Abstract
Antitumor effects of CD40 ligation appear to involve distinct antitumor effector cells in different experimental models. In this study, we tested whether T cells were required for antitumor effects of agonistic anti-CD40 mAb (αCD40) against immunogenic versus poorly immunogenic tumors. Treatment of mice bearing poorly immunogenic B16 melanoma and its more immunogenic variant, B16-hsp72.1, with αCD40 resulted in a similar level of tumor growth suppression. Depletion of T cells did not reduce the antitumor effects in these 2 tumor models. To generate antitumor T cell responses, C57BL/6 mice were immunized with irradiated B16-hsp72.1. Treatment of these vaccinated mice challenged with a high dose of B16-hsp72.1 tumor cells with αCD40 induced tumor growth suppression, which was reduced by T-cell depletion, demonstrating that T cells were involved in the antitumor effect of αCD40. However, immunized mice depleted of T cells and treated with αCD40 were still able to suppress tumor growth as compared to tumor growth in immunized, T cell-depleted mice not treated with αCD40, suggesting that T cells were not required for the antitumor effect of αCD40. To confirm a lack of correlation between tumor immunogenicity and T-cell requirement in antitumor effects of CD40 ligation, we found that αCD40 induced tumor growth suppression in nude and SCID/beige mice bearing highly immunogenic tumors such as Meth A sarcoma, suggesting that macrophages may play a role. Indeed, both poorly immunogenic and highly immunogenic tumors were sensitive to in vitro growth inhibition by macrophages from αCD40-treated mice. Taken together, our results indicate that antitumor effects induced by αCD40, even against immunogenic tumors, can be observed in the absence of T cells and may involve macrophages.
Keywords: Tumor Immunity, T cells, Monocytes/macrophages, Anti-CD40 mAb
Introduction
CD40–CD40 ligand (CD40L3) interaction has been found to be a crucial mechanism involved in generation of the immune response [1, 21, 23]. While the steps involved in the CD40–CD40L interaction between APC and T cells have been well characterized, the mechanisms of the antitumor effects of CD40 ligation are not yet fully understood. Several lines of in vitro evidence suggest that direct CD40–CD40L interaction might be involved in the antitumor effects of CD40 ligation in some models of CD40+ tumors. For example, it has been shown that for some tumors including B-cell lymphomas [7], human breast carcinomas [9], and human melanomas [13], CD40-ligation can directly induce proliferation inhibition and apoptosis of tumor cells in vitro [9, 13]. However, in vivo studies have shown that agonistic anti-CD40 mAb (αCD40) was much more effective in inducing antitumor effects against CD40-positive B-cell lymphomas in immunocompetent mice than in SCID mice [7]. This observation indicates that ligation of tumor cell surface CD40 in vivo is not, by itself, responsible for the antitumor effect and suggests that the CD40-induced antitumor effects are immunologically mediated, and likely involve T cells in this system [7]. In addition to a strong antitumor effect of αCD40 against CD40-positive B-lymphomas [7, 29], CD40 ligation can also induce antitumor effects against CD40-negative tumors, including highly immunogenic tumors [7, 27, 30]. These data support the predominant view that antitumor effects induced by CD40-triggering agents are indirect and mediated by tumor-reactive T cells that have been activated by the CD40-stimulated APC. Indeed, in many studies with immunogenic tumors the therapeutic effects of CD40 ligation have been shown to involve CD8+ T cells [6, 7, 16, 17, 25, 29, 30].
We have identified two separate antitumor pathways for CD40 ligation that do not appear to work through the activation of systemic CD8+ T-cell immunity. First, we have shown that in vivo treatment of tumor-bearing mice with αCD40 results in activation of NK cells and causes T-cell-independent antitumor and antimetastatic effects against weakly immunogenic mouse tumors [28]. Second, even in the absence of both T cells and NK cells, αCD40 treatment activated macrophages (Mϕ) mediated antitumor effects in vitro and in vivo against weakly immunogenic tumors [2, 14]. While it is clear that APC such as Mϕ can be directly activated by CD40 ligation, as we [2] and others [5, 11] have shown, the direct role of these cells in CD40 ligation-induced antitumor effects, particularly against highly immunogenic tumors, has not been elucidated.
To analyze the role of T cells in antitumor effects of CD40 ligation, we tested whether T cells were required for antitumor effects of agonistic αCD40 against highly immunogenic versus weakly immunogenic tumors. We hypothesized that αCD40 would induce primarily T cell-mediated responses against highly immunogenic tumors, and NK and/or Mϕ-mediated responses against weakly immunogenic tumors. In these studies, we operationally define the “immunogenicity” by the ability of a primary tumor or tumor vaccine to induce an immune response that is protective to subsequent challenge with the same tumor cells. Unexpectedly, our results showed that CD40 ligation could induce T-cell-independent antitumor effects even against immunogenic tumors.
Materials and methods
Mice and tumor cell lines
C57BL/6, BALB/C, DBA/2, A/J, nude and C.B.-17 SCID/beige female mice between 8 and 12 weeks of age were obtained from Harlan Sprague Dawley, Madison, WI, USA. All animals were housed in university-approved facilities and were handled according to National Institutes of Health and University of Wisconsin-Madison Research Animal Resource Center guidelines. The tumor cell lines used in this study were weakly immunogenic B16 melanoma (H-2b), and highly immunogenic L5178Y lymphoma (H-2d), Meth A sarcoma (H-2d), CMT-93 carcinoma (H-2b) and Renca carcinoma (H-2d). In addition, a more immunogenic variant of B16 melanoma, B16-hsp72.1, was used. The B16-hsp72.1 clone was obtained by transfecting B16 cells to express the 72-kD human heat shock protein (hsp), which resulted in increased MHC class I expression and immunogenicity (34).
