Abstract
We have previously reported that a single-chain T cell receptor/IL-2 fusion protein (scTCR-IL2) exhibits potent targeted antitumor activity in nude mice bearing human tumor xenografts that display cognate peptide/HLA complexes. In this study, we further explore the mechanism of action of this molecule. We compared the biological activities of c264scTCR-IL2, a scTCR-IL2 protein recognizing the aa264–272 peptide of human p53, with that of MART-1scTCR-IL2, which recognizes the MART-1 melanoma antigen (aa27–35). In vitro studies showed that c264scTCR-IL2 and MART-1scTCR-IL2 were equivalent in their ability to bind cell-surface IL-2 receptors and stimulate NK cell responses. In mice, MART-1scTCR-IL2 was found to have a twofold longer serum half-life than c264scTCR-IL2. However, despite its shorter serum half-life, c264scTCR-IL2 showed significantly better antitumor activity than MART-1scTCR-IL2 against p53+/HLA-A2+ tumor xenografts. The more potent antitumor activity of c264scTCR-IL2 correlated with an enhanced capacity to promote NK cell infiltration into tumors. Similar differences in antigen-dependent tumor infiltration were observed with activated splenocytes pre-treated in vitro with c264scTCR-IL2 or MART-1scTCR-IL2 and then transferred into p53+/HLA-A2+ tumor bearing recipients. The data support a model where c264scTCR-IL2 activates immune cells to express IL-2 receptors. Following stable interactions with cell-surface IL-2 receptors, c264scTCR-IL2 fusion molecule enhances the trafficking of immune cells to tumors displaying target peptide/HLA complexes where the immune cells mediate antitumor effects. Thus, this type of fusion molecule could be used directly as a targeted immunotherapeutic or in adoptive cell transfer approaches to activate and improve the anti-cancer activities of immune cells by providing them with pre-selected antigen recognition capability.
Keywords: T cell receptors, p53, Cytokines, Tumor immunity, Natural killer cells
Introduction
IL-2 is a pleiotropic cytokine produced by activated T lymphocytes that regulates survival, proliferation and/or differentiation of B cell, T cells, monocytes, macrophages, and natural killer (NK) cells [11, 26]. Recombinant human IL-2 (rhIL-2, aldesleukin) immunotherapy has been approved for treatment of patients with metastatic melanoma and renal cell carcinoma based on partial and complete, durable responses observed in a subset of patients and is currently a standard agent for these diseases [1, 29]. While the precise mechanisms by which rhIL-2 exerts its antitumor effects are not fully understood, it is likely that the expansion of NK cells, T cells and other lymphokine activated killer (LAK) cells and stimulation of their cytolytic activities by rhIL-2 plays a significant role. Unfortunately, standard high dose rhIL-2 treatment is associated with acute toxicity due in part to a capillary leak syndrome that restricts its use to relatively healthy patients in closely monitored clinical settings such as intensive care units [29].
Several different strategies have been explored to reduce the systemic toxic side effects of rhIL-2 by targeting the immunostimulatory activity of the cytokine to the tumor site. Administration of rhIL-2 directly into or around the tumor has been tested in animal studies and in patients with metastatic melanoma and other cancers [13, 24]. In a phase II trial, intratumoral IL-2 was found to be well tolerated and had activity against soft-tissue melanoma metastases, indicating that high concentrations of the tumor-localized cytokine may be beneficial [24]. Unfortunately, intratumoral IL-2 treatment did not affect the appearance or progression of distant metastases or micrometastases, potentially limiting this treatment to readily identified, superficially accessible tumors. An alternative means of delivering IL-2 intratumorally is to target it to the tumor site with antibody recognition domains directed against tumor associated antigens [7]. Antibody–cytokine fusion proteins comprised of IL-2 genetically linked to Ab domains specific for a variety of cell surface antigens have been generated and characterized in vitro and in vivo [2, 6, 18, 32, 33]. Studies in tumor bearing mice have demonstrated that systemic administration of these fusion proteins resulted in specific localization of IL-2 to tumors expressing the target antigen and provided more effective antitumor responses than were observed with equivalent doses of IL-2 alone or in combination with free Ab. The antitumor responses of the Ab-cytokine fusions were found to be mediated by T-cells and/or NK cells and in some cases, Ab-cytokine treatment resulted in the development of long-lived and transferable antitumor immunity [2, 17, 32]. Adoptive cell transfer therapies also provide evidence for a tumor targeted mechanism of action mediated by IL-2 stimulated immune effector cells. For example, clinical protocols involving ex vivo expansion of autologous tumor infiltrating lymphocytes followed by their adoptive transfer and expansion in vivo have shown promising results in the regression of large metastases in patients with metastatic melanoma [10].
In developing a broad based approach for treating cancer, we have been particularly interested in targeting the human p53 protein. This intracellular tumor suppressor protein is mutated in approximately half of all human cancers, leading to its increased protein stability and subsequent accumulation [16]. In addition, p53 mutation/overexpression correlates with tumor transformation and aggression and is associated with lower overall survival rates and resistance to chemotherapeutic intervention in cancer patients [28]. Since p53 protein is not expressed on the tumor cell surface, most immunotherapeutic strategies have focused on augmenting cellular responses to p53 derived peptide antigens. Indeed, p53 peptides displayed on the tumor cell surface in the context of MHC can be recognized by T-cells from cancer patients [12]. In addition, we have recently confirmed that the peptide epitope spanning the amino acid residues 264–272 of the p53 protein presented by HLA-A2 is significantly elevated in a wide range of human tumor tissues [36]. As a result, we have been exploring approaches using soluble p53 peptide-reactive TCRs as immunotherapeutic reagents for targeting p53+ cancers [3, 4, 21].
In one such approach, we have created a soluble fusion protein comprising rhIL-2 linked to a three domain HLA-A2.1-restricted single-chain TCR (scTCR) specific for p53 (aa264–272). We have demonstrated that this fusion protein exhibits potent activity against p53+/HLA-A2+ human melanoma (A375), mammary adenocarcinoma (MDA-MB-231) and pancreatic (PANC1) tumors in xenograft models and that this activity is likely mediated by NK cell activation [3, 4]. However, the underlying mechanism of action of this type of targeted immunotherapeutic agent is largely unknown. The studies reported here reveal a novel mechanism of action of the scTCR-IL2 fusion construct that challenges the concept that tumor-specific immunotherapeutic agents first bind target antigens in the tumor microenvironment and then provide localized effector function by attracting cytotoxic immune cells.
Materials and methods
Materials
Athymic nude mice (Nu/Nu) (females, 5–6 week old) were purchased from Harlan (Indianapolis, Indiana). CHO, T2, IL-2-dependent CTLL-2, human melanoma A375 (HLA-A2+/p53+), BF1, and W4F hybridoma cell lines were obtained from American Type Culture Collection (Rockville, MD). BF1 and W4F hybridoma cell lines produce mAbs that recognize different epitopes in the human TCR beta constant region. All other Abs were obtained from BD Bioscience (San Diego, CA), unless otherwise noted. HLA-A2.1/Kb transgenic mice, kindly provided by Dr. L. Sherman (Scripps Research Institute, La Jolla, CA), were bred and maintained at Altor’s animal facility. Cell culture media were purchased from CellGro/Mediatech (Herndon, VA) and fetal bovine serum was obtained from HyClone (Logan, Utah). Human PBMCs were isolated from human blood buffy coats (Community Blood Center, Miami, FL) by gradient centrifugation through HistoPague (Sigma-Aldrich, St Louis, MO). Cell lines were grown at 37°C with 5% CO2 in complete culture medium (IMDM supplemented with 10% FBS plus 2 mM l-glutamine). CTLL-2 cells were maintained in the same medium with the addition of 50 ng/ml rhIL-2. Mouse splenocytes were maintained in RPMI medium supplemented with 10% FBS (RPMI-10).
