Abstract
Purpose
Electrochemotherapy (ECT) is an effective local therapy of human cutaneous cancers but has no effect on distant untreated tumors. We addressed whether tumor-associated antigens released after ECT could induce an efficient systemic immunity when associated with an appropriate immunoadjuvant.
Methods and results
We first studied the nature of the cellular recruitment and the expression of various toll-like receptors (TLRs) in tumors treated by ECT. We found that ECT induced a massive recruitment of CD11c and CD11b positive cells in the tumors and a strong increase of TLR9 expression. We then tested antitumor effects of the combination: ECT followed by TLR-9 ligands, CpG oligodeoxynucleotides (CpG ODN), in three murine tumor models. We found that this combination triggered both potent local synergistic antitumor effects, on the ipsi-lateral ECT-treated tumor, and more interestingly, a systemic antitumor response on the contra-lateral untreated tumor, in the three models. The systemic protection was T-cell dependent as it was not observed in nude littermates. The combination induced tumor-specific T cell effectors in the tumor-draining lymph nodes and in the spleen which secreted significantly more gamma-interferon upon activation than with ECT or CpG ODN alone.
Conclusions
Our data show that ECT and CpG ODN synergize and induce a significant increase of the local effect and a systemic T-dependent antitumor response. Such combination constitutes a potential innovative vaccination strategy using in situ tumor-associated antigens that could eventually be translated into the clinic.
Keywords: Electrochemotherapy, Immunotherapy, CpG oligodeoxynucleotides, Melanoma
Introduction
A decade ago electrochemotherapy (ECT) emerged as a new method to cure cutaneous cancers [26]. It is based on the permeabilization of the tumor cell membrane by means of short and intense electric pulses allowing entry of a highly cytotoxic non-permeant drug, like bleomycin, into the cytosol [24, 30]. It is now established as an efficient, local, inexpensive treatment that can be offered to patients with unresectable malignant melanoma, basal cell carcinoma, squamous cell carcinoma, adenocarcinoma and Kaposi’s sarcoma [20, 32]. Nonetheless, ECT alone is a local treatment with only palliative effects and is therefore of limited utility for metastatic patients. However, ECT induces a rapid and massive cell death in the tumor in vivo [21], with dying cells and thus tumor-associated antigens (TAA) remaining in situ. We and others [19] have already demonstrated that various cell destruction processes, like γ-irradiation or anthracyclines, can induce T cell-dependent antitumor effects in addition to their cytotoxic effects [9, 29]. In a recent paper, den Brok and colleagues showed that the induction of tumor protective immunity is dependent on the presence of the cryo-destructed tumor material since mice were not immunized if the cryo-ablated tumors were excised [11]. However, most of these processes do not trigger efficient immune responses when used alone. Likewise, in the context of ECT alone, TAA do not seem to be presented efficiently to immune cells since no significant distant antitumor effect could be observed in animal [28] or in human [32]. Therefore our goal was to take advantage of this “on-site” tumor destruction and to increase the tumor immunogenicity after ECT.
Our first step was to identify an immune adjuvant that could be associated to ECT and induce a danger signal which could optimize cross-presentation of ECT-induced TAA by antigen-presenting cells (APC). We demonstrated by immunohistochemistry that ECT induced a recruitment of myeloid cells and by RT-PCR that TLR9 expression was strongly increased in the tumor after ECT. Accordingly, we chose to associate ECT to a potent TLR9 ligand, i.e., oligodeoxynucleotides containing unmethylated CpG motifs (CpG ODN) which are known to mimic the effect of bacterial DNA and to stimulate APC to induce a favourable environment to the development of a Th1 response and the generation of cytotoxic T lymphocytes [10, 31, 34].
We report here the antitumor effect of the combination of ECT plus CpG ODN in three tumor models and demonstrate that CpG ODN not only enhance the local effects of ECT but also trigger a systemic immunity mediated by T cells.
Materials and methods
Mice
C57BL/6 Female (H-2b) were obtained from the Center d’Elevage Janvier (Le Genest, St Isle, France) and male nude swiss mice were produced locally. All the animals used at 8–20 weeks of age were handled in the animal housing facility of the Institut Gustave Roussy according to the Experimental Animal Ethics Committee Guidelines (Val de Marne, France).
Cell lines
All the cell lines (LPB, B16OVA and B16F10) were maintained in vitro at 37°C with 5% of CO2. LPB cells were derived from a methylcholanthrene induced-sarcoma in C57BL/6 mice [3]. These cells were cultivated in minimal essential medium (Gibco, Cergy-Pontoise, France) supplemented with 8% of heat inactivated foetal calf serum (Gibco), 100 U/ml of penicillin and 100 μg/ml of streptomycin (Gibco). The stable line B16OVA was kindly provided by the Dr K.L. Rock. B16OVA cells were cultivated in RPMI 1640 medium (Gibco) supplemented with 8% of foetal calf serum, 100 U/ml of penicillin and 100 μg/ml of streptomycin and 1% of the following substances: l-glutamine, sodium pyruvate, non essential amino-acids (Gibco).
Murine tumor models
Mice were inoculated with 106 LPB or 5.105 B16OVA or B16F10 cells into the left flank. Three days later, the same cells were injected into the right flank. Electrochemotherapy was performed at day zero on the left side when the tumor size reached 3–5 mm mean diameter. At day zero, the untreated right tumors were smaller but already palpable and easy to measure. Animals were followed up by measuring the two largest perpendicular tumor diameters a and b (a > b). Tumor volumes (V) were calculated using the V = ab²π/6 formula.
Anaesthesia
Mice were anaesthetized with 150 μl of a mixture composed of xylazine 12.5 mg/kg (Bayer Phama, Puteaux, France) and ketamine 125 mg/kg (Parke Davis, Courbevoie, France) injected intraperitoneally before the treatment by electroporation or electrochemotherapy.
