Abstract
Known for years as the principal messengers of the immune system, dendritic cells (DC) represent a heterogeneous population of antigen presenting cells critically located at the nexus between innate and adaptive immunity. DC play a central role in the initiation of tumor-specific immune responses as they are endowed with the unique ability to take up, process and present tumor antigens to naïve CD4+ or CD8+ effector T lymphocytes. By virtue of the cytokines they produce, DC also regulate the type, strength and duration of T cell immune responses. In addition, they can participate in anti-tumoral NK and NKT cell activation and in the orchestration of humoral immunity. More recent studies have documented that besides their primary role in the induction and regulation of adaptive anti-tumoral immune responses, DC are also endowed with the capacity to directly kill cancer cells. This dual role of DC as killers and messengers may have important implications for tumor immunotherapy. First, the direct killing of malignant cells by DC may foster the release and thereby the immediate availability of specific tumor antigens for presentation to cytotoxic or helper T lymphocytes. Second, DC may participate in the effector phase of the immune response, potentially augmenting the diversity of the killing mechanisms leading to tumor elimination. This review focuses on this non-conventional cytotoxic function of DC as it relates to the promotion of cancer immunity and discusses the potential application of killer DC (KDC) in tumor immunotherapy.
Keywords: Killer dendritic cells, Tumor, Cancer immunotherapy
Introduction
The primary objective of cancer immunotherapy is to promote tumor eradication through the activation of innate and adaptive immune responses. The unique property of dendritic cells (DC) to act as professional antigen presenting cells (APC) has positioned them as central players in the orchestration and control of the complex interacting cellular networks that govern these immune responses. Such a cardinal role has also been largely the basis for the utilization of these cells as promising tools to induce and sustain anti-cancer immunity [1, 2]. DC represent a heterogeneous population of cells with specific subsets defined by their anatomic distribution, phenotype, mode of antigen presentation and cytokine production profile [3–5]. They continuously gather antigens in peripheral tissues, process them and, following migration to the secondary lymphoid organs, present them on major histocompatibility complexes (MHC) Class I or Class II to CD8+ cytotoxic (CTL) or CD4+ helper (Th) T lymphocytes, respectively [4, 6]. The resulting clonal expansion and activation of CTL and Th cells by DC represents a critical event for the elimination of the source of antigens. The type of DC subset, the level of activation of these cells and the nature of the cytokines they produce dictate the differentiation of effector T lymphocytes toward a defined polarized subset (Th-1, Th-2, Th-17…) [4–8]. DC also modulate the function of numerous other immune cells, including NK, B or NKT cells [9–12]. Although critical for the development of adaptive immune response, DC may also contribute to the mechanisms of immune tolerance, thus playing a central role in the control of autoimmunity [13–17]. These so-called tolerogenic DC may silence or anergize effector T lymphocytes [14, 18, 19], induce FoxP3+ regulatory T cells (Treg) or drive the differentiation of anergic IL-10-secreting immunosuppressive Tr-1 cells [20–23].
The current vision of cancer immunosurveillance relies on a multi-stage process tightly enforced and regulated by DC and which requires the initial release of tumor antigens [2, 24, 25]. A principal source of available antigens is generated in the form of apoptotic or necrotic debris that result from spontaneous tumor cell death or from tumor cell killing by macrophages, NK, NKT or other cytotoxic innate immune cells or by chemotherapeutic drugs [26–29]. The initial step consists of the capture of tumor antigens by immature DC attracted to the tumor site. DC subsequently enter a maturation and activation phase, process the captured antigens into peptides and present them to T lymphocytes on MHC Class I or Class II molecules. Following activation, tumor-specific CD8+ CTL, the dominant effector killer cells, leave the secondary lymphoid organs and home to the tumor site where they can eliminate tumor cells by multiple killing mechanisms extensively reviewed in [24]. CD4+ T helper lymphocytes primed by DC may further support CTL responses and provide help to other cytotoxic innate and adaptive immune cells such as NK or macrophages [29, 30]. Activated NKT cells that recognize tumor-derived glycolipids associated with CD1d expressed by DC may also lead to tumor cell destruction [31]. Thus, DC can theoretically orchestrate the immune attack against cancer at virtually all of its stages (initiation, maintenance and regulation, activation of diverse effector anti-tumoral cells). As such, they represent strategic targets for immune intervention strategies and have successfully been used in animals and humans to induce specific anti-cancer immunity after being loaded with tumor antigens [2, 32–39].
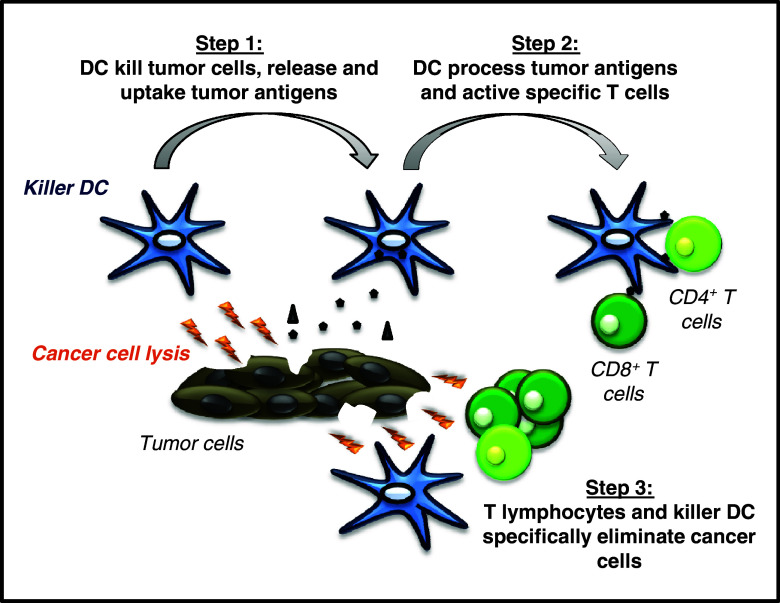
Although the direct elimination of tumor cells has primarily been attributed to highly specialized killer cells such as CTL, NKT, NK, or macrophages, many studies conducted mainly in rodents and humans have highlighted the capacity of multiple DC subsets to exert direct cytotoxic activity against cancer cells in vitro and in vivo [24, 25, 40–42]. These killer DC (KDC) may foster the release of tumor antigens and consequently their rapid acquisition and presentation to T lymphocytes. Additionally KDC by virtue of their direct tumoricidal activity may augment the diversity and thereby the efficiency of the cytotoxic effector mechanisms responsible for tumor destruction (Fig. 1). In this review we examine the cytotoxic property of DC as it pertains to their role in the promotion of cancer immunity and the potential interest of these multitasking cells for cancer immunotherapy.
Fig. 1.
Role of KDC in immunity against cancer. KDC initially kill directly cancer cells by multiple mechanisms, leading to the production of dead tumor cells or cellular debris that represent an ideal source of antigens available for immediate uptake by KDC. In a second phase, KDC process tumor-specific antigens and associate them with MHC Class I or Class II molecules, mature and migrate to the lymph nodes where they activate tumor specific CD4+ T helper cells and CD8+ CTL that in turn migrate to the tumor site. The final step involves the massive elimination of tumor cells by CTL and other killer cells such as macrophages supported by activated CD4+ T lymphocytes, with the participation of KDC as cytotoxic effectors
KDC are heterogeneous in phenotype, tissue distribution, function and mode of target cell killing
Multiple subsets of KDC have been described in rodent and human with conflicting results with regard to the mode of induction of their cytotoxic activity (spontaneous or induced) and to their mechanism of target cell killing [24, 25, 40–42] (Table 1).