Preparation of c264scTCR-IL2 and MART-1scTCR-IL2
The c264scTCR-IL2 fusion protein was prepared as described previously [3]. The MART-1 (aa27–35)/HLA-A2.1 specific TCR alpha and beta genes from the human CTL clone F5 were kindly provided by Drs. Richard A. Morgan and Steven A Rosenberg, National Cancer Institute [14]. To generate the MART-1scTCR-IL2 expression construct, two primers (5′-CCAGGTTCGACCGGTCAGAAGGAGGTGGAGCAGAATTCTGG-3′ and 5′-CCGCCACCGCTAGCACCACCCCCGCTCCCACCCCCACCACTAGTGTCGGGTTTCACAGATAACTCCGTTCCCTG-3′) were used to amplify the TCR alpha chain and two primers (5′-GGTGGTGCTAGCGGTGGCGGCGGTTCTGGCGGTGGCGGTTCCTCCAGCATTGCAGGGATCACCCAGGCACCAAC-3′ and 5′-GACCGCCATCGATGTTAACGTCTGCTCTACCCCAGGCCTCG-3′) were used to amplify the TCR beta chain. The amplified products were ligated into a vector containing sequences encoding a signal peptide and human IL-2. The DNA fragment, encoding the signal peptide—scTCR (TCR Vα–linker–TCR Vβ–TCR Cβ)—IL-2, was isolated and ligated into pNEF38 expression vector [27]. The MART-1scTCR-IL2 fusion protein was produced by CHO cells transfected with the expression vector construct and purified by antibody immunoaffinity chromatography, as described previously [3].
In vitro assays
Bioassays for the IL-2 activity of the scTCR-IL2 fusion proteins were conducted using CTLL-2 proliferation as an end point as described previously [4] with the modification that 2 × 103 cells/well were incubated with fusion protein for 2 days prior to determination of cell proliferation using WST-1 reagent. To measure binding activity of the TCR domain, T2 cells (2 × 106 cells/ml) were loaded with 20 μg/ml of p53 aa264–272 or MART-1 aa27–35 peptides in the presence of peptide-loading enhancer (PLE) (Altor BioScience Corp., Miramar, FL) for 3 h at 37°C. Peptide loaded T2 cells or unmanipulated A375 tumor cells (2 × 105 cells/50 μl) were incubated with 0.5 μg of PE-conjugated 264scTCR or MART-1scTCR multimer for 60 min at room temperature. Cells were washed once in PBS containing 0.5% BSA and 0.05% sodium azide and analyzed with flow cytometry on a FACScan flow cytometry instrument (BD Biosciences). Data were analyzed by the CellQuest software, version 3.3 (BD Biosciences).
Cytotoxicity assay
Murine splenocytes were prepared from female athymic nude mice and cultured (1 × 107 cells/well) in the presence of c264scTCR-IL2 (800 ng/ml) or MART-1scTCR-IL2 (800 ng/ml) for 4 days to generate LAK cells. A375 and YAC-1 target cells were labeled with 50 μg/ml calcein AM (Molecular Probes, Eugene, OR) for 60 min at 37°C and washed twice. The activated LAK cells were mixed with 2 × 104 target cells in 0.2 ml of RPMI-10 medium and incubated at 37°C. The fluorescent intensity (FI) of the supernatant was measured after 2 h with a fluorescent plate reader (excitation wavelength = 485 nm; emission wavelength = 538 nm; cutoff = 530 nm). The specific cytotoxicity was calculated using the following formula: percentage of cytotoxicity = (FI of test sample − FI of target cells with medium)/(FI of target cells treated with 0.04% Triton X-100 − FI of target cells with medium) × 100. In the case where the FI of target cells with medium (spontaneous release) exceeded the value measured in the test sample (containing target cells and effector cells), the percent cytotoxicity was assumed to be zero.
Binding half-life determination of fusion proteins to IL-2 receptor (IL-2R) bearing CTLL-2 cells
CTLL-2 cells or activated human PBMC (incubated with 50 ng/ml of rhIL-2 for 10 days) were washed twice with culture medium and incubated for 30 min at 37°C to further remove the residual IL-2. Cells were then incubated with c264scTCR-IL2 or MART-1scTCR-IL2 at 24 μg/ml, or rhIL-2 at 6 μg/ml (molar equivalent concentration as scTCR-IL2 fusion proteins) for 20 min at 4°C in order to saturate the IL-2R with ligands. Following a wash step, cells were incubated for 0–180 min at 37°C to allow internalization or disassociation of the ligands from the IL-2R. At each time point, cells were transferred to 4°C to stop the receptor internalization. PE-conjugated mouse anti-human IL-2 mAb (4 μg/ml) was then added and cells were incubated for 15 min at 4°C. The cells were washed with PBS containing 0.5% BSA and 0.05% sodium azide and analyzed by flow cytometry. Mean fluorescence intensity (MFI) was determined for each sample and was corrected for background staining of control cells incubated with PE-conjugated anti-human IL-2 Ab alone. Percent cells staining positive at each time point was calculated as (corrected MFI at the time point)/(corrected MFI at time zero) × 100. Data were analyzed using one phase exponential decay curve-fitting model (GraphPad Prism version 4, GraphPad Software, Inc., San Diego, CA) to determine the cell surface residence time of the fusion proteins or rhIL-2.
In vivo activation and pharmacokinetic studies
Female athymic nude mice were subcutaneously injected with 1 × 106 A375 tumor cells on the dorsal side of the animal. Tumors were allowed to grow to about 3 mm in diameter (13.5 mm3) prior to treatment and mice with established tumors were randomized. Mice were then intravenously injected daily with c264scTCR-IL2 or MART-1scTCR-IL2 at 32 μg/mouse at the time points indicated in the figures. Mice were sacrificed at each sampling time point (2 h after treatment unless otherwise stated) and tissues (spleen, lymph nodes, bone marrow and primary tumor) were collected. Some of the collected tissues were frozen and stored for subsequent immunohistochemistry (IHC). For other samples, single cell suspensions were prepared for flow cytometric analyses by homogenizing tissues in Hank’s Balanced Salt Solution through a metal screen and passing through a 19-gauge needle three times. The red blood cells were lysed in lysing buffer (0.16 M NH2Cl, 0.17 M Tris, pH 7.6). Cell surface markers were monitored by flow cytometry with antibodies against mouse CD25, CD122 or CD49b (pan-NK, eBioscience, San Diego, CA). Isotype matched control mAbs were used as controls.
The pharmacokinetic behavior of MART-1scTCR-IL2 was evaluated in female HLA-A2.1/Kb transgenic mice (4–6 mice/time point) as previously described for c264scTCR-IL2 and rhIL-2 [3]. MART-1scTCR-IL2 serum concentration was evaluated in assays that detected either the intact fusion protein (anti-IL2 ELISA) or the TCR domain of the molecule only (W4F ELISA) [3].
