Abstract
The feasibility and safety of immunotherapy mediated by intentionally mismatched rIL-2 activated killer lymphocytes (IMAK) with no prior stem cell engraftment was investigated in patients with advanced chemotherapy-resistant hematological malignancies and metastatic solid tumors. Our goals were to maximize anti-cancer activity by using intentionally mismatched donor lymphocytes; amplify killing of target cancer cells by rIL-2 activation of killer cells in vitro and in vivo, and avoid the risk of graft-versus-host disease (GVHD) by anticipated rejection of alloreactive donor lymphocytes. Conditioning consisted of 5 days of fludarabine 25 mg/m2 or a single dose of cyclophosphamide 1,000 mg/m2, 2 subcutaneous injections of alpha interferon (IFN) 3 × 106 and COX2 inhibitors, followed by administration of IMAK (65 ± 5 CD3+CD56−; 17 ± 5 CD3−CD56+) in conjunction with low dose subcutaneous rIL-2 (6 × 106 IU/m2/day) for 5 days for continuous activation of alloreactive donor lymphocytes prior to their anticipated rejection. Here, we present our phase 1 clinical study data in a cohort of 40 high-risk patients with metastatic solid tumors and hematological malignancies. Treatment was accompanied by some malaise and occasional self-limited fever but otherwise well tolerated on an outpatient basis. Transient engraftment of donor cells was documented in two patients and only one developed self-limited grade 1 GVHD. Among patients with chemotherapy-resistant disease, long-term progression-free survival was recorded in 5 of 21 evaluable patients with metastatic solid tumors and in four of five patients with hematological malignancies. We conclude that the proposed procedure is feasible, safe, and potentially effective, with some otherwise resistant cancer patients long-term disease-free, thus justifying larger Phase II studies in patients with hematological malignancies and metastatic solid tumors, preferably at a stage of minimal residual disease with the goal in mind to eradicate all malignant cells at an early stage of the disease.
Keywords: Metastatic solid tumors Graft-versus-tumor (GVT) effects; Hematological malignancies (leukemia, lymphoma); Cell-mediated immunotherapy; Killer T cells; Natural killer (NK) cells
Introduction
Although the large majority of patients with hematological malignancies and solid tumors reach a stage of minimal disease following conventional chemotherapy or surgical debulking of visible tumor mass, disease recurrence represents a major cause of failure. Patients with hematological malignancies can be cured despite resistance to chemo-radiotherapy due to immune mediated graft-versus-tumor (GVT) effects induced by alloreactive donor T lymphocytes [1–6]. Unfortunately, in most cases successful GVT effects inducible by alloreactive donor lymphocytes occur in association with graft versus host disease (GVHD), which may result in severe acute and chronic disease with significant morbidity and mortality [1–6]. The therapeutic role of alloreactive donor lymphocyte infusion (DLI) following induction of host-versus-graft unresponsiveness represents a most effective tool for eradication of malignant hematopoietic cells despite their resistance to maximally tolerated doses of chemoradiotherapy [5–10]. Unfortunately, despite the remarkable GVL effects induced by DLI, the procedure fails to cure the large majority of patients with relapsed acute leukemia and lymphoma [5, 6, 10] and nearly all patients with metastatic solid tumors [11]. We have previously documented that GVT effects induced by DLI can be further amplified by stimulation of both autologous and especially allogeneic donor lymphocytes with IL-2 in vitro and/or in vivo in mice [12–17] and human hosts [6, 18–20]. Donor lymphocytes may even be effectively activated in vitro against specific host alloantigens, thus potentially amplifying the GVT effects inducible by donor lymphocytes in rodents [21] and in clinical practice [22]. More recently, GVT effects previously documented in rodents [23–26], were also documented against certain metastatic solid tumors suggesting the potential use of alloreactive donor lymphocytes against residual malignant cells in patients with metastatic solid tumors following induction of host versus graft unresponsiveness by engraftment of donor stem cells [11, 27–29]. Unfortunately, GVT effects induced by MHC-compatible donor lymphocytes, may have limited capacity against resistant hematological malignancies and certainly against bulky metastatic solid tumors, despite the presence of severe acute and chronic GVHD [11]. Consequently, despite the existence of GVHD, tumor occurrence may not be effectively controlled. Using HLA-compatible donor lymphocytes, induction of GVT effects in successfully treated cases requires durable anti-host alloreactivity for several months up to one year to allow sufficient time for the eradication of residual malignant cells of host origin even in most responsive patients with hematological malignancies [30] and particularly in metastatic solid tumors [11]. Although GVT effects were previously documented without durable engraftment of donor lymphocytes in patients with residual disease following high-dose chemoradiotherapy supported by autologous SCT in patients with lymphoma [19] and metastatic breast cancer [20], and more recently following lymphocytes depletion also in patients with acute myeloid leukemia [31], as a rule, engraftment of donor stem cells is a mandatory preconditioning to allow durable engraftment of donor lymphocytes [32, 33]. Furthermore, eradication of malignant cells is unlikely to be accomplished either because the intensity of GVT effect mediated by MHC compatible donor lymphocytes is insufficient, or because of prohibitive GVHD, which necessitate concomitant administration of immunosuppressive agents that negate GVT effects [11, 28, 29]. Besides, conceptually, it seems unlikely that a complicated, hazardous, and expensive procedure involving allogeneic SCT would represent a desirable approach for the treatment of such a common disease like cancer. Simpler, more practical, and safer approaches for control of minimal residual disease following initial tumor debulking seem much more logical and urgently indicated.
