL)-17/IL-23 and Th17 cells in cancer and discusses their potential roles in cancer development. Finally, we note several issues in this research area that must be resolved before the design of novel therapeutic approaches specifically targeting Th17 cells in cancer become feasible.
Keywords: Th17 cells, IL-17, IL-23, RORγt, Tumor immunity
Introduction
CD4+ T cells are essential in regulating the immune response in that they coordinate the functions of other immune cell types. Following activation, naive CD4+ T cells can be induced to differentiate into various Th subsets. Approximately 20 years ago, it was shown that CD4+ T helper cells differentiate into Th1 or Th2 subsets with distinct cytokine profiles and functions [1]. Th1 cells typically produce interferon (IFN)-γ and are involved in autoimmune diseases and immunity against intracellular pathogens. Th2 cells produce interleukin (IL)-4, IL-5, and IL-13 and participate in humoral immunity against parasites and in allergic reactions [2–4]. CD4+ Th cells can also develop into T regulatory (Treg) cells as defined by expression of forkhead box P3 (FoxP3). They play an anti-inflammatory role and maintain tolerance to self-components by contact-dependent suppression or by releasing anti-inflammatory cytokines such as transforming growth factor (TGF) β, IL-10 [5–9].
More recently, a novel type of CD4+ Th cell, Th17, was identified in both mice and human [10–18]. Th17 cells are characterized by the production of IL-17A, IL-17F, IL-21, IL-22, IL-26 (in humans), and CCL20 [19–21]. There is growing evidence that in both mice and humans Th17 cells are pathogenic in inflammation and in autoimmune diseases [13, 22–27]. However, there is little information on the prevalence and regulation of Th17 cells in cancer. This review provides a summary of the development and transcriptional programming of Th17 cells and highlights our current knowledge on IL-17/IL-23 and Th17 cells in cancer immunity.
Development and plasticity of Th17 cells
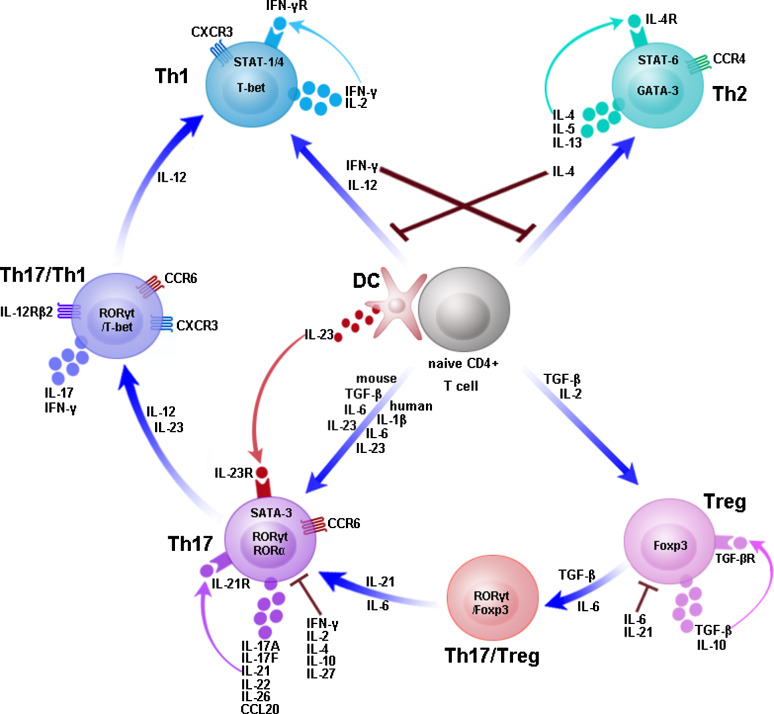
The development of Th17 cells is independent of the cytokines required for Th1 or Th2 differentiation; indeed, IFN-γ and IL-4 inhibit IL-23-dependent IL-17 production (Fig. 1). Several groups have found differences in the development and regulation of Th17 cells in humans versus mice [15, 28, 29]. TGF-β and IL-6 are required to induce the development of Th17 cells in mice [30–33]. Although IL-23 is not needed for the early differentiation of mice Th17 cells, it seems to be important in the expansion, maintenance, and/or survival of the Th17 cell subset [30–33]. However, the precise conditions for human Th17 cell development are still not completely understood and remain controversial. Some groups reported that IL-1β, IL-6, and IL-23 were sufficient to promote Th17 cell differentiation in humans and that TGF-β is not needed [15, 29, 34]. In contrast, subsequent studies showed a requirement for TGF-β in the induction of human Th17 cells [35–37]. It has been recently shown in both mice and humans that TGF-β is not essential in Th17 cell development, in fact it has been demonstrated that TGF-β plays only an indirect role by suppressing Th1 and Th2 cell development [38, 39]. Nonetheless, it is largely agreed that, in contrast to mice, the combination of TGF-β and IL-6 alone is not sufficient to drive human Th17 cell differentiation. IL-1β and IL-23 have critical functions in the progress of human Th17 cell development while other cytokines direct the development and regulation of these cells. The discrepancy between humans and mice has been attributed to the different cell origins. So far, all studies agree that mouse Th17 cells share a common origin with Treg cells. However, Cosmi et al. have recently shown that CD161 is expressed by human Th17 cells and that Th17 cells originate from a naive CD4+ CD161+ cell present in umbilical cord blood (UCB) and thymus [40]. IL-21, a member of the IL-2 cytokine family, was not only produced by Th17 cells but also shown to be a component of an autocrine loop driving mouse and human Th17 cell development [36, 41–43]. IL-2, a growth factor for most T cells, has been shown to promote Foxp3 expression in Th17 cells and to inhibit cellular differentiation [44]. Two groups demonstrated that IL-10 negatively regulates the differentiation of Th17 cells in mice and humans [45, 46]. IL-27 suppressed Th17 differentiation by a mechanism involving signal transducers and activators of transcription (STAT) 1 [47, 48].
