Abstract
Patients with cancer may develop paraneoplastic neurological syndromes (PNS) in which onconeural antibodies are important diagnostic findings. As the functional role of onconeural antibodies is largely unknown, insight gained by identifying associated antibodies may help to clarify the pathogenesis of the PNS. In this study, we identified patients with Yo antibodies who also had antibodies to an uncharacterized protein called coiled-coil domain-containing protein 104 (CCDC104). We found a significant association between CCDC104 and Yo antibodies (4 of 38, 10.5%), but not other onconeural antibodies (0 of 158) (P = 0.007, Fisher’s exact test). The prevalence of CCDC104 antibodies was approximately similar in patients with cancer (8 of 756, 1.1%) and in healthy blood donors (2 of 300, 0.7%). CCDC104 antibodies were not associated with PNS, as this was found in only two of the ten CCDC104-positive patients. The CCDC104 protein, whose function is unknown, is expressed in various human tissues, including the brain, and is localized mainly to the nucleus, but is also found in the cytoplasm. The association between Yo and CCDC104 antibodies may indicate functional similarities.
Keywords: CCDC104, CDR2, Onconeural antibody, Paraneoplastic neurological syndrome
Introduction
Paraneoplastic neurological syndromes (PNS) are rare side effects of cancer, and can be found in patients with various types of cancer. Onconeural antibodies recognize antigens in the cancer that are similar to neuronal proteins and can be detected both in the serum and in the cerebrospinal fluid of patients with PNS. These antibodies are, therefore, of great importance. Specific antibodies are often associated with specific PNS and types of cancer; Hu antibodies are commonly associated with paraneoplastic encephalomyelitis and small-cell lung cancer whereas Yo antibodies are often associated with paraneoplastic cerebellar degeneration (PCD) and ovarian cancer [1]. The detection of onconeural antibodies usually relies on immunohistochemistry and immunoblotting, but we have also developed an in vitro transcription and translation assay that is very sensitive and specific for detecting onconeural antibodies [2–4].
Some patients with PNS also harbor antibodies to other antigens [5, 6]. For example, a significant association between glial nuclear protein SOX1 antibodies and those against voltage-gated calcium channels has been found in patients with paraneoplastic Lambert–Eaton myasthenic syndrome [7]. As the functional role of onconeural antibodies is largely unknown, insight gained by identifying associated antibodies may help to clarify the pathogenesis of the PNS. In the present study, we looked for other antibodies in patients with anti-Yo and found a subgroup that carried additional antibodies against a coiled-coil domain-containing protein 104 (CCDC104). The function of this protein, however, remains to be characterized.
Materials and methods
Patients and controls
Serum containing Yo antibodies from a patient with PCD and ovarian cancer was screened with immunohistochemistry, Western blot of rat cerebellar extract, and line blot with recombinant onconeural proteins (Ravo Diagnostika GmbH, Freiburg, Germany). In addition to Yo antibodies, Western blot of cerebellar extract also detected another distinct band of approximately 39 kDa. This serum was used to screen a cDNA expression library from rat cerebellum. Sera were obtained from 756 patients with cancer (261 breast cancer, 255 lung cancer, 205 ovarian/uterus cancer and 35 other cancers) and from 300 blood donors. 196 of the 756 cancer patients had onconeural antibodies (38 patients with Yo, 13 patients with amphiphysin, 33 patients with CRMP5, 86 patients with Hu, 11 patients with Ri and 15 patients with Ma2 antibodies), previously identified by a radioactive in vitro transcription-translation (ITT) and immunoprecipitation method [8–10].
Construction and screening of cDNA expression library
We constructed and screened a cDNA expression library as described by Bredholt et al. [6]. Briefly, we purified total RNA and generated cDNA from rat cerebellum. A primary λ phage expression library was titrated by plaque assay and underwent one round of amplification in the E. coli strain XL1 MRF′ in NZY soft agar to obtain sufficient material for screening.
A single colony of XL1 MRF′ grown on Luria Bertani (LB) agar containing tetracycline (15 μg/ml) was grown in LB broth containing 10 mM MgSO4 and 0.2% maltose at 30°C overnight. The bacteria were pelleted the next day and were resuspended in 10 mM MgSO4. Recombinant phages were added to the resuspended bacteria, incubated at 37°C for 15 min and plated out in soft agar on NZY agar plates. The plates were incubated at 42°C until plaques could be visualized and overlaid by HyBond C nitrocellulose filters (Amersham Pharamcia Biotech, Uppsala, Sweden). After incubation at 37°C for 3.5 h, the filters were washed and unspecific binding was blocked before the filters were incubated overnight with the serum from the Yo-positive patient diluted 1:500 in 20 mM Tris–HCl pH 7.5, 0.1% gelatin, 0.05% Tween-20 (TBS-GT). Alkaline phosphatase-conjugated affinity-purified goat anti-human IgG (Sigma-Aldrich) diluted 1:3,000 in TBS-GT was used as secondary antibody. Color development was done with 0.3 mg/ml p-nitrobluetetrazolium chloride (NBT) (BioRad, Sundbyberg, Sweden) and 0.15 mg/ml 5-bromo-4-chloro-3-indolyl phosphate (BCIP) (BioRad) in 0.1 M NaHCO3 pH 9.8 and 1 mM MgCl2. Positive clones were screened again until pure isolates were obtained. We excised the recombinant pBK-CMV phagemids from the ZAP Express vector by in vivo excision using a helper phage and purified them by using a Plasmid Maxi Kit (Qiagen).