The approach presented in the present article is based on induction of short-term GVT effects in patients with hematological malignancies and metastatic solid tumors by intentionally mismatched activated donor lymphocytes with no prior engraftment of donor stem cells. Our phase 1 clinical trial suggests that treatment with short acting intentionally mismatched activated killers (IMAK), activated by IL-2 prior to cell infusion in vitro and following administration in vivo, may be used safely as effective anti-cancer killer cells with no major risk of GVHD due to anticipated consistent rejection of intentionally mismatched donor T cells.
Patients and methods
Patients
A cohort of 40 consenting patients, 16 males, and 24 females, aged 5–73 (median 52) years, all with advanced chemotherapy-resistant malignancies despite optimal conventional treatment were recruited between February 1998 and April 2006 for a phase I clinical study to assess feasibility and safety of cell therapy with in vitro rIL-2 activated intentionally mismatched donor lymphocytes. A total of 35 patients [31 evaluable; 4 lost to follow up) had chemotherapy-resistant metastatic solid tumors and five patients had hematological malignancies with no option for allogeneic stem cell transplantation. All patients have previously failed ≥ 2 lines of conventional chemotherapy. Each patient signed an informed consent approved by the Ethical Committee of the Hadassah Medical Center and the Ministry of Health. Patient characteristics are shown in Table 1.
Table 1.
Disease categories of patients with metastatic solid tumors [n = 35] and patients with chemotherapy-resistant hematological malignancies [n = 5]
| Metastatic solid tumors | Number of patients | Hematological malignancies | Number of patients |
|---|---|---|---|
| Breast cancer | 12 | Multiple myeloma | 2 |
| Colorectal cancer | 3 | Non-Hodgkin’s lymphoma | 2 |
| (1 with renal cancer) | |||
| Gastric cancer | 3 | Resistant Hodgkin’s disease | 1 |
| Pancreatic cancer | 3 | ||
| Malignant melanoma | 3 | ||
| Cervical cancer | 2 | ||
| Glioblastoma multiforme | 2 | ||
| Metastatic carcinoid | 1 | ||
| Ovarian cancer | 1 | ||
| Prostate cancer | 1 | ||
| Thyroid cancer | 1 | ||
| Squamos cell cancer with lung metastases | 1 | ||
| PNET | 1 | ||
| Head and neck (Tonsilar) | 1 | ||
| Total | 35 | Total | 5 |
Preparation of activated lymphocytes obtained from unrelated fully mismatched or haploidentically mismatched family members
Lymphocytes were obtained by aphaeresis using a COBE spectra cell separator from related haploidentically mismatched related (n = 32) or fully mismatched unrelated volunteers (n = 8). Cells were transferred to 250 ml tubes and loaded over ficoll gradient at room temperature and spun for 20 min at 2,000 rpm using a Sorval centrifuge. Cells were cultured in a 1 L Lifecell bag (Baxter, USA) at a concentration of 2 × 106 cells/ml in RPMI supplemented with 10% heat inactivated human AB serum, glutamine 1% and antibiotics (Gentamicine 0.1%). Recombinant human IL-2 (Chiron, The Netherlands) was added at 6,000 IU/ml. Cells were placed in a 5% CO2 in air incubator at 37°C, at a maximum volume of 1,500 ml in 3 L Lifecell bags for 4 days. Upon termination of incubation, cells were transferred to 600 ml transfer pack by plasma transfer set. Cells were spun at 1,200 rpm for 15 min. Cells were washed and re-suspended in saline in a 150 ml transfusion bag prior to infusion. A total of 1.5–18.0 (median 4.0) × 107 cells/kg were injected intravenously with no depletion of T cells. The phenotype of activated donor lymphocytes is shown in Table 2.
Table 2.
Phenotypic analysis and cytotoxicity of intentionally mismatched donor killer cells (IMAK)—lymphocytes activated with rIL-2
| %CD56+/CD3− cells | %CD3+/C56− cells | %CD56+/CD3+ cells | Lytic unitsa |
