Abstract
Purpose: We investigated granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-12 (IL-12) infused into the injection site of irradiated tumor vaccine (TV) as therapy for gliomas. Methods: Rats with subcutaneous RT-2 gliomas were treated with irradiated TV and/or subcutaneous infusion of GM-CSF and/or IL-12 via osmotic minipump 5 days after tumor-cell inoculation. Cytotoxic T lymphocyte (CTL) and natural killer (NK) cell activity were analyzed to investigate immune responses. Rats with intracerebral gliomas were treated with irradiated TV and infused GM-CSF/IL-12 3 days after tumor-cell inoculation. Tumor growth rates and animal survival were followed. Survivors were re-challenged with wild-type RT-2 cells subcutaneously or intracerebrally to study long-term anti-tumor immunity. Results: Rats with subcutaneous gliomas treated with GM-CSF and IL-12 or TV plus GM-CSF or IL-12 did not have increased survival rate (P>0.2), but did have prolonged survival time (P<0.05); in contrast, rats treated with TV plus GM-CSF/IL-12 had increased survival rate (P<0.05) and prolonged survival time (P<0.05) compared with controls. These treatment strategies showed enhanced CTL and NK cell activities. Rats with intra-cerebral gliomas treated with TV plus GM-CSF/IL-12 did not have increased survival rate (P=0.11), but did have prolonged survival time (P<0.0001). Survivors in each group were re-challenged with wild-type RT-2 cells, and all had long-term survival. Conclusions: Irradiated TV plus continuous localized infusion of GM-CSF/IL-12 may induce a tumor-specific anti-tumor immune response on established subcutaneous or intra-cerebral gliomas, and such a treatment strategy deserves consideration as adjuvant treatment for glioma.
Keywords: Glioma, Immunotherapy, GM-CSF, IL-12, Tumor vaccine
Introduction
Patients with malignant gliomas usually demonstrate immuno-suppression including reduced T-cell response to mitogens, decreased secretion of interleukin-2 (IL-2), interleukin-12 (IL-12), interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), and tumor necrosis factor-α in activated T cells, reduced expression of high affinity IL-2 receptors in T cells, and impaired activity of natural killer (NK) cells [1, 2]. Such immuno-suppression is closely correlated with tumor progression and recurrence [3–5]. Because of host immuno-deficiency, immuno-therapy that enhances immune system’s ability to exert anti-tumor effects is considered a feasible treatment strategy for gliomas. To reach a maximal immune response and activation of cytotoxic T cells, multiple steps and many regulatory molecules such as interaction among antigen, major histo-compatibility complex (MHC), antigen-presenting cells (APCs), T cells, costimulatory molecule CD28, and cytokines are generally required [6–8]. GM-CSF, an important cytokine for enhancement of anti-tumor immune response [9–11], can activate hematopoietic progenitor cells and stimulate committed progenitors to differentiate into granulocytes and mononuclear phagocytes [12, 13]. In addition, GM-CSF shows high affinity with dendritic cells (DCs), and can stimulate DC precursors to differentiate into DCs, eventually stimulating presentation of host tumor antigen [12–14]. Furthermore, IL-12 is an important cytokine for induction of anti-tumor immunity [6, 15–18]. IL-12 is produced by APCs such as macrophages, monocytes, and DCs [19, 20], and it plays a significant role in regulation of the balance between type 1 and type 2 T helper cells (Th1 and Th2 cells) [21]. IL-12 stimulates naïve T cells to differentiate into Th1 cells, enhances Th1 immune responses, stimulates T cells and NK cells, enhances secretion of IFN-γ by these cells, activates NK/lymphokine-activated killer (NK/LAK) cells, and promotes specific cytotoxic T lymphocyte (CTL) responses and delayed-type hypersensitivity (DTH) [21–23]. The functions of GM-CSF and IL-12 indicate that they could be used to enhance host-initiated anti-tumor effects [9–11, 21–26].
Immunotherapy using either GM-CSF or IL-12 has been demonstrated to have anti-tumor effects on various cancers including malignant gliomas [6, 10, 12, 15, 17, 18, 27 and 30]. Previous studies have revealed that the combination of various immuno-regulatory strategies may have better therapeutic effects than single immunotherapy [18, 30 and–32]. However, few studies to date have investigated the effects of immuno-therapy using both GM-CSF and IL-12 on neoplasms, and the results have been inconsistent [33–35]. In addition, cytokine immunotherapy can be done as immuno-gene therapy; immunotherapy using cytokine only, etc.; these methods have inherent advantages and disadvantages [36, 37]. Immuno-gene therapy for each individual patient usually requires harvesting and culturing of tumor cells followed by gene transfer; thus, it is time-consuming, costly, and safety is a problem [38]. For immunotherapy using cytokine only, side effects of a single large dose of cytokine are major obstacles to clinical use [36, 37]. Recently, treatment with tumor vaccine (TV) and continuously infused cytokine using an osmotic mini pump to offer tumor antigen and long-term release of cytokine has been proposed as an alternate immuno-therapy method, one that might avoid the side effects of a single large dose of cytokine and one with a concept similar to that of gene- therapy [16, 38 and–40]. This treatment strategy has been found to provide anti-tumor effects on neuro-blastoma or glioma [16, 38–40]. In addition, treatment with TV and infused GM-CSF and IL-12 has shown enhanced anti-tumor effects on 9L gliomas [40]. However, all these reports involved treatment either several days before tumor-cell inoculation or simultaneously with inoculation [16, 38 and–40]. These animal models do not represent actual conditions because tumors exist clinically before any treatment is initiated. In the literature, no reports have investigated the effects of such a treatment strategy on established gliomas. More data about the effects of such a treatment strategy on different animal models, especially on established gliomas in animals, are needed before clinical application can be considered. Thus, in this study, we intended to investigate the effects of irradiated TV and continuous localized infusion of both GM-CSF and IL-12 on established subcutaneous and intra-cerebral gliomas in rats, with the goal of studying induced immune responses in the host animals.
Materials and methods
Tissue culture and cell lines
The cell line used in this study was the rat RT-2 glioma cell line, which is derived from an avian sarcoma virus-induced brain tumor in the Fischer 344 rat [41]. All cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum (FCS) at 37°C in a 5% CO2 incubator. Irradiation of RT-2 cells at 4,360 cGy from a 137Cs source inhibited cell proliferation and led to complete cell death by day 5.
Cytokines
The cytokines used in this study included recombinant murine GM-CSF purchased from Sigma Chemical Co. (Missouri, USA) and recombinant murine IL-12 purchased from PeproTech EC Ltd. (London, UK). As previous studies had demonstrated that 10 ng/kg GM-CSF had a stronger anti-tumor effect on neuro-blastoma or glioma than 1 ng/kg GM-CSF [38–40] and 1 ng/kg of IL-12 has been demonstrated to have a stronger anti-tumor effect on gliomas than 10 ng/kg IL-12 [16], we used 10 ng/day GM-CSF and 1 ng/kg IL-12 in this study. Both cytokines were diluted with phosphate buffered saline (PBS) containing 0.1% bovine serum albumin (Biochrom KG, Berlin, Germany) before placement into the osmotic mini pump (Model 2002, Alza Co., Palo Alto, CA, USA).
Animals
Animal experiments were approved by the Committee on Laboratory Animal Research of the College of Medicine of the National Taiwan University Hospital, Taiwan, and conducted according to the guidelines of the Laboratory Animal Center of the National Taiwan University College of Medicine. Fischer rats weighing 200–350 g each were used for experiments. The rats were housed with free access to food and water on a 12:12 h day–night cycle (lights on between 0600 and 1800 h) with room temperature maintained around 20°C. Rats were anesthetized with intraperitoneal injection of 10 mg/kg xylazine and 80 mg/kg ketamine hydrochloride. Animals were put to euthanasia with thiopental sodium before harvesting of tumor specimens.
Continuous localized infusion of cytokines via osmotic mini pump
Rats were treated with irradiated TV and/or continuous infusion of cytokines (GM-CSF and/or IL-12) initiated on day 5 after subcutaneous implantation or on day 3 after intra-cerebral implantation of RT-2 glioma cells. A continuous infusion of cytokine was delivered via an osmotic mini pump implanted subcutaneously in the left flank of the animal; the osmotic mini pump was connected to the TV injection site at the left flank through a No. 60 polyethylene tube (Clay Adams, Parsipanny, NJ, USA). The pump delivered its content at a rate of 0.5 μl/h over a total of 14 days [16, 38, 39]. The volume of the osmotic minipump was 200 μl.