Purification of recombinant protein
We expressed full-length CCDC104 fused to an N-terminal 10× histidine affinity tag by subcloning the open reading frame (ORF) of the cDNA from the pBK-CMV phagemid into the NdeI and BamHI restriction sites of pET-19b (Novagen, Madison, WI, USA). Full-length coding cDNA was amplified by PCR using a forward linker-primer containing an NdeI restriction site and a reverse linker-primer containing a BamHI restriction site. The fusion protein was expressed in the E. coli strain Rosetta (DE3)pLysS (Novagen). Protein expression was induced with 1 mM IPTG at an OD600 of about 0.6. We harvested the cells 2 h later by centrifugation and separated soluble and insoluble proteins by using B-PER Bacterial Protein Extraction Reagent (Pierce, Rockford, IL, USA) with added EDTA-free Protease Inhibitor Cocktail Tablets (Roche, Basel, Switzerland). The soluble fraction was centrifuged at 10,000g and filtered through a 0.45-μm syringe filter followed by immobilized metal affinity chromatography (IMAC) under native conditions using the ÄKTAprime chromatography system and HiTrap Chelating HP columns (Amersham Pharmacia Biotech) loaded with nickel ions. Briefly, we loaded 40 ml of protein extract onto the column followed by washing with 20 column volumes of a buffer containing 20 mM Na2HPO4, 500 mM NaCl and 20 mM imidazol pH 7.6. We washed the column with 10 ml of 20 mM Na2HPO4, 500 mM NaCl and 150 mM imidazol, pH 7.6. The protein was eluted with a buffer containing 20 mM Na2HPO4, 500 mM NaCl and 500 mM imidazol pH 7.6. Protein purity was evaluated by SDS-PAGE followed by individual Coomassie blue staining and Western blot. The protein was concentrated using Amicon (Millipore, Billerica, MA, USA).
Antibodies
Recombinant protein was diluted 1:1 in PBS pH 7.4 and dialyzed towards PBS in a Slide-A-Lyzer Dialysis cassette (extra strength), 10,000 MWCO” (Pierce) for 24 h. The dialyzed protein was used to produce polyclonal antibodies in rabbits (Genosphere Biotech, Paris, France). We also produced a peptide antibody, raised towards two epitopes, aa127–142 (IIQERNGVLPDCLTDG) and aa291–305 (RTKQIQNMEQKGKPT), in rabbits (Medprobe/Eurogentec, Liège, Belgium).
In vitro transcription-translation (ITT) and immunoprecipitation
All sera were screened by a radioactive ITT and immuno-precipitation method [2, 10, 11]. Pooled sera from 100 blood donors were used as negative control and polyclonal CCDC104 antibody was used as positive control. The cut-off for CCDC104 antibodies was 44 (mean index of the 300 blood donors ± 5 SD).
Immunoblot
Premade ReadyBlot Brain Protein Explorer (New Born Rat) and Adult Human Tissues ReadyBlot Tissue Protein Explorer was purchased from Alpha Diagnostic International (San Antonio, TX, USA). The blots were treated according to the manufacturer’s instructions and were incubated with preimmune rabbit serum or polyclonal CCDC104 antibody, diluted 1:500 (newborn rat brain blot) or 1:1,000 (human tissues blot) in PBS-Tween containing 0.05% dry milk for 1 h at room temperature. The blots were washed in PBS-Tween followed by incubation with pig anti-rabbit IgG HRP (Dako, Glostrup, Denmark) diluted 1:1,000 in PBS-Tween/dry milk for 1 h at room temperature. The blots were washed twice in PBS-Tween and twice in PBS before detection by chemiluminescence (Amersham ECL™ Western Blotting Detection Reagents, GE Healthcare, Chalfont St. Giles, UK). We visualized the bonds on Amersham Hyperfilm ECL (GE Healthcare).
Western blot
An extract of rat cerebellum was prepared according to the Total protein extraction kit manual (Chemicon International, Billerica, MA, USA). Cerebellum extract (40 μg) or recombinant CCDC104 protein (100 ng) was separated on a 10% SDS-PAGE at 200 V. The proteins were then blotted onto a nitrocellulose membrane, Transblot Transfer Medium (Bio-Rad). The blots were incubated in 5% dry milk in PBS for 1 h before washing 3 × 10 min in PBS-Tween. We used patient serum or polyclonal CCDC104 antibody, diluted 1:100 in PBS-Tween containing 0.5% dry milk, as the primary antibodies. HRP-conjugated rabbit anti-human IgG antibody (Dako) or pig anti-rabbit IgG (Dako), diluted 1:1,000 in PBS-Tween with 0.5% dry milk, was used as secondary antibody. Development was done with 4-chloro-1-naphthol (Sigma Aldrich, St. Louis, MO, USA) and H2O2 in PBS.