|---|---|---|---|
| 17 ± 5 | 65 ± 5 | 7 ± 4 | 251 ± 4 |
aLytic units were calculated per 106 cells for 20% lysis of K562 cells as previously described [36]. Percent cytoxcity was determined by 51CR—release assay. Results are presented as mean ± SE of 9 MAK cultures
Conditioning of patients prior to cell infusion
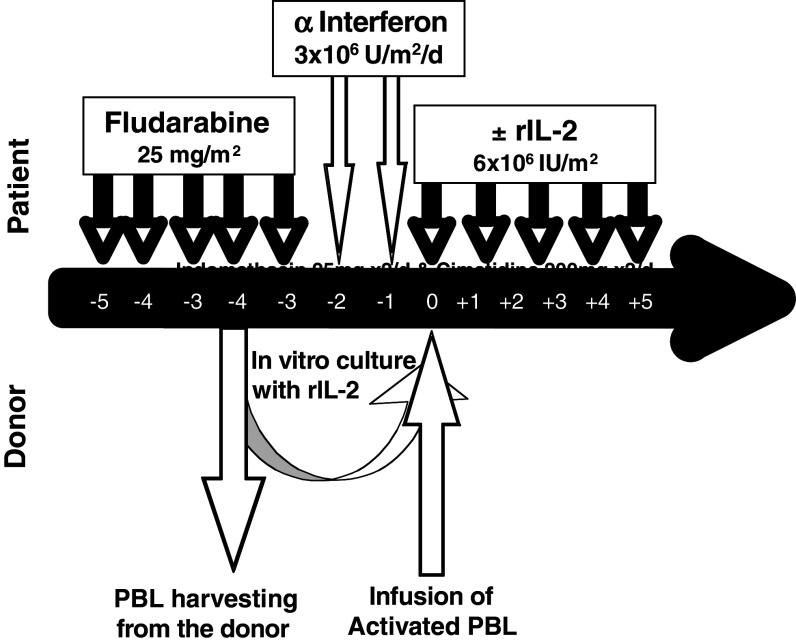
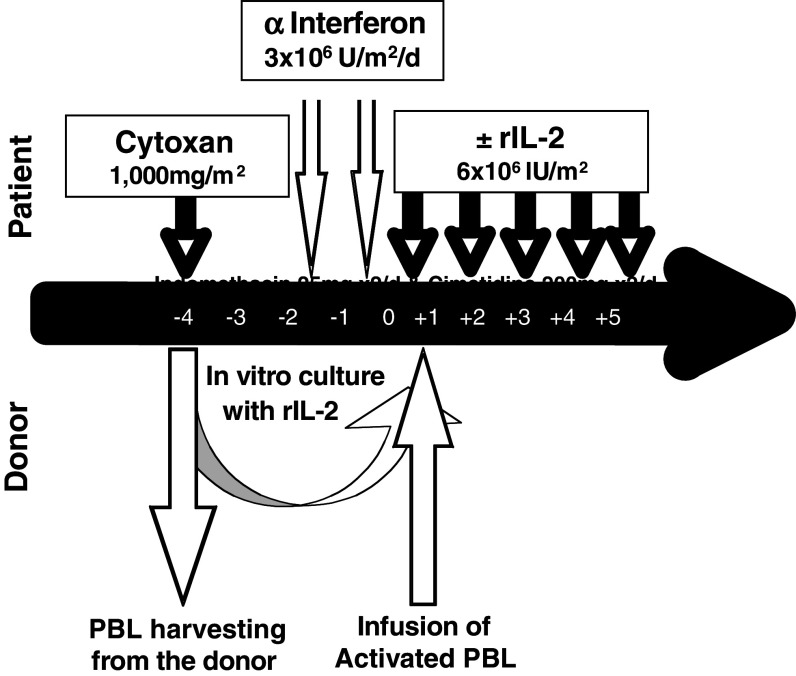
In order to prolong the survival of donor lymphocytes and possibly induce transient chimerism in the host, immunosuppressive treatment with Fludarabine (Schering AG) was given to the first 21 patients at a dose of 25 mg/m2 from day −7 to day −3 followed by two subcutaneous (SC) injections of alpha interferon (Roferon A, Hoffman LaRoche) (IFN) in a dose of 3 × 106 IU/day from day −2 to day −1 in an attempt to upregulate immunogenic cell surface antigens (Fig. 1). A total of 19 patients were conditioned with a single dose of intravenous cyclophosphamide 1,000 mg/m2 on day −4 (Fig. 2). Such a conditioning is mildly immunosuppressive and unlikely to result in durable engraftment of mismatched donor lymphocytes, while in parallel, it may also eliminate regulatory T cells.
Fig. 1.
Treatment of high-risk patients with chemotherapy-resistant malignancies treated with intentionally mismatched activated killer cells (IMAK)—donor lymphocytes activated with rIL-2 using fludarabine-based conditioning
Fig. 2.
Treatment of high-risk patients with chemotherapy-resistant malignancies treated with intentionally mismatched activated killer cells (IMAK)—donor lymphocytes activated with rIL-2 using cyclophosphamide-based conditioning
On day 0, lymphocytes obtained 4 days earlier from haploidentically mismatched related or fully mismatched unrelated donor were infused after in vitro activation with rIL-2 for 4 days. Starting on the day of lymphocyte infusion, rIL-2 was administered for five consecutive days SC in a dose of 6 × 106 IU/m2 daily.
Cell infusion
Activated lymphocytes were harvested following 4 days of culturing and just prior to cell infusion patients received phenergan 12.5 mg against potential allergic reaction. Starting on the day of cell infusion and daily for 5 days, each patient received rIL-2 6 × 106 IU subcutaneously on an outpatient basis in an attempt to continue the activation of killer cells. Indomethacin 25 mg was administered twice daily as supportive treatment to block COX 2 for down-regulation of prostaglandin E2 and to prevent fever and inflammatory reactions induced by rIL-2 treatment. Cimetidine 200 mg or Zantac 150 mg were administered twice daily as prophylaxis against indomethacin-induced gastric irritation.
Observing for engraftment and GVHD
All patients were closely monitored for toxicity and clinical and laboratory signs of acute and later on chronic GVHD. Chimerism in female recipients of male cells was checked using male-specific amelogenin gene PCR [34]. Donor-specific VNTR-PCR was used in female-to-female, male-to-male, or female to male cases for detection of circulating donor cells [35].
Results
Conditioning prior to cell infusion was uneventful except for mild malaise in some patients following injections of IFN. Except for shaking chills and mild transient fever in some patients no other side effects were noticed during the cell infusion. On day 0, a total of 1.5–18.0 (median 4.0) × 107 cells/kg were infused. Overall, treatment was well tolerated. Administration of rIL-2 following cell infusion was accompanied by local erythema at the site of injection, as well as self-limited malaise and fever. All procedures were carried out in the outpatient setting and no patient required admission to the hospital. Due to subjective intolerance to rIL-2, the dose was reduced to 0.5 × 106 IU daily in one patient and rIL-2 administration was reduced from 5 to 3 days in five patients.