Treatment of subcutaneous gliomas with various treatment strategies
Cultured log phase RT-2 cells (1×105) in 10 μl PBS were injected subcutaneously into the shaved right flank of Fischer rats. Rats were treated with irradiated TV and continuously infused cytokines (GM-CSF and/or IL-12) via osmotic mini pump starting on day 5 after RT-2 cell implantation. The experiments consisted of 9 groups with 10 rats in each group. Group A (No Tx) was a control group of rats that received no treatment. Group B (Saline) was treated with a continuous infusion of PBS via osmotic minipump. Group C (TV) was treated with a subcutaneous injection of five doses of irradiated TV (1×106 irradiated RT-2 cells in each dose on days 5, 8, 11, 14, and 17 after tumor-cell implantation) in the left flank. Group D (GM) was treated with a continuous infusion of GM-CSF (10 ng/day) via osmotic mini pump. Group E (IL-12) was treated with a continuous infusion of IL-12 (1 ng/day) via osmotic mini pump. Group F (GM + IL-12) was treated with a continuous infusion of GM-CSF and IL-12 via osmotic mini pump. Group G (TV + GM) was treated with irradiated TV as in Group C combined with a continuous infusion of GM-CSF via osmotic mini pump. Group H (TV + IL-12) was treated with five doses of irradiated TV as in Group C combined with a continuous infusion of IL-12 via osmotic mini pump. The final group, Group I (TV + GM + IL-12) was treated with five doses of irradiated TV combined with a continuous infusion of GM-CSF and IL-12 via osmotic mini pump. Animal survival time and survival rate were recorded and compared among groups.
Observation of tumor growth rates and animal survival
Growth rates of subcutaneous tumors were monitored; tumor size was measured weekly until each rat died. A blinded observer measured tumor length and width. Tumor volume was calculated from the formula V = 1/2(d1 × d2 × d3), where d1, d2, and d3 were tumor diameters measured with calipers in mutually perpendicular directions. Average daily tumor volumes from each group were compared throughout the course of the experiment. Group averages were not compared after one or more animals in the group died.
Cytotoxic T lymphocyte assay
After rats had been inoculated subcutaneously with 1×105 RT-2 cells and treated with a treatment strategy, spleens were harvested on day 21 after tumor-cell inoculation. Each group consisted of three rats. The cytotoxic activity of activated spleen cells was tested in vitro in a standard 51Cr-release assay. In vitro stimulation was conducted by culturing 2×106 splenocytes with 1×105 irradiated RT-2 cells per well on 24-well plates for 5 days at 37°C in the presence of recombinant human IL-2 (10 IU/ml). Target cells (RT-2 cells) were 51Cr-labeled for 1 h and then washed extensively with RPMI medium. The labeled target cells (1×104 cells) were mixed with effector cells at the effector-to-target (E/T) ratio indicated on 96-well U-bottomed plates. Mixtures were incubated at 37°C for 4 h, after which released 51Cr radioactivity was measured in 100-μl aliquots of supernatant. All determinations were made in triplicate; the percentage of lysis was calculated using the formula [(experimental cpm - spontaneous cpm)/(maximum cpm - spontaneous cpm)] × 100% (cpm, counts per minute).
NK cell assay
After rats had been inoculated subcutaneously with 1×105 RT-2 cells and treated with a treatment strategy, spleens were harvested on day 21 after tumor-cell inoculation. Each group consisted of three rats. Single cell suspensions of splenocytes were made and co-cultured with 51Cr-labeled YAC-1 cells (American Tissue Type Culture, Rockville, MD, USA) at 100:1 E/T ratio for 10–12 h. YAC-1 lysis was determined by chromium release into supernatant as measured by a scintillation counter. Percent specific lysis was calculated as described for CTL assays.
Treatment of intra-cerebral gliomas with TV plus continuously infused GM-CSF and IL-12
Intra-cerebral tumor was induced by implanting tumor cells into the brain of Fischer 344 rats by stereotactic surgery. There were ten rats in each group. Each rat was fixed in a stereotactic frame after anesthesia, a burr hole was drilled, and tumor cells were injected into the right caudate-putamen (coordinates: 2.5 mm lateral, 1 mm anterior to the bregma, 4 mm below the dura) via a Hamilton syringe. Typically, 5×103 tumor cells were suspended in 5 μl PBS. Injection was accomplished over 3 min with the syringe remaining in place for another 3 min; it was then slowly withdrawn for a third 3-min period. These rats received either no treatment (Group A-1) or received five doses of irradiated TV combined with continuously infused GM-CSF (10 ng/kg) and IL-12 (1 ng/day) via osmotic mini pump, with treatment initiated 3 days after tumor-cell inoculation (Group I-1). The difference in animal survival rate and survival time between these two groups was compared. Brains were harvested when animals died, and tumor size was measured.
Immuno-histochemical staining of the tumors treated with tumor vaccine plus GM-CSF and IL-12
Rats bearing intra-cerebral tumors were treated with no treatment (Group A-1) or TV plus GM-CSF and IL-12 (Group I-1), and were sacrificed on day 21 after tumor-cell inoculation. The brains included the tumors were removed and embedded in AMES ornithine carbamyl transferase embedding compound (Miles, Elkhart, IN, USA) and frozen at −70°C. For immuno-histochemical analysis, 10-μm cryostat tissue sections were air dried at room temperature for 1 h, fixed in acetone at 4°C for 5 min, washed with PBS, and then incubated with 3% H2O2 (in methanol) for 30 min. The sections were then incubated in a blocking solution for 30 min, followed by incubation with specific antibodies that were diluted with 1% bovine serum albumin in PBS at an optimal concentration as suggested by the manufacturer. Mouse anti-rat antibodies to cytotoxic T cells (CD8a, MRC OX-8) and NK cells (CD161, 10/78) (Serotec, Kidlinton, Oxford, UK) were used in this study. The antibodies were layered onto the section and incubated at 4°C for 12 h. After two washes with PBS, sections were incubated with a secondary antibody. The sections were washed and processed by the avidin–biotin peroxidase method. The slides were then counterstained with hematoxylin, mounted, coverslipped, and viewed under a light microscope.
Long-term anti-tumor immunity
To assess long-term anti-tumor immunity, surviving animals with subcutaneous gliomas (regardless of treatment) were re-challenged with subcutaneous inoculation of 1×105 RT-2 cells 150 days after the initial tumor-cell inoculation. In addition, surviving animals with intra-cerebral gliomas were re-challenged with intra-cerebral inoculation of 5×103 RT-2 cells into the left hemisphere 100 days after initial tumor-cell inoculation. Tumor growth and animal survival were followed.
Statistical analyses
Tumor size was compared using analysis of variance and post hoc Scheffe’s multiple comparisons. The Fischer exact test was used to analyze animal survival rates; the Kaplan–Meier method was used to assess the animal survival time; and the log-rank statistic was used to test differences between groups. P values of <0.05 were considered to be statistically significant.
Results
Effects of irradiated TV and/or continuous infusion of cytokines (GM-CSF and/or IL-12) on tumor growth and survival of rats with subcutaneous gliomas
The survival rate of rats with subcutaneous gliomas undergoing each of the various treatments was analyzed. All rats in Groups A (No Tx), B (Saline), C (TV), D (GM), and E (IL-12) died, with survival times of 43.3±4.3 [mean ± standard deviation (SD)], 45.1±3.8, 46.2±6.5, 48.1±9.6, and 45.3±11.9 days, respectively. In contrast, Groups F (GM + IL-12), G (TV + GM), and H (TV + IL-12) had 20% long-term survival, and Group I (TV + GM + IL-12) had 40% long-term survival. There was no difference in survival rate among the eight groups A–H (P>0.2). The survival rate of Group I was significantly higher than that of Groups A, B, C, D, and E (P=0.04), but was not significantly different from that of Groups F, G, and H (P>0.3). Animal survival time among various groups was analyzed as described below. Tumor size among groups receiving one of the various treatment strategies showed no difference on or before day 28 after tumor-cell inoculation (P>0.05). Further, group averages were not compared after day 35 post tumor-cell inoculation because animals had died after this time point; thus, only the statistics of tumor size on day 35 are shown below.