Immunofluorescence
Intracellular localization of CCDC104 was done in the neuroblastoma cell line SK-N-SH (ATCC, Manassas, VA, USA). We seeded about 7,200 cells per chamber onto eight-welled Lab-Tek™ Chamber Permanox Slides (Nunc, Roskilde, Denmark) and grew the cells for 3 days in Eagle’s minimum essential medium, modified (EMEM; ATCC) containing 10% fetal calf serum (FCS; Sigma Aldrich). The cells were fixed in 4% ice-cold formaldehyde (Ted Pella, Redding, CA, USA) for 20 min, rinsed in PBS and permeabilized in 0.2% Triton X-100 (v/v) (VWR, Poole, UK) for 15 min at room temperature. We washed the cells in PBS and blocked them for 1 h in EMEM/FCS. After the cells had been washed in PBS, we incubated them with preimmune serum or peptide CCDC104 antibody diluted 1:200 in EMEM/FCS for 1 h at room temperature. The cells were washed for 3 × 10 min with 0.1% Triton X-100 and rinsed once with PBS before 1 h of incubation with secondary antibody Alexa Fluor 488 goat-anti-rabbit (Invitrogen Molecular Probes, Carlsbad, CA, USA) diluted 1:200 in EMEM/FCS. We then washed the cells for 3 × 10 min with 0.1% Triton X-100 and once with PBS. The slides were mounted with Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Indirect immunofluorescence was done on Zeiss LSM 510 Meta.
Bioinformatic analysis
A protein multiple sequence alignment for CCDC104 was build by the following method. Information about CCDC104 amino acid composition was gathered for Pan troglodytes (XP_001156193.1), Canis familiaris (XP_852317.1), Bos taurus (NP_001029625.1), Mus musculus (NP_080016.1), Rattus norvegicus (NP_001020037.1) and Gallus gallus (XP_419284.2) from Uniprot (http://www.uniprot.org). The sequences were collected in FastA format. Where necessary, we converted the sequences to FastA format using Don Gilberts readseq algorithm (http://searchlauncher.bcm.tmc.edu/seq-util.html). We aligned and analyzed the sequences by using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2).
Results
Identification of CCDC104
Western blot of rat cerebellum extract probed with serum of patients with Yo antibodies and PCD revealed that one patient had a distinct band of approximately 39 kDa in addition to the Yo band of 52 kDa (CDR2 protein) (Fig. 1). We characterized this 39 kDa protein by using serum from this patient to screen a rat cerebellum library from which a positive clone was isolated and sequenced. The nucleotide sequence showed that the clone contained the gene for an unidentified protein called CCDC104 (Uniprot accession number Q96G28).
Fig. 1.
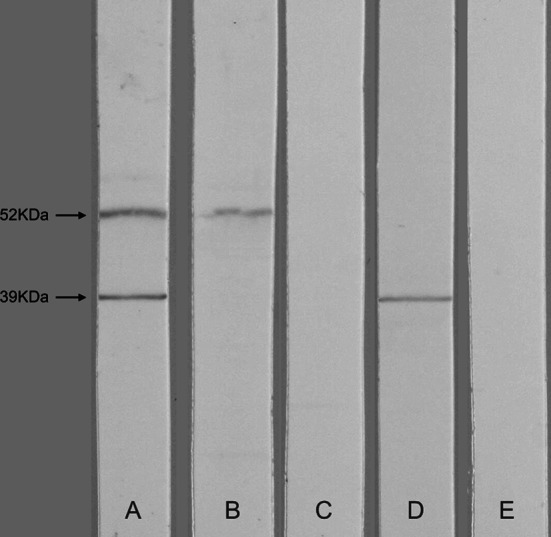
Western blot of rat cerebellum extract probed with various sera. a Yo serum with an additional 39 kDa band used for cDNA library screen. The 52 kDa band represents the CDR2 protein. b Yo serum without 39 kDa band. c Blood donor as negative control. d Rabbit anti-CCDC104. e Preimmune rabbit serum as negative control
Using the ClustalW2 multiple sequence alignment, we found that CCDC104 is highly conserved among mammalians with 85% sequence identity and 93% amino acid sequence conservation. Human CCDC104 also shares 59% sequence identity with Gallus gallus (XP_419284.2).
