Abstract
Within cancer research, phage display libraries have been widely used for the identification of tumor targeting peptides and antibodies. Additionally, phages are known to be highly immunogenic; therefore we evaluated the immunotherapeutic potential of tumor specific phages to treat established solid tumors in a mouse model of melanoma. We developed two tumor specific phages, one derived from a peptide phage display library and one Fab expressing phage with known specificity, for the treatment of mice bearing palpable B16-F10 or B16/A2Kb tumors. Therapy in B16-F10 tumor bearing mice with tumor specific phages was superior to treatment with non-tumor specific phages and lead to delayed tumor growth and increased survival. In B16/A2Kb tumor bearing mice, therapy with tumor specific phages resulted in complete tumor regression and long-term survival in 50% of the mice. Histological analysis of tumors undergoing treatment with tumor specific phages revealed that phage administration induced a massive infiltration of polymorphonuclear neutrophils. Furthermore, phages induced secretion of IL-12 (p70) and IFN-γ as measured in mouse splenocyte culture supernatants. These results demonstrate a novel, immunotherapeutic cancer treatment showing that tumor specific phages can promote regression of established tumors by recruitment of inflammatory cells and induction of Th1 cytokines.
Keywords: Phage display, Cancer immunotherapy, Targeting, Innate immunity
Introduction
Monoclonal antibody based immunotherapy has resulted in successful clinically available products such as Herceptin [6] and Rituxan [2]. Some of the initial drawbacks of anti-cancer antibody treatments were the development of human anti-mouse antibodies, short circulating half-life, limited penetration into tumor sites and limitations in the production process. With the development of phage display [30], many of these problems can be circumvented. Through a process called in vivo panning, an aliquot representing the whole phage library is injected into the animal. Phages with affinity for various exposed epitopes in the animal will stick to those structures. Such target specific phage clones are subsequently captured by dissection of the different tissue and organs of the animal, and propagated in Escherichia coli. The resulting phage population, enriched for target specific clones, is then used for an additional round of in vivo selection. This procedure can be repeated for several rounds allowing positive selection, while non-specific clones are discarded (negative selection). Although in vitro selections of phage display libraries often yield antibodies and peptides with high affinity for their targets they may, due to low stability or bio-availability, not bind their targets in vivo. On the other hand, phage particles selected in vivo are positively selected for their ability to bind and/or internalize to their targets within a complete organism, such as a tumor mass. The procedure enables rapid selection of human high affinity antibodies and peptides against specific targets such as tumor associated antigens [22, 26, 27, 33], angiogenic vessels [3, 24], whole organs [25, 34] and subsets of cells of the immune system [11], from libraries of huge diversities.
Since tumor specific antibody fragments (Fab/scFv) identified by phage display screenings lack the Fc region, they cannot stimulate immune responses via antibody-dependent cell-mediated cytotoxicity or complement dependent cytotoxicity. Therefore, their use in cancer therapeutics is restricted, and they are commonly transformed to whole IgG format, or fused to a broad variety of molecules, e.g., cytotoxic drugs, radionuclides, immunotoxins and cytokines [28]. However, one interesting and overlooked possibility regarding phage display based selection and identification of tumor specific antibody fragments and peptides is to keep high affinity antibody fragments or peptides linked to the phage particle and use it as an immunotherapeutic agent instead of fusing it to a cytotoxic molecule.
Phages are highly immunogenic and known to induce humoral [14, 36] and cellular [37] immune responses. These immune responses are directed against phage proteins or against foreign epitopes displayed on the surface of the phage particle. In addition to their immunogenic properties, phages are good candidates for therapy because they extravasate, penetrate into tissue when administered i.v. [38], are non-toxic to animals [21] and, hence, unlikely to cause side effects. The combination of rapid identification of tumor specific antibody fragments or peptides by phage display library screenings and the immunological and biological properties of phage particles makes it worthwhile to evaluate their capacity in a targeted tumor immunotherapy approach. Therefore, we explored the potential of tumor specific phages to induce tumor regression in a mouse model of melanoma.
This work describes the use of a M13 filamentous phage display particle as a combined immunogenic and tumor targeting agent, with the hypothesis that localization of immunogenic bacteriophages to the tumor will create an anti-phage immune response resulting in the destruction of phage–tumor complexes by mechanisms similar to the actions of tumor reactive antibodies. We evaluated the tumor reduction properties of a B16-F10 mouse melanoma specific peptide phage and a HLA-A2 specific Fab-phage against established B16-F10 and B16/A2Kb tumors. We showed that peritumoral administration of tumor specific phages can inhibit tumor growth and prolong survival of tumor bearing mice. This work reveals a novel tumor treatment reagent that has potent anti-tumor properties.
Materials and methods
Cell culture
The HB-54 hybridoma cell line, which produces anti-HLA-A2 antibodies and the B16-F10 mouse melanoma cell line were obtained from ATCC (Rockville, MD, USA). Cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Bio Whittaker, East Rutherford, NJ, USA) supplemented with 2 mM l-glutamine (Sigma, St Louis, MO, USA), 10 mM HEPES, 25 μg/ml gentamycin and 10% heat-inactivated fetal calf serum (FCS) (Invitrogen, Carlsbad, CA, USA). The A2Kb expressing B16-F10 cell line (B16/A2Kb) was generated by stable transfection with a plasmid encoding the A2/Kb molecule [19]. The melan-a cell line was a gift from Dr Bennett et al. [7] and cultured in the above medium containing 200 nM phorbol 12-myristate 13-acetate (Sigma).
In vivo phage selection on established tumors
Female C57BL/6 mice (6- weeks) were purchased from Taconic Farms (Rockville, MD, USA) and cared for in accordance with the guidelines set forth by the Animal Research Advisory Committee of the National Institutes of Health (NIH). The NIH is accredited by the American Association for the Accreditation of Laboratory Animal Care-accredited Institution. C57BL/6 mice bearing single, subcutaneous B16-F10 tumors were injected i.p. with 1.5 × 1011 pfu of the Ph.D.-12-filamentous bacteriophage phage display peptide library (New England Biolabs, Beverly, MA, USA) in 100 μl of sterile PBS (Invitrogen) supplemented with 1% bovine serum albumin (BSA) (Sigma) (PBSA). After 1- h, the animals were euthanized and the tumor tissues were immediately resected and placed in a sterile, pre-chilled (-0°C) mortar. The tumor tissue was frozen at -0°C for 2 h and homogenized with a mortar and pestle in 10 ml of 2× YT (20 g tryptone, 10 g yeast extract and 5 g NaCl per liter, pH 7.0) E. coli culture medium supplemented with tetracycline and 50 μl E. coli (XL-1 Blue). The homogenate was transferred into a 50-ml conical tube, and then the mortar was washed twice with 10 ml of 2× YT medium. The 30 ml of tumor slurry was incubated shaking at 37°C overnight for phage amplification. The overnight culture was centrifuged at 1,500g for 30 min to pellet the tumor tissue. Seven milliliters of 30% PEG 8000-.5 M NaCl (Sigma) solution and 28 ml of the supernatant from the overnight culture were mixed thoroughly in a new 50-ml conical tube and cooled to +4°C. The mixture was centrifuged at 10,000g for 20 min, the supernatant was decanted, and the container was inverted for at least 30 min to drain excess fluid. The pellet was resuspended in 1 ml of PBSA, heated at 70°C for 10 min, vortexed while hot for 1 min, and centrifuged at 20,000g for 10-5 min to pellet any residual bacteria.
