Abstract
Mutations in the small GTPase R-Ras that promote constitutive activation of this signaling molecule have been observed in a variety of invasive cancer cell types. We previously reported that expression of an oncogenic form of R-Ras (R-Ras87L) in a cell line of cervical cancer (C33A cells) augments cell growth in vitro and tumorigenicity in vivo. Because increased tumorigenicity in vivo often precedes metastasis, we now examined whether the expression of R-Ras87L also increased the metastatic potential of C33A cells. Accelerated tumor growth was observed in athymic mice after subcutaneous injection of R-Ras87L-expressing C33A cells. In addition, increased metastasis to the liver, in immunodeficient SCID mice, was observed after intravenous injection of R-Ras87L-expressing C33A cells. Also, R-Ras87L-expressing cells presented decreased membrane expression of MHC class I molecules, and β1 integrins, but increased levels of PI 3-K and Akt activities. C33A cells expressing R-Ras87L also migrated more over collagen I in wound assays. Inhibition of the PI 3-K/Akt/mTOR pathway by pharmacological means blocked R-Ras87L-induced accelerated growth and migration over collagen I. These results suggest oncogenic R-Ras has a central role in cancer progression towards a metastatic phenotype, through the activation of the PI 3-K/Akt/mTOR signaling pathway.
Keywords: Oncogene, Migration, PI 3-K, Akt
Introduction
Cervical carcinoma is the second most common malignant disease among women worldwide, accounting for 15% of all deaths from malignant disease [35, 40]. The highest incidence rates are observed in parts of Africa, Southeast Asia, and Latin America [29]. Over 95% of all cervical carcinomas contain DNA of some human papillomavirus [20, 21, 35]. Cervical tumors usually are invasive and cause many deaths each year, close to 2,000 in the United Kingdom [41] and 12,000 in Mexico [40]. Thus there is a lot of interest in understanding the adhesion and migration properties of epithelial tumor cells in an effort to control the most invasive and malignant forms of this type of carcinoma.
The oncogene R-Ras, a member of the super family of small GTPases, has been implicated in several cell functions [36]. The R-Ras protein is 55% identical to H-Ras and has an extension of 26 amino acids in its amino terminal end [17]. R-Ras reportedly influences integrin activation by both direct [44] and indirect mechanisms [37]. Oncogenic R-Ras induced cell transformation in fibroblasts [3, 25] but not in other cell types [36]. This difference is due to the fact that distinct molecular activators from those that control H-Ras [13] regulate R-Ras. Activated R-Ras also was reported to promote migration and invasion in vitro, of breast epithelial cells [16]. Moreover, the effects of R-Ras on cell migration seem to involve changes in the cytosqueleton through activation of the GTPases Rho and Rac [10, 14, 42]. We previously reported that cervical epithelial cells expressing a constitutively active form of R-Ras grew, as tumors in nude mice, faster than control cells [32]. These cells also presented a marked increase in cell spreading and migrated more over collagen I-coated filters [32].
Because increased tumorigenicity in vivo often precedes metastasis, we now examined whether the expression of an active form of R-Ras also increased the metastatic potential of cervical epithelial cells. We found increased metastasis to the liver in immunodeficient SCID mice, injected intravenously with cervical epithelial cells expressing active R-Ras. Also, these cells presented decreased membrane expression of MHC class I molecules, and β1 integrins, and increased levels of phosphatidylinositol 3-kinase (PI 3-K) and Akt activities; all markers of a more malignant phenotype. In addition, pharmacological inhibition of the PI 3-K/Akt/mTOR pathway blocked active R-Ras-induced accelerated cell growth and migration over collagen I. These data suggest that oncogenic R-Ras has a central role in cancer progression towards a metastatic phenotype, through activation of the PI 3-K-Akt-mTOR signaling pathway.
Materials and methods
Cell culture
The C33A cervical carcinoma epithelial cell line (catalog no. HTB-31; American Type Culture Collection; Manassas, VA, USA) was grown in Dulbecco modified Eagle medium (DMEM; Gibco BRL, Grand Island, NY, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS; Gibco BRL) and 20 μM glutamine.
Plasmids and reagents
The following antibodies were used: monoclonal antibody (mAb) TS2/16, anti-β1 integrin (donated by Dr. Martin Hemler; Dana Farber Cancer Research Institute, Boston, MA, USA); mAb IB4, anti-β2 integrin (a gift from Dr. Eric J. Brown; University of California, San Francisco, CA, USA); mAb W6/32, anti-major histocompatibility complex (MHC) class I (from American Type Culture Collection). mAb AP3, anti-β3 integrin; mAb R6G9, anti-β6 integrin; mAb P1E6, anti-α2 integrin; mAb P1B5, anti-α3 integrin; mAb P4G9, anti-α4 integrin; mAb P1D6, anti-α5 integrin; and mAb VNR147, anti-αv integrin, were from Gibco, BRL (Grand Island). Anti-PI 3-K p110β rabbit polyclonal IgG (catalog no. sc-7189) was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Rabbit polyclonal anti-Akt (catalog no. 9272) and rabbit polyclonal anti phospho-Akt (catalog no. 4058) were from Cell Signaling Technology (Beverly, MA, USA). FITC-conjugated F(ab’)2 goat anti-mouse IgG (catalog no. 55522), and F(ab’)2 goat anti-rabbit IgG (catalog no. 55665) were from ICN-Cappel (Aurora, OH, USA). Plasmids that direct the synthesis of normal (wild-type) or mutant forms of R-Ras were a generous gift from Dr. Adrienne D. Cox (University of North Carolina, Chapel Hill, NC, USA) and have been previously described [3, 13, 16]. The constitutively active PI 3-K p110 K227E [33] was a generous gift from Dr. Julian Downward (The Imperial Cancer Research Fund, London, UK). Collagen I was donated by Dr. Jesus Chimal (Instituto de Investigaciones Biomédicas, UNAM). Fibronectin was from Roche Molecular Biochemicals (Indianapolis, IN, USA). The specific PI 3-K inhibitor LY294002 was obtained from Calbiochem (San Diego, CA, USA). All other chemicals were from Sigma Chemical Company (St. Louis, MO, USA).
