Abstract
Patients and animals bearing tumors have increased levels of CD34+ progenitor cells, which are capable of developing into dendritic cells. However, addition of medium conditioned by murine Lewis lung carcinoma cells increases the cellularity of the CD34+ cell cultures and redirects their differentiation into endothelial cells. The resulting cells resemble endothelial cells phenotypically as well as functionally by their capacity to reorganize into cord structures. Mechanisms by which tumors induced the increased cellularity and skewing toward endothelial cells were examined. Tumor-derived VEGF contributed to the increase in cellularity, but not to the redirection of differentiation. Differentiation into endothelial cells was blocked with sTie-2, suggesting tumor-derived angiopoietins in skewing differentiation. These studies show the capacity of tumors to skew progenitor cell development toward endothelial cells and define the mediators that contribute to endothelial cell development.
Keywords: Angiogenesis, Angiopoietins, Endothelial cells, Tumor, Vasculogenesis, VEGF
Introduction
Vascularization is required for progressive tumor growth. Classically, vascularization has been considered to occur through the processes of angiogenesis, which involves outgrowth of new endothelial cells from mature preexisting endothelial cells. More recent studies have suggested that vasculogenesis, which occurs more prominently during embryonic development, can contribute to vessel formation in adults [24]. Vasculogenesis is the development of vessels from precursor cells that migrate to the site of vascularization. Vasculogenesis may also occur concomitantly with angiogenesis [1, 7, 13]. In models of neovascularization in adults, such as in response to ischemia, i.v.-infused human CD34+ progenitor cells have been shown to become incorporated as endothelial cells into newly growing vessels at the site of injury-induced angiogenesis [5].
In addition to postneonatal vasculogenesis being observed in instances of ischemia, vasculogenesis has been suggested to contribute to tumor vascularization. Mice bearing human breast cancers had increased levels of endothelial precursor cells in the bone marrow, peripheral blood, and within the tumor [22]. Progenitor cells staining brightly positive for CD34 are readily detectable within human squamous cell carcinomas of the head and neck [29, 34, 37]. This contrasts to the more dimly staining CD34+ endothelial cells [31, 35]. The brightly staining CD34+ cells in head and neck cancers are progenitor cells as they form colonies in soft agar and, depending on the cytokines within the agar, can grow into colonies composed of neutrophils and monocytes [34]. These CD34+ cells also have the capacity to differentiate in vitro into antigen-presenting dendritic cells [10, 17] or into cells that incorporate into the tumor vasculature [28].
The factors that contribute to vasculogenesis in adults are currently under investigation. Mobilization of endothelial cell precursors from the bone marrow into the peripheral blood can be stimulated by GM–CSF [24]. VEGF can also mobilize circulating endothelial progenitor cells [21]. VEGF is important in both stimulation of proliferation and accumulation of endothelial precursor cells [12, 23]. While the role of angiopoietins in vasculogenesis is not well defined, expression of the angiopoietin decoy sTie-2 reduces in vivo tumor vascularization and growth [19].
The process that leads to the accumulation of CD34+ cells within tumor tissue has not been fully defined, but it is likely to involve multiple steps. GM–CSF production by head and neck cancers or by murine Lewis lung carcinoma (LLC) cells has been shown to coincide with increased peripheral blood levels of progenitor cells [17, 18, 30, 32]. Besides the GM–CSF-induced mobilization of CD34+ cells from the bone marrow into the periphery [34, 36], another step is the influx of CD34+ cells into the tumor tissue. Accumulation of progenitor cells within human and animal tumors has been attributed to chemoattraction by tumor-derived VEGF [6, 33]. Other cytokines, such as interleukin-3 (IL-3) or stromal cell-derived factor-1 (SDF-1), have been shown to be chemoattractants for normal bone marrow CD34+ progenitor cells [2, 20], but their contribution to the accumulation of progenitor cells in tumor has not yet been defined [2, 8, 14].
Our prior studies have shown that both human and animal tumors can mobilize and chemoattract CD34+ progenitor cells [6, 17, 33]. The present studies were conducted to define the steps through which tumors can skew CD34+ cell differentiation into endothelial cells. These studies show that when cultured under conditions to facilitate myeloid cell development and to yield high proportions of dendritic cells, CD34+ progenitor cells are instead redirected by tumor to differentiate into endothelial cells. The resulting cells resemble endothelial cells phenotypically as well as functionally by their capacity to be stimulated to reorganize into cord structures. Tumor-derived VEGF was a prominent contributor to the increase in cellularity, but not to the redirection of differentiation. Differentiation into endothelial cells was blocked with sTie-2, suggesting tumor-derived angiopoietins in skewing differentiation into endothelial cells.
Methods
Tumor cells
A cloned metastatic LLC line was used for generating tumor-conditioned medium. These cells were maintained in RPMI-1640 culture medium containing 10% endotoxin-free defined fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.02 M Hepes buffer, 5×10−5 M 2-mercaptoethanol, and 2 mM L-glutamine. To generate tumor-conditioned medium, subconfluent LLC cells were seeded in fresh medium. After 24 h, the LLC-conditioned medium was collected, filtered, and used at a 40% concentration (v/v) unless otherwise indicated in specific experiments.
CD34+ cell cultures
Femoral CD34+ progenitor cells were isolated immunomagnetically by using antibodies to CD34. Briefly, femoral bone marrow cells of normal mice were distributed into a single cell suspension, washed, and then incubated for 45 min on ice with anti-CD34 antibody (RAM34; BD Pharmingen, San Diego, CA, USA). After incubating with the antibodies, femoral bone marrow cells were washed and incubated with biotinylated goat antirat secondary antibody coupled to magnetic microbeads (Militenyi Biotec, Auburn, CA, USA). Cells were then washed and resuspended in biotin-free BSA/PBS and subjected to magnetic column fractionation (Militenyi Biotec). The CD34+ cells that were retained in the magnetic column were collected. The purity of this progenitor cell population was verified by incubating an aliquot of cells overnight, immunostaining with anti-CD34 antibody and then analyzing by flow cytometry. The collected CD34+ cells were cultured in wells of 6-well plates or in microscope chamber slides (Nalge Nunc International, Rochester, NY, USA) at a concentration of 3×105 cells/ml. To support CD34+ cell viability and to promote myeloid cell differentiation, all cultures were supplemented with 20 ng/ml GM–CSF (R & D Systems‘) and 50 ng/ml stem cell factor (SCF; R & D Systems). Experimental wells also contained 40% (v/v) LLC-conditioned medium. In some studies, neutralizing VEGF antibody that is capable of recognizing murine VEGF isoforms 120 and 164 (500 ng/ml; R & D Systems) or sTie-2-Fc (50 μg/ml; Regeneron Pharmaceuticals, Inc., Tarrytown, NY, USA) were first admixed with the LLC-conditioned medium.