Fig. 1.
Development and plasticity of T helper cell subsets. Naive CD4+ T cells can differentiate into Th1, Th2, Treg, or Th17 cells under the influence of different cytokines. IL-12 enhances expression of T-bet and STAT-1/4 and promotes development of Th1 cells, which secrete IFN-γ and IL-2. IL-4 enhances expression of GATA-3 and STAT-6 and promotes development of Th2 cells, which secrete IL-4, IL-5, and IL-13. TGF-β and IL-2 promote induction of Treg cells, which secrete TGF-β and IL-10. TGF-β, IL-6, IL-23 or IL-1β promote development of Th17 cells, which secrete IL-17A, IL-17F, IL-21, IL-22, IL-26 (in humans), and CCL20. IL-23 produced by dendritic cell (DC) allows the expansion, maintenance, and/or survival of Th17 cells. IFN-γ, IL-4, IL-21, and TGF-β drive T helper cell subset development in an autocrine manner. Th17/Th1 and Th17/Treg cell intermediates display intermediate phenotypes. Th17 cells can arise from Treg cells and convert into Th1 cells
The discovery that the early differentiation of Treg and Th17 cells from naive CD4+ T cells shares a requirement for TGF-β indicated that there is substantial plasticity in the early and late stages of Th17 and Treg cell development [30, 49]. In the two lineages, TGF-β-induced differentiation appears to be reciprocally related, since naive CD4+ T cells promote the development of Treg by TGF-β whereas both TGF-β and IL-6 promote Th17 development. A number of groups have demonstrated that Th17 cells can arise from Treg cells in both mice and humans [50–54]. Transient coexpression of retinoid-related orphan receptor gamma-t (RORγt) and Foxp3 was found in the early response stages of naive T cells that had been stimulated with TGF-β alone or in combination with IL-6 [55–57]. Xu et al. showed that mature Treg cells themselves differentiate into Th17 cells in the presence of IL-6 [51]. IL-6 and IL-21 may be involved in the switch of Th17/Treg cells into Th17 cells. A potential role of Foxp3 in the suppression of Th17 cell development through inhibition of both retinoid-related orphan receptor alpha (RORα) and RORγt has been reported. The populations of Treg cells that co-express Foxp3 and IL-17 can retain their suppressive function [54, 58].
To date, several reports have shown that Th17 cells can shift to Th1 cells but that the reverse is not true [59–62]. Th17 cells producing both IL-17 and IFN-γ (Th17/Th1) were detected in significant numbers in the gut of patients with Crohn’s disease and uveitis [16, 63, 64]. Th17/Th1 cells have also been described in murine models of graft versus host disease [65]. Th17 cells up-regulated T-bet expression and shift to the production of IFN-γ in the presence of IL-12 and/or IL-23 [16, 59, 60]. IL-12 may be involved in the switch of Th17/Th1 cell into Th1. Th1 cells express CCR5 and CXCR3, whereas Th2 cells express chemokine receptor CCR4 [66–69]. The expression of CCR6 and IL-23R reportedly defines a population of Th17 cells that selectively express IL-17 but not IFN-γ, whereas cells expressing CCR6 and CXCR3 produce IL-17 and IFN-γ (Th17/Th1) [14, 16, 70].
Transcriptional control of the Th17 cell lineage
After antigen-specific activation, differentiation of the CD4+ Th cell subset requires a series of transcription factors, specifically T-bet for Th1 cells and STAT-6 and GATA binding protein 3 (GATA-3) for Th2 cells [71]. Foxp3 was identified as the specific transcription factor for Treg cells, controlling the expression of multiple genes that mediate important cellular functions [9, 72].
RORγt was the first transcription factor to be selectively expressed in Th17 cells [73]. Gene profiling analysis of Th17 cells showed that the overexpression of RORγt promotes Th17 differentiation and substantially up-regulates IL-17, whereas RORγt-deficient cells produce very little IL-17 [73, 74]. Two groups have shown that, in the human system, the overexpression of RORC2 (the human ortholog of RORγt) in naive T cells induces the expression of IL-17A, IL-17F, IL-26, and CCR6 [35, 75]. A recent report established that RORα was up-regulated in Th17 cells [76]. Although RORα deletion had minimal effects on IL-17 production, a deficiency in both RORγt and RORα completely abolished IL-17 production and completely inhibited EAE disease. This suggests that the two receptors functionally synergize to promote Th17 cell differentiation.