The cell lines were grown in RPMI-1640 media supplemented with penicillin and streptomycin (100 U/ml), l-glutamine (2 mM) (both from Life Technologies, Inc., Grand Island, NY, USA) and 10% fetal calf serum (FCS, Sigma Chemicals, St Louis, MO, USA). The B16-hsp72.1 line was grown in the presence of G18 antibiotic to maintain selective pressure. Cells were maintained at 37°C in a humidified 5% CO2 atmosphere. When tested for surface expression of CD40 by flow cytometry, B16, MethA, Renca and CMT-93 tumor cells were and found to be negative, while L51718Y cells had a negligible level of CD40 expression (not shown).
Agonistic αCD40 and tumor therapy
The FGK 45.5 hybridoma producing the agonistic rat anti-mouse CD40 mAb [22] was a gift from Dr. Fritz Melchers (Basel Institute for Immunology, Basel, Switzerland). αCD40 was purified and confirmed to be specific as previously described [28]. For in vivo studies, mice were injected s.c. with the indicated numbers of tumor cells in 100 μl PBS in the middle of the abdominal wall. Mice were injected intraperitoneally (i.p.) with 0.25–0.5 mg of either αCD40 or control rat IgG (Sigma) on days 5, 12 and 19 (unless stated otherwise) after tumor cell implantation. Tumor size was measured twice a week with a caliper, and volume was calculated by applying the formula [volume (mm3) = length × width × width/2].
Vaccination
C57BL/6 mice were injected s.c. in the left and right flanks with 106 γ-irradiated (200 Gy) B16-hsp72.1 cells in 0.1 PBS on days −42 and −21. On day 0, these vaccinated and naïve mice were injected s.c. in the middle of the abdominal wall with 7.5 × 105 viable B16-hsp72.1 tumor cells.
In vivo cell depletion
C57BL/6 mice were depleted of T and NK cells by i.p. injection of a mixture of 0.3 mg anti-CD4 mAb (clone GK1.5), 0.3 mg anti-CD8 mAb (clone 2.43), and 0.3 mg anti-NK1.1 mAb (clone PK136) in 0.5 ml PBS on days 2, 7, 11 and 16 after tumor cell implantation. The efficacy of cell depletion was confirmed by us in previous studies [3].
Concomitant immunity model
Mice were injected s.c. with 106 B16 or B16-hsp72.1 tumor cells (C57BL/6 mice) or MethA cells (Balb/c mice) in the right side of the abdomen. Nine days later, these tumor-bearing mice were injected s.c. with 4–5 × 105 tumor cells in the left side of the abdomen. As a control, naïve C57BL/6 or Balb/c mice were also injected with the same tumor cells in the left side of the abdomen.
Tumor growth was followed.
Flow cytometric analysis
B16 and B16-Hsp72.1 (0.5–1 × 106) were stained for 40 min at 4° C with FITC-conjugated anti-H-2Kb and H-2Db mAbs (PharMingen, San Diego, CA, USA). Propidium iodide was added to stain dead cells that subsequently were excluded from the analysis. Stained cells were analyzed using a FACScan cytofluorometer (Becton Dickinson, San Jose, CA, USA) and data collected for 10,000 events/sample.
Cytostatic assay by peritoneal Mϕ
Antitumor cytostatic activity of Mϕ was determined by the inhibition of 3H-thymidine (3H-TdR)-incorporation in tumor cells, as described previously [3, 28]. In brief, peritoneal exudate cells (PEC) were collected by peritoneal lavage from groups of 3–4 C57BL/6 mice treated 5 days earlier with 0.5 mg αCD40 or control rat IgG. PEC were plated at 3 × 105 cells in 0.1 ml/well in a 96-microwell flat bottom plate (Corning Inc, Corning, NY, USA). After 2 h, the monolayer was washed 3 times with warm cell culture medium to remove nonadherent cells. Flow cytometry revealed that 95% of adherent cells were Mϕ, based on F4/80 expression. L5178Y, Renca, CMT-93 or MethA tumor cell targets (1 × 104/well) were added in triplicate for 48 h to wells with or without Mϕ in the presence of 10 ng/ml of lipopolysaccharide (LPS) (Sigma). Cells were pulsed with 3H-TdR (1 μCi/well, PerkinElmer, Boston, MA, USA) during the last 6 h of incubation. 3H-TdR incorporation was determined by β-scintillation of total cells harvested from the wells onto glass fiber filters (Packard, Meriden, CT, USA), using the Packard Matrix 9600 Direct β-counter (Packard). Results are expressed as 5-min mean counts of triplicate wells ± SEM. In these assays, PEC showed negligible [3H]-TdR incorporation (data not shown), as published previously [2].
Statistical analysis
A two-tailed Student’s t test was used to determine significance of differences between experimental and relevant control values. Data are presented as mean ± SEM.