Out of the first cohort of 35 patients treated for metastatic solid tumors with IMAK, 4 were lost to follow up; of the remaining 31, 10 are alive. All five patients with hematological malignancies were available for long-term follow up; four are alive. The procedure was well tolerated by most patients and only three patients featured procedure-related toxicity > grade 2 (Table 3). All procedures were done in the outpatient clinic and none of the patients required admission to the hospital. Clinical details and outcomes of the first cohort of patients with chemotherapy-resistant solid tumors and hematological malignancies treated with IMAK cells are shown in Tables 3 and 4, respectively.
Table 3.
Toxicity of treatment with intentionally mismatched activated killer cells (IMAK) donor lymphocytes activated with rIL-2, using WHO criteria
| Grade I | Grade II | Grade III | Grade IV | |
|---|---|---|---|---|
| Hemoglobin | 4 | 0 | 0 | 0 |
| Leucocytes | 0 | 0 | 0 | 0 |
| Platelets | 1 | 1 | 0 | 0 |
| ALT | 3 | 5 | 0 | 0 |
| AST | 4 | 1 | 0 | 0 |
| GGTP | 1 | 5 | 3 | 0 |
| Alk. phosphatase | 3 | 1 | 0 | 0 |
Table 4.
Survival of patients with chemotherapy-resistant metastatic solid tumors treated with intentionally mismatched activated killer cells (IMAK)—donor lymphocytes activated with rIL-2
| Number of patients (n) | Survival in months | |||||
|---|---|---|---|---|---|---|
| Alive-PFS | 5 | >93 BC | >80 BC | >14 BC | >12 SCLC | >10 PrC |
| Alive-SD/PD | 5 | >24 PaC | >13 OC | >12 PaC | >11 BC | >6 BC |
| Lost to follow up | 4 | |||||
| Died | 21 | 1−54 [median 14] months | ||||
PFS progression-free survival, SD stable disease, PD progressive disease, BC breast cancer, SCLC small cell lung cancer, OC ovarian cancer, PrC prostate cancer, PaC pancreas cancer
One patient with metastatic colon cancer demonstrated transient documented engraftment of donor cells on day +6 by VNTR-PCR and partial response for 16 months. No sign of engraftment could be documented at 1 month. A second patient with metastatic breast cancer had self-limited macular erythematosus rash resembling grade 1 cutaneous GVHD with transient elevation of liver enzymes. This patient showed a small signal of circulating male cells on day +6 but showed no evidence of chimerism at 1-month post cell therapy. She had stable disease for 23 months. No chimerism was detected in all other patients on day 6 or 1-month post cell infusion. As shown in Tables 4, 5 patients with solid tumors, most with no bulky disease to start with, showed no evidence of disease progression for more than 10 to 93 months. An additional 5 were alive with progressive disease and 21 were dead at the time of reporting. Among the patients with hematological malignancies, two patients with multiple myeloma treated at the stage of minimal residual disease had no evidence of disease for the entire observation period of 88 and 63 months (Table 5). Two additional patients with non-Hodgkin’s lymphoma were also free of disease at 24 and 14 months following allogeneic cell therapy and the only disease progression was noted in the patient with Hodgkin’s disease who died at 38 months following allogeneic cell therapy (Table 5).
Table 5.
Survival of patients with chemotherapy-resistant hematological malignancies treated with intentionally mismatched activated killer cells (IMAK)—donor lymphocytes activated with rIL-2
| Number of patients (n) | Survival in months | ||||
|---|---|---|---|---|---|
| Alive | 4 | >88 MM | >63 MM | >24 NHL | >14 NHL |
| Lost to follow up | 1 | ||||
| Died | 1 | Died after 38 months | |||
MM multiple myeloma, NHL non-Hodgkin’s lymphoma
A general summary of the outcome of total cohort of 40 patients suggests that the therapeutic protocol using IMAK is reasonably well tolerated.
A general summary of the outcome of total cohort of 40 patients suggests that using IMAK as a new treatment modality appears to be reasonably well tolerated. No patient developed > grade 2 toxicity and only three patients (7.5%) developed grade 3 toxicity as judged by a transient rise of their GGTP. Grade 2 toxicity was reported in 13 patients (32.5%) and grade 1 toxicity was reported in 16 patients. Most important, no patient developed any severe or ongoing acute or chronic GVHD, due to rejection of donor lymphocytes, because no patient developed stable chimerism.
Discussion
The main purpose of the experimental procedure presented here was to design a safe, simple, and inexpensive protocol for large-scale clinical application of immunotherapy, aiming to eliminate minimal residual disease in high-risk patients and delay progression in patients with advanced hematological malignancies and metastatic solid tumors. The present protocol represents a summary of a pilot clinical trial that started in 1992 in leukemia and continued until 1998 in patients with solid tumors, using intentionally mismatched donor lymphocytes activated with rIL-2 for eradication of cancer cells resistant to conventional anti-cancer modalities. In this phase 1 study, all consenting patients with advanced cancer were considered eligible. Consequently, most patient included in this study were treated at advanced stages of their disease, aiming to document feasibility and safety of the use of rIL-2 activated, intentionally mismatched lymphocytes including an unmodified mixture of T and NK cells, in an attempt to maximize tumor cell killing by a combination of immunotherapeutic procedures. The results based on a small cohort of 40 patients suggest that the procedure proposed is reasonably safe and well tolerated, since side effects were moderate and the mild toxicity was self-limited. Remarkably, a significant proportion of patients with hematological malignancy and some with advanced solid tumors appear to have benefited from the treatment proposed, presenting longer than expected progression free survival.