Transfection
Cells were transfected with the calcium phosphate-DNA coprecipitation method using 10 μg of plasmid for each 60-mm culture dish and individual clones selected in the presence of G418 as described [32].
PI 3-K activity assay
PI 3-K activity was determined by in vitro kinase assays as previously described [31].
Tumor growth in nude mice
R-Ras-, PI 3-K p110-, or empty vector-transfected C33A cells (5 × 106 cells) were injected subcutaneously into athymic mice (Harlam, Mexico City, Mexico). Five mice were used for each cell type. Tumor growth was evaluated by measuring tumor size in two dimensions with a calibrated caliper every week.
In vivo metastasis assay
A total of 10 × 106 C33A cells expressing one of the different forms of R-Ras, or the active PI 3-K p110 were injected into the tail vein of 6-week old female Fox Chase C.B-17-scid mice (Taconic; Hudson, NY, USA). Three mice were injected with each cell type. After 42 days, mice were sacrificed, dissected, and analyzed by gross examination. The livers were excised and placed in saline solution. Metastatic foci were counted at the liver surface under a dissecting microscope.
Flow cytometry
Staining of cells with various antibodies for flow cytometry analysis was done exactly as previously described [30].
Cell migration/wound assay
Six-well tissue culture plaques (Costar; Corning, NY, USA) were coated with a 10 μg/ml solution of either collagen I or fibronectin, and incubated for 8 h at 4°C. Wells were then washed and one million cells were seeded into each well. After cells have reached confluency, the cell monolayer was “wounded” by scraping it with a 200 μl pipette tip, and covered with fresh serum-supplemented DMEM medium. Cell migration over the monolayer wound was analyzed every 12 h. In selected experiments, cells were treated with 25 μM LY294002, or with 5 nM rapamycin in serum-free DMEM for 2 h before wound infliction.
Western blotting
PI 3-K, Akt, and phospho Akt were detected by immunoblotting with the corresponding antibody: anti-PI 3-K at 0.05 μg/ml, anti-Akt at 1/1,000 dilution, and anti-phospho-Akt at 1/1,000 dilution, exactly as previously described [7, 30].
Statistical analysis
Data were compared with an unpaired Student t and ANOVA tests using the computer program KaleidaGraph version 3.6.2 (Synergy Software; Reading, PA, USA). Differences were considered statistically significant when P values ≤ 0.01.
Results
R-Ras promoted accelerated in vitro growth of cervical epithelial cells via PI 3-K
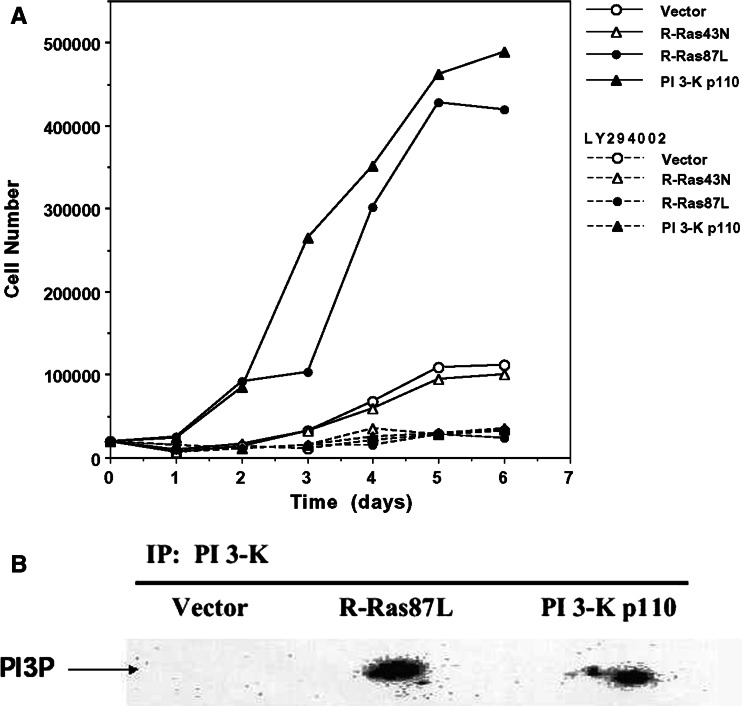
We have previously shown that the expression of an oncogenic form of R-Ras (R-Ras87L) in a cervical epithelial cell line (C33A cells) promotes accelerated cell growth in vitro and increased tumorigenicity in vivo [32]. It has also been shown for various cell types that PI 3-K can act as a down-stream effector of R-Ras [18, 36], controlling cellular functions such as cell survival, migration, and adhesion. We thus explored the possibility that PI 3-K was acting as an R-Ras effector for increased tumorigenicity. C33A cells were transfected with a constitutively active form of PI 3-K (p110) [33], and then selected for stable expression of this enzyme. Similarly to expression of R-Ras87L in C33A cells, expression of constitutively active PI 3-K resulted in accelerated cell growth in vitro (Fig. 1a). Pharmacological inhibition of PI 3-K with the drug LY294002 blocked accelerated growth both in R-Ras87L- and PI 3-K p110-expressing cells (Fig. 1a). This suggested that in these cells PI 3-K is indeed a downstream effector of oncogenic R-Ras. To confirm this idea we directly measured the activity of PI 3-K in R-Ras87L-expressing cells. A nearly tenfold increase in PI 3-K activity was observed in C33A cells expressing R-Ras87L, over C33A control cells (Fig. 1b). As expected, an increase in PI 3-K activity was observed in C33A cells expressing PI 3-K p110 (Fig. 1b). Taken together these results indicate that accelerated in vitro cell growth, induced by the expression of oncogenic R-Ras, involves a PI 3-K-dependent mechanism.
Fig. 1.