Recently, STAT-3, the major signal transducer for IL-6, IL-21, and IL-23, was identified as a crucial transcription factor regulating Th17 cell lineage development [20, 77–79]. Retroviral expression of a hyperactive STAT-3 enhanced Th17 cell differentiation, while STAT3 deficiency impaired Th17 cell differentiation through blunted RORγt expression [41, 77]. It was recently shown that interferon regulatory factor 4 (IRF4) is also critical in Th17 cell differentiation, not only through the conventional IL-6 and TGF-β pathway but also through the IL-21-mediated pathway [80–82]. In Irf4−/− Th cells or wild-type Th cells transfected with IRF4 siRNA, the expression of RORγt was decreased whereas Foxp3 expression increased. Most recently, aryl hydrocarbon receptor (AHR), a ligand-dependent transcription factor, was shown to participate in Th17 cell differentiation [83–85]. In addition, Ets-1, STAT-5, and the suppressor of cytokine signaling 3 (SOCS3) were found to negatively regulate Th17 cell differentiation [44, 86, 87]. However, the mechanism by which Ets-1 and SOCS3 inhibit Th17 cell differentiation remains unclear.
The paradox of Th17 cell functions in tumors
Th17 cells have been shown to play important roles in inflammation and autoimmune diseases, but relatively little is known about their specific roles in tumor immunity. Both experimental animal models and clinical studies have suggested functions for Th17 cells and Th17-related cytokines, such as IL-17 and IL-23, in tumor development (Table 1), but it is not yet clear whether Th17 cells promote or inhibit tumor progression, and the mechanism of their involvement in tumor immunity is unknown.
Table 1.
List of Th17 cells and IL-17/IL-23 in cancers
| Cancer type | Experimental approaches | Reference |
|---|---|---|
| Lymphoma | Clinical research | [93, 106] |
| Ovarian cancer | Clinical research | [88, 97, 105, 122] |
| Breast cancer | Clinical research; animal model | [89, 107] |
| Colorectal cancer | Clinical research; animal mode | [90, 91, 101, 108, 112, 127] |
| Lung cancer | Animal model | [92, 95], |
| Myeloma | Clinical research | [102, 103] |
| Renal cell carcinoma | Clinical research | [117] |
| Cervical carcinoma | Animal mode | [110] |
| Fibrosarcoma | Animal model | [114, 124] |
| Gastric cancer | Clinical research | [99] |
| Hepatocellular carcinoma | Clinical research | [100] |
| Acute myeloid leukemia | Clinical research | [104] |
| Prostate cancer | Clinical research | [109, 133] |
| Melanoma | Animal model | [95, 112, 130, 131] |
Evidence of IL-17/IL-23 and Th17 cells in tumors
In previous studies, IL-17 mRNA and protein were detected in a considerable proportion of ovarian, breast, and colorectal cancers, as well as in non-small cell lung cancer (NSCLC), prostate cancer, and Sezary syndrome [88–93]. IL-23 is a member of the proinflammatory heterodimeric cytokine family and consists of a p19 subunit and a p40 subunit that is shared with IL-12 [94]. IL-23 but not IL-12 was shown to be an important molecular link between tumor-promoting and proinflammatory processes. IL-23p19 mRNA was found to be significantly overexpressed in the majority of cancer samples from various organ types, including colon, ovarian, head and neck, lung, and stomach cancers as well as melanoma [95]. An increase in Th17 cells has been detected in the peripheral blood, the tumor microenvironment and tumor-draining lymph nodes of several different human and mouse tumor types [96]. One study found a high percentage of CD4+ Th17 cells at sites of ovarian cancer but a low percentage of Th17 cells in peripheral blood mononuclear cells from healthy donors and cancer patients [97]. A recent study showed that the number of Th17 cells increased in the tumor-infiltrating lymphocytes (TILs) from melanoma and breast and colon cancers [98]. Patients with gastric cancer had a higher proportion of Th17 cells in peripheral blood and in tumor-draining lymph nodes both of which were associated with clinical stage [99]. Th17 cells were also suggested as a prognostic marker in hepatocellular carcinoma (HCC) [100]. Another study proved that the Th17 response directly contributes to enterotoxigenic Bacteroides fragilis (ETBF)-induced colon carcinogenesis [101]. In contrast to data on solid tumors, little is known about Th17 cells in hematological malignancies. Recently, serum IL-17 levels were shown to be elevated in patients with multiple myeloma, especially in stages II and III of the disease. Thus, current data confirm a role for IL-17 in the promotion of angiogenesis and in the progression of multiple myeloma [102]. In myeloma patients, Th17 cells are enriched in the bone marrow compared with the marrow in preneoplastic monoclonal gammopathy of undetermined significance (MGUS) [103]. Th17 cell frequencies and IL-17 concentrations were significantly higher in peripheral blood samples from untreated patients with acute myeloid leukemia (AML) than in those from healthy volunteers and were reduced in the former after chemotherapy [104].