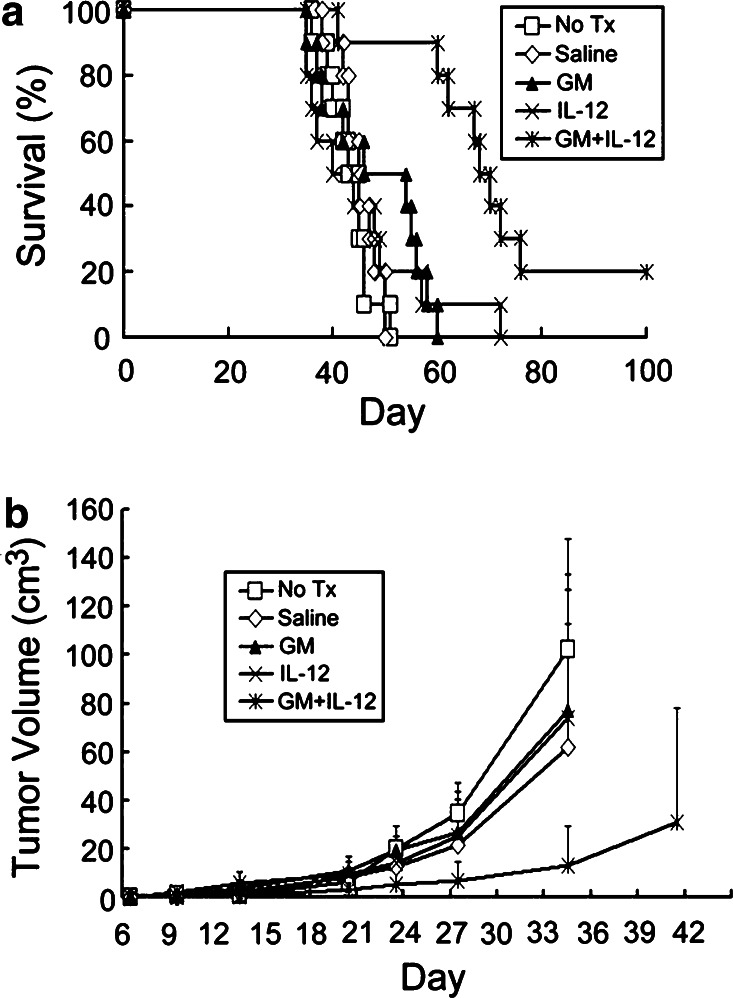
Effects of continuously infused GM-CSF and IL-12 on survival time and tumor growth in rats with subcutaneous gliomas
Animal survival (Fig. 1a) and tumor growth rate (Fig. 1b) of rats with subcutaneous gliomas treated with GM-CSF and/or IL-12 were analyzed. There was no difference in survival time among Groups A (No Tx), B (Saline), D (GM), and E (IL-12) (P>0.11). In contrast, Group F (GM + IL-12) had significantly longer survival time than the other four groups (P<0.003), although survival rate was not significantly different among these five groups (P>0.23). Tumor size on day 35 among these five groups was further analyzed, and we found that tumor size in Group F (GM + IL-12) was significantly smaller than that in Groups A, B, D, and E (P<0.05). The results indicated that continuously infused GM-CSF or IL-12 only had no anti-tumor effects on the subcutaneous gliomas; however, continuous infusion of both GM-CSF and IL-12 had anti-tumor effects on the gliomas.
Fig. 1.
Survival curves and tumor growth curves for rats with subcutaneous gliomas treated with GM-CSF and/or IL-12. Rats were implanted with RT-2 cells subcutaneously and treatment began 5 days after inoculation. Treatments included no treatment (No Tx, Group A), continuous infusion of phosphate buffered saline (Saline, Group B), continuous infusion of GM-CSF (GM, Group D), continuous infusion of IL-12 (IL-12, Group E), or continuous infusion of GM-CSF and IL-12 (GM + IL-12, Group F). a Animal survival curves. Fischer exact test was used to analyze survival rates; the Kaplan–Meier method was used to assess survival time; and the log-rank statistic was used to test differences between groups. Survival time, Group F vs. Group A, B, D, or E, P<0.003; Group A vs. Group B vs. Group D vs. Group E, P>0.11). b Tumor growth rates. Each point represents the average volume (cm3) of tumor in each of the five groups. Daily average tumor volumes from each group were compared throughout the course of the experiment using analysis of variance and post hoc Scheffe’s multiple comparison. Group averages were not compared after one or more animals died (>35 days after tumor-cell inoculation). No difference was found among various groups in tumor size on or before 28 days after tumor cell inoculation (P>0.05). Tumor size at 35 days post tumor-cell inoculation, Group F vs. Group A, B, D, or E, P<0.05; Group A vs. Group B vs. Group D vs. Group E, P>0.05
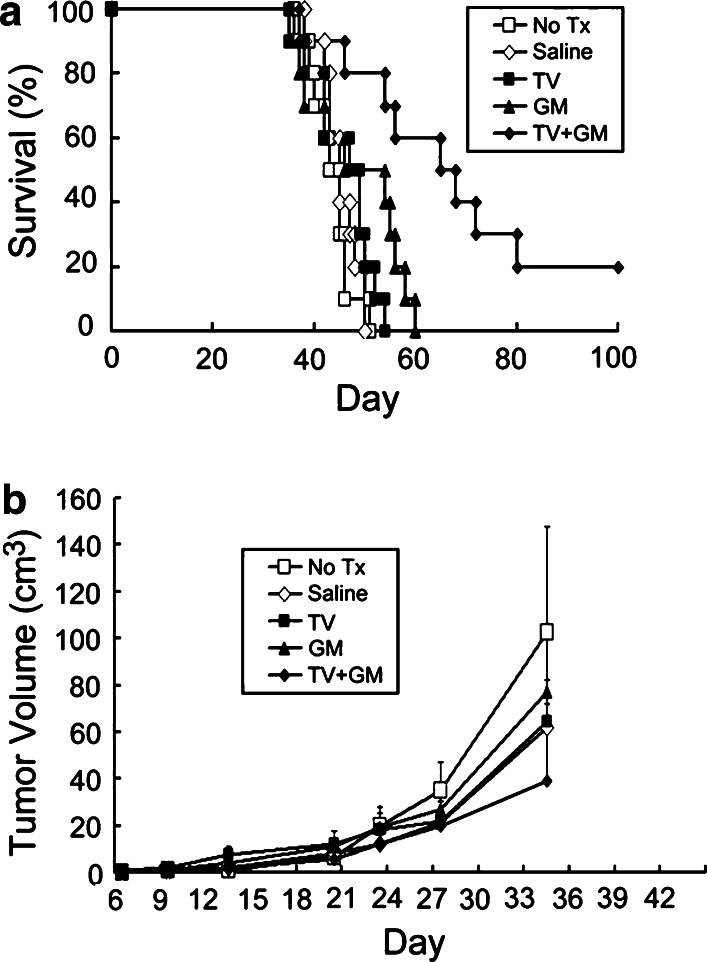
Effects of combined irradiated TV and continuously infused GM-CSF on survival time and tumor growth in rats with subcutaneous gliomas
Animal survival and tumor growth rate in rats with subcutaneous gliomas undergoing treatment with TV and/or GM-CSF were analyzed (Fig. 2). There was no difference in survival time among Groups A (No Tx), B (Saline), C (TV), and D (GM) (P>0.11). The survival time of Group G (TV + GM) was significantly longer than that of Group A, B, or D (P<0.04). In addition, tumor size on day 35 in Group G was significantly smaller than that in Groups A, B, C, and D (P<0.05). The results indicated that combination of irradiated TV with continuously infused GM-CSF had stronger anti-tumor effects than infusion of GM-CSF only. However, such combination had only slightly stronger anti-tumor effects than TV only, although the tumor growth rate in the former was slower than in the latter, and there was 20% long-term animal survival in the former.
Fig. 2.