Results
αCD40 induces similar T cell-independent antitumor effects against B16 melanoma and its more immunogenic variant, B16-hsp72.1
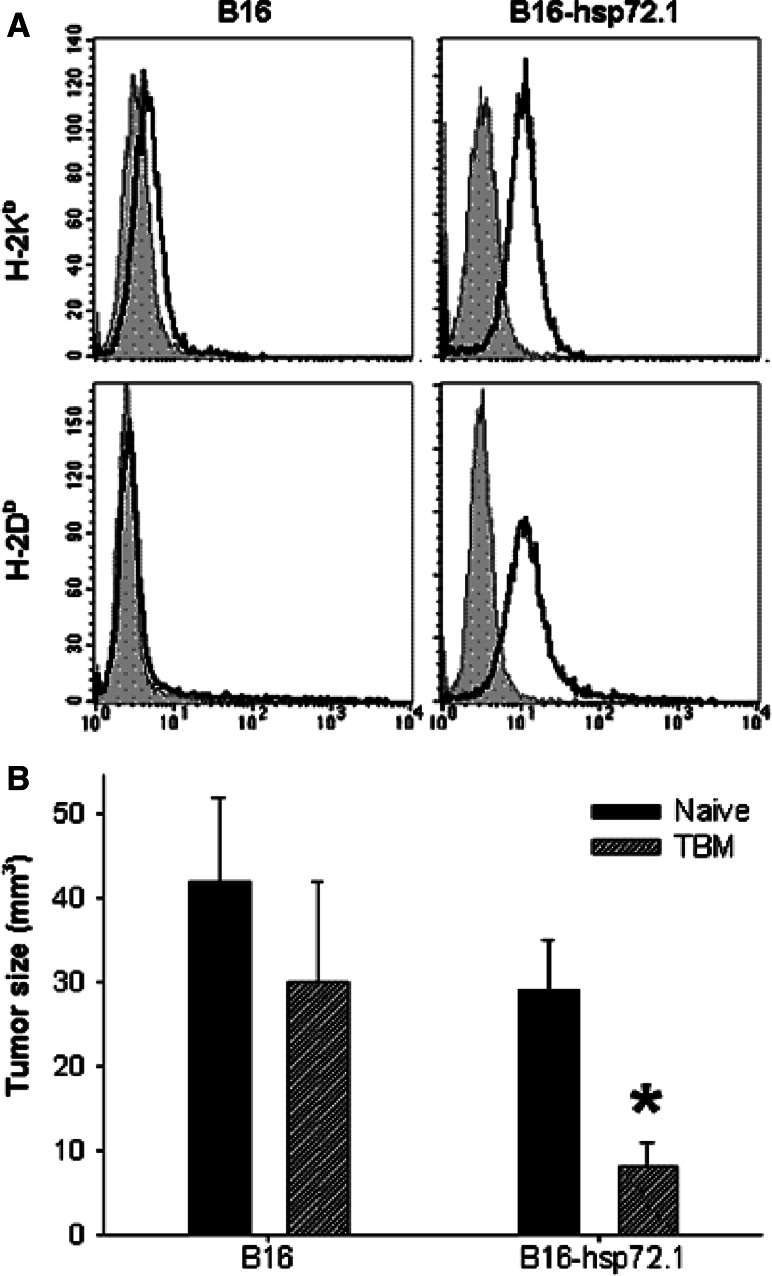
We hypothesized that the initial immune mechanisms induced by growing tumor cells, namely highly immunogenic tumors preferentially activating T cells and weakly immunogenic tumors activating NK cells or Mϕ, could be further stimulated by CD40 ligation. To address this hypothesis, we compared the in vivo growth of the weakly immunogenic wild-type B16 cells with that of the more immunogenic variant of this same B16 tumor, B16-hsp72.1, in response to αCD40 treatment. The B16-hsp72.1 cells were far more effective than are parental B16 cells at immunizing syngeneic mice [34]. The higher immunogenicity was associated with augmented MHC-class-I expression on B16-hsp72.1 cells as shown previously [34] and confirmed here (Fig. 1a). In addition, using a model of concomitant immunity, we confirmed that B16-hsp.72.1 cells are more immunogenic than parental B16 cells. In this model, growing primary tumors induce immune response, which depending on tumor immunogenicity, can inhibit or prevent growth of a second inoculum of tumor cells. The results in Fig. 1b show that the growth of secondary tumors implanted 9 days after the primary tumors was significantly reduced in mice bearing B16-hsp72.1 tumors, but not B16 tumors.
Fig. 1.
Immunogenicity of B16-hsp72.1 versus B16 tumor. a Expression of MHC class I antigens. B16 and B16-hsp72.1 tumor cells were stained with FITC-conjugated anti-H-2Kb mAb and PE-conjugated H-2Db mAb (gray) or isotype controls (black). b Concomitant immunity in mice bearing B16 and B16-hsp72.1 tumors. C57BL/6 mice were injected s.c. with 106 B16 or B16-hsp72.1 tumor cells in the right side of the abdomen. Nine days later, these same tumor-bearing mice (TBM) were injected s.c. with 4 × 105 B16 or B16-hsp72.1 tumor cells in the left side of the abdomen, respectively. At that same time, naïve C57BL/6 mice were also injected with B16 or B16-hsp72.1 tumor cells in the left side as well. Tumor volumes are presented as Mean ± SEM on day 8 post secondary tumor challenge. *P < 0.025
To determine if tumor sensitivity to αCD40 therapy depends on tumor immunogenicity, we compared the effects of αCD40 treatment on parental B16 tumor and B16-hsp72.1 tumor growing in syngeneic C57BL/6 mice. Because B16-hsp72.1 cells formed tumors slower than B16 cells in preliminary experiments, C57BL/6 mice were injected s.c. with 1 × 105 B16 cells or 2 × 105 B16-hsp72.1 cells. The results presented in Fig. 2 show that B16 tumors and B16-hsp72.1 tumors had a similar high sensitivity to αCD40 therapy. In addition, our results show that the antitumor effects of αCD40 against either tumor did not require T cells: tumor growth suppression induced by αCD40 therapy was not inhibited by depletion of CD4+ and CD8+ T cells (Fig. 2).
Fig. 2.
Effect of αCD40 against B16 and B16-hsp72.1 melanomas: Role of T cells. C57BL/6 mice were injected s.c. with 1 × 105 B16 cells (a) or 2 × 105 B16-hsp72.1 cells (b) on day 0. αCD40 (0.5 mg) was injected i.p. on days 5, 12 and 19. αCD40-treated mice were depleted of T cells as described in “Materials and methods”. Control mice received rat IgG. Tumor volumes are presented as Mean ± SEM
Role of T cells in CD40 ligation-induced antitumor effects in B16-hsp72.1-vaccinated mice
To increase T-cell immunity in mice at the time of αCD40 therapy, we decided to immunize mice with irradiated B16-hsp72 tumor cells and challenge them with a tumorogenic dose of B16-hsp72 tumor. In an initial series of experiments, C57BL/6 mice were vaccinated with γ-irradiated B16-hsp72 tumor cells, and boosted 3 weeks later. This immunization regimen resulted in complete protection against the tumor challenge with 105 B16-hsp72.1 tumor cells. In contrast, a larger dose of B16-hsp72.1 tumor cells (7.5 × 105) induced tumor growth in all immunized mice, although these tumors grew at a slower pace than in non-vaccinated mice (data not shown). The rate of B16-hsp72.1 tumor growth in vaccinated mice varied between the experiments (e.g., Fig. 3a vs. 4a), probably due to variability from experiment to experiment in the strength of the immune response generated.