Selection on cells
The in vivo selected phages (above) were in some instances also subjected to further selections using tumor cells in suspension. Harvested B16-F10 cells were resuspended at a concentration of 3 × 106 cells per milliliter in complete DMEM described above with 1% FCS. Five milliliters of the cell suspension was incubated with 100 μl of the purified in vivo selected phages and lightly mixed for 3 h at room temperature. A separation solution of PBS containing 50% Ficoll-Paque Plus, lymphocyte isolation (GE Healthcare, Piscataway, NJ, USA) was prepared and cooled to +4°C. The cells were pelleted by centrifugation at 150g, resuspended in 1 ml of complete medium, and added to the top of 10 ml of separation solution. This solution was centrifuged at 150g for 20 min and all but 1 ml of the separation solution was carefully removed so that non-cell adherent phage was discarded. The cell pellet was collected by pipetting and the phages were amplified and rescued as described above. Phages were quantified using a plaque forming unit assay on IPTG/X-gal/tetracycline plates and blue plaques were selected and sequenced according to the manufacturer’s instructions.
In this experiment, the phage library went through either four rounds of selection on established B16 tumors (in vivo selection) or a combination of selection on tumors and cells (in vivo/in vitro selection). In the latter case, two selections were performed on tumors, one on cells, another on tumor cells, and finally one round on cells. Phage clones representing more than 25% of the total pfu count after the last round of selection were included in the study. Phages were resuspended in PBS, and to exclude any involvement of LPS in the tumor treatment studies endotoxin was removed by phase separation using Triton X-114 [1], and LPS content was measured using the QCL-1000® Chromogenic LAL endpoint assay (Cambrex, Walkersville, MD, USA). After phase separation, the levels of endotoxin were <0.2 endotoxin units and regarded as endotoxin free.
RNA isolation and cDNA synthesis
HB-54 cells were subjected to RNA isolation using the RNeasy kit (Qiagen Inc., Valencia, CA, USA) according to the manufacturer’s instructions. After isolation, the concentration was measured by spectroscopy and the purity was analyzed by RNA-gel electrophoresis in 1% agarose in 1× TAE buffer (10× TAE buffer: 400 mM Tris, 20 mM Na2EDTA·2H2O, 200 mM sodium acetate and 296 mM glacial acetic acid, pH 8.0). Prior to analysis, the RNA was treated in denaturing buffer (65% formamide, 20% formaldehyde and 15% MOPS). cDNA was synthesized using SUPERSCRIPT-II (Invitrogen) reverse transcriptase as per manufacturer.
Construction of HB-54 Fab phagemid vector
The HB-54 immunoglobulin Κ-light chain genes were amplified by the polymerase chain reaction (PCR) using a primer set according to Huse et al. [18]. The primers introduce a SacI site in the 5-end and an XbaI site in the 3-end of the product which allows insertion into the pComb-3H phagemid cloning vector [4]. The primers used for amplification were the forward primer mVK2a (5-CCAGTTCCGAGCTCCAGATGACCCAGTCTCCA-3- SacI site underlined) and the reverse primer mCKa (5-GCGCCGTCTAGAAATAACACTCATTCCTGTTGAA-3- XbaI site underlined). The amplified PCR product was purified by the QIAquick PCR kit (Qiagen) and directly ligated into the pGEM-T easy TA cloning vector (Promega). The ligated product was transformed into competent Top 10 cells (Invitrogen) and the transfected bacteria were plated onto LB plates containing ampicillin, IPTG and X-gal. After overnight incubation, white colonies were screened for the light chain insert through colony PCR. Positive clones were propagated in LB medium overnight and plasmid isolation was performed using the QIAquick plasmid mini kit (Qiagen). The pGEM-T easy vector was cleaved with SacI and XbaI and the light chain fragment was gel purified and recovered using the QIAquick gel extraction kit (Qiagen). The fragment was inserted into the SacI-XbaI sites of the phagemid vector pComb-3H.
PCR amplification of the HB54 variable heavy-chain gene and CH1 domain was carried out using the forward primer mVH4d (5-GAGGTTCAGCTGCTCGAGTCTGGGGC(A/T)GAG-3-XhoI site underlined) and the reverse primer mCG1c (5-TATGCAAGGCTTACTAGTACAATCTCTGGGCACAATTTTCTTGTC-3-SpeI site underlined) (primers from Ersoy and Persson, unpublished). The insert was cleaved with XhoI and SpeI and inserted into the corresponding site of the pComb-3H-LC vector and thus fused to a truncated form of the phage gene III. The phagemid was transformed into Top 10 bacteria and positive clones were identified using colony PCR. Clones containing the insert were propagated and the vector was isolated and purified as described above. The phagemid was analyzed on gel and the inserts were sequenced. Expression of Fab fragments was verified using ELISA. Briefly, colonies containing the phagemid were cultured in 0.5 ml super broth (SB) [5] supplemented with 50 μg/ml ampicillin and 1% glucose. When the cultures became dense they were diluted to 4 ml, when OD600 reached approximately 1.0, the cultures were centrifuged and the media was changed to SB, 2 mM IPTG, and 20 mM MgCl2 and cultures were incubated overnight. Overnight cultures were freeze-thawed and centrifuged. Supernatants were added to ELISA plates pre-coated with goat anti-mouse Fab (Pierce, Rockford, IL, USA), for detection of Fab fragments a secondary alkaline phosphatase labeled anti-mouse Fab (Pierce) was added. After addition of p-nitrophenylphosphate (Sigma), the plate was read at 405 nm.
