Abstract
Gonadotrophin-releasing hormone (GnRH) is the prime decapeptide hormone in the regulation of mammalian reproduction. Active immunization against GnRH has been a good treatment option to fight against hormone-dependent disease such as breast cancer. We designed and purified a novel protein vaccine Hsp65–GnRH6 containing heat shock protein 65 (Hsp65) and six copies of GnRH in linear alignment. Immunization with Hsp65–GnRH6 evoked strong humoral response in female mice. The generation of specific anti-GnRH antibodies was detected by ELISA and verified by western blot. In addition, anti-GnRH antibodies effectively neutralized endogenous GnRH activity in vivo, as demonstrated by the degeneration of the ovaries and uteri in the vaccinated mice. Moreover, the growth of EMT-6 mammary tumor allografts was inhibited by anti-GnRH antibodies. Histological examinations have shown that there was increased focal necrosis in tumors. Taken together, our results showed that immunization with Hsp65–GnRH6 elicited high titer of specific anti-GnRH antibodies and further led to atrophy of reproductive organs. The specific antibodies could inhibit the growth of EMT-6 murine mammary tumor probably via an indirect mechanism that includes the depletion of estrogen. In view of these results, the protein vaccine Hsp65–GnRH6 appears to be a promising candidate vaccine for hormone-dependent cancer therapy.
Keywords: GnRH, Hsp65, Vaccine, Cancer immunotherapy, Hormone-dependent cancer
Introduction
GnRH, also known as luteinizing hormone-releasing hormone (LHRH), is the key decapeptide hormone in the regulation of mammalian reproduction. It is released from hypothalamus in a pulsatile manner and stimulates pituitary gonadotropes to synthesize and release the gonadotropins, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The gonadotropins are members of the glycoprotein hormone family and stimulate folliculogenesis, ovulation, and spermatogenesis [1, 2]. It is well known that several tumors, most commonly tumors of the breast and prostate, are considered hormone-dependent. In such cases, cancer patients might benefit from endocrine therapy, mainly based on deprivation of hormone by different procedures [3]. There is currently a wide and growing armamentarium of hormone-therapy procedures, which mainly includes surgical castration and medical castration. Surgical castration is relatively simple and effective, but it is permanent and cannot be reversed which made it difficult to be accepted by many people [4]. Medical castration is minimally invasive and reversible and is an effective alternative of surgical castration, but the patients would suffer from some upset side effects and expensive cost. There are many classes of drugs such as gonadotropin-releasing hormone (GnRH) analogs, antiandrogen, antiestrogen, sex hormone receptor antagonists, and aromatase inhibitors [3]. Among these, GnRH analogs are most commonly used as hormone therapy and there are currently a number of different GnRH analogs available [4, 5]. GnRH agonists and antagonists have been the first-line drugs and the future development trend for the treatment of prostate and breast cancer [6].
At the same time, increasing knowledge has accumulated about active immunization for cancer treatment. Though GnRH is a self hapten with inherently weak immunogenicity, it has been shown that it is possible to provoke strong humoral immune responses by various methods, such as the usage of powerful adjuvants, linear alignment of the hapten, retro-inverso strategy and fusion or conjugation to defined T helper epitopes, and/or carrier proteins [7–9]. Linear repeats of GnRH fused to the receptor-binding domain of pseudomonas exotoxin A elicited high-titer GnRH-specific antibodies and promoted degeneration of ovaries in female rabbits [10]. In our laboratory, we also have successfully improved the immunogenicity of GnRH. First, we prepared a recombinant peptide vaccine GnRH3-hinge-MVP, containing three copies of GnRH, hinge region of human IgG, and T helper epitope of measles virus protein (MVP). The double-chain miniprotein obtained by oxidizing GnRH3-hinge-MVP induced anti-GnRH antibody responses in rats in the presence of Freund’s adjuvant [11]. However, Freund’s adjuvant is not acceptable for human use due to contamination with non-metabolizable oil and mycobacteria [12]. Various systems are being developed to circumvent the need for strong and often toxic adjuvant [9]. Certain carrier proteins such as mycobacterial heat shock protein 65 (Hsp65) also have adjuvant-like properties and can be used efficiently as carriers in an adjuvant-free system [13, 14]. Our previous results shown the conjugates of GnRH3-hinge-MVP and Hsp65 could elicit strong humoral immune responses against GnRH, and inhibit the growth of H22 hepatocellular carcinoma and RM-1 prostate cancer in mice [7, 15]. However, we had to express and purify two proteins: recombinant peptide vaccine GnRH3-hinge-MVP and recombinant Hsp65. Meanwhile, the efficiency and purity of chemical conjugation also must be taken into account. To simplify the processes, we fused six copies of GnRH to the C-terminus of Hsp65 by genetic technology in this study. Vaccination with the fusion protein Hsp65–GnRH6 evoked strong humoral response and further led to atrophy of ovary and uterus. Moreover, our results also show here that the immunization with Hsp65–GnRH6 would inhibit the growth of EMT-6 mammary tumor cells.