R-Ras promotes accelerated in vitro growth of cervical epithelial cells via PI 3-K. a 2 × 104 vector-transfected C33A cells (open circles), activated R-Ras87L-transfected C33A cells (solid circles), dominant negative R-Ras43N-transfected C33A cells (open triangles), or activated PI 3-K p110-transfected C33A cells (closed triangles), were plated in wells of a six-well plate and cultured for 1 week. Cells from each well were trypsinized, resuspended, and counted every day. Some cells were cultured in the presence of 25 μM LY294002 (dashed lines). Data are from one of four different experiments that yielded similar results. b PI 3-K was immunoprecipitated from cell lysates of 1 × 107 vector-transfected C33A cells (vector), activated R-Ras-transfected C33A cells (R-Ras87L), or activated PI 3-K p110-transfected C33A cells (PI 3-K p110). PI 3-K was then measured by an in vitro kinase assay. Phosphatidylinositol-3-phosphate (PI3P). Data are representative of two separate experiments
Expression of oncogenic R-Ras promoted accelerated tumor growth in vivo
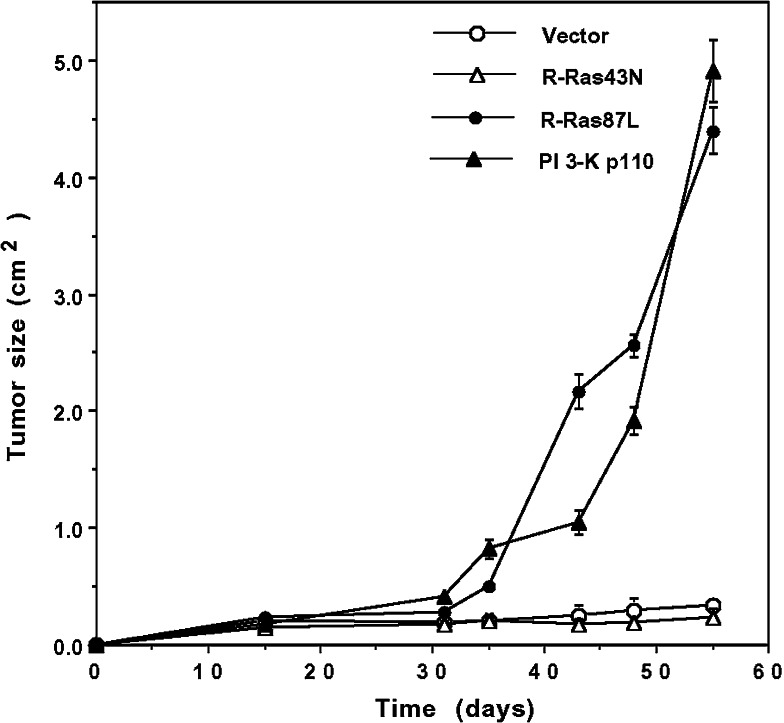
To determine whether PI 3-K was also involved in R-Ras87L-mediated accelerated tumor growth in vivo, we injected subcutaneously R-Ras87L- and PI 3-K p110-expressing C33A cells into athymic mice. All animals developed subcutaneous solid tumors (Fig. 2). Tumors in mice injected with R-Ras87L- and PI 3-K p110-expressing C33A cells, grew at a faster rate than those tumors formed by untransfected C33A cells or cells expressing the dominant negative form of R-Ras, R-Ras43N (Fig. 2). These results thus indicated that expression of oncogenic R-Ras could also promote increased tumor growth in vivo through a mechanism involving PI 3-K.
Fig. 2.
Activated R-Ras increased tumor growth of cervical epithelial cells in athymic mice. 5 × 106 vector-transfected C33A cells (open circles), activated R-Ras87L-transfected C33A cells (solid circles), dominant negative R-Ras43N-transfected C33A cells (open triangles), or activated PI 3-K p110-transfected C33A cells (closed triangles), were injected subcutaneously into athymic mice. Tumor size was measured every week in two dimensions with a calibrated caliper. Data are the mean ± standard error values from five animals for each cell type
Expression of oncogenic R-Ras promoted metastasis of C33A cells
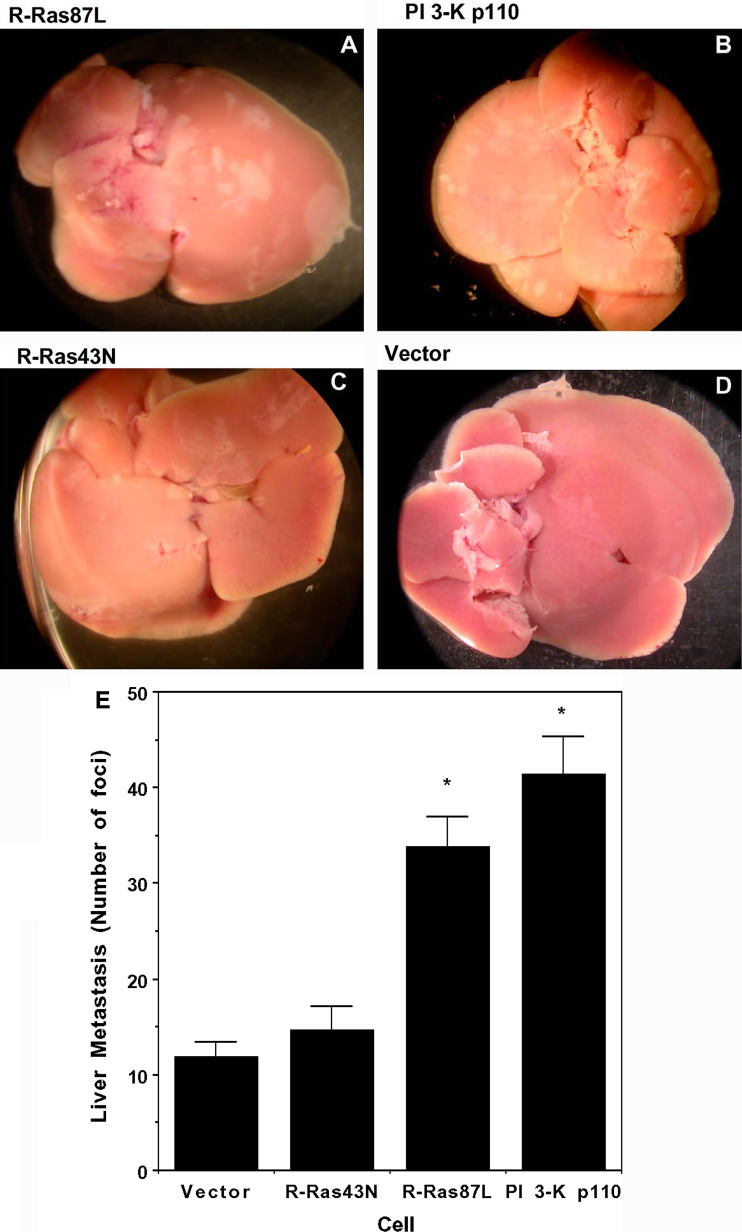
The observation that expression of oncogenic R-Ras in C33A cells promoted increased tumorigenicity in vivo raised the question as to whether oncogenic R-Ras could also promote metastasis. To explore this idea we directly evaluated the metastasis-promoting potential of R-Ras87L by injecting C33A cells expressing this form of R-Ras into the tail vein of SCID mice. Forty-two days after injection, mice were sacrificed and dissected to look for tumor formation. Several organs, including spleen, kidney, heart, stomach, intestines, lungs, muscle, and brain were free of tumors (data not shown). In contrast, liver of SCID mice injected with R-Ras87L-expressing C33A cells were enlarged and presented extensive metastatic nodules (Fig. 3a). Similarly, intravenous injection of PI 3-K p110- expressing C33A cells resulted in altered liver morphology and extensive nodule formation (Fig. 3b). In contrast, intravenous injection of dominant negative R-Ras43N-expressing C33A cells (Fig. 3c) or empty-vector-expressing C33A cells (Fig. 3d) resulted in almost normal liver morphology with few metastatic nodules. Liver metastasis was quantified by counting the number of nodules in various liver preparations (Fig. 3e). These results thus indicate that expression of oncogenic R-Ras in C33A cells promotes a more metastatic phenotype, and also suggest that a PI 3-K-dependent mechanism is involved.
