Abstract
Several vectors, viral and bacterial, have been developed over the past few years for means of generating an effective antitumor immune response. We have developed and studied a “model for immunotherapy” using a viral vector disabled infectious single cycle-herpes simplex virus (DISC-HSV), which efficiently transduces various tumor cell lines and offers a useful vehicle for the further development of cell-based vaccines. The immunotherapeutic potential of DISC-HSV encoding granulocyte macrophage colony stimulating factor (GM-CSF) was demonstrated in a number of murine carcinoma models, leading to complete regression of well-established tumors in up to 70% of the mice. Moreover, the therapeutic potential of DISC-HSV-GM-CSF was significantly enhanced when used in combination therapy with either OX40L or dendritic cells (DC), even in a poorly immunogenic tumor model. The ability of this vector to accept large gene inserts, its good safety profile, its ability to undergo only a single round of infection, the inherent viral immunostimulatory properties and its ability to infect various tumor cell lines efficiently, make DISC-HSV an ideal candidate vector for immunotherapy. The DISC- CT-26 tumor model was used to investigate the mechanisms associated with immunotherapy induced tumor rejection. Although CTL induction, was positively correlated with regression, MHC class I down regulation and accumulation of immature Gr1+ myeloid cells were shown to be the main immuno-suppressor mechanisms operating against regression and associated with progressive tumor growth. The CTL response was associated with the immuno-dominant AH-1 peptide of the retroviral glycoprotein gp70. This model of immunotherapy has provided an opportunity to dissect further the immunological events associated with tumor-rejection and escape. Since other antigens may be important in initiating tumor rejection, we have investigated the expression of MTA-1, an antigen that appears to be expressed widely in human and murine tumors. The immunogenicity of MTA-1 was studied and its potential as a tumor rejection antigen is under investigation.
Keywords: Herpes Simplex Virus, Granulocyte Macrophage Colony Stimulate Factor, Laryngeal Squamous Cell Carcinoma, Peptide Specific CTLs, OX40 Molecule
General introduction
The relatively recent identification of human and murine tumor antigens has led to a resurgence of interest in immunotherapy, previously directed at the activation of antigen-specific T-cells [9, 28, 32]. It is therefore important to gain insight into the contributing factors governing rejection versus tumor escape from both human and model systems. A limited number of models exist which allows detailed analysis of the immune systems in mice exhibiting progressing or regressing tumors following immunotherapy. In this review, we describe the use of a potent viral vector, disabled infectious single cycle-herpes simplex virus (DISC-HSV), as a useful vehicle for the efficient transduction of cytokine genes into tumor cells and capable of promoting immunotherapy against established tumors. The DISC-HSV/CT26 tumor model has proved to be useful for investigating immune escape mechanisms. The immune response in this model was correlated with CTL responses against an endogenous murine leukemia virus antigen gp70 and the following summarizes our recent finding that suggests possible pathways for immune escape and the identity of additional cancer-associated target proteins.
DISC-HSV as a vector for immunotherapy
Over the past few years, a number of viral vectors have been investigated not only for their ability to transfect autologous or allogeneic cell lines for cell-based vaccines but also for directly delivering the tumor antigens to antigen presenting cells (APC) in vivo in an effort to generate an immune response. An ideal vector should be able to infect a broad range of cell lines efficiently, have a very good safety profile, should not be highly immunogenic itself, should be able to accept large cDNA inserts and should be able to infect dividing and non-dividing cells [8]. DISC-HSV is a herpes simplex virus (HSV) in which the gene for glycoprotein H (gH) is deleted and hence this virus is only capable of a single round of infection. This property allows the virus to propagate after infecting the susceptible cells but the progeny are non-infectious, thus preventing the subsequent infection of other cells [13]. Moreover, HSV virus was very well characterized and has extensive safety profile in human clinical trials [23, 50]. Also, DISC-HSV has a 150 kb genome, which makes it convenient vector for the insertion of large amounts, of approximately 30 kb, of genetic material [8].
Genes encoding a variety of cytokine or co-stimulatory molecules can be inserted into DISC-HSV, and subsequently used to infect cell lines and tissues, of both murine and human origin [51, 62]. The transduction efficiency of this virus was found to be comparable to adenovirus. A panel of tumor cell lines was efficiently infected with DISC-HSV encoding the gene for granulocyte macrophage colony stimulating factor (GM-CSF) and GM-CSF secretion was observed continually for up to 72 h post infection [62]. Moreover, it was observed that DISC-HSV infection of tumor cells inhibited cell growth and induced necrotic, rather than apoptotic cell death; thereby providing the immune system with the essential ‘danger signals’ [37, 62]. Moreover, herpes infections are known to cause inflammation that could activate the APC, and induce the up regulation of IL-12 expression, [25, 41].
Considering the in vitro efficiency of DISC-HSV to infect the cells, its in vivo potential for treatment of established tumors was studied. DISC-HSV encoding mGM-CSF, when injected intra-tumorally induced the complete regression in up to 70% of the animals and immunity to further challenge with the parental tumors [3, 62]. These results were confirmed in other tumor models including renal and breast carcinoma [4, 34, 48, 62]. Interestingly, “empty” DISC-HSV, or virus expressing an irrelevant gene could also generate an antitumor immune response [62]. This therapeutic effect could be due to the inherent immuno-stimulatory properties of the virus and necrotic death of the infected cells, and subsequent activation of dendritic cells. Interestingly, intra-tumor administration of DCs together with DISC-HSV–mGM-CSF proved more effective than virus alone.