However, some studies have found that the number of Th17 cells is decreased in several types of tumor. The levels of tumor-infiltrating Th17 cells and IL-17 in ascites were reduced in a group of ovarian cancer patients with more advanced disease and seemed to positively predict outcome [105]. A low number of Th17 cell is present in the tumor microenvironment of non-Hodgkin’s lymphoma because malignant B cells may up-regulate Treg cells and inhibit Th17 cells [106]. Th17 cells are present in much lower numbers in HER2-positive breast cancer patients than in either healthy controls, or HER2-negative patients [107]. Tumor growth and lung metastasis were enhanced in IL-17-deficient mice while in vitro TGF-β and IL-6 polarized Th17 cells induced tumor regression [108]. One study in prostate cancer demonstrated that Th17 cells infiltrating the tumor correlated inversely with the Gleason score [109]. This implied that Th17 cells mediate an antitumor effect in the development of prostate cancer. One group found that IL-17 promoted the tumorigenicity of human cervical tumors in nude mice but inhibited the growth of hematopoietic tumors, mastocytoma P815, and plasmocytoma in immunocompetent mice [110, 111]. Although endogenous IL-23 expression has been reported to promote tumor incidence and growth, the most recent studies have shown that IL-23 induces antitumor immune responses. In IL-23-transduced tumor-cells, IL-23 was shown to be very effective in inhibiting tumor growth and lung metastases by eliciting a strong CTL memory response [112]. It has also been reported that antitumor immunity was promoted in dendritic cells transduced with IL-23 [113]. Following in vivo electroporation of IL-23 plasmid DNA into the pretibial muscles of C57BL/6 mice, the growth of pre-existing MCA205 fibrosarcoma was suppressed and the survival of the treated mice prolonged [114].
The pro and contra functions of Th17 cells in tumors
It is well established that IL-17 acts as an angiogenic factor that stimulates the migration and cord formation of vascular endothelial cells in vitro and elicits vessel formation in vivo [115, 116]. Additionally, IL-17 may promote tumor growth and metastasis through de novo carcinogenesis and tumor neovascularization via STAT-3 signaling and other mechanisms. T cell-secreted IL-17 dramatically increased tumor-cell release of IL-8, which has both chemoattractant and angiogenic activities [117]. IL-23 may up-regulate IL-17 and matrix metalloprotease 9 (MMP-9) to stimulate angiogenesis and reduce the number of CD8+ T cells in the tumor microenvironment. The mechanism of upregulation Th17 cells in tumor is not clear. Tumor-associated monocytes and some cytokines such as IL-2, IL-6 and TNF-α in the tumor microenvironment probably regulate Th17 cells. Charles et al. found that TNF-α enhanced tumor growth via the inflammatory cytokine IL-17 in a mouse model of ovarian cancer and in patients with advanced cancer [118]. A paper by Su et al. demonstrated that tumor cells and tumor-derived fibroblasts secrete monocyte chemotactic protein 1(MCP-1) and RANTES that mediate the recruitment of Th17 cells [98]. Furthermore, the group also showed that inflammatory Toll-like receptor (TLR) and nucleotide oligomerization binding domain (Nod) 2 signaling promote the generation and expansion of Th17 cells. More recently, Kuang et al. showed that tumor-activated monocytes promote expansion of Th17 cells through secreting a set of key proinflammatory cytokines in the peritumoral stroma of HCC tissues [119]. It is clear that Treg cells efficiently suppressed the function of antitumor CD8+ T cells [120, 121]. A recent study reported that IL-2 regulates the balance between tumor Treg and Th17 cells by stimulating the differentiation of the former and inhibiting that of the latter in the tumor microenvironment [96]. However, in vitro cultures of epithelial ovarian cancer (EOC) tumor samples in the presence of IL-2 contained high frequencies of Th17 cells among the tumor-infiltrating lymphocytes and tumor-associated lymphocytes [122]. Most recently, Vicari et al. have revealed that paclitaxel combined with PF-3512676 (formerly CpG 7909) could increase IL-17 and decrease IL-10 associated with decreased Treg in mouse tumor models [123].
