Abstract
The interleukin-8 (IL-8) network is involved in the colorectal cancer (CRC) progression. However, its role during the adenoma–carcinoma transition to date has not been fully investigated. To evaluate the dynamic changes of IL-8 network along the colorectal adenoma–carcinoma sequence, we examined the tissue IL-8 mRNA level in colorectal biopsies from 53 colorectal adenomas, 44 CRCs and 18 controls by quantitative real-time PCR (Q-PCR), and the expressions of IL-8 and its receptors (IL-8RA and IL-8RB) in the tumor microenvironment by immunohistochemistry (IHC) and double IHCs. The results showed that the tissue IL-8 mRNA level began to increase in the precancerous lesions (adenomas) as compared with the controls and became even higher in the CRCs. Significantly, the increase of IL-8 mRNA levels was associated with the increase of dysplastic grades in the adenomas, and also paralleled to the increase of Duke’s stages in the CRCs. IHC results revealed that IL-8 and its receptors, IL-8RA and IL-8RB, were observed both in the stroma and in the adenomatous/cancerous cells. By double IHCs, the IL-8 expression was characterized in macrophages, lymphocytes and myofibroblasts in the tumor stroma. Further double IHC identified the co-expression of IL-8 receptors (IL-8RA and IL-8RB) with CD34 positive tumor-associated microvessels in both the adenomas and CRCs. We, therefore, conclude that activated IL-8 network in the tumor microenvironment may function as a significant regulatory factor for the adenoma progression and the adenoma–carcinoma transition.
Keywords: Chemokine, Carcinogenesis, Adenoma–carcinoma sequence
Introduction
Colorectal cancer (CRC) is one of the leading worldwide cancers with high mortality. According to the theory of adenoma–carcinoma sequence, CRC develops through a multi-step process that initially begins from colorectal adenoma with a low-grade dysplasia (LGD) through a high-grade dysplasia (HGD) and finally to carcinoma over many years [1, 2]; therefore, the adenoma has been recognized as the main cancerous lesion for CRC [3–7]. The clarification of the factors involved in the adenoma–carcinoma transition has been a question of great interest, because the biological targeting of factors involved in this progression procedure is currently an interesting clinical approach in the prevention of CRC. Although a number of environmental and genetic factors that contribute to adenoma formation and progression have been postulated to be involved in the process [2], the full mechanisms are still not understood.
Interleukin (IL)-8 is a multifunctional inflammatory cytokine that was originally identified on the basis of its chemotactic activity in both normal and pathological conditions including human cancers [8, 9]. It plays an important role in tracking immune cells, i.e. neutrophils, monocytes, dendritic cells to the inflammatory site and tumor site. In addition to its chemotactic functions, it has been now recognized that IL-8 is both the potent angiogenic factor and growth factor in many human cancers including CRC [8, 10–13]. In CRC, one of the main effects of IL-8 is to stimulate CRC cell proliferation and growth, in vitro evidence has shown that IL-8 can stimulate the proliferation and migration of CRC cells via heparin-binding epidermal growth factor (HB-REGF) [14], and cancer cells-derived IL-8 can act as a autocrine factors in stimulating CRC cell growth and metastasis [12, 15, 16]. However, most current studies in human CRCs have focused on the measurement of serum level of IL-8 in the established stage of CRC [12, 17, 18]. There are few studies to address the potential role of IL-8 during the colorectal neoplastic transformation. Recently, Rubie et al. [19] showed that IL-8 mRNA level has been found to be elevated in adenoma tissues as compared with that in chronic inflammation mucosa from inflammatory bowel diseases (IBD) and suggested that IL-8 could be an important factor involved in the pathological procedure of adenomas.
An essential for tumor growth and spreading is the formation of new blood vessels (angiogenesis), the significant regulatory effect of IL-8 on tumor angiogenesis has been reported, which could be one of main mechanisms for IL-8 in promoting CRC invasion and metastasis [8, 20]. The actions of IL-8 are mediated by its receptors, and two IL-8 receptors A (IL-8RA) and B (IL-8RB) (also known as CXCR1 and CXCR2) have been implicated in the tumor growth and angiogenesis response regulated by IL-8 [21]. IL-8RA is a selective receptor for IL-8, whereas IL-8RB may also interact with other chemokines [22]. The expression of IL-8 receptors has been reported in both some types of cancer and endothelial cells. Particularly, IL-8RB is postulated as the main receptor in mediating IL-8’s regulating effect on angiogenesis [10, 11]. To directly regulate angiogenesis swift, the expression of IL-8RA and IL-8RB must be present in the tumor-associated microvessels. In the CRC patients, serum IL-8 level has been found to be increased and associated with the increased angiogenesis and poor clinical outcomes [13, 18, 23]. However, the expression of IL-8 receptors in the tumor microenvironment along the adenoma–carcinoma sequence is still little known.