Table 1.
Major subsets of KDC
| Subset | Killing mechanism | Induction | Targets | Reference |
|---|---|---|---|---|
| Mouse | ||||
| Bone marrow-derived | Fas-L | Spontaneous | Jurkat | [93] |
| NO | Spontaneous | Tumor cells | [96] | |
| TRAIL | Enhanced by LPS | T cells | [97] | |
| TNF-α, TRAIL | IL-12, IL-18 | Tumor cells | [95] | |
| FasL, TNF, TRAIL | Spontaneous | Tumor cells | [94] | |
| Spleen CD8α+ | Fas-L | Spontaneous | CD4+ T cells | [43] |
| Spleen NKDC (CD11c+CD49b+) | ND | LCMV + anti-CD40 | RMAS, YAC | [51] |
| Spleen NKDC (CD11c+NK1.1+) | Perforin/Granzyme | CpG/IL-18 | NK-sensitive cell line | [53, 57] |
| IKDC (CD11c+ NK1.1+B220+) | TRAIL | Imatinib + IL-2 | Tumor cells | [54] |
| IKDC (CD11cintCD49+ B220+) | Perforin/Granzyme | CpG, listeria | YAC | [49] |
| Langerhans cells | Fas-L | Anti-CD40 | T cells | [44] |
| Lymph Node DC | Fas-L | Influenza virus | T cells | [118] |
| Rat | ||||
| Bone marrow-derived | NO | NKG2D (anti-NKRP2) | Histiocytic tumor | [73] |
| NO | LPS, IFN-γ | Tumor cells | [99] | |
| Spleen CD103+ | Perforin/Granzyme | Spontaneous | YAC | [68] |
| Osteosarcoma | [69] | |||
| TNF-α | NKG2D (anti-NKRP2) | Tumor Cells | [72, 73] | |
| Spleen and Lymph Node (CD103+CD4+) | ND | Spontaneous | Tumor cells, Endothelial cells | [70, 71] |
| Thymic DC | NO | Spontaneous | Thymocytes | [117] |
| Human | ||||
| Blood CD11c+ mDC | TRAIL | IFN-α, IFN-γ | Tumor cells | [76] |
| TNF-α | LPS, IFN-γ, IL-15 | Tumor cells | [79] | |
| Perforin/Granzyme | TLR7/8 (Imiquimod) | K562 | [80] | |
| Blood M-DC8+ mDC | TNF-α | IFN-γ | Tumor cells | [84] |
| Blood (CD4+HLADR+Lin−) |
TNF-α, TRAIL FasL, LTα1β2 |
Spontaneous | Tumor cells, Endothelial cells | [77, 78] |
| Blood pDC | TRAIL | TLR7/8 (Imiquimod) | Jurkat | [80] |
| Influenza virus CpG, R848 | A549, Mel | [75] | ||
| HIV, IFN Imiquimod | T cells | [74] | ||
| CD34-derived | TRAIL | IFN-β | Tumor cells | [102] |
| Monocyte-derived | TRAIL | Measles virus | MDA231 | [104] |
| CD40-L | Tumor cells | [104] | ||
| IFN-α, IFN-γ | Tumor cells | [109] | ||
| LPS, IFN-γ | Tumor cells | [112] | ||
| IFN-β | Tumor cells | [102] | ||
| dsRNA | MDA231 | [104] | ||
| Spontaneous | Tumor cells | [103] | ||
|
TNF-α, TRAIL FasL, LTα1β2 |
Spontaneous | Tumor cells | [78] | |
| TNF-α | LPS, IFN-γ | Tumor cells | [79] | |
| CD40L | MDA231 | [104] | ||
KDC described in vivo
Native mouse KDC
In mouse, initial evidence indicating that DC may harbor cytotoxic properties came from a study by Suss et al. [43]. A CD11chighCD8α+Fas-ligand(FasL)+ splenic DC subpopulation was identified with the capacity to kill CD4+ T lymphocytes in a Fas/FasL-dependent manner without additional stimulation [43]. In line with this study, FasL expression by mouse Langerhans DC that became capable of triggering Jurkat cell apoptosis was reported, but required activation of the cells with CD40 ligand [44]. A non-conventional population of CD11cintCD11bint DC expressing inducible nitric oxide synthase (iNOS) and producing large amounts of TNF-α was described in the spleens of mice infected with Listeria Monocytogenes [45]. This DC subset critically contributed to innate responses controlling bacterial infection but was not capable of priming naïve T cells [45].
Mouse NKDC and IKDC
Multiple reports have documented the existence in mouse of hybrid NK-DC cells that may first behave as killers and then acquire APC properties leading to the efficient priming T cell immune responses. This concept of NKDC, IFN-producing KDC or IKDC has raised numerous questions and has been an area of extensive controversy [46–65]. Initially characterized phenotypically by the co-expression of DC and NK markers (CD11c and NK1.1, respectively), these cells were shown to produce high levels of IFN-γ following exposure to tumor cells or activation with CpG and IL-4 or IL-12 and IL-18 [53, 57]. Their expansion was proposed to be fostered by Flt3-ligand [50]. When isolated from mouse spleen, liver, lymph nodes and thymus and appropriately activated, NKDC were reported to exert cytotoxic effects against tumor cells and to subsequently present antigens to naïve T cells [50, 53]. They have thus been involved in anti-tumor immune responses [50, 53, 57, 58], but also in the control of infection [59]. This concept of a unique hybrid NK-DC cell type unifying phenotypic and functional properties of NK and DC has, however, been questioned based on the fact that the markers used to identify these cells (CD11c and NK1.1) may not unambiguously distinguish them from classical NK cells [24, 42, 66] and possible cross-contamination by conventional DC or NK may have occurred in these studies.
One particular subset of NKDC termed interferon producing killer dendritic cells or IKDC has recently been the focus of a more refined characterization [47–49, 52, 54, 60–65]. IKDC were phenotypically defined as CD11c+B220+NK1.1+CD3−CD19−Gr1−Ly6C− cells. Non-activated IKDC were characterized by the absence of MHC Class II or co-stimulatory molecule expression. Following activation with Toll-like Receptor Ligands (primarily TLR3, TLR4 or TLR9 ligands), cytokines or after contact with tumor cells or virus-infected cells (but not normal cells) IKDC were reported to up-regulate MHC Class II, CD40 and CD86 and to produce large amounts of IFN-γ or IFN-α, or IL-12 [49, 54]. IKDC killing activity was documented as transient and associated with the overexpression of NKG2D and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). The loss of IKDC cytotoxic function coincided with acquisition of MHC class II, costimulatory molecule expression and antigen presenting function [24, 25, 49, 54]. IKDC were proposed to play an important role in tumor immunosurveillance through cancer cell killing by TRAIL or/and perforin-granzyme-dependent mechanisms [54, 62–65] and with a major role of IL-15 to support their expansion and function in vivo [52, 60]. However, compelling evidence has recently revealed that these cells may not belong to the DC lineage but are rather a subset of NK [46, 55, 67]. In addition, and inconsistent with previous studies, IKDC appeared to be very poor APC, did not produce IFN-α [46], were absent in Rag2−/−IL2Rγ−/− mice [46, 55] and did not express PU.1, a transcription factor involved in hematopoiesis and expressed by DC but not by NK cells [46].