The observation that, DISC-HSV, when combined with dendritic cells (DC) in a combination therapy, caused regression in up to 100% of the animals signifies the role of the antigen presenting cells in the development of the immune response against tumors [3]. The immune rejection of tumors treated with DISC-HSV-based immunotherapy was mediated by CD8+ cytotoxic T lymphocytes (CTLs), thus no tumor regression or protection was observed in Balb/c nude mice or on depletion of CD8+ T cells [3].
One of the major concerns of immunotherapy using a viral vector is the presence of pre-existing immunity to the vector, which might limit the efficacy of the vaccine. However, experimentally previous exposure to HSV-1 virus did not limit the therapeutic efficacy of the DISC-HSV therapy in the CT26 tumor model [3]. Moreover, the systemic immunity developed in these tumor models in response to DISC-HSV therapy prevented the tumor growth at distant sites. This systemic immunity to CT26 tumors was observed to be mediated by CTLs directed mainly against the AH-1 peptide of the gp70 tumor antigen, which is naturally processed by the CT-26 tumor cells. CD4+ T cells were shown to be an essential component of tumor rejection, as their depletion decreased the level of antitumor immunity. Adoptive transfer experiments using AH-1 peptide specific CTLs into tumor-bearing animals led to regression of tumors in these animals whereas administration of CTLs directed against an irrelevant peptide did not influence tumor growth. Not only was the presence of these CTLs observed in the tumors by immunohistochemistry, but their specific trafficking into the tumors was also confirmed by real time in vivo microscopy (IVM) using fluorescently labeled CTLs [4, 5].
In conclusion, DISC-HSV could not only proved to be an important vector for delivery of cytokine genes into tumor cells but also act as an immune adjuvant capable of generating tumor specific immune responses. Moreover, pre-existing immunity to HSV does not affect the efficacy of DISC-HSV therapy, which has also been reported to have an excellent safety profile as shown in clinical trials for primary and recurrent HSV-2 [23].
DISC-HSV and OX40L as a combination therapy
Optimal activation and development of naïve T cells into effector and/or memory cells requires two signals. The first signal is provided by the binding of T cell receptor with MHC-peptide complex on the APC, whereas the second co-stimulatory signal is generated by interaction between molecules such as CD40-CD40L, CD28-CD80/CD86 and CD27-CD70. This co-stimulatory signal is essential for an effective immune response development, as its absence can lead to anergy and apoptosis of antigen specific T cells. Interaction of CD28 with its ligand occurs early during this process and leads to up regulation of other co-stimulatory molecules such as OX40 and 4-1BBL.
OX40 (CD134) is a member of the TNF receptor superfamily. OX40 is an interesting and unique molecule to target in cancer immunotherapy as it is only expressed on activated T cells. OX40 ligation leads to clonal expansion and proliferation of the activated T cells and OX40 expression peaks on the activated T cells after approximately 48 h [57]. OX40 ligand is expressed on the APCs and OX40 ligation, was shown to be essential for the generation of the memory T cells and their survival. More studies in OX40/OX40L knock out mice clearly show an impaired CD4+ T-cell as well as defective dendritic cell mediated cytokine production and co-stimulation [12].
One of the main aims of cancer immunotherapy is to generate memory T cell responses in order to prevent relapse of the disease after initial clearance of the tumor. OX40 molecules seem to be an ideal target to achieve this aim. Indeed, OX40 therapy in tumor-bearing animals, through agonist antibodies or OX40L-immunoglobulin fusion proteins, enhances antitumor immune response and increased tumor-free survival in various tumor models [6, 27, 40]. It has been speculated that OX40 mediated stimulation helps in the generation and survival of CD4+ T cells with a memory phenotype and provides help to generate effector CTLs [38]. Also, these CD4+ T cells might have a more direct role in tumor cell killing as reported by various studies [15, 49]. Tumor infiltrating CD8+ T cells have also been observed to express OX40 molecules, which suggest that OX40 might directly or indirectly enhance CTL function [26, 57].
As OX40 is mainly involved in proliferation of activated T cells and the generation of a memory phenotype, it could serve as an agent of value in promoting tumor antigen specific responses when used in a combination immuno therapy. OX40L was recently used in a combination therapy with another co-stimulatory molecule anti-4-1BB and adenovirus encoding for IL-12, which led to regression of large established hepatic metastasis. Interestingly, this model required the presence of all three agents to achieve maximum benefit, whereas eliminating any of the three agents from the combination severely compromised the efficacy of the therapy [47]. Another study demonstrated that C26 carcinoma cells co-transfected with GM-CSF and OX40L were rejected by 85% of the animals, whereas injection of either GM-CSF or OX40L transfectants alone only achieved a delay in tumor onset [19]. The advantage of using OX40L for combination cancer immunotherapy was clearly demonstrated in both studies. Moreover, in both these models, CD4+ T cells were essential and long-term memory was established as immunized animals rejected the challenge of parental tumor cells after therapy. However, none of the studies investigated the possible effector functions of the CD4+ T cells. The therapeutic efficacy of OX40 mediated therapy is dependant on the anatomic site of the tumor growth, tumor burden and the intrinsic immunogenicity of the tumor [26].