Adoptive transfer of in vivo activated and scTCR-IL2 fusion protein-coated mouse splenocytes
A375 primary tumor bearing athymic nude mice were treated with c264scTCR-IL2 (32 μg/dose) or MART-1scTCR-IL2 (32 μg/dose) for four consecutive daily injections to activate the immune cells. Single cell suspensions of splenocytes from the donor mice were prepared. Depending on the previous in vivo treatment, the splenocytes (1 × 107 cells) were incubated with 0.5 mg/ml of either c264scTCR-IL2 or control MART-1scTCR-IL2 for 30 min on ice. The fusion protein-coated splenocytes (5 × 106 cells) were washed and injected into A375 tumor-bearing nude mice recipients i.v. through the lateral tail vein. Tumor xenografts were collected 24 h after adoptive transfer and tumor cryosections were stained with anti-mouse CD45R and anti-human TCR constant domain antibodies.
IHC staining of tissue infiltrating immune cells
Tissue and tumors collected from treated mice were frozen in OCT compound (Sakura Finetek USA, Inc., Torrance, CA) and stored at −70°C. Cryosections (5 μm) were prepared with a Cryostat (Cryocut 1800, Leica Microsystems, Nossloch, Germany). To stain the cryosections, the slides were washed in TBST before quenching endogenous peroxidase activity with 3% H2O2 and blocking endogenous biotin with avidin/biotin reagents (Biotin Blocking System, DAKO, Carpinteria, CA). Primary antibodies, rat anti-mouse CD45R or CD19 mAbs (0.3 μg/ml) or biotinylated BF-1 mAb (10 μg/ml), in dilution buffer (5% normal mouse serum, 1% BSA in PBS) were applied onto the slides and incubated for 1 h. The slides were washed twice with TBST before addition of secondary detection reagents (2.5 μg/ml biotinylated mouse polyclonal anti-rat IgG for anti-CD45R or anti-CD19 mAb) for 1 h. After wash steps, the cryosections were incubated with peroxidase-conjugated streptavidin solution and visualized with a DAB substrate chromogen solution (DAKO). The slides were counterstained with hematoxylin and mounted. The immunohistochemistry evaluation of immune cell staining was carried out independently by J.H. and Z.X. blinded for treatment status. At least 10 fields of view per slide were counted for the number of positively stained cells.
In vivo efficacy in xenograft mouse model
Antitumor activity of c264scTCR-IL2 or MART-1scTCR-IL2 was assessed in the A375 xenograft tumor model described previously [3]. Briefly, A375 tumor bearing female nude mice (8 per group) were treated intravenously with c264scTCR-IL2 or MART-1scTCR-IL2 at 10 μg/dose daily for four days followed by a 10-day rest and four additional daily injections. Tumors were measured using a caliper and the tumor volumes were calculated using an equation of L × W 2/2, where L is the length (mm) and W the width (mm) of the tumor. The Altor Bioscience Corp. Institutional Animal Care and Use Committee approved all animal studies.
Statistical analysis
Results are expressed as mean ± SE or median and range when indicated. Two-tailed Mann–Whitney test or ANOVA with Bonferroni posttest was used to compare groups. P values of 0.05 or less were considered statistically significant.
Results
TCR-IL2 fusion proteins exhibited comparable IL-2 potency in vitro.
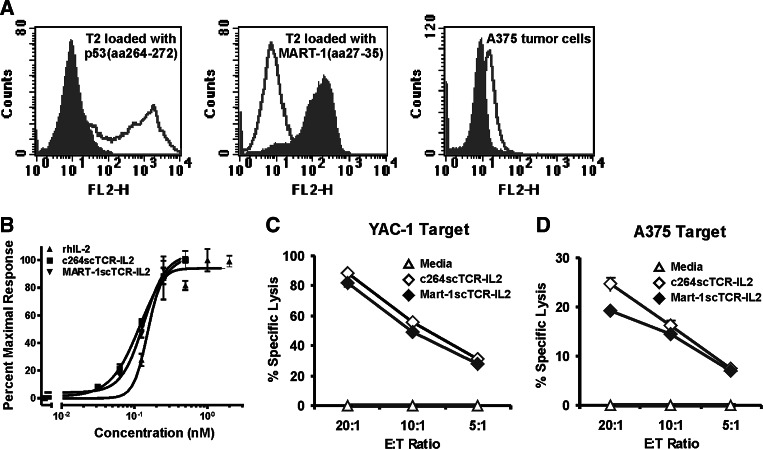
We have previously demonstrated that the bispecific scTCR-IL2 fusion protein (c264scTCR-IL2) is capable of binding p53 (aa264–272) peptide in the context of HLA-A2.1 displayed on p53-positive A375 melanoma cells. This fusion protein also exhibits potent antitumor activity against primary subcutaneous A375 tumor xenografts in athymic nude mice. To better understand the role of tumor antigen targeting in the activity of this protein, we created a control fusion protein (MART-1scTCR-IL2) comprising rhIL-2 genetically linked to a scTCR molecule specific to the melanoma peptide antigen, MART-1 (aa27–35), presented in the context of HLA-A2.1. This control MART-1scTCR fusion protein was shown to recognize HLA-A2.1-positive T2 cells loaded with MART-1 (aa27–35) peptide but not cells loaded with p53 (aa264–272) peptide (Fig. 1a). In addition, MART-1scTCR does not recognize human A375 melanoma cells, which do not express MART-1 [35], whereas 264scTCR-based reagents detected p53 (aa264–272)/HLA-A2.1 complexes presented on these cells (Fig. 1a) [36]. Thus, the MART-1scTCR-IL2 protein has utility as a non-targeted control reagent in determining the antitumor mechanism of action of the p53-specific scTCR-IL2 fusion protein.
Fig. 1.
Binding and stimulatory activity of MART-1scTCR and c264scTCR fusion proteins. a Flow cytometry analyses was carried out on T2 antigen presenting cells loaded with p53 (aa264–272) peptide (left) or MART-1 (aa27–35) peptide (middle) and on unmanipulated A375 tumor cells (right) stained with PE-conjugated 264scTCR (dark line) or MART-1scTCR multimer (gray shading). b CTLL-2 cells were subjected to WST-1 assays after 2 days of culture with c264scTCR-IL2, MART-1scTCR-IL2 or rhIL-2. Each curve represents normalized proliferative responses. Data are mean ± SE of triplicates. EC50 and 95% CI interval were estimated by four-parameter logistic curve fitting analysis. c, d Lytic activity of scTCR-IL2 fusion protein-activated nude mouse splenocytes was compared to control splenocytes using YAC-1 (c) or A375 (d) target cells at different E:T ratios. Data are mean ± SE of triplicates and are representative results of two independent experiments
To verify the control MART-1scTCR-IL2 construct retains IL-2 bioactivity previously demonstrated for c264scTCR-IL2 [3], CTLL-2 cells were cultured with either MART-1scTCR-IL2, 264scTCR/IL-2 or rhIL-2 at various concentrations, and cell proliferation was assessed using WST-1. For each protein, a dose-dependent response of CTLL-2 proliferation was observed and the protein concentration providing a half-maximal effect (EC50) was determined (Fig. 1b). Given the fourfold difference in molecular weight between rhIL-2 and the scTCR-IL2 fusion proteins, the three proteins exhibited similar activity on an equal molar basis (EC50: rhIL-2, 0.15 nM; c264scTCR-IL2, 0.12 nM; and MART-1scTCR-IL2, 0.13 nM), indicating that c264scTCR-IL2 and MART-1scTCR-IL2 fusion proteins have equivalent IL-2 bioactivity.