For studies assessing cellular expansion in the CD34+ cell cultures, a tetrazolium-based colorimetric assay (Promega, Madison, WI) was used on days that are indicated in specific experiments. Results are shown as mean absorbance values ± SEM of multiple experiments. When conducting morphological or phenotypic analyses, cells were incubated for 7 days unless otherwise indicated. For microscopic analysis of resulting cultures, cells were subjected to immunohistochemical staining with primary antibodies and staining was then visualized with the Vectastain ABC system. When analyzed by flow cytometry, endothelial cells were detached by scraping and immunofluorescent-stained with FITC-conjugated CD31 antibody (BD Biosciences). Scrapped endothelial cells were also recognized by indirect immunostaining first with mouse CD144 (BD Biosciences) and then with FITC-conjugated secondary antibody. Dendritic cells were recognized by indirect staining with CD205 antibody (Serotec) and then with FITC-conjugated rabbit antimouse antibody. The frequency of positive-staining cells was quantitated with a FACS 420 (Becton Dickinson, Sunnyvale, CA, USA). Dendritic cell functional analysis was indicated by secretion of IL-12. Cultures were first stimulated with 100 ng/ml LPS (E. coli 055:B55) for the last 24 h of culture. Supernatants were then collected and used to measure IL-12 p70 levels by ELISA (R & D Systems). In studies to assess the capacity of CD34+-derived cells to form cord structures, they were detached after 7 days and seeded over a layer of Matrigel. The Matrigel layer was prepared by a 1:4 dilution in iced medium and allowing the Matrigel to solidify in microscope chamber slides at 37°C. The solidified Matrigel was then soaked with fresh medium, which was removed prior to the addition of cells derived from CD34+ cultures. After 24 h, the medium was removed and cells were immunostained.
Analysis of data
The Student’s t test was used to determine the significance of differences between control and experimental values. Data shown are mean values of multiple experiments or representative results of these analyses.
Results
Effect of tumor-conditioned medium on cellularity and dendritic cell development from CD34+ cells cultured under conditions to support myeloid cell development
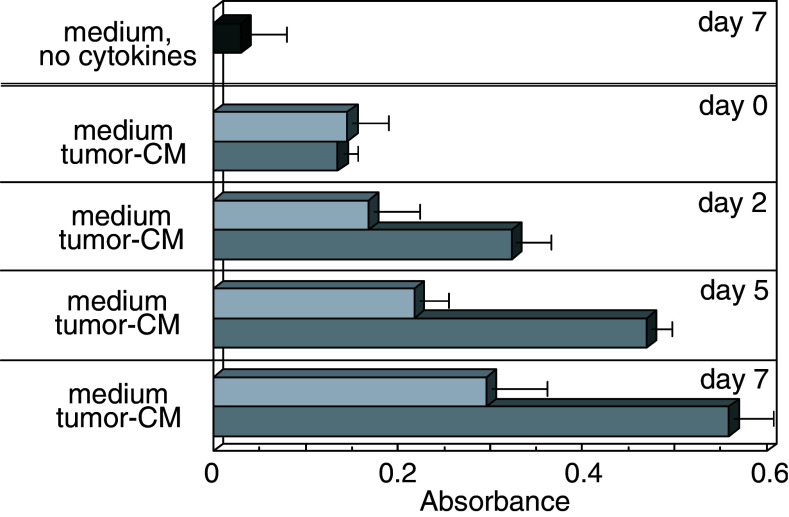
Prior studies had shown the importance of tumor vascularization in progressive tumor growth and metastasis. However, tumor vascularization can involve not only the processes of angiogenesis, but also vasculogenesis where endothelial cells develop from immature precursor cells [21, 22]. While tumors have been shown to mobilize CD34+ progenitor cells from the bone marrow into the peripheral blood and tumor tissue [17, 34], the processes that regulate expansion and differentiation of these cells into endothelial cells are not well defined. In determining the impact of tumor on differentiation of these CD34+ progenitor cells, they were isolated and cultured under conditions that stimulate development of myeloid cells and that have been used to generate dendritic cells. Addition of tumor-conditioned medium to these CD34+ cell cultures resulted in an early and sustained increase in cellularity (Fig. 1). This increase in cellularity in cultures containing tumor-conditioned medium was observed at 2 days of culture and became more prominent at 5 days of culture. Cultures that similarly contained cytokines to support myeloid cell development but which did not contain tumor-conditioned medium also exhibited an increase in cellularity. However, the increase was delayed compared to cultures containing the tumor-conditioned medium and did not attain the levels seen with LLC-conditioned medium. In the absence of myeloid-cell-supporting conditions, the viability of the CD34+ cell cultures declined. Therefore, cultures used in all subsequent studies contained the myeloid-cell-supporting cytokines.
Fig. 1.
Kinetic analysis of the stimulation of proliferation of CD34+ cell cultures by tumor-conditioned medium. CD34+ cells were seeded under conditions to support myeloid cell growth (GM–CSF and SCF) in the presence or absence of 40% tumor-conditioned medium. At various times, the effect of tumor-conditioned medium on cellularity was approximated by a tetrazolium-based colorimetric assay. Data shown are mean absorbance values ± SEM from four separate experiments
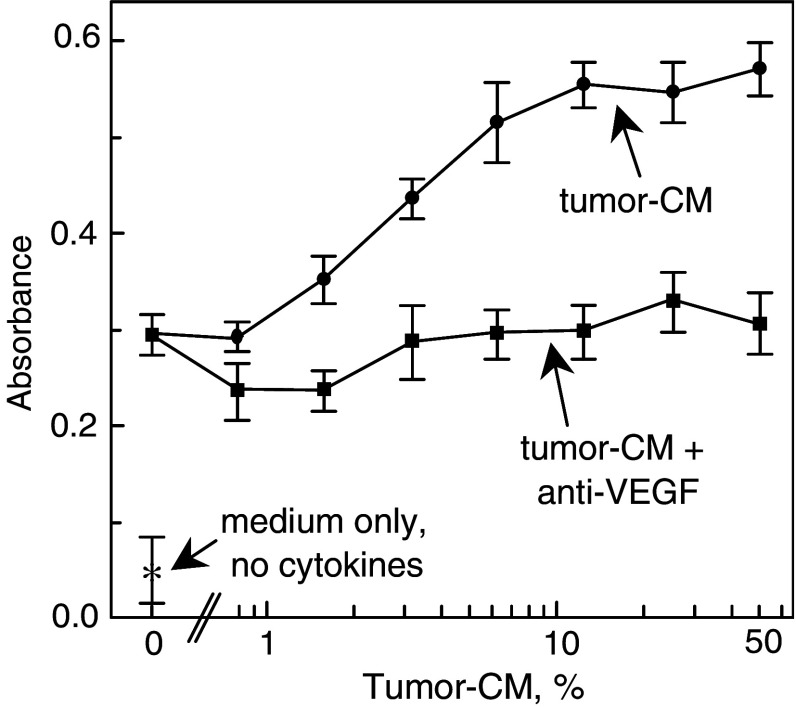
Since tumor-derived VEGF has been shown to be important in stimulating angiogenesis, the possibility that tumor-derived VEGF mediated the expansion of cells in the CD34+ cell cultures was assessed. Addition of increasing concentration of tumor-conditioned medium to CD34+ cells that were cultured under conditions to support myeloid cell development resulted in a dose-dependent increase in cellularity (Fig. 2). Neutralization of VEGF in the tumor-conditioned with antibody diminished the tumor-stimulated expansion of the CD34+ cell cultures to levels seen in cultures to which tumor-conditioned medium had not been added. This suggested that tumor-derived VEGF was responsible for the expansion of the CD34+ progenitor cells.