Fig. 3.
Activated R-Ras increased metastatic potential of cervical epithelial cells. Livers of female Fox Chase C.B-17-scid mice 42 days after i.v. injection of 10 × 106 C33A cells transfected with a R-Ras87L, b PI 3-K p110, c R-Ras43N, or d empty vector. e Number of metastatic foci at the liver surface. Data shown are the mean ± standard error values from three animals for each cell type. Differences were statistically significant (*) at P ≤ 0.003 in ANOVA test
Expression of oncogenic R-Ras promoted an altered expression pattern of membrane receptors
Altered integrin expression patterns have been found in various types of cancer cells [15, 26] and these changes are often related to a more metastatic phenotype [5, 11, 19]. We thus explored the possibility that expression of R-Ras87L could alter the pattern of integrin expression in C33A cells. Expression of the β1 intregrin subunit, a molecule with a major role in cell attachment to the extracellular matrix, was considerably reduced in cells expressing R-Ras87L or PI 3-K p110 (Table 1), but not in R-Ras43N-expressing cells. In addition, R-Ras87L- and PI 3-K p110-expressing cells also had a significant reduction in membrane expression of MHC class I molecules (Table 1). Expression of integrin α2, α3, and α6, but not α5 subunits was also reduced in cells expressing R-Ras87L, and PI 3-K p110 (Table 1). Our results strongly suggest that expression of oncogenic R-Ras induces alterations in the expression pattern of membrane receptors that contribute to an increased malignant phenotype of cervical epithelial cells.
Table 1.
Integrin and MHC class I expression on C33A cells transfected with R-Ras or PI 3-K
| Molecule | Empty-vector | R-Ras43N | R-Ras87L | PI 3-K p110 |
|---|---|---|---|---|
| α2 | 61.3 | 60.6 | 40.9 | 28.3 |
| α3 | 62.2 | 40.4 | 19.9 | 15.8 |
| α4 | – | – | – | – |
| α5 | 60.3 | 50.1 | 58.7 | 57.5 |
| α6 | 58.3 | 71.2 | 25.9 | 21.8 |
| αv | – | – | – | – |
| β1 | 74.8 | 56.7 | 25.5 | 19.3 |
| β2 | – | – | – | – |
| β3 | – | – | – | – |
| β4 | – | – | – | – |
| β6 | – | – | – | – |
| MHC-I | 280.6 | 223.7 | 96.1 | 55.8 |
Mean fluorescence intensity. Average of two independent measurements
– Molecule not expressed on cell membrane
Expression of oncogenic R-Ras promoted increased cell migration over collagen I
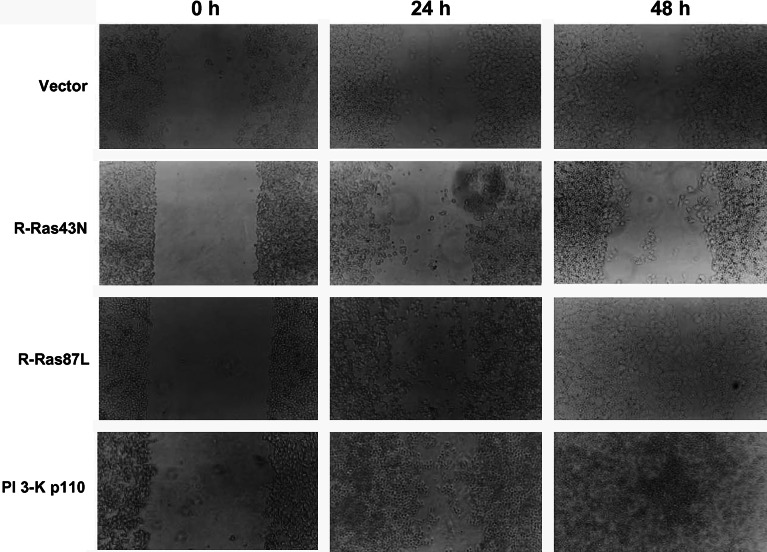
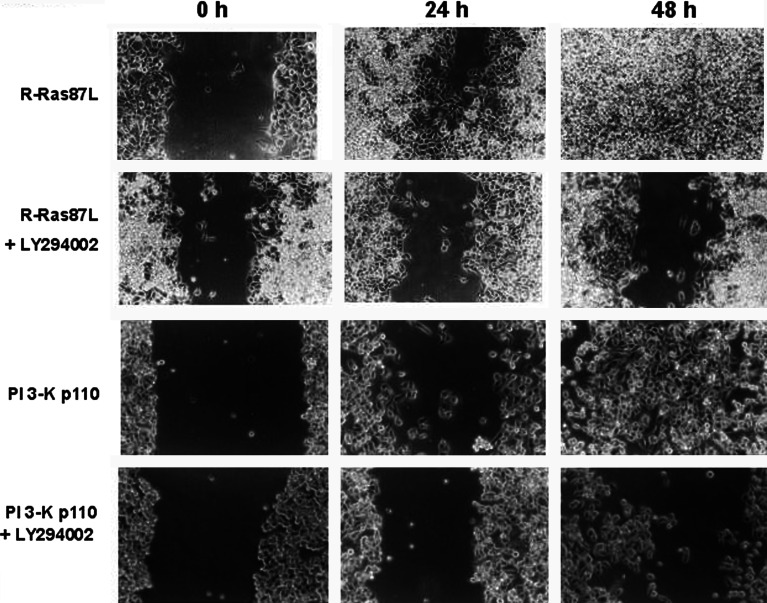
Metastatic cells often show increased migratory capacity over a variety of substrates. Expression of R-Ras87L resulted in elevated cell migration over collagen I in wound assays, compared to untransfected C33A cells and cells expressing R-Ras43N (Fig. 4). Similarly, expression of PI 3-K p110 in C33A cells also resulted in increased cell migration over collagen I (Fig. 4). Consistent, with a PI 3-K-dependent mechanism involved in R-Ras87L-induced metastasis, this increase in migration was blocked by the PI 3-K inhibitor LY294002 (Fig. 5). In contrast, cell migration over fibronectin was not observed in untransfected cells, nor in C33A cells transfected with either R-Ras87L, R-Ras43N, or PI 3-K p110 (data not shown).