Tissue expression of CCDC104
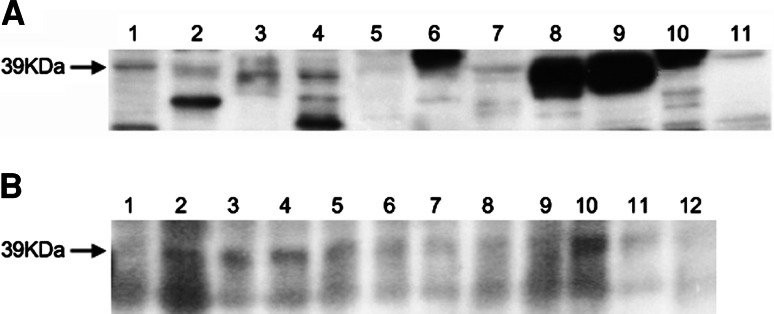
The 39 kDa CCDC104 protein was expressed in various human tissues, especially in the testis and spleen, but could also be observed in the brain (Fig. 2a). Isoforms of CCDC104 were also identified; a 42 kDa band was most pronounced in the lung and pancreas, whereas a 36 kDa band was most pronounced in the heart. The CCDC104 protein was widely expressed in newborn rat brain, but less in the frontal cortex and in the spinal cord (Fig. 2b). Blots incubated with preimmune rabbit serum were negative.
Fig. 2.
Immunoblot of human and rat tissue probed with rabbit CCDC104 antibody. a Human tissue. 1 brain, 2 heart, 3 small intestine, 4 kidney, 5 liver, 6 lung, 7 skeletal muscle, 8 testis, 9 spleen, 10 pancreas, 11 ovary. b Newborn rat brain. 1 frontal cortex, 2 posterior cortex, 3 cerebellum, 4 hippocampus, 5 olfactory bulb, 6 striatum, 7 thalamus, 8 midbrain, 9 entorhinal cortex, 10 pons, 11 medulla, 12 spinal cord. The arrow indicates the CCDC104 39 kDa protein
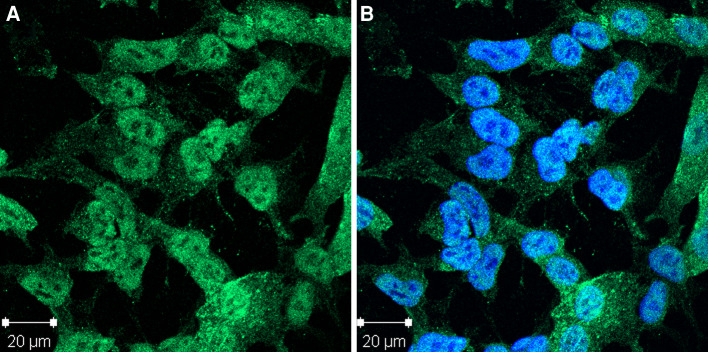
The CCDC104 rabbit serum stained mainly the nucleus (not the nucleolus), but also parts of the cytoplasm of neuroblastoma cells (Fig. 3). The staining was speckled, indicating that CCDC104 was located in special compartments in the cytosol. Cells incubated with preimmune rabbit serum were negative. The CCDC104 antibody did not react with rat cerebellar tissue, not even after antigen retrieval by boiling the tissue slides in citrate buffer.
Fig. 3.
Indirect immunofluorescence of neuroblastoma cells stained with rabbit CCDC104 antibody. a The CCDC104 antibody mainly stains the nucleus, but also parts of the cytoplasm. b Anti-CCDC104 and DAPI nuclear staining
Antibodies to CCDC104 in patients and controls
CCDC104 antibodies were present in 4 of 38 (10.5%) of the Yo-positive sera. One of the four patients was the patient with PCD used for screening the cDNA library. None of the 158 patients with Hu, CRMP5, amphiphysin, Ri or Ma2 antibodies had CCDC104 antibodies. The association between Yo and CCDC104 antibodies compared to CCDC104 antibodies and the other paraneoplastic antibodies was statistically significant (P = 0.007, Fisher’s exact test). We found that 8 of 756 (1.1%) patients with cancer and 2 of 300 (0.7%) blood donors had CCDC104 antibodies.
Nine of the ten patients showed reactivity with the recombinant CCDC104 protein in Western blot (Table 1). Only one of the patients being positive by ITT was negative in Western blot, indicating that this patient had CCDC104 antibodies to a conformational epitope. Patients with an index below the cut-off value of the immunoprecipitation assay were negative on Western blots. The patients with both Yo and CCDC104 antibodies showed reactivity with the corresponding antigens in rat cerebellar extract.
Table 1.