Rat KDC detected in vivo
In rat, a subset of spleen and thymus DC that express the NK marker NKR-P1 was identified more than 10 years ago [68]. Freshly isolated splenic DC upregulated NKR-P1 after culture and were able to kill the NK-sensitive YAC-1 target cell by a Ca2+-dependent mechanism [68]. The same team later characterized splenic and lymph node KDC as CD4−NKp46−CD11b+CD103+CD200+MHC Class II+ and established that these cells were not related to the NK lineage [69–71]. Freshly isolated immature rat KDC triggered apoptotic death of NK-sensitive but also and more importantly NK-resistant tumor cell lines, further arguing against a NK-type lineage [70, 71]. Interestingly KDC killing activity was spontaneous (no exogenous activation was required), restricted to immature cells, did not involve the perforin-granzyme system and was not dependent on the death receptor family molecules Fas-L, TRAIL or TNF-α or on caspase activation, but required cell contact [70]. Following killing of tumor cells, these KDC were capable to specifically take up the resulting apoptotic bodies [70]. Interestingly, KDC activation resulted in the loss of their tumoricidal and endocytic ability [70]. A more recent study provided some relevance of these findings in vivo as it pertains to tumor growth control [69]. In this report, splenic KDC driven to kill and take up an osteosarcoma tumor cell line in vitro were shown to promote tumor regression when administered to rats bearing the same osteosarcoma. Since the beneficial effects of KDC vaccination were abrogated in CD8 T cell depleted animals, the authors suggested that following killing and capture of cancer cells, KDC were capable to efficiently cross-presenting tumor antigens to specific CTL [69]. It has independently been reported that rat CD103+ DC from spleen or lymph nodes of tumor-bearing rats expressed NKR-P2 (NKG2D). Agonistic NKG2D antibodies promoted the tumoricidal activity of KDC in vitro and hindered tumor progression in vivo, while KDC killing potential was impaired by blocking the interaction of this molecule with its ligand [72]. NKR-P2/NKG2D engagement led to KDC activation evidenced by the upregulation of MHC Class II, NKR-P2 and costimulatory molecule expression, production of cytokines such as TNF-α and secretion of high amounts of nitric oxide (NO) leading to apoptosis of tumor cells [72, 73].
Native KDC described in human
In humans, several studies have reported on the cytotoxicity of different subsets of native DC endowed with a wide variety of killing mechanisms and modes of induction. Human blood DC may be classified into two subpopulations: CD11c+HLA-DR+ myeloid DC (mDC) or CD123+HLA-DR+ plasmacytoid DC (pDC). Following exposure to viruses (such as HIV or the influenca virus) pDC stimulated through TLR7 or TLR-9 may acquire TRAIL-dependent killing properties [74, 75]. IFN-α or IFN-γ may also promote TRAIL expression on freshly isolated peripheral blood CD11c+ DC, but not IL-3 receptor α+ (IL-3Rα+) pre-DC, endowing them with the capacity to kill TRAIL-sensitive tumor cells [76]. Additional studies have suggested that native immature HLA-DR+CD4+lin− DC from peripheral blood induced apoptosis of various cancer cells but not normal cells through TNF, Fas-L, TRAIL or Lymphotoxin-α1β2 [77, 78]. TNF-α-dependent anti-tumor effects of blood DC were also described against breast cancer cell lines which were enhanced with LPS, IFN-γ or IL-15 [79]. Of clinical relevance, the seminal work of Stary et al. in patients with basal cell carcinoma demonstrated that a TLR7/8 agonist (Imiquimod) promoted the recruitment to the tumor site of CD123+HLA-DR+ pDC and CD11c+HLA-DR+ mDC both endowed with cytotoxic activity [80]. Interestingly, mDC were located at the tumor periphery and expressed perforin and granzyme B while pDC were infiltrating the tumor beds and expressed TRAIL [80]. In this study, blood-derived mDC were shown to also express perforin and granzyme B (10–15%) but not TRAIL upon TLR-7/8 stimulation and were capable of killing K562 leukemia cells but not perforin/granzyme-resistant Jurkat cells [80]. Conversely, expression of granzyme B was detected in the cytoplasm of a majority of unstimulated pDC isolated from the peripheral blood but was significantly reduced following activation with TLR7 or TLR7/8 ligands [80]. However, these TLR agonists induced TRAIL expression on blood-derived pDC which gave them the ability to trigger TRAIL-dependent apoptosis of Jurkat cells [80].
CD11c+CD123− mDC are commonly subdivided into three subpopulations based on the expression of CD16, CD1b/c, and CD141 (BDCA-3). The CD16+ subset encompasses cells expressing or not the M-DC8 antigen (M-DC8+ or M-DC8− DC) [81, 82]. Freshly isolated blood M-DC8+ cells have been reported to express FcγRIII and FcγRII and to induce tumor-directed antibody dependent cell-mediated cytotoxicity (ADCC) [83]. These blood myeloid M-DC8+ DC may also exert cytotoxic effects against different tumor cell lines but not against normal cells when activated with IFN-γ [84].
Besides the above mentioned reports, Langerhans cells have additionally been shown to express the death receptor ligand Fas-L [85], but the physiological relevance of this finding remains unclear. Whether other subsets of human DC may be capable of killing target cells remains poorly explored.
These studies thus demonstrate the existence in vivo in rodents and humans of different subsets of DC with a potential cytotoxic activity that, in most cases, is dependent on exogenous activation signals.
Generation of KDC ex vivo
The paucity of DC in vivo has been a key-limiting factor for the application of DC-based vaccines in cancer therapy. This limitation has been overcome by the development of ex vivo differentiation and expansion procedures that allow the generation of these cells in large numbers. The primary source of DC currently used in clinical trials and in most animal studies consists in monocyte-derived DC [33, 86–89]. Other techniques of generating DC include the expansion of CD34+ bone marrow or blood-derived precursors [90–92]. Although relatively limited data are available regarding the cytotoxic function of freshly isolated native DC (see above), numerous studies have reported on the killing activity of ex vivo generated DC, essentially monocyte-derived human DC.
Rodent KDC differentiated from bone marrow cultures
Murine bone marrow-derived DC (BMDC) have been reported to exert killing activity against cancer cells [93–98]. In most reports, this tumoricidal function was spontaneously acquired during their differentiation in culture and did not seem to require pro-inflammatory signals [93, 94, 98] but could be enhanced by LPS [97]. DC produced from bone marrow cells cultured in the presence of GM-CSF and IL-4 were shown to express Fas-L and to mediate apoptosis of Jurkat cells [93]. The involvement of the death receptor ligands Fas-L, TRAIL and TNF-α in bone marrow-derived KDC cytotoxic activity has been reported in additional studies [93, 94, 97]. However, in contradiction with these reports, others found that apoptosis of syngeneic and allogenic tumor cells by bone marrow-derived KDC did not depend on death receptors or on IFN-α or TNF-α and did not require cell contact but was partially dependent on NO and on undefined pro-apoptotic factor(s) [96]. Interestingly, our own recent data indicated that mouse CD11c+ BMDC acquired killing capabilities toward tumor cells only when activated with the TLR-4 ligand LPS or the TLR-2 agonist Pam3Cys-SK4. The cytotoxic potential of these KDC was not mediated by Fas-L, TNF or TRAIL, but was dependent on peroxynitrites, the main metabolites of nitrite oxide (NO). Consistent with this observation, we further demonstrated that the tumoricidal activity of BMDC from iNOS or GP91 knock-out mice was significantly reduced. Importantly, after killing of cancer cells KDC were capable of engulfing dead tumor cell fragments and of presenting tumor antigens to specific T lymphocytes (J. Fraszczak et al., manuscript in revision). The interest in using the anti-tumoral potential of KDC generated from bone marrow to treat established cancers was highlighted in a study by Tatsumi et al. [95]. These authors demonstrated that the adenoviral infection of BMDC with IL-12 or IL-18 or a combination of both strongly enhanced TNF-α and Fas-L expression and resulted in improved tumoricidal activity [95]. Intratumoral injection with these genetically modified bone-marrow KDC promoted the regression of sarcoma, which was dependent upon the presence of CD4+ and CD8+ T cells [95]. Beside these studies in mice, we have reported that in rat, KDC generated from the bone marrow were endowed with cytotoxic properties against syngeneic, allogenic and xenogenic tumor cell lines [99]. BMDC killing activity was significantly enhanced with IFN-γ and LPS and was mediated by a NO-dependent non-apoptotic mechanism [99]. It is thus not yet clear, from the aforementioned conflicting reports, whether in rodents the cytotoxic function of KDC generated ex vivo from bone marrow cells is spontaneous or may require some form of exogenous activation.