Materials and methods
Construction of the expression plasmid
DNA fragment encoding GnRH3-hinge-MVP which contains three copies of GnRH was amplified by anchor PCR using forward primer HGP1: 5′-ACC AGT ACG GCT AGC GAA CAT TGG-3′ (with NheI site) and reverse primer HGP2: 5′-CCG CAA GCT TAT TTA GCA AC-3′ (with HindIII site). The plasmid pEDG (pET28a-ansB-C-GnRH3-hinge-MVP) constructed by our laboratory previously was used as a template [16]. The PCR product was double-digested by NheI and HindIII and subsequently inserted into the plasmid pET28a–Hsp65 to generate a new plasmid pET28a-Hsp65-GnRH3-hinge-MVP. Then, another DNA fragment containing three copies of GnRH (GnRH3) was also synthesized by anchor PCR with forward primer HGP3: 5′-ACC AGT ACG GGT ACC GAA CAT TGG-3′ (with KpnI site), reverse primer HGP4: 5′-CGG CGC GCA AAG CTT AAC CCG GAC GCA G-3′ (with HindIII site), and the template plasmid pEDG. Then, the fragment hinge-MVP of the plasmid pET28a-Hsp65-GnRH3-hinge-MVP was substituted by GnRH3. The resulting expression plasmid was designated as pETHG6 (pET28a–Hsp65–GnRH6).
Expression and purification of the fusion protein Hsp65–GnRH6
The plasmid pETHG6 was transformed into E. coli. BL21 (DE3), and a single resultant colony was inoculated into 100 mL of LB medium (containing 50 μg/mL kanamycin) and grown overnight at 37°C with shaking at 200 r/min. The seed was inoculated into fresh medium at the ratio of 1:50. When OD600 reached 0.6–0.8, lactose was added to a final concentration of 5 mmol/L. After induction, 1 mL aliquots of bacteria culture were sampled hourly and analyzed by 12% SDS-PAGE to detect the expression level of fusion protein Hsp65–GnRH6.
The cells were harvested by centrifugation and resuspended in cell lysis buffer (50 mmol/L Tris–Cl, pH 8.0; 0.5% Triton-X 100; 0.2 mg/mL lysozyme and 0.01 mg/mL DNase I) by stirring for 0.5 h at 37°C. After centrifugation, the pellets were discarded and the supernatant containing soluble Hsp65–GnRH6 proteins was undergone ammonium sulfate fractionation. The proteins precipitated with 20–40% saturated (NH4)2SO4 were analyzed by 12% SDS-PAGE to detect the content of fusion protein Hsp65–GnRH6. Then the proteins precipitated with 40% saturated (NH4)2SO4 were resolved and dialysed against 25 mmol/L Tris–Cl (pH 7.5) for 24 h. The solution was loaded on a pre-equilibrated DEAE-cellulose (Whatman, USA) column and the proteins were eluted with a linear gradient of 0–0.4 mol/L NaCl in the equilibration buffer 25 mmol/L Tris–Cl (pH 7.5). The peak fraction containing Hsp65–GnRH6 (determined by SDS-PAGE) was pooled and dialysed against equilibration buffer for 12 h and thereafter water for 12 h. Finally, the inner solution was collected and lyophilized.
Immunization procedure
Female Balb/c mice were randomized into three groups, with eight mice per group. The mice were administered subcutaneously in the right flank with 100 μL PBS (placebo), 50 μg Hsp65 (a gift from Dr. Liang Jin) and 50 μg Hsp65–GnRH6 four times at biweekly intervals. Sera were collected biweekly for immunoassay from the following week after initial immunization.
Western blot assay
This assay was conducted as described previously [17]. Briefly, fusion protein VEGF–GnRH, VEGF and prestained protein marker were electrophoresed on 15% SDS-polyacrylamide gel and transferred onto nitrocellulose membrane (Millipore, USA). Then the membrane was blocked with 5% BSA for 2 h and probed with 1:50 diluted sera for 1 h at 37°C. Thereafter, the membrane was rinsed four times and then hybridized with HRP-conjugated goat anti-mouse IgG (Boster, China) diluted 1:200. After washing again, the protein bands were developed using DAB and H2O2 at room temperature.
ELISA for anti-GnRH antibodies
The titers of antibodies against GnRH in immunized animals were detected using ELISA as described previously [17, 18]. In brief, 96-well ELISA plates (Costar, USA) were coated with 10 μg/well VEGF–GnRH and kept overnight at 4°C. Plates were blocked with 5% BSA for 1 h and then incubated with 100 μL/well 1:100 dilution of sera which collected from immunized animals for another 1 h at 37°C. After incubation, wells were washed six times with PBST and then incubated with 100 μL/well of HRP-conjugated goat anti-mouse IgG (Boster, China) diluted 1:20,000 in PBS containing 2% BSA for 1 h at 37°C. Wells were washed intensively six times with PBST and then incubated with 100 μL/well of the horseradish peroxidase substrate (0.01% TMB and 0.24% H2O2–urea) for 20 min at 37°C. The reaction was halted by H2SO4 and then measurement of OD450 value was performed. Each measurement was carried out in duplicate.