The mechanism of Th17 cells’ antitumor activity remains largely unknown. One publication has reported antitumor activity of IL-17 by means of a T cell-dependent mechanism [111]. Transfection of IL-17 into human tumor cell lines was shown to augment the expression of MHC class I and II antigens, thereby inducing tumor-specific antitumor immunity [124]. Two studies by Benatar et al. demonstrated that IL-17E, a novel family of cytokines that possess significant homology to IL-17, has antitumor activity in multiple tumor models, and eosinophils and B cells are involved in the antitumor mechanism of action of IL-17E [125, 126]. Further studies should be performed to examine the potential role of IL-17E in the context of IL-17 and/or Th17 antitumor activities. The expression of IL-23 in tumors produces T cell-dependent antitumor effects and induces systemic immunity [127]. Th17 cells may contribute to protective human tumor immunity by inducing Th1-type chemokines and stimulating CXCL9 and CXCL10 production to recruit effector cells to the tumor microenvironment. Leveque et al. [122] suggested that human EOC-associated Th17 cells cosecreted both IFN-γ and TNF-α. A recent study has also demonstrated that almost half of IL-17-producing CD4+ T cells isolated from HCC tissues simultaneously produced IFN-γ [119]. An interesting work of the Gaudernack group has demonstrated that IL-17-secreting T cell clones obtained from long-term survivor patients after immunotherapy also secreted IFN-γ, IL-4, IL-5, and IL-13 [128]. More recently, it was shown that Th17 cells and IL-17 participate in antitumor immunity by facilitating dendritic cell recruitment into tumor tissues and promoting the activation of tumor-specific CD8+ T cells [129]. In addition, Th17 cells eradicated established melanoma in a model of adoptive transfer of T cell receptor transgenic CD4+ T cells specific for the shared self-tumor antigen tyrosinase-related protein 1 (TRP1) [130]. Th17 frequencies increased during treatment with trastuzumab in patients with breast cancer [107]. Th17 cells were significantly increased in patients with metastatic melanoma treated with the anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA4) antibody tremelimumab [131]. Alvarez et al. demonstrated that fusion of dendritic cells and tumor cells (FC) transduced with adenovirus encoding CD40L (Adv-CD40L) increased Th17-type immune response and enhances the antitumor effect of FC vaccines in a murine lymphoma model [132]. Derhovanessian et al. has observed a highly significant correlation between a higher frequency of IL-17-producing T cells prevaccination and a shorter time to metastatic progression after immunotherapy [133]. These data imply the important involvement of Th17 cells in the response to cancer immunotherapy.
Conclusion
Th17 cells, a recently identified distinct lineage, seem to have critical functions in inflammation and autoimmune diseases. In contrast to the differentiation of Th1 and Th2 cells, Th17 cell differentiation requires TGF-β and IL-6. IL-23 may be important for the expansion and survival of Th17 cells, while RORγt, RORα, and STAT3 are crucial transcription factors regulating Th17 cell development. In addition, Th17 cells are reciprocally related to Foxp3+ Treg cells and can undergo a lineage shift to Th1 cells in both mice and humans. The magnitude of the data regarding Th17 cells in experimental animal models of cancer as well as in human cancers suggests that this T cell subset is involved in cancer immunity. However, the role of Th17 cells is no doubt highly complex, and it remains controversial whether these cells promote tumor growth or regulate antitumor responses. An understanding of the exact mechanism that regulates Th17 cells in vivo may help to resolve many issues in cancer development. This includes an understanding of the molecular regulation of plasticity between committed Th17 cells, Treg cells, and Th1 cells in vivo, especially in the tumor microenvironment. Finally, whether Th17 cells play the same roles in the different types and stages of cancers remains to be determined. A comprehensive approach to unraveling the role of Th17 cells in tumor development and/or immunity could help in the design of novel therapeutic approaches specifically targeting Th17 cells in cancer.
Conflict of interest statement
The authors declare that they have no conflict of interest.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 4.Romagnani S. The Th1/Th2 paradigm. Immunol Today. 1997;18:263–266. doi: 10.1016/S0167-5699(97)80019-9. [DOI] [PubMed] [Google Scholar]
- 5.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+ CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudensky AY, Campbell DJ. In vivo sites and cellular mechanisms of T reg cell-mediated suppression. J Exp Med. 2006;203:489–492. doi: 10.1084/jem.20060214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6:338–344. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 8.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+ CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 11.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 15.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 16.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang GX, Gran B, Yu S, Li J, Siglienti I, Chen X, Kamoun M, Rostami A. Induction of experimental autoimmune encephalomyelitis in IL-12 receptor-beta 2-deficient mice: IL-12 responsiveness is not required in the pathogenesis of inflammatory demyelination in the central nervous system. J Immunol. 2003;170:2153–2160. doi: 10.4049/jimmunol.170.4.2153. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Oukka M, Kuchroo VK. T-H-17 cells in the circle of immunity and autoimmunity. Nature Immunology. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 20.Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 21.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–688. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 22.Dong G, Ye R, Shi W, Liu S, Wang T, Yang X, Yang N, Yu X. IL-17 induces autoantibody overproduction and peripheral blood mononuclear cell overexpression of IL-6 in lupus nephritis patients. Chin Med J (Engl) 2003;116:543–548. [PubMed] [Google Scholar]