Phage production and rescue
The phagemid was electroporated into the E. coli strain XL-1 Blue (Stratagene, La Jolla, CA, USA) using a GenePulser (Bio Rad, Richmond, CA, USA) at 200 Ohm, 25 μF and 12.5 kV/cm. After suspension in SOC medium and 1 h incubation at 37°C, cells were plated on LB-ampicillin plates. After overnight incubation, colonies were put into 10 ml SB-ampicillin and incubated for 16 h. Five milliliters of the culture was diluted to 1 l SB supplemented with 10 μg/ml tetracycline, 50 μg/ml ampicillin and 1% glucose. The culture was grown to an OD600 of 0.4 and infected with helper phage VCSM13 (Stratagene) (20 times excess) and further incubated for 30 min at 37°C without shaking. The culture was centrifuged for 10 min at 3,500g and the pellet was resuspended in SB containing antibiotics and 1 mM IPTG (Invitrogen) and grown at 30°C for 16 h. The culture was centrifuged twice at 4,000g for 30 min and phages were recovered from the medium by 20% PEG 8000-.5 M NaCl precipitation as described [10]. The phage pellet was resuspended in PBS and quantified using a standard colony counting assay. Endotoxin was removed as described (see above).
Flow cytometry
Phage binding properties and specificity to B16-F10, B16/A2Kb and melan-a cells were analyzed using flow cytometry. Phage suspensions containing 1 × 1011 cfu/pfu were blocked in 200 μl PBS containing 2.5% milk for 1 h at RT. The phage/milk solution was added to 2 × 105 cells in a 96-well plate and incubated for 1.5 h at 4°C and then washed twice in 200 μl staining buffer (PBS containing 0.5% BSA and 0.05% NaN3). Phage-labeled cells were incubated for 30 min with biotinylated anti-M13 phage antibody (1:100, Accurate, Westbury, NY, USA) washed twice in PBS, and then incubated in straptavidin–phycoerythrin (1:100, Jackson Laboratories). The cells were transferred to FACS tubes, washed twice and resuspended in 500 μl PBS for flow cytometry. Data acquisition and analysis were carried out using a FACSCalibur cytometer (Becton Dickinson, San Jose, CA, USA) and the CellQuest Pro software (Becton Dickinson).
Animal experiments
Female C57BL/6 mice 6-0 weeks old, obtained from Taconic M&B (Bomholt, Denmark) were used in all of the experiments. The animals, ten per cage, were housed at Microbiology and Tumor Biology Center at the Karolinska Institute (Stockholm, Sweden). All experiments using mice were approved by the Swedish National Board for Laboratory Animals.
Mice were injected subcutaneously (s.c.) in the right flank with 5 × 104 B16-F10 cells. Once the tumors were palpable (- mm diameter) endotoxin-free tumor specific phage, 1 × 1011 pfu in 100 μl PBS, was injected s.c. peritumorally every 3- days for two consecutive weeks. Control mice received either HLA-A2 specific Fab-phage or PBS. The mice were treated individually, and no specific time points for treatment beginning or end were set in advance. Tumor progression was monitored three times per week throughout the experiment and clinical signs were recorded. For all tumor treatment experiments, the mice were euthanized by cervical dislocation once the tumor reached 250 mm3. In another set of experiments, mice received s.c. injections of 1 × 106 B16/A2Kb cells. When tumors reached a diameter of 5 mm mice were treated with B16-F10 specific peptide phage or HLA-A2 specific Fab-phage as described above. Wild-type phage and PBS were used as negative controls. The number of cells injected was based on tumor titration experiments and gives a consistent 100% tumor take within 7-0 days after tumor cell inoculation.
Morphological analysis and immunohistochemistry
B16/A2Kb established tumors were treated with tumor specific or wild-type phages. Tumors were removed 24, 48 and 72 h after phage administration and fixed in 4% buffered formalin phosphate (Apoteksbolaget, Stockholm, Sweden) for 6- h and transferred to 70% ethanol. Sections (5 μm) from paraffin-embedded tumors were placed on glass-slides. Deparaffinization, rehydration and H&E staining were performed using standard procedures. Neutrophils were characterized by identification of segmented nuclei and by immunohistochemical staining. Briefly, sections were depariffinized in xylene and rehydrated by washes in graded alcohol concentrations followed by antigen retrieval in a microwave oven for 20 min in sodium-citrate buffer (10 mM, pH 6.0). Slides were washed in PBS and blocked by incubation in normal rabbit serum (1:20 in PBS) for 30 min and subsequently incubated with a 1:200 monoclonal, rat anti-mouse neutrophils antibody, which recognizes a polymorphic 40 kD cell surface antigen expressed by polymorphonuclear neutrophils (PMN) (Serotec, Hamar, Norway) overnight at +4°C. Sections were then incubated for 30 min with a 1:200 secondary biotinylated anti-rat antibody (Vector Laboratories, Burlingame, CA, USA) diluted in PBS with 1% BSA. Slides were incubated in 0.3% hydrogen peroxide to quench endogenous peroxidase for 30 min, washed in PBS and incubated with avidin-biotin-complex-PO using the VECTASTAIN® Elite® ABC Kit (Vector Laboratories) according to the manufacturers-instructions. Diaminobenzidine tetrahydrocholride (Sigma) substrate was added in the presence of H2O2 to the slides, and finally, sections were counterstained with hematoxylin, dehydrated and mounted.
Cytokine ELISA
To investigate the ability of phages to induce secretion of cytokines, IFN-γ and IL-12p70 ELISA kits (MABTECH, Stockholm, Sweden) were used according to the manufacturers-instructions. Single-cell suspensions were prepared from mouse spleens. Erythrocytes were removed by ammonium chloride lysis and the splenocytes were cultured in DMEM supplemented with 2 mM l-glutamine, 10 mM HEPES, 1% non-essential amino acids, 5 × 10- M 2-mercaptoethanol, 25 μg/ml gentamycin and 10% FCS, at 1 × 106 splenocytes/well in a 96-well plate. Wild-type phages, crude fractions or cleared from endotoxin, at 5 × 1010 pfu/150 μl were added to the cells to a total volume of 300 μl/well. After 48 h incubation at 37°C, culture supernatants were collected and cytokine content was measured.