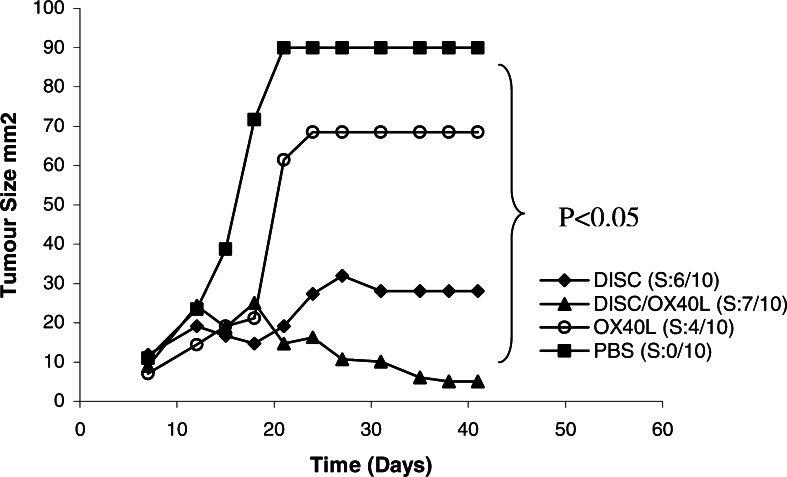
The potential of OX40L and OX40L/DISC-HSV combination therapy in inducing the regression of established tumors is shown in Fig. 1 and was previously reported [6]. In two histologically different tumors, it was observed that intra peritoneal injection of mOX40L-immunoglobulin fusion protein led to a significantly reduced tumor growth in tumor-bearing animals and up to 50% of the animals remained tumor-free for the duration of the experiments, whereas no protection was observed in animals given control therapy in the form of either PBS or human OX40L. This protection was dependant on the timing of the therapy, the dose and the route of administration of OX40L. Five doses of 250 μg mOX40L given intra-muscularly significantly improved the response. Moreover, timing was crucial in generating the maximal benefit of OX40L therapy, as OX40L administration three days after tumor cell inoculation protected seven out of ten mice whereas significantly less protection was observed, when the therapy was started before or after three days [6]. This may be due to the transient expression of OX40 on the activated T cells, which peaks at approximately 3 days coinciding with the maximal activation of these T cells [56]. Moreover, OX40L also reduced the development of the experimental metastases in 50% of the animals that remained tumor-free for the duration of the experiment. Combined OX40L and DISC-HSV therapy in a moderately immunogenic 4T1 tumor model, inhibited tumor growth rate and caused tumor regression in up to 70% of animals, which were immune to further challenge by the parental tumor cells. Furthermore, in a poorly immunogenic tumor model, RENCA, DISC-HSV on its own provides little if any therapeutic benefit; but when combined with OX40L, tumor regression was observed in approximately 40% of the animals. Investigating the tumor-rejection mechanism in CT-26 tumor model confirmed the involvement of both CD4+ and CD8+ T cells, as therapy proved ineffective in CD4+ or CD8+ T cell depleted mice. This again suggests that CD8+ CTLs mediate the effector function but require CD4+ T cells for optimal activation. Additionally, CD4+ T cells might have a direct antitumor role since the depletion of CD8+ T cells does not abrogate the response completely [6].
Fig. 1.
Combination therapy of DISC-HSV/mGM-CSF and OX40L in a CT-26 tumor model. Four groups of 10 BALB/c mice were implanted s.c. with 1×104 CT26 cells. Therapy was initiated when tumors reached the size of 0.09–0.36 cm2, which is usually on days 8–10. DISC mice were injected twice at 2 days intervals with 2.5 ×107 pfu DISC/mGM-CSF virus. OX40L mice were injected i.p. with 250 g per mouse on days 8and 14. DISC + OX40L mice were first injected intra-tumorally with DISC virus and concomitantly injected i.p. with OX40L fusion protein as above. PBS mice were injected intra-tumorally and i.p. with PBS as control. (Adapted from [5])
Immuno escape mechanisms in DISC-HSV/CT26 model
Cancerous cells are genetically unstable, and may change their phenotypic characteristics in order to evade the immune system. In fact, it was suggested that the immune system helps tumor cells to develop a more aggressive phenotype by eliminating the more vulnerable cells from a heterogeneous population, a phenomenon termed as ‘immunoediting’ [14]. Some mechanisms employed by tumors in order to evade immune attack are: down regulation of their MHC molecule expression, loss of the expression of an immuno-dominant antigen, recretion of immuno-suppressive cytokines such as TGF-β and IL-10, expression of FasL that may induce apoptosis of activated T cells and the activation of the immuno-regulatory cells such as natural killer T (NKT) cells and CD4+CD25+ T regulatory cells [1, 11, 22, 60]. Hence, immune response generated against a tumor may only lead to temporary regression, unless it is accompanied by a strategy to counteract immuno-escape mechanisms. It is clear from research in this field that different tumors employ different mechanisms to evade the immune system.
As discussed previously, DISC-HSV therapy in CT-26-bearing animals leads to tumor regression in a high proportion of animals, but approximately 40% of treated mice will continue to progress their tumors [3, 62]. This model has allowed the study of immune-escape mechanisms employed by the progressor mice and develop strategies to prevent them. It was observed that the majority of progressor tumors from mice receiving DISC-HSV therapy had low (55%) or no (45%) expression of the MHC class I molecules whereas only 50% of tumor-bearing animals (not receiving therapy) had partial down regulation of MHC antigens, the remaining 50% showing no loss of MHC class I expression [1]. Interestingly, the tumor cells isolated from progressor or tumor bearer mice up-regulated their MHC class I expression following overnight culture in vitro, showing this phenomenon to be reversible and demonstrating the dynamic nature of phenotypic change associated with their growth environment.