Human KDC generated from peripheral blood monocytes
Numerous studies have reported that human DC generated from monocytes cultured in vitro may act as potent cytotoxic effectors, with a range of divergent reports describing their mode of killing and requirement for activation of their tumoricidal activity [77, 78, 84, 100–107]. Immature DC generated from peripheral blood monocytes were shown to induce caspase-dependent apoptosis of several cancer cell lines as well as freshly isolated non-cultured cancer cells and endothelial cells but only of a minority of tested normal cells [77]. The basis of normal cell resistance to DC killing activity, however, has not been determined. Interestingly, following activation with CD40L these monocyte-derived DC lost their tumoricidal activity [77]. Consistent with this result immature monocyte-derived DC were observed to be cytostatic and cytotoxic against tumor cells, however, KDC activation with IFN-α, IFN-β, IFN-γ or LPS did not affect their killing potential [100, 101]. Others have reported that the inhibition of the growth of several human tumor cell lines by DC generated from blood monocytes was enhanced by LPS and IFN-γ, but not by TNF-α or CD40L [106]. It has conversely been shown that CD1a+ monocyte-derived immature DC stimulated with soluble CD40L, LPS or both promoted growth inhibition of breast cancer cells [105]. The promotion of DC killing activity by CD40L or double stranded RNA (dsRNA) has been attributed to two different mechanisms [104]. In fact, dsRNA, but not CD40L, were capable of indirectly triggering TRAIL expression by DC through the induction of IFN-α production, while CD40L-mediated KDC cytotoxic function depended on TNF-α and other unidentified mechanisms [104]. Providing further insights into the role of CD40 and CD40L, a study has recently described the tumoricidal properties of immature human myeloid-derived DC exposed to OK432, a penicillin-inactivated lyophilized preparation of Streptococcus pyogenes. The observed killing properties of these KDC were associated with OK432-induced up-regulation of CD40L and depended on the expression of CD40 on tumor cells [108]. As described for their rodent counterparts, the mechanisms underlying the killing activity of human KDC are subjects of controversy. These cells were shown to promote the death of their targets by TNF-α, FasL or TRAIL-independent pathways [100, 103]. These observations were challenged by other reports indicating that TNF, Fas-L, TRAIL or lymphotoxin-α1β2, acting alone or in concert, were involved in the tumoricidal activity of human KDC, which was induced or enhanced after IFN-α, IFN-β or CD40L stimulation [78, 102, 104, 109]. However, it is to be underlined that the role of CD40L added in DC-tumor cell co-cultures is unclear since this molecule may directly affect the survival or growth of some CD40-expressing cancers [110]. Our own data indicated that human CD14+ monocyte-derived DC generated from the peripheral blood acquired a killing activity against different type of cancer cells following activation with LPS. We established that, similar to our observations in mice, the cytotoxic function of these cells was mediated by a peroxynitrite-dependent, death receptor ligand-independent mechanism (D. Lakomy et al., unpublished data).
Human KDC produced from cord blood, CD34+ cells or from other sources
Besides KDC generated from peripheral blood precursors, immature DC prepared from monocytes isolated from ascitic fluid of patients with ovarian carcinomas were identified as potent tumor cell killers [111]. These cells triggered apoptotic cell death in autologous and allogeneic ovarian cancer cells through a Ca2+-independent, Fas-L-dependent mechanism and were capable of capturing dying tumor cells [111]. In addition, DC generated from CD34+ cells after 8 days in culture were reported to express TRAIL following stimulation with IFN-β while LPS treatment actually reduced the expression of this molecule [102]. It was further determined that this induction of TRAIL partially contributed to the enhanced cytotoxic effects of IFN-β-stimulated CD34+ stem cell-derived DC [102]. Human cord blood monocyte-derived DC were additionally reported to exert selective cytotoxic effects against hematological tumor cells but not against normal cells following activation with IFN-γ or LPS [112]. Both, IFN-γ or LPS stimulation up-regulate the expression of intracellular, but not cell-surface TRAIL [112].
In summary, multiple subsets of KDC have been described with a considerable variability with regard to the modalities of induction of their cytotoxic function and to the mechanisms of tumor cell killing.
Role and therapeutic potential of KDC in cancer: a double edge sword?
Cancer immunotherapy strategies have traditionally been designed to induce specific anti-tumoral T lymphocytes through different vaccination approaches, one of them using DC loaded with tumor antigens of diverse sources [113–115]. In most of these approaches, the interest in DC-based vaccines has centered on the antigen presenting and immunostimulatory function of the DC. However, even if proven clinically safe and efficient to prime and sustain immune responses, conventional DC-based immunotherapy has only sparked moderate enthusiasm because of the relatively limited objective clinical responses that have been observed in cancer patients. Therefore, the non-conventional ability of DC to act as direct tumor cell killers certainly opens new avenues for the design of future cancer immunotherapies.
Different aspects of KDC properties should be considered with regard to their potential clinical benefits. Firstly, advocating in favor of the implementation of KDC in cancer immunotherapies, these cells could directly participate in tumor cell elimination. Considering the fact that tumors have evolved multiple strategies to resist killing inflicted by NK or CTL through classical pathways (such as perforin/granzyme, Fas-L…), the wide variety of the mechanisms used by KDC to kill their targets may provide a significant advantage insofar as it increases the diversity and therefore the efficiency of cytotoxic effector responses. Secondly, the direct killing of tumor cells by KDC is of considerable relevance for the acquisition of tumor-derived material in a rapid and efficient manner. Indeed, not only it quantitatively fosters the release of available tumor antigens, but the direct elimination of cancer cells allows for their rapid uptake by DC and thus prevents their clearance by scavenger neutrophils or macrophages. Thirdly, following killing and capture of cancer cells debris, KDC are capable of switching their function from killers to messengers capable of processing and presenting or cross-presenting acquired tumor antigens to CD4+ or CD8+ T lymphocytes. The unification of these properties therefore makes KDC highly desirable for the induction of specific anti-tumoral immunity. Finally, the fundamental observation that KDC cytotoxic activity is mainly directed toward tumor cells implies their specific recognition through cell surface receptors (such as NKG2D or other unidentified molecules) and importantly provides these cells with the ability to spare non-malignant cells. One may therefore logically expect relatively limited side-effects associated with the exploitation of the killing potential of KDC in clinic.