Fig. 4.
Activated R-Ras and activated PI 3-K increased migration of cervical epithelial cells on collagen I. Monolayers of C33A cells transfected with empty plasmid (vector), dominant negative R-Ras (R-Ras43N), activated R-Ras (R-Ras87L), or activated PI 3-K (PI 3-K p110), were grown on collagen I-covered six-well plates, and scratched (wounded) with a pipette tip (0 h). Cells were then covered with fresh medium and monolayers were allowed to recover. Wound area was photographed 24 and 48 h later. Data are from one of three different experiments that yielded similar results
Fig. 5.
Activated R-Ras increased migration of cervical epithelial cells via PI 3-K. Monolayers of C33A cells transfected with activated R-Ras (R-Ras87L), or activated PI 3-K (PI 3-K p110), were grown on collagen I-covered six-well plates, and scratched (wounded) with a pipette tip (0 h). Cells were then covered with fresh medium and monolayers were allowed to recover. Wound area was photographed 24 and 48 h later. Some cells were cultured in the presence of 25 μM LY294002. Data are from one of three different experiments that yielded similar results
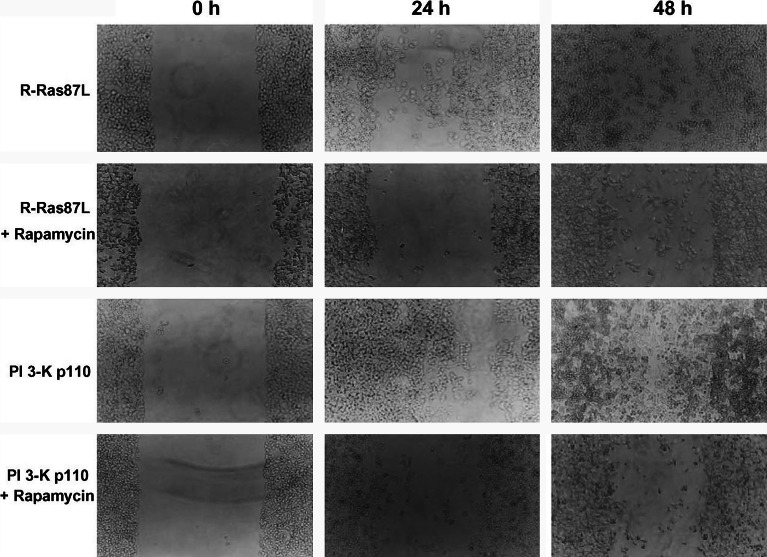
Expression of oncogenic R-Ras promotes increased cell motility through activation of Akt/mTOR
PI 3-K-dependent activation of Akt and of its downstream effector mammalian Target of Rapamycin (mTOR), has been reported to be of importance in the regulation of cell migration in various systems [4, 6, 39]. Western-blotting experiments showed that, compared to untransfected C33A cells, cells expressing either R-Ras87L or PI 3-K p110 had higher levels of active (phosphorylated) Akt (data not shown). To directly evaluate the participation of mTOR in R-Ras-dependent migration of C33A cells, we repeated the wound assays in the presence of the mTOR inhibitor rapamycin. Migration of R-Ras87L- or PI 3-K p110-expressing cells over collagen I was indeed blocked by rapamycin (Fig. 6). In contrast, rapamicyn had no effect on the slow migration of untransfected C33A cells, or cells transfected with R-Ras43N (data not shown). These results suggested that induction of a more metastatic phenotype by oncogenic R-Ras might be related, at least in part, to increased cell migration through activation of the PI-3K/Akt/mTOR pathway.
Fig. 6.
Activated R-Ras increased migration of cervical epithelial cells via mTOR. Monolayers of C33A cells transfected with activated R-Ras (R-Ras87L), or activated PI 3-K (PI 3-K p110), were grown on collagen I-covered six-well plates, and scratched (wounded) with a pipette tip (0 h). Cells were then covered with fresh medium and monolayers were allowed to recover. Wound area was photographed 24 and 48 h later. Some cells were cultured in the presence of 5 nM Rapamycin. Data are from one of three different experiments that yielded similar results
Discussion
In this report, we have investigated the ability of the oncogene R-Ras to induce a more metastatic phenotype in human cervical epithelium cells. We found that the expression of an active mutant of R-Ras (R-Ras87L) resulted in faster tumor growth in nude mice, and in augmented metastasis to the liver in SCID mice. In addition, cells that expressed the active oncogene R-Ras87L had an important reduction of integrin and major histocompatibility complex (MHC) class I molecules on their membrane. R-Ras87L-expressing cells also presented increased cell migration over collagen I. The increase in cell migration was dependent on PI 3-K and mTOR. Moreover, the activity of PI 3-K and Akt was augmented in cells that expressed R-Ras87L. These results indicate that the oncogene R-Ras depends on PI 3-K, Akt, and mTOR to induce cell migration and tumor metastasis of cervical epithelial cells.