To address the endpoint titer of specific anti-GnRH antibody, the serum collected a week after the last booster immunization was diluted by twofold serial dilutions from an initial dilution of 1:100 in PBS. Aliquots of 100 μL serially diluted samples were performed ELISA as described earlier. Endpoint titers were expressed as the reciprocal log 2 of the last sample dilution giving an OD450 value above 0.1.
Tumor challenge experiment
Tumor challenge experiment was performed by subcutaneous injection of EMT-6 mammary tumor cells (5 × 107 cells in 0.2 mL PBS) in the left flank of all mice on the eighth week after the initial immunization. On the tenth week, all mice were killed. The tumors and the uteri with ovaries attached were dissected out carefully and excess fat was removed. The combined weight of uterus and ovaries, and the weight of tumor were determined for individual mice in each group.
Histological evaluation
Following necropsy, the tumors, ovaries, and uteri from the vaccinated and control mice were fixed in 10% buffered neutral formalin immediately and then embedded in paraffin wax according to standard procedures. The 5-μm sections were cut and stained by standard protocols with hematoxylin and eosin (HE). Histological examination of the tissues was performed by light microscopy. All histological sections were examined by a consultant pathologist who was not informed which treatment the mice had been given.
Statistical analysis
The statistical analysis was carried out using the Student’s t test to determine differences between the groups. p value of < 0.05 was considered statistically significant.
Results
Preparation of the fusion protein Hsp65–GnRH6
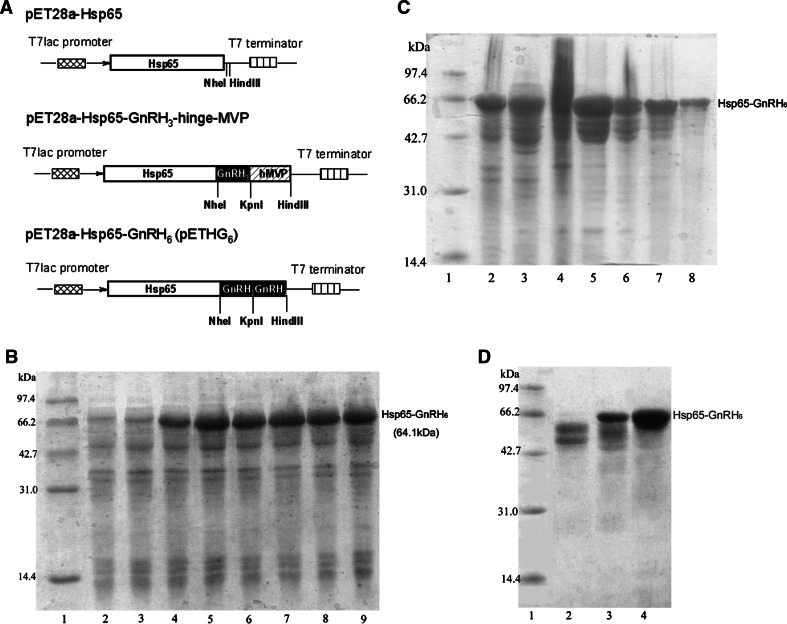
The plasmid pEDG (pET28a-ansB-C-GnRH3-hinge-MVP) which contained three linear repeats of GnRH had been constructed by our laboratory [16]. By using it as template, anchor PCR was performed to obtain two different DNA fragments containing three copies of GnRH. Then the PCR products were inserted into plasmid pET28a–Hsp65 in turn to generate plasmid pETHG6 (Fig. 1a).
Fig. 1.
Expression and purification of the fusion protein Hsp65–GnRH6. a Schematic diagrams of the construction process of the expression plasmid pETHG6. Upper the Hsp65 (represented by open box) gene was placed under the control of T7lac promoter (represented by cross box); middle DNA fragment encoding GnRH3-hinge-MVP was ligated to Hsp65 gene through the restriction enzymes NheI and HindIII; lower the fragment hinge-MVP (represented by bias box) was substituted by another DNA fragment encoding three copies of GnRH (represented by dark box). The resulting plasmid was designated as pETHG6. b Expression level of the fusion protein Hsp65–GnRH6. Total cell proteins were analyzed on a 12% polyacrylamide gel and stained with Coomassie Brilliant Blue R-250. Lane 1 marker proteins with molecular masses in kilodaltons indicated at left margin; lanes 2–9 total cell proteins from E. coli BL21 with plasmid pETHG6 after induction 0, 1, 2, 3, 4, 5, 6 and 7 h, respectively. c SDS-PAGE analysis of partially purified HSP65–GnRH6 by ammonium sulfate precipitation. Lane 1 marker proteins; lanes 2, 3 total cell proteins from E. coli BL21 with plasmid pETHG6 after induction 7 h; lanes 4–8 the pellets precipitated by 20, 25, 30, 35 and 40% saturated ammonium sulfate, respectively. d SDS-PAGE analysis of purified HSP65–GnRH6. Lane 1 marker proteins; lane 2 the flow-through of DEAE column; lane 3 partially purified HSP65–GnRH6 precipitated by 40% saturated ammonium sulfate; lane 4 purified HSP65–GnRH6 by further DEAE anion exchange chromatography
After being reinoculated for 3.5 h, the bacteria population entered into the logarithmic growth phase and then lactose was added to induce the expression of fusion protein Hsp65–GnRH6. The expression level of Hsp65–GnRH6 increased with time reaching its maximum about 3 h after induction (Fig. 1b). Since the culture attained the stationary phase at about 10 h after reinoculation, the cells were harvested at that time to obtain high yield production of the fusion protein. Hsp65–GnRH6 was expressed as soluble proteins, and purified to apparent homogeneity in the SDS-PAGE by ammonium sulfate fractionation and DEAE anion exchange chromatography (Fig. 1c, d).