However, despite these undeniable theoretical advantages of using KDC as powerful killers–gatherers–messengers, one should consider yet another face of these cells: their potential role as inducers of immune tolerance. It has been proposed that immature DC may take advantage of their cytotoxic function to acquire self-antigens from apoptotic normal cells and present them to autoreactive T cells, leading to self tolerance [42, 116]. As previously described some studies indicate that KDC may also promote the killing thus the deletion of T lymphocytes or thymocytes [42, 43, 117, 118]. In cancer, DC are defective in their maturation and immunostimulatory function and thus are capable of inducing T cell tolerance to specific tumor antigens [13, 14, 17, 20–22, 116]. It should therefore be taken into account that tumors may subvert the tumoricidal properties of KDC to escape immune detection and elimination. It is indeed conceivable that immature KDC that had killed cancer cells may, in absence of activation signals, present processed tumor antigens to T lymphocytes without co-stimulation or pro-inflammatory cytokine secretion, leading to T cell anergy or deletion. Based on this consideration, it is thus critical to clearly determine whether tumor cell killing by KDC may lead to their maturation. This potential deleterious effect of KDC in anti-tumoral immune response may very probably be avoided by appropriately promoting the full maturation of KDC into potent APC using exogenous stimuli such as TLR ligands, interferons or a combination of these molecules as described [76, 80, 84, 95, 99, 102, 104–106, 109, 112].
Several immune intervention strategies may be envisioned to evaluate the potential of KDC in human cancer immunotherapy. First, KDC generated in vitro that are allowed to kill, capture and process tumor cells in culture may be administered as conventional DC vaccines. A second approach may consist in the systemic or intra-/peri-tumoral injection of KDC generated in vitro. Encouraging results of this approach have been reported by Triozzi et al. [119]. Third, therapies can be designed to promote the tumoricidal activity of DC in vivo and/or the recruitment of these KDC to the tumor site, as evidenced by the seminal work of Stary using the TLR-7 ligand Imiquinod [80]. In all these approaches, the choice of the type of DC activation signal(s) is critical since it may determine the nature of the killing mechanism. This is an important point to consider since tumor cells may develop resistance to specific death pathways. It may therefore, be advantageous to promote simultaneously the tumoricidal activities of multiple KDC subsets (capable of inducing tumor cell killing by different mechanisms) to overcome the emergence of resistant tumor variants. Finally, the genetic engineering of DC to render them cytotoxic (expression of TRAIL, Fas-L…) or to enhance their tumoricidal properties, thus creating a “customized” killer APC, may represent an additional useful approach. However, it is likely that complete tumor elimination will not solely depend on the anti-tumoral activity of KDC but will require the ultimate induction of tumor-specific T lymphocyte activation.
A major obstacle for successful cancer immunotherapy is the expansion of CD4+CD25+Treg that occurs during tumor progression [38, 120–124]. Tumor-induced Treg compromise the function of anti-tumor effector CD8+ T cells, curtail CD4+ T cell help and impede antigen-presenting cell activity [37, 124, 125]. Studies in humans and in animal models have demonstrated that attempts to disrupt Treg suppressive activity promote anti-tumoral immunity [38, 121, 126, 127]. Therefore, associating KDC-based therapy with Treg elimination or inactivation strategies, and more generally with approaches aimed at overcoming tumor-induced tolerance (inhibition of immunosuppressive molecules or cells such as TGF-β or myeloid-derived suppressor cells…), may further enhance the clinical efficiency of these killer-APC.
Conclusion
Besides their primary role as potent inducers of tumor-specific CD4+ and CD8+ T lymphocytes or of NK cells, numerous reports have extensively demonstrated that DC are also endowed with the capacity to directly kill cancer cells. This concept integrates KDC in a multi-step process in which they may generate their own source of tumor-derived material by virtue of their cytotoxic activity, then switch function to present captured and processed tumor antigens to specific T lymphocytes. This notion of a ‘multitasking’ cell population that can act at virtually all levels of anti-tumor immune response has sparked considerable interest for the development of novel cancer immunotherapeutic strategies in humans.
However, multiple questions remain unanswered particularly as they relate to the induction and regulation of DC killing function, the kinetics in acquisition of their killing and APC potential and the mechanisms by which they trigger death of their targets. For instance, a major challenge is to establish whether recognition and killing of tumor cells in vivo may drive the differentiation of KDC into powerful APC capable of inducing anti-cancer immunity, or whether immature KDC that had killed tumor cells may behave as tolerogenic APC responsible for effector T cell deletion or Treg induction. It will also be critical to address the mechanism underlying the specific recognition and killing of tumor cells and why, in most scenarios, normal cells are spared by KDC. The outcome of these studies may contribute to further understanding and developing this novel generation of tumor killer cells and expand their application beyond their primary role as inducers and regulators of immune responses against cancers.
Acknowledgments
EK and NL are supported by NIH grant R01 CA104926, the Leukemia and Lymphoma Society Fellow Award 5188-07 (NL), The Alex’s Lemonade Stand Foundation for Childhood Cancer (NL), the Tee Up for Tots, and Raise a Racquet for Kids Funds (EK and NL).
Contributor Information
Nicolas Larmonier, Phone: +1-520-6260012, FAX: +1-520-6266986, nrlarmon@email.arizona.edu.
Emmanuel Katsanis, Phone: +1-520-6260012, FAX: +1-520-6266986, Email: katsanis@peds.arizona.edu.