CCDC104-positive patients and controls
| Sex/age (years) | Cancer/PNS | Onconeural antibodies | CCDC104 index | CCDC104 immunoblot |
|---|---|---|---|---|
| F/65 | Breast/– | – | 58 | + |
| F/52 | Ovary/– | – | 136 | + |
| F/66 | Lung/– | – | 75 | – |
| M/60 | Lung/– | – | 99 | + |
| M/63 | Lung/– | Yo | 75 | + |
| M/77 | HL/AN | Yo | 68 | + |
| M/77 | Prostate/– | Yo | 197 | + |
| F/58 | Ovary/PCD | Yo | 845 | + |
| M/53 (BD) | –/– | – | 72 | + |
| F/30 (BD) | –/– | – | 99 | + |
PNS paraneoplastic neurological syndrome, PCD paraneoplastic cerebellar degeneration, AN Axonal neuropathy, HL Hodgkin lymphoma, BD blood donor
Discussion
As the functional role of onconeural antibodies is largely unknown, insight gained by identifying associated antibodies may help to clarify the pathogenesis of the PNS. In this study, we found that CCDC104 and Yo antibodies were significantly associated. CCDC104 antibodies were not associated with other paraneoplastic antibodies, such as Hu, Ri, amphiphysin, CRMP5 or Ma2 antibodies, however, larger studies should be performed to confirm this finding. CCDC104 antibodies were detected in 1.1% of the sera from the cancer patients and in 0.7% of blood donor sera, indicating that CCDC104 antibodies are not tumor specific. Two of the Yo- and CCDC104-positive patients had PNS, whereas the rest of the patients did not, suggesting that CCDC104 antibodies are not associated with PNS. Among the Yo- and CCDC104-positive patients, three of four were men. This is of interest since Yo antibodies have only rarely been described in men [12, 13]. However, the overall sex distribution among the CCDC104-positive patients was similar.
We found that CCDC104 is expressed in different human tissues. It has been shown that several tissues express low levels of CCDC104 mRNA, but the mRNA expression is increased in several areas of the brain and in the testis (ArrayExpress Warehouse 8.10 database). This is of interest, since the paraneoplastic antigen CDR2, which is identified by Yo antibodies, is restricted to brain and testis [14] and various types of cancer [15]. Genome analysis suggests 5 possible splice variants of CCDC104 (Ensembl geneID: ENSG00000163001), and 2 of these, coding for the 39 and 42 kDa proteins, have been verified (Uniprot accession number Q96G28) [16–18]. Our results show that human tissues express different isoforms of CCDC104. The main isoform, which was detected in various parts of the brain and in testis, was the CCDC104 of approximately 39 kDa. Other human tissues expressed several CCDC104 isoforms in the range of 25–42 kDa. The function of CCDC104 is largely not known. The fact that CCDC104 is well conserved among mammalians implies that it is a biologically important protein in which little change is allowed to preserve its biological function. Various paraneoplastic antigens, such as CDR2, show similar traits (data not shown).
We found that the CCDC104 protein was located mainly in the nuclei of neuroblastoma cells, but was also present in the cytoplasm. Whether the CCDC104 protein shows similar localization in other cells remains to be shown. Unfortunately, our CCDC104 antibody did not react with tissue sections. Other localization studies by the Human Protein Atlas Project have shown that normal and malignant tissue show weak to moderate cytoplasmic expression of CCDC104. Furthermore, CCDC104 is present in the cytoplasm and luminal membranes of intestinal epithelia. Spermatocytes, spermatids, and alveolar cells show both strong cytoplasmic and nuclear staining, whereas strong nuclear staining is present in hematopoietic cells (http://www.proteinatlas.org).
It has been reported that Yo antibodies also recognize a 34 kDa protein known as CDR1 [19]. CCDC104 is different from CDR1, however, and the CDR1 gene has been mapped to Xq27.1-q27.2. The CDR1 protein has a 262 amino acid sequence with many repeats, but no coiled-coil domains [20, 21]. The CCDC104 gene has been mapped to 2p16.1 and encodes a 342 amino acid protein with few recognizable domains [22]. It has a potential coiled-coil domain (aa 150–187) and a phosphoserine at aa201 (Swiss-Prot entry Q96G28). CCDC104 has been shown to be phosphorylated at Ser201 by ataxia telangiectasia-mutated (ATM) or ATM-Rad3-related (ATR) proteins [23]. ATM and ATR are protein kinases that are activated as responses to various forms of DNA damage [24]. Patients with ATM mutations are more susceptible to various forms of cancer [25] suggesting a potential role for CCDC104 in cancer control. Patients with ATM mutations also develop progressive ataxia, with loss of Purkinje cells and thinning of the granule cell layer [26]. Loss of Purkinje cells is the typical pathological finding in PCD [27] and may be caused by Yo antibodies and/or CDR2-specific T cells [28]. The biological function of CDR2 is not known. However, CDR2, through its leucine zipper motif, has been demonstrated to interact with c-myc [29], with cell cycle-related proteins [30, 31] and with a protein kinase [32], indicating that CDR2 is involved in signal transduction and gene transcription. The CDR2 protein has also potential coiled-coil domains (Swiss-Prot entry Q01850), as found for CCDC104. The association between Yo and CCDC104 antibodies may therefore indicate some functional similarities.