Fig. 3.
Effect of αCD40 on B16-hsp72.1 tumor growth in vaccinated and non-vaccinated mice: role of T and NK cells. C57BL/6 mice were injected s.c. with 106 γ-irradiated B16-hsp72.1 cells on days −42 and −21. On day 0, these vaccinated mice (a) and naïve mice (b) were injected s.c. with 7.5 × 105 B16-hsp72.1 tumor cells. αCD40 (0.5 mg) or rat IgG were injected i.p. on days 5, 12 and 19. αCD40-treated mice were depleted of T cells or NK cells as described in “Materials and methods”. Control mice received rat IgG instead of anti-CD4/CD8 mAbs. Tumor volumes are presented as Mean ± SEM. *P < 0.05
Fig. 4.
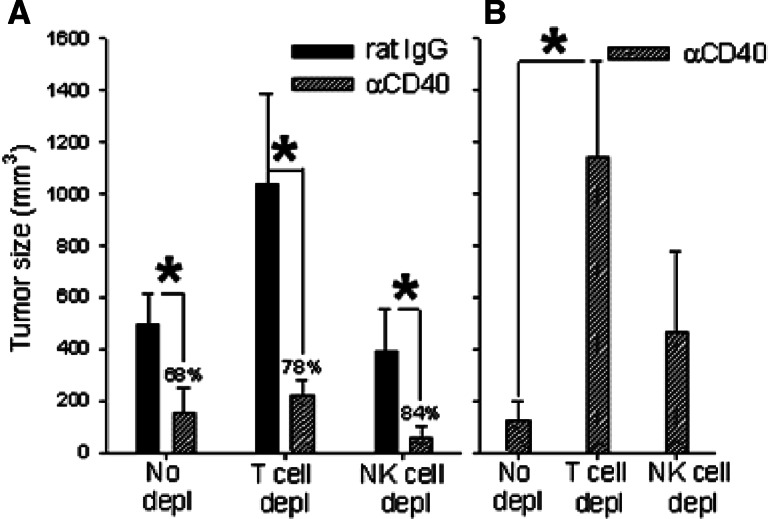
Effect of αCD40 on B16-hsp72.1 tumor growth in vaccinated mice: role of T and NK cells. C57BL/6 mice were vaccinated on day −42 and −21 with 1 × 106 γ-irradiated B16-hsp72.1 cells. On day 0, these mice were challenged with 7.5 × 105 B16-hsp72.1 cells. Anti-CD4 mAb + anti-CD8 mAb and anti-NK1.1 mAb were injected on day 2,7 and 11. αCD40 (0.5 mg) or rat IgG were injected on day 5 and 12. Tumor volumes are shown as Mean ± SEM on day 16 (a) and 23 (b). By day 23, the tumors in the rat IgG groups (no depl, T cell depl, and NK depl) had grown too large so that all animals had been euthanized, and thus data for tumor size could not be shown. Percentages above each pair of bars in A indicate index of tumor growth inhibition for each group [i.e., 100 × (tumor size in rat IgG treated mice) − (tumor size in αCD40 treated mice)/(tumor size in rat IgG treated mice)]. *P < 0.05
We then used these conditions to evaluate αCD40 treatment effects. C57BL/6 mice were again vaccinated twice with irradiated B16-hsp72.1 cells, challenged with 7.5 × 105 of B16-hsp72.1 cells, and then treated with αCD40 on days 5, 12 and 19 after tumor implantation. As noted above, the B16-hsp72.1 tumors grew more slowly in vaccinated mice than in naive mice; note the difference in tumor sizes (different scales on y axis) for the “control” tumor bearing animals in immunized mice (Fig. 3a) versus non-immunized mice (Fig. 3b). To determine the role of T cell and NK cells in αCD40-induced antitumor effects, αCD40-treated mice were depleted of T cells or NK cells by injections of anti-CD4 mAb + anti-CD8 mAb, or anti-NK1.1 mAb, respectively. The results in Fig. 3 show that depletion of T cells reduced the antitumor effect of CD40 ligation in vaccinated mice (P < 0.05), but not in non-vaccinated mice, suggesting that T cells were involved in the antitumor effects induced by αCD40 in the vaccinated mice. Depletion of NK cells also reduced the antitumor effect of CD40 ligation in vaccinated mice, but this reduction was not statistically significant.
To further elucidate the role of T cells and NK cells in CD40 ligation-induced antitumor effects in vaccinated mice, in the next experiment, we depleted T cells and NK cells in both αCD40-treated and rat IgG-treated mice. The results show that αCD40 can induce antitumor effects in the absence of T cells, as seen 16 days after tumor cell challenge (Fig. 4a). However, the involvement of T cells in the antitumor effect of αCD40 in these same animals was evident 1 week later: αCD40-treated mice depleted of T cells had significantly larger tumors than αCD40-treated non-depleted mice (Fig. 4b, day 23 post tumor-cell transplantation).