References
- 1.Adema GJ. Dendritic cells from bench to bedside and back. Immunol Lett. 2009;122:128–130. doi: 10.1016/j.imlet.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ueno H, Klechevsky E, Morita R, et al. Dendritic cell subsets in health and disease. Immunol Rev. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 5.Dubsky P, Ueno H, Piqueras B, et al. Human dendritic cell subsets for vaccination. J Clin Immunol. 2005;25:551–572. doi: 10.1007/s10875-005-8216-7. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Pulendran B, Smith JL, Caspary G, et al. Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA. 1999;96:1036–1041. doi: 10.1073/pnas.96.3.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci USA. 2007;104:12099–12104. doi: 10.1073/pnas.0705268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jego G, Palucka AK, Blanck JP, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/S1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 10.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 11.Walzer T, Dalod M, Robbins SH, et al. Natural-killer cells and dendritic cells: “l’union fait la force”. Blood. 2005;106:2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 12.Fujii S, Shimizu K, Hemmi H, et al. Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity. Immunol Rev. 2007;220:183–198. doi: 10.1111/j.1600-065X.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 13.Steinbrink K, Jonuleit H, Muller G, et al. Interleukin-10-treated human dendritic cells induce a melanoma-antigen-specific anergy in CD8(+) T cells resulting in a failure to lyse tumor cells. Blood. 1999;93:1634–1642. [PubMed] [Google Scholar]
- 14.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 15.Monti P, Leone BE, Zerbi A, et al. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10 high IL-12 low regulatory dendritic cell. J Immunol. 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 16.Ohnmacht C, Pullner A, King SB, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonuleit H, Schmitt E, Schuler G, et al. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman RM, Hawiger D, Liu K, et al. Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Ann N Y Acad Sci. 2003;987:15–25. doi: 10.1111/j.1749-6632.2003.tb06029.x. [DOI] [PubMed] [Google Scholar]
- 19.Tuettenberg A, Huter E, Hubo M, et al. The role of ICOS in directing T cell responses: ICOS-dependent induction of T cell anergy by tolerogenic dendritic cells. J Immunol. 2009;182:3349–3356. doi: 10.4049/jimmunol.0802733. [DOI] [PubMed] [Google Scholar]
- 20.Banerjee DK, Dhodapkar MV, Matayeva E, et al. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki S, Steinman RM. Dendritic cells as controllers of antigen-specific Foxp3+ regulatory T cells. J Dermatol Sci. 2009;54:69–75. doi: 10.1016/j.jdermsci.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roncarolo MG, Gregori S, Battaglia M, et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.Chan CW, Housseau F. The ‘kiss of death’ by dendritic cells to cancer cells. Cell Death Differ. 2008;15:58–69. doi: 10.1038/sj.cdd.4402235. [DOI] [PubMed] [Google Scholar]
- 25.Ullrich E, Chaput N, Zitvogel L. Killer dendritic cells and their potential role in immunotherapy. Horm Metab Res. 2008;40:75–81. doi: 10.1055/s-2007-1022554. [DOI] [PubMed] [Google Scholar]
- 26.Larmonier N, Billerey C, Rebe C, et al. An atypical caspase-independent death pathway for an immunogenic cancer cell line. Oncogene. 2002;21:6091–6100. doi: 10.1038/sj.onc.1205738. [DOI] [PubMed] [Google Scholar]
- 27.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 28.Kepp O, Tesniere A, Zitvogel L, et al. The immunogenicity of tumor cell death. Curr Opin Oncol. 2009;21:71–76. doi: 10.1097/CCO.0b013e32831bc375. [DOI] [PubMed] [Google Scholar]
- 29.Bonnotte B, Larmonier N, Favre N, et al. Identification of tumor-infiltrating macrophages as the killers of tumor cells after immunization in a rat model system. J Immunol. 2001;167:5077–5083. doi: 10.4049/jimmunol.167.9.5077. [DOI] [PubMed] [Google Scholar]
- 30.Zitvogel L, Tesniere A, Kroemer G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat Rev Immunol. 2006;6:715–727. doi: 10.1038/nri1936. [DOI] [PubMed] [Google Scholar]
- 31.Shimizu K, Kurosawa Y, Taniguchi M, et al. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–2653. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilboa E, Nair SK, Lyerly HK. Immunotherapy of cancer with dendritic-cell-based vaccines. Cancer Immunol Immunother. 1998;46:82–87. doi: 10.1007/s002620050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nestle FO, Farkas A, Conrad C. Dendritic-cell-based therapeutic vaccination against cancer. Curr Opin Immunol. 2005;17:163–169. doi: 10.1016/j.coi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Palucka AK, Laupeze B, Aspord C, et al. Immunotherapy via dendritic cells. Adv Exp Med Biol. 2005;560:105–114. doi: 10.1007/0-387-24180-9_14. [DOI] [PubMed] [Google Scholar]
- 35.Mayordomo JI, Zorina T, Storkus WJ, et al. Bone marrow-derived dendritic cells pulsed with synthetic tumour peptides elicit protective and therapeutic antitumour immunity. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu J, Suda T, Yoshioka T, et al. Induction of tumor-specific in vivo protective immunity by immunization with tumor antigen-pulsed antigen-presenting cells. J Immunol. 1989;142:1053–1059. [PubMed] [Google Scholar]
- 37.Larmonier N, Cantrell J, Lacasse C, et al. Chaperone-rich tumor cell lysate-mediated activation of antigen-presenting cells resists regulatory T cell suppression. J Leukoc Biol. 2008;83:1049–1059. doi: 10.1189/jlb.0907635. [DOI] [PubMed] [Google Scholar]
- 38.Larmonier N, Janikashvili N, LaCasse CJ, et al. Imatinib mesylate inhibits CD4+ CD25+ regulatory T cell activity and enhances active immunotherapy against BCR-ABL-tumors. J Immunol. 2008;181:6955–6963. doi: 10.4049/jimmunol.181.10.6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larmonier N, Merino D, Nicolas A, et al. Apoptotic, necrotic, or fused tumor cells: an equivalent source of antigen for dendritic cell loading. Apoptosis. 2006;11:1513–1524. doi: 10.1007/s10495-006-8765-0. [DOI] [PubMed] [Google Scholar]
- 40.Bonmort M, Dalod M, Mignot G, et al. Killer dendritic cells: IKDC and the others. Curr Opin Immunol. 2008;20:558–565. doi: 10.1016/j.coi.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Wesa AK, Storkus WJ. Killer dendritic cells: mechanisms of action and therapeutic implications for cancer. Cell Death Differ. 2008;15:51–57. doi: 10.1038/sj.cdd.4402243. [DOI] [PubMed] [Google Scholar]
- 42.Chauvin C, Josien R. Dendritic cells as killers: mechanistic aspects and potential roles. J Immunol. 2008;181:11–16. doi: 10.4049/jimmunol.181.1.11. [DOI] [PubMed] [Google Scholar]
- 43.Suss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996;183:1789–1796. doi: 10.1084/jem.183.4.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shibaki A, Katz SI. Activation through CD40 ligation induces functional Fas ligand expression by Langerhans cells. Eur J Immunol. 2001;31:3006–3015. doi: 10.1002/1521-4141(2001010)31:10<3006::AID-IMMU3006>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 45.Serbina NV, Salazar-Mather TP, Biron CA, et al. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/S1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 46.Caminschi I, Ahmet F, Heger K, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shortman K, Villadangos JA. Is it a DC, is it an NK? No, it’s an IKDC. Nat Med. 2006;12:167–168. doi: 10.1038/nm0206-167. [DOI] [PubMed] [Google Scholar]
- 48.Vremec D, O’Keeffe M, Hochrein H, et al. Production of interferons by dendritic cells, plasmacytoid cells, natural killer cells, and interferon-producing killer dendritic cells. Blood. 2007;109:1165–1173. doi: 10.1182/blood-2006-05-015354. [DOI] [PubMed] [Google Scholar]