Fig. 2.
Neutralization of VEGF blocks the expansion of CD34+ cell cultures by tumor-conditioned medium. Isolated CD34+ cells were seeded into culture with increasing concentrations of tumor-conditioned medium together with isotype control or 250 ng/ml neutralizing VEGF antibodies. After 7 days, the relative cell number was colorimetrically measured with a tetrazolium-based assay. Data shown are mean absorbance values ± SEM from four separate experiments
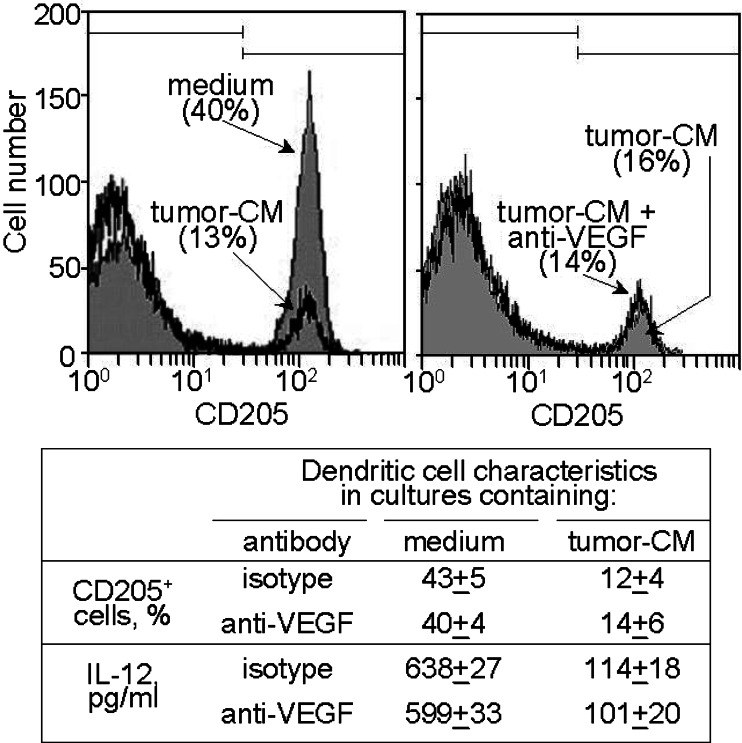
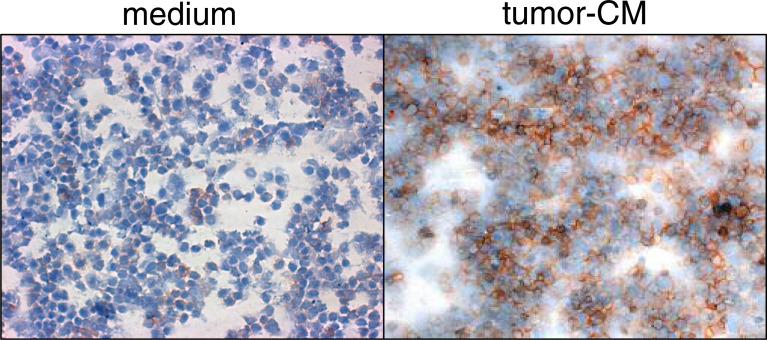
Since the conditions under which the CD34+ cells were cultured to facilitate myeloid cell development and had previously been use to generate CD34+-cell-derived dendritic cells, the cells that were cultured in the presence or absence of tumor-conditioned medium were analyzed after 7 days by flow cytometry for development of dendritic cells. In the absence of tumor-conditioned medium, almost half of the cells that developed in the cytokine-containing cultures stained positive for the dendritic cells marker CD205 (Fig. 3, left panel), similar to what has previously been published [10]. However, the addition of tumor-conditioned medium markedly reduced the development of dendritic cells to one-third the levels without tumor-conditioned medium. Whether or not the tumor-induced decline in dendritic cell development was due to VEGF was assessed because of the above studies demonstrating its role in increasing cellularity. While adding tumor-conditioned medium to CD34+ cell cultures containing cytokines to facilitate myeloid cell development minimized dendritic cell development; this was not mediated by VEGF since antibody neutralization of VEGF did not restore development of dendritic cells from CD34+ cells (Fig. 3, right panel). This phenotypic analysis was confirmed by functional analysis for levels of IL-12 secreted by the cultures. The presence of tumor-conditioned medium diminished the levels of IL-12 released by the cells that were developed from the CD34+ cells.
Fig. 3.