Survival curves and tumor growth curves for rats with subcutaneous gliomas treated with TV and/or GM-CSF. Rats were implanted with RT-2 cells subcutaneously and treatment began 5 days after inoculation. Treatments included no treatment (No Tx, Group A), continuous infusion of phosphate buffered saline (Saline, Group B), five doses of irradiated tumor vaccine (TV, Group C), continuous infusion of GM-CSF (GM, Group D), or five doses of irradiated TV plus GM-CSF (TV + GM, Group G). a Survival curves. Fischer exact test was used to analyze survival rates; the Kaplan–Meier method was used to assess survival time; and the log-rank statistic was used to test differences between groups. Survival time, Group G vs. Group A, B, or D, P<0.007; Group G vs. Group C, P=0.08; Group C vs. Group A or B, P<0.04; Group C vs. Group D, P=0.13; Group A vs. Group B vs. Group D, P>0.11). b Tumor growth rates. Each point represents the average tumor volume (cm3) in each of the five groups. Group averages were not compared after one or more animals in the group died (>35 days post tumor-cell inoculation). Tumor size at or before 28 days after tumor-cell inoculation, no difference among various groups, P>0.05. Tumor size at 35 days post tumor-cell inoculation, Group G vs. Group A, B, C, or D, P<0.05; Group A vs. Group B vs. Group C vs. Group D, P>0.05
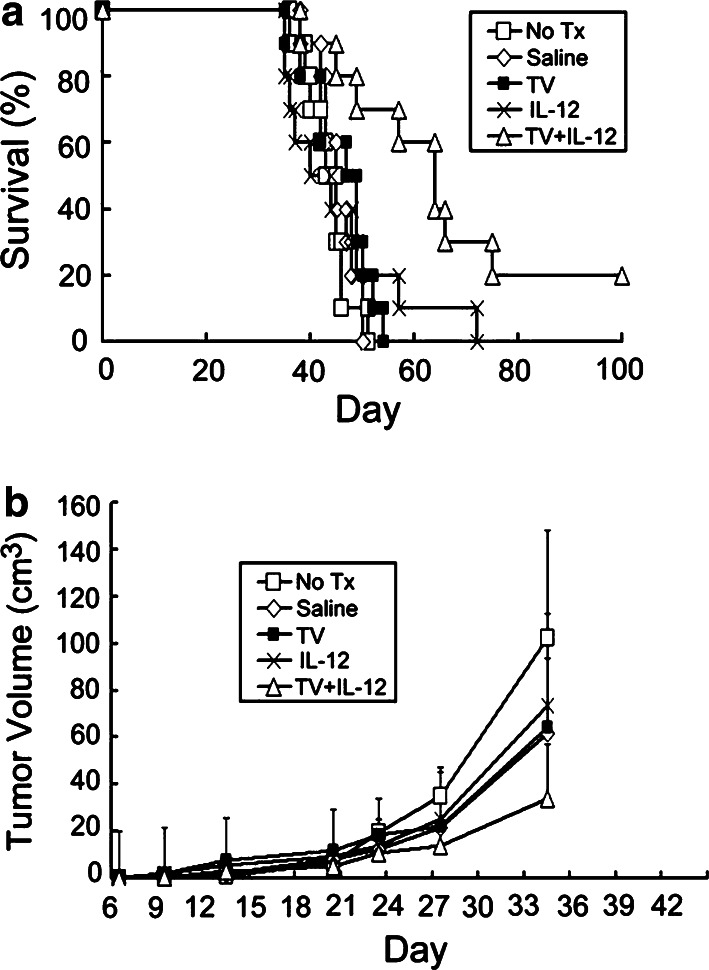
Effects of combined irradiated TV and continuously infused IL-12 on survival time and tumor growth in rats with subcutaneous gliomas
Animal survival and tumor growth rate in rats with subcutaneous gliomas undergoing treatment with TV and/or IL-12 were analyzed (Fig. 3). There was no difference in survival time among Groups A (No Tx), B (Saline), C (TV), and E (IL-12) (P>0.09). Survival time of Group H (TV + IL-12) was significantly longer than that of Group A, B, or E (P<0.05). Tumor size on day 35 in Group H was significantly smaller than that in Groups A, B, C, and E (P<0.05). The results indicated that combination of irradiated TV with continuously infused IL-12 had stronger anti-tumor effects than infusion of IL-12 only. However, such combination had only slightly stronger anti-tumor effects than TV only, although the tumor growth rate in the former was slower than in the latter, and there was 20% long-term animal survival in the former.
Fig. 3.
Survival curves and tumor growth curves for rats with subcutaneous gliomas treated with TV and/or IL-12. Rats were implanted with RT-2 cells subcutaneously and treatment began 5 days after inoculation. Treatments included no treatment (No Tx, Group A), continuous infusion of phosphate buffered saline (Saline, Group B), five doses of irradiated tumor vaccine (TV, Group C), continuous infusion of IL-12 (IL-12, Group E), or five doses of irradiated TV plus IL-12 (TV + IL-12, Group H). a Animal survival curves. Fischer exact test was used to analyze survival rates; the Kaplan–Meier method was used to assess survival time; and the log-rank statistic was used to test differences between groups. Survival time, Group H vs. Group A, B, or E, P<0.011; Group H vs. Group C, P=0.25; Group C vs. Group A or B, P<0.04; Group C vs. Group E, P=0.09; Group A vs. Group B vs. Group D, P>0.68). b Tumor growth rates. Each point represents the average tumor volume (cm3) in each of the five groups. Group averages were not compared after one or more animals in the group died (>35 days post tumor-cell inoculation). Tumor size at or before 28 days after tumor-cell inoculation, no difference among groups, P>0.05. Tumor size at 35 days post tumor-cell inoculation, Group H vs. Group A, B, C, or E, P<0.05; Group A vs. Group B vs. Group C vs. Group E, P>0.05
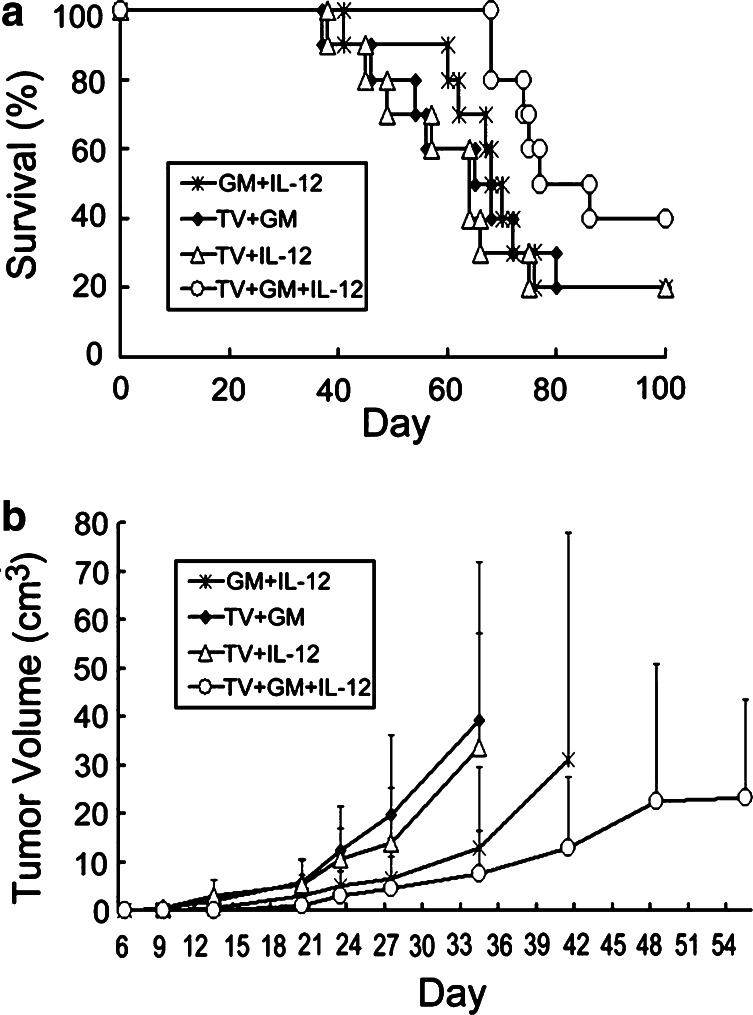
Effects of combined irradiated TV and continuously infused GM-CSF and IL-12 on survival time and tumor growth in rats with subcutaneous gliomas
Animal survival and tumor growth rate in rats with subcutaneous gliomas undergoing treatment with TV plus GM-CSF, TV plus IL-12, GM-CSF and IL-12, or TV plus GM-CSF and IL-12 were analyzed (Fig. 4). There was no difference in survival time among Groups F (GM + IL-12), G (TV + GM), and H (TV + IL-12) (P>0.1). In contrast, Group I (TV + GM + IL-12) had significantly longer survival time than the other three groups (P<0.05), although animal survival rate was not different, as was described above (40% vs. 20%). In addition, Group I had significantly longer survival time than Group A, B, C, D, or E (P<0.0001). Tumor size on day 35 among these four groups was further analyzed, and we found that Group I had significantly smaller tumor than Groups F and G (P<0.05), but not smaller than Group H (P=0.28). The results indicated that combination of irradiated TV with continuously infused GM-CSF and IL-12 had stronger anti-tumor effects than combination of two cytokines, TV plus GM-CSF, or TV plus IL-12. Further, we found that Group G (TV + GM) had longer survival time than Group E (IL-12) (P=0.01), and Group H (TV + IL-12) had longer survival time than Group D (GM) (P=0.007). However, there was no difference in survival time among Groups F (GM + IL-12), G, and H (P>0.1). The data indicated that irradiated TV plus continuous infusion of one cytokine exerted stronger anti-tumor effect on the subcutaneous gliomas than infusion of cytokine only, irrespective of GM-CSF or IL-12.