R-Ras reportedly induces cell transformation in fibroblasts [3, 34] and induces a more invasive phenotype in breast epithelial cells [16]. NIH-3T3 cells that express an active R-Ras could form foci, although they did so much less efficiently than cells expressing the better-studied oncogne H-Ras. R-Ras-expressing fibroblasts [3, 25] also grew in nude mice and had elevated levels of ERK kinases [3]. These data suggested that R-Ras, like H-Ras, plays an important role in cell growth control. We found that active R-Ras87L-expressing C33A cells grew faster than control cells or dominant negative R-Ras43N-expressing cells, in vitro and in nude mice. The mechanism whereby R-Ras induces cell cycle progression and a more malignant phenotype remains unknown. However, it does not seem to involve ERK kinases, because C33A cells that expressed active R-Ras87L did not have increased ERK activity [32], although ERK could be easily activated by phorbol esters in these cells [32]. Other groups also have not been able to detect R-Ras-induced ERK activation [16, 18, 36].
Although, most cervical carcinomas contain DNA of some human papillomavirus (HPV) [20, 21, 35], we used the C33A cervical carcinoma epithelial cell line precisely because it does not have HPV. This choice was made to look at the effects of R-Ras on a cervical epithelial cell without interference from the effects of viral oncoproteins. We found that oncogenic R-Ras87L indeed induced cell cycle progression and a more malignant phenotype in cervical epithelial cells. However, there is not information on the level of R-Ras activity in HPV-infected cells. R-Ras has been found altered in other types of tumors. For example, elevated levels of R-Ras are reported to be sufficient for inducing estrogen-independent proliferation of breast cells [43] and the progression of breast cancer cells to tamoxifen resistance [9]. Also, upregulation of R-Ras was found in transformed colorectal crypt cells [45] and in gastric cancers [23]. In addition, functional blocking of R-Ras in these cells resulted in the disappearance of adhered cells, confirming the role of R-Ras in clinal gastric tumors [23]. Moreover, R-Ras expression and phosphorylation correlated with increasing grade of gliomas in human brain tumor specimens [22]. These reports show that R-Ras has a relevant role in tumor progression of varios types of cancer. Our data show that activated (oncogenic) R-Ras can also induce a malignant phenotype in human cervical cells. It would be now very interesting to determine the status of R-Ras, either overexpression or mutants, in clinical HPV-positive cervical tumors to determine a possible connection between R-Ras and HPV oncoproteins E6 and E7.
Early studies suggested that R-Ras could increase the migration and invasion potential of breast epithelial cells [16]. Later, we found that R-Ras could also increase migration in vitro of cervical epithelial cells [32]. More recently several reports have indicated that R-Ras is an important regulator of cell migration in other cell types [10, 12, 24, 42]. These reports underline the fact that R-Ras is an important regulator of the migratory capacity of many cell types. Now, we also report here that active R-Ras87L indeed promotes a more migratory and metastatic phenotype in cervical epithelial cells. The mechanism by which R-Ras induces augmented cell migration is complex and remains obscure. The effects of R-Ras are clearly multifactorial involving adhesion molecules such as integrins [11], signaling molecules such as PI 3-K [36], and GTPases to regulate the cytoskeleton [10, 42].
Expression of the active form R-Ras87L resulted in profound changes in integrin surface expression. β1 integrins were reduced on the cell membrane in about 2/3. This reduction may correspond to the integrins formed with the α2, α3, and α6 chains, as their surface expression was also reduced (Table 1). Interestingly, the expression level of α5 integrins did not change. Because the α5β1 integrins bind mainly fibronectin, and α2β1 and α3β1 integrins bind mainly collagen, reduction of the latter integrins may explain in part the increased migration R-Ras87L-expressing cells had over collagen I, and the total absence of migration over fibronectin. It is likely that less collagen receptors results in weaker cell attachment over this matrix protein, and leads to increased cell migration. In addition, R-Ras87L-expressing cells presented a significant reduction in major histocompatibility complex (MHC) class I molecules on their membranes (Table 1). Reduction of integrin expression is associated with a more migratory phenotype [11, 15], and is also a prerequisite for metastasis [15, 26, 28]. Reduction of MHC class I molecules often correlates with impaired recognition and killing by natural killer cells [8, 38]. Thus, R-Ras contributes to cancer development by altering expression of MHC class I molecules and integrins on cervical epithelial cells.
Elucidating the signaling molecules downstream from R-Ras is an active line of research. Several groups have indicated that R-Ras functions through PI 3-K. [1, 2, 16, 18, 25, 32]. Expression of R-Ras87L caused a strong constitutive activation of PI 3-K in C33A cells (Fig. 1 and [32]). This enzymatic activity seems to be responsible for the effect of R-Ras87L on cell proliferation and migration, because treatment with the PI 3-K inhibitor LY294002 prevented cell growth in culture and inhibited cell migration over collagen I. Moreover, C33A cells expressing a constitutively activated form of PI 3-K (p110) mimicked completely the phenotype observed in R-Ras87L-expressing C33A cells. In addition, the kinase Akt was also activated in R-Ras87L- and PI 3-K p110-expressing C33A cells. Thus, R-Ras, via PI 3-K induces Akt, but not ERK activation. Because a major downstream target of Akt is mTOR, we reasoned that inhibition of mTOR would have a similar effect on cell migration as the inhibition of PI 3-K. Treatment with rapamycin, a specific inhibitor of mTOR, indeed blocked increased cell migration of PI 3-K p110- and R-Ras87L-expressing C33A cells. Because mTOR regulates p70 ribosomal S6 kinase leading to cell cycle progression [27], it is likely that R-Ras87L-mediated increased tumorigenicity is also related to mTOR activation. Taken together, these results suggest that induction of a more metastatic phenotype by oncogenic R-Ras may be related, at least in part, to increased cell migration through activation of the PI-3K/Akt/mTOR pathway. The mTOR pathway has also been implicated in increased proliferation of mammary and [4] and prostate tumors [6].