Electrochemotherapy
Ten (LPB model) or 30 μg (B16OVA and B16F10 models) of bleomycin (Roger Bellon, Neuilly, France) were injected intravenously in the anaesthetized mice. Four minutes after the injection, eight square electric pulses of 100 μs and 1,300 V/cm were delivered at a frequency of 5,000 Hz by 5 mm apart plate electrodes placed on both sides of the tumor and connected to an electropulsator (Cliniporator™, Igea, Carpi, Italy).
Immunotherapy
Purified phosphorothioate single-stranded sequences were 5′-TAAACGTTATAACGTTATGACGTCAT-3′ for the unmethylated CpG motif-containing oligodeoxynucleotides (CpG ODN) and 5′-TGACTGTGAAGGTTAGAGATGA-3′ for the control sequence (control ODN) in which the CpG motifs have been mutated (Eurogentech, Seraing, Belgium). Thirty or 50 μg of CpG ODN were diluted in NaCl 0.9% and 100 μl were injected respectively in LPB tumors or B16OVA and B16F10 tumors at days two and nine after the ECT.
Immunohistochemistry
Mice bearing LPB tumor on the flank were treated by ECT and tumors were surgically removed at different time points after ECT. Tumors were snap-frozen in OCT and stored at −80°C. Frozen sections (10 μm) were thawed, fixed in acetone at 4°C and incubated for 30 min with anti-CD4 (1:3,000, clone H129.19), anti-CD8 (1:1,000, clone 53-6.7), anti-CD86 (1:50, clone GL1), anti-CD11b (1:600, clone M1/70), anti-CD80 (1:50, clone 16-10A1) (BD Pharmingen) and anti-CD11c (1:50, clone N418, Caltag Laboratories, Burlingame, CA) monoclonal antibodies (mAbs). The first four mAbs were then incubated for 30 min with a rabbit anti-rat mAb (DakoCytomation, Glostrup, Denmark) followed by rat alkaline phosphatase anti-alkaline phosphatase (APAAPcomplexes; DakoCytomation). The last two mAbs were incubated with a biotinylated anti-hamster mAb (Vector Laboratories Inc., Burlingame, CA) for 30 min followed by incubation with avidin biotinylated alkaline phosphatase (ABComplex/AP; DakoCytomation). Enzyme reaction was developed with Fast Red substrate (DakoCytomation).
TLRs expression in tumors by quantitative RT-PCR
To study TLRs tumor RNA expression after electrochemotherapy we used a quantitative RT-PCR. Samples were obtained from mice bearing a subcutaneous LPB tumor on the flank treated by electrochemotherapy. Human samples were obtained from cutaneous metastases from patients with metastatic melanoma treated at the Institut Gustave Roussy. Informed consents were obtained from all patients. RNA isolation from tumors was performed with TRI Reagent (Sigma–Aldrich, St-Louis, MO) following the manufacturers recommendations. One microgram total RNA was reverse-transcribed in a 20 μl volume reaction. The complementary DNAs (cDNAs) were then diluted 1/20 in nuclease-free water. cDNA equivalent to 25 ng total RNA were used to carry out PCR for mTLR3, mTLR4, mTLR7 and mTLR9 with ready to use Gene Expression Products (Applied Biosystems) in a unique PCR master mix (Applied Biosystems). Real-time PCR was developed on the ABI Prism 7700 sequence Detector. For each experiment, amplification of 18 s ribosomal DNA was carried out simultaneously as an endogenous control of the total RNA quantity in the reaction.
Specific immune response analysis
The specific immune-response was studied in the draining lymph nodes of mice bearing a B16OVA tumor in the leg. Electrochemotherapy was performed when the tumor reached 5 mm average diameter. Fifty microgram of CpG ODN were diluted in NaCl 0.9% and 50 μl were injected into the tumors at day two after the ECT. The day after the intratumoral injection of CpG ODN, mice were killed and inguinal lymph nodes were harvested and crushed in cell strainers. Cells were stained with PE H-2Kb OVA257–264 tetramers (Beckman Coulter, Fullerton, CA) for 30 min at room temperature and then with FITC anti-CD3 mAb and APC anti-CD8α mAb (BD Pharmingen, San Diego, CA) in the same conditions. Stained cells were washed in PBS1X before analysis with a FACSCalibur (Becton Dickinson). Naïve mice and mice immunized into the right footpad with 45 μg of OVA257–264 peptide mixed with 30 μg of CpG ODN were used respectively as negative and positive controls of the tetramer staining. Alternatively, 105 lymph node cells per well were restimulated in vitro with 1 mg/ml of ovalbumin (Calbiochem, San Diego, CA) in complete medium for 48 h prior to the collection of the supernatants to quantify IFN γ production by ELISA (BD Pharmingen, San Diego, CA). Mice bearing a B16OVA tumor in the flank were treated and killed ten days after the second injection of CpG ODN to remove spleens. 5 × 105 splenocytes per well were restimulated in vitro as above.
Statistics
Statistical significance (P < 0.05) was determined by the two tailed Mann–Whitney’s t test or by Fisher’s exact test.