Role of T cells in anti-CD40 mAb therapy of highly immunogenic tumors
The results of the previous experiment showed that αCD40 induced growth inhibition of B16-hsp72.1 tumors in vaccinated mice, even in the absence of T cells. It appeared, therefore, that T cells are not essential in CD40-ligation-induced antitumor effects even in the case of a more immunogenic tumor. To further test this hypothesis, we determined the effect of αCD40 against four intrincically immunogenic tumors (L5178Y lymphoma, Renca carcinoma, CMT-93 carcinoma and MethA sarcoma) growing in immunocompetent and T-cell-deficient mice. Some of these tumors, such as CMT-93, have been shown by others to respond to αCD40 with a T-cell-mediated immune response [27].
We determined the antitumor effects of αCD40 against CMT-93 carcinoma. The preliminary experiments established that the minimal tumorigenic dose of these cells in syngeneic C57BL/6 mice was 7.5–10 × 106 cells (data not shown). C57BL/6 mice injected with such high numbers of tumor cells normally developed small, slowly growing tumors that often spontaneously regressed (data not shown). This is consistent with the CMT-93 carcinoma being a highly immunogeneic tumor [27]. CMT-93 cells were implanted in C57BL/6 mice and the mice received the treatment with αCD40. The results in Fig. 5a show that CD40 ligation induced more rapid regression of CMT-93 tumors in immunocompetent mice. Spontaneous or CD40 ligation-induced tumor regression required T cells, as it was not observed in T cell depleted mice (Fig. 5b). However, T cells were not solely responsible for the antitumor effect induced by αCD40, because CMT-93 tumor growth suppression was still observed in T-cell-depleted mice following the anti-CD40 mAb treatment (Fig. 5b).
Fig. 5.
Effect of αCD40 therapy on immunogenic tumors in vivo. a C57BL/6 mice were injected s.c. with 8 × 106 CMT-93 tumor cells on day 0. b Another group of C57BL/6 mice received i.p. injections of anti-CD4 mAb + anti-CD8 mAb on days −1, 3, 7, 11 and 15, and these mice received 1 × 106 CMT-93 tumor cells s.c. on day 0. Both groups of mice received αCD40 (0.5 mg) or rat IgG i.p. on days 5 and 12. Tumor volumes are presented as mean ± SEM for all data points. Numbers indicate tumor-free mice/total mice per group. *P < 0.001; **P < 0.05. c Nude mice were injected s.c. with 1 × 106 L5178Y lymphoma, Renca carcinoma, CMT-93 carcinoma or MethA sarcoma cells on day 0. αCD40 (0.25 mg) or rat IgG were injected i.p. on days 5 and 12. Tumor volumes are presented as Mean ± SEM on days 18–19. *P < 0.05 for tumor size in treated vs. control groups, evaluated independently for each tumor type shown. d Scid/beige mice were injected s.c. with 7 × 105 MethA cells (day 0). αCD40 or rat IgG (0.5 mg) was injected i.p. on day 4, 8 and 12. Mean ± SEM of 6–7 mice per group. *P < 0.05; **P < 0.001
Experiments conducted in nude mice confirmed that αCD40-induced growth suppression of intrinsically immunogenic tumors does not require T cells. Figure 5c shows that αCD40 treatment of nude mice induced tumor growth suppression in each of four tested tumor models: L5178Y lymphoma, Renca carcinoma, CMT-93 carcinoma and MethA sarcoma. Because nude mice still have peripheral T cells which in some cases may be functional [10, 20], we tested the effect of αCD40 in SCID/beige mice which lack T cells, B cells and cytotoxic NK cells. The results in Fig. 5d show that CD40 ligation significantly suppressed MethA tumor growth in SCID/beige mice, confirming that αCD40 can induce T cell-independent antitumor effects against highly immunogenic tumors.
In the previous experiments αCD40 therapy was started on day 5 post tumor cell implantation when the tumors were very small. We next asked whether αCD40 could induce growth suppression of advanced tumors. Treatment of Balb/c mice with αCD40 on days 15, 17, 19 and 21 post s.c. injection of 106 MethA cells (average tumor diameter was 8.3–9.5 mm on day 14) resulted in substantial antitumor effect as measured by tumor volumes ± SEM on day 22 (453 ± 181 vs. 2017 ± 454 mm3 in αCD40 vs. rat IgG-treated mice, P < 0.01).
Anti-CD40 mAb can inhibit concomitant antitumor immune response in vivo
In the experiments described above, αCD40 induced suppression of MethA tumor growth, especially in immunodeficient mice. However, in some experiments with immunocompetent mice when MethA tumors grew slowly or were spontaneously regressing due to their high immunogenicity, αCD40 therapy could actually facilitate tumor growth (not shown). Therefore, we suggested that in some instances αCD40 can suppress ongoing T-cell antitumor responses. To test this hypothesis in vivo, we employed a model of concomitant immunity similar to that shown in Fig. 1b. In this model, the strength of antitumor immunity in tumor-bearing mice is determined by rejection of a smaller inoculum of the same tumor. In immunocompetent mice bearing a primary MethA tumor, concomitant immunity reaches a peak on day 9 [18]. Therefore, Balb/c mice were injected with MethA tumor cells, treated with αCD40 or rat IgG 4 and 8 days later, and injected at a distant site with MethA cells 9 days after the first tumor injection. As expected, secondary tumors did not grow in rat IgG-treated mice due to concomitant immunity (Fig. 6a). In contrast, secondary tumors emerged, although temporarily, in mice treated with αCD40 (Fig. 6b), indicating that CD40 ligation can transiently suppress T cell-mediated [18] concomitant antitumor responses against MethA sarcoma.
Fig. 6.