- 49.Chan CW, Crafton E, Fan HN, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhry UI, Katz SC, Kingham TP, et al. In vivo overexpression of Flt3 ligand expands and activates murine spleen natural killer dendritic cells. FASEB J. 2006;20:982–984. doi: 10.1096/fj.05-5411fje. [DOI] [PubMed] [Google Scholar]
- 51.Homann D, Jahreis A, Wolfe T, et al. CD40L blockade prevents autoimmune diabetes by induction of bitypic NK/DC regulatory cells. Immunity. 2002;16:403–415. doi: 10.1016/S1074-7613(02)00290-X. [DOI] [PubMed] [Google Scholar]
- 52.Mignot G, Ullrich E, Bonmort M, et al. The critical role of IL-15 in the antitumor effects mediated by the combination therapy imatinib and IL-2. J Immunol. 2008;180:6477–6483. doi: 10.4049/jimmunol.180.10.6477. [DOI] [PubMed] [Google Scholar]
- 53.Pillarisetty VG, Katz SC, Bleier JI, et al. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 54.Taieb J, Chaput N, Menard C, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 55.Vosshenrich CA, Lesjean-Pottier S, Hasan M, et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guimont-Desrochers F, Cappello ZJ, Chagnon M, et al. Cutting edge: genetic characterization of IFN-producing killer dendritic cells. J Immunol. 2009;182:5193–5197. doi: 10.4049/jimmunol.0803969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chaudhry UI, Kingham TP, Plitas G, et al. Combined stimulation with interleukin-18 and CpG induces murine natural killer dendritic cells to produce IFN-gamma and inhibit tumor growth. Cancer Res. 2006;66:10497–10504. doi: 10.1158/0008-5472.CAN-06-1908. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhry UI, Plitas G, Burt BM, et al. NK dendritic cells expanded in IL-15 exhibit antitumor responses in vivo. J Immunol. 2007;179:4654–4660. doi: 10.4049/jimmunol.179.7.4654. [DOI] [PubMed] [Google Scholar]
- 59.Plitas G, Chaudhry UI, Kingham TP, et al. NK dendritic cells are innate immune responders to Listeria monocytogenes infection. J Immunol. 2007;178:4411–4416. doi: 10.4049/jimmunol.178.7.4411. [DOI] [PubMed] [Google Scholar]
- 60.Ullrich E, Bonmort M, Mignot G, et al. Trans-presentation of IL-15 dictates IFN-producing killer dendritic cells effector functions. J Immunol. 2008;180:7887–7897. doi: 10.4049/jimmunol.180.12.7887. [DOI] [PubMed] [Google Scholar]
- 61.Zitvogel L, Mignot G, Bonmort M, et al. IKDC: killer dendritic cells or antigen-presenting NK cells? Med Sci (Paris) 2008;24:525–528. doi: 10.1051/medsci/2008245525. [DOI] [PubMed] [Google Scholar]
- 62.Bonmort M, Ullrich E, Mignot G, et al. Interferon-gamma is produced by another player of innate immune responses: the interferon-producing killer dendritic cell (IKDC) Biochimie. 2007;89:872–877. doi: 10.1016/j.biochi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 63.Ullrich E, Bonmort M, Mignot G, et al. Therapy-induced tumor immunosurveillance involves IFN-producing killer dendritic cells. Cancer Res. 2007;67:851–853. doi: 10.1158/0008-5472.CAN-06-3766. [DOI] [PubMed] [Google Scholar]
- 64.Himoudi N, Nabarro S, Buddle J, et al. Bone marrow-derived IFN-producing killer dendritic cells account for the tumoricidal activity of unpulsed dendritic cells. J Immunol. 2008;181:6654–6663. doi: 10.4049/jimmunol.181.9.6654. [DOI] [PubMed] [Google Scholar]
- 65.Jiang Q, Wei H, Tian Z. IFN-producing killer dendritic cells contribute to the inhibitory effect of poly I:C on the progression of murine melanoma. J Immunother. 2008;31:555–562. doi: 10.1097/CJI.0b013e31817d8e75. [DOI] [PubMed] [Google Scholar]
- 66.Spits H, Lanier LL. Natural killer or dendritic: what’s in a name? Immunity. 2007;26:11–16. doi: 10.1016/j.immuni.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Blasius AL, Barchet W, Cella M, et al. Development and function of murine B220+ CD11c+ NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Josien R, Heslan M, Soulillou JP, et al. Rat spleen dendritic cells express natural killer cell receptor protein 1 (NKR-P1) and have cytotoxic activity to select targets via a Ca2+-dependent mechanism. J Exp Med. 1997;186:467–472. doi: 10.1084/jem.186.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chauvin C, Philippeau JM, Hemont C, et al. Killer dendritic cells link innate and adaptive immunity against established osteosarcoma in rats. Cancer Res. 2008;68:9433–9440. doi: 10.1158/0008-5472.CAN-08-0104. [DOI] [PubMed] [Google Scholar]
- 70.Trinite B, Chauvin C, Peche H, et al. Immature CD4-CD103+ rat dendritic cells induce rapid caspase-independent apoptosis-like cell death in various tumor and nontumor cells and phagocytose their victims. J Immunol. 2005;175:2408–2417. doi: 10.4049/jimmunol.175.4.2408. [DOI] [PubMed] [Google Scholar]
- 71.Trinite B, Voisine C, Yagita H, et al. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202–4208. doi: 10.4049/jimmunol.165.8.4202. [DOI] [PubMed] [Google Scholar]
- 72.Alli R, Savithri B, Das S, et al. Involvement of NKR-P2/NKG2D in DC-mediated killing of tumor targets: indicative of a common, innate, target-recognition paradigm? Eur J Immunol. 2004;34:1119–1126. doi: 10.1002/eji.200324793. [DOI] [PubMed] [Google Scholar]
- 73.Srivastava RM, Varalakshmi C, Khar A. Cross-linking a mAb to NKR-P2/NKG2D on dendritic cells induces their activation and maturation leading to enhanced anti-tumor immune response. Int Immunol. 2007;19:591–607. doi: 10.1093/intimm/dxm024. [DOI] [PubMed] [Google Scholar]
- 74.Hardy AW, Graham DR, Shearer GM, et al. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci USA. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chaperot L, Blum A, Manches O, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 76.Fanger NA, Maliszewski CR, Schooley K, et al. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Janjic BM, Lu G, Pimenov A, et al. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 78.Lu G, Janjic BM, Janjic J, et al. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha(1)beta(2), Fas ligand, and TNF-related apoptosis-inducing ligand. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 79.Manna PP, Mohanakumar T. Human dendritic cell mediated cytotoxicity against breast carcinoma cells in vitro. J Leukoc Biol. 2002;72:312–320. [PubMed] [Google Scholar]
- 80.Stary G, Bangert C, Tauber M, et al. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schakel K, Mayer E, Federle C, et al. A novel dendritic cell population in human blood: one-step immunomagnetic isolation by a specific mAb (M-DC8) and in vitro priming of cytotoxic T lymphocytes. Eur J Immunol. 1998;28:4084–4093. doi: 10.1002/(SICI)1521-4141(199812)28:12<4084::AID-IMMU4084>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 82.Schakel K, Kannagi R, Kniep B, et al. 6-Sulfo LacNAc, a novel carbohydrate modification of PSGL-1, defines an inflammatory type of human dendritic cells. Immunity. 2002;17:289–301. doi: 10.1016/S1074-7613(02)00393-X. [DOI] [PubMed] [Google Scholar]
- 83.Schmitz M, Zhao S, Schakel K, et al. Native human blood dendritic cells as potent effectors in antibody-dependent cellular cytotoxicity. Blood. 2002;100:1502–1504. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 84.Schmitz M, Zhao S, Deuse Y, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174:4127–4134. doi: 10.4049/jimmunol.174.7.4127. [DOI] [PubMed] [Google Scholar]
- 85.De Panfilis G, Venturini M, Lavazza A, et al. The tolerogenic molecule CD95-L is expressed on the plasma membrane of human activated, but not resting, Langerhans’ cells. Exp Dermatol. 2003;12:692–699. doi: 10.1034/j.1600-0625.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 86.Figdor CG, de Vries IJ, Lesterhuis WJ, et al. Dendritic cell immunotherapy: mapping the way. Nat Med. 2004;10:475–480. doi: 10.1038/nm1039. [DOI] [PubMed] [Google Scholar]