Analysis of specific anti-GnRH antibodies
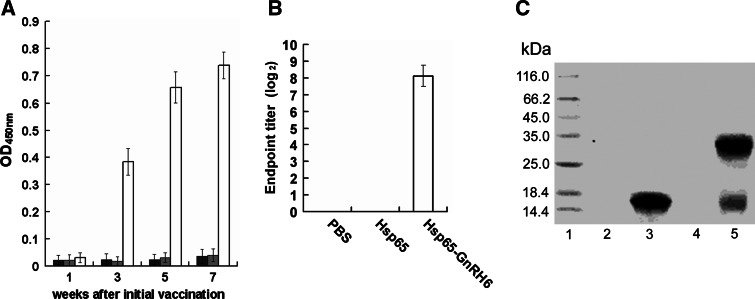
To determine antibody responses, mice were immunized s.c. with Hsp65–GnRH6, Hsp65, and PBS, sera were sampled at designated time points and GnRH-specific IgG were monitored by ELISA (Fig. 2a). The antibody was not detectable in all mice at 1 week after the initial immunization, while at 1 week after the second immunization, the mice treated with Hsp65–GnRH6 had produced high-titer anti-GnRH antibodies (p < 0.001 vs. Hsp65 or PBS). And the titer of anti-GnRH antibodies rose after successive administration. Our results suggested that Hsp65–GnRH6 was immunogenic enough to elicit strong humoral response in mice, and the level of anti-GnRH antibodies was positively related to the vaccination times. During the course of experiment, no antibodies responses against GnRH were observed in mice immunized with PBS or Hsp65.
Fig. 2.
Assay of specific anti-GnRH antibodies. a ELISA results of anti-GnRH antibodies in sera of the mice immunized by subcutaneous injection (n = 8, mean ± SD). Balb/c male mice were immunized four times at biweekly intervals. Sera samples were collected biweekly after initial immunization and subjected to ELISA (PBS dark bar; Hsp65 gray bar; Hsp65–GnRH6 empty bar). b Endpoint titer of anti-GnRH antibody. Endpoint titers were represented as the reciprocal log2 of the last sample dilution giving an OD450 value above 0.1. c Western blot analysis of anti-GnRH antibodies. Antibodies from the mice reacted with VEGF–GnRH rather than VEGF. Lane 1 prestained protein marker; lane 2 VEGF with DTT; lane 3 VEGF–GnRH with DTT; lane 4 VEGF without DTT; lane 5 VEGF–GnRH without DTT
To address the endpoint titer of specific anti-GnRH antibody, twofold serial dilutions methods were performed. Our results showed that after being diluted 25,600 times with PBS, the serum still can give a positive signal (Fig. 2b).
To further demonstrate the specificity of the antibodies against GnRH, the serum from mice immunized with Hsp65–GnRH6 was subjected to western blot. The results suggested that antibodies from immunized mice could bind to VEGF–GnRH (lanes 3 and 5) rather than VEGF (lanes 2 and 4), regardless of under reducing or non-reducing conditions (Fig. 2c).
Effect of anti-GnRH antibodies on the reproductive organs
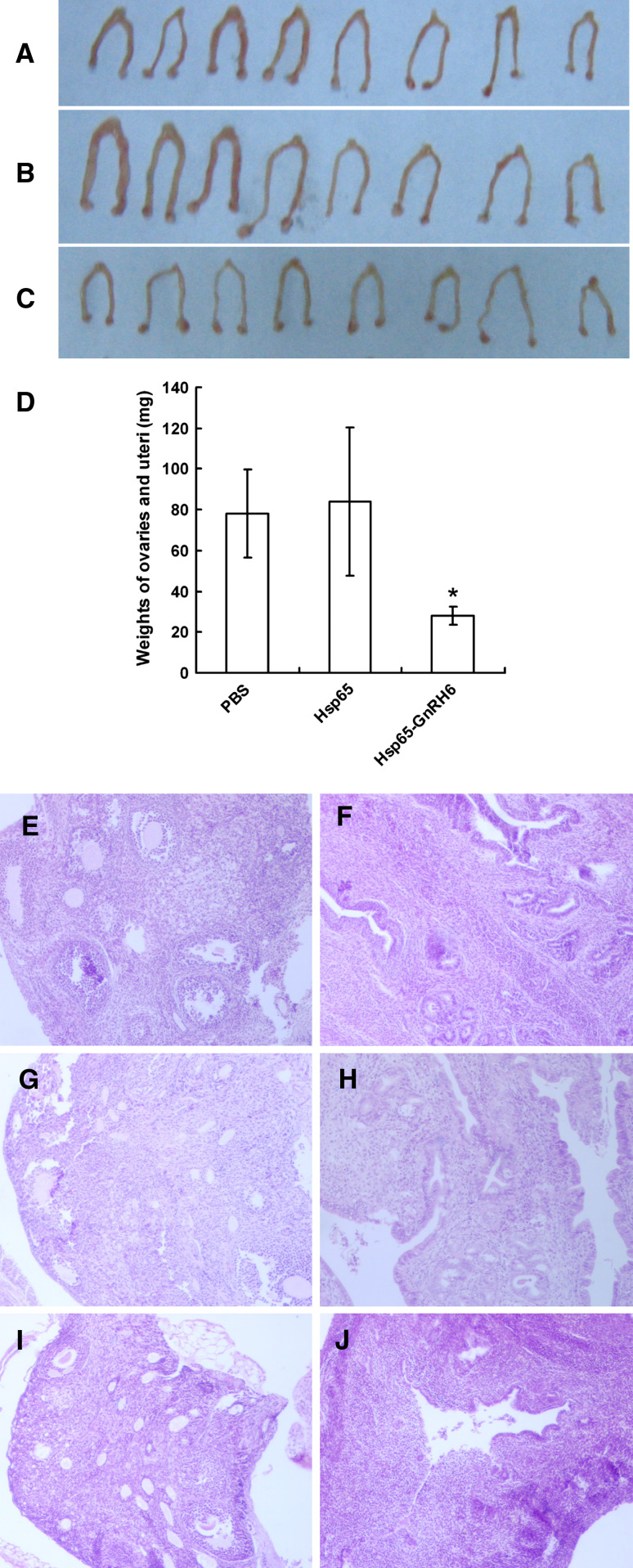