Based on the above background, we hypothesized that IL-8 network may be involved in the colorectal adenoma–carcinoma transition. Therefore, the objective of this study was to examine the IL-8 network and its potential role in regulating tumor growth and angiogenesis along the adenoma–carcinoma sequence.
Materials and methods
Patients
Colorectal biopsies obtained from 53 colorectal adenomas excised completely by endoscopic polypectomy (age 43–92 years); 44 CRC excised by surgery (age 42–89 years) and 18 morphological normal colorectal mucosa without pathological evidence by colonoscopic and microscopic examinations (age 30–77 years) from the Departments of Gastrointestinal Surgery and Gastroenterology, University Hospital of North Norway between August 2003 and November 2008 were included in this study (for detailed information, see Table 1). No patients or control subjects had a history of regular use of immunomodulatory treatments or chemotherapy. The biopsies were prepared and embedded in paraffin routinely. Sections were cut at 4 μm, and then stained with hematoxylin and eosin (H&E). The conventional histological diagnosis for all the biopsies was examined at Department of Pathology, University Hospital of North Norway. The study was approved by the Regional Ethical Committee of Northern Norway, the permission for the storage of human tissues and data was given by the Norwegian Department of Health and the Norwegian Bureau of Data Surveillance, the written informed consent was obtained from the patients.
Table 1.
Histological data of specimens from patients and normal controls
| Gender | Pathology | Dysplasia | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Tubular | Tubulovillous | Villous | LGD | MGD | HGD | |
| Normal | 12 | 6 | ||||||
| Adenoma | 34 | 19 | 24 | 17 | 2 | 24 | 21 | 8 |
| Gender | Pathology | Duke’s stage | ||||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Adenocarcinoma | Mucinous | Signet-ring | A | B | C | |
| Cancer | 37 | 7 | 41 | 2 | 1 | 12 | 16 | 16 |
LGD lower grade dysplasia, MGD moderate grade dysplasia, HGD high-grade dysplasia
Quantification of IL-8 mRNA in the tissues from normal, adenoma and CRC by real-time PCR
Biopsies were collected in RNAlater solution (Ambion Europe, Cambridgeshire, UK) and total RNA was extracted by the Trizol method (Invitrogen Life Tech., Carlsbad, MA, USA) and reverse transcription was performed with SuperScript II (Invitrogen Life Tech.) [24]. Real-time PCR was performed on an ABI-prism 7900 sequence detector with TaqMan Gold™ PCR core reagents kit (Applied Biosystems/Roche, Branchburg, NJ, USA) in 25 μL volume according to our previously published method [24]. The primer sequences for IL-8 and house keeping gene (beta-actin) have been published previously [24, 25]. IL-8 mRNA expressions in adenomas and cancers were measured by cycle threshold cross-point (CT) value relative to that of normal mucosa as fold difference (N) = 2−ΔΔCT, ΔCT = CTIL-8 gene − CTbeta-actin, ΔΔCT = ΔCTCRA or CRC − average ΔCTnormal as described in our recent publication [26, 27]. The difference among normal controls, colorectal adenoma and CRC was compared by ΔCT values.
Immunohistochemical examinations of IL-8 (+) cells, IL-8 receptors IL-8RA and IL-8RB (+) cells in the tumor microenvironment
Immunohistochemistry (IHC) was performed in 4 μm paraffin sections from controls, adenomas and CRC with Vectastatin Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions and our published methods [28]. The following primary antibodies were used: mouse anti-human IL-8 polyclonal antibody (working dilution 1:100; R&D system, Minneapolis, MN, USA), mouse anti-human IL-8RA monoclonal antibody (working dilution 1:100; BD Pharmingen, San Diego, CA, USA) and mouse anti-human IL-8RB monoclonal antibody (working dilution 1:50, R&D system). Antibodies were incubated at 4°C over night. 3-Amino-9-ethylcarbazole (AEC; Vector Laboratories) was used as chromogen and slides were slightly counterstained with Mayer’s hematoxylin. Negative control slides for IHCs were performed routinely: (1) primary antibodies were substituted with the isotype-matched control antibodies; (2) secondary antibody was substituted with phosphate-buffered saline (PBS).
Evaluation of IL-8 expressing cell type and the expressions of IL-8 receptors (IL-8RA and IL-8RB) in tumor-associated microvessels by double IHCs
To define the cellular types of IL-8 expression in the stroma, double IHC staining with antibodies IL-8/CD68 (to label macrophages), IL-8/CD3 (to label lymphocytes) and IL-8/SMA-alpha (to label myofibroblasts) was performed with EnVision Doublestain System kit (DAKO, Carpinteria, CA, USA) as described previously [29]. To identify the expression of IL-8 receptors (IL-8RA and IL-8RB) in tumor-associated microvessels, double IHC staining with antibodies IL-8RA/CD34 (to label tumor-associated microvessels; working dilution 1:50, purchased from DAKO) and IL-8RB/CD34 was performed with EnVision Doublestain System kit [29]. In brief, the slides were incubated for 18 h at 4°C with anti-IL-8 or IL-8RA/IL-8RB antibodies after antigen retrieval, then incubated with labeled polymer-horseradish peroxidase-anti-mouse and anti-rabbit antibodies for 30 min. Peroxidase activity was detected with the enzyme substrate 3,3′-diaminobenzidine tetrachloride (DAB). After quenching the enzyme reaction, the slides were incubated in Doublestain Block for 5 min to block endogenous phosphatase. The slides were then incubated with anti-CD68, anti-CD3, anti-SMA-alpha and anti-CD34 antibodies individually for 60 min at room temperature. After washing, the slides were incubated with labeled polymer-alkaline phosphatase anti-mouse and anti-rabbit antibody for 30 min at room temperature. Fast Red chromogen substrate solution was used for the visualization. Nuclear counterstaining was not applied.