Results
Development of tumor specific phages
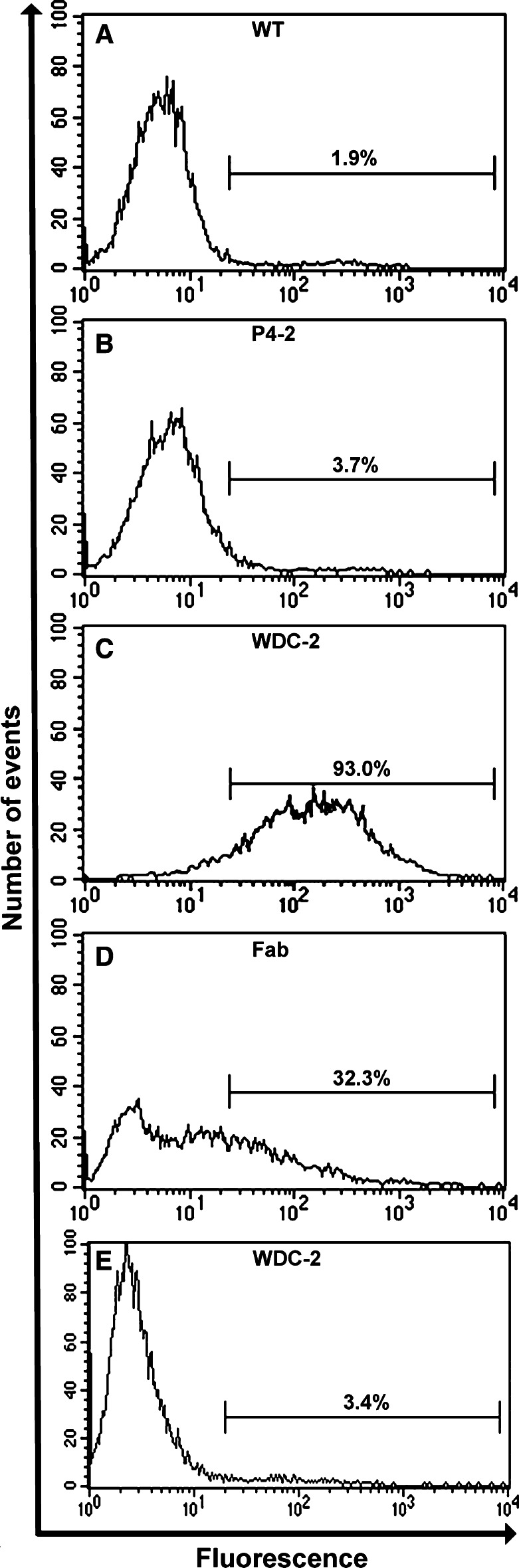
Two peptide expressing phages, designated as P4-2 (peptide sequence: RRKRMTILKSRM, in vivo selection only) and WDC-2 (peptide sequence: TRTKLPRLHLQS, in vivo/in vitro selection), were chosen due to their enrichment during the selection process. They were then analyzed for tumor cell specificity by flow cytometry. The wild-type M13 control and P4-2 phages showed very little specificity and stained 1.9 and 3.7% (Fig. 1a, b) of the tumor cells, respectively; whereas, the WDC-2 phage stained 93.0% (Fig. 1c). Therefore, the WDC-2 phage was chosen for subsequent tumor therapy studies on B16 tumors.
Fig. 1.
Phage specificity for B16/A2Kb mouse melanoma cells. Flow cytometry histograms of B16/A2Kb cells incubated with control phage (a), phage selected on B16-F10 mouse melanoma tumors by in vivo panning (b), combined in vitro/in vivo panning on B16-F10 cells/tumors (c), HLA-A2 specific Fab-phage (d) and non-tumorigenic melan-a cells incubated with WDC-2 phage (e). The numbers indicated represent the percentage of cells bound by phage
Restriction endonuclease analysis and sequencing of the anti-HLA-A2 Fab phage phagemid verified the insertion of the light chain and the Fd heavy-chain domains of the HB-54 into the pComb-3H vector. Expression of the Fab fragments was confirmed by ELISA (data not shown). The HLA-A2 specific Fab-phage stained 32.3% of B16/A2Kb cells (Fig. 1d), and neither the WDC-2 nor the Fab-phages showed any specificity to irrelevant cell lines, e.g., MC57 and 293 cells (data not shown). Additionally, WDC-2 did not react above background levels to the syngeneic, non-tumorigenic, melanocyte cell line, melan-a (Fig. 1e) further demonstrating the melanoma specificity of the phage. These data show that tumor specificity of phages can be achieved by either in vitro/in vivo panning or cloning of antibody fragments with known specificity into phagemid vectors.
Tumor specific phages delay progression of B16-F10 tumors
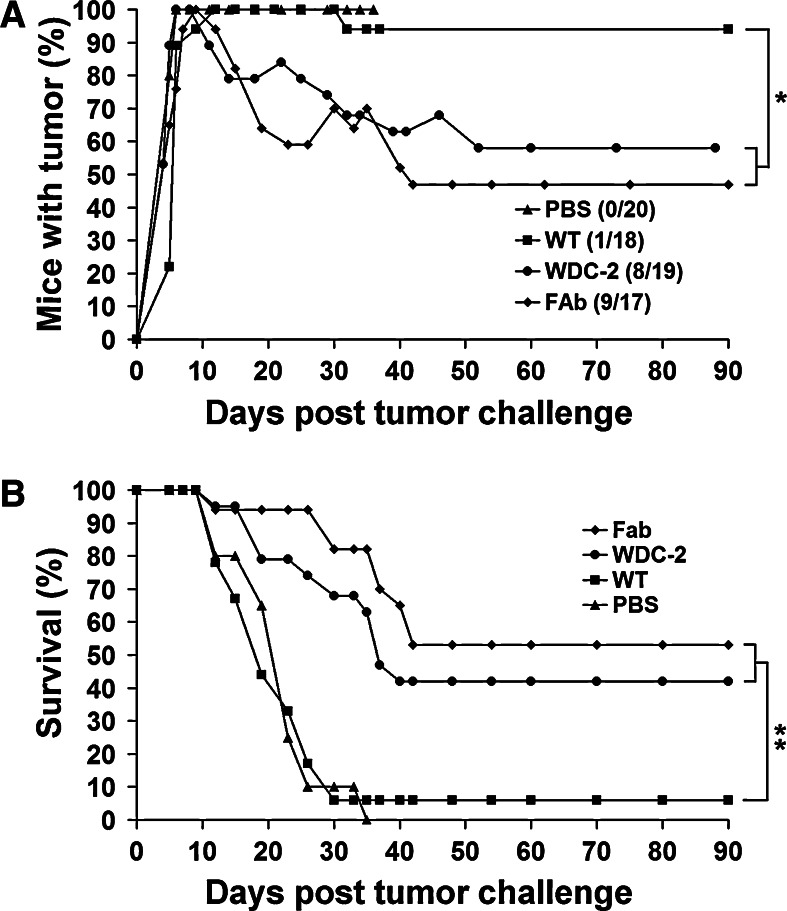
We evaluated the ability of tumor specific phages to treat the highly aggressive and poorly immunogenic B16-F10 tumor. Mice were injected adjacent to the tumor site with endotoxin-free non-tumor specific Fab-phage or tumor specific WDC-2 phages when the B16-F10 tumors reached a diameter of - mm, typically 7-0 days post tumor inoculation. The treatment was administered every 3- days for two consecutive weeks and tumor progression was monitored. Clinical signs of phage administration included swelling at the site of injection (days 1-), scab formation (days 3-), skin ulceration (days 7-4), suppuration, and enlargement of tumor draining lymph nodes, and these signs occurred only in mice treated with the WDC-2 tumor specific phages. Treatment with the WDC-2 tumor specific phage controlled tumor growth in mice bearing B16-F10 tumors for 2 weeks compared to the non-specific Fab-phage and PBS treated controls, where the tumors progressed rapidly from day 10 post tumor challenge (Fig. 2a). The controlled tumor growth lead to an increase in survival of the mice between day 10 and day 16. No mice in the Fab-phage or PBS treated groups survived more than 18 days compared to the WDC-2 treated group, where 20% of the mice survived for 30 days post tumor challenge (Fig. 2b).