In mice immunized with Hsp65–GnRH6, the reproductive organs were smaller than those in mice treated with PBS or Hsp65 (Fig. 3a–c). The total weights of ovaries and uteri were also significantly lighter than those in controls (Fig. 3d, p < 0.001 vs. PBS or Hsp65). Moreover, the microscopic observation of H&E-stained sections from mice treated with PBS showed normal follicular development and luteinization in ovaries together with normal endometrial development of glandular structures and active stroma in uteri (Fig. 3e–h). Meanwhile, the reproductive organs from animals administered with Hsp65–GnRH6 revealed reduced follicular development in the ovaries and atrophy of the uteri with fewer glandular components and inactive stroma (Fig. 3i, j). These results indicated that the reproductive organs would degenerate owing to the depletion of endogenous GnRH, which was neutralized by Hsp65–GnRH6 induced antibodies.
Fig. 3.
Effect of anti-GnRH antibodies on the reproductive organs. The morphology of ovaries and uteri of mice immunized with PBS (a), Hsp65 (b) or Hsp65–GnRH6 (c). d Comparsion of the total weights of ovaries and uteri immunized with PBS, Hsp65 or Hsp65–GnRH6. e–j The microscopic observation of H&E-stained sections of ovaries and uteri from mice treated with PBS (e, f), Hsp65 (g, h) and Hsp65–GnRH6 (i, j)
Effect of anti-GnRH antibodies on tumor growth
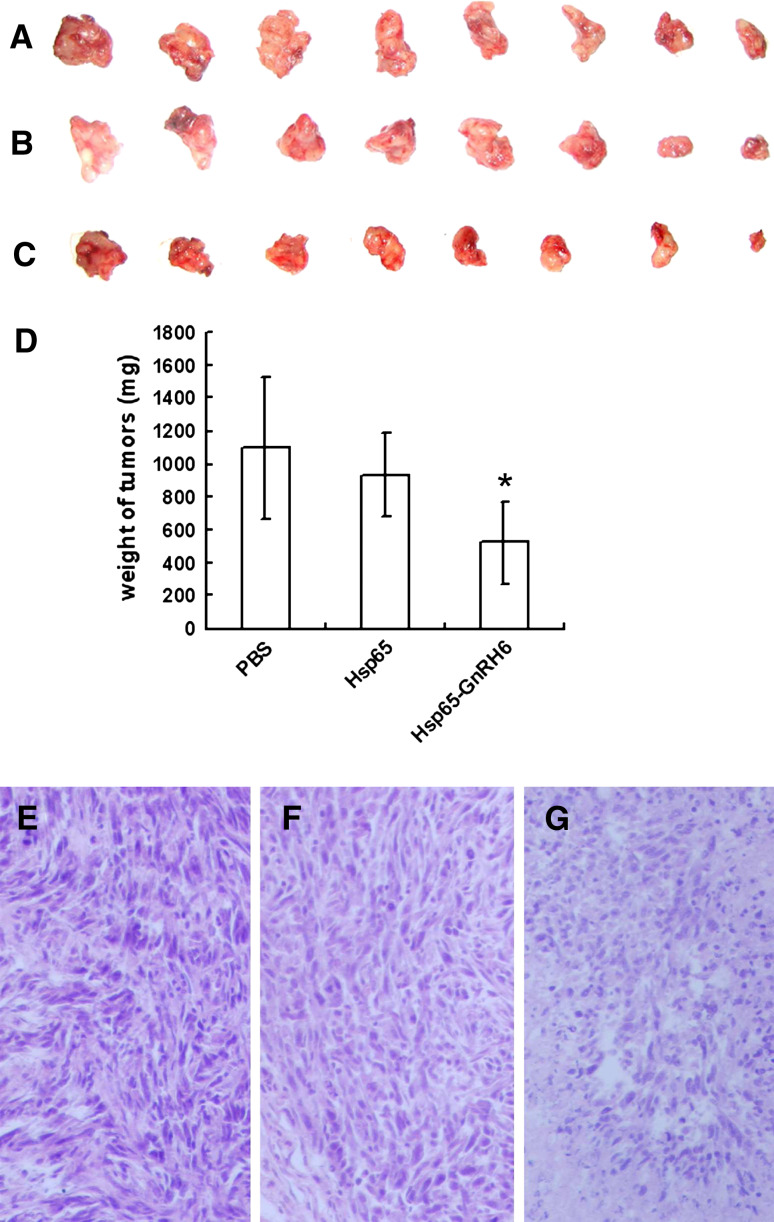
After four continuous immunizations at biweekly intervals, the mice were challenged with mammary tumor cells. The rate of tumor growth was significantly inhibited in mice immunized with Hsp65–GnRH6 compared with that in mice treated with PBS or Hsp65. All mice were killed on day 14 after tumor challenge. The tumors were carefully removed and weighed. A significant inhibition of tumor growth was achieved by immunized with Hsp65–GnRH6, the weight of the tumor was 522.4 ± 252.0 mg, in contrast to that of 1100.6 ± 429.6 mg in PBS group (p < 0.01) or that of 931.5 ± 249.9 mg in Hsp65 group (p < 0.01) (Fig. 4a–d). These results demonstrated that tumor growth was inhibited by active immunization with fusion protein Hsp65–GnRH6. To further demonstrate the anti-tumor effect of Hsp65–GnRH6, the tumor sections were observed under an optical microscope and showed decrease of tumor volume and severe focal necrosis (Fig. 4g), while the tumor cells of mice treated with PBS or Hsp65 grew well and had little necrosis (Fig. 4e, f).