Tumor-conditioned medium diminishes CD34+ cell development into CD205+ dendritic cells through a VEGF-independent pathway. CD34+ cells were seeded under conditions to support myeloid cell growth in the presence or absence of 40% tumor-conditioned medium. After 7 days, they were detached and immunostained for CD205. Shown in the top panels are representative histograms from five similarly conducted studies. In the panel below is a summary of the mean percentages of CD205 cells ± SEM from five different analyses
Tumor-conditioned medium increases the frequency and absolute number of endothelial cells that develop from CD34+ progenitor cells
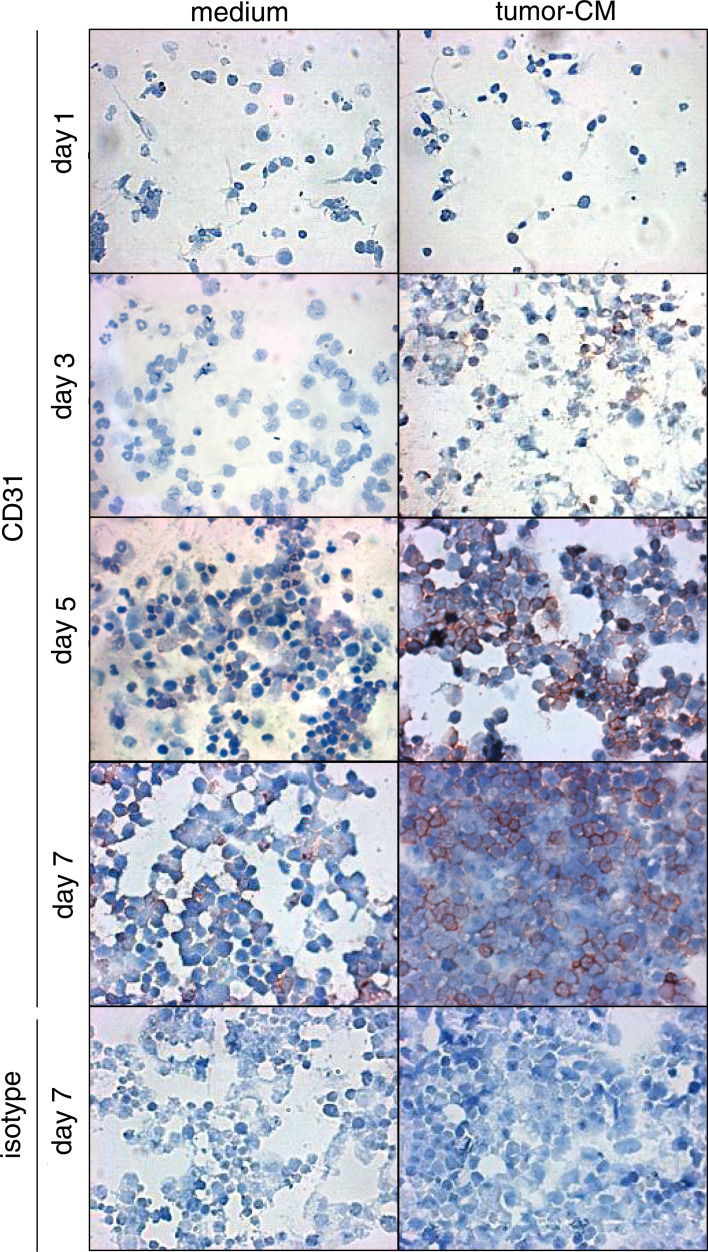
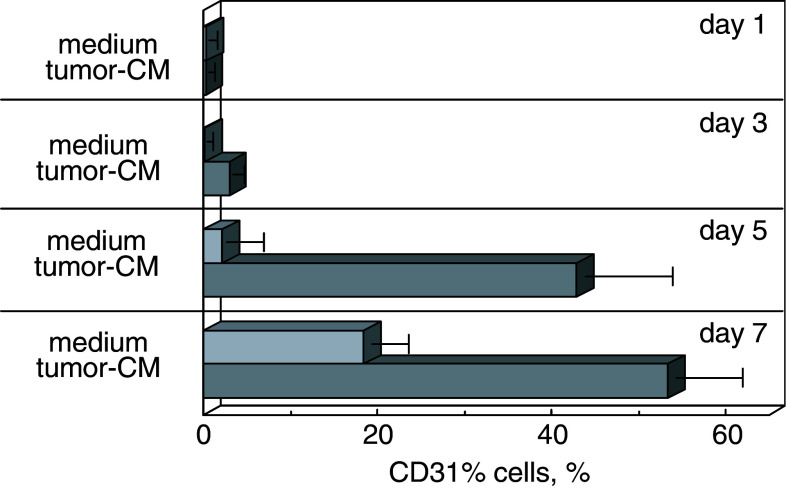
Microscopic evaluation of the CD34+ cells that were cultured under conditions which support myeloid cell growth, but in the presence or absence of tumor-conditioned medium demonstrated morphological differences and confirmed the above-described tetrazolium-based colorimetric analyses. At 3 days of culture in the absence of tumor-conditioned medium, there was a prominent appearance of cells resembling immature neutrophils, although these cells were largely replaced by day 5 with a more heterogeneous-appearing culture (Fig. 4). Neutrophil-like cells were less prominent in cultures containing tumor-conditioned medium. By day 7, the cultures containing tumor-conditioned medium clearly had a greater cell density than did cultures without tumor-conditioned medium.
Fig. 4.
Kinetic analysis of the stimulation by tumor-conditioned medium of CD31+ cell development from CD34+ cell cultures. CD34+ cells were seeded under conditions to support myeloid cell growth (GM–CSF and SCF) in the presence or absence of 40% tumor-conditioned medium. At various times, cultures were subjected to immunocytochemical staining for CD31-expressing cells. Cells were counterstained for visualization
The above studies showing that cultures containing tumor-conditioned medium yielded fewer dendritic cells, together with the potential of CD34+ cells to develop into endothelial cells, prompted assessment of whether the cells whose levels increased in cultures containing tumor-conditioned medium could be endothelial cells. This was accomplished by immunostaining cells that were cultured under myeloid-supportive conditions in the presence or absence of tumor-conditioned medium for the CD31 endothelial cell marker. As a control for CD31 expression by CD34+ progenitor cells, CD34+ cells were immunostained for CD31 after 1 day of culture. These newly seeded cultures contained few cells that stained for CD31 (Fig. 4). During the 7 days of culture with cytokines that support myeloid cell growth but in the absence of tumor-conditioned medium, few CD31+ cells developed from the CD34+ cells. In contrast, when these cultures were supplemented with tumor-conditioned medium, a greater number of CD31+ cells developed as compared to cultures without added tumor-conditioned medium. This increase in cell expression of CD31 became prominent at day 5.
The frequency of CD31+ cells that developed from CD34+ cells in the presence or absence of tumor-conditioned medium was quantitated by digital image analysis of at least 5 fields from 3 separate experiments. These analyses (Fig. 5) showed that the addition of tumor-conditioned medium significantly increased the proportion of CD31+ cells per field after 5 days (P<0.001) and 7 days (P<0.001) of culture. Thus, tumor-conditioned medium increased the absolute number of endothelial cells by both increasing the proportion of CD31+ endothelial cells as well as increasing cellularity by approximately twofold.
Fig. 5.