Fig. 2.
Treatment of mice with established s.c. B16-F10 melanoma tumors. a Mice were treated peritumorally with tumor specific peptide phage (WDC-2), non-tumor specific phage (Fab) (1 × 1011 pfu/cfu in 100 μl PBS), or PBS alone (100 μl) for up to 2 weeks. WDC-2 phage (filled circle) was able to control tumor growth for 2 weeks compared to the Fab-phage (filled diamond) and PBS (filled triangle) treatment. The difference in the mean tumor volume on day 13, comparing WDC-2 treated to Fab-phage and PBS treated mice was significantly different as determined by Student’s t-test (star indicates P < 0.01). The graph shows tumor development from treatment start (arrow). Horizontal bars represent mean values. A minor, but not significant (P = 0.057) increase of survival in mice treated with tumor specific phage was also observed (b), as determined by log-rank analysis. The data is representative of two experiments
Antibody and peptide phages have similar treatment outcomes
In order to improve the treatment, i.e., to achieve better treatment results than those obtained with tumor specific peptide phages (Fig. 2), we explored the potential of antibody phages. The hypothesis was that an antibody possesses higher affinity to its target compared to a peptide and a stronger phage–tumor interaction may promote a stronger anti-tumor response. Therefore, we evaluated the treatment of established B16/A2Kb tumors with an A2 specific Fab-phage or the B16-F10 tumor specific WDC-2 peptide phage. Approximately 7 days post tumor challenge, established 5 mm tumors were treated as described earlier. Treated B16/A2Kb tumor bearing mice displayed the same side effects of treatment as B16-F10 tumor bearing mice. The signs occurred in all mice treated with tumor specific phages (Fab and WDC-2) and in mice where the treatment resulted in complete tumor regression. Typically, responders were tumor-free after 1 week of treatment. When the treatment was stopped, based on the absence of a palpable tumor, the adverse symptoms disappeared within 2 weeks and no mouse was sacrificed due to adverse effects of the treatment. The non-tumor specific controls were mice treated with wild-type phage. These mice did not show adverse symptoms like Fab-phage or WDC-2 phage treated mice, with the exception of three mice that developed scabs, and one of these had complete tumor regression. PBS treated control mice never developed any side effects of treatment and succumbed to the tumor challenge within 5 weeks. Treatment with HLA-A2 specific Fab-phage and B16 specific WDC-2 phage resulted in complete regression of established tumors and long-term survival in 53 and 42% of the mice, respectively (Fig. 3a, b). Treated mice remained tumor-free for 2 months. To further prove that treatment failure was not due to tumor escape through the loss of A2 antigen, flow cytometry analysis revealed that recurring B16/A2Kb tumor cells exhibited the same level (>90%) of HLA-A2Kb expression as prior to phage treatment (data not shown). Although the treatment outcome of the Fab treated animals was superior, it did not significantly augment the treatment compared to WDC-2 treatment. These observations strongly suggest that tumor specificity of the phages is critical for mounting a potent anti-tumor response and promoting tumor regression and in some cases clearance. The data also suggest that the treatment efficacy is more robust on more immunogenic tumors, which was further supported since Fab-phage treatment of B16/A2Kb tumors in A2Kb transgenic mice (not shown) yielded the same results as WDC-2 phage treatment of B16-F10 in wild-type C57BL/6 mice.
Fig. 3.
Treatment of mice with established s.c. B16/A2Kb melanoma tumors. a Mice were treated with tumor specific phage (Fab and WDC-2), wild-type M13 (1 × 1011 pfu/cfu in 100 μl PBS) or PBS alone (100 μl) for 2 weeks. The numbers of long-term surviving mice with complete tumor regression are indicated in parenthesis. The difference between tumor free mice, comparing those who received tumor specific phage versus wild-type phage, was statistically significant (star indicates P < 0.01), as determined by Chi-square testing. b Tumor specific phage treatment significantly increases survival compared to control phage treatment. Survival was monitored 2- times per week, and plotted as a Kaplan–Meier survival curve based on the experiment from (a). Mice were sacrificed when the tumors reached a volume of 250 mm3. WDC-2 and HLA-A2 specific phage treatment significantly increased survival of mice with established tumors (two stars indicate P < 0.0001) compared to wild-type phage treatment, as determined by log-rank analysis
Phage immunogenicity
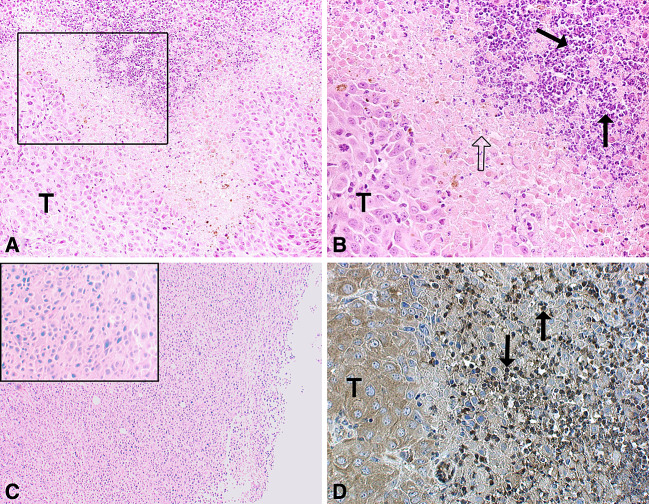
Since phages are known to be immunogenic and since the symptoms of treatment were evident within 24 h post treatment, we wanted to evaluate what cell populations are present that might be contributing to the tumor regression. B16/A2Kb tumor bearing mice were sacrificed 24 and 72 h after phage treatment and the tumors were removed and subjected to morphological analysis. Hematoxylin/eosin staining of the sections revealed a massive infiltration of PMN within 24 h (Fig. 4a, b), which was also confirmed by immunohistochemical staining (Fig. 4d). The infiltration was accompanied by necrosis, after 72 h considerable damage to the tumor tissue was observed (not shown), and only few tumor cells were still viable. Only moderate cell infiltration was observed after wild-type phage treatment (Fig. 4c).
Fig. 4.