Fig. 4.
Survival curves and tumor growth curves for rats with subcutaneous gliomas undergoing various combination treatments with TV plus GM-CSF and/or IL-12, or combination treatment with GM-CSF and IL-12. Rats were implanted with RT-2 cells subcutaneously and treatment began 5 days after inoculation. Treatments included continuous infusion of GM-CSF and IL-12 (GM + IL-12, Group F), five doses of irradiated TV plus GM-CSF (TV + GM, Group G), five doses of irradiated TV plus IL-12 (TV + IL-12, Group H), and five doses of irradiated TV plus GM-CSF and IL-12 (TV + GM + IL-12, Group I). a Animal survival curves. Survival time, Group I vs. Group F, G, or H, P<0.05; Group F vs. Group G vs. Group H, P>0.6. b Tumor growth rates. Each point represents the average tumor volume (cm3) in each of the four groups. Group averages were not compared after one or more animals in the group died (>35 days post tumor-cell inoculation). Tumor size on or before 28 days after tumor-cell inoculation, no difference among various groups, P>0.05. Tumor size at 35 days post tumor-cell inoculation, Group I vs. Group F or G, P<0.05; Group I vs. Group H, P=0.28; Group F vs. Group G vs. Group H, P>0.05
All these data described above suggested that treatment with TV plus infusion of one cytokine or combined two cytokines had stronger anti-tumor effects to prolong the animal survival time than treatment with single cytokine or TV alone. In addition, combination of TV plus infusion of GM-CSF and IL-12 had the strongest anti-tumor effects among all the treatment strategies used in this study, shown as increased animal survival rate, prolonged animal survival time, and reduced tumor growth rate.
Cytotoxic T lymphocyte assay
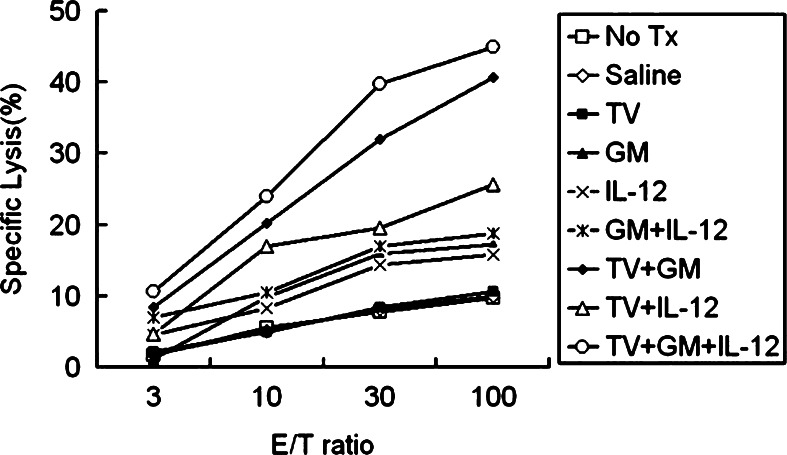
Tumor-specific CTL activity was measured using 51Cr-release assay from spleens of animals with subcutaneous gliomas treated with one of the various treatment strategies. Results shown in Fig. 5 indicate that Group I (TV + GM + IL-12) and Group G (TV + GM) generated the most prominent tumor-specific CTL response, with the former slightly stronger than the latter. CTL activity in these two groups was stronger than that in the other seven groups including Group H (TV + IL-12). Further, CTL activity in groups treated with one or two cytokines (Groups D, E, and F) was lower than that in groups treated with TV plus one or two cytokines (Groups G, H, and I). Groups A (No Tx), B (PBS), and C (TV) had lower CTL activity than the other six groups. The results indicated that the group treated with irradiated TV plus continuously infused GM-CSF and IL-12 induced the strongest CTL activity among all tested treatment strategies; and TV and GM-CSF were important for the enhanced CTL activity.
Fig. 5.
Cytotoxic T lymphocyte (CTL) assay. Cytotoxic activity of activated spleen cells was tested in vitro with a standard 51Cr-release assay 3 weeks after tumor-cell inoculation. Splenocytes were co-cultured with irradiated RT-2 cells for 5 days; then, cells were harvested and cytotoxic T cell (CTL) activity was determined with the 51Cr-labeled RT-2 cells as target cells. Three independent experiments were done for each group, and representative data were presented
NK cell assay
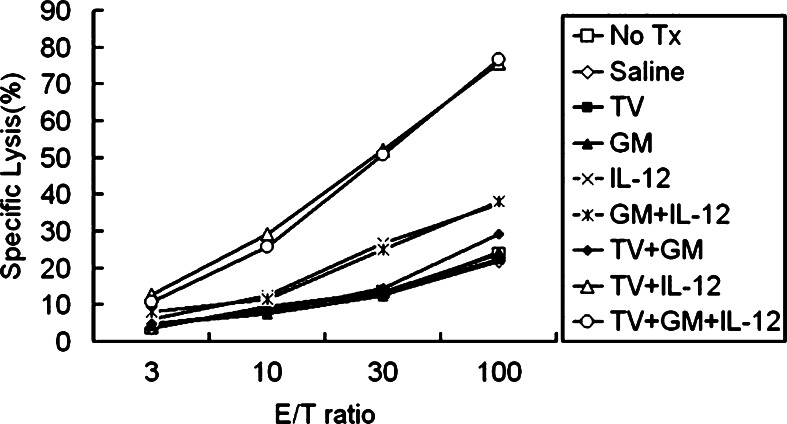
NK cell activity was measured using 51Cr-release assay from spleens of animals with subcutaneous gliomas treated with one of the various treatment strategies. Results shown in Fig. 6 indicate that the NK cell activity of Groups H (TV + IL-12) and I (TV + GM + IL-12) was the highest among all studied groups. NK cell activity in Groups E (IL-12) and F (GM + IL-12) was lower than in Groups H and I but higher than in Groups A (No Tx), B (Saline), C (TV), D (GM), and E (TV + GM). The results indicated that the group treated with TV plus continuously infused GM-CSF and IL-12 and the group treated with TV plus infused IL-12 had the highest NK activity among all tested treatment strategies; and TV and IL-12 were important for the enhanced NK cell activity.
Fig. 6.
Natural killer (NK) cell assay. NK cell activity of activated spleen cells was tested in vitro with a standard 51Cr-release assay 3 weeks after tumor-cell inoculation. Splenocytes were co-cultured with irradiated RT-2 cells for 5 days; then, cells were harvested and NK cell activity was determined with 51Cr-labeled YAC-1 cells as target cells. Three independent experiments were done for each group, and representative data were presented
Effects of irradiated TV and continuously infused GM-CSF and IL-12 on intracerebral gliomas
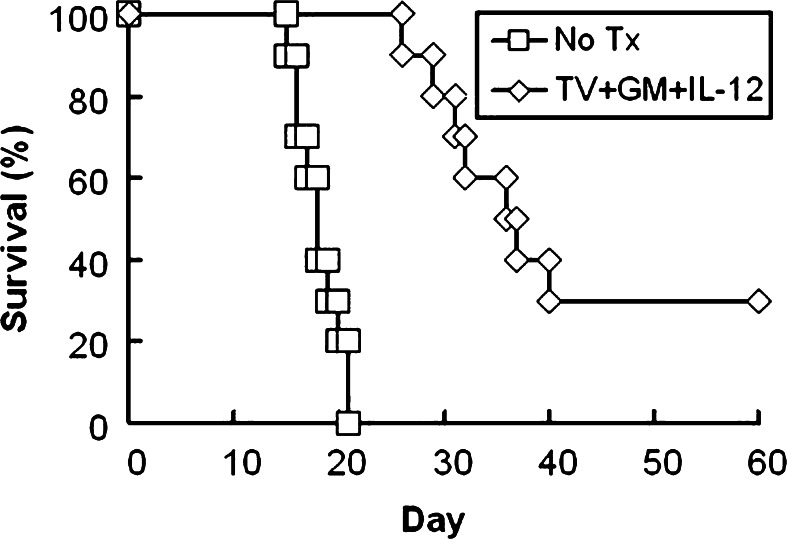
Figure 7 shows survival curves of rats with intra-cerebral gliomas receiving no treatment (No Tx, Group A-1) or treated with irradiated TV plus GM-CSF and IL-12 (Group I-1, TV + GM + IL-12). All rats in Group A-1 died, with a survival time of 18.1±2.1 days. Group I-1 had 30% long-term survival, with survival time of rats that died longer than 25 days after tumor-cell inoculation. Although the survival rate of Group I-1 was not significantly different from that of Group A-1 (P=0.11), Group I-1 had significantly longer survival time than Group A-1 (P<0.0001). The results indicated that treatment with irradiated TV and continuously infused GM-CSF and IL-12 exerted anti-tumor effects on the intra-cerebral gliomas to prolong animal survival time.