- 23.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traves SL, Donnelly LE. Th17 cells in airway diseases. Curr Mol Med. 2008;8:416–426. doi: 10.2174/156652408785160998. [DOI] [PubMed] [Google Scholar]
- 25.Yamada H. Th17 cells in human rheumatoid arthritis. Nihon Rinsho Meneki Gakkai Kaishi. 2009;32:249–255. doi: 10.2177/jsci.32.249. [DOI] [PubMed] [Google Scholar]
- 26.Oukka M. Th17 cells in immunity and autoimmunity. Ann Rheum Dis. 2008;67(Suppl 3):iii26–iii29. doi: 10.1136/ard.2008.098004. [DOI] [PubMed] [Google Scholar]
- 27.Cooke A. Th17 cells in inflammatory conditions. Rev Diabet Stud. 2006;3:72–75. doi: 10.1900/RDS.2006.3.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Z, Tato CM, Muul L, Laurence A, O’Shea JJ. Distinct regulation of interleukin-17 in human T helper lymphocytes. Arthritis Rheum. 2007;56:2936–2946. doi: 10.1002/art.22866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 30.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 31.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Stritesky GL, Yeh N, Kaplan MH. IL-23 promotes maintenance but not commitment to the Th17 lineage. J Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chizzolini C, Chicheportiche R, Alvarez M, de Rham C, Roux-Lombard P, Ferrari-Lacraz S, Dayer JM. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 2008;112:3696–3703. doi: 10.1182/blood-2008-05-155408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang L, Anderson DE, Baecher-Allan C, Hastings WD, Bettelli E, Oukka M, Kuchroo VK, Hafler DA. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 2008;454:350–352. doi: 10.1038/nature07021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volpe E, Servant N, Zollinger R, Bogiatzi SI, Hupe P, Barillot E, Soumelis V. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat Immunol. 2008;9:650–657. doi: 10.1038/ni.1613. [DOI] [PubMed] [Google Scholar]
- 38.Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207–215. doi: 10.1002/eji.200838748. [DOI] [PubMed] [Google Scholar]
- 39.Das J, Ren G, Zhang L, Roberts AI, Zhao X, Bothwell AL, Van Kaer L, Shi Y, Das G. Transforming growth factor beta is dispensable for the molecular orchestration of Th17 cell differentiation. J Exp Med. 2009;206:2407–2416. doi: 10.1084/jem.20082286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+ CD4+ T cell precursor. J Exp Med. 2008;205:1903–1916. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 42.Korn T, Bettelli E, Gao W, Awasthi A, Jager A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 44.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’Shea JJ. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Gu Y, Yang J, Ouyang X, Liu W, Li H, Bromberg J, Chen SH, Mayer L, Unkeless JC, Xiong H. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heo YJ, Joo YB, Oh HJ, Park MK, Heo YM, Cho ML, Kwok SK, Ju JH, Park KS, Cho SG, Park SH, Kim HY, Min JK. IL-10 suppresses Th17 cells and promotes regulatory T cells in the CD4+ T cell population of rheumatoid arthritis patients. Immunol Lett. 2010;127:150–156. doi: 10.1016/j.imlet.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 47.Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 48.Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, Saris CJ, O’Shea JJ, Hennighausen L, Ernst M, Hunter CA. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 49.Veldhoen M, Stockinger B. TGFbeta1, a “Jack of all trades”: the link with pro-inflammatory IL-17-producing T cells. Trends Immunol. 2006;27:358–361. doi: 10.1016/j.it.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 50.Akimzhanov AM, Yang XO, Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J Biol Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 51.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 52.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 53.Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol. 2009;131:298–307. doi: 10.1016/j.clim.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Ayyoub M, Deknuydt F, Raimbaud I, Dousset C, Leveque L, Bioley G, Valmori D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Du J, Huang C, Zhou B, Ziegler SF. Isoform-specific inhibition of ROR alpha-mediated transcriptional activation by human FOXP3. J Immunol. 2008;180:4785–4792. doi: 10.4049/jimmunol.180.7.4785. [DOI] [PubMed] [Google Scholar]
- 57.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, Feng XH, Jetten AM, Dong C. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Voo KS, Wang YH, Santori FR, Boggiano C, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E, Zheng B, Littman DR, Liu YJ. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Annunziato F, Romagnani S. Do studies in humans better depict Th17 cells? Blood. 2009;114:2213–2219. doi: 10.1182/blood-2009-03-209189. [DOI] [PubMed] [Google Scholar]
- 60.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bending D, De La Pena H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A (2009) Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest doi:10.1172/JCI37865 [DOI] [PMC free article] [PubMed]