Results
In situ recruitment of immune cells after ECT
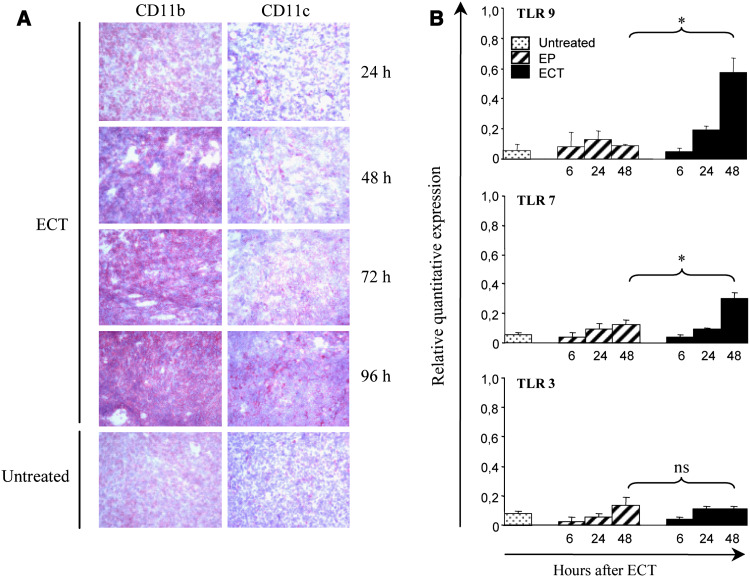
In situ, ECT induces a massive cell death of the tumor cells within 24 h after treatment [21] but also induces a recruitment of mononuclear leucocytes with high density in the area where a large fraction of apoptotic cells was also present [21]. The nature of these infiltrating leucocytes has not yet been identified. To characterize the immune cells recruited after ECT, mice bearing a 5 mm diameter LPB fibrosarcoma were treated by ECT and the tumors were surgically removed at different time points after the ECT and examined for the CD11b, CD11c, CD80, CD86, CD4 and CD8 cells infiltration. We found that tumor-infiltrating CD11b and CD11c positive cells were largely recruited from 48 to 96 h after ECT, when compared to basic infiltration of untreated tumors (Fig. 1a). This recruitment was associated with a similar increase of CD80 and CD86 positive cells (Table 1). CD8 cells were mostly present into the tumors at later time points (72 and 96 h), while the number of CD4 cells remained stable (Table 1). Therefore ECT treatment provokes the recruitment of DCs and myeloid cells, both known to be capable of antigen presentation.
Fig. 1.
In situ recruitment of myeloid cells and expression of TLRs mRNA in tumors after ECT. ECT was performed on mice bearing an established LPB tumor on the flank. a non-treated tumors or ECT-treated tumors were surgically removed 24, 48, 72 and 96 h after the treatment. Frozen histological sections were stained with anti-CD11c and anti-CD11b antibodies and representative sections are shown here (magnification ×200). b Expression of TLRs mRNA was determined by quantitative RT-PCR in non-treated tumors, tumors electroporated without bleomycin (EP) and ECT-treated tumors obtained 6, 24 and 48 h after the treatment. Each value was calculated as fold increase relative to value obtained from spleen mRNA. Means with SE from three mice per group are shown in a representative experiment out of three
Table 1.
Immunohistochemical analyses of tumor-infiltrating cells after ECT
| CD11b | CD11c | CD80 | CD86 | CD8 | CD4 | |
|---|---|---|---|---|---|---|
| ECT 24 h | ++ | + | + | ++ | − | + |
| ECT 48 h | ++ | ++ | ++ | ++ | + | + |
| ECT 72 h | +++ | +++ | +++ | ++ | ++ | + |
| ECT 96 h | ++++ | +++ | ++++ | +++ | ++ | + |
| Untreated | + | + | + | + | − | + |
Semi-quantitative evaluation of antigen expression in tumor sections. Immunohistochemical staining was performed with various antibodies in two independent experiments. Histological sections were obtained from non-treated LPB tumors (n = 3) or treated tumors removed 24, 48, 72 and 96 h after ECT (n = 3)
In order to identify a suitable immunologic adjuvant that could efficiently bind to receptors expressed by cells recruited in situ, and since dendritic cells and macrophages are known to express various TLRs according to their subtype [15], we studied the expression of various TLRs, i.e. TLR3, TLR4, TLR7 and TLR9, by quantitative RT-PCR in the context of tumors treated by ECT. Messenger RNAs extracted from non-treated tumors or tumors electroporated without bleomycin were used as controls for basal expression of TLRs. As described in Fig. 1b, TLR9 expression was strongly enhanced in tumors 48 h after ECT, whereas TLR4 (data not shown) and TLR3 signal did not increase and TLR7 was slightly enhanced.
Thus, ECT induced at 48 h a massive infiltration of potential APC expressing CD11c or CD11b associated with a significant increase of the TLR9 mRNA expression. We therefore chose to combine ECT to injections of CpG ODN directly into the tumor.
Potentiation of local and induction of systemic antitumor effects of ECT by CpG ODN
We used a model in which two tumors were inoculated subcutaneously on each flank of the mice, but only one was treated by either ECT alone, intratumoral CpG ODN injections or the combination of these two treatments. With this model we could study both local and systemic effects of the treatment by following tumor growth on each flank.