The capability of these proteins to stimulate LAK/NK cell responses was also examined. Athymic nude mouse splenocytes were incubated for four days in media containing equivalent amounts of c264scTCR-IL2 or MART-1scTCR-IL2. Splenocytes were then mixed with target cells at different ratios to determine cell-mediated cytotoxic activity. Both c264scTCR-IL2 and MART-1scTCR-IL2 stimulated the lytic activity of splenocytes to a similar degree against either the NK-sensitive YAC-1 target cells or the p53-positive human A375 tumor cell line (Fig. 1c, d). Induced expression of degranulation markers, CD107a/b, on the scTCR-IL2-activated splenocytes was also observed following incubation with target cells and correlated with the in vitro cytotoxicity (data not shown). Recombinant human IL-2 also could stimulate nude mouse splenocyte cytotoxicity against the A375 tumor cells (data not shown) indicating that tumor antigen targeting per se is not required for LAK cell activity in vitro.
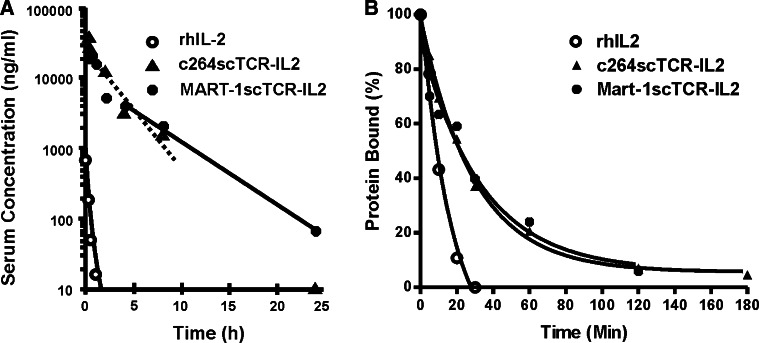
Comparison of the pharmacokinetic profiles of c264scTCR-IL2 and MART-1scTCR-IL2
We had previously determined that the c264scTCR-IL2 fusion protein had approximately a fivefold longer terminal half-life than rhIL-2 in a HLA-A2/Kb-transgenic mouse strain [3]. Prolonged serum half-lives have also been observed with other IL-2 fusion proteins [7, 19]. To assess whether the control MART-1scTCR-IL2 fusion protein has similar properties, its pharmacokinetic parameters were determined in HLA-A2.1/Kb transgenic mice. These mice were used to provide a more “humanized” model since the presence of the HLA-A2.1 domain, for which MART-1scTCR is restricted, may affect the pharmacokinetics of the fusion protein. As shown in Table 1, the terminal half-lives of rhIL-2, c264scTCR-IL2 and MART-1scTCR-IL2 were determined to be about 0.33, 1.84 and 3.50 h, respectively. Thus, MART-1scTCR-IL2 has a serum half-life about 1.9 times longer than c264scTCR-IL2 and about 11 times longer that rhIL-2 (Fig. 2a; Table 1). Consistent with the results previously observed for c264scTCR-IL2 [3], pharmacokinetics of MART-1scTCR-IL2 were equivalent whether assessed by the anti-IL-2 or W4F Ab-based ELISAs, indicating that the bi-functional fusion protein was not cleaved apart in vivo.
Table 1.
Pharmacokinetic parameters of rhIL-2, c264scTCR-IL2 and MART-1scTCR-IL2 in HLA-A2/Kb-transgenic mice
| Parameter | rhIL-2 | c264scTCR-IL2 | MART-1scTCR-IL2 | ||
|---|---|---|---|---|---|
| W4F ELISA | α-IL2 ELISA | W4F ELISA | α-IL2 ELISA | ||
| Dose (mg/kg) | 0.4 | 1.60 | 1.60 | 1.60 | 1.60 |
| AUC∞(obs.) (h ng/ml) | 213.08 | 64,185.37 | 72,605.84 | 67,068.41 | 59,414.29 |
| AUC∞(obs.)/D (h ng/ml/ng) | 0.03 | 2.01 | 2.27 | 2.10 | 1.86 |
| Cl(obs.) (ml/h) | 37.55 | 0.50 | 0.44 | 0.48 | 0.54 |
| Cmax (ng/ml) | 1,637.52 | 43,684.77 | 51,722.81 | 27,795.81 | 22,456.62 |
| Rsq (adj.) | 1.00 | 0.92 | 0.92 | 0.99 | 1.00 |
| t1/2 terminal (h) | 0.33 | 1.83 | 1.84 | 3.61 | 3.32 |
| Vss(obs.) (ml) | 7.47 | 1.24 | 1.10 | 2.02 | 2.13 |
| Vz(obs.) (ml) | 17.78 | 1.32 | 1.17 | 2.49 | 2.58 |
Fig. 2.
Pharmacokinetic profile and cellular IL-2R binding properties of rhIL-2, c264scTCR-IL2 and MART-1scTCR-IL2. a c264scTCR-IL2, MART-1scTCR-IL2 and rhIL-2 were administered intravenously to female HLA-A2.1/Kb-transgenic mice and serum levels were measured by a human TCR-specific ELISA. Data are mean ± SE of five serum samples per time point. Equivalent results were observed when serum protein concentrations were determined with the anti-IL2 ELISA format (see Table 1). Curves represent fit of data to a non-compartmental model. b Cell surface binding of rhIL-2, c264scTCR-IL2 and MART-1scTCR-IL2 to IL-2R+ CTLL-2 cells was assessed by flow cytometry. Curves represent fit of data to a one-phase exponential decay model. Representative results of four independent experiments
TCR-IL2 fusion proteins bind to IL-2R bearing murine and human cells with an extended residency time
The cell surface residency time of the scTCR-IL2 fusion protein on the IL-2R-bearing cells may influence the ability of the fusion protein to target or bridge effector cells with the TCR-specific tumor cells. To investigate this, binding of the scTCR-IL2 fusion proteins and rhIL-2 to high affinity IL-2R bearing CTLL-2 cells was assessed by flow cytometry. Figure 2b shows the results of such a study in which c264scTCR-IL2, MART-1scTCR-IL2 or rhIL-2-coated cells were incubated in media at 37°C for up to 180 min and the level of proteins remaining on the cell surface was detected with anti-IL-2 mAb. As shown at the initial time point (t = 0), addition of equivalent molar amounts of c264scTCR-IL2, MART-1scTCR-IL2 or rhIL-2 to CTLL-2 cells resulted in specific staining with PE-labeled anti-IL-2 mAb. Incubation at 37°C resulted in cellular internalization and/or dissociation of the proteins as measured by a decrease in anti-IL-2 mAb binding. The cell surface half-life of rhIL-2 was determined to be 8.5 min by this method, a value similar to the 10 min t 1/2 reported by others [25]. The calculated half-lives of c264scTCR-IL2 and MART-1scTCR-IL2 interaction with CTLL-2 IL-2R were both 22.6 min. Similar studies done with activated human PBMC indicated that c264scTCR-IL2 specifically binds to the human IL-2R bearing cells with a half-life of about 25 min (data not shown), equivalent to the half-life seen for CTLL-2 cells. The results indicated that the IL-2 domain of c264scTCR-IL2 and MART-1scTCR-IL2 showed equivalent binding to IL-2R on cells with a residency time about three times that of rhIL-2. Interestingly, the difference in cell surface binding between the scTCR-IL2 fusion proteins and rhIL-2 did not result in a significant difference in bioactivity, consistent with previous studies of IL-2 mutants showing no correlation between IL-2/IL-2R internalization-dissociation and IL-2 signaling [5].