Fig. 7.
Survival curve for rats with intracerebral gliomas undergoing combination treatment with TV plus GM-CSF and IL-12. Rats were intra-cerebrally inoculated with 5×103 RT-2 cells and received no treatment (Group A-1) or five doses of irradiated TV plus GM-CSF and IL-12 infusion via osmotic minipump (TV + GM + IL-12, Group I-1). There were ten rats in each group. Fischer exact test was used to analyze the animal survival rates; the Kaplan–Meier method was used to assess the animal survival time; and the log-rank statistic was used to test differences between groups. Survival rate, P=0.04; survival time, P<0.0001
Immuno-histochemical staining of the CD8+ T cells and NK cells in the intra-cerebral gliomas
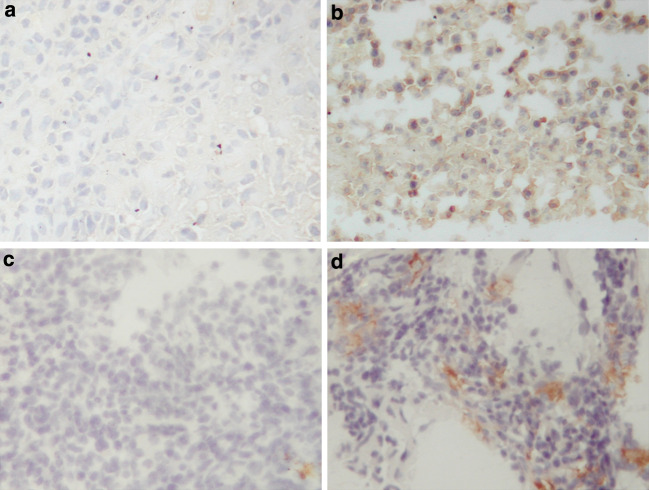
To study local immune responses in more detail, immuno-histochemical staining was performed for the intra-cerebral tumors treated with no treatment (Group A-1) or irradiated TV plus GM-CSF and IL-12 (Group I-1, TV + GM + IL-12) on day 21 after tumor-cell inoculation (Fig. 8). The tumor in the Group I-1 showed more prominent infiltration of CD8+ T cells and NK cells, as compared with scanty immune cell infiltration in the Group A-1.
Fig. 8.
Immuno-histochemical analyses of intra-cerebral gliomas treated with TV plus GM-CSF and IL-12. Rats with intra-cerebral gliomas were treated with no treatment (Group A-1) or five doses of irradiated TV plus GM-CSF and IL-12 (Group I-1). Staining was performed on day 21 after tumor-cell inoculation. Immune cells analyzed included CD8+ T cells (a Group A-1; b Group I-1; 200×) and NK cells (c Group A-1; d Group I-1; 200×)
Long-term anti-tumor immunity
Survivors in Groups F (GM + IL-12, 2 rats), G (TV + GM, 2 rats), H (TV + IL-12,2 rats), and I (TV + GM + IL-12, 4 rats) were re-challenged with subcutaneous inoculation of 1×105 RT-2 cells 150 days after initial tumor-cell inoculation. In addition, survivors in Group I-1 (TV + GM + IL-12, 3 rats) were re-challenged with intra-cerebral inoculation of 5×103 RT-2 cells into the left hemisphere 100 days after initial tumor-cell inoculation. All rats did not subsequently develop tumors, and all survived longer than 100 days following re-challenge with wild-type tumor cells. The results indicate that the anti-tumor response induced by infusion of GM-CSF and IL-12, TV plus infusion of GM-CSF, TV plus infusion of IL-12, or TV plus infusion of GM-CSF and IL-12 stimulated immunological memory.
Discussion
In this study, we treated established gliomas in rats using irradiated TV and/or continuous infusion of GM-CSF and/or IL-12. A pre-existing glioma usually induces host immuno-suppression [3, 5, 36], thus the animal model with established glioma is considered a mimic of the immuno-deficiency seen clinically in patients with malignant gliomas; we found that infusion of GM-CSF or IL-12 alone had no anti-tumor effects; however, infusion of both GM-CSF and IL-12 had anti-tumor effects. These data suggest that immuno-therapy using only one cytokine (GM-CSF or IL-12) is not strong enough to suppress glioma growth; in contrast, combination of these two cytokines exerted slightly stronger anti-tumor effects with our rat model. The immune response induced by cytokines is tumor-nonspecific and the negative or mild anti-tumor effects were consistent with previously reported data [36, 37]. We also found that TV alone exerted no anti-tumor effects on the established gliomas; in contrast, 9L TV had been found to exert some anti-tumor effects in other studies [16, 38, and 40]. Such difference might be related to factors such as timing of treatment (preventive or immediate treatment versus treatment of established gliomas); immuno-genicity of the tested glioma cells, animal models, and dosage of TVs. Past researchers have considered 9L glioma cells to be highly immuno-genic [42, 43]. The immuno-genicity of RT-2 glioma cells is not well studied; however, we had found wild-type RT-2 glioma grew in the subcutaneous tissue of rats previously vaccinated with irradiated TV (data not shown). Thus, we concluded that RT-2 cells have relatively low immuno-genicity. A low-immuno-genic tumor cell usually induces a less powerful immune reaction than a high-immunogenic cell, and this might contribute to difference in therapeutic effects seen with different animal models.
Although in the current study, one cytokine or TV alone had no therapeutic effects on established gliomas, the combination of TV and infusion of one cytokine (either GM-CSF or IL-12) could suppress tumor growth and prolong survival time. These results are consistent with those of reports using similar treatment strategies [16, 38, and 39]; they suggest that combined TV and continuously infused cytokine is an alternate strategy for gliomas because treatment provides tumor antigen and long-term release of cytokine in a manner that avoids side effects seen with a single large dose of cytokine and employs a concept similar to gene therapy. It is well known that multiple steps and regulatory molecules are generally required for a maximal immune response [6, 44], and combination of various strategies involving different immuno-regulatory responses may lead to better therapeutic effects than single immunotherapy [18, 30 and–32]. In addition, this study found that the combination of GM-CSF and IL-12 or combination of TV and GM-CSF (or IL-12) had synergistic anti-tumor effects. Thus, the combination of TV and GM-CSF, and IL-12 to up-regulate immune responsiveness is reasonably expected to have enhanced anti-tumor effects against gliomas. In the literature, few studies have investigated the effects of immunotherapy using both GM-CSF and IL-12 on the neo-plasms, and results have been inconsistent [33–35]. Of any single kind of TV, local injection of GM-CSF-secreting and IL-12-secreting TVs to tumor draining lymph nodes (TDLNs) can stimulate TDLN effector cells with resulting stronger cytotoxicity and better therapeutic effect for metastatic melanoma [33]. Similarly, the combination of CD40 ligand, CD80, and GM-CSF-expressing leukemia TV and recombinant IL-12 has been found to have better therapeutic effect for mouse leukemia than any single treatment strategy [34]. In contrast, patients vaccinated with idiotype-specific antigen of multiple myeloma and local injection of GM-CSF and IL-12 had no difference in reduction of circulating clonal tumor B cells compared with patients vaccinated with idiotype-specific antigen of multiple myeloma and local injection of IL-12 [35]. One study used combined TV and continuously infused GM-CSF and IL-12 to treat 9L gliomas in animals and found the treatment strategy had good therapeutic effects [40]. However, the authors started treatment either immediately at or 3 days before tumor-cell implantation, which does not represent clinical conditions with pre-existing gliomas. Thus, the good therapeutic response seen in that study might not necessarily indicate that a similar treatment strategy is effective for established gliomas. In our study, we treated subcutaneous gliomas 5 days and intra-cerebral gliomas 3 days after tumor-cell implantation and found that the combination of TV and GM-CSF plus IL-12 exerted stronger anti-tumor effects than any two combinations (infusion of two cytokines or combined TV and one cytokine).