Another potential mechanism of immune-escape associated with the CT26 model is the accumulation of immature Gr1+ myeloid cells. Gr1+ cells were significantly increased in the spleens and the blood of animals with progressive tumors, and their presence was also detected within the tumors of mice failing to respond to DISC-HSV or OX40L therapy [2]. Interestingly, these immature forms of myeloid Gr1+/CD11b+ cells have also been identified in other mouse models and cancer patients [7, 10, 17] and shown to inhibit the activation of the primary T cells [30]. However, the exact role of Gr1+ cells in suppressing the immune response to tumors is not yet known. Immature myeloid Gr1+/CD11b+ cells can be induced to differentiate into mature DC through intratumoral gene delivery of GM-CSF leading to activation of the natural killer cells and CTLs in mice treated with combination therapy of IL-12 and anti 4-1BB [33]. Another study has recently shown that administration of all trans retinoic acid (ATRA) along with antigen specific vaccination could induce differentiation of immature myeloid Gr1+ cells into mature DCs improving the functional ability of antitumor CD4+ and CD8+ T cells and leading to objective regression of large tumors [31]. Tumor inhibition was also enhanced by co-administration of ATRA with DISC-mGM-CSF therapy in CT26 tumor model (Ali et al. unpublished observation).
Natural killer T cells have recently generated substantial interest as CD4+ or double negative (CD4–CD8-) sub-populations of T cells, which also express NK cell markers, can produce large amounts of cytokines, especially IL-13 and act as immunosuppressive cells [18, 39, 55]. However, some studies indicate that NKT cells might be important in antitumor immunity [55, 58]. Recently, a model for negative regulation of immune response against cancer was proposed by Terabe et al. [61], briefly: the NKT cells upon activation by glycolipid antigen on APCs secrete IL-13, which acts on the Gr1+/CD11b+ cells, inducing them to secrete TGF-β which suppresses antigen-specific CTL responses. Initial studies in the DISC-HSV/CT26 model failed to confirm the involvement of NKT cells or IL-13 production from CD4+ cells isolated from progressor mice splenocytes. However, CD4+ T cells isolated from the splenic parenchymal tissue of mice failing to respond to DISC-HSV therapy can be immunosuppressive and shown to inhibit antigen specific CTL activity [2]. Although these T cells were CD4+CD25-, initial results suggest that they are not NKT cells as no significant difference in IL-13 production was observed.
Limitations of the DISC-HSV/CT26 model
Although, the CT-26 model allows us to study the antitumor associated immune responses and the escape mechanisms, it has certain limitations. Firstly, the CT26 murine colon carcinoma was established by sub-cutaneous implantation and tumors develop rapidly, whereas most human cancers grow slowly over a period of years, allowing multiple mutations to accumulate and the likely development of “immune escape” tumor variants. Also, the immuno-dominant epitope in the CT-26 cell line is derived from murine leukemia virus gp70, whereas most tumor antigens are over-expressed or mutated ‘self antigens’. However, recently it has been shown that certain human cancers express endogenous retroviral sequences although it is not known as to whether they are associated with malignant transformation [16, 43].
MTA1-a potentially novel metastasis associated antigen for immunotherapy
In a search for “novel” cancer-associated antigens that are broadly expressed in cancers, metastasis associated tumor antigen 1 (MTA1) can be proposed as a suitable candidate. Originally identified using a differential cDNA library screening using rat mammary adenocarcinoma cells [63] the MTA1 gene encodes an 82 kDa protein. Analysis of the gene sequence of MTA1 revealed that it has a proline rich region (SH3 binding motif), putative zinc finger DNA binding motif and a leucine zipper motif. Moreover, the human MTA1 protein is also rich in SPXX motifs, which are usually expressed in gene regulatory and DNA binding proteins. Also, it contains three nuclear localization signals [44, 46]. Although consisting of motifs usually found in transcription factors, it was shown recently that MTA1 might be a part of the nucleosome remodeling (NuRD) and histone deacetylase complex (HDAC) and thus inhibit transcription [65]. Histone proteins help in the organization of the DNA into nucleosomes, which are regular repeating structures in the chromatin and the acetylation status of the histone proteins effect gene expression by altering the transcription of the genes [36]. Moreover, the expression level of MTA1 correlated inversely with the acetylation status of histone H4 in invasive oesophageal carcinomas, which correlated positively with the prognosis of the patients [66]. This evidence suggests that MTA1 might be localized in the nucleus and act to repress transcription [44].
Other members of MTA1 family have recently been identified, MTA2, MTA3 and MTA1s. MTA2 and MTA3 might possibly have similar functions to MTA1 in terms of histone modification and transcriptional regulation [54, 68], whereas MTA1s is a naturally occurring shorter variant of MTA1, expressed in breast cancer tissues and is capable of sequestering estrogen receptor-α in the cytoplasm, making these cells unresponsive to hormonal therapy [29].
Although MTA1 is expressed at very low levels in most tissues of the body, various studies have confirmed the association of MTA1 over-expression with the increased tumor growth and invasive potential; gastric and colorectal carcinomas over-expressing MTA1 showed significantly higher rates of invasion and lymph node metastasis [64]. In similar studies, DNA microarray analysis was used to investigate MTA1 expression in 300 patient samples comprising of metastatic prostrate cancer, clinically localized prostrate cancer and benign prostrate tissue and MTA1 expression was correlated positively with malignant potential [24]. Human laryngeal squamous cell carcinomas, lung cancers, ovarian carcinomas, hepatocellular carcinomas and breast cancers also demonstrate MTA1 to be associated with tumor aggression [21, 45, 53, 59, 69].