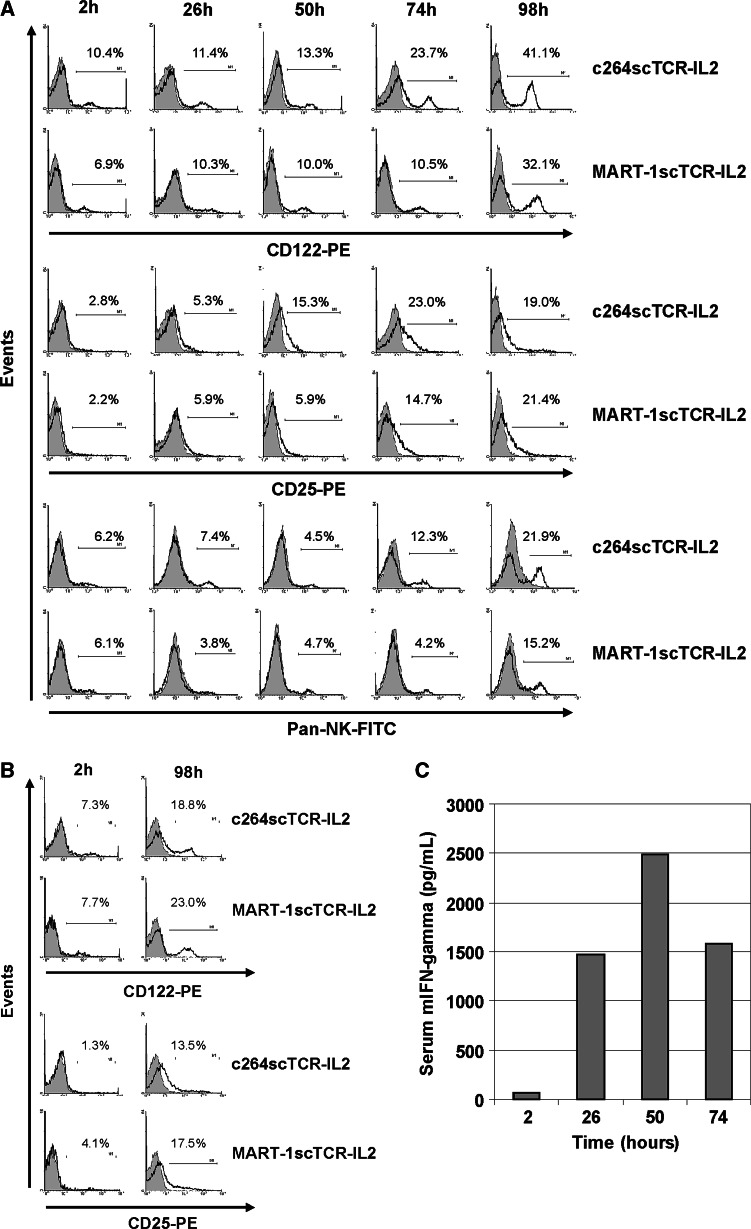
c264scTCR-IL2 and MART-1scTCR-IL2 exhibited comparable potency for activating immune cells in vivo
The effect of the scTCR-IL2 fusion proteins on immune cells was further investigated in A375 tumor bearing nude mice. Changes in IL-2R subunit expression were observed in immune cells isolated from the spleens, lymph nodes and bone marrow of mice treated with c264scTCR-IL2 or MART-1scTCR-IL2. Splenocytes expressing IL-2β (CD122) increased fourfold with repeated daily treatments of scTCR-IL2 such that over 30% of splenocytes were CD122+ 24 h following the fourth treatment of c264scTCR-IL2 or MART-1scTCR-IL2 (Fig. 3a). Comparable results were observed for cells of the lymph node (Fig. 3b) and bone marrow (data not shown). An eightfold increase in immune cells expressing IL-2Rα (CD25) was also observed following c264scTCR-IL2 or MART-1scTCR-IL2 treatment, with the highest percentage of CD25+ splenocytes detected at one day after the fourth day of daily injections. In addition, repeated administration of c264scTCR-IL2 or MART-1scTCR-IL2 resulted in a treatment-dependent increase in NK cells. For both fusion proteins, the levels of both IL-2R+ cells and NK cells showed a similar changes over the treatment time course. Repeated daily intravenous administration of c264scTCR-IL2 in these mice also resulted in an increase in serum IFN-γ levels further indicating immune cell activation (Fig. 3c). These findings are consistent with the known biological activities of IL-2 and suggest that repeated scTCR-IL2 fusion protein administration augments immune responsiveness via IL-2R-positive cells to the fusion protein [8, 9, 23]. These results also indicate that c264scTCR-IL2 and MART-1scTCR-IL2 have equivalent activity in stimulating immune cells in vivo.
Fig. 3.
In vivo activation of immune cells in A375 tumor bearing nude mice following c264scTCR-IL2 treatment. a, b Activation of immune cells was compared following repeated administration of c264scTCR-IL2 or MART-1scTCR-IL2 in nude mice bearing A375 xenografts. A375 tumor bearing nude mice injected i.v. with rhIL-2, c264scTCR-IL2 or MART-1scTCR-IL2 at 0, 24, 48, and 72 h. Splenocytes (a) and lymph node cells (b) were isolated at the indicated times and analyzed by flow cytometry for expression of CD25, CD122 or pan-NK markers. The plots indicate the percentage of CD25, CD122 or pan-NK-positive staining (dark line) compared with cells staining with an isotype control mAb (gray shading). c c264scTCR-IL2 (32 μg/dose) was administered intravenously into A375 tumor bearing nude mice as described above. Two hours after treatment, serum was collected and mouse interferon-gamma levels were measured by ELISA
In vivo retargeting of effector cells to tumor sites
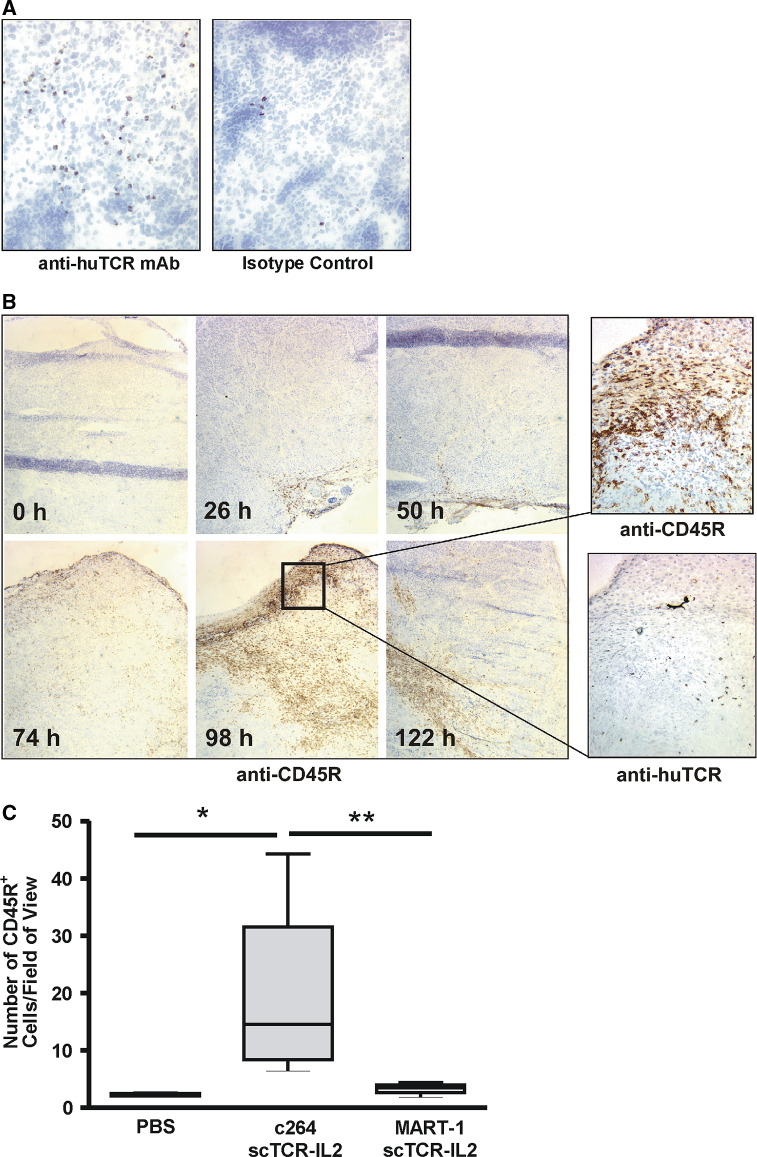
Previous studies indicated that treatment of nude mice bearing subcutaneous A375 tumor with 264scTCR-IL2 resulted in accumulation of CD45R+ NK cells within the tumor [3]. Moreover, NK cell infiltration was not observed in A375 tumors from mice treated with rhIL-2 or in non-targeted HLA-A2-negative AsPC1 primary tumors from mice treated with either 264scTCR-IL2 or rhIL-2. Thus, the levels of CD45R+ NK cell accumulation in p53+/HLA-A2+ tumors correlate well with the ability of the scTCR-IL2 fusion protein to bind tumor antigen and mediate antitumor responses.