Our working hypothesis was that intentionally mismatched rIL-2 activated donor lymphocytes (especially NK cells) from related or unrelated donors, introduced into a tumor-bearing host may “reject” cancer cells recognized as non-self according to the “missing self” hypothesis [36]. As such, cytotoxic activity mediated by IMAK could last for as long as donor cells circulated in the host, before their anticipated rejection. Fully mismatched lymphocytes were employed to generate anti-tumor cytotoxicity by maximizing alloreactivity between effectors and target cells. However, in the absence of prior stem cell transplantation for induction of host-versus-graft unresponsiveness or lymphoablative conditioning, it seems of paramount importance to induce anti-cancer effects as soon as donor lymphocytes were injected intravenously, prior to their anticipated rejection, with an estimated circulation time of less than 1 week. Indeed, it was previously documented that target cell killing by activated NK cells occurs within minutes or few hours [37]. In addition, it was assumed that non-specific activation of host immune system with allogeneic cells used for immunotherapy may provide an additional adjuvant effect, thus possibly inducing long-lasting anti-cancer effects by a “mirror effect” due to induction of host immune response against residual malignant cells as recently suggested [38, 39]. Effective elimination of tumor cells in patients with minimal residual disease following myeloablative conditioning in preparation for autologous stem cell transplantation, with no prior engraftment of donor stem cells thus resulting in limited circulation time of donor lymphocytes, was previously documented in mice inoculated with murine leukemia [40] or metastatic breast cancer [25, 26], and in clinical practice as well [41, 42].
The concept of fast and potent killing of malignant cells despite resistance to maximally tolerated doses of chemoradiotherapy without donor stem cell engraftment using IMAK, even if such lymphocytes were eventually rejected, was pioneered in 1992 on a compassionate basis in a young girl with chemotherapy-resistant acute myelogenous leukemia (M3) relapsing following myeloablative conditioning and autologous stem cell transplantation. This patient had documented persistent disease as early as 28 days after myeloablative chemotherapy. Since no matched donor was available and based on our prior experience with DLI, she was treated with haploidentically mismatched maternal lymphocytes activated in vitro and in vivo with rIL-2. In response to treatment with transient circulation of donor lymphocytes and with no clinical evidence of GVHD, leukemia was eliminated, and remained undetectable with RT-PCR. With an observation period of more than 17 years with no evidence of disease, it can be concluded that this patient was cured by IMAK [41]. We have subsequently demonstrated that myeloablative conditioning precluded immediate rejection of haploidentically mismatched donor lymphocytes, and such rIL-2 activated lymphocytes induced potent GVT effects against resistant cancer cells; however, such treatment also resulted in lethal acute GVHD, similar to blood transfusion reactions in immunocompromised recipients, thus suggesting that rIL-2-activated mismatched donor lymphocytes are most potent against cancer but must be eliminated before development of irreversible GVHD [42]. Based on our cumulative experience in preclinical animal models and considering the fast and effective GVT effects inducible by intentionally mismatched donor lymphocytes we hypothesized that a similar procedure could be utilized safely provided that donor cells will be consistently rejected before causing irreversible GVHD on the one hand, or using donor NK cells that cannot mediate GVHD, on the other. Our prior clinical experience using HLA-compatible donor lymphocytes in patients with high-risk hematological malignancies and metastatic solid tumors, aiming for induction of GVT effects without concomitant engraftment of donor stem cells resulted in significant but limited GVT effects [19, 20]. Encouraged by effective immunotherapy potential inducible by allogeneic lymphocytes, but confronted with the need to induce faster and more effective GVT effects, it seemed justified to try and use much more potent modality based on the use of IMAK.
Although the focus of our protocol was based on induction of maximal GVT effects by short-lived donor lymphocytes, non-MHC restricted T cells, and NK cells reacting against malignant cells that may express down regulated MHC or missing class 1 relative to donor NK cells, additional components were also introduced. First, IMAK was applied following administration of a single low dose of cyclophosphamide or fludarabine, to optimize adoptive immunotherapy by (1) creating a temporary “niche” for donor lymphocytes for optimal homeostatic proliferation; and (2) attempting to eliminate or down-regulate regulatory T cells known to suppress immune-mediated anti-cancer effects [43, 44]. Furthermore, in this protocol we also used indomethacin administered in conjunction with agents to prevent gastrointestinal discomfort and mucosal erosion. Prior investigations in pre-clinical animal models confirmed that the efficacy of immunotherapy against cancer could be significantly improved by COX-2 inhibition with indomethacin, probably due to the well-known role of prostaglandin E2 as one of the factors blocking development of anti-cancer immunity [45]. Second, administration of two doses of IFN prior to infusion of IMAK was aimed to increase cell surface expression of cancer-specific or cancer-associated antigens for better recognition by the killer cells on the one hand, and to augment the activation of host killer cells in synergy with rIL-2 on the other. The use of IFN alone and in combination with rIL-2 for immunotherapy of cancer is based on published observations based on a previous clinical trial [18]. Third, in order to amplify the capacity of donor lymphocytes to kill cancer cells, donor lymphocytes were also activated in vivo with low doses of rIL-2 administered subcutaneously for 3–5 days starting on the day of cell infusion as previously described for autologous lymphocytes [46], assuming that rIL-2 will amplify alloreactivity by IMAK for maximizing the anticipated anti-cancer effects. It was also assumed that effective killing of cancer cells mediated by rIL-2-activated NK cells may be facilitated by IFN, while non-MHC restricted killing of tumor cells by rIL-2-activated T cells could be amplified by over-expression of cancer-specific or cancer-associated antigens as a result of activation of malignant cells with IFN. Finally, the most important assumption was based on the hypothesis that in the absence of profound immunosuppression pre-treatment, and considering the intentional MHC mismatch, circulation of donor lymphocytes will be restricted to less than a week due to mandatory rejection of killer cells, thus avoiding the risk of severe GVHD. Our working hypothesis was based on the fact that while killing by in vitro preactivated killer cells (including activated T and NK cells) was expected to occur within minutes or a few hours [37], persistence of activated donor cells for a few days would allow effective anti-cancer effects before anti-cancer effector cells will be rejected.