Recently it has been demonstrated that inhibition of MTA1 expression in MDA-MB-231 breast cancer cells by anti-sense phosphorothioate oligonucleotides resulted in inhibition of their growth and invasiveness [46]. Another recent study investigated the functional role of MTA1 expression in cancer cells [35]. MTA1 overexpression in an immortalized human keratinocyte cell line increased its migratory and invasive potential, allowed cell survival in the anchorage independent state and also led to increased expression of anti-apoptotic Bcl-2 family member Bcl-xl. Thus, MTA1 over expression might support various steps in the transformation process of normal cells [35].
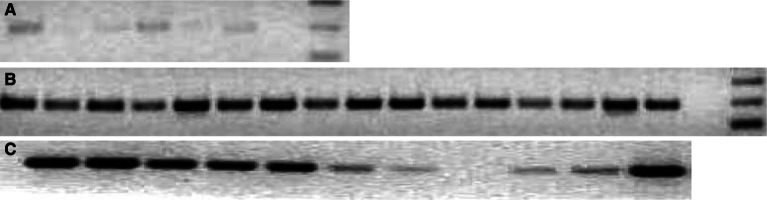
Overexpressed cancer-associated proteins, particularly those involved in cell transformation represent potential targets for immunotherapy. Considering the expression pattern and the diversity of MTA1 expression in different tumors, MTA1 might serve as a target for immunotherapy. MTA1 is expressed in normal tissues at lower levels and hence there is a risk of inducing auto-immunity. We have confirmed the low level expression of MTA1 in various normal murine tissues compared to very high levels of expression in almost all murine tumor cells lines that were tested (Fig. 2). However, similar self-antigens, for example, like p53 and survivin, were used in vaccines without severe adverse effects [42, 52, 67].
Fig. 2.
Determination and comparison of expression of MTA1 in normal human tissues, human tumor cell lines, murine tissues and murine tumor cell lines using RT-PCR. a From left to right, normal human tissues, lanes 1–6 = brain, heart, lung, trachea, liver, kidney. b From left to right, Human tumor cell lines, lanes 1–16, HT29, ISUL, T47D, BB49, SENY, FM487, Jurkat, MCF 7, MEL 270, T2, JY, GERL 4.3, FM3, DBTRG, 293, T98G. c From left to right, murine tumor cell lines and tissues, lanes 1–11, CT26, A20, Renca, CMT 93, B16, liver, lung, muscle, spleen, kidney, testis
Metastasis associated tumor antigen 1 was identified as a SEREX antigen and hence it is likely to be capable of inducing a T-cell response in cancer patients [32]. The protein is highly conserved between mouse and humans, 86% and 96% identity of the nucleotide and protein sequences respectively, and appears to perform similar functions in both species. This provides an opportunity to establish models for investigating MTA1 immunotherapy where initial studies have demonstrated the presence of immunogenic MHC class I restricted peptides (Assudani et al. unpublished observations). In a search for peptides, it is important to consider that the CTL repertoire against high affinity binding peptides is partially tolerated, which may not be the case for moderate to low affinity peptides [20].
Conclusions and future directions
Disabled infectious single cycle herpes simplex virus could be a potential vector, not only for in vitro transduction of cells but also as a vector for the in vivo delivery of “immune response” genes or antigens. Therapy with DISC-HSV is safe and is not affected by previous exposure to HSV infection. Regression of established tumors in approximately two-thirds of animals receiving DISC-HSV immunotherapy can lead to the generation of tumor specific immunity.
In combination with cell-based or cytokine immunotherapies DISC-HSV can promote high levels of CTL effectors and immunity to tumor antigens. It will be important to design future immunotherapy strategies to target antigens, such as MTA1, that are correlates of tumor aggression.
Acknowledgements
We would like to take this opportunity to acknowledge the John and Lucille van Geest Foundation, Nottingham Trent University and Xenova Group plc for their financial support in these studies. We gratefully acknowledge the technical assistance of Mr. Stephen Reeder and Mr. Robert Davy. Lastly, we would like to thank Glenda Kill for her help in preparation of this manuscript.
Abbreviations
- DISC-HSV
Disabled infectious single cycle-herpes simplex virus
- mGM-CSF
Murine granulocyte macrophage colony stimulating factor
- Gr1
Granulocyte marker
- CTL
Cytotoxic T lymphocytes
- APC
Antigen presenting cells
- MTA1
Metastasis associated antigen 1
Footnotes
This article is a symposium paper from the conference "Progress in Vaccination against Cancer 2004 (PIVAC 4)", held in Freudenstadt-Lauterbad, Black Forest, Germany, on 22–25 September 2004.