Quantitation of the proportion of CD31+ cells that develop from CD34+ cells in the presence of tumor-conditioned medium. CD34+ cells were seeded under conditions to support myeloid cell growth (GM–CSF and SCF) in the presence or absence of 40% tumor-conditioned medium. At various times, cultures were subjected to immunocytochemical staining for CD31-expressing cells. The proportion of positive-staining cells was digitally quantitated by MetaMorph microscopic analysis. Results shown are mean values from at least five fields enumerated in each of three separate analyses ± SEM
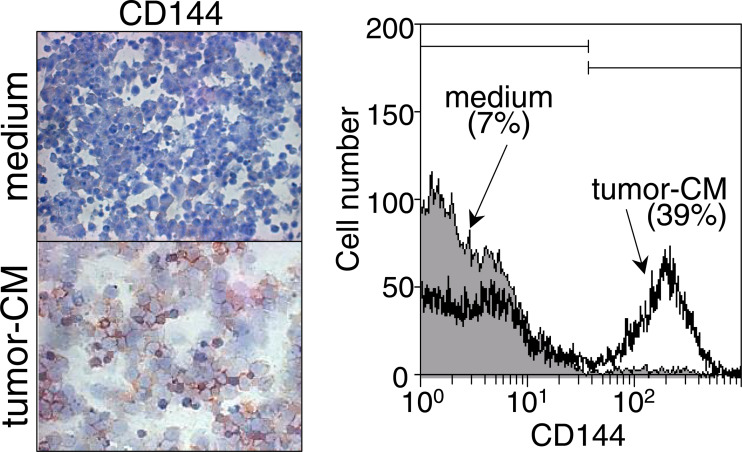
As an alternative means to determine if tumor-conditioned medium directs CD34+ cells to develop into cells that phenotypically resemble endothelial cells, cultures were immunohistochemically stained for CD144 (Fig. 6, left panel). Similar to results of CD31 staining, the presence of tumor-conditioned medium increased the number of cells staining positive for CD144. The results of immunohistochemical analyses were confirmed by flow cytometric analysis of immunostained cells (Fig. 6, right panel). The results of the above proliferation and phenotypic analyses showed that tumor-conditioned medium stimulates an early increase in proliferation, and a more prominent increase in CD34+ cell differentiation into cells that phenotypically resemble endothelial cells.
Fig. 6.
Appearance of CD144+ cells in the presence of tumor-conditioned medium. CD34+ cells were seeded under conditions to support myeloid cell growth in the presence or absence of 40% tumor-conditioned medium. After 7 days, CD144+ endothelial cells were detected by immunohistochemical staining (left panels) or by flow cytometric analysis of immunofluorescent-stained cells (right panel)
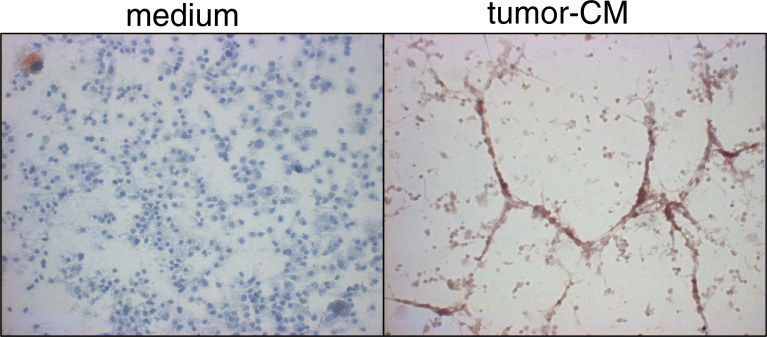
In addition to the phenotypic analyses described above, analyses were conducted to determine if tumors skew development of CD34+ cells into cells with functional characteristics of endothelial cells. This was accomplished by culturing cells for 7 days under conditions that support myeloid cell development in the presence or absence of tumor-conditioned medium, and then examining cultures for their ability to reorganize into cord structures. Formation of cord structures was stimulated by subculturing cells during the last 24 h on Matrigel. Cells were then immunostained with CD31 antibody to localize the CD34+-cell-derived endothelial cells. The onset of this reorganization was visible within 4 h of addition of Matrigel (not shown) and was prominent at 24 h (Fig. 7, right panel). Cells that had been cultured in the absence of tumor-conditioned medium were more uniformly distributed throughout the culture (Fig. 7, left panel). While the reorganization of cells that had been cultured in tumor-conditioned medium into larger structures on Matrigel was prominent, these structures were composed of large multilayers of cells. Consequently, studies were also conducted in which the cells that developed from CD34+ cells in the presence of tumor-conditioned medium were seeded at a tenfold lower density onto Matrigel. This resulted in a rapid reorganization into elongated cells in an end-to-end configuration that is characteristically seen of mature endothelial cells (Fig. 8). In combination with the analyses described above, these studies indicate that tumor-conditioned medium skews CD34+ cell differentiation into cells that phenotypically and functionally resemble endothelial cells.
Fig. 7.
Cells derived from CD34+ cells that were cultured in the presence of tumor-conditioned medium reorganize into cord structures on Matrigel. CD34+ cells were seeded under conditions to support myeloid cell growth (GM–CSF and SCF) in the presence or absence of 40% tumor-conditioned medium. After 7 days, they were detached and seeded onto Matrigel-coated wells. After 24 h, CD31+ cells were detected by immunocytochemical staining
Fig. 8.