Acknowledgments
We thank the Western Norway Regional Health Authority (Helse Vest) and the Gerda Meyer, Nyquist Guldbranson and Gerdt Meyer Nyquist legacy for financial support. We thank Kibret Mazengia, Emilia Lohndal and Sue Olsen for technical assistance and Jan Aarseth for statistical help. We also thank Lars Drivsholm, Storstrømmens Hospital, Helga Salvesen and Per E. Lønning, Haukeland University Hospital, for sera and clinical information regarding the different tumor patients.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
References
- 1.Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Smitt PS, Vedeler C, Verschuuren JJ, Vincent A, Voltz R. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–1140. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen A, Monstad SE, Dorum A, Lonning PE, Salvesen HB, Drivsholm L, Aarseth JH, Vedeler CA. Ri antibodies in patients with breast, ovarian or small cell lung cancer determined by a sensitive immunoprecipitation technique. Cancer Immunol Immunother. 2006;55:1280–1284. doi: 10.1007/s00262-006-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monstad SE, Storstein A, Dorum A, Knudsen A, Lonning PE, Salvesen HB, Aarseth JH, Vedeler CA. Yo antibodies in ovarian and breast cancer patients detected by a sensitive immunoprecipitation technique. Clin Exp Immunol. 2006;144:53–58. doi: 10.1111/j.1365-2249.2006.03031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monstad SE, Drivsholm L, Skeie GO, Aarseth JH, Vedeler CA. CRMP5 antibodies in patients with small-cell lung cancer or thymoma. Cancer Immunol Immunother. 2008;57:227–232. doi: 10.1007/s00262-007-0369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boe AS, Bredholt G, Knappskog PM, Storstein A, Vedeler CA, Husebye ES. Pyridoxal phosphatase is a novel cancer autoantigen in the central nervous system. Br J Cancer. 2004;91:1508–1514. doi: 10.1038/sj.bjc.6602142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bredholt G, Storstein A, Haugen M, Krossnes BK, Husebye E, Knappskog P, Vedeler CA. Detection of autoantibodies to the BTB-kelch protein KLHL7 in cancer sera. Scand J Immunol. 2006;64:325–335. doi: 10.1111/j.1365-3083.2006.01821.x. [DOI] [PubMed] [Google Scholar]
- 7.Sabater L, Titulaer M, Saiz A, Verschuuren J, Gure AO, Graus F. SOX1 antibodies are markers of paraneoplastic Lambert–Eaton myasthenic syndrome. Neurology. 2008;70:924–928. doi: 10.1212/01.wnl.0000281663.81079.24. [DOI] [PubMed] [Google Scholar]
- 8.Monstad SE, Drivsholm L, Storstein A, Aarseth JH, Haugen M, Lang B, Vincent A, Vedeler CA. Hu and voltage-gated calcium channel (VGCC) antibodies related to the prognosis of small-cell lung cancer. J Clin Oncol. 2004;22:795–800. doi: 10.1200/JCO.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 9.Monstad SE, Vedeler CA. An immunoprecipitation assay for the detection of onconeural antibodies. Acta Neurol Scand Suppl. 2006;183:71–72. doi: 10.1111/j.1600-0404.2006.00622.x. [DOI] [PubMed] [Google Scholar]
- 10.Monstad SE, Knudsen A, Salvesen HB, Aarseth JH, Vedeler CA (2009) Onconeural antibodies in sera from patients with various types of tumours. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed]
- 11.Storstein A, Monstad SE, Nakkestad HL, Husebye ES, Vedeler CA. Paraneoplastic antibodies against HuD detected by a sensitive radiobinding assay. J Neurol. 2004;251:197–203. doi: 10.1007/s00415-004-0303-9. [DOI] [PubMed] [Google Scholar]
- 12.Debes JD, Lagarde SM, Hulsenboom E, Sillevis Smitt PA, ten Kate FJ, Sulter GA, van Lanschot JJ. Anti-Yo-associated paraneoplastic cerebellar degeneration in a man with adenocarcinoma of the gastroesophageal junction. Dig Surg. 2007;24:395–397. doi: 10.1159/000107782. [DOI] [PubMed] [Google Scholar]