References
- 1.Ahmad M, Rees RC, Ali SA. Escape from immunotherapy: possible mechanisms that influence tumor regression/progression. Cancer Immunol Immunother. 2004;53:844. doi: 10.1007/s00262-004-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmad M, Rees RC, McArdle SE, Li G, Mian S, Entwisle C, Loudon P, Ali SA (2005) Regulation of CTL responses to MHC-restricted class I peptide of the gp70 tumour antigen by splenic parenchymal CD4(+) T cells in mice failing immunotherapy with DISC-mGM-CSF. Int J Cancer. February 18 Epub ahead of print [DOI] [PubMed]
- 3.Ali SA, Lynam J, McLean CS, Entwisle C, Loudon P, Rojas JM, McArdle SE, Li G, Mian S, Rees RC. Tumor regression induced by intratumor therapy with a disabled infectious single cycle (DISC) herpes simplex virus (HSV) vector, DISC/HSV/murine granulocyte-macrophage colony-stimulating factor, correlates with antigen-specific adaptive immunity. J Immunol. 2002;168:3512. doi: 10.4049/jimmunol.168.7.3512. [DOI] [PubMed] [Google Scholar]
- 4.Ali SA, Rees RC, Anderson DQ, Reed MW, Goepel JR, Brown NJ. Trafficking of ‘immune’ CD4(+)/CD8(+)T-lymphocytes into the RENCA tumour microcirculation in vivo in mice. Br J Cancer. 2000;83:1061. doi: 10.1054/bjoc.2000.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali S, Ahmad M, Lynam J, Rees RC, Brown N. Trafficking of tumor peptide-specific cytotoxic T lymphocytes into the tumor microcirculation. Int J Cancer. 2004;110:239. doi: 10.1002/ijc.20113. [DOI] [PubMed] [Google Scholar]
- 6.Ali SA, Ahmad M, Lynam J, McLean CS, Entwisle C, Loudon P, Choolun E, McArdle SE, Li G, Mian S, Rees RC. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 7.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 8.Bonnet MC, Tartaglia J, Verdier F, Kourilsky P, Lindberg A, Klein M, Moingeon P. Recombinant viruses as a tool for therapeutic vaccination against human cancers. Immunol Lett. 2000;74:11. doi: 10.1016/S0165-2478(00)00244-3. [DOI] [PubMed] [Google Scholar]
- 9.Boon T, Cerottini JC, Vanden Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 10.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838. [PMC free article] [PubMed] [Google Scholar]
- 11.Casares N, Arribillaga L, Sarobe P, Dotor J, Lopez-Diazde Cerio A, Melero I, Prieto J, Borras-Cuesta F, Lasarte JJ. CD4+/CD25+ regulatory cells inhibit activation of tumor-primed CD4+ T cells with IFN-gamma-dependent antiangiogenic activity, as well as long-lasting tumor immunity elicited by peptide vaccination. J Immunol. 2003;171:5931. doi: 10.4049/jimmunol.171.11.5931. [DOI] [PubMed] [Google Scholar]
- 12.Chen AI, McAdam AJ, Buhlmann JE, Scott S, Lupher ML, Jr, Greenfield EA, Baum PR, Fanslow WC, Calderhead DM, Freeman GJ, Sharpe AH. OX40-ligand has a critical costimulatory role in dendritic cell:T cell interactions. Immunity. 1999;11:689. doi: 10.1016/S1074-7613(00)80143-0. [DOI] [PubMed] [Google Scholar]
- 13.Dilloo D, Rill D, Entwistle C, Boursnell M, Zhong W, Holden W, Holladay M, Inglis S, Brenner M. A novel herpes vector for the high-efficiency transduction of normal and malignant human hematopoietic cells. Blood. 1997;89:119. [PubMed] [Google Scholar]
- 14.Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 15.Egilmez NK, Hess SD, Chen FA, Takita H, Conway TF, Bankert RB. Human CD4+ effector T cells mediate indirect interleukin-12- and interferon-gamma-dependent suppression of autologous HLA-negative lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2002;62:2611. [PubMed] [Google Scholar]
- 16.Ford CE, Faedo M, Crouch R, Lawson JS, Rawlinson WD. Progression from normal breast pathology to breast cancer is associated with increasing prevalence of mouse mammary tumor virus-like sequences in men and women. Cancer Res. 2004;64:4755. doi: 10.1158/0008-5472.CAN-03-3804. [DOI] [PubMed] [Google Scholar]
- 17.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 18.Godfrey DI, Hammond KJ, Poulton LD, Smyth MJ, Baxter AG. NKT cells: facts, functions and fallacies. Immunol Today. 2000;21:573. doi: 10.1016/S0167-5699(00)01735-7. [DOI] [PubMed] [Google Scholar]
- 19.Gri G, Gallo E, Di Carlo E, Musiani P, Colombo MP. OX40 ligand-transduced tumor cell vaccine synergizes with GM-CSF and requires CD40-APC signaling to boost the host T cell antitumor response. J Immunol. 2003;170:99. doi: 10.4049/jimmunol.170.1.99. [DOI] [PubMed] [Google Scholar]
- 20.Gross D, Graff-Dubois S, Opolon P, Cornet S, Alves P, Bennaceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S, Lemonnier F, Davoust J, Miconnet I, Vonderheide R, Kosmatopoulos K. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425. doi: 10.1172/JCI200419418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamatsu T, Rikimaru T, Yamashita Y, Aishima S, Tanaka S, Shirabe K, Shimada M, Toh Y, Sugimachi K. The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol Rep. 2003;10:599. [PubMed] [Google Scholar]