Effect of αCD40 on concomitant immunity. a, b Balb/c mice were injected s.c. with 1 × 106 MethA cells in the right side of abdomen (day −9). Rat IgG (a) or αCD40 (b) at the dose of 0.5 mg per mouse was injected i.p. on day −5 and −1. On day 0, these and naïve control mice (c) were injected s.c. with 5 × 105 MethA cells in the left side of abdomen. Individual tumor volumes for the tumors on the left side of the abdomen for 4 (a, b) or 5 (c) mice per group are shown
CD40 ligation can activated Mϕ to suppress tumor cell proliferation in vitro
The results in Fig. 5d showed that αCD40 induced antitumor effects against MethA tumor in SCID/beige mice, indicating that the cells other than T, B or cytotoxic NK cells are responsible for these effects. Based on our recent studies with weakly immunogeneic tumors showing the role of Mϕ in the antitumor effects [3, 14], we suggested that Mϕ can be activated by CD40 ligation to kill highly immunogeneic tumors. We show here that Mϕ activated with αCD40 in combination with LPS suppressed proliferation of 4 separate immunogenic tumor cell lines in vitro (Fig. 7), suggesting that Mϕ activated in vivo with αCD40 may control growth of immunogeneic tumors even in the absence of T cells.
Fig. 7.
In vitro sensitivity of immunogenic tumor cell lines to αCD40-activated Mϕ. C57BL/6 mice were injected i.p. with either αCD40 (0.5 mg/mouse) or rat IgG (control). Five days later, PEC were harvested, seeded into 96 well plates (3 × 105 cells/well), and enriched for Mϕ by adherence to plastic. Indicated tumor cells (1 × 104cells/well) were co-cultured with Mϕ with or without LPS (10 ng/ml) for 48 h. Mϕ-mediated inhibition of tumor cell proliferation was measured by 3H-TdR incorporation. Results are presented as mean ± SEM of triplicate wells. *P < 0.001. **P < 0.005
Discussion
CD40–CD40L interaction between APC and CD4+ T cells is an intrinsic mechanism of a normal immune response resulting in CD8+ T-cell activation [1, 21, 23]. CD40 ligation has been shown to be involved in generation of antitumor T-cell immunity using cancer vaccines [16]. Based on this activation pathway, different strategies to directly activate APC either with CD40L or αCD40 have been used for inducing therapeutic T-cell responses against established tumors [6, 7, 25, 27, 29, 30]. However, we [3, 14, 28] and others [8] demonstrated that CD40 ligation could induce antitumor effects against weakly immunogenic tumors even in the absence of T cells. These antitumor effects could be mediated by NK cells activated indirectly [8, 28] or directly activated Mϕ [3, 14]. As such, in vivo antitumor effects could be achieved by CD40 ligation, and these effects could involve distinct antitumor effector cells (Mϕ, NK or T cells) in different experimental models. It is not clear why different effectors seemed to be involved in the antitumor response of CD40 ligation in different model systems. We hypothesized that T cells could be primarily involved in the antitumor effects of CD40 ligation against more immunogenic tumors, whereas innate immune cells could be the major effector cells against weakly immunogenic tumors. Thus we have here presented the analyses of CD40 ligation in mice bearing weakly or strongly immunogenic tumors and characterized the phenotypes of cells involved. The results of this study show that even with immunogenic tumors, αCD40 therapy can induce T-cell-independent growth suppression.
Our results do not imply that T cells had no contribution to the antitumor effects induced by anti-CD40 mAb. In fact, our data with highly immunogenic CMT-93 tumors show that complete tumor resolution, both spontaneous and CD40 ligation-facilitated, was achieved in immunocompetent mice, but not in T-cell-deficient nude mice. These results indicate that T cells were essential for complete tumor eradication in this model. In agreement with the study by Todryk et al. [27], αCD40 facilitated CMT-93 tumor eradication in immunocompetent mice, but also caused significant tumor growth suppression in nude mice, indicating that CD40 ligation can induce T cell-independent antitumor effects. Mϕ activated in vivo by CD40 ligation were able to suppress proliferation of immunogenic tumor cells in the presence of LPS in vitro. Together with the finding that NK cells did not play a substantial role in the antitumor effects analyzed in this study, these results suggest that αCD40-activated Mϕ can mediate antitumor effects against immunogenic tumors in vivo. This is consistent with our recent findings demonstrating a role of Mϕ in CD40 ligation-induced antitumor effects in vivo against weakly immunogenic tumors such as B16 melanoma and NXS2 neuroblastoma [3, 14].
It is not clear why in some models T cells, in particular CD8+ T cells, appear to be crucial for CD40-ligation-induced antitumor effects, whereas in our studies they do not seem to be essential. Our findings suggest that, in part, these differences may be due to the design and interpretation of depletion experiments. For example, Fig. 3a illustrates that depletion of CD8+ T cells in vaccinated B16-hsp72.1-bearing mice treated with αCD40 abrogated the antitumor effect of αCD40. These results would support the conclusion that CD8+ T cells are essential for the antitumor effect of αCD40. However, when we asked a different question in this same tumor model, i.e., whether αCD40 could induce antitumor effects against an immunogenic tumor in the absence of T cells, we used a different experimental design and the conclusion from our experiments was different. Specifically, T cells were depleted not only in αCD40-treated mice, but also in control tumor-bearing mice (Fig. 4a). In comparison with T-cell-depleted, rat IgG-treated mice, there was a significant antitumor effect seen on day 16 in T-cell depleted, αCD40-treated mice, suggesting that CD40 ligation may induce tumor reduction in the absence of T cells. Nevertheless, compared with immunocompetent αCD40-treated mice, T-cell-depleted αCD40-treated mice showed a significant reduction of the antitumor effect, especially at the later time points, indicating that T cells are also involved in the antitumor effects of CD40 ligation against immunogenic tumors.
It is also possible that the duration of αCD40 treatment may influence the level of contribution of T cell and innate immunity to the observed antitumor effects. Thus, Stumbles et al. showed that continued treatment with αCD40 given every other day was required to maintain CD8+ T-cell-mediated tumor regression in their model system [26]. Also, it appears that the efficacy of αCD40 therapy and possibly involvement of T cells may depend on the dose of tumor cell inoculum [29] and tumor location—for example, tumors injected i.v. were more responsive to αCD40 treatment than s.c. tumors [27, 29]. Another study has shown that αCD40 accelerated deletion of tumor-specific CD8+ T cells, unless it was coupled to the tumor vaccine [12]. This negative effect of αCD40 on CD8+ T cells may explain the temporary inhibition of CD8+ T cell-mediated concomitant antitumor immunity [18] in our experiments with the Meth A tumor model (Fig. 6).