Fig. 4.
Effect of anti-GnRH antibodies on tumor growth. Solid tumors from mice were aligned and their photos were taken. a PBS; b Hsp65 and c Hsp65–GnRH6. d The wet weights of tumor of mice treated with PBS, Hsp65 or Hsp65–GnRH6 e–g The microscopic observation of H&E-stained sections of tumor from mice treated with PBS (e), Hsp65 (f) and Hsp65–GnRH6 (g)
Discussions
GnRH is the prime regulator of the hypothalamic-pituitary-gonadal axis in all mammals. It is secreted in a pulsatile manner into the pituitary portal vessels and acts upon gonadotrophs in the pituitary to stimulate the production of the gonadotropins, luteinizing hormone (LH), and follicle-stimulating hormone (FSH), thus playing a central role in maturation of ovarian follicles or spermatogenesis [1, 4]. Over the past three decades, first-, second-, and third-generation GnRH analogs have been developed to combat the hormone-dependent diseases, especially breast and prostate cancers [5, 19]. Although current GnRH agonists and antagonists have some excellent therapeutic properties in these applications, they have limitations in poor oral bioavailability and rapid metabolic clearance [20, 21]. Among the various approaches investigated in GnRH activity suppression, active immunotherapy has gained widespread acceptance. Immunoneutralization of GnRH resulted in a decrease in estrogen levels and subsequent mammary tumor suppression in a rat model [22]. In nude mice, anti-GnRH antibodies blocked successfully the hormone function which led to atrophy of reproductive organs and inhibited the growth of MCF7 human breast cancer allografts [23]. Active immunization with D17DT (GnRH linked to diphtheria toxoid) in patients with locally advanced prostate cancer induced castrate levels of testosterone and might have advantages over conventional forms of hormonal therapy [24]. Our previous results suggested that immunization with conjugates of GnRH and Hsp65 could induce atrophy of reproductive organs and inhibit the growth of H22 hepatocellular carcinoma and RM-1 prostste cancer in mice [7, 15]. In this study, the fusion protein Hsp65–GnRH6 consisting of six tandem GnRH repeats and Hsp65 from M. bovis BCG was used as a candidate vaccine for treating breast cancer.