- 22.Hicklin DJ, Wang Z, Arienti F, Rivoltini L, Parmiani G, Ferrone S. beta2-Microglobulin mutations, HLA class I antigen loss, and tumor progression in melanoma. J Clin Invest. 1998;101:2720. doi: 10.1172/JCI498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickling JK, Chishlom SE, Duncan IE, Taylor EJ, Boswell C, McLean CS, Uttridge J, Roberts JS, Tomasi A, Stanberry L, Bernstein DI, Boursnell M, Inglis SC (1998) Immunogenicity of a disabled infectious single cycle HSV-2 vaccine in phase I clinical trials in HSV-2 seropositive and seronegative volunteers. In: 18th international congress on infectious diseases, Boston, Abs 22.008
- 24.Hofer M, Menke A, Genze F, Gierschik P, Giehl K. Expression of MTA1 promotes motility and invasiveness of PANC-1 pancreatic carcinoma cells. Br J Cancer. 2004;90:455. doi: 10.1038/sj.bjc.6601535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanangat S, Thomas J, Gangappa S, Babu JS, Rouse BT. Herpes simplex virus type 1-mediated up-regulation of IL-12 (p40) mRNA expression. Implications in immunopathogenesis and protection. J Immunol. 1996;156:1110. [PubMed] [Google Scholar]
- 26.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514. [PubMed] [Google Scholar]
- 27.Kjaergaard J, Peng L, Cohen PA, Drazba JA, Weinberg AD, Shu S. Augmentation versus inhibition: effects of conjunctional OX-40 receptor monoclonal antibody and IL-2 treatment on adoptive immunotherapy of advanced tumor. J Immunol. 2001;167:6669. doi: 10.4049/jimmunol.167.11.6669. [DOI] [PubMed] [Google Scholar]
- 28.Krackhardt AM, Witzens M, Harig S, Hodi FS, Zauls AJ, Chessia M, Barrett P, Gribben JG. Identification of tumor-associated antigens in chronic lymphocytic leukemia by SEREX. Blood. 2000;100:2123. doi: 10.1182/blood-2002-02-0513. [DOI] [PubMed] [Google Scholar]
- 29.Kumar R, Wang RA, Mazumdar A, Talukder AH, Mandal M, Yang Z, Bagheri-Yarmand R, Sahin A, Hortobagyi G, Adam L, Barnes CJ, Vadlamudi RK. A naturally occurring MTA1 variant sequesters oestrogen receptor-alpha in the cytoplasm. Nature. 2002;418:654. doi: 10.1038/nature00889. [DOI] [PubMed] [Google Scholar]
- 30.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 31.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441. [PubMed] [Google Scholar]
- 32.Li G, Miles A, Line A, Rees RC. Identification of tumour antigens by serological analysis of cDNA expression cloning. Cancer Immunol Immunother. 2004;53:139. doi: 10.1007/s00262-003-0471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Pan PY, Gu P, Xu D, Chen SH. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 2004;64:1130. doi: 10.1158/0008-5472.CAN-03-1715. [DOI] [PubMed] [Google Scholar]
- 34.Loudon PT, McLean CS, Martin G, Curry J, Leigh Shaw M, Hoogstraten C, Verdegaal E, Osanto S. Preclinical evaluation of DISC–GMCSF for the treatment of breast carcinoma. J Gene Med. 2003;5:407. doi: 10.1002/jgm.354. [DOI] [PubMed] [Google Scholar]
- 35.Mahoney M, Simpson A, Jost M, Noe M, Kari C, Pepe D, Choi Y, Uitto J, Rodeck U. Metastasis-associated protein (MTA)1 enhances migration, invasion, and anchorage-independent survival of immortalized human keratinocytes. Oncogene. 2002;21:2161. doi: 10.1038/sj.onc.1205277. [DOI] [PubMed] [Google Scholar]
- 36.Marks P, Rifkind R, Richon V, Breslow R, Miller T, Kelly W. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 37.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 38.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164:107. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 39.Moodycliffe AM, Nghiem D, Clydesdale G, Ullrich SE. Immune suppression and skin cancer development: regulation by NKT cells. Nat Immunol. 2000;1:521. doi: 10.1038/82782. [DOI] [PubMed] [Google Scholar]
- 40.Morris A, Vetto JT, Ramstad T, Funatake CJ, Choolun E, Entwisle C, Weinberg AD. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71. doi: 10.1023/A:1010649303056. [DOI] [PubMed] [Google Scholar]
- 41.Mu J, Zou JP, Yamamoto N, Tsutsui T, Tai XG, Kobayashi M, Herrmann S, Fujiwara H, Hamaoka T. Administration of recombinant interleukin 12 prevents outgrowth of tumor cells metastasizing spontaneously to lung and lymph nodes. Cancer Res. 1995;55:4404. [PubMed] [Google Scholar]
- 42.Murakami T, Tokunaga N, Waku T, Gomi S, Kagawa S, Tanaka N, Fujiwara T. Antitumor effect of intratumoral administration of bone marrow-derived dendritic cells transduced with wild-type p53 gene. Clin Cancer Res. 2004;10:3871. doi: 10.1158/1078-0432.CCR-03-0599. [DOI] [PubMed] [Google Scholar]
- 43.Muster T, Waltenberger A, Grassauer A, Hirschl S, Caucig P, Romirer I, Fodinger D, Seppele H, Schanab O, Magin-Lachmann C, Lower R, Jansen B, Pehamberger H, Wolff K. An endogenous retrovirus derived from human melanoma cells. Cancer Res. 2003;63:8735. [PubMed] [Google Scholar]
- 44.Nawa A, Nishimori K, Lin P, Maki Y, Moue K, Sawada H, Toh Y, Fumitaka K, Nicolson GL. Tumor metastasis-associated human MTA1 gene: its deduced protein sequence, localization, and association with breast cancer cell proliferation using antisense phosphorothioate oligonucleotides. J Cell Biochem. 2000;79:202. doi: 10.1002/1097-4644(20001101)79:2<202::AID-JCB40>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 45.Nicolson GL, Moustafa AS. Metastasis-associated genes and metastatic tumor progression. In Vivo. 1998;12:579. [PubMed] [Google Scholar]