Overall, our results suggest a two-phase process of CD40 ligation-induced antitumor effector mechanisms. First, αCD40 directly activates Mϕ, which are capable of exerting antitumor effects in vitro via nitric oxide, TNF-α [15] and tumor cell apoptosis [2]. These activated Mϕ also appear to induce antitumor effects in vivo [3, 14]. In addition, CD40 ligation-activated Mϕ and dendritic cells secrete cytokines such as IL-12 [5, 19], resulting in activation of NK cells [8, 28]. These CD40 ligation-activated Mϕ and dendritic cells also express co-stimulatory molecules, leading to activation of antigen-specific CD8+ T effector cells [1, 21, 23, 31] that can recognize and destroy immunogenic tumors. This mechanism of CD40 ligation-induced antitumor effects is compatible with other studies. Thus, Stumbles et al. demonstrated the role of CD8+ T cells in tumor regression following systemic CD40 activation, but also noted the existence of another mechanism capable of suppressing tumor growth in CD8+ T cell-depleted mice [26]. Similarly to this report, as well as to the results of the present study, it was shown that whereas CD8+ T cells were essential for complete resolution of CMT93 tumors, CD40 ligation still induced some level of tumor growth suppression in nude mice [27]. The exact mechanism of T cell-independent, αCD40-induced inhibition of growth of immunogeneic tumors in immunodeficient mice is yet to be elucidated.
It has long been known that chemotherapy and radiotherapy, common clinical treatments for cancer, can result in immunosuppression [4]. However, these treatments are not equally immunosuppressive to all components of the immune system. For example, while chemotherapy in breast cancer patients caused reduction in CD3+ T cell number, the total number of CD14+ monocytes/Mϕ was not affected [24]. Therefore, approaches such as CD40 ligation aimed at activating cells of innate immunity in the absence of T cells may be clinically relevant for treating patients whose T cell immunity is compromised by chemotherapy or radiation. Given the promising antitumor effects of CD40L [32] and anti-CD40 mAb [33] in recent clinical trials, future studies are required to rationally design improved therapeutic strategies employing CD40 ligation. For example, we have recently shown that an addition of CpG to anti-CD40 mAb resulted in synergistic Mϕ activation and enhanced antitumor effects in mice [3]. Further testing is required to determine if this combination may be appropriate for future clinical testing as a means to augment the antitumor efficacy of CD40 ligation.
Acknowledgments
The authors thank Drs. Jackie Hank, Jacek Gan, and Hillary Lum for helpful discussions.
Abbreviations
- CD40L
CD40 ligand
- αCD40
Anti-CD40 monoclonal antibody
- Mϕ
Macrophages
- PEC
Peritoneal exudate cells
- IgG
Immunoglobulin G
- 3H-TdR
3H-thymidine
Footnotes
This work was supported by National Institutes of Health Grants CA87025, CA032685, grants from the Midwest Athletes Against Childhood Cancer Fund, and support from the Crawdaddy Foundation.
References
- 1.Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFA, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 2.Buhtoiarov IN, Lum HD, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation induces antitumor reactivity of murine macrophages via an IFN gamma-dependent mechanism. J Immunol. 2005;174:6013–6022. doi: 10.4049/jimmunol.174.10.6013. [DOI] [PubMed] [Google Scholar]
- 3.Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–318. doi: 10.4049/jimmunol.176.1.309. [DOI] [PubMed] [Google Scholar]
- 4.Campbell AC, Hersey P, MacLennan IC, Kay HE, Pike MC. Immunosuppressive consequences of radiotherapy and chemotherapy in patients with acute lymphoblastic leukaemia. BMJ. 1973;2:385–388. doi: 10.1136/bmj.2.5863.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeKruyff RH., Gieni RS, Umetsu DT. Antigen-driven but not lipopolysaccharide-driven IL-12 production in macrophages requires triggering of CD40. J Immunol. 1997;158:359–366. [PubMed] [Google Scholar]
- 6.Diehl L, Boer AT, Schoenberger SP, Voort E, Schumacher TNM, Melief CJM, Offringa R, Toes REM. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments antitumor vaccine efficacy. Nat Med. 1999;5:774–779. doi: 10.1038/10495. [DOI] [PubMed] [Google Scholar]
- 7.French RR, Chan HTC, Tutt AL, Glennie MJ. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/5505. [DOI] [PubMed] [Google Scholar]
- 8.Gruber TA, Skelton DC, Kohn DB. Requirement for NK cells in CD40 ligand-mediated rejection of Philadelphia chromosome-positive acute lymphoblastic leukemia cells. J Immunol. 2002;168:73–80. doi: 10.4049/jimmunol.168.1.73. [DOI] [PubMed] [Google Scholar]
- 9.Hirano A, Longo DL, Taub DD, Ferris DK, Young LS, Eliopoulos AG, Agathanggelou A, Cullen N, Macartney J, Fanslow WC, Murphy WJ. Inhibition of human breast carcinoma growth by a soluble recombinant human CD40 ligand. Blood. 1999;93:2999–3007. [PubMed] [Google Scholar]
- 10.Hunig T. T-cell function and specificity in athymic mice. Immunol Today. 1983;4:84–87. doi: 10.1016/0167-5699(83)90125-1. [DOI] [PubMed] [Google Scholar]
- 11.Imaizumi K, Kawabe T, Ichiyama S, Kikutani H, Yagita H, Shimokata K, Hasegawa Y. Enhancement of tumoricidal activity of alveolar macrophages via CD40–CD40 ligand interaction. Am J Physiol. 1999;277:L49–L57. doi: 10.1152/ajplung.1999.277.1.L49. [DOI] [PubMed] [Google Scholar]