Considering the fact that anti-cancer effects against large tumor mass is time consuming, and may last for several months up to 1 year [11, 30], we would like to hypothesize that the therapeutic protocol based on IMAK is likely to be more effective when applied at the stage of minimal residual disease, preferably at an earlier stage of the disease, like previously documented in patients with relapsed leukemia [41], resistant lymphoma [19], and metastatic breast cancer [20] treated successfully with allogeneic cells.
For patients severely immunosuppressed by aggressive chemoradiotherapy, safer immunotherapy with IMAK may be accomplished by administration of T cell-depleted activated NK cells to avoid any potential risk of GVHD that may be caused by engraftment or delayed rejection of donor lymphocytes. Such NK cell preparations can be obtained by positive selection of CD56-positive NK cells or negative selection of CD3-positive T cells using immunomagnetic beads using CliniMACS. The use of isolated NK cells to generate GVT reactivity is, in our opinion, the only safe approach for using allogeneic lymphocytes while avoiding the risk of GVHD as recently suggested for patients with hematological malignancies [31, 47]. Instead of using a very costly procedure available in few medical centers [48, 49], “poor man” GVT effects may be accomplished with minimal risk without the expensive immunomagnetic purification of NK cells and possibly also enhanced by alloreactive T cells, as suggested by the findings presented here. The role of KIR/KIR-Ligand mismatched NK cells was originally discussed by Velardi et al. [50] following the beneficial role of KIR mismatching following haploidentical stem cell transplantation. They have not studied the therapeutic role of IMAK activated with rIL-2, and they did not attempt to use mismatched lymphocytes, matched or mismatched for KIR with no prior stem cell transplantation based on GVT effects induced by transient circulation of donor lymphocytes.
In summary, the use of short-term immunotherapy with IMAK appears to be well tolerated by patients with hematological malignancies and metastatic solid tumors. This procedure is relatively inexpensive and applicable for all cancer patients on an outpatient basis, as it primarily employs readily available mismatched lymphocytes activated by rIL-2 that are short lived due to consistent rejection of donor lymphocytes. Such treatment represents an attractive logical future approach that needs to be further examined for elimination of minimal residual disease in patients with hematological malignancies and metastatic solid tumors.
Acknowledgments
We wish to thank Mr. Yuri Verkholevsky for helping summarize patient records.
References
- 1.Weiden PL, Flournoy N, Sanders JE, Sullivan KM, Thomas ED. Antileukemic effect of graft-versus-host disease contributes to improved survival after allogeneic marrow transplantation. Transplant Proc. 1981;13:248–251. [PubMed] [Google Scholar]
- 2.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 3.Slavin S, Or R, Naparstek E, Ackerstein A, Weiss L. Cellular-mediated immunotherapy of leukemia in conjunction with autologous and allogeneic bone marrow transplantation in experimental animals and man. Blood. 1988;72(Suppl 1):407a. [Google Scholar]
- 4.Slavin S, Or R, Kapelushnik Y, Drakos P, Ackerstein A, Vourka-Karussis U, et al. Immunotherapy of minimal residual disease in conjunction with autologous and allogeneic bone marrow transplantation (BMT) Leukemia. 1992;6(Suppl 4):164–166. [PubMed] [Google Scholar]
- 5.Slavin S, Naparstek E, Nagler A, Ackerstein A, Kapelushnik J, Or R. Allogeneic cell therapy for relapsed leukemia after bone marrow transplantation with donor peripheral blood lymphocytes. Exp Hematol. 1995;23:1553–1562. [PubMed] [Google Scholar]
- 6.Slavin S, Naparstek E, Nagler A, Ackerstein A, Samuel S, Kapelushnik J, et al. Allogeneic cell therapy with donor peripheral blood cells and recombinant human interleukin-2 to treat leukemia relapse after allogeneic bone marrow transplantation. Blood. 1996;87:2195–2204. [PubMed] [Google Scholar]
- 7.Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 8.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 9.Mackinnon S, Papadopoulos EB, Carabasi MH, Reich L, Collins NH, Boulad F, et al. Adoptive immunotherapy evaluating escalating doses of donor leukocytes for relapse of chronic myeloid leukemia after bone marrow transplantation: separation of graft-versus-leukemia responses from graft-versus-host disease. Blood. 1995;86:1261–1268. [PubMed] [Google Scholar]
- 10.Collins RH, Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–444. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 11.Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. doi: 10.1056/NEJM200009143431101. [DOI] [PubMed] [Google Scholar]
- 12.Slavin S, Eckerstein A, Weiss L. Adoptive immunotherapy in conjunction with bone marrow transplantation–amplification of natural host defence mechanisms against cancer by recombinant IL-2. Nat Immun Cell Growth Regul. 1988;7:180–184. [PubMed] [Google Scholar]