- 13.Matschke J, Kromminga A, Erbersdobler A, Lamszus K, Anders S, Kofuncu E. Paraneoplastic cerebellar degeneration and anti-Yo antibodies in a man with prostatic adenocarcinoma. J Neurol Neurosurg Psychiatry. 2007;78:775–777. doi: 10.1136/jnnp.2006.112961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corradi JP, Yang C, Darnell JC, Dalmau J, Darnell RB. A post-transcriptional regulatory mechanism restricts expression of the paraneoplastic cerebellar degeneration antigen cdr2 to immune privileged tissues. J Neurosci. 1997;17:1406–1415. doi: 10.1523/JNEUROSCI.17-04-01406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Darnell JC, Albert ML, Darnell RB. Cdr2, a target antigen of naturally occuring human tumor immunity, is widely expressed in gynecological tumors. Cancer Res. 2000;60:2136–2139. [PubMed] [Google Scholar]
- 16.Strausberg RL, Feingold EA, Grouse LH, Derge JG, Klausner RD, Collins FS, Wagner L, Shenmen CM, Schuler GD, Altschul SF, Zeeberg B, Buetow KH, Schaefer CF, Bhat NK, Hopkins RF, Jordan H, Moore T, Max SI, Wang J, Hsieh F, Diatchenko L, Marusina K, Farmer AA, Rubin GM, Hong L, Stapleton M, Soares MB, Bonaldo MF, Casavant TL, Scheetz TE, Brownstein MJ, Usdin TB, Toshiyuki S, Carninci P, Prange C, Raha SS, Loquellano NA, Peters GJ, Abramson RD, Mullahy SJ, Bosak SA, McEwan PJ, McKernan KJ, Malek JA, Gunaratne PH, Richards S, Worley KC, Hale S, Garcia AM, Gay LJ, Hulyk SW, Villalon DK, Muzny DM, Sodergren EJ, Lu X, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Young AC, Shevchenko Y, Bouffard GG, Blakesley RW, Touchman JW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Krzywinski MI, Skalska U, Smailus DE, Schnerch A, Schein JE, Jones SJ, Marra MA. Generation and initial analysis of more than 15, 000 full-length human and mouse cDNA sequences. Proc Natl Acad Sci USA. 2002;99:16899–16903. doi: 10.1073/pnas.242603899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clark HF, Gurney AL, Abaya E, Baker K, Baldwin D, Brush J, Chen J, Chow B, Chui C, Crowley C, Currell B, Deuel B, Dowd P, Eaton D, Foster J, Grimaldi C, Gu Q, Hass PE, Heldens S, Huang A, Kim HS, Klimowski L, Jin Y, Johnson S, Lee J, Lewis L, Liao D, Mark M, Robbie E, dSanchez C, Schoenfeld J, Seshagiri S, Simmons L, Singh J, Smith V, Stinson J, Vagts A, Vandlen R, Watanabe C, Wieand D, Woods K, Xie MH, Yansura D, Yi S, Yu G, Yuan J, Zhang M, Zhang Z, Goddard A, Wood WI, Godowski P, Gray A. The secreted protein discovery initiative (SPDI), a large-scale effort to identify novel human secreted and transmembrane proteins: a bioinformatics assessment. Genome Res. 2003;13:2265–2270. doi: 10.1101/gr.1293003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhard DS, Wagner L, Feingold EA, Shenmen CM, Grouse LH, Schuler G, Klein SL, Old S, Rasooly R, Good P, Guyer M, Peck AM, Derge JG, Lipman D, Collins FS, Jang W, Sherry S, Feolo M, Misquitta L, Lee E, Rotmistrovsky K, Greenhut SF, Schaefer CF, Buetow K, Bonner TI, Haussler D, Kent J, Kiekhaus M, Furey T, Brent M, Prange C, Schreiber K, Shapiro N, Bhat NK, Hopkins RF, Hsie F, Driscoll T, Soares MB, Casavant TL, Scheetz TE, Brown-stein MJ, Usdin TB, Toshiyuki S, Carninci P, Piao Y, Dudekula DB, Ko MS, Kawakami K, Suzuki Y, Sugano S, Gruber CE, Smith MR, Simmons B, Moore T, Waterman R, Johnson SL, Ruan Y, Wei CL, Mathavan S, Gunaratne PH, Wu J, Garcia AM, Hulyk SW, Fuh E, Yuan Y, Sneed A, Kowis C, Hodgson A, Muzny DM, McPherson J, Gibbs RA, Fahey J, Helton E, Ketteman M, Madan A, Rodrigues S, Sanchez A, Whiting M, Madari A, Young AC, Wetherby KD, Granite SJ, Kwong PN, Brinkley CP, Pearson RL, Bouffard GG, Blakesly RW, Green ED, Dickson MC, Rodriguez AC, Grimwood J, Schmutz J, Myers RM, Butterfield YS, Griffith M, Griffith OL, Krzywinski MI, Liao N, Morin R, Palmquist D, Petrescu AS, Skalska U, Smailus DE, Stott JM, Schnerch A, Schein JE, Jones SJ, Holt RA, Baross A, Marra MA, Clifton S, Makowski KA, Bosak S, Malek J. The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC) Genome Res. 2004;14:2121–2127. doi: 10.1101/gr.2596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunningham J, Graus F, Anderson N, Posner JB. Partial characterization of the Purkinje cell antigens in paraneoplastic cerebellar degeneration. Neurology. 1986;36:1163–1168. doi: 10.1212/wnl.36.9.1163. [DOI] [PubMed] [Google Scholar]