To effectively neutralize the endogenous hormone GnRH, high titer of specific anti-GnRH antibodies should be raised. This goal can be achieved by lots of manipulations, such as tandem repeat methods, chemical conjugation, and fusion expression [7, 8, 10]. Since chemical conjugation often has low efficiency and the conjugates are usually highly heterogeneous, we chose the fusion expression strategy. Recent reports shown that Hsp65 had numerous B and T cell epitopes and had powerful intrinsic ability to enhance the immune response to the associated antigens in the absence of any other adjuvant when used as a carrier molecule [13]. So we fused six tandem GnRH repeats to the C-terminus of Hsp65. The fusion protein Hsp65–GnRH6 was expressed as soluble protein and purified to more than 90% homogeneity without degradation (Fig. 1c). Our results shown that Hsp65–GnRH6 had evoked high titer of specific anti-GnRH antibodies in the absence of any classic adjuvants (Fig. 2a–c), which indicated that Hsp65 could function as a suitable carrier molecule for delivering B cell epitopes to the immune system in vivo. We continued to ask whether the production of anti-GnRH antibodies would lead to atrophy of reproductive organs. In mice treated with Hsp65–GnRH6, the reproductive organs were smaller and lighter than those in mice treated with PBS or Hsp65 (Fig. 3a–d). Histologic examinations further confirmed the opinion (Fig. 3e–j). These results indicated that the endogenous GnRH had been neutralized by Hsp65–GnRH6 induced antibodies. The depletion of GnRH led to the decrease of estrogen concentration, and then resulted in degeneration of ovaries and uteri. Then, the antitumor activity of anti-GnRH antibodies was assessed. Our results showed that the volumes and weights of estrogen-dependent EMT-6 mouse mammary tumor allografts were inhibited significantly [25] (Fig. 4a–d). Microscopic examinations indicated that active immunization with Hsp65–GnRH6 would lead to increased focal necrosis in tumors allografts (Fig. 4e–g). Taken together, our results indicated that GnRH immunoneutralization might induce the depletion of estrogen and then result in suppression of the growth of EMT-6 allografts.
In summary, active immunization with Hsp65–GnRH6 resulted in significant suppression of tumor growth together with increased necrosis in the mice model harboring EMT-6 allografts. The mechanisms of antitumor activity might, at least in part, associate with the depletion of estrogen, supported by the atrophy of female reproductive organs. Hsp65–GnRH6 appears to be a promising candidate vaccine for hormone-dependent cancer therapy in view of its ability to inhibit the growth of EMT-6 murine mammary tumor by immunization efficiently.
Acknowledgments
The authors would like to thank Dr. Liang Jin and Dr. Yan Kai Zhang for provision of purified protein Hsp65. This work was supported by the National Natural Science Foundation of China (No: 30500458). The authors declare that they have no conflict of interest.
Abbreviations
- FSH
Follicle stimulating hormone
- GnRH
Gonadotrophin-releasing hormone
- HE
Hematoxylin and eosin
- Hsp65
Heat shock protein 65
- LH
Luteinizing hormone
- MVP
Measles virus protein
Contributor Information
Xue Jun Wang, Phone: +86-25-86862729, FAX: +86-25-86862728, Email: wangxuejun1979728@hotmail.com.
Jing Jing Liu, Phone: +86-25-83271369, FAX: +86-25-83271242, Email: liujingj@public1.ptt.js.cn.
References
- 1.Naor Z. Signaling by G-protein-coupled receptor (GpCR): studies on the GnRH receptor. Front Neuroendocrinol. 2009;30(1):10–29. doi: 10.1016/j.yfrne.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Plant TM. Hypothalamic control of the pituitary-gonadal axis in higher primates: key advances over the last two decades. J Neuroendocrinol. 2008;20(6):719–726. doi: 10.1111/j.1365-2826.2008.01708.x. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez G, Lage A. Cancer vaccines for hormone/growth factor immune deprivation: a feasible approach for cancer treatment. Curr Cancer Drug Targets. 2007;7(3):229–241. doi: 10.2174/156800907780618310. [DOI] [PubMed] [Google Scholar]
- 4.Persson BE, Kold Olesen T, Jensen JK. Degarelix: a new approach for the treatment of prostate cancer. Neuroendocrinology. 2009;90(3):235–244. doi: 10.1159/000228832. [DOI] [PubMed] [Google Scholar]
- 5.Doehn C, Sommerauer M, Jocham D. Degarelix for prostate cancer. Expert Opin Investig Drugs. 2009;18(6):851–860. doi: 10.1517/13543780902954713. [DOI] [PubMed] [Google Scholar]