- 62.Shi G, Cox CA, Vistica BP, Tan C, Wawrousek EF, Gery I. Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol. 2008;181:7205–7213. doi: 10.4049/jimmunol.181.10.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amadi-Obi A, Yu CR, Liu X, Mahdi RM, Clarke GL, Nussenblatt RB, Gery I, Lee YS, Egwuagu CE. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 64.van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, Kapsenberg ML, de Jong EC. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Vodanovic-Jankovic S, Johnson B, Keller M, Komorowski R, Drobyski WR. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loetscher P, Uguccioni M, Bordoli L, Baggiolini M, Moser B, Chizzolini C, Dayer JM. CCR5 is characteristic of Th1 lymphocytes. Nature. 1998;391:344–345. doi: 10.1038/34814. [DOI] [PubMed] [Google Scholar]
- 67.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Siveke JT, Hamann A. T helper 1 and T helper 2 cells respond differentially to chemokines. J Immunol. 1998;160:550–554. [PubMed] [Google Scholar]
- 69.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. The phenotype of human Th17 cells and their precursors, the cytokines that mediate their differentiation and the role of Th17 cells in inflammation. Int Immunol. 2008;20:1361–1368. doi: 10.1093/intimm/dxn106. [DOI] [PubMed] [Google Scholar]
- 71.Rengarajan J, Szabo SJ, Glimcher LH. Transcriptional regulation of Th1/Th2 polarization. Immunol Today. 2000;21:479–483. doi: 10.1016/S0167-5699(00)01712-6. [DOI] [PubMed] [Google Scholar]
- 72.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 73.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 74.Leppkes M, Becker C, Ivanov II, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 75.Crome SQ, Wang AY, Kang CY, Levings MK. The role of retinoic acid-related orphan receptor variant 2 and IL-17 in the development and function of human CD4+ T cells. Eur J Immunol. 2009;39:1480–1493. doi: 10.1002/eji.200838908. [DOI] [PubMed] [Google Scholar]
- 76.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 78.Egwuagu CE. STAT3 in CD4+ T helper cell differentiation and inflammatory diseases. Cytokine. 2009;47:149–156. doi: 10.1016/j.cyto.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kapur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-secreting Th cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- 80.Brustle A, Heink S, Huber M, Rosenplanter C, Stadelmann C, Yu P, Arpaia E, Mak TW, Kamradt T, Lohoff M. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8:958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 81.Huber M, Brustle A, Reinhard K, Guralnik A, Walter G, Mahiny A, von Low E, Lohoff M. IRF4 is essential for IL-21-mediated induction, amplification, and stabilization of the Th17 phenotype. Proc Natl Acad Sci USA. 2008;105:20846–20851. doi: 10.1073/pnas.0809077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Q, Yang W, Gupta S, Biswas P, Smith P, Bhagat G, Pernis AB. IRF-4-binding protein inhibits interleukin-17 and interleukin-21 production by controlling the activity of IRF-4 transcription factor. Immunity. 2008;29:899–911. doi: 10.1016/j.immuni.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 84.Veldhoen M, Hirota K, Christensen J, O’Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 86.Moisan J, Grenningloh R, Bettelli E, Oukka M, Ho IC. Ets-1 is a negative regulator of Th17 differentiation. J Exp Med. 2007;204:2825–2835. doi: 10.1084/jem.20070994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z, Laurence A, Kanno Y, Pacher-Zavisin M, Zhu BM, Tato C, Yoshimura A, Hennighausen L, O’Shea JJ. Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc Natl Acad Sci USA. 2006;103:8137–8142. doi: 10.1073/pnas.0600666103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282:735–738. doi: 10.1006/bbrc.2001.4618. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X, Mulcahy LA, Mohammed RA, Lee AH, Franks HA, Kilpatrick L, Yilmazer A, Paish EC, Ellis IO, Patel PM, Jackson AM. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Le Gouvello S, Bastuji-Garin S, Aloulou N, Mansour H, Chaumette MT, Berrehar F, Seikour A, Charachon A, Karoui M, Leroy K, Farcet JP, Sobhani I. High prevalence of Foxp3 and IL17 in MMR-proficient colorectal carcinomas. Gut. 2008;57:772–779. doi: 10.1136/gut.2007.123794. [DOI] [PubMed] [Google Scholar]
- 91.Lee JW, Wang P, Kattah MG, Youssef S, Steinman L, DeFea K, Straus DS. Differential regulation of chemokines by IL-17 in colonic epithelial cells. J Immunol. 2008;181:6536–6545. doi: 10.4049/jimmunol.181.9.6536. [DOI] [PubMed] [Google Scholar]
- 92.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177–6189. doi: 10.4049/jimmunol.175.9.6177. [DOI] [PubMed] [Google Scholar]
- 93.Ciree A, Michel L, Camilleri-Broet S, Jean Louis F, Oster M, Flageul B, Senet P, Fossiez F, Fridman WH, Bachelez H, Tartour E. Expression and activity of IL-17 in cutaneous T-cell lymphomas (mycosis fungoides and Sezary syndrome) Int J Cancer. 2004;112:113–120. doi: 10.1002/ijc.20373. [DOI] [PubMed] [Google Scholar]
- 94.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu Y, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 95.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 96.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 97.Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci USA. 2008;105:15505–15510. doi: 10.1073/pnas.0710686105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 99.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–537. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 100.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 101.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, Housseau F, Pardoll DM, Sears CL. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat Med. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alexandrakis MG, Pappa CA, Miyakis S, Sfiridaki A, Kafousi M, Alegakis A, Stathopoulos EN. Serum interleukin-17 and its relationship to angiogenic factors in multiple myeloma. Eur J Intern Med. 2006;17:412–416. doi: 10.1016/j.ejim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 103.Dhodapkar KM, Barbuto S, Matthews P, Kukreja A, Mazumder A, Vesole D, Jagannath S, Dhodapkar MV. Dendritic cells mediate the induction of polyfunctional human IL17-producing cells (Th17-1 cells) enriched in the bone marrow of patients with myeloma. Blood. 