- 13.Ackerstein A, Kedar E, Slavin S. Use of recombinant human interleukin-2 in conjunction with syngeneic bone marrow transplantation in mice as a model for control of minimal residual disease in malignant hematologic disorders. Blood. 1991;78:1212–1215. [PubMed] [Google Scholar]
- 14.Weiss L, Reich S, Slavin S. Use of recombinant human interleukin-2 in conjunction with bone marrow transplantation as a model for control of minimal residual disease in malignant hematological disorders: I. Treatment of murine leukemia in conjunction with allogeneic bone marrow transplantation and IL-2-activated cell-mediated immunotherapy. Cancer Invest. 1992;10:19–26. doi: 10.3109/07357909209032785. [DOI] [PubMed] [Google Scholar]
- 15.Vourka-Karussis U, Karussis D, Ackerstein A, Slavin S. Enhancement of GVL effect with rhIL-2 following BMT in a murine model for acute myeloid leukemia in SJL/J mice. Exp Hematol. 1995;23:196–201. [PubMed] [Google Scholar]
- 16.Slavin S, Ackerstein A, Kedar E, Weiss L. IL-2 activated cell-mediated immunotherapy: control of minimal residual disease in malignant disorders by allogeneic lymphocytes and IL-2. Bone Marrow Transplant. 1990;6(Suppl 1):86–90. [PubMed] [Google Scholar]
- 17.Slavin S, Ackerstein A, Weiss L, Nagler A, Or R, Naparstek E. Immunotherapy of minimal residual disease by immunocompetent lymphocytes and their activation by cytokines. Cancer Invest. 1992;10:221–227. doi: 10.3109/07357909209032764. [DOI] [PubMed] [Google Scholar]
- 18.Nagler A, Ackerstein A, Or R, Naparstek E, Slavin S. Immunotherapy with recombinant human interleukin-2 and recombinant interferon-alpha in lymphoma patients postautologous marrow or stem cell transplantation. Blood. 1997;89:3951–3959. [PubMed] [Google Scholar]
- 19.Or R, Ackerstein A, Nagler A, Amar A, Naparstek E, Varadi G, et al. Allogeneic cell-mediated and cytokine-activated immunotherapy for malignant lymphoma at the stage of minimal residual disease after autologous stem cell transplantation. J Immunother. 1998;21:447–453. doi: 10.1097/00002371-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Or R, Ackerstein A, Nagler A, Kapelushnik J, Naparstek E, Samuel S, et al. Allogeneic cell-mediated immunotherapy for breast cancer after autologous stem cell transplantation: a clinical pilot study. Cytokines Cell Mol Ther. 1998;4:1–6. [PubMed] [Google Scholar]
- 21.Ji YH, Weiss L, Zeira M, Abdul-Hai A, Reich S, Schuger L, et al. Allogeneic cell-mediated immunotherapy of leukemia with immune donor lymphocytes to upregulate antitumor effects and downregulate antihost responses. Bone Marrow Transplant. 2003;32:495–504. doi: 10.1038/sj.bmt.1704150. [DOI] [PubMed] [Google Scholar]
- 22.Slavin S, Ackerstein A, Morecki S, Gelfand Y, Cividalli G. Immunotherapy of relapsed resistant chronic myelogenous leukemia post allogeneic bone marrow transplantation with alloantigen pulsed donor lymphocytes. Bone Marrow Transplant. 2001;28:795–798. doi: 10.1038/sj.bmt.1703223. [DOI] [PubMed] [Google Scholar]
- 23.Moscovitch M, Slavin S. Anti-tumor effects of allogeneic bone marrow transplantation in (NZB X NZW)F1 hybrids with spontaneous lymphosarcoma. J Immunol. 1984;132:997–1000. [PubMed] [Google Scholar]
- 24.Morecki S, Moshel Y, Gelfend Y, Pugatsch T, Slavin S. Induction of graft vs. tumor effect in a murine model of mammary adenocarcinoma. Int J Cancer. 1997;71:59–63. doi: 10.1002/(SICI)1097-0215(19970328)71:1<59::AID-IJC11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Morecki S, Yacovlev E, Gelfand Y, Vilensky A, Slavin S. Allogeneic versus syngeneic killer splenocytes as effector cells for the induction of graft-versus-tumor effect. Biol Blood Marrow Transplant. 2004;10:40–48. doi: 10.1016/j.bbmt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Panigrahi S, Yacovlev E, Gelfand Y, Schuger L, Slavin S, Morecki S. Intraportal and systemic allogeneic cell therapy in a murine model of hepatic metastatic breast cancer. Cytokines Cell Mol Ther. 2002;7:99–106. doi: 10.1080/13684730310001661. [DOI] [PubMed] [Google Scholar]
- 27.Ben-Yosef R, Or R, Nagler A, Slavin S. Graft-versus-tumour and graft-versus-leukaemia effect in patient with concurrent breast cancer and acute myelocytic leukaemia. Lancet. 1996;348:1242–1243. doi: 10.1016/S0140-6736(05)65517-1. [DOI] [PubMed] [Google Scholar]
- 28.Eibl B, Schwaighofer H, Nachbaur D, Marth C, Gachter A, Knapp R, et al. Evidence for a graft-versus-tumor effect in a patient treated with marrow ablative chemotherapy and allogeneic bone marrow transplantation for breast cancer. Blood. 1996;88:1501–1508. [PubMed] [Google Scholar]
- 29.Ueno NT, Rondon G, Mirza NQ, Geisler DK, Anderlini P, Giralt SA, et al. Allogeneic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol. 1998;16:986–993. doi: 10.1200/JCO.1998.16.3.986. [DOI] [PubMed] [Google Scholar]