Formation of cord structures by cells derived from CD34+ cell cultures containing tumor-conditioned medium. Procedures for cell cultures were the same as for Fig. 7, except cells were diluted to 105 cells/ml before being seeded onto Matrigel-coated plates
Identification of the tumor-derived mediators that skew CD34+ cell differentiation into CD31+ endothelial cells
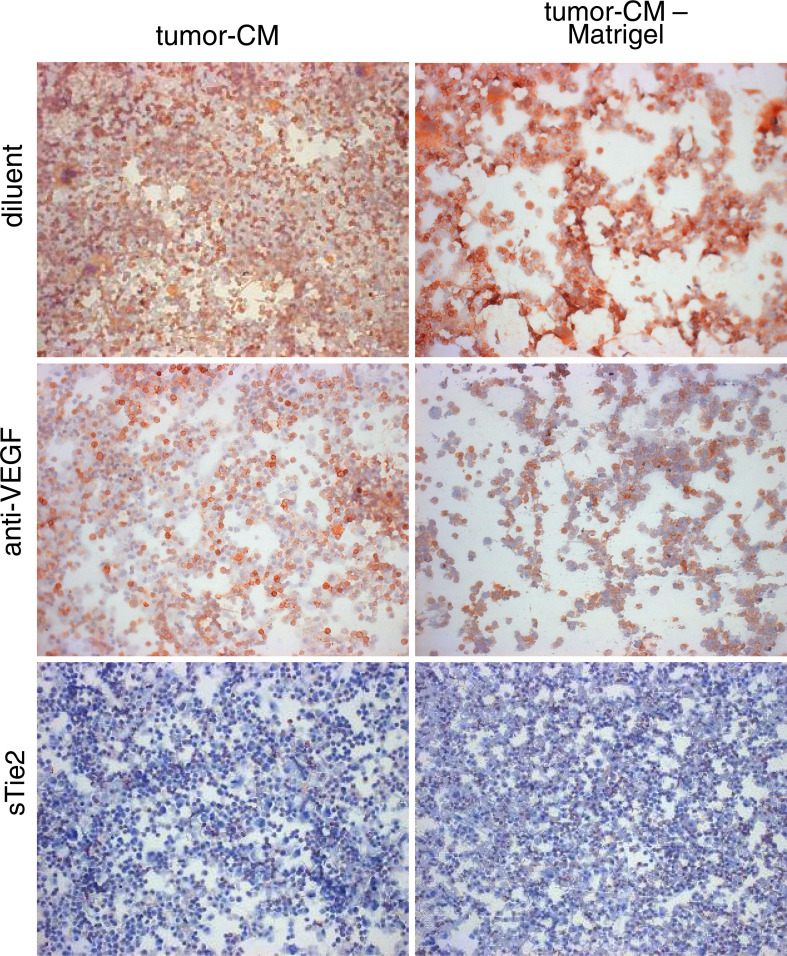
Several mediators were considered as candidates by which LLC tumor cells skewed CD34+ cell development into CD31+. VEGF was considered as a candidate based on the above studies showing that it was the prominent mediator by which tumor cells stimulated expansion of CD34+ cell cultures. Whether tumor skewing of CD34+ cell differentiation into endothelial cells mediated through VEGF was assessed by determining if it was blocked with neutralizing VEGF antibody. As shown in the studies described above, tumor-conditioned medium skewed CD34+ cell differentiation into cells staining positive for the CD31 endothelial cell marker, despite culture conditions to facilitate myeloid cell development (Fig. 9, top row). The addition of neutralizing VEGF antibody to tumor-conditioned medium did not alter the endothelial cell skewing since a high proportion of CD34+ cells developed into cells that stained strongly for CD31 (Fig. 9, middle row). This was not due to the inability to neutralize VEGF since addition of VEGF antibody was effective at reducing cellularity of the CD34+ cell cultures.
Fig. 9.
Assessing the roles of VEGF and the Tie-2 pathway on tumor skewing of CD34+ cells into CD31+ endothelial cells. CD34+ cells were seeded under conditions to support myeloid cell growth in the presence or absence of 40% tumor-conditioned medium which had been admixed with neutralizing VEGF antibody or sTie-2-Fc. After 7 days, cells were detached and seeded onto Matrigel-coated wells. After 24 h, CD31+ cells were detected by immunocytochemical staining
The possibility that angiopoietins could be the tumor-derived mediators which skew progenitor cell differentiation into endothelial cells was assessed by determining the effect of the Tie-2 decoy, sTie-2, on the development of endothelial cells in CD34+ cell cultures containing tumor-conditioned medium. The addition of sTie-2 to the tumor-conditioned medium diminished the number of CD31+-staining endothelial cells that developed from the CD34+ cell cultures (Fig. 9, bottom row). The cultures containing tumor-conditioned medium plus either diluent or sTie-2 were then added to Matrigel-coated plates to determine the effect of sTie-2 on the formation of cord structures. In the presence of tumor-conditioned medium, the CD34+ cells developed into CD31-staining cells that reorganized on Matrigel into cord structures (Fig. 9). When sTie-2 was added to the tumor-conditioned medium, the percentage of cells that stained positive for CD31 was reduced and the formation of cord structures was blocked. These studies show the importance of the Tie-2 pathway in skewing CD34+ cell differentiation into CD31+ cells, and suggest a role for tumor-derived angiopoietins in this process.
Discussion
Tumor vascularization has generally been considered to result from tumor stimulation of angiogenesis. The angiogenic factors that have been shown to be released from tumors include, but are not limited to, VEGF, TGFβ, bFGF, and PGE2 [11, 25–27]. Studies with models of ischemia have led to the recognition that postnatal vasculogenesis can also be involved in the process of vascularization [13, 24]. However, few studies have examined whether vasculogenesis accompanies angiogenesis in the process of tumor vascularization. The present study demonstrates that tumor-conditioned medium can redirect the pathway of progenitor cell differentiation from a pathway that results in a high proportion of dendritic cells into cells that phenotypically and functionally resemble endothelial cells. Tumor-derived VEGF contributed to increased cellularity in the cultures derived from CD34+ progenitor cells, but was not responsible for skewing differentiation of CD34+ cells toward endothelial cells. In contrast, differentiation into endothelial cells was mediated through the Tie-2 pathways, suggesting the involvement of tumor-derived angiopoietins.
That tumor-conditioned medium redirects differentiation of CD34+ cells from developing into dendritic cells instead of becoming endothelial cells was shown using multiple confirmatory approaches. First, the demonstration that the presence of tumor-conditioned medium diminishes levels of dendritic cells was shown by both flow cytometry and functionally by the reduced levels of IL-12 release. Demonstration that tumor-conditioned medium increases development of endothelial cells from CD34+ cells was shown by microscopic kinetic analyses of immunocytochemically stained cells, flow cytometric analysis of immunostained cells, and use of several different antibodies against distinct surface markers of endothelial cells. Functional analyses showed cells that developed in tumor-conditioned medium acquired the capacity to reorganize into cords upon exposure to Matrigel. When examined at high density, these structures were large multilayered cell structures containing CD31-staining cells. However, when the cells that developed from the CD34+ cell cultures were seeded at low density on Matrigel, they formed the elongated cord structures that are characteristics of mature endothelial cells. There is the possibility that the endothelial cells in the cultures containing tumor-conditioned medium arose from contaminating endothelial cells. While this is a possibility, the extent to which it contributes to the prominent appearance of endothelial cells in the cultures containing tumor-conditioned medium is lessened by the absence of detectable cells with an endothelial cell morphology or cells that immunostain for the endothelial cell marker CD31 in the CD34+ cells at early time points following their seeding into culture.