Our results provide stronger evidence of the feasibility of such a treatment strategy in the treatment of established gliomas than the data obtained from models of involving immediate or preventive treatment [16, 38, and 40]. The weaker anti-tumor effect on intra-cerebral gliomas than on subcutaneous gliomas in the current study was probably related to factors such as strength of the immune response and the presence of blood–brain barrier, however, further studies are necessary to elucidate the mechanisms underlying observed differences. In addition to anti-tumor effects, the immune response induced by TV and infused cytokines protected rats from subsequent re-challenge with wild-type RT-2 glioma cells subcutaneously or intra cerebrally, a finding that suggests such treatment induces long-lasting immunity.
To understand the mechanisms of the treatment strategies used in this study, we analyzed post-treatment CTL and NK cell activity. Enhanced CTL activity was noted in rats treated with strategies including TV and GM-CSF. In contrast, enhanced NK cell activity was noted in rats treated with strategies including TV and IL-12 infusion. The level of NK cell activity was similar in rats treated with TV + GM + IL-12 and those treated with TV + IL-12, although the former showed slightly higher CTL activity than the latter (Fig. 5 and 6). The data suggest that IL-12 infusion slightly enhances CTL activity, but GM-CSF infusion does not affect NK cell activity significantly. Combined TV plus GM-CSF infusion or TV plus IL-12 infusion increased CTL activity and NK cell activity respectively, which indicates that tumor-specific immune responses involve stronger CTL and NK cell activity than tumor-nonspecific immune responses. The enhanced CTL and NK activity was further confirmed by the infiltration of CD8+ T cells and NK cells in the intra-cerebral tumors of the rats treated with TV plus GM-CSF and IL-12. As a whole, five doses of irradiated TV delivered in 12 days together with 2-week delivery of GM-CSF and IL-12 via osmotic mini-pump elicited synergistic anti-tumor effects on established gliomas to suppress tumor growth, prolong survival time; and provide long-term anti-tumor immunity. The underlying mechanisms for such anti-tumor immunity might be related to enhanced CTL and NK cell activity, as well the previously demonstrated increase in infiltration of CD4+ and CD8+ T cells in tumor, along with enhanced DTH [40]. The immune reactions induced by the treatment strategy using TV + GM + IL-12 were thought to be initiated from detection of tumor antigens in TV and phagocytosis of antigens by GM-CSF-stimulated APCs [45, 46]. Under this scenario, APCs differentiated into peripheral DCs and migrated to regional lymph nodes, where they became a major source of epitopes for activation of T cells, ultimately activating CTL lysis of tumor cells [45, 46]. Furthermore, IL-12 produced by GM-CSF-activated APCs or delivered via osmotic pump stimulated naïve T cells to differentiate into Th1 cells, enhanced Th1 immune responses, stimulated T cells and NK cells and enhanced their secretion of IFN-γ, activated NK/LAK cells, promoted specific CTL responses [21–23], and eventually killed tumor cells. Such an anti-tumor immunity induced DTH and proved long-lasting anti-tumor protection.
In conclusions, the present study demonstrated that treatment using continuous subcutaneous infusion of GM-CSF and IL-12 localized to the site of injection of irradiated tumor cells used as a source of tumor antigens can initiate an immune response that suppresses tumor growth of established subcutaneous or intra-cerebral gliomas. The anti-tumor effects of this treatment strategy are thought to be related to enhanced CTL and NK cell activities. The effectiveness of the GM-CSF/IL-12 infusion-based immunotherapy on established gliomas offers an approach to treatment of glioma that avoids the need for genetically engineered cells, and thus it should be considered as an adjuvant treatment for glioma. However, further research is necessary to refine this treatment technique and to improve therapeutic effects on gliomas.
Acknowledgments
This study was supported by research grants NTUH91-S040 from National Taiwan University Hospital and NSC91-2314-B-002-338 from the National Science Council, Taiwan, R.O.C. awarded to Dr. Sheng-Hong Tseng.
References
- 1.Urbani F, Maleci A, La Sala A, Lande R, Ausiello CM. Defective expression of interferon-γ, granulocyte-macrophage colony stimulating factor, tumor necrosis factor α, and interleukin-6 in activated peripheral blood lymphocytes from glioma patients. J Interferon Cytokine Res. 1995;15:421. doi: 10.1089/jir.1995.15.421. [DOI] [PubMed] [Google Scholar]
- 2.Zou JP, Morford LA, Chougnet C, Dix AR, Brooks AG, Torres N, Shuman JD, Coligan JE, Brooks WH, Roszman TL, Shearer GM. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882. [PubMed] [Google Scholar]
- 3.Couldwell WT, Dore-Duffy P, Apuzzo MLJ, Antel JP. Malignant glioma modulation of immune function: relative contribution of different soluble factors. J Neuroimmunol. 1991;33:89. doi: 10.1016/0165-5728(91)90052-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahaley MS, Brooks WH, Roszman TL, Bigner DD, Duka L, Richardson S. Immunobiology of primary intracranial tumors Part I: studies of the cellular and humoral general immune competence of the brain-tumor patients. J Neurosurg. 1977;46:467. doi: 10.3171/jns.1977.46.4.0467. [DOI] [PubMed] [Google Scholar]
- 5.Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991;12:370. doi: 10.1016/0167-5699(91)90068-5. [DOI] [PubMed] [Google Scholar]
- 6.Chong H, Todryk S, Hutchinson G, Hart IR, Vile RG. Tumor cell expression of B7 costimulatory molecules and interleukin-12 of granulocyte-macrophage colony-stimulating factor induces a local antitumor response and may generate systemic protective immunity. Gene Ther. 1998;5:223. doi: 10.1038/sj.gt.3300584. [DOI] [PubMed] [Google Scholar]
- 7.Freeman GJ, Gray GS, Gimmi CD, Lombard DB, Zhou LJ, White M, Fingeroth JD, Gribben JG, Nadler LM. Structure, expression and T cell costimulatory activity of the murine homologue of the human B lymphocyte activation antigen B7. J Exp Med. 1991;174:625. doi: 10.1084/jem.174.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward SG. CD28: a signaling perspective. Biochem J. 1996;6:361. doi: 10.1042/bj3180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parney IF, Petruk KC, Zhang C, Farr-Jones M, Sykes DB, Chang LJ. Granulocyte-macrophage colony-stimulating factor and B7-2 combination immunogene therapy in an allogenic Hu-PBL-SCID/beige mouse–human glioblastoma multiforme model. Hum Gene Ther. 1997;8:1073. doi: 10.1089/hum.1997.8.9-1073. [DOI] [PubMed] [Google Scholar]
- 10.Tseng SH, Hwang LH, Lin SM. Induction of antitumor immunity by intracerebrally implanted rat C6 glioma cells genetically engineered to secrete cytokines. J Immunother. 1997;20:334. doi: 10.1097/00002371-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Wakimoto H, Abe J, Tsunoda R, Aoyagi M, Hirakawa K, Hamada H. Intensified antitumor immunity by a cancer vaccine that produces granulocyte-macrophage colony-stimulating factor plus interleukin 4. Cancer Res. 1996;56:1823. [PubMed] [Google Scholar]
- 12.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting antitumor immunity. Proc Natl Acad Sci U S A. 1993;90:3539. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. Paracrine cytokine adjuvants in cancer immunotherapy. Annu Rev Immunol. 1995;13:399. doi: 10.1146/annurev.iy.13.040195.002151. [DOI] [PubMed] [Google Scholar]
- 14.Paglia P, Girolomoni G, Robbiati F, Granucci F, Ricciardi-Castagnoli P. Immortalized dendritic cell line fully competent in antigen presentation initiates primary T cell responses in vivo. J Exp Med. 1993;178:1893. doi: 10.1084/jem.178.6.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davidoff AM, Kimbrough SA, Ng CYC, Shochat SJ, Vanin EF. Neuroblastoma regression and immunity induced by transgenic expression if interleukin-12. J Pediatr Surg. 1999;34:902. doi: 10.1016/S0022-3468(99)90395-0. [DOI] [PubMed] [Google Scholar]