- 30.Raanani P, Dazzi F, Sohal J, Szydlo RM, van Rhee F, Reiter A, et al. The rate and kinetics of molecular response to donor leucocyte transfusions in chronic myeloid leukaemia patients treated for relapse after allogeneic bone marrow transplantation. Br J Haematol. 1997;99:945–950. doi: 10.1046/j.1365-2141.1997.4683272.x. [DOI] [PubMed] [Google Scholar]
- 31.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 32.Slavin S. Immunotherapy of cancer with alloreactive lymphocytes. N Engl J Med. 2000;343:802–803. doi: 10.1056/NEJM200009143431109. [DOI] [PubMed] [Google Scholar]
- 33.Slavin S. Immunotherapy of cancer with alloreactive lymphocytes. Lancet Oncol. 2001;2:491–498. doi: 10.1016/S1470-2045(01)00455-7. [DOI] [PubMed] [Google Scholar]
- 34.Pugatsch T, Oppenheim A, Slavin S. Improved single-step PCR assay for sex identification post-allogeneic sex-mismatched BMT. Bone Marrow Transplant. 1996;17:273–275. [PubMed] [Google Scholar]
- 35.Nakamura Y, Leppert M, O’Connell P, Wolff R, Holm T, Culver M, et al. Variable number of tandem repeat (VNTR) markers for human gene mapping. Science. 1987;235:1616–1622. doi: 10.1126/science.3029872. [DOI] [PubMed] [Google Scholar]
- 36.Karre K. Natural killer cell recognition of missing self. Nat Immunol. 2008;9:477–480. doi: 10.1038/ni0508-477. [DOI] [PubMed] [Google Scholar]
- 37.Arnon TI, Markel G, Mandelboim O. Tumor and viral recognition by natural killer cells receptors. Semin Cancer Biol. 2006;16:348–358. doi: 10.1016/j.semcancer.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Har-Noy M, Slavin S. The anti-tumor effect of allogeneic bone marrow/stem cell transplant without graft vs. host disease toxicity and without a matched donor requirement? Med Hypotheses. 2008;70:1186–1192. doi: 10.1016/j.mehy.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Har-Noy M, Zeira M, Weiss L, Slavin S. Completely mismatched allogeneic CD3/CD28 cross-linked Th1 memory cells elicit anti-leukemia effects in unconditioned hosts without GVHD toxicity. Leuk Res. 2008;32:1903–1913. doi: 10.1016/j.leukres.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Weiss L, Nusair S, Reich S, Sidi H, Slavin S. Induction of graft versus leukemia effects by cell-mediated lymphokine-activated immunotherapy after syngeneic bone marrow transplantation in murine B cell leukemia. Cancer Immunol Immunother. 1996;43:103–108. doi: 10.1007/s002620050309. [DOI] [PubMed] [Google Scholar]
- 41.Slavin S. Allogeneic cell-mediated immunotherapy at the stage of minimal residual disease following high-dose chemotherapy supported by autologous stem cell transplantation. Acta Haematol. 2005;114:214–220. doi: 10.1159/000088412. [DOI] [PubMed] [Google Scholar]
- 42.Nagler A, Ackerstein A, Or R, Naparstek E, Slavin S. Adoptive immunotherapy with haploidentical allogeneic peripheral blood lymphocytes following autologous bone marrow transplantation. Exp Hematol. 2000;28:1225–1231. doi: 10.1016/S0301-472X(00)00533-6. [DOI] [PubMed] [Google Scholar]
- 43.Muranski P, Boni A, Wrzesinski C, Citrin DE, Rosenberg SA, Childs R, et al. Increased intensity lymphodepletion and adoptive immunotherapy–how far can we go? Nat Clin Pract Oncol. 2006;3:668–681. doi: 10.1038/ncponc0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallimore A, Godkin A. Regulatory T cells and tumour immunity—observations in mice and men. Immunology. 2008;123:157–163. doi: 10.1111/j.1365-2567.2007.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morecki S, Yacovlev L, Slavin S. Effect of indomethacin on tumorigenicity and immunity induction in a murine model of mammary carcinoma. Int J Cancer. 1998;75:894–899. doi: 10.1002/(SICI)1097-0215(19980316)75:6<894::AID-IJC12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Chang AE, Rosenberg SA. Overview of interleukin-2 as an immunotherapeutic agent. Semin Surg Oncol. 1989;5:385–390. doi: 10.1002/ssu.2980050604. [DOI] [PubMed] [Google Scholar]
- 47.Miller JS, Weisdorf DJ, Burns LJ, Slungaard A, Wagner JE, Verneris MR, et al. Lymphodepletion followed by donor lymphocyte infusion (DLI) causes significantly more acute graft-versus-host disease than DLI alone. Blood. 2007;110:2761–2763. doi: 10.1182/blood-2007-05-090340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slavin S, Shapira M, Morecki S, Samuel S, Ackerstein A, Gelfand Y, et al. Immunotherapy for resistant hematologic malignancies using matched or mismatched rIL-2 activated donor lymphocytes positively selected for CD56+ after allogeneic stem cell transplantation for allogeneic cell therapy without GVHD. Blood. 2003;102:5400b. doi: 10.1182/blood-2003-09-3004. [DOI] [Google Scholar]
- 49.Slavin S, Or R, Aker M, Shapira MY, Resnick I, Bitan M, et al. Treatment of resistant leukemia by rIL-2 activated NK cells in recipients of HLA matched and haploidentically mismatched stem cell allografts while avoiding GVHD. Blood. 2004;104:379b. [Google Scholar]
- 50.Velardi A, Ruggeri L, Mancusi A, Aversa F, Christiansen FT. Natural killer cell allorecognition of missing self in allogeneic hematopoietic transplantation: a tool for immunotherapy of leukemia. Curr Opin Immunol. 2009;21:525–530. doi: 10.1016/j.coi.2009.07.015. [DOI] [PubMed] [Google Scholar]