Neutrophils accumulate in tumor specific phage treated B16/A2Kb tumors. a Tumor rejection area of a WDC-2 phage treated tumor 24 h after tumor specific phage injection (20× lens magnification). The tumor is massively infiltrated by PMN. b Lens magnification (40×) of the box in (a) showing, from left to right, viable tumor cells (T), necrotic or damaged tumor cells (open arrow) and infiltrating PMN (arrows). c Tumor section 24 h after administration of wild-type phage. Lens magnification (4×) with a representative 20× lens magnification insert showing the absence of infiltrating PMN. d Immunohistochemical staining of tumor infiltrating neutrophils 24 h after tumor specific phage administration (×20 lens magnification)
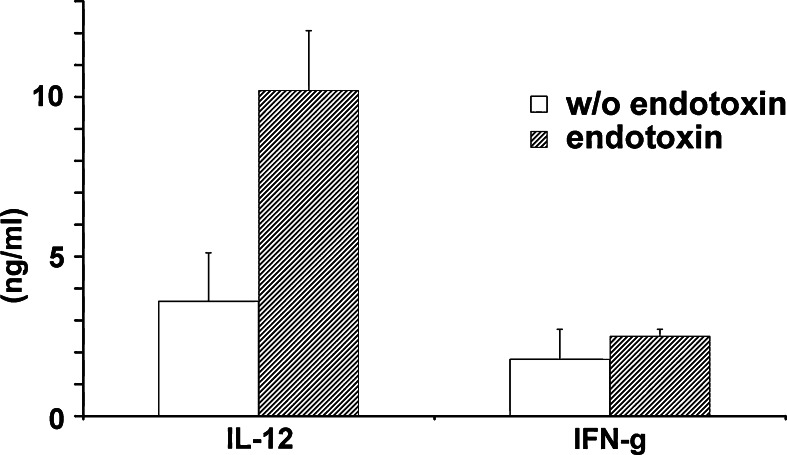
Since Th1 cytokines are known to augment anti-tumor immune responses, the ability of phages to induce Th1 cytokines was explored. In the production of phages, endotoxin is a contaminant which may be responsible for the induction of cytokines. To exclude unwanted effects due to contamination, endotoxin was removed prior to all in vivo experiments, as described in Sect. Materials and methods. Phages with or without endotoxin were found to induce IL-12 and IFN-γ production in mouse splenocyte cultures (Fig. 5). Although endotoxin contamination increased the levels of both cytokines, it was evident that phages can induce secretion of cytokines also in its absence.
Fig. 5.
Phage induced cytokine production. Wild-type phage preparations, with and without endotoxin, were added to mouse splenocyte cultures. In collected supernatants, IFN-γ and IL-12 were detected by ELISA. Experiments were performed twice in triplicate and one representative experiment is shown. Statistically significant differences were observed in IL-12 levels between cultures that received phage preparations with or without endotoxin (P < 0.05), as determined by Student’s t-test
Discussion
In this study, phage display technology enabled the identification and production of tumor specific phage particles which are able to promote regression of solid tumors and prolong survival in melanoma bearing mice. Tumor specific phages, either selected from a peptide phage library or developed from a hybridoma with known antigen specificity, were compared in their anti-tumor activities on poorly immunogenic and highly immunogenic established tumors. The significance of developing tumor specific phages is to localize highly immunogenic phage particles to the tumor site and thus attract an inflammatory response to the vicinity of the tumor.
Little is known about phage interactions with mammalian cells and especially tumor cells. In the 1940s, Bloch [8] showed that bacteriophages inhibited growth of tumors in various animals, and these results were recently confirmed by Dabrowska et al. [12, 13] who suggested that phages inhibit metastatic growth of B16 tumors by blocking integrins necessary for expansion and metastasis. However, we suggest that in palpable, 3- mm, B16 tumors the anti-tumor activities are mediated by the host immune system and mainly through tumor infiltrating neutrophils, as indicated in Fig. 4, and that the recruitment of the immune system is highly dependent on phage accumulation at the tumor site. Flow cytometry analysis of phage–tumor interactions demonstrated that our approach with a combined in vitro/in vivo selection scheme yielded a peptide phage with high specificity (93%) for the tumor (Fig. 1) compared with the in vivo selected and wild-type M13 phage. The relatively low percentage (32%) of staining of the HLA-A2 specific Fab-phage could be explained by the fact that when using a phagemid system the main fraction of phages produced in the bacteria are insert-less [31] due to less efficient incorporation of pIII-fusions into the phage particle. Therefore, less copies of the pIII-fusion are displayed per phage in a phagemid system compared to phage display vectors where all phages produced express the fusion on all copies of the pIII protein. A reduction of the WDC-2 phage titer from 1011 pfu down to 1010 pfu by mixing it with irrelevant phages yielded staining percentages approaching those for Fab-phage staining on B16/A2Kb cells (data not shown). This suggests that the number of phages per cell is important for the staining assay. Additionally, peptide phages have the capacity of multivalent binding to antigens and most likely have a lower off rate, and hence they are more resistant to the washing steps during the staining protocol, which results in a higher signal upon flow cytometry analysis. Also, shed Fab-fragments competing with recombinant Fab-phage for binding cannot be excluded. Another factor that can influence Fab-phage binding is the level of expressed antigen. In other words, the unidentified ligand for the WDC-2 phage may be present at a higher quantity than the A2 antigen on the B16/A2Kb tumor cells, thus providing a higher mean fluorescent intensity (MFI). Although the vast majority (>90%) of the B16/A2Kb cells express the A2 antigen, the MFI of the HLA-A2 positive human melanoma cell line DFW upon HB-54 antibody staining is up to 100-fold higher. Accordingly, Fab-phage staining of DFW cells clearly enhanced the signal compared to B16/A2Kb cell staining (data not shown). Taken together, these results clearly demonstrate that, even though all cells express the antigen, the staining intensity is highly dependent on: (1) the number of recombinant phages per target cell, (2) the level of expressed antigen on the target cells, and (3) the number of fusion proteins displayed on the phage surface.