- 16.Jean WC, Spellman SR, Wallenfriedman MA, Hall WA, Low WC. Interleukin-12-based immunotherapy against rat 9L glioma. Neurosurgery. 1998;42:850. doi: 10.1097/00006123-199804000-00097. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi T, Joki T, Abe T, Ohno T. Antitumor activity of killer cells stimulated with both interleukin-2 and interleukin-12 on mouse glioma cells. J Immunother. 1999;22:245. doi: 10.1097/00002371-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Zitvogel L, Robbins PD, Storkus WJ, Clarke MR, Maeurer MJ, Campbell RL, Davis CG, Tahara H, Schreiber RD, Lotze MT. Interleukin-12 and B7.1 co-stimulation cooperate in the induction of effective antitumor immunity and therapy of established tumors. Eur J Immunol. 1996;26:1335. doi: 10.1002/eji.1830260624. [DOI] [PubMed] [Google Scholar]
- 19.D’Andrea A, Rengaraju M, Valiante NM, Chehimi J, Kubin M, Aste M, Chen SH, Kobayashi M, Young D, Nickbarg E, et al. Production of natural killer cell stimulatory factor (interleukin-12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macatonia SE, Hosken NA, Litton M, Vieira P, Hsieh CS, Culpepper JA, Wysocka M, Trinchieri G, Murphy KM, O’Garra A. Dendritic cells produce IL-12 and direct the development of Th1 cells from naïve CD4+ T cells. J Immunol. 1995;154:5071. [PubMed] [Google Scholar]
- 21.Hendrzak JA, Brunda MJ. Interleukin-12: biologic activity, therapeutic utility, and role in disease. Lab Invest. 1995;72:619. [PubMed] [Google Scholar]
- 22.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12 deficient mice are defective in IFNγ production and type 1 cytokine responses. Immunity. 1996;4:471. doi: 10.1016/S1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 23.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cell type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008. [PubMed] [Google Scholar]
- 24.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, Koda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(Suppl.):S52. doi: 10.1007/PL00014051. [DOI] [PubMed] [Google Scholar]
- 25.Ohmi Y, Shiku H, Nishimura T. Tumor-specific targeting of T helper type 1 (Th1) cells by anti-CD3 × anti-c-ErbB-2 bispecific antibody. Cancer Immunol Immunother. 1999;48:456. doi: 10.1007/s002620050622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 27.Ausielo CM, Maleci A, Cassone A. Modulation of immune function by glioma. Immunol Today. 1992;13:148. doi: 10.1016/0167-5699(92)90113-L. [DOI] [PubMed] [Google Scholar]
- 28.Colombo MP, Ferrari G, Stoppacciaro A, Parenza M, Rodolfo M, Marmiani G. Granulocyte colony-stimulating factor gene transfer suppresses tumorigenicity of a murine adenocarcinoma in vivo. J Exp Med. 1991;173:889. doi: 10.1084/jem.173.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishima H, Shimizu K, Miyao Y, Mabuchi E, Tamura K, Tamura M, Sasaki M, Hakakawa T. Systemic interleukin 12 displays anti-tumor activity in the mouse central nervous system. Br J Cancer. 1998;78:446. doi: 10.1038/bjc.1998.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tseng SH, Hsieh CL, Lin SM, Hwang LH. Regression of orthotopic brain tumors by cytokine-assisted tumor vaccines primed in the brain. Cancer Gene Ther. 1999;6:302. doi: 10.1038/sj.cgt.7700057. [DOI] [PubMed] [Google Scholar]
- 31.Cayeux S, Beck C, Dorken B, Blankenstein T. Coexpression of interleukin-4 and B7.1 in murine tumor cells leads to improved tumor rejection and vaccine effect compared to single gene transfectants and a classical adjuvant. Hum Gene Ther. 1996;7:525. doi: 10.1089/hum.1996.7.4-525. [DOI] [PubMed] [Google Scholar]
- 32.Kikuchi T, Joki T, Saitoh S, Hata Y, Abe T, Kato N, Kobayashi A, Miyazaki T, Ohno T. Antitumor activity of interleukin-2-producing tumor cells and recombinant interleukin 12 against mouse glioma cells located in the central nervous system. Int J Cancer. 1999;82:425. doi: 10.1002/(SICI)1097-0215(19990129)80:3<425::AID-IJC15>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 33.Aruga A, Tanigawa K, Aruga E, Yu Hua Chang AE. Enhanced adjuvant effect of granulocyte-macrophage colony-stimulating factor plus interleukin-12 compared with either alone in vaccine-induced tumor immunity. Cancer Gene Ther. 1999;6:89. doi: 10.1038/sj.cgt.7700010. [DOI] [PubMed] [Google Scholar]
- 34.Gruber TA, Skelton DC, Kohn DB. Requirement for NK cells in CD40 ligand-mediated rejection of Philadelphia chromosome-positive acute lymphoblastic leukemia cells. J Immunol. 2002;168:73. doi: 10.4049/jimmunol.168.1.73. [DOI] [PubMed] [Google Scholar]
- 35.Rasmussen T, Hansson L, Osterborg A, Johnsen HE, Mellstedt H. Idiotype vaccination in multiple myeloma induced a reduction of circulating clonal tumor B cells. Blood. 2003;101:4607. doi: 10.1182/blood-2002-06-1925. [DOI] [PubMed] [Google Scholar]
- 36.Mahaley MS, Bertsch L, Cush S, Gillespie GY. Systemic gamma-interferon therapy for recurrent gliomas. J Neurosurg. 1988;69:826. doi: 10.3171/jns.1988.69.6.0826. [DOI] [PubMed] [Google Scholar]
- 37.Merchant RE, Merchant LH, Cook SHS, McVicar DW, Young HF. Intralesional infusion of lymphokine-activated killer (LAK) cells and recombinant interleukin-2 (rIL-2) for the treatment of patients with malignant tumor. Neurosurgery. 1988;23:725. doi: 10.1097/00006123-198812000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Wallenfriedman MA, Conrad JA, DelaBarre L, Graupman PC, Lee G, Garwood M, Gregerson DS, Jean WC, Hall WA, Low WC. Effects of continuous localized infusion of granulocyte-macrophage colony-stimulating factor and inoculations of irradiated glioma cells on tumor regression. J Neurosurg. 1999;90:1064. doi: 10.3171/jns.1999.90.6.1064. [DOI] [PubMed] [Google Scholar]
- 39.Chen Y, Lin SM, Lai HS, Tseng SH, Chen WJ. Effects of irradiated tumor vaccine and continuous localized infusion of granulocyte-macrophage colony-stimulating factor on neuroblastomas in mice. J Pediatr Surg. 2002;37:1298. doi: 10.1053/jpsu.2002.34995. [DOI] [PubMed] [Google Scholar]
- 40.Jean WC, Spellman SR, Wallenfriedman MA, Flores CT, Kurtz BP, Hall WA, Low WC. Effects of combined granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin-2, and interleukin-12 based immunotherapy against intracranial glioma in the rat. J Neurooncol. 2004;66:39. doi: 10.1023/B:NEON.0000013477.94568.0f. [DOI] [PubMed] [Google Scholar]
- 41.Copeland DD, Talley FA, Bigner DD. The fine structure of intracranial neoplasms induced by the inoculation of avian sarcoma virus in neonatal and adult rats. Am J Pathol. 1976;83:149. [PMC free article] [PubMed] [Google Scholar]
- 42.Heuer JG, Tucker-McClung C, Gonin R, Hock RA. Retrovirus-mediated gene transfer of B7-1 and MHC class II converts a poorly immunogenic neuroblastoma into a highly immunogenic one. Hum Gene Ther. 1996;7:2059. doi: 10.1089/hum.1996.7.17-2059. [DOI] [PubMed] [Google Scholar]
- 43.Smilowitz HM, Micca PL, Nawrocky MM, Slatkin DN, Tu W, Coderre JA. The combination of boron neutron-capture therapy and immunoprophylaxis for advanced intracerebral gliosarcomas in rats. J Neurooncol. 2000;46:231. doi: 10.1023/A:1006409721365. [DOI] [PubMed] [Google Scholar]
- 44.Colombo MP, Forni G. Immunotherapy 1: Cytokine gene transfer strategies. Cancer Metastasis Rev. 1996;15:317. doi: 10.1007/BF00046345. [DOI] [PubMed] [Google Scholar]
- 45.Bannerji R, Arroyo CD, Cordon-Cardo C, Gilboa E. The role of IL-2 secreted from genetically modified tumor cells in the establishment of antitumor immunity. J Immunol. 1994;152:2324. [PubMed] [Google Scholar]
- 46.Huang AY, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone-marrow-derived cells in presenting major histocompatibility complex class I-restricted tumor antigens. Science. 1994;264:961. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]