In summary, the present report shows that oncogenic R-Ras has a central role in cancer progression of cervical epithelial cells, towards a metastatic phenotype, through activation of the PI 3-K-Akt-mTOR signaling pathway.
Acknowledgments
We thank Dr. Adrienne D. Cox for R-Ras constructs, Dr. Julian Downward for the active PI 3-K construct, and Dr. Martin Hemler, and Dr. Eric J. Brown for anti-integrin antibodies. We also thank Dr. Jesus Chimal for helping taking pictures of mouse livers, and Jose Alejandro Marmolejo Valencia for technical assistance. This work was supported by grant 36407-M from Consejo Nacional de Ciencia y Tecnología, Mexico, and by grant IN220703 from DGAPA, Universidad Nacional Autónoma de México, Mexico.
References
- 1.Berrier AL, Mastrangelo AM, Downward J, Ginsberg M, LaFlamme SE. Activated R-Ras, Rac-1, PI 3-kinase and PKCe can each restore cell spreading inhibited by isolated integrin b1 cytoplasmic domains. J Cell Biol. 2000;151:1549–1560. doi: 10.1083/jcb.151.7.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cole AL, Subbanagounder G, Mukhopadhyay S, Berliner JA, Vora DK. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler Thromb Vasc Biol. 2003;23:1384–1390. doi: 10.1161/01.ATV.0000081215.45714.71. [DOI] [PubMed] [Google Scholar]
- 3.Cox AD, Brtva TR, Lowe DG, Der CJ. R-Ras promotes malignant, but not morphologic, transformation of NIH3T3 cells. Oncogene. 1994;9:3281–3288. [PubMed] [Google Scholar]
- 4.Debnath J, Walker SJ, Brugge JS. Akt activation disrupts mammary acinar architecture and enhances proliferation in an mTOR-dependent manner. J Cell Biol. 2003;163:315–326. doi: 10.1083/jcb.200304159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA. 2001;98:1853–1888. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gao N, Zhang Z, Jiang B-H, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Garcia E, Sanchez-Mejorada G, Rosales C. Phosphatidylinositol 3-kinase and ERK are required for NF-kB activation, but not for phagocytosis. J Leukoc Biol. 2001;70:649–658. [PubMed] [Google Scholar]
- 8.Ghim SJ, Sundberg J, Delgado G, Jenson AB. The pathogenesis of advanced cervical cancer provides the basis for an empirical therapeutic vaccine. Exp Mol Pathol. 2001;71:181–185. doi: 10.1006/exmp.2001.2393. [DOI] [PubMed] [Google Scholar]
- 9.Gotoh T, Cai D, Tian X, Feig LA, Lerner A. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J Biol Chem. 2000;275:30118–30123. doi: 10.1074/jbc.M003074200. [DOI] [PubMed] [Google Scholar]
- 10.Holly SP, Larson MK, Parise LV. The unique N-terminus of R-Ras is required for Rac activation and precise regulation of cell migration. Mol Biol Cell. 2005;16:2458–2469. doi: 10.1091/mbc.E03-12-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hood JD, Cheresh DA. Role of integrins in cell invation and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y, Rangwala F, Fulkerson PC, Ling B, Reed E, Cox AD, Kamholz J, Ratner N. Role of TC21/R-Ras2 in enhanced migration of neurofibromin-deficient Schwann cells. Oncogene. 2004;23:368–378. doi: 10.1038/sj.onc.1207075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huff SY, Quilliam TR, Cox AD, Der CJ. R-Ras is regulated by activators and effectors distinct from those that control Ras function. Oncogene. 1997;14:133–143. doi: 10.1038/sj.onc.1200815. [DOI] [PubMed] [Google Scholar]
- 14.Jeong H-W, Nam J-O, Kim I-S. The COOH-terminal end of R-Ras alters the motility and morphology of breast epithelial cells through Rho/Rho-kinase. Cancer Res. 2005;65:507–515. [PubMed] [Google Scholar]
- 15.Juliano RL, Varner JA. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-T. [DOI] [PubMed] [Google Scholar]
- 16.Keely PJ, Rusyn EV, Cox AD, Parise LV. R-Ras signals through specific integrin alpha cytoplasmic domains to promote migration and invasion of breast epithelial cells. J Cell Biol. 1999;145:1077–1088. doi: 10.1083/jcb.145.5.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe DG, Capon DJ, Delwart E, Sakaguchi AY, Naylor SL, Goeddel DV. Structure of the human and murine R-ras genes, novel genes closely related to ras proto-oncogenes. Cell. 1987;48:137–146. doi: 10.1016/0092-8674(87)90364-3. [DOI] [PubMed] [Google Scholar]
- 18.Marte BM, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. R-Ras can activate the phosphoinositide 3-kinase but not the MAP kinase arm of the Ras effector pathways. Curr Biol. 1996;7:63–70. doi: 10.1016/S0960-9822(06)00028-5. [DOI] [PubMed] [Google Scholar]
- 19.Maschler S, Wirl G, Spring H, Bredow DV, Sordat I, Beug H, Reichmann E. Tumor cell invasiveness correlates with changes in integrin expression and localization. Oncogene. 2005;24:2032–2041. doi: 10.1038/sj.onc.1208423. [DOI] [PubMed] [Google Scholar]
- 20.Motoyama S, Ladines-Llave CA, Luis-Villanueva S, Maruo T. The role of human papilloma virus in the molecular biology of cervical carcinogenesis. Kobe J Med Sci. 2004;50:9–19. [PubMed] [Google Scholar]