- 20.Chen YT, Rettig WJ, Yenamandra AK, Kozak CA, Chaganti RS, Posner JB, Old LJ. Cerebellar degeneration-related antigen: a highly conserved neuroectodermal marker mapped to chromosomes X in human and mouse. Proc Natl Acad Sci USA. 1990;87:3077–3081. doi: 10.1073/pnas.87.8.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siniscalco M, Oberle I, Melis P, Alhadeff B, Murray J, Filippi G, Mattioni T, Chen YT, Furneaux H, Old LJ, et al. Physical and genetic mapping of the CDR gene with particular reference to its position with respect to the FRAXA site. Am J Med Genet. 1991;38:357–362. doi: 10.1002/ajmg.1320380239. [DOI] [PubMed] [Google Scholar]
- 22.Hillier LW, Graves TA, Fulton RS, Fulton LA, Pepin KH, Minx P, Wagner-McPherson C, Layman D, Wylie K, Sekhon M, Becker MC, Fewell GA, Delehaunty KD, Miner TL, Nash WE, Kremitzki C, Oddy L, Du H, Sun H, Bradshaw-Cordum H, Ali J, Carter J, Cordes M, Harris A, Isak A, van Brunt A, Nguyen C, Du F, Courtney L, Kalicki J, Ozersky P, Abbott S, Armstrong J, Belter EA, Caruso L, Cedroni M, Cotton M, Davidson T, Desai A, Elliott G, Erb T, Fronick C, Gaige T, Haakenson W, Haglund K, Holmes A, Harkins R, Kim K, Kruchowski SS, Strong CM, Grewal N, Goyea E, Hou S, Levy A, Martinka S, Mead K, McLellan MD, Meyer R, Randall-Maher J, Tomlinson C, Dauphin-Kohlberg S, Kozlowicz-Reilly A, Shah N, Swearengen-Shahid S, Snider J, Strong JT, Thompson J, Yoakum M, Leonard S, Pearman C, Trani L, Radionenko M, Waligorski JE, Wang C, Rock SM, Tin-Wollam AM, Maupin R, Latreille P, Wendl MC, Yang SP, Pohl C, Wallis JW, Spieth J, Bieri TA, Berkowicz N, Nelson JO, Osborne J, Ding L, Sabo A, Shotland Y, Sinha P, Wohldmann PE, Cook LL, Hickenbotham MT, Eldred J, Williams D, Jones TA, She X, Ciccarelli FD, Izaurralde E, Taylor J, Schmutz J, Myers RM, Cox DR, Huang X, McPherson JD, Mardis ER, Clifton SW, Warren WC, Chinwalla AT, Eddy SR, Marra MA, Ovcharenko I, Furey TS, Miller W, Eichler EE, Bork P, Suyama M, Torrents D, Waterston RH, Wilson RK. Generation and annotation of the DNA sequences of human chromosomes 2 and 4. Nature. 2005;434:724–731. doi: 10.1038/nature03466. [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER, 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, Shiloh Y, Gygi SP, Elledge SJ. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 24.Hurley PJ, Bunz F. ATM and ATR: components of an integrated circuit. Cell Cycle. 2007;6:414–417. doi: 10.4161/cc.6.4.3886. [DOI] [PubMed] [Google Scholar]
- 25.Staropoli JF. Tumorigenesis and neurodegeneration: two sides of the same coin? Bioessays. 2008;30:719–727. doi: 10.1002/bies.20784. [DOI] [PubMed] [Google Scholar]
- 26.Crawford TO. Ataxia telangiectasia. Semin Pediatr Neurol. 1998;5:287–294. doi: 10.1016/S1071-9091(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 27.Storstein A, Krossnes BK, Vedeler CA. Morphological and immunohistochemical characterisation of paraneoplastic cerebellar degeneration associated with Yo antibodies. Acta Neurol Scand. 2009;120(1):64–67. doi: 10.1111/j.1600-0404.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- 28.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7:327–340. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okano HJ, Park WY, Corradi JP, Darnell RB. The cytoplasmic Purkinje onconeural antigen cdr2 down-regulates c-Myc function: implications for neuronal and tumor cell survival. Genes Dev. 1999;13:2087–2097. doi: 10.1101/gad.13.16.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakai K, Kitagawa Y, Saiki S, Saiki M, Hirose G. Effect of a paraneoplastic cerebellar degeneration-associated neural protein on B-myb promoter activity. Neurobiol Dis. 2004;15:529–533. doi: 10.1016/j.nbd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Sakai K, Shirakawa T, Li Y, Kitagawa Y, Hirose G. Interaction of a paraneoplastic cerebellar degeneration-associated neuronal protein with the nuclear helix-loop-helix leucine zipper protein MRG X. Mol Cell Neurosci. 2002;19:477–484. doi: 10.1006/mcne.2001.1059. [DOI] [PubMed] [Google Scholar]
- 32.Takanaga H, Mukai H, Shibata H, Toshimori M, Ono Y. PKN interacts with a paraneoplastic cerebellar degeneration-associated antigen, which is a potential transcription factor. Exp Cell Res. 1998;241:363–372. doi: 10.1006/excr.1998.4060. [DOI] [PubMed] [Google Scholar]