- 87.Inaba K, Inaba M, Romani N, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schreurs MW, Eggert AA, de Boer AJ, et al. Generation and functional characterization of mouse monocyte-derived dendritic cells. Eur J Immunol. 1999;29:2835–2841. doi: 10.1002/(SICI)1521-4141(199909)29:09<2835::AID-IMMU2835>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 89.Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Caux C, Vanbervliet B, Massacrier C, et al. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to GM-CSF+TNF alpha. J Exp Med. 1996;184:695–706. doi: 10.1084/jem.184.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fay JW, Palucka AK, Paczesny S, et al. Long-term outcomes in patients with metastatic melanoma vaccinated with melanoma peptide-pulsed CD34(+) progenitor-derived dendritic cells. Cancer Immunol Immunother. 2006;55:1209–1218. doi: 10.1007/s00262-005-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Paczesny S, Banchereau J, Wittkowski KM, et al. Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells. J Exp Med. 2004;199:1503–1511. doi: 10.1084/jem.20032118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lu L, Qian S, Hershberger PA, et al. Fas ligand (CD95L) and B7 expression on dendritic cells provide counter-regulatory signals for T cell survival and proliferation. J Immunol. 1997;158:5676–5684. [PubMed] [Google Scholar]
- 94.Huang J, Tatsumi T, Pizzoferrato E, et al. Nitric oxide sensitizes tumor cells to dendritic cell-mediated apoptosis, uptake, and cross-presentation. Cancer Res. 2005;65:8461–8470. doi: 10.1158/0008-5472.CAN-05-0654. [DOI] [PubMed] [Google Scholar]
- 95.Tatsumi T, Huang J, Gooding WE, et al. Intratumoral delivery of dendritic cells engineered to secrete both interleukin (IL)-12 and IL-18 effectively treats local and distant disease in association with broadly reactive Tc1-type immunity. Cancer Res. 2003;63:6378–6386. [PubMed] [Google Scholar]
- 96.Shimamura H, Cumberland R, Hiroishi K, et al. Murine dendritic cell-induced tumor apoptosis is partially mediated by nitric oxide. J Immunother. 2002;25:226–234. doi: 10.1097/00002371-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y, Liu S, Wang W, et al. Involvement of tumour necrosis factor-alpha-related apoptosis-inducing ligand in enhanced cytotoxicity of lipopolysaccharide-stimulated dendritic cells to activated T cells. Immunology. 2002;106:308–315. doi: 10.1046/j.1365-2567.2002.01431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lu L, Qian S, Starzl TE, et al. Blocking of the B7-CD28 pathway increases the capacity of FasL+ (CD95L+) dendritic cells to kill alloactivated T cells. Adv Exp Med Biol. 1997;417:275–282. doi: 10.1007/978-1-4757-9966-8_45. [DOI] [PubMed] [Google Scholar]
- 99.Nicolas A, Cathelin D, Larmonier N, et al. Dendritic cells trigger tumor cell death by a nitric oxide-dependent mechanism. J Immunol. 2007;179:812–818. doi: 10.4049/jimmunol.179.2.812. [DOI] [PubMed] [Google Scholar]
- 100.Vanderheyde N, Aksoy E, Amraoui Z, et al. Tumoricidal activity of monocyte-derived dendritic cells: evidence for a caspase-8-dependent, Fas-associated death domain-independent mechanism. J Immunol. 2001;167:3565–3569. doi: 10.4049/jimmunol.167.7.3565. [DOI] [PubMed] [Google Scholar]
- 101.Vanderheyde N, Vandenabeele P, Goldman M, et al. Distinct mechanisms are involved in tumoristatic and tumoricidal activities of monocyte-derived dendritic cells. Immunol Lett. 2004;91:99–101. doi: 10.1016/j.imlet.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 102.Liu S, Yu Y, Zhang M, et al. The involvement of TNF-alpha-related apoptosis-inducing ligand in the enhanced cytotoxicity of IFN-beta-stimulated human dendritic cells to tumor cells. J Immunol. 2001;166:5407–5415. doi: 10.4049/jimmunol.166.9.5407. [DOI] [PubMed] [Google Scholar]
- 103.Ayres FM, Narita M, Takahashi M, et al. Human dendritic cells mediate anti-tumor activity against hematopoietic tumor cells without direct contact and Fas/FasL killing pathway. Oncol Rep. 2004;11:1017–1023. [PubMed] [Google Scholar]
- 104.Vidalain PO, Azocar O, Yagita H, et al. Cytotoxic activity of human dendritic cells is differentially regulated by double-stranded RNA and CD40 ligand. J Immunol. 2001;167:3765–3772. doi: 10.4049/jimmunol.167.7.3765. [DOI] [PubMed] [Google Scholar]
- 105.Joo HG, Fleming TP, Tanaka Y, et al. Human dendritic cells induce tumor-specific apoptosis by soluble factors. Int J Cancer. 2002;102:20–28. doi: 10.1002/ijc.10656. [DOI] [PubMed] [Google Scholar]
- 106.Chapoval AI, Tamada K, Chen L. In vitro growth inhibition of a broad spectrum of tumor cell lines by activated human dendritic cells. Blood. 2000;95:2346–2351. [PubMed] [Google Scholar]
- 107.Korthals M, Safaian N, Kronenwett R, et al. Monocyte derived dendritic cells generated by IFN-alpha acquire mature dendritic and natural killer cell properties as shown by gene expression analysis. J Transl Med. 2007;5:46. doi: 10.1186/1479-5876-5-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hill KS, Errington F, Steele LP, et al. OK432-activated human dendritic cells kill tumor cells via CD40/CD40 ligand interactions. J Immunol. 2008;181:3108–3115. doi: 10.4049/jimmunol.181.5.3108. [DOI] [PubMed] [Google Scholar]
- 109.Griffith TS, Wiley SR, Kubin MZ, et al. Monocyte-mediated tumoricidal activity via the tumor necrosis factor-related cytokine, TRAIL. J Exp Med. 1999;189:1343–1354. doi: 10.1084/jem.189.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hirano A, Longo DL, Taub DD, et al. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 111.Yang R, Xu D, Zhang A, et al. Immature dendritic cells kill ovarian carcinoma cells by a FAS/FASL pathway, enabling them to sensitize tumor-specific CTLs. Int J Cancer. 2001;94:407–413. doi: 10.1002/ijc.1484. [DOI] [PubMed] [Google Scholar]
- 112.Shi J, Ikeda K, Fujii N, et al. Activated human umbilical cord blood dendritic cells kill tumor cells without damaging normal hematological progenitor cells. Cancer Sci. 2005;96:127–133. doi: 10.1111/j.1349-7006.2005.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 114.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 115.Schuler G, Steinman RM. Dendritic cells as adjuvants for immune-mediated resistance to tumors. J Exp Med. 1997;186:1183–1187. doi: 10.1084/jem.186.8.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 117.Aiello S, Noris M, Piccinini G, et al. Thymic dendritic cells express inducible nitric oxide synthase and generate nitric oxide in response to self- and alloantigens. J Immunol. 2000;164:4649–4658. doi: 10.4049/jimmunol.164.9.4649. [DOI] [PubMed] [Google Scholar]
- 118.Legge KL, Braciale TJ. Lymph node dendritic cells control CD8+ T cell responses through regulated FasL expression. Immunity. 2005;23:649–659. doi: 10.1016/j.immuni.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 119.Triozzi PL, Khurram R, Aldrich WA, et al. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89:2646–2654. doi: 10.1002/1097-0142(20001215)89:12<2646::AID-CNCR18>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 120.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/S0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 121.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+ CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–344. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 122.Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169:2756–2761. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 123.Viguier M, Lemaitre F, Verola O, et al. Foxp3 expressing CD4+ CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol. 2004;173:1444–1453. doi: 10.4049/jimmunol.173.2.1444. [DOI] [PubMed] [Google Scholar]
- 124.Larmonier N, Marron M, Zeng Y, et al. Tumor-derived CD4(+)CD25 (+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Prasad SJ, Farrand KJ, Matthews SA, et al. Dendritic cells loaded with stressed tumor cells elicit long-lasting protective tumor immunity in mice depleted of CD4+ CD25+ regulatory T cells. J Immunol. 2005;174:90–98. doi: 10.4049/jimmunol.174.1.90. [DOI] [PubMed] [Google Scholar]
- 127.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest. 2005;115:3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]