The present report is the second of a series that aims to dissect how tumor-conditioned medium influences the contribution of CD34+ cells toward tumor vascularization. In our prior study [28], we had shown that tumor-conditioned medium is chemotactic for CD34+ cells and this chemotactic activity is mediated through tumor-derived VEGF. The present study shows that VEGF is not only chemotactic for CD34+ cells, but it is the dominant mediator in tumor-conditioned medium of CD34+ cell proliferation. Like the present study, the prior study also showed that the presence of tumor-conditioned medium skews CD34+ cell differentiation into cells that phenotypically resemble endothelial cells. The present study extends these prior studies by showing both phenotypically and functionally that this skewing is at the expense of development of dendritic cells. When cultured under conditions that would otherwise yield significant levels of dendritic cells, the addition of tumor-conditioned medium results in a decline in the levels of cells that express the dendritic cell marker CD205, and diminishes the production of IL-12 from these cultures. This study also extends the prior study by showing that the cells which develop in the cultures containing tumor-conditioned medium exhibit functions of endothelial cells, namely the capacity to reorganize into cord structures. The results of the present functional analyses demonstrating that the formation of these cord structures is dependent on the Tie-2 pathway and extends the results of our prior studies showing that the presence of antibodies to angiopoietin-1 blocks development of cells that phenotypically resemble endothelial cells.
While various studies have tested multiple treatment approaches by which to block tumor-stimulated vascularization, they have targeted the steps of the process of angiogenesis and have generally not considered vasculogenesis. However, in a recent study, endostatin was suggested to have the potential to interfere in vasculogenesis since it diminished the number of circulating endothelial progenitor cells that were mobilized in response to treatment of mice with VEGF [21]. Not known is the relative contribution of angiogenesis versus vasculogenesis in the formation of the tumor vasculature. There are several fundamental requirements that are likely to overlap between the processes of angiogenesis and vasculogenesis. Among these is the requirement for shared soluble mediators of vascularization, cell proliferation, motility, and reorganization into new vessel structures. For example, VEGF, angiopoietins, and TGF-β are important in both angiogenesis and prenatal vasculogenesis [1, 7]. The role of angiopoietins-1 and −2 in these processes is complicated as they have opposing effects on maturation and stability of vessels [15]. The capacity of endothelial precursor cells to give rise to cord formation that is similar to that seen for mature endothelial cells suggests that the treatment approaches to block angiogenesis can also diminish vasculogenesis [9, 21].
The demonstration that tumor can skew differentiation of progenitor cells into endothelial cells could have multiple impacts. The first is that vasculogenesis could further facilitate vascularization, which is required for tumor growth to progress. The second possible impact is that the redirection of progenitor cell differentiation from a myeloid to endothelial cell lineage could limit potential antitumor immune reactivity. Under the culture conditions used in the absence of tumor-conditioned medium, a significant proportion of CD34+ cells develops into dendritic cells. We had previously shown such dendritic cells to be functional as antigen-presenting cells [16, 17]. The potential antigen-presenting capability of dendritic cells has led to their use in tumor vaccine strategies. However, the redirection of cellular differentiation into endothelial cells could contribute to the immune dysfunction that is characteristic of cancer patients and could lead to a failure in effectiveness of dendritic cells in stimulating antitumor immune reactivity. In fact, several studies have shown cancer patients to have defects in maturation of myeloid cells, a reduced number of mature dendritic cells, and associated suppressed immune capabilities [3, 4].
The present study showed that tumors can redirect differentiation of progenitors that are cultured under conditions to give rise to myeloid and a significant number of dendritic cells toward cells that phenotypically and functionally resemble endothelial cells. Also, these studies further dissected some of mediators that could contribute to tumor-stimulated vasculogenesis. Prior studies had shown the importance of tumor-derived VEGF in chemoattracting CD34+ progenitor cells [6, 29]. However, the present study showed that VEGF is also important in mediating the tumor-induced proliferation of CD34+ cell cultures, although it was not important in redirecting differentiation of these cells into endothelial cells. Instead, differentiation was skewed toward endothelial cells by Tie-2-dependent pathways. The levels of LLC production of angiopoetin-1 versus angiopoietin-2, and their relative contributions to tumor skewing of CD34+ cells toward endothelial cells are currently under study. Also being examined is the extent to which tumor vascularization can be blocked by interfering in the Tie-2 pathway in vivo.
Acknowledgements
This work was supported by the Medical Research Service of the Department of Veterans Affairs, and by grants CA97813 and CA85266 from the National Institutes of Health (MRIY).
References
- 1.Achen MG, Stacker SA. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int J Exp Pathol. 1998;79:255–265. doi: 10.1046/j.1365-2613.1998.700404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185:111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 4.Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, Carbone DP, Gabrilovich DI. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Banich JC, Kolesiak K, Young MR. Chemoattraction of CD34+ progenitor cells and dendritic cells to the site of tumor excision as the first step of an immunotherapeutic approach to target residual tumor cells. J Immunother. 2003;26:31–40. doi: 10.1097/00002371-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Beck LJ, D’Amore PA. Vascular development: cellular and molecular regulation. FASEB J. 1997;11:365–373. [PubMed] [Google Scholar]
- 8.Dutt P, Wang JF, Groopman JE. Stromal cell-derived factor-1 alpha and stem cell factor/kit ligand share signaling pathways in hemopoietic progenitors: a potential mechanism for cooperative induction of chemotaxis. J Immunol. 1998;161:3652–3658. [PubMed] [Google Scholar]
- 9.Feraud O, Cao Y, Vittet D. Embryonic stem cell-derived embryoid bodies development in collagen gels recapitulates sprouting angiogenesis. Lab Invest. 2001;81:1669–1681. doi: 10.1038/labinvest.3780380. [DOI] [PubMed] [Google Scholar]