- 46.Nicolson G, Nawa A, Toh Y, Taniguchi S, Nishimori K, Moustafa A. Tumor metastasis-associated human MTA1 gene and its MTA1 protein product: role in epithelial cancer cell invasion, proliferation and nuclear regulation. Clin Exp Metastasis. 2003;20:19. doi: 10.1023/A:1022534217769. [DOI] [PubMed] [Google Scholar]
- 47.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6:528. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 48.Parkinson RJ, Mian S, Bishop MC, Gray T, Li G, McArdle SE, Ali S, Rees RC. Disabled infectious single cycle herpes simplex virus (DISC-HSV) is a candidate vector system for gene delivery/expression of GM-CSF in human prostate cancer therapy. Prostate. 2003;56:65. doi: 10.1002/pros.10207. [DOI] [PubMed] [Google Scholar]
- 49.Qin Z, Blankenstein T. CD4+ T cell–mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 2000;12:677. doi: 10.1016/S1074-7613(00)80218-6. [DOI] [PubMed] [Google Scholar]
- 50.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 51.Rees RC, McArdle S, Mian S, Li G, Ahmad M, Parkinson R, Ali SA. Disabled infectious single cycle-herpes simplex virus (DISC-HSV) as a vector for immunogene therapy of cancer. Curr Opin Mol Ther. 2002;4:49. [PubMed] [Google Scholar]
- 52.Reker S, Meier A, Holten-Andersen L, Svane I, Becker J, Straten Pt P, Andersen M. Identification of novel survivin-derived CTL epitopes. Cancer Biol Ther. 2004;3:173. doi: 10.4161/cbt.3.2.611. [DOI] [PubMed] [Google Scholar]
- 53.Sasaki H, Moriyama S, Nakashima Y, Kobayashi Y, Yukiue H, Kaji M, Fukai I, Kiriyama M, Yamakawa Y, Fujii Y. Expression of the MTA1 mRNA in advanced lung cancer. Lung Cancer. 2002;35:149. doi: 10.1016/S0169-5002(01)00329-4. [DOI] [PubMed] [Google Scholar]
- 54.Simpson A, Uitto J, Rodeck U, Mahoney M. Differential expression and subcellular distribution of the mouse metastasis-associated proteins Mta1 and Mta3. Gene. 2001;273:29. doi: 10.1016/S0378-1119(01)00563-7. [DOI] [PubMed] [Google Scholar]
- 55.Smyth M, Thia K, Street S, Cretney E, Trapani J, Taniguchi M, Kawano T, Pelikan S, Crowe N, Godfrey D. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuber E, Strober W. The T cell-B cell interaction via OX40-OX40L is necessary for the T cell-dependent humoral immune response. J Exp Med. 1996;183:979. doi: 10.1084/jem.183.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4:420. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 58.Takeda K, Hayakawa Y, Atsuta M, Hong S, Van Kaer L, Kobayashi K, Ito M, Yagita H, Okumura K. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000;12:909. doi: 10.1093/intimm/12.6.909. [DOI] [PubMed] [Google Scholar]
- 59.Tang Q, Ji W, Pan Z, Zheng Y, Guan C. Expression of the metastasis-associated gene 1 in laryngeal squamous cell carcinoma: correlation with cervical lymph node metastasis. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2003;38:213. [PubMed] [Google Scholar]
- 60.Terabe M, Matsui S, Noben-Trauth N, Chen H, Watson C, Donaldson DD, Carbone DP, Paul WE, Berzofsky JA. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat Immunol. 2000;1:515. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 61.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Todryk S, McLean C, Ali S, Entwistle C, Boursnell M, Rees R, Vile R. Disabled infectious single-cycle herpes simplex virus as an oncolytic vector for immunotherapy of colorectal cancer. Hum Gene Ther. 1999;10:2757. doi: 10.1089/10430349950016492. [DOI] [PubMed] [Google Scholar]
- 63.Toh Y, Pencil S, Nicolson G. A novel candidate metastasis-associated gene, mta1, differentially expressed in highly metastatic mammary adenocarcinoma cell lines. cDNA cloning, expression, and protein analyses. J Biol Chem. 1994;269:22958. [PubMed] [Google Scholar]
- 64.Toh Y, Oki E, Oda S, Tokunaga E, Ohno S, Maehara Y, Nicolson GL, Sugimachi K. Overexpression of the MTA1 gene in gastrointestinal carcinomas: correlation with invasion and metastasis. Int J Cancer. 1997;74:459. doi: 10.1002/(SICI)1097-0215(19970822)74:4<459::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 65.Toh Y, Kuninaka S, Endo K, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Nicolson G, Sugimachi K. Molecular analysis of a candidate metastasis-associated gene, MTA1: possible interaction with histone deacetylase 1. J Exp Clin Cancer Res. 2000;19:105. [PubMed] [Google Scholar]
- 66.Toh Y, Ohga T, Endo K, Adachi E, Kusumoto H, Haraguchi M, Okamura T, Nicolson G. Expression of the metastasis-associated MTA1 protein and its relationship to deacetylation of the histone H4 in esophageal squamous cell carcinomas. Int J Cancer. 2004;110:362. doi: 10.1002/ijc.20154. [DOI] [PubMed] [Google Scholar]
- 67.Vierboom M, Nijman H, Offringa R, van der Voort E, van Hall T, van den Broek L, Fleuren G, Kenemans P, Kast W, Melief C. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186:695. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yao YL, Yang WM. The metastasis-associated proteins 1 and 2 form distinct protein complexes with histone deacetylase activity. J Biol Chem. 2003;278:42560. doi: 10.1074/jbc.M302955200. [DOI] [PubMed] [Google Scholar]
- 69.Yi S, Guangqi H, Guoli H. The association of the expression of MTA1, nm23H1 with the invasion, metastasis of ovarian carcinoma. Chin Med Sci J. 2003;18:87. [PubMed] [Google Scholar]