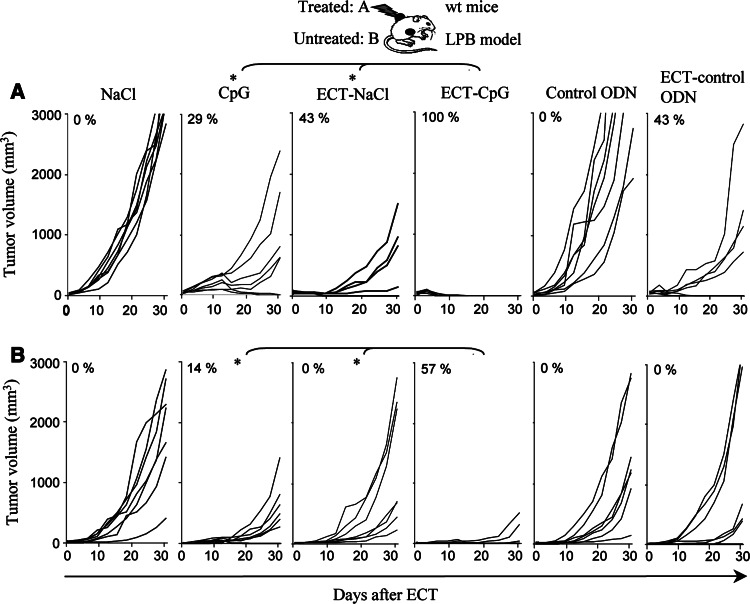
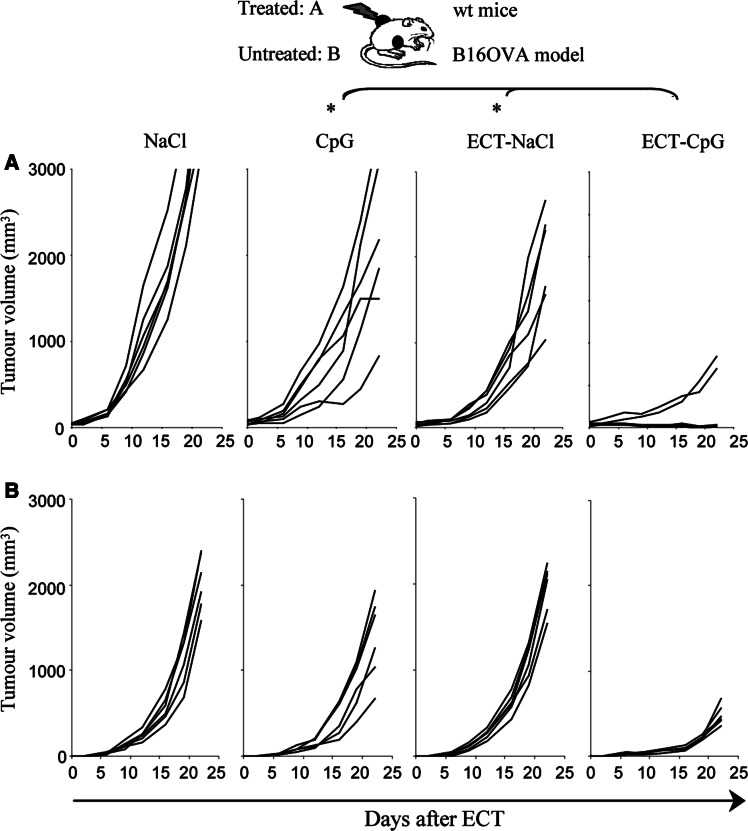
ECT efficacy was dramatically enhanced by CpG ODN as this combination resulted in the complete regression of all local treated LPB tumors (Fig. 2a). In contrast, ECT or CpG ODN performed alone were less efficient locally and resulted in only 43 and 29% of complete tumor regressions, respectively (Fig. 2a). This demonstrated a synergistic effect of this association in curing established tumors. Moreover the combined treatment induced a systemic antitumor effect with 57% of rejections of the distant untreated tumors (Fig. 2b). Mice that had rejected LPB tumors on both sides remained tumor-free for two months. Finally, the combined treatment resulted in a specific memory immunity as these mice were protected against a rechallenge with the same tumor but not with an unrelated B16F10 tumor 2 months after the end of the treatment (data not shown). On the contrary, ECT alone did not trigger any regression in the distant tumor and injections of CpG ODN alone cured only 14% of the distant tumors (Fig. 2b). Experiments were also performed with a control ODN without CpG motifs which had no local or systemic activity neither as single treatment nor in combination with ECT (Fig. 2). We also investigated the efficiency of the combined treatment in B16OVA and B16F10 melanomas. In these models, both the local and distant effects were significantly superior with the association than with ECT or CpG ODN alone (Fig. 3 and data not shown). In the B16OVA model, animals treated by ECT-CpG ODN had a mean tumor volume of 270 mm3 ± 158 at day 22 whereas those treated by CpG ODN or ECT had a higher mean tumor volume of 2,329 mm3 ± 323 (P < 0.05) and 1,929 mm3 ± 252 (P < 0.05), respectively (Fig. 3a). Systemic effect was also significantly greater with the combined treatment (mean tumor volume ± SE, 415 mm3 ± 75) than with ECT (1,963 mm3 ± 114, P < 0.05) or CpG ODN (1,305 mm3 ± 110, P < 0.05) alone (Fig. 3b).
Fig. 2.
Local and systemic potentiation of ECT by CpG ODN in the LPB fibrosarcoma. Mice were inoculated with 106 LPB cells on each side and were only treated on the left side when the tumor reached 5 mm diameter. ECT was performed as a single session treatment at day 0. A dose of 30 μg of CpG ODN or control CpG was delivered into the left tumors at days 2 and 9. Results show the monitoring of the treated tumors (a) and the non treated tumors (b) in a representative experiment out of three and values indicate the percentage of complete regression (n = 7 per group, *P < 0.05)
Fig. 3.
Local and systemic potentiation of ECT by CpG ODN in the B16OVA melanoma. Mice were inoculated with 5.105 B16OVA cells on each side and were only treated on the left side when the tumor reached 5 mm diameter. ECT was performed as a single session treatment at day zero. A dose of 50 μg of CpG ODN was delivered into the left tumors at days 2 and 9. Results show the monitoring of the treated tumors (a) and the non-treated tumors (b) in a representative experiment out of two (n = 7 per group, *P < 0.05)
Altogether, these results indicate that in three tumor models, combination of ECT plus CpG ODN is highly efficient to reduce local tumor growth and leads to a systemic antitumor response.