- 21.Münger K, Baldwin A, Edwards KM, Hayakawa H, Nguyen CL, Owens M, Grace M, Huh K. Mechanisms of human papillomavirus-induced oncogenesis. J Virol. 2004;78:11451–11460. doi: 10.1128/JVI.78.21.11451-11460.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakada M, Niska JA, Tran NL, McDonough WS, Berens ME. EphB2/R-Ras signaling regulates glioma cell adhesion, growth, and invasion. Am J Pathol. 2005;167(2):565–576. doi: 10.1016/S0002-9440(10)62998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishigaki M, Aoyagi K, Danjoh I, Fukaya M, Yanagihara K, Sakamoto H, Yoshida T, Sasaki H. Discovery of aberrant expression of R-RAS by cancer-linked DNA hypomethylation in gastric cancer using microarrays. Cancer Res. 2005;65:2115–2124. doi: 10.1158/0008-5472.CAN-04-3340. [DOI] [PubMed] [Google Scholar]
- 24.Oinuma I, Ishikawa Y, Katoh H, Negishi M. The Semaphorin 4D receptor Plexin-B1 is a GTPase activating protein for R-Ras. Science. 2004;305:862–965. doi: 10.1126/science.1097545. [DOI] [PubMed] [Google Scholar]
- 25.Osada M, Tolkachera T, Li W, Chan TO, Tsichlis PN, Saez R, Kimmelman AC, Chan AM. Differential roles of Akt, Rac, and Raf in R-Ras-mediated cellular transformation adhesion and survival. Mol Cell Biol. 1999;9:6333–6344. doi: 10.1128/mcb.19.9.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parise LV, Weon J, Juliano RL. New aspects of integrin signaling in cancer. Sem Cancer Biol. 2000;10:407–414. doi: 10.1006/scbi.2000.0337. [DOI] [PubMed] [Google Scholar]
- 27.Pene F, Claessens YE, Muller O, Viguie F, Mayeux P, Dreyfus F, Lacombe C, Bouscary D. Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma. Oncogene. 2002;21:6587–6597. doi: 10.1038/sj.onc.1205923. [DOI] [PubMed] [Google Scholar]
- 28.Ponta H, Sleeman J, Herrlich P. Tumor metastasis formation: cell-surface proteins confer metastasis-promoting or -suppressing properties. Biochim Biophys Acta. 1994;1198:1–10. doi: 10.1016/0304-419x(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 29.Potter JD (1997) Food, nutrition and the prevention of cancer: A global perspective. The American Institute for Cancer Research, Washington DC, p 670
- 30.Reyes-Reyes M, Mora N, Gonzalez G, Rosales C. b1 and b2 integrins activate different signalling pathways in monocytes. Biochem J. 2002;363:273–280. doi: 10.1042/0264-6021:3630273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reyes-Reyes M, Mora N, Zentella A, Rosales C. Phosphatidylinositol 3-kinase mediates integrin-dependent NF-kB and MAPK activation through separate signaling pathways. J Cell Sci. 2001;114:1579–1589. doi: 10.1242/jcs.114.8.1579. [DOI] [PubMed] [Google Scholar]
- 32.Rincón-Arano H, Rosales R, Mora N, Rodríguez-Castañeda A, Rosales C. R-Ras promotes tumor growth of cervical epithelial cells. Cancer. 2003;97:575–585. doi: 10.1002/cncr.11093. [DOI] [PubMed] [Google Scholar]
- 33.Rodríguez Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- 34.Saez R, Chan AM-L, Miki T, Aaronson SA. Oncogenic activation of human R-Ras by point mutations analogous to those of prototype H-Ras. Oncogene. 1994;9:2977–2982. [PubMed] [Google Scholar]
- 35.Schorge JO, Knowles LM, Lea JS. Adenocarcinoma of the cervix. Curr Treat Options Oncol. 2004;5:119–127. doi: 10.1007/s11864-004-0044-0. [DOI] [PubMed] [Google Scholar]
- 36.Self AJ, Caron E, Peterson HF, Hall A. Analysis of R-Ras signalling pathways. J Cell Sci. 2001;114:1357–1366. doi: 10.1242/jcs.114.7.1357. [DOI] [PubMed] [Google Scholar]
- 37.Sethi T, Ginsberg MH, Downward J, Hughes PE. The small GTP-binding protein R-Ras can influence integrin activation by antagonizing a Ras/Raf-initiated intetgrin supression pathway. Mol Biol Cell. 1999;10:1799–1809. doi: 10.1091/mbc.10.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sirianni MC, Libi F, Campagna M, Rossi D, Capello D, Sciaranghella G, Carbone A, Simonelli C, Monini P, Gaidano G, Ensoli B. Downregulation of the major histocompatibility complex class I molecules by human herpesvirus type 8 and impaired natural killer cell activity in primary effusion lymphoma development. Br J Haematol. 2005;130:92–95. doi: 10.1111/j.1365-2141.2005.05581.x. [DOI] [PubMed] [Google Scholar]
- 39.Sugatani T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem. 2005;280:3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- 40.Torres Lobaton A, Rojo Herrera G, Torres Rojo A, Hurtado Estrada G, Roman Bassaure E. Cervical cancer. Current view of its epidemiology and risk factors. Ginecol Obstet Mex. 2004;72:466–474. [PubMed] [Google Scholar]
- 41.Woodman CBJ, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001;357:1831–1836. doi: 10.1016/S0140-6736(00)04956-4. [DOI] [PubMed] [Google Scholar]
- 42.Wozniak MA, Kwong L, Chodniewicz D, Klemke RL, Keely PJ. R-Ras controls membrane protrusion and cell migration through the spatial regulation of Rac and Rho. Mol Biol Cell. 2005;16:84–96. doi: 10.1091/mbc.E04-04-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Feig LA. Involvement of R-Ras and Ral GTPases in estrogen-independent proliferation of breast cancer cells. Oncogene. 2002;21:7557–7568. doi: 10.1038/sj.onc.1205961. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Z, Vouri K, Wang HG, Reed JC, Rouslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/S0092-8674(00)81082-X. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Zhong X, Zheng S, Ge Z, Du Q, Zhang S. Transformation of immortalized colorectal crypt cells by microcystin involving constitutive activation of Akt and MAPK cascade. Carcinogenesis. 2005;26:1207–1214. doi: 10.1093/carcin/bgi069. [DOI] [PubMed] [Google Scholar]