The mechanism behind the infiltration and regression and how it is induced by phages is not yet fully understood. Since phages are viruses and regarded as foreign by the immune system, it is likely that the inflammatory process is mediated through similar mechanisms as other microbial infections. The phenomenon could partly be explained by the “danger model-[16] which suggests that tumor trauma and presence of infectious agents may result in regression of tumors. In our study, we observed regression of tumors after treatment with wild-type phages with no specificity for the tumor, supporting the danger model. The phages may also attract and locally activate dendritic cells which then present tumor antigens to T cells in a more efficient manner and induce an immune response. The involvement of the complement system and especially the non-classical pathway in eliciting both innate and adaptive anti-viral responses have been demonstrated in several studies [20, 32] and the release of complement factor C5a is known to attract neutrophils to inflammatory sites [9]. Our initial studies revealed that immunization with phage particles prior to tumor challenge and phage treatment start did not augment the treatment efficacy, suggesting a role of the alternative pathway where antibodies are not involved. Alternatively, the phages may promote inflammation by activation of toll-like receptor 9 (TLR-9) on antigen presenting cells and neutrophils. It was shown that M13 phages possess anti-viral activity through the induction of IFN-γ secretion in a mouse model of vaccinia virus infection [23], most likely due to the presence of CpG sequences in their genome. Although the ability of CpG motifs to activate TLR-9 on neutrophils have been debated [17, 35], the phages may activate TLR-9 on other subsets of immune cells such as macrophages and dendritic cells. These statements are supported by the fact that phages, regardless of any cell specificity, induce production of pro-inflammatory cytokines when added to splenocyte cultures in vitro, as shown in Fig. 5, and after s.c. injections in vivo (not shown). Additionally, other studies have reported the secretion of IL-6 and TNF-α upon phage addition [39].
Neutrophils and NK-cells provide the first innate cellular defense against invading pathogens and are also potent mediators of inflammation. These cells kill and phagocytose infectious agents and secrete pro-inflammatory cytokines and chemotactic factors that recruit other subsets of immune cells to the inflammatory site [29]. The release of various cytotoxic mediators by these cells efficiently kills invading organisms, but can also, under certain conditions, cause damage to host tissue by a bystander mechanism. After administration of tumor specific phages, we have observed the appearance of skin ulceration and various degrees of scabbing, followed by the regression of the tumors. We consider this an indirect effect of the actions of cells of innate immunity attracted to the tumor area by the phages. As demonstrated in Fig. 5, addition of phages to splenocyte culture induces secretion of cytokines such as IFN-γ and IL-12. This could further explain the occurrence of tissue damage since other studies have shown that in the presence of various cytokines, including IFN-γ, neutrophils are activated to secrete reactive oxygen species and other cytotoxic agents [15].
In summary, this study describes a novel experimental approach for treatment of established large tumors in B16-F10 models. As demonstrated in Figs. 2 and 3, administration of tumor specific phages is required to induce a complete tumor regression in initial phases of treatment. Regardless of tumor type, the specific phage treatment initially results in the same signs of inflammation, retardation of tumor growth and prolonged survival. However, the long-term survival is enhanced by the immunogenicity and possibly induction of adaptive immunity associated with the B16/A2Kb tumors. Tumor treatment with tumor specific phages thus offers a novel treatment modality for established tumors and warrants further evaluation.
Acknowledgments
Financial support: This work was supported partly by grants from the Cancer Society in Stockholm, the Swedish Cancer Society, Karolinska Institute Funds, the Swedish Research Council (Medical Branch), the EU 6-FP “ALLOSTEM-(LSHB-CT-2004-503319), the EU 6-FP “ENACT-and US Department of Defense Prostate Cancer Research Program (PC030958).
References
- 1.Aida Y, Pabst MJ. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J Immunol Methods. 1990;132:191–195. doi: 10.1016/0022-1759(90)90029-U. [DOI] [PubMed] [Google Scholar]
- 2.Anderson DR, Grillo-Lopez A, Varns C, Chambers KS, Hanna N. Targeted anti-cancer therapy using rituximab, a chimaeric anti-CD20 antibody (IDEC-C2B8) in the treatment of non-Hodgkin’s B-cell lymphoma. Biochem Soc Trans. 1997;25:705–708. doi: 10.1042/bst0250705. [DOI] [PubMed] [Google Scholar]
- 3.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, Ellerby HM, Bredesen DE, Pasqualini R, Ruoslahti E. Targeting the prostate for destruction through a vascular address. Proc Natl Acad Sci USA. 2002;99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barbas CF, 3rd, Wagner J. Synthetic human antibodies: selecting and evolving functional proteins. Methods. 1995;8:94–103. doi: 10.1006/meth.1995.9997. [DOI] [Google Scholar]
- 5.Barbas CF, 3rd, Kang AS, Lerner RA, Benkovic SJ. Assembly of combinatorial antibody libraries on phage surfaces: the gene III site. Proc Natl Acad Sci USA. 1991;88:7978–7982. doi: 10.1073/pnas.88.18.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselga J. Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivotal trials. Oncology. 2001;61(Suppl. 2):14–21. doi: 10.1159/000055397. [DOI] [PubMed] [Google Scholar]
- 7.Bennett DC, Cooper PJ, Hart IR. A line of non-tumorigenic mouse melanocytes, syngeneic with the B16 melanoma and requiring a tumour promoter for growth. Int J Cancer. 1987;39:414–418. doi: 10.1002/ijc.2910390324. [DOI] [PubMed] [Google Scholar]
- 8.Bloch H. Experimental investigation of the relationship between bacteriophage and malignant tumors. Arch Gesamte Virusforsch. 1940;1:481–496. doi: 10.1007/BF01240654. [DOI] [Google Scholar]
- 9.Blue CE, Spiller OB, Blackbourn DJ. The relevance of complement to virus biology. Virology. 2004;319:176–184. doi: 10.1016/j.virol.2003.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton DR, Barbas CF, 3rd, Persson MA, Koenig S, Chanock RM, Lerner RA. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curiel TJ, Morris C, Brumlik M, Landry SJ, Finstad K, Nelson A, Joshi V, Hawkins C, Alarez X, Lackner A, Mohamadzadeh M. Peptides identified through phage display direct immunogenic antigen to dendritic cells. J Immunol. 2004;172:7425–7431. doi: 10.4049/jimmunol.172.12.7425. [DOI] [PubMed] [Google Scholar]