Morphometric analysis
All the stained slides were examined under light microscopy; the semi-quantified density grading of IL-8 immunoreactivity (IR) (+) cells, IL-8 receptor, IL-8RA IR (+) and IL-8RB IR (+), cells in the stroma and epithelium, respectively, was performed according to the method described in our previous publication [30]. In the stroma, semi-quantified scoring was done in at least five optional fields with abundant distribution from each slide under 400× high-power magnifications and scored as: nil (0), 1–19 cells/field (1+), 20–49 cells/field (2+) and over 50 cells/field (3+). In the epithelium, IL-8 IR (+) cells were graded on a scale of 0–3, with 0 representing no detectable staining and 3+ representing the strongest staining. The average values were used for statistic analysis.
Statistical analysis
The results were expressed as mean ± SEM unless otherwise stated. Statistical significance was evaluated by the Mann–Whitney test and the Kruskal–Wallis test. Values of P < 0.05 or P < 0.01 were considered significant.
Results
Gradual increasing of tissue IL-8 mRNA level paralleled the colorectal adenoma–carcinoma sequence
By quantitative real-time PCR, a gradual increasing trend of IL-8 mRNA expression level in the local tissues was shown along the adenoma–carcinoma sequence (Fig. 1a). The tissue IL-8 mRNA level began to increase in the precancerous lesions (adenomas) and became even higher in the CRCs. As compare to the controls, tissue IL-8 mRNA expression in the adenomas was significantly increased to a ~420-fold higher level. Most significantly, the increased IL-8 mRNA level was associated with the increasing grading degree of dysplasia in the adenomas (see Fig. 1b, P < 0.01, the non-parametric Kruskal–Wallis test) and not with histological types (tubular type vs. tubulovillous type: 10.49 ± 0.47 vs. 12.39 ± 0.65; P > 0.05, the Mann–Whitney test. Villous type adenoma was excluded for analysis due to the limited number in this study). The relative tissue IL-8 mRNA level was increased continually toward the cancer stage to a higher level (~2,140 folder in relative to the control) than that in the adenoma stage (Fig. 1, P < 0.0001, the Mann–Whitney test). Interestingly, the increase of tissue IL-8 mRNA level was also paralleled to the increase of the Duke’s stages (see Fig. 1c), although the statistical significance was not reached.
Fig. 1.
a Quantification of relative tissue IL-8 mRNA level (expressed by 2−ΔΔCT method) by real-time PCR along the colorectal adenoma–carcinoma sequence. In relative to the controls, patients with colorectal adenomas showed a ~420 folder high tissue IL-8 mRNA level and patients with cancers showed an even higher level and reached to ~2,140 folder (adenoma vs. cancer, P < 0.0001, the Mann–Whitney test). b The gradual increased IL-8 mRNA level was significantly associated with increased degree of dysplasia in the adenomas (P < 0.05, the Kruskal–Wallis test). c The increase trend of tissue IL-8 mRNA level was also found to be paralleled to the increase of the Duke’s stages in the CRCs, although the statistical significance was not reached (P > 0.05, the Kruskal–Wallis test)
IL-8, IL-8 receptors (IL-8RA and IL-8RB) were expressed in both the tumor stromal cells and adenomatous/cancerous cells
To further examine the expressions of IL-8 and its receptors (IL-8RA and IL-8RB) in the adenomas and CRCs, IHCs for IL-8, IL-8RA and IL-8RB were performed. The IHC results revealed that IL-8 IR was observed both in the stroma (arrows in Fig. 2a–c) and epithelium (arrowheads in Fig. 2a, b, d) in all three groups (the control, adenoma and cancer). In the stained CRC slides, the IL-8 positive stromal cells were relatively polarized in the invading edges (Fig. 2c); there were no significant differences of IL-8 positive cell density in the stroma among the three groups when the density was scored (Table 2, P > 0.05, the Kruskal–Wallis test). However, a significant change of the IL-8 immunoreactivity (IR) in the epithelium was observed: the grading scores for IL-8 in the epithelium were slightly increased in the adenoma cells and greatly increased in the cancer cells (Table 2, P < 0.05, the non-parametric Kruskal–Wallis test). When the associations between the scores of IL-8 expressed in the epithelium with dysplasia degrees in the adenomas and Duke’s stages in the CRCs were analyzed, no statistical significance could be found (data not shown).
Fig. 2.
Examinations of IL-8 and IL-8 receptor (IL-8RA/IL-8RB) expression in the tissues of colorectal adenomas and cancers examined with immunohistochemistry (IHC). In the controls, the IL-8 expression could be observed in both the stroma (arrows in a) and the epithelium (arrowheads in a). In the adenomas, the IL-8 positive cells were mostly distributed in the upper stroma toward the epithelium (arrows in b) and some in the adenomatous epithelium (arrowheads in b). In the cancers, the abundant IL-8 positive cells were shown in the invading edge of cancers (arrows in c) and increased expression of IL-8 in the cancer cells was also observed (arrowheads in d). The expression of IL-8 receptor (IL-8RA and IL-8RB) was observed in both the stroma (arrow) and epithelium (arrowhead) in all three groups as well (IL-8RA: e for the control, f for the adenoma, g for the cancer; IL-8RB: h for the control, i for the adenoma, j for the cancer). Counterstained with hematoxylin, original magnification ×400; scale bar 50 μm
Table 2.
The expression of IL-8, IL-8 receptors (IL-8RA/IL-8RB) immunoreactivities in controls, adenomas and cancers
| Control | Adenoma | Cancer | P value | |
|---|---|---|---|---|
| IL-8 in stroma | 2.13 ± 0.25 | 2.38 ± 0.17 | 2.27 ± 0.18 | >0.05 |
| IL-8 in epithelium | 1.46 ± 0.28 | 1.66 ± 0.21 | 2.32 ± 0.28 | <0.05 |
| IL-8RA in stroma | 1.62 ± 0.15 | 1.64 ± 0.13 | 2.32 ± 0.26 | >0.05 |
| IL-8RA in epithelium | 1.27 ± 0.18 | 1.11 ± 0.13 | 1.02 ± 0.20 | >0.05 |
| IL-8RB in stroma | 2.44 ± 0.16 | 2.66 ± 0.11 | 2.42 ± 0.16 | >0.05 |
| IL-8RB in epithelium | 0.75 ± 0.25 | 1.20 ± 0.21 | 0.92 ± 0.21 | >0.05 |
Statistical significance was evaluated by the non-parametric Kruskal–Wallis test
IHC results also showed that the IL-8RA and IL-8RB IRs were also observed in both the stroma and the epithelium (Fig. 2e–g for IL-8RA and h–j for IL-8RB). Analysis of IHCs revealed that the grading score for IL-8RA in the stroma was similar in the adenomas and the controls (see Table 2). However, the grading score for IL-8RA in the stroma of CRCs was slightly increased as compared with the adenomas and controls, although statistical significance was not reached (Table 2, P > 0.05, the non-parametric Kruskal–Wallis test). In the epithelium, the grading scores for IL-8RA were similar in the adenomas, CRCs and controls (Table 2). Finally, analysis of IL-8RB IHCs showed that the grading scores for IL-8RB were also not different among all three groups (Table 2).
The phenotypic characterization of IL-8 expressing cells in the tumor stroma and IL-8 receptors (IL-8RA/IL-8RB) in tumor-associated microvessels
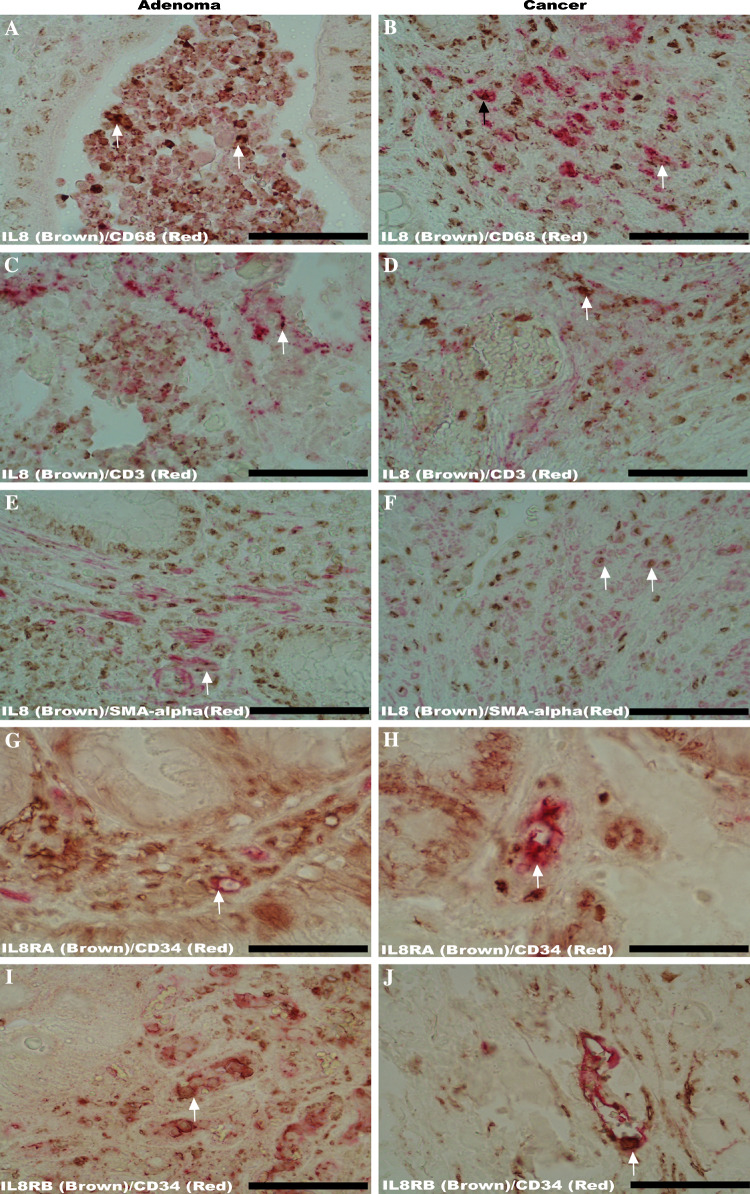
Double IHCs demonstrated that most IL-8 positive cells in the stroma of adenomas and cancers were macrophages, lymphocytes and myofibroblasts: the IL-8 immunoreactivity (Fig. 3a–f; brown color) in the stroma could be observed in macrophages (Fig. 3a for adenomas, b for cancers, labeled by CD68, red color), lymphocytes (Fig. 3c for adenomas, d for cancers; labeled by CD3, red color) and myofibroblasts (Fig. 3e for adenomas, f for cancers; labeled by SMA-alpha, red color) in both the adenomas and cancers.
Fig. 3.
Double immunohistochemistry (IHC) to examine the IL-8 expressing cellular phenotypes in the tumor stroma and IL-8 receptors IL-8RA/IL-8RB in tumor-associated microvessels. Double IHCs revealed that IL-8 immunoreactivity (brown color) was co-localized with CD3 immunoreactivity (to label infiltrated lymphocytes, red color), CD68 immunoreactivity (to label infiltrated macrophages, red color) and SMA-alpha (to label myofibroblasts, red color) in both the adenomas (arrows point to the co-localization in a, c, e) and cancer (arrows point to the co-localization in b, d, f). Further, double IHCs demonstrated the co-localizations (arrows) of IL-8RA and IL-8RB immunoreactivities (brown color) with CD34 positive tumor-associated microvessels (labeled with CD34 immunoreactivity, red color) in representative sections from both the adenomas (g, i) and cancers (h, j). Original magnification ×400; scale bar 50 μm
In addition to the promoting effect on tumor growth [8, 9], IL-8 has also been found to have a strong effect in modulating angiogenesis via IL-8RA and/or IL-8RB recently [8–11]. The co-expression of IL-8 receptors (IL-8RA and IL-8RB) with tumor-associated microvessels was, therefore, examined. The results showed that both the IL-8RA and IL-8RB (brown color in Fig. 3g–j) were present in the CD34 positive tumor-associated microvessels (red color in Fig. 3g–j) in the adenomas (Fig. 3g, i) and cancers (Fig. 3h, j).
Discussion
Previously, we and other groups have demonstrated disrupted cytokine profile in colorectal adenoma and CRC [13, 18, 26, 30], suggesting that cytokine alternation is a common phenomena and may play a role in the adenoma–carcinoma transition. In this study, we were able to demonstrate a gradually increased IL-8 mRNA level throughout the adenoma–carcinoma sequence, IL-8 and its receptors (IL-8RA and IL-8RB) were expressed in both the stromal cells and tumor cells. In the tumor stroma, the IL-8RA and IL-8RB could be observed in the tumor-associated microvessels in adenomas and cancers. This indicated that increased IL-8 might play a potential role to be involved in the adenoma–carcinoma transition via regulating tumor cell growth and angiogenesis.
According to the adenoma–carcinoma sequence theory, CRCs arise primarily in a setting where a multi-step carcinogenesis event progresses from colorectal adenoma with a LGD to high dysplasia and finally to carcinoma [1, 2]. Thus, the colorectal adenoma has been recognized as the main precancerous lesion for most CRCs, and many molecular and genetic alterations that contribute to the adenoma formation and cancer progression have been identified [1, 2]. The colorectal adenoma–carcinoma transition develops slowly over many years and the microenvironment inevitably changes so as to establish a supportive environment. Some cytokines and chemokines derived from CRC and immune cells may participate in the establishment of such supportive microenvironment. Most significantly, a number of recent studies have shown that IL-8, released from both the tumor cells and non-tumor cells, can promote the development of CRCs [9, 21, 31]. Our current results, consistent with a recent published report [19], have demonstrated that tissue IL-8 mRNA along the adenoma–carcinoma sequence was gradually increased in relative to the controls. Particular interesting finding was that the gradual increased IL-8 mRNA level was significantly associated with increased degree of dysplasia in the adenomas. Since in vitro studies have shown that IL-8 can serve as a important growth factor for CRC cells [32] and tumor cell-derived IL-8 may act as an autocrine factor in stimulating CRC cell growth [15, 16, 33], our current observations may suggest that increased IL-8 is a common feature of the adenoma–carcinoma sequence and could play a pivotal role in the formation and progression of adenoma. Furthermore, it has been found that the IL-8 level in serum of CRC patients is elevated and associated with disease stages and metastasis potential [18, 19, 34]. In this study, we have also observed an even higher tissue IL-8 mRNA in CRCs as compared with the adenomas and was related to the Duke’s stages, and suggested that increased IL-8 may be an important contributor to the cancer progression.
Extensive evidence now suggests that cancer cells can produce many factors (including cytokines and chemokines) to stimulate and/or create a favorite microenvironment for the cancer cell survival, proliferation and metastasis [12, 16, 35]. In the current study, a significant increased IL-8 expression was observed in the cancer cells, this finding suggested that CRC derived IL-8 may significantly contribute to the increased tissue IL-8 level in CRC patients and act as an autocrine factor in stimulating cancer cell growth, such observation has been confirmed in CRC cell line in vitro study [15]. It is well accepted that the function of infiltrated immune cells and the stromal myofibroblasts are capable of remodeling tumor stroma and play a key role in controlling tumor cell growth and angiogenesis [36, 37]. In our previous study, a general increase numbers of macrophage and lymphocyte infiltration and fibroblast/myofibroblasts activation throughout the adenoma–carcinoma sequence have been observed [38]; all these cells are the important cytokine source and play a critical role in mediating the tumor growth and angiogenesis in many types of human cancers [8, 9]. In the current study, by IHCs and double IHCs, we demonstrated that both the adenomatous/cancerous cells and several types of cells (including macrophages, lymphocytes and myofibroblasts) present in the tumor stroma can express IL-8 (Figs. 2, 3), and indicated that IL-8 was from a mixture cellular source (both the adenomatous/cancerous epithelium and stroma) in colorectal adenomas/cancers. One of the prerequisites for IL-8 in performing its biological role is the presenting of IL-8 and its receptors IL-8RA and IL-8RB in the tumor microenvironment. Our current IHC results clearly showed that IL-8 and both IL-8RA and IL-8RB were expressed in adenomatous/cancerous cells and stromal cells. Accordingly, the expression of both IL-8 and its receptors in the adenomatous/cancerous cells further supported an autocrine and/or paracrine loop existed in the tumor microenvironment.
In addition to the direct effect in stimulating CRC cell growth, another promoting effect of IL-8 during the carcinogenesis is to enhance the angiogenesis in the tumor microenvironment, which provides the necessary nutrients for cancer growth and invasion [8]. Angiogenic switch during the transformation in colorectal mucosa has been demonstrated [39–41], and more recently such swift is found to be an early occurring event in the early stage of adenomas [6]. IL-8 is one of the potential proangiogenic factors and stimulates angiogenesis via IL-8 receptors particularly IL-8RB defined in the microvessels both in vivo and in vitro [8–10]. In this study, double IHCs with IL-8RA/CD34 and IL-8RB/CD34 antibodies revealed that both IL-8RA and IL-8RB immunoreactivities were observed in CD34 positive tumor-associated microvessel. This finding suggested that both IL-8RA and IL-8RB may be involved in the IL-8’s effect in modulating angiogenesis during the adenoma–carcinoma transition.
Taken together, our data outline a potential role of IL-8 network in promoting adenoma–carcinoma transition. This role could be via a direct stimulating effect on colorectal mucosal cell transformation and indirect enhancing effect on angiogenesis. Therefore, we propose that IL-8 and its receptors may have important implication for serving as interesting therapeutic targets in modulating the adenoma–carcinoma transition, which has recently been discussed in other types of cancer [21].
Acknowledgments
This work was financially supported by grants from Medical Research Program, Northern Norway Regional Health Authority (SFP-44-04) and Erna & Olav Aakres Foundation (A4783) to G. Cui.
Conflict of interest statement
None.
Abbreviations
- IL-8
Interleukin-8
- CRC
Colorectal cancer
- Q-PCR
Quantitative real-time PCR
- IHC
Immunohistochemistry
References
- 1.Khosraviani K. Colorectal adenoma–carcinoma sequence. Gut. 1996;39:342. doi: 10.1136/gut.39.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie A, Carey FA, Pratt NR, Steele RJ. The colorectal adenoma–carcinoma sequence. Br J Surg. 2002;89:845–860. doi: 10.1046/j.1365-2168.2002.02120.x. [DOI] [PubMed] [Google Scholar]
- 3.Young J, Jass JR. The case for a genetic predisposition to serrated neoplasia in the colorectum: hypothesis and review of the literature. Cancer Epidemiol Biomark Prev. 2006;15:1778–1784. doi: 10.1158/1055-9965.EPI-06-0164. [DOI] [PubMed] [Google Scholar]
- 4.Habermann JK, Paulsen U, Roblick UJ, Upender MB, McShane LM, Korn EL, Wangsa D, Kruger S, Duchrow M, Bruch HP, Auer G, Ried T. Stage-specific alterations of the genome, transcriptome, and proteome during colorectal carcinogenesis. Genes Chromosomes Cancer. 2007;46:10–26. doi: 10.1002/gcc.20382. [DOI] [PubMed] [Google Scholar]
- 5.Mizoshita T, Tsukamoto T, Inada KI, Hirano N, Tajika M, Nakamura T, Ban H, Tatematsu M. Loss of MUC2 expression correlates with progression along the adenoma–carcinoma sequence pathway as well as de novo carcinogenesis in the colon. Histol Histopathol. 2007;22:251–260. doi: 10.14670/HH-22.251. [DOI] [PubMed] [Google Scholar]
- 6.Staton CA, Chetwood AS, Cameron IC, Cross SS, Brown NJ, Reed MW. The angiogenic switch occurs at the adenoma stage of the adenoma carcinoma sequence in colorectal cancer. Gut. 2007;56:1426–1432. doi: 10.1136/gut.2007.125286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee S, Bang S, Song K, Lee I. Differential expression in normal–adenoma–carcinoma sequence suggests complex molecular carcinogenesis in colon. Oncol Rep. 2006;16:747–754. [PubMed] [Google Scholar]
- 8.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 9.Xie K. Interleukin-8 and human cancer biology. Cytokine Growth Factor Rev. 2001;12:375–391. doi: 10.1016/S1359-6101(01)00016-8. [DOI] [PubMed] [Google Scholar]
- 10.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 11.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 12.Li A, Varney ML, Singh RK. Expression of interleukin 8 and its receptors in human colon carcinoma cells with different metastatic potentials. Clin Cancer Res. 2001;7:3298–3304. [PubMed] [Google Scholar]
- 13.Chung YC, Chang YF. Significance of inflammatory cytokines in the progression of colorectal cancer. Hepatogastroenterology. 2003;50:1910–1913. [PubMed] [Google Scholar]
- 14.Itoh Y, Joh T, Tanida S, Sasaki M, Kataoka H, Itoh K, Oshima T, Ogasawara N, Togawa S, Wada T, Kubota H, Mori Y, Ohara H, Nomura T, Higashiyama S, Itoh M. IL-8 promotes cell proliferation and migration through metalloproteinase-cleavage proHB-EGF in human colon carcinoma cells. Cytokine. 2005;29:275–282. doi: 10.1016/j.cyto.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 15.Brew R, Erikson JS, West DC, Kinsella AR, Slavin J, Christmas SE. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- 16.Brew R, Southern SA, Flanagan BF, McDicken IW, Christmas SE. Detection of interleukin-8 mRNA and protein in human colorectal carcinoma cells. Eur J Cancer A. 1996;32:2142–2147. doi: 10.1016/S0959-8049(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 17.Lurje G, Zhang W, Schultheis AM, Yang D, Groshen S, Hendifar AE, Husain H, Gordon MA, Nagashima F, Chang HM, Lenz HJ. Polymorphisms in VEGF and IL-8 predict tumor recurrence in stage III colon cancer. Ann Oncol. 2008;19:1734–1741. doi: 10.1093/annonc/mdn368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 19.Rubie C, Frick VO, Pfeil S, Wagner M, Kollmar O, Kopp B, Graber S, Rau BM, Schilling MK. Correlation of IL-8 with induction, progression and metastatic potential of colorectal cancer. World J Gastroenterol. 2007;13:4996–5002. doi: 10.3748/wjg.v13.i37.4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates RC, DeLeo MJ, III, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–324. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 21.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 22.Miller MD, Krangel MS. Biology and biochemistry of the chemokines: a family of chemotactic and inflammatory cytokines. Crit Rev Immunol. 1992;12:17–46. [PubMed] [Google Scholar]
- 23.Terada H, Urano T, Konno H. Association of interleukin-8 and plasminogen activator system in the progression of colorectal cancer. Eur Surg Res. 2005;37:166–172. doi: 10.1159/000085964. [DOI] [PubMed] [Google Scholar]
- 24.Cui G, Olsen T, Christiansen I, Vonen B, Florholmen J, Rasmus G. Improvement of real-time PCR for quantifying TNF-a mRNA expression in inflamed colorectal mucosa—an approach to optimize procedures for clinical use. Scand J Clin Lab Invest. 2006;66:249–259. doi: 10.1080/00365510600590472. [DOI] [PubMed] [Google Scholar]
- 25.Goll R, Gruber F, Olsen T, Cui G, Raschpichler G, Buset M, Asfeldt AM, Husebekk A, Florholmen J. Helicobacter pylori stimulates a mixed adaptive immune response with a strong T-regulatory component in human gastric mucosa. Helicobacter. 2007;12:185–192. doi: 10.1111/j.1523-5378.2007.00495.x. [DOI] [PubMed] [Google Scholar]
- 26.Cui G, Yuan A, Goll R, Olsen T, Husebekk A, Vonen B, Florholmen J. Distinct changes of dendritic cell number and IL-12 mRNA level in adjacent mucosa throughout the colorectal adenoma–carcinoma sequence. Cancer Immunol Immunother. 2007;56:1993–2001. doi: 10.1007/s00262-007-0345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan A, Steigen SE, Goll R, Vonen B, Husbekk A, Cui G, Florholmen J. Dendritic cell infiltration pattern along the colorectal adenoma–carcinoma sequence. Apmis. 2008;116:445–456. doi: 10.1111/j.1600-0463.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 28.Cui G, Koh TJ, Chen D, Zhao CM, Takaishi S, Dockray GJ, Varro A, Rogers AB, Fox JG, Wang TC. Overexpression of glycine-extended gastrin inhibits parietal cell loss and atrophy in the mouse stomach. Cancer Res. 2004;64:8160–8166. doi: 10.1158/0008-5472.CAN-04-0876. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Liu J, Tang F, Liu Y, Waldum HL, Cui G. Expression of non-mast cell histidine decarboxylase in tumor-associated microvessels in human esophageal squamous cell carcinomas. Apmis. 2008;116:1034–1042. doi: 10.1111/j.1600-0463.2008.01048.x. [DOI] [PubMed] [Google Scholar]
- 30.Cui G, Goll R, Olsen T, Steigen SE, Husebekk A, Vonen B, Florholmen J. Reduced expression of microenvironmental Th1 cytokines accompanies adenomas–carcinomas sequence of colorectum. Cancer Immunol Immunother. 2007;56:985–995. doi: 10.1007/s00262-006-0259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cacev T, Radosevic S, Krizanac S, Kapitanovic S. Influence of interleukin-8 and interleukin-10 on sporadic colon cancer development and progression. Carcinogenesis. 2008;29:1572–1580. doi: 10.1093/carcin/bgn164. [DOI] [PubMed] [Google Scholar]
- 32.Brew R, Erikson JS, West DC, Flanagan BF, Christmas SE. Interleukin-8 as a growth factor for human colorectal carcinoma cells in vitro. Biochem Soc Trans. 1997;25:264S. doi: 10.1042/bst025264s. [DOI] [PubMed] [Google Scholar]
- 33.Wigmore SJ, Maingay JP, Fearon KC, Ross JA. Endogenous production of IL-8 by human colorectal cancer cells and its regulation by cytokines. Int J Oncol. 2001;18:467–473. doi: 10.3892/ijo.18.3.467. [DOI] [PubMed] [Google Scholar]
- 34.Haraguchi M, Komuta K, Akashi A, Matsuzaki S, Furui J, Kanematsu T. Elevated IL-8 levels in the drainage vein of resectable Dukes’ C colorectal cancer indicate high risk for developing hepatic metastasis. Oncol Rep. 2002;9:159–165. [PubMed] [Google Scholar]
- 35.Fantini MC, Pallone F. Cytokines: from gut inflammation to colorectal cancer. Curr Drug Targets. 2008;9:375–380. doi: 10.2174/138945008784221206. [DOI] [PubMed] [Google Scholar]
- 36.Almholt K, Johnsen M. Stromal cell involvement in cancer. Recent Results Cancer Res. 2003;162:31–42. doi: 10.1007/978-3-642-59349-9_3. [DOI] [PubMed] [Google Scholar]
- 37.De Wever O, Mareel M. Role of tissue stroma in cancer cell invasion. J Pathol. 2003;200:429–447. doi: 10.1002/path.1398. [DOI] [PubMed] [Google Scholar]
- 38.Cui G, Yuan A, Vonen B, Florholmen J (2009) Progressive cellular response in the lamina propria of the colorectal adenoma–carcinoma sequence. Histopathology (in press) [DOI] [PubMed]
- 39.Hanrahan V, Currie MJ, Gunningham SP, Morrin HR, Scott PA, Robinson BA, Fox SB. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma–carcinoma sequence during colorectal cancer progression. J Pathol. 2003;200:183–194. doi: 10.1002/path.1339. [DOI] [PubMed] [Google Scholar]
- 40.Shpitz B, Gochberg S, Neufeld D, Grankin M, Buklan G, Klein E, Bernheim J. Angiogenic switch in earliest stages of human colonic tumorigenesis. Anticancer Res. 2003;23:5153–5157. [PubMed] [Google Scholar]
- 41.Takahashi Y, Ellis LM, Mai M. The angiogenic switch of human colon cancer occurs simultaneous to initiation of invasion. Oncol Rep. 2003;10:9–13. [PubMed] [Google Scholar]