2008;112:2878–2885. doi: 10.1182/blood-2008-03-143222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wu C, Wang S, Wang F, Chen Q, Peng S, Zhang Y, Qian J, Jin J, Xu H. Increased frequencies of T helper type 17 cells in the peripheral blood of patients with acute myeloid leukaemia. Clin Exp Immunol. 2009;158:199–204. doi: 10.1111/j.1365-2249.2009.04011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin’s lymphoma. Cancer Res. 2009;69:5522–5530. doi: 10.1158/0008-5472.CAN-09-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Horlock C, Stott B, Dyson PJ, Morishita M, Coombes RC, Savage P, Stebbing J. The effects of trastuzumab on the CD4 + CD25 + FoxP3 + and CD4+ IL17A+ T-cell axis in patients with breast cancer. Br J Cancer. 2009;100:1061–1067. doi: 10.1038/sj.bjc.6604963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009;114:357–359. doi: 10.1182/blood-2008-09-177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, Meeker AK, Isaacs WB, Drake CG. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res. 2008;14:3254–3261. doi: 10.1158/1078-0432.CCR-07-5164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tartour E, Fossiez F, Joyeux I, Galinha A, Gey A, Claret E, Sastre-Garau X, Couturier J, Mosseri V, Vives V, Banchereau J, Fridman WH, Wijdenes J, Lebecque S, Sautes-Fridman C. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 111.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114–2121. doi: 10.1182/blood.V99.6.2114. [DOI] [PubMed] [Google Scholar]
- 112.Lo CH, Lee SC, Wu PY, Pan WY, Su J, Cheng CW, Roffler SR, Chiang BL, Lee CN, Wu CW, Tao MH. Antitumor and antimetastatic activity of IL-23. J Immunol. 2003;171:600–607. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 113.Hu J, Yuan X, Belladonna ML, Ong JM, Wachsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Can Res. 2006;66:8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 114.Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol. 2007;178:7571–7580. doi: 10.4049/jimmunol.178.12.7571. [DOI] [PubMed] [Google Scholar]
- 115.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunol Lett. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 116.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 117.Inozume T, Hanada K, Wang QJ, Yang JC. IL-17 secreted by tumor reactive T cells induces IL-8 release by human renal cancer cells. J Immunother. 2009;32:109–117. doi: 10.1097/CJI.0b013e31819302da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR, Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154–164. doi: 10.1002/hep.23291. [DOI] [PubMed] [Google Scholar]
- 120.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, Rosenberg SA, Restifo NP. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leveque L, Deknuydt F, Bioley G, Old LJ, Matsuzaki J, Odunsi K, Ayyoub M, Valmori D. Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother. 2009;32:101–108. doi: 10.1097/CJI.0b013e318195b59e. [DOI] [PubMed] [Google Scholar]
- 123.Vicari AP, Luu R, Zhang N, Patel S, Makinen SR, Hanson DC, Weeratna RD, Krieg AM. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol Immunother. 2009;58:615–628. doi: 10.1007/s00262-008-0586-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hirahara N, Nio Y, Sasaki S, Minari Y, Takamura M, Iguchi C, Dong M, Yamasawa K, Tamura K. Inoculation of human interleukin-17 gene-transfected Meth-A fibrosarcoma cells induces T cell-dependent tumor-specific immunity in mice. Oncology. 2001;61:79–89. doi: 10.1159/000055357. [DOI] [PubMed] [Google Scholar]
- 125.Benatar T, Cao MY, Lee Y, Li H, Feng N, Gu X, Lee V, Jin H, Wang M, Der S, Lightfoot J, Wright JA, Young AH. Virulizin induces production of IL-17E to enhance antitumor activity by recruitment of eosinophils into tumors. Cancer Immunol Immunother. 2008;57:1757–1769. doi: 10.1007/s00262-008-0502-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Benatar T, Cao MY, Lee Y, Lightfoot J, Feng N, Gu X, Lee V, Jin H, Wang M, Wright JA, Young AH (2009) IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol Immunother doi:10.1007/s00262-009-0802-8 [DOI] [PMC free article] [PubMed]
- 127.Wang YQ, Ugai S, Shimozato O, Yu L, Kawamura K, Yamamoto H, Yamaguchi T, Saisho H, Tagawa M. Induction of systemic immunity by expression of interleukin-23 in murine colon carcinoma cells. Int J Cancer. 2003;105:820–824. doi: 10.1002/ijc.11160. [DOI] [PubMed] [Google Scholar]
- 128.Kyte JA, Trachsel S, Risberg B, thor Straten P, Lislerud K, Gaudernack G. Unconventional cytokine profiles and development of T cell memory in long-term survivors after cancer vaccination. Cancer Immunol Immunother. 2009;58:1609–1626. doi: 10.1007/s00262-009-0670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31:787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.von Euw E, Chodon T, Attar N, Jalil J, Koya RC, Comin-Anduix B, Ribas A. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med. 2009;7:35. doi: 10.1186/1479-5876-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alvarez E, Moga E, Barquinero J, Sierra J, Briones J (2009) Dendritic and tumor cell fusions transduced with adenovirus encoding CD40L eradicate B-cell lymphoma and induce a Th17-type response. Gene Ther doi:10.1038/gt.2009.150 [DOI] [PubMed]
- 133.Derhovanessian E, Adams V, Hahnel K, Groeger A, Pandha H, Ward S, Pawelec G. Pretreatment frequency of circulating IL-17 + CD4 + T-cells, but not Tregs, correlates with clinical response to whole-cell vaccination in prostate cancer patients. Int J Cancer. 2009;125:1372–1379. doi: 10.1002/ijc.24497. [DOI] [PubMed] [Google Scholar]