To further examine the effects of scTCR-IL2 on immune cell trafficking, time course studies were carried out. Nude mice carrying subcutaneous A375 tumors were treated i.v. with c264scTCR-IL2 (32 μg/dose) at 0, 24, 48, 72 and 120 h. Two hours after each treatment, tumors were obtained and sectioned for IHC staining with antibodies specific to TCR domain of the fusion protein and to various murine immune cell markers. Following three daily treatments, c264scTCR-IL2 was detectable on cells in lymph nodes (Fig. 4a) and spleen (data not shown), suggesting that the functional IL-2 portion of the fusion molecule stably binds to IL-2R-bearing immune cells. At this time point (2 h after the third dose), little or no c264scTCR-IL2 was detected in the tumors indicating that the fusion protein preferentially binds to immune effector cells rather than tumor cells. In addition, no marked changes in the immune cells within the tumor were found at this time point. However, as daily administration of c264scTCR-IL2 continued, changes in immune cell infiltration were observed such that an increase in CD45R+ cells was detected in the A375 tumors following 4 days of treatment with a maximum level of CD45R+ cell accumulation seen approximately one day after the fourth treatment (98 h time point). These cells were not detected with an anti-CD19 mAb (B cell marker), indicating that they are primarily NK cells. Tumor cryosections from the 98 h time point also showed positive staining with the anti-human TCR mAb, indicating localization of cells bearing the c264scTCR-IL2 fusion protein (see higher magnification insets in Fig. 4b).
Fig. 4.
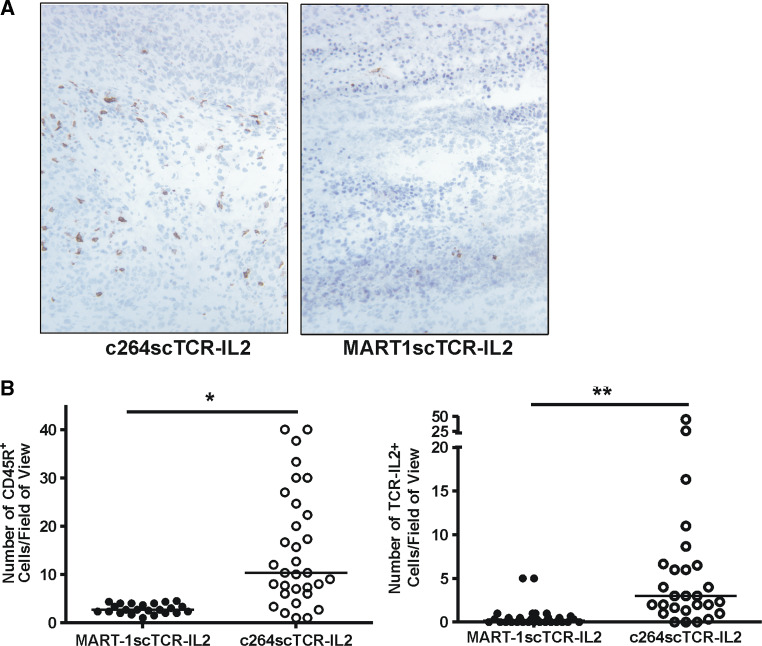
Treatment-dependent infiltration of NK cells into A375 tumors in nude mice. a A375 tumor bearing nude mice were treated with c264scTCR-IL2 as described. At 50 h, lymph nodes were collected and lymph node cryosections were stained with anti-human TCR mAb (upper panel) or an isotype control mAb (lower panel). Original magnification, ×200. b, CD45R+ cells were stained by IHC in A375 tumors obtained from nude mice treated with c264scTCR-IL2 at 0, 24, 48, 72 and 120 h. Increasing levels of CD45R+ cell infiltration were observed in the A375 tumors following 3–5 days of 264scTCR-IL2 treatment with a maximum level of infiltration seen at 98 h (left panels). Original magnification, ×50. Tumor from the 98 h sample also showed positive staining with the anti-human TCR mAb indicating localization of cells bearing the c264scTCR-IL2 fusion protein (higher magnification panels on right). Original magnification, ×400. c CD45R+ cell density was quantified in A375 tumors obtained from nude mice 24 h after last of four daily treatments with c264scTCR-IL2, MART-1scTCR-IL2 or PBS. Boxes represent median ± interquartile range of distribution (25th–75th percentile) and the error bars show the lowest the highest values. *P = 0.028, **P = 0.028. At least three tumors per group and ten fields per tumor were analyzed
In a subsequent study, tumor infiltration of CD45R+ cells was compared in A375 tumor bearing nude mice treated with c264scTCR-IL2 (10 μg/dose), control MART-1scTCR-IL2 (10 μg/dose) or PBS. Following four days of daily treatment, significantly more CD45R+ cell accumulation was observed in tumors of c264scTCR-IL2 treated mice (14.5 CD45R+ cells/field of view) compared with that seen in tumors from mice treated with either MART-1scTCR-IL2 (4.0 CD45R+ cells/field of view) or PBS (2.5 CD45R+ cells/field of view) (Fig. 4c). Collectively, these findings suggest that administration of the tumor-specific c264scTCR-IL2 fusion protein leads to the activation and targeted accumulation of CD45R+ effector cells into p53+/HLA-A2+ tumors.