- 10.Garrity T, Pandit R, Wright MA, Benefield J, Young MRI. Increased presence of CD34+ cells in the peripheral blood of head and neck cancer patients and their differentiation into CD1a+ cells. Int J Cancer. 1997;73:663–669. doi: 10.1002/(SICI)1097-0215(19971127)73:5<663::AID-IJC9>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 11.Gupta RA, Tejada LV, Tong BJ, Das SK, Morrow JD, Dey SK, DuBois RN. Cyclooxygenase-1 is overexpressed and promotes angiogenic growth factor production in ovarian cancer. Cancer Res. 2003;63:906–911. [PubMed] [Google Scholar]
- 12.Hirashima M, Kataoka H, Nishikawa S, Matsuyoshi N. Maturation of embryonic stem cells into endothelial cells in an in vitro model of vasculogenesis. Blood. 1999;93:1253–1263. [PubMed] [Google Scholar]
- 13.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103:1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CH, Broxmeyer HE. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood. 1998;91:100–110. [PubMed] [Google Scholar]
- 15.Koga K, Todaka T, Morioka M, Hamada J, Kai Y, Yano S, Okamura A, Takakura N, Suda T, Ushio Y. Expression of angiopoietin-2 in human glioma cells and its role for angiogenesis. Cancer Res. 2001;61:6248–6254. [PubMed] [Google Scholar]
- 16.Lathers DM, Achille N, Young MR. Dendritic cell development from mobilized peripheral blood CD34+ cells. Methods Mol Biol. 2003;215:409–415. doi: 10.1385/1-59259-345-3:409. [DOI] [PubMed] [Google Scholar]
- 17.Lathers DMR, Achille N, Kolesiak K, Hulett K, Sparano A, Young MRI. Increased levels of immune inhibitory CD34+ progenitor cells in the peripheral blood of patients with node positive head and neck cancer and the ability of the CD34+ cells to differentiate into antigen presenting dendritic cells. Clin Cancer Res. 2001;125:205–212. doi: 10.1067/mhn.2001.117871. [DOI] [PubMed] [Google Scholar]
- 18.Lathers DMR, Lubbers E, Wright MA, Young MRI. Dendritic cell differentiation pathways of CD34+ cells from the peripheral blood of head and neck cancer patients. J Leukoc Biol. 1999;65:623–628. doi: 10.1002/jlb.65.5.623. [DOI] [PubMed] [Google Scholar]
- 19.Melani C, Stoppacciaro A, Foroni C, Felicetti F, Care A, Colombo MP. Angiopoietin decoy secreted at tumor site impairs tumor growth and metastases by inducing local inflammation and altering neoangiogenesis. Cancer Immunol Immunother. 2004;53:600–608. doi: 10.1007/s00262-004-0500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schofield KP, Rushton G, Humphries MJ, Dexter TM, Gallagher JT. Influence of interleukin-3 and other growth factors on α4β1 integrin-mediated adhesion and migration of human hematopoietic progenitor cells. Blood. 1997;90:1858–1866. [PubMed] [Google Scholar]
- 21.Schuch G, Heymach JV, Nomi M, Machluf M, Force J, Atala A, Eder JP, Jr, Folkman J, Soker S. Endostatin inhibits the vascular endothelial growth factor-induced mobilization of endothelial progenitor cells. Cancer Res. 2003;63:8345–8350. [PubMed] [Google Scholar]
- 22.Shirakawa K, Furuhata S, Watanabe I, Hayase H, Shimizu A, Ikarashi Y, Yoshida T, Terada M, Hashimoto D, Wakasugi H. Induction of vasculogenesis in breast cancer models. Br J Cancer. 2002;87:1454–1461. doi: 10.1038/sj.bjc.6600610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM. VEGF gene delivery to muscle: potential role for vasculogenesis in adults. Mol Cell. 1998;2:549–558. doi: 10.1016/S1097-2765(00)80154-9. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/8462. [DOI] [PubMed] [Google Scholar]
- 25.Tanaka F, Ishikawa S, Yanagihara K, Miyahara R, Kawano Y, Li M, Otake Y, Wada H. Expression of angiopoietins and its clinical significance in non-small cell lung cancer. Cancer Res. 2002;62:7124–7129. [PubMed] [Google Scholar]
- 26.Tsuda S, Ohtsuru A, Yamashita S, Kanetake H, Kanda S. Role of c-Fyn in FGF-2-mediated tube-like structure formation by murine brain capillary endothelial cells. Biochem Biophys Res Commun. 2002;290:1354–1360. doi: 10.1006/bbrc.2002.6345. [DOI] [PubMed] [Google Scholar]
- 27.Vinals F, Pouyssegur J. Transforming growth factor β1 (TGF-β1) promotes endothelial cell survival during in vitro angiogenesis via an autocrine mechanism implicating TGF-α signaling. Mol Cell Biol. 2001;21:7218–7230. doi: 10.1128/MCB.21.21.7218-7230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Young MR. Tumor skewing of CD34+ progenitor cell differentiation into endothelial cells. Int J Cancer. 2004;109:516–524. doi: 10.1002/ijc.20003. [DOI] [PubMed] [Google Scholar]
- 29.Young MR, Kolesiak K, Wright MA, Gabrilovich DI. Chemoattraction of femoral CD34+ progenitor cells by tumor-derived vascular endothelial cell growth factor. Clin Exp Metastasis. 1999;17:881–888. doi: 10.1023/A:1006708607666. [DOI] [PubMed] [Google Scholar]
- 30.Young MR, Wright MA, Lathers DM, Messingham KA. Increased resistance to apoptosis by bone marrow CD34+ progenitor cells from tumor-bearing mice. Int J Cancer. 1999;82:609–615. doi: 10.1002/(SICI)1097-0215(19990812)82:4<609::AID-IJC23>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 31.Young MR, Young ME, Kim K. Regulation of tumor-induced myelopoiesis and the associated immune suppressor cells in mice bearing metastatic Lewis lung carcinomas by prostaglandin E2 . Cancer Res. 1988;48:6826–6831. [PubMed] [Google Scholar]
- 32.Young MRI, Halpin J, Wang J, Wright MA, Matthews J, Schmidt-Pak A. 1α,25-dihydroxyvitamin D3 plus γ-interferon blocks lung tumor production of granulocyte-macrophage colony-stimulating factor and induction of immunosuppressor cells. Cancer Res. 1993;53:6006–6010. [PubMed] [Google Scholar]
- 33.Young MRI, Petruzzelli G, Kolesiak K, Lathers DMR, Lingen MW, Gabrilovich D. Human squamous cell carcinomas of the head and neck chemoattract immune suppressive CD34+ progenitor cells. Hum Immunol. 2001;62:332–341. doi: 10.1016/S0198-8859(01)00222-1. [DOI] [PubMed] [Google Scholar]
- 34.Young MRI, Schmidt-Pak A, Wright MA, Matthews JP, Collins SL, Petruzzelli G. Mechanisms of immune suppression in patients with head and neck cancer: Presence of immune suppressive CD34+ cells in cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 35.Young MRI, Wright MA. Myelopoiesis-associated immune suppressor cells in mice bearing metastatic Lewis lung carcinoma tumors: interferon-γ plus tumor necrosis factor-α synergistically reduce immune suppressor and tumor growth-promoting activities of bone marrow cells, and diminish tumor recurrence and metastasis. Cancer Res. 1992;52:6335–6340. [PubMed] [Google Scholar]
- 36.Young MRI, Wright MA, Lozano Y, Matthews JP, Benefield J, Prechel MM. Mechanisms of immune suppression in patients with head and neck cancer: influence on the immune infiltrate of the cancer. Int J Cancer. 1996;67:333–338. doi: 10.1002/(SICI)1097-0215(19960729)67:3<333::AID-IJC5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 37.Young MRI, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL, Petruzzelli GJ. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. doi: 10.1002/(SICI)1097-0215(19970220)74:1<69::AID-IJC12>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]