- 12.Dabrowska K, Opolski A, Wietrzyk J, Switala-Jelen K, Boratynski J, Nasulewicz A, Lipinska L, Chybicka A, Kujawa M, Zabel M, Dolinska-Krajewska B, Piasecki E, Weber-Dabrowska B, Rybka J, Salwa J, Wojdat E, Nowaczyk M, Gorski A. Antitumor activity of bacteriophages in murine experimental cancer models caused possibly by inhibition of beta3 integrin signaling pathway. Acta Virol. 2004;48:241–248. [PubMed] [Google Scholar]
- 13.Dabrowska K, Opolski A, Wietrzyk J, Switala-Jelen K, Godlewska J, Boratynski J, Syper D, Weber-Dabrowska B, Gorski A. Anticancer activity of bacteriophage T4 and its mutant HAP1 in mouse experimental tumour models. Anticancer Res. 2004;24:3991–3995. [PubMed] [Google Scholar]
- 14.Fogelman I, Davey V, Ochs HD, Elashoff M, Feinberg MB, Mican J, Siegel JP, Sneller M, Lane HC. Evaluation of CD4+ T cell function In vivo in HIV-infected patients as measured by bacteriophage phiX174 immunization. J Infect Dis. 2000;182:435–441. doi: 10.1086/315739. [DOI] [PubMed] [Google Scholar]
- 15.Fossati G, Bucknall RC, Edwards SW. Insoluble and soluble immune complexes activate neutrophils by distinct activation mechanisms: changes in functional responses induced by priming with cytokines. Ann Rheum Dis. 2002;61:13–19. doi: 10.1136/ard.61.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs EJ, Matzinger P. Is cancer dangerous to the immune system? Semin Immunol. 1996;8:271–280. doi: 10.1006/smim.1996.0035. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi F, Means TK, Luster AD. Toll-like receptors stimulate human neutrophil function. Blood. 2003;102:2660–2669. doi: 10.1182/blood-2003-04-1078. [DOI] [PubMed] [Google Scholar]
- 18.Huse WD, Sastry L, Iverson SA, Kang AS, Alting-Mees M, Burton DR, Benkovic SJ, Lerner RA. Generation of a large combinatorial library of the immunoglobulin repertoire in phage lambda. Science. 1989;246:1275–1281. doi: 10.1126/science.2531466. [DOI] [PubMed] [Google Scholar]
- 19.Irvine KR, Parkhurst MR, Shulman EP, Tupesis JP, Custer M, Touloukian CE, Robbins PF, Yafal AG, Greenhalgh P, Sutmuller RP, Offringa R, Rosenberg SA, Restifo NP. Recombinant virus vaccination against “self-antigens using anchor-fixed immunogens. Cancer Res. 1999;59:2536–2540. [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang H, Wang Z, Serra D, Frank MM, Amalfitano A. Recombinant adenovirus vectors activate the alternative complement pathway, leading to the binding of human complement protein C3 independent of anti-ad antibodies. Mol Ther. 2004;10:1140–1142. doi: 10.1016/j.ymthe.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Krag DN, Fuller SP, Oligino L, Pero SC, Weaver DL, Soden AL, Hebert C, Mills S, Liu C, Peterson D. Phage-displayed random peptide libraries in mice: toxicity after serial panning. Cancer Chemother Pharmacol. 2002;50:325–332. doi: 10.1007/s00280-002-0489-4. [DOI] [PubMed] [Google Scholar]
- 22.Landon LA, Deutscher SL. Combinatorial discovery of tumor targeting peptides using phage display. J Cell Biochem. 2003;90:509–517. doi: 10.1002/jcb.10634. [DOI] [PubMed] [Google Scholar]
- 23.Mori K, Kubo T, Kibayashi Y, Ohkuma T, Kaji A. Anti-vaccinia virus effect of M13 bacteriophage DNA. Antiviral Res. 1996;31:79–86. doi: 10.1016/0166-3542(96)00951-5. [DOI] [PubMed] [Google Scholar]
- 24.Mutuberria R, Satijn S, Huijbers A, Van Der Linden E, Lichtenbeld H, Chames P, Arends JW, Hoogenboom HR. Isolation of human antibodies to tumor-associated endothelial cell markers by in vitro human endothelial cell selection with phage display libraries. J Immunol Methods. 2004;287:31–47. doi: 10.1016/j.jim.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 26.Popkov M, Rader C, Barbas CF., 3rd Isolation of human prostate cancer cell reactive antibodies using phage display technology. J Immunol Methods. 2004;291:137–151. doi: 10.1016/j.jim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 27.Romanov VI, Durand DB, Petrenko VA. Phage display selection of peptides that affect prostate carcinoma cells attachment and invasion. Prostate. 2001;47:239–251. doi: 10.1002/pros.1068. [DOI] [PubMed] [Google Scholar]
- 28.Sanz L, Cuesta AM, Compte M, Alvarez-Vallina L. Antibody engineering: facing new challenges in cancer therapy. Acta Pharmacol Sin. 2005;26:641–648. doi: 10.1111/j.1745-7254.2005.00135.x. [DOI] [PubMed] [Google Scholar]
- 29.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 30.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 31.Soltes G, Barker H, Marmai K, Pun E, Yuen A, Wiersma EJ. A new helper phage and phagemid vector system improves viral display of antibody Fab fragments and avoids propagation of insert-less virions. J Immunol Methods. 2003;274:233–244. doi: 10.1016/S0022-1759(02)00294-6. [DOI] [PubMed] [Google Scholar]
- 32.Suresh M, Molina H, Salvato MS, Mastellos D, Lambris JD, Sandor M. Complement component 3 is required for optimal expansion of CD8 T cells during a systemic viral infection. J Immunol. 2003;170:788–794. doi: 10.4049/jimmunol.170.2.788. [DOI] [PubMed] [Google Scholar]
- 33.Tordsson JM, Ohlsson LG, Abrahmsen LB, Karlstrom PJ, Lando PA, Brodin TN. Phage-selected primate antibodies fused to superantigens for immunotherapy of malignant melanoma. Cancer Immunol Immunother. 2000;48:691–702. doi: 10.1007/s002620050018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trepel M, Arap W, Pasqualini R. Modulation of the immune response by systemic targeting of antigens to lymph nodes. Cancer Res. 2001;61:8110–8112. [PubMed] [Google Scholar]
- 35.Trevani AS, Chorny A, Salamone G, Vermeulen M, Gamberale R, Schettini J, Raiden S, Geffner J. Bacterial DNA activates human neutrophils by a CpG-independent pathway. Eur J Immunol. 2003;33:3164–3174. doi: 10.1002/eji.200324334. [DOI] [PubMed] [Google Scholar]
- 36.Willis AE, Perham RN, Wraith D. Immunological properties of foreign peptides in multiple display on a filamentous bacteriophage. Gene. 1993;128:79–83. doi: 10.1016/0378-1119(93)90156-W. [DOI] [PubMed] [Google Scholar]
- 37.Wu Y, Wan Y, Bian J, Zhao J, Jia Z, Zhou L, Zhou W, Tan Y. Phage display particles expressing tumor-specific antigens induce preventive and therapeutic anti-tumor immunity in murine p815 model. Int J Cancer. 2002;98:748–753. doi: 10.1002/ijc.10260. [DOI] [PubMed] [Google Scholar]
- 38.Yip YL, Hawkins NJ, Smith G, Ward RL. Biodistribution of filamentous phage-Fab in nude mice. J Immunol Methods. 1999;225:171–178. doi: 10.1016/S0022-1759(99)00044-7. [DOI] [PubMed] [Google Scholar]
- 39.Zimecki M, Weber-Dabrowska B, Lusiak-Szelachowska M, Mulczyk M, Boratynski J, Pozniak G, Syper D, Gorski A. Bacteriophages provide regulatory signals in mitogen-induced murine splenocyte proliferation. Cell Mol Biol Lett. 2003;8:699–711. [PubMed] [Google Scholar]