Adoptive transfer of scTCR-IL-2 fusion protein activated and coated immune cells into p53/HLA-A2 tumor bearing mice
To further demonstrate the targeting effect of c264scTCR-IL2 in vivo, nude mouse splenocytes were activated by treating mice with c264scTCR-IL2 or MART-1scTCR-IL2 at 32 μg/dose daily for 4 days. The activated splenocytes were isolated and incubated with the respective fusion proteins prior to transfer into A375 tumor bearing nude mouse recipients. Significantly more accumulation of CD45R+ cells was observed in tumors from nude mice receiving splenocytes incubated with c264scTCR-IL2 (10.3 CD45R+ cells/field of view) than in tumors from mice treated with splenocytes preincubated with the control MART-1scTCR-IL2 (2.7 CD45R+ cells/field of view) (Fig. 5a, b). In addition, when these sections were stained for either c264scTCR-IL2 or MART-1scTCR-IL2 fusion proteins, significantly more cells bound with detectable levels of c264scTCR-IL2 were observed infiltrating into the tumors (c264scTCR-IL2: 3.0 BF1+ cells/field of view; MART-1scTCR-IL2; 0.2 BF1+ cells/field of view) (Fig. 5b). These data provide additional evidence that activated NK cells bound with c264scTCR-IL2 molecules have greater trafficking capability to the p53+/HLA-A2+ tumors than that of MARTscTCR-IL-2 activated and coated NK cells.
Fig. 5.
Targeted trafficking of adoptively transferred effector cells bearing c264scTCR-IL2 to A375 tumors in nude mice. a In vivo activated splenocytes were treated with c264scTCR-IL2 or MART-1scTCR-IL2 in vitro and transferred into A375 tumor bearing nude mice. Twenty-four hours after adoptive transfer, tumor were isolated and stained by IHC for NK cells. An increase in CD45+ NK cell infiltration was observed in tumor sections from mice administered c264scTCR-IL2-coated cells (left panel) as compared those treated with MART-1scTCR-IL2-coated cells (right panel). Original magnification, ×200. b CD45R+ cell density (left) and human TCR-coated cells (right) were quantified in A375 tumors obtained from nude mice 24 h after adoptive transfer of c264scTCR-IL2 or MART-1scTCR-IL2 treated splenocytes. Symbols represent number of positively staining cells per field of view and bars represent median value. *P < 0.0001, **P < 0.0001. At least three tumors per group and ten fields per tumor were analyzed
Antitumor efficacy of c264scTCR-IL2 and MART-1scTCR-IL2 against primary tumor xenografts in nude mice
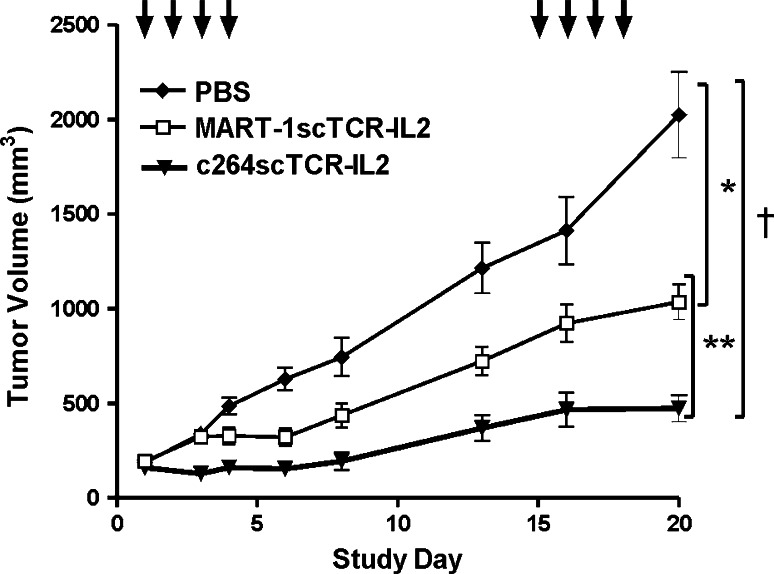
To compare the observed immune cell responses and the antitumor activity of the scTCR-IL2 fusion proteins, efficacy studies were conducted in which subcutaneous A375 tumor bearing nude mice were treated with c264scTCR-IL2 or the control MART-1scTCR-IL2 fusion protein. In each case, treatment consisted of four daily i.v. injections followed by a 10-day rest period and then four more daily injections at 10 μg/dose TCR/IL-2 fusion protein or an equivalent volume of PBS. Treatment of c264scTCR-IL2 resulted in a significantly better tumor growth inhibition than was observed following MART-1scTCR-IL2 treatment. The antitumor activity of the c264scTCR-IL2 fusion protein correlates with the timing and relative levels of CD45R+ cell accumulation in the tumors (Fig. 4). These results support our hypothesis that the bifunctional c264scTCR-IL2 molecules have the capability to bind and then retarget immune cells to the tumor site where the TCR component of the molecule specifically recognizes its tumor associated antigen.
The control MART-1scTCR-IL2 fusion proteins were also effective in delaying tumor growth when compared with PBS (Fig. 6). Since we have previously shown that IL-2 did not inhibit A375 tumor growth in this model [3], these results suggest that increasing the biological half-life of IL-2 by the MART-1scTCR-IL2 fusion (without specific tumor binding) may lead to effective antitumor responses. Slightly elevated levels of CD45R+ cell infiltration in the tumors were also seen following treatment with the MART-1scTCR-IL2 fusion (Fig. 4). This further illustrates that the combination of improved IL-2 serum half-life and tumor recognition activity of the TCR domain could create a very potent antitumor agent as exemplified by the c264scTCR-IL2 fusion protein.
Fig. 6.
Effect of scTCR-IL2 fusion protein targeting on growth of primary p53+ HLA-A2+ tumors in nude mice. Subcutaneous A375 tumor-bearing nude mice (8 per group) were treated with PBS, MART1scTCR-IL2 or c264scTCR-IL2 on the study days indicated by the arrows. Tumor volumes were measured over time. Data are mean ± SE (n = 8). *P < 0.0001, MART-1scTCR-IL2 compared to PBS (day 13 to day 20, P < 0.001 by Bonferroni posttest), †P < 0.0001, c264scTCR-IL2 compared to PBS (day 6 to day 20, P < 0.001 by Bonferroni post-test), **P < 0.0001, c264scTCR-IL2 compared to MART-1scTCR-IL2 (day 13, P < 0.05; day 16, P < 0.01; day 20, P < 0.001 by Bonferroni post-test)
Discussion
Bi-functional fusion molecules comprised of genetically linked single-chain TCR and cytokine domains were created to exploit the tumor antigen recognition activity of TCR to localize cytokine activity to the tumor microenvironment. One such fusion protein, c264scTCR-IL2, recognizing a p53 peptide antigen was found to exhibit potent antitumor activity against human p53+ tumors in various mouse xenograft models [3, 4]. Although c264scTCR-IL2 has longer serum half-life than rhIL-2, the fusion molecule offered no advantage over rhIL-2 in inhibiting growth of p53-negative tumors, suggesting that increased stability alone may not account for the improved in vivo efficacy of c264scTCR-IL2 [3]. To address this question more directly, we constructed a control scTCR-IL2 fusion protein that does not bind p53 antigen. This control, MART-1scTCR-IL2, exhibited a serum half-life that was about 2-times longer than that of c264scTCR-IL2 and 11-times longer than that of rhIL-2. When compared to the lack of efficacy observed previously for IL-2 [3], the significant antitumor activity of MART-1scTCR-IL2 against A375 tumor xenografts suggests that this tumor model is sensitive to the more stable scTCR-IL2 fusion protein. However, MART-1scTCR-IL2 showed significantly less antitumor activity than the p53 antigen-specific c264scTCR-IL2 fusion protein against p53+/HLA-A2+ tumors, even though the two scTCR-IL2 fusion proteins were indistinguishable in terms of their in vitro activity in stimulating NK cell responses and ability to bind IL-2 receptors on the immune cell surface. These data strongly support the proposal based on our previous studies that the ability of c264scTCR-IL2 to mediate antitumor activity is dependent on the p53 and HLA-A2 status of the tumor xenograft and that the scTCR component provides the targeting capability [3]. We also observed the differences in antitumor activity correlated with fusion protein-dependent increase in CD45R+ NK cell accumulation into p53+/HLA-A2+ tumors. This suggests that the c264scTCR-IL2-mediated trafficking of NK cells in the tumors is also dependent on the display of p53 peptide as previously proposed [3].