- 12.Kedl RM, Jordan M, Potter T, Kappler J, Marrack P, Dow S. CD40 stimulation accelerates deletion of tumor-specific CD8(+) T cells in the absence of tumor-antigen vaccination. Proc Natl Acad Sci USA. 2001;98:10811–10816. doi: 10.1073/pnas.191371898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leoprechting VA, Bruggen VDP, Pahl HL, Aruffo A, Simon JC. Stimulation of CD40 on immunogenic human malignant melanomas augments their cytotoxic T lymphocyte-mediated lysis and induces apoptosis. Cancer Res. 1999;59:1287–1294. [PubMed] [Google Scholar]
- 14.Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. In vivo CD40 ligation can induce T cell-independent antitumor effects that involve macrophages. J Leuk Biol. 2006;79:1181–1192. doi: 10.1189/jlb.0405191. [DOI] [PubMed] [Google Scholar]
- 15.Lum HD, Buhtoiarov IN, Schmidt BE, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. Tumoristatic effects of anti-CD40 mAb-activated macrophages involve nitric oxide and tumor-necrosis factor-α. Immunol. 2006;118:261–270. doi: 10.1111/j.1365-2567.2006.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey MF, Gunn JR, Ting P, Kikutani H, Dranoff G, Noelle RJ, Barth RJ. Protective immunity induced by tumor vaccines requires interaction between CD40 and its ligand, CD154. Cancer Res. 1997;57:2569–2574. [PubMed] [Google Scholar]
- 17.Nakajima A, Kodama T, Morimoto S, Azuma M, Takeda K, Oshima H, Yoshino S, Yagita H, Okumura K. Antitumor effect of CD40 ligand: elicitation of local and systemic antitumor responses by IL-12 and B7. J Immunol. 1998;161:1901–1907. [PubMed] [Google Scholar]
- 18.North RJ, Bursuker I. Generation and decay of the immune response to a progressive fibrosarcoma. I. Ly-1 + 2- suppressor T cells down-regulate the generation of Ly-1–2+ effector T cells. J Exp Med. 1984;159:1295–1311. doi: 10.1084/jem.159.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan B, Thomas R. CD40 and dendritic cell function. Crit Rev Immunol. 2003;23:83–107. doi: 10.1615/CritRevImmunol.v23.i12.50. [DOI] [PubMed] [Google Scholar]
- 20.Payer E, Strohal R, Kutil R, Elbe A, Stingl G. Demonstration of a CD3+ lymphocyte subset in the epidermis of athymic nude mice. Evidence for T cell receptor diversity. J Immunol. 1992;149:413–420. [PubMed] [Google Scholar]
- 21.Ridge JP, Rosa FD, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 22.Rolink A, Melchers F, Andersson J. The SCID but not the RAG-2 gene product is required for 5 mu-S epsilon heavy chain class switching. Immunity. 1996;5:319–330. doi: 10.1016/S1074-7613(00)80258-7. [DOI] [PubMed] [Google Scholar]
- 23.Schoenberger SP, Toes REM, van der Voort EIH, Offringa R, Melief CJM. T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 24.Solomayer EF, Feuerer M, Bai L, Umansky V, Beckhove P, Meyberg GC, Bastert G, Schirrmacher V, Diel IJ. Influence of adjuvant hormone therapy and chemotherapy on the immune system analysed in the bone marrow of patients with breast cancer. Clin Cancer Res. 2003;9:174–180. [PubMed] [Google Scholar]
- 25.Sotomayor EM, Borrello I, Tubb E, Rattis FM, Bien H, Lu Z, Fein S, Schoenberger S, Levitsky H. Conversion of tumor-specific CD4+ T-cell tolerance to T-cell priming through in vivo ligation of CD40. Nat Med. 1999;5:780–787. doi: 10.1038/10503. [DOI] [PubMed] [Google Scholar]
- 26.Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol. 2004;173:5923–5928. doi: 10.4049/jimmunol.173.10.5923. [DOI] [PubMed] [Google Scholar]
- 27.Todryk SM, Tutt AL, Green MH, Smallwood JA, Halanek N, Dalgleish AG, Glennie MJ. CD40 ligation for immunotherapy of solid tumours. J Immunol Meth. 2001;248:139–147. doi: 10.1016/S0022-1759(00)00349-5. [DOI] [PubMed] [Google Scholar]
- 28.Turner JG, Rakhmilevich AL, Burdelya L, Neal Z, Imboden M, Sondel PM, Yu H. Anti-CD40 antibody induces antitumor and anti-metastatic effects: role of natural killer cells. J Immunol. 2001;166:89–94. doi: 10.4049/jimmunol.166.1.89. [DOI] [PubMed] [Google Scholar]
- 29.Tutt AL, O’Brien L, Hussain A, Crowther GR, French RR, Glennie MJ. T cell immunity to lymphoma following treatment with anti-CD40 monoclonal antibody. J Immunol. 2002;168:2720–2728. doi: 10.4049/jimmunol.168.6.2720. [DOI] [PubMed] [Google Scholar]
- 30.van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, Melief CJ, Toes RE. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci USA. 2002;99:5561–5566. doi: 10.1073/pnas.082107699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesosky B, Hurwitz AA. Modulation of costimulation to enhance tumor immunity. Cancer Immunol Immunother. 2003;52:663–669. doi: 10.1007/s00262-003-0424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, Garl S, Butine MD, Perry VP, Armitage RJ, Ghalie R, Caron DA, Gribben JG. Phase I study of recombinant human CD40 ligand in cancer patients. J Clinic Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 33.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, Sullivan P, Mahany JJ, Gallagher M, Kramer A, Green SJ, O’Dwyer PJ, Running KL, Huhn RD, Antonia SJ. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clinic Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 34.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609–617. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]