T cells are essential effectors for the combined treatment distant efficacy
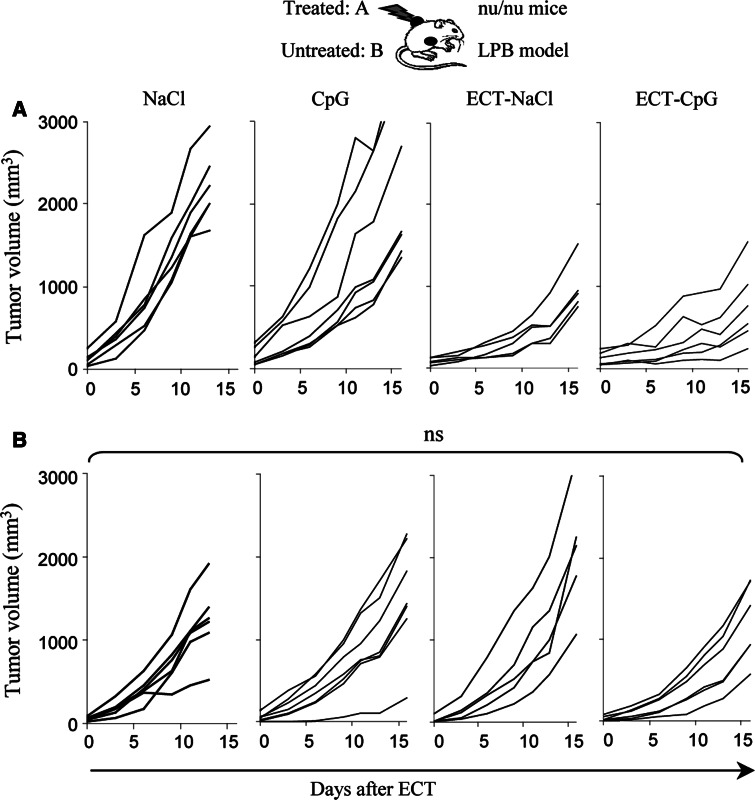
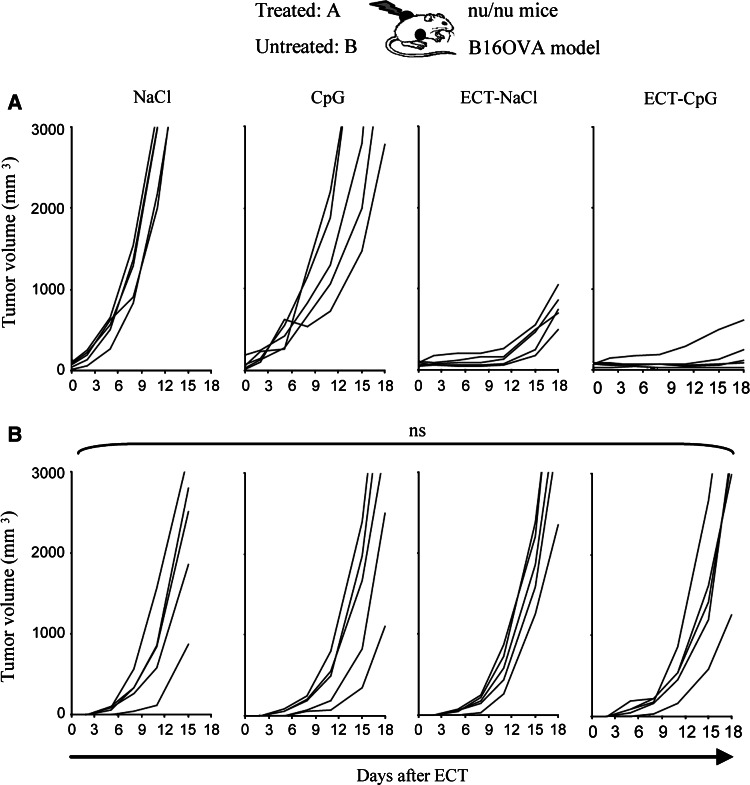
We investigated the role of T cells in the antitumor effects described above by reproducing the same experiments in nude mice. In the LPB model, the local synergistic effect of the association was not achieved and no tumor were rejected (Fig. 4a). Moreover, the systemic effect was totally abolished in these T cell deficient mice (Fig. 4b). In the B16OVA model, the local effect of the association was not significantly different from what was observed in immunocompetent mice, however, here again, the systemic effect of the combined treatment was abrogated (Fig. 5b). Thus, the systemic antitumor effect of the association ECT plus CpG ODN is dependent on the presence of T cells.
Fig. 4.
Effects of ECT-CpG ODN in nude mice bearing two LPB tumors. Nude mice were inoculated with 106 LPB cells on each side and were only treated on the left side when the tumor reached 5 mm diameter. ECT was performed as a single session treatment at day zero. A dose of 30 μg of CpG ODN was delivered into the left tumors at days 2 and 9. Results show the monitoring of the treated (a) and non-treated (b) tumors (n = 5–7 per group)
Fig. 5.
Effects of ECT-CpG ODN in nude mice bearing two B16OVA tumors. Nude mice were inoculated with 5.105 B16OVA cells on each side and were only treated on the left side when the tumor reached 5 mm diameter. ECT was performed as a single session treatment at day zero. A dose of 50 μg of CpG ODN was delivered into the left tumors at days 2 and 9. Results show the monitoring of the treated (a) and non-treated (b) tumors (n = 5–7 per group)
Specific immune response analysis
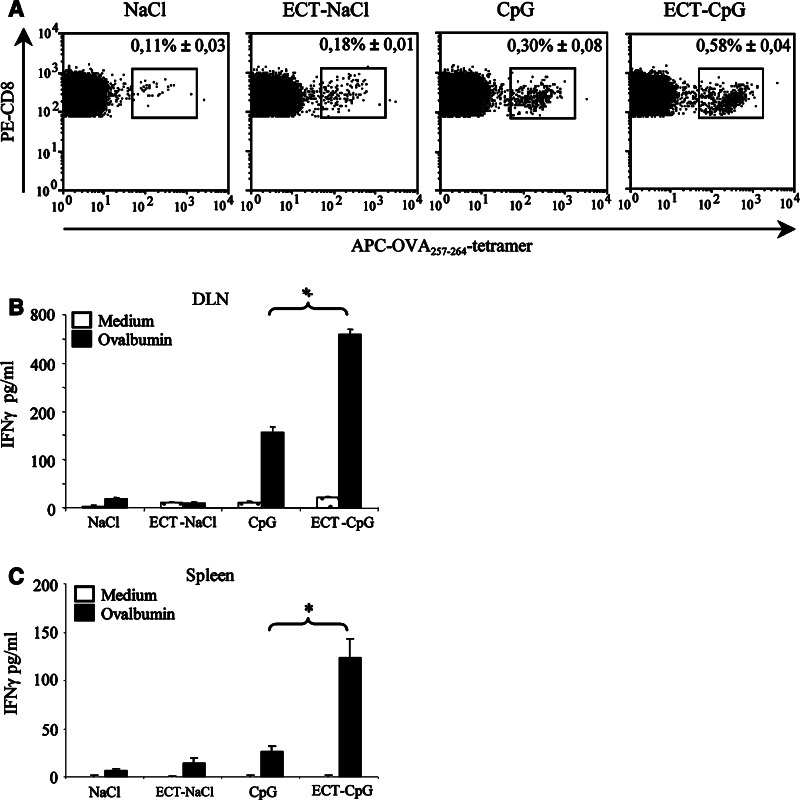
In order to identify antigen-specific T cells we used the B16OVA model. Treatments were performed on mice bearing a subcutaneous B16OVA tumor in the leg and we examined the frequency of OVA257–264-specific CD8 T cells within inguinal tumor-draining lymph nodes by tetramer staining. As shown in Fig. 6a, ECT-CpG ODN induced an increased frequency of tetramer positive CD8 T cells (0.58 ± 0.04%) compared to ECT alone (0.18 ± 0.01%) or CpG ODN alone (0.30 ± 0.08%). Tetramer staining of lymphocytes from non-tumor-bearing naïve mice revealed no non-specific fixation and mice immunized with OVA257–264 peptide plus CpG ODN into the footpad induced 0.45 ± 0.14% of positive tetramer cells among CD3 CD8 T cells in the draining lymph node (data not shown).
Fig. 6.
Specific immune response analysis. a Mice bearing a subcutaneous B16OVA tumor in the leg were treated by ECT, CpG ODN or their association. One day after intratumor injection of CpG ODN, mice were killed to remove the tumor-draining inguinal lymph nodes. Cells were harvested and freshly stained with OVA257–264-H-2Kb tetramers, anti-CD3 and anti-CD8 mAbs. Values indicate the percentage of tetramer-positive cells within CD3 and CD8 double positive cells. b After an in vitro stimulation of 48 h by ovalbumin, IFNγ production by T lymphocytes was determined in culture supernatants by ELISA. c Mice bearing a B16OVA tumor in the flank were treated and killed 10 days after the second injection of CpG ODN to remove spleens. Splenocytes were restimulated in vitro as above. Results represent the mean with SE from three mice per group in a representative experiment out of two (*P < 0.05)
More important than the number of specific T cells induced by this treatment, is the function of these T cells. Therefore, we studied the IFNγ producing capability of these cells both in the tumor-draining lymph node and in the spleen of treated animals. Our results showed that IFNγ production after a two days in vitro stimulation in the presence of ovalbumin was significantly increased in tumor-draining lymph nodes and in the spleen of the mice treated with the combined treatment compared to the mice receiving ECT or CpG ODN alone (Fig. 6b, c). These findings suggest that the combination of ECT and CpG ODN induce a specific functional activation of T cells both regionally (draining lymph node) and systemically (spleen).
Discussion
Association of tumor destruction and immune stimulation is an attractive way of tumor vaccination. The underlying rational is that tumor-associated-antigens (TAA) available on the tumor site after tumor destruction can be the source of vaccine antigens and that the combined use of an appropriate immune adjuvant can induce an immunogenic environment, allowing an efficient cross-presentation of TAA to the immune system. This paradigm has been shown to be efficient in several animal tumor models using various ways of tumor destruction like radiotherapy, chemotherapy or cryoablation combined with different immune-stimulating agents like anti-CTLA-4 antibodies [12] or CpG ODN [11, 22, 23, 35]. One major advantage of these endogenous vaccination strategies combining TAA exposure and APC activation is that they dispend from TAA preparation or administration, and from the difficult and expensive dendritic cell preparation from the patients’ monocytes or CD34 progenitors.
ECT represents an alternative method of tumor destruction applicable to non resectable cutaneous and subcutaneous primary or metastatic tumors. Since the first clinical trial [4] ECT has demonstrated high response rates on localized metastases. The multicentric ESOPE clinical study establishing the standard operating procedures of ECT [25] (the drug and its route of administration, the type of electrodes) reached an objective response rate of 85% including 73.7% of complete responses [20]. This study demonstrated that ECT efficacy is independent of the histology and the volume of the tumor. Nevertheless, ECT is a local treatment without any effect on the non-treated metastases. However, since the first in vivo applications of ECT to treat established tumors [26], it appeared that the immune system was partially involved as ECT was less efficient in nude mice than in immunocompetent animals [26, 27]. Similar results were found with ECT using cisplatin [33]. These results are in accordance with our data showing that several cytotoxic agents, like γ-irradiation or anthracyclines, also induce T cell-dependent antitumor effects (9, 29). In humans, ECT combined to IL-2 did not induce any effect on distant metastases [1]. We looked for an adjuvant delivering a danger signal and stimulating APC in situ which seems to us more suitable than IL-2, especially since IL-2 has been shown to amplify regulatory T lymphocytes in human [36].
First, we showed that ECT induced the recruitment of immune cells including activated DCs on the site of tumor damage (Fig. 1a; Table 1). We then tested by quantitative RT-PCR, the expression of various TLRs in the tumor after ECT and found that expression of TLR9 increased significantly more than the other TLRs tested, namely TLR3, TLR4 and TLR7. However it remains to be established whether dendritic cells and/or macrophages may account for tumor TLR9 increase among inflammatory cells recruited in situ. Furthermore, CpG ODN are known to induce a Th 1 type immune response and have already been proved to be efficient in several animal tumor models [7, 8, 14, 16]. Altogether, the results from our post-ECT tumor TLR9 expression and DCs recruitment led us to test the combined treatment: ECT followed by intratumoral CpG ODN injections.
Our results demonstrated both local and systemic effects of ECT-CpG ODN in several murine tumor models. On the one hand, ECT-CpG ODN demonstrated a local synergistic efficacy in treating established tumors. Complete regressions of LPB tumors were enhanced from 43 to 100% when CpG ODN were combined to ECT (Fig. 2a). In a more aggressive tumor model like B16OVA melanoma, ECT-CpG ODN combination induced a growth arrest of the tumors whereas ECT or CpG ODN alone remained poorly efficient (Fig. 3a). More importantly, ECT-CpG ODN triggered a systemic antitumor immune response. Indeed, the combined treatment induced complete rejections in 57% of the distant untreated LPB tumors (Fig. 2b) and induced a specific immune memory, protecting the mice against a tumor rechallenge 2 months after tumor rejection. In the B16OVA model, ECT-CpG ODN combination was able to significantly reduce tumor growth of the distant tumor (Fig. 3b). As expected, CpG ODN or ECT alone had respectively little or no effect on the growth of the distant tumor in the two models.
Disappearance of this systemic effect in nude mice (Figs. 4, 5b) illustrated the critical role of T cell mediated immune response in the distant antitumor effect. We showed that the combined treatment could enhance the frequency of tumor specific CD8 T cells in the tumor-draining lymph nodes (Fig. 6a). Moreover these T cells were able to secrete a large amount of IFNγ in response to an in vitro specific stimulation (Fig. 6b). To explore the systemic T cell specific response, we performed the same functional assay in the spleen and found a highly significant increase of INFγ production by splenocytes from mice treated with the combination compared to mice treated by ECT or CpG ODN alone (Fig. 6b). Therefore, the combined treatment induces a distant antitumor response dependent on functional tumor specific T cells. We can hypothesize that tumor-antigens available after ECT-induced cell death could be efficiently taken-up by DCs recruited following ECT that are locally activated by CpG ODN. These activated DCs might further migrate to draining lymph nodes to induce an efficient systemic immune response.
Both ECT and CpG ODN are already available for treatment in humans. ECT is simple, reproducible and can be performed under local anaesthesia. CpG ODN tested in cancer patients seemed to show promising results with a good tolerance profile in preliminary studies [5, 18]. However, in the case of large tumors CpG ODN efficiency decreases [17] suggesting that this adjuvant must be used with additional tumor reduction strategies. The combination of immunotherapy with tumor reduction treatments in order to use the tumor as a vaccine could be a new challenge for the next years.
Since TLR9 is differentially expressed in mice and humans, i.e. on myeloid DCs and monocytes/macrophages in mice and plasmacytoid DCs and B cells in humans, it is possible that other TLR ligands might be more adapted than TLR9 ligands for a therapeutic use in association with ECT in humans. Furthermore, several studies have also suggested that the optimal CpG motif might differ between mice and humans [2, 13]. However, our preliminary results showed an increase of TLR9 mRNA expression 24 h after ECT, compared to non-treated tumors, in two out of three patients presenting skin melanoma metastases (data not shown). The CpG ODN we used here harbor the 5′-AACGTT motif that has a good efficacy in both human and rodents [6]. Therefore, we think that this particular association is promising and should be evaluated for its efficacy in patients presenting with cutaneous or subcutaneous metastases.
Acknowledgments
We acknowledge Véronique Scott and Stéphanie Esselin for their technical assistance in the development of RT-PCRs and Elisabeth Connault for excellent technical assistance with immunohistochemistry. Grant support CNRS and Institut Gustave Roussy. Stéphan Roux was supported by Fondation pour la Recherche Médicale.
References
- 1.Andersen MH, Gehl J, Reker S, et al. Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol. 2003;13:449–459. doi: 10.1016/j.semcancer.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 2.Bauer S, Kirschning CJ, Hacker H, et al. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci USA. 2001;98:9237–9242. doi: 10.1073/pnas.161293498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jr Belehradek J, Barski G, Thonier M. Evolution of cell-mediated antitumor immunity in mice bearing a syngeneic chemically induced tumor. Influence of tumor growth, surgical removal and treatment with irradiated tumor cells. Int J Cancer. 1972;9:461–469. doi: 10.1002/ijc.2910090302. [DOI] [PubMed] [Google Scholar]
- 4.Belehradek M, Domenge C, Luboinski B, Orlowski S, Jr Belehradek J, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I–II trial. Cancer. 1993;72:3694–3700. doi: 10.1002/1097-0142(19931215)72:12<3694::AID-CNCR2820721222>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A, Laigle-Donadey F, Zohar S, et al. Phase 1 trial of a CpG oligodeoxynucleotide for patients with recurrent glioblastoma. Neuro Oncol. 2006;8:60–66. doi: 10.1215/S1522851705000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpentier AF, Auf G, Delattre JY. CpG-oligonucleotides for cancer immunotherapy: review of the literature and potential applications in malignant glioma. Front Biosci. 2003;8:115. doi: 10.2741/934. [DOI] [PubMed] [Google Scholar]
- 7.Carpentier AF, Chen L, Maltonti F, Delattre JY. Oligodeoxynucleotides containing CpG motifs can induce rejection of a neuroblastoma in mice. Cancer Res. 1999;59:5429–5432. [PubMed] [Google Scholar]
- 8.Carpentier AF, Xie J, Mokhtari K, Delattre JY. Successful treatment of intracranial gliomas in rat by oligodeoxynucleotides containing CpG motifs. Clin Cancer Res. 2000;6:2469–2473. [PubMed] [Google Scholar]
- 9.Casares N, Pequignot MO, Tesniere A, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chu RS, Targoni OS, Krieg AM, Lehmann PV, Harding CV. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.den Brok MH, Sutmuller RP, Nierkens S, et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 2006;66:7285–7292. doi: 10.1158/0008-5472.CAN-06-0206. [DOI] [PubMed] [Google Scholar]
- 12.den Brok MH, Sutmuller RP, van der Voort R, et al. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann G, Krieg AM. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J Immunol. 2000;164:944–953. doi: 10.4049/jimmunol.164.2.944. [DOI] [PubMed] [Google Scholar]
- 14.Heckelsmiller K, Rall K, Beck S, et al. Peritumoral CpG DNA elicits a coordinated response of CD8 T cells and innate effectors to cure established tumors in a murine colon carcinoma model. J Immunol. 2002;169:3892–3899. doi: 10.4049/jimmunol.169.7.3892. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 16.Kawarada Y, Ganss R, Garbi N, Sacher T, Arnold B, Hammerling GJ. NK- and CD8(+) T cell-mediated eradication of established tumors by peritumoral injection of CpG-containing oligodeoxynucleotides. J Immunol. 2001;167:5247–5253. doi: 10.4049/jimmunol.167.9.5247. [DOI] [PubMed] [Google Scholar]
- 17.Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6:88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 18.Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 2006;5:471–484. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 19.Lake RA, Robinson BW. Immunotherapy and chemotherapy—a practical partnership. Nat Rev Cancer. 2005;5:397–405. doi: 10.1038/nrc1613. [DOI] [PubMed] [Google Scholar]
- 20.Marty M, Sersa G, Garbay JR, et al. Electrochemotherapy—an easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur J Cancer Supp. 2006;4:3–13. doi: 10.1016/j.ejcsup.2006.08.002. [DOI] [Google Scholar]
- 21.Mekid H, Tounekti O, Spatz A, Cemazar M, El Kebir FZ, Mir LM. In vivo evolution of tumour cells after the generation of double-strand DNA breaks. Br J Cancer. 2003;88:1763–1771. doi: 10.1038/sj.bjc.6600959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meng Y, Carpentier AF, Chen L, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116:992–997. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- 23.Milas L, Mason KA, Ariga H, et al. CpG oligodeoxynucleotide enhances tumor response to radiation. Cancer Res. 2004;64:5074–5077. doi: 10.1158/0008-5472.CAN-04-0926. [DOI] [PubMed] [Google Scholar]
- 24.Mir LM. Bases and rationale of the electrochemotherapy. Eur J Cancer Supp. 2006;4:38–44. doi: 10.1016/j.ejcsup.2006.08.005. [DOI] [Google Scholar]
- 25.Mir LM, Gehl J, Sersa G, et al. Standard operating procedures of the electrochemotherapy: instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses delivered by the CliniporatorTM by means of invasive or non-invasive electrodes. Eur J Cancer Supp. 2006;4:14–25. doi: 10.1016/j.ejcsup.2006.08.003. [DOI] [Google Scholar]
- 26.Mir LM, Orlowski S, Belehradek JJr, Paoletti C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer. 1991;27:68–72. doi: 10.1016/0277-5379(91)90064-K. [DOI] [PubMed] [Google Scholar]
- 27.Mir LM, Orlowski S, Poddevin B, Belehradek JJr Electrochemotherapy tumor treatment is improved by interleukin-2 stimulation of the host’s defenses. Eur Cytokine Netw. 1992;3:331–334. [PubMed] [Google Scholar]
- 28.Mir LM, Roth C, Orlowski S, et al. Systemic antitumor effects of electrochemotherapy combined with histoincompatible cells secreting interleukin-2. J Immunother Emphasis Tumor Immunol. 1995;17:30–38. doi: 10.1097/00002371-199501000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13(1):54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 30.Orlowski S, Mir LM. Cell electropermeabilization: a new tool for biochemical and pharmacological studies. Biochim Biophys Acta. 1993;1154:51–63. doi: 10.1016/0304-4157(93)90016-h. [DOI] [PubMed] [Google Scholar]
- 31.Roman M, Martin-Orozco E, Goodman JS, et al. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat Med. 1997;3:849–854. doi: 10.1038/nm0897-849. [DOI] [PubMed] [Google Scholar]
- 32.Sersa G. The state-of-the-art of electrochemotherapy before the ESOPE study; advantages and clinical uses. Eur J Cancer Supp. 2006;4:52–59. doi: 10.1016/j.ejcsup.2006.08.007. [DOI] [Google Scholar]
- 33.Sersa G, Miklavcic D, Cemazar M, Belehradek JJr, Jarm T, Mir LM. Electrochemotherapy with CDDP on LPB sarcoma: comparison of the anti-tumor effectiveness in immunocompetent and immunodeficient mice. Bioelectrochem Bioenerg. 1997;43:279–283. doi: 10.1016/S0302-4598(96)05194-X. [DOI] [Google Scholar]
- 34.Wagner H. Bacterial CpG DNA activates immune cells to signal infectious danger. Adv Immunol. 1999;73:329–368. doi: 10.1016/S0065-2776(08)60790-7. [DOI] [PubMed] [Google Scholar]
- 35.Weigel BJ, Rodeberg DA, Krieg AM, Blazar BR. CpG oligodeoxynucleotides potentiate the antitumor effects of chemotherapy or tumor resection in an orthotopic murine model of rhabdomyosarcoma. Clin Cancer Res. 2003;9:3105–3114. [PubMed] [Google Scholar]
- 36.Zhang H, Chua KS, Guimond M, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4 + CD25+ regulatory T cells. Nat Med. 2005;11:1238–1243. doi: 10.1038/nm1312. [DOI] [PubMed] [Google Scholar]