There are two possible underlying mechanisms for targeting with this type of fusion molecule. First, in a fusion protein-based mechanism, the TCR domain of the fusion protein binds to the target antigen displayed by the tumor cells, followed by the attraction of immune cells bearing cytokine receptors to the tumor microenvironment via interaction with the cytokine domain of the bound fusion molecule. The tumor-localized immune cells subsequently carry out their effector functions. In a second cell-mediated mechanism, the cytokine domain of the fusion protein activates immune cells to proliferate and express intermediate and high-affinity cytokine receptors. Following stable interactions between the cytokine domain and the induced cytokine receptors, the scTCR domain of the fusion molecule acts as a new cell surface receptor localizing the immune cells in tumors displaying target peptide/HLA complexes. Alternatively, it is possible that the IL-2 domain of the fusion protein induces the expression of adhesion molecules and/or chemokine receptors. The chemokine receptors increase the trafficking of the fusion protein-coated immune cells to the tumors which are known to be sources of inflammatory chemokines. Once at the tumor site, the surface-bound scTCR-IL2 fusion protein acts as an adhesion molecule to increase the immune cell’s residence time within the tumor. Interestingly, this possibility is consistent with two of our observations: (1) the fusion protein was shown to have the capability of conjugating the cells carrying the IL-2 receptors and cells displaying the target antigen [4], and (2) the fusion protein induces high expression of the CX3CR1 chemokine receptor on purified murine NK cells in vitro (data not shown). CX3CR1 and its ligand, CX3CL1 have been shown to play a major role of lymphocyte trafficking to the tumor site and antitumor responses in various animal models [15, 34].
The results of our adoptive transfer studies with c264scTCR-IL-2 and MART-1scTCR-IL-2 treated nude mouse splenocytes also strongly favor the cell-mediated mechanism. As we have shown in this report, the IL-2 domain of c264scTCR-IL2 and MART-1scTCR-IL2 fusion proteins had equivalent biological and IL-2 receptor binding activities. Thus, it is conceivable that the in vivo activated immune cells bind equivalent amounts of c264scTCR-IL2 and MART-1scTCR-IL2. However, activated NK cells pretreated with c264scTCR-IL2 showed significantly more infiltration into the p53+/HLA-A2+ tumor within 24 h of adoptive transfer than NK cells pretreated with MART-1scTCR-IL2. In a separate study, we found that very little c264scTCR-IL2 fusion protein dissociated from the adoptively transferred cells in vivo (c264scTCR-IL2 serum concentration = ~1 nmol/l per 107 treated splenocytes 15 min after transferred). Thus, it is unlikely that soluble fusion molecules from the adoptively transferred cells play a role in the observed tumor targeting.
The cell-mediated mechanism is also consistent with other observations. During the initial 36 h period of treatment, c264scTCR-IL2 preferentially localized in the lymphoid organs. This is likely due to the presence of immune cells bearing IL-2R molecules that interact with the IL-2 domain of the fusion protein at nanomolar (or lower) binding affinity. In contrast, the p53 (aa264–272)/HLA-A2.1 complex interacts monomeric 264scTCR domain with a K d of 0.5 to 2 μmol/l [21, 36]. At a dose of 32 μg c264scTCR-IL2, the maximum observed serum concentration in mice was ~0.8 μmol/L with a serum half-life of ~2 h. This would provide saturating binding conditions for IL-2R-bearing cells for 10 to 12 h post injection [22], but much less favorable binding conditions for tumor cells displaying p53 (aa264–272)/HLA-A2.1 complex. In fact, marked preferential binding to IL-2R-bearing cells would be anticipated at the 2–10 μg/dose range observed for c264scTCR-IL2 antitumor efficacy [3]. Consistent with the cell-mediated mechanism, in vitro binding of c264scTCR-IL2 fusion protein to IL-2R-bearing cells was much more readily observed than interactions with p53 peptide loaded T2 cells (data not shown). In addition, repeated daily injection of c264scTCR-IL2 in nude mice elevated IL-2R subunit expression and NK cell proliferation indicating enhanced responsiveness to IL-2. Interestingly, NK cells are known to constitutively express several thousand IL-2 receptors on their surface [30, 31]. Binding of c264scTCR-IL2 to these receptors could effectively coat the NK cells with the c264scTCR recognition domain at sufficient density to permit binding to p53 (aa264–272)/HLA-A2 complexes on tumor cells. The timing of the appearance of CD45R+ NK cells and scTCR-IL2 fusion protein observed within p53+/HLA-A2+ tumors in the c264scTCR-IL2 treated mice is consistent with such a model. Once localized in the tumor site the IL-2-activated NK cells should be capable of mediating their cytolytic effector functions. Indeed, we have observed that c264scTCR-IL-2 and rhIL-2 activated splenocytes are equally effective at killing A375 tumor cells in vitro.
Several factors may create a favorable environment for immune effector cells to participate in such a cell-mediated mechanism of action. First, the pharmacological half-life of scTCR-IL2 fusion proteins is significantly longer than rhIL-2. This increased in vivo stability allows the scTCR-IL2 fusion protein to be present at levels favorable for IL-2R binding (i.e. serum concentration > 10 pM) for 10–12 h post injection. Second, the scTCR-IL2 fusion proteins remain bound to the IL-2R-positive immune cell surface for a half-life about twice that of rhIL-2, suggesting a decreased rate of internalization. The stable complex may provide additional time for the TCR-coated cells to traffic to the tumor site and interact with the p53 (aa264–272)/HLA-A2.1 target. Third, repeated administration of the scTCR-IL2 fusion protein results in expansion in the number of cells expressing IL-2R. These responses are consistent with the activity of rhIL-2 in mice and are expected to further potentiate any effects of the fusion protein-targeted cells against the tumor.
Based on its tumor targeted activity, scTCR-IL2 fusion molecules described here could be employed as a replacement for IL-2 in several different clinical settings. In various subcutaneous xenograft p53+ tumor models, c264scTCR-IL2 treatment provided much more potent antitumor activity than an equal molar dosing of rhIL-2 [3]. Further, once daily administration of c264scTCR-IL2 in two-four-day cycles was as effective in reducing tumor growth in nude mice as a 12-fold higher cumulative dose of rhIL-2 given in the standard high-dose IL-2 regimen (e.g. every 8 h in two-five-day cycles). In this study, toxicity observed in mice during IL-2 treatment was much more significant than that seen in the c264scTCR-IL2 treated group. Based on these and other preclinical studies, we have recently initiated a clinical trial with c264scTCR-IL2 fusion protein administration in patients with tumors presenting p53 (aa264–272)/HLA-A2 complexes. The goal of this study is to determine whether reduced levels of tumor targeted scTCR-IL2 activity can provide improved clinical benefit with less toxicity than current high-dose rhIL-2 therapy.
Use of soluble scTCR-IL2 fusion proteins in ex vivo cell-based therapies may also be of considerable interest. Recent improvements to adoptive immunotherapies with antigen-specific T-cells have resulted in effective treatments for metastatic melanoma [10]. However, a major difficulty with this approach is the requirement that patients have pre-existing tumor reactive T-cells that can be