- 6.Huhtaniemi I, White R, McArdle CA, Persson BE. Will GnRH antagonists improve prostate cancer treatment? Trends Endocrinol Metab. 2009;20(1):43–50. doi: 10.1016/j.tem.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Zhu Z, Wu J, Liu W, Shen X, Zhang Y, Hu Z, Zhu D, Roque RS, Liu J. Immunization with a recombinant GnRH vaccine conjugated to heat shock protein 65 inhibits tumor growth in orthotopic prostate cancer mouse model. Cancer Lett. 2008;259(2):240–250. doi: 10.1016/j.canlet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Junco JA, Peschke P, Zuna I, Ehemann V, Fuentes F, Bover E, Pimentel E, Basulto R, Reyes O, Calzada L, Castro MD, Arteaga N, Lopez Y, Garay H, Hernandez H, Bringas R, Guillen GE. Immunotherapy of prostate cancer in a murine model using a novel GnRH based vaccine candidate. Vaccine. 2007;25(50):8460–8468. doi: 10.1016/j.vaccine.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Fromme B, Eftekhari P, Van Regenmortel M, Hoebeke J, Katz A, Millar R. A novel retro-inverso gonadotropin-releasing hormone (GnRH) immunogen elicits antibodies that neutralize the activity of native GnRH. Endocrinology. 2003;144(7):3262–3269. doi: 10.1210/en.2002-221135. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CT, Ting CY, Ting CJ, Chen TY, Lin CP, Whang-Peng J, Hwang J. Vaccination against gonadotropin-releasing hormone (GnRH) using toxin receptor-binding domain-conjugated GnRH repeats. Cancer Res. 2000;60(14):3701–3705. [PubMed] [Google Scholar]
- 11.Jinshu X, Jingjing L, Duan P, Zheng Z, Ding M, Jie W, Rongyue C, Zhuoyi H. The immunogenicity of recombinant and dimeric gonadotrophin-releasing hormone vaccines incorporating a T-helper epitope and GnRH or repeated GnRH units. J Immunol Methods. 2004;289(1–2):111–122. doi: 10.1016/j.jim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Carelli C, Audibert F, Gaillard J, Chedid L. Immunological castration of male mice by a totally synthetic vaccine administered in saline. Proc Natl Acad Sci USA. 1982;79(17):5392–5395. doi: 10.1073/pnas.79.17.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang BF, Zhao HL, Xue C, Xiong XH, Zhang W, Yao XQ, Liu ZM. Recombinant heat shock protein 65 carrying hepatitis b core antigen induces HBcAG-specific CTL response. Vaccine. 2007;25(22):4478–4486. doi: 10.1016/j.vaccine.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Guojun W, Wei G, Kedong O, Yi H, Yanfei X, Qingmei C, Yankai Z, Jie W, Hao F, Taiming L, Jingjing L, Rongyue C. A novel vaccine targeting gastrin-releasing peptide: Efficient inhibition of breast cancer growth in vivo. Endocr Relat Cancer. 2008;15(1):149–159. doi: 10.1677/ERC-07-0224. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Xu J, Zhao R, Liu J, Wu J. Inhibition effects on liver tumors of BALB/c mice bearing h22 cells by immunization with a recombinant immunogen of GnRH linked to heat shock protein 65. Vaccine. 2007;25(39–40):6911–6921. doi: 10.1016/j.vaccine.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Zhu Z, Duan P, Li W, Zhang Y, Wu J, Hu Z, Roque RS, Liu J. Cloning, expression, and purification of a highly immunogenic recombinant gonadotropin-releasing hormone (GnRH) chimeric peptide. Protein Expr Purif. 2006;50(2):163–170. doi: 10.1016/j.pep.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Wang XJ, Gu K, Xiong QY, Shen L, Cao RY, Li MH, Li TM, Wu J, Liu JJ. A novel virus-like particle based on hepatitis b core antigen and substrate-binding domain of bacterial molecular chaperone DnaK. Vaccine. 2009;27(52):7377–7384. doi: 10.1016/j.vaccine.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Wang XJ, Gu K, Xu JS, Li MH, Cao RY, Wu J, Li TM, Liu JJ. Preparation of a peptide vaccine against GnRH by a bioprocess system based on asparaginase. Vaccine. 2010;28(31):4984–4988. doi: 10.1016/j.vaccine.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Emons G, Grundker C, Gunthert AR, Westphalen S, Kavanagh J, Verschraegen C. GnRH antagonists in the treatment of gynecological and breast cancers. Endocr Relat Cancer. 2003;10(2):291–299. doi: 10.1677/erc.0.0100291. [DOI] [PubMed] [Google Scholar]
- 20.Ratcliffe KE, Fraser HM, Sellar R, Rivier J, Millar RP. Bifunctional gonadotropin-releasing hormone antagonist-progesterone analogs with increased efficacy and duration of action. Endocrinology. 2006;147(1):571–579. doi: 10.1210/en.2004-1481. [DOI] [PubMed] [Google Scholar]
- 21.Handelsman DJ, Swerdloff RS. Pharmacokinetics of gonadotropin-releasing hormone and its analogs. Endocr Rev. 1986;7(1):95–105. doi: 10.1210/edrv-7-1-95. [DOI] [PubMed] [Google Scholar]
- 22.Ferro VA, Stimson WH. Immunoneutralisation of gonadotrophin releasing hormone: a potential treatment for oestrogen-dependent breast cancer. Eur J Cancer. 1997;33(9):1468–1478. doi: 10.1016/S0959-8049(97)00126-3. [DOI] [PubMed] [Google Scholar]
- 23.Jacobs E, Watson SA, Michaeli D, Ellis IO, Robertson JF. Anti-gonadotrophin releasing hormone antibodies inhibit the growth of MCF7 human breast cancer xenografts. Br J Cancer. 1999;80(3–4):352–359. doi: 10.1038/sj.bjc.6690362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simms MS, Scholfield DP, Jacobs E, Michaeli D, Broome P, Humphreys JE, Bishop MC. Anti-GnRH antibodies can induce castrate levels of testosterone in patients with advanced prostate cancer. Br J Cancer. 2000;83(4):443–446. doi: 10.1054/bjoc.2000.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teicher BA. Preclinical In vivo models of drug resistance. In: Bernal SD, editor. Drug resistance in oncology. New York: Marcel Dekker Inc; 1997. pp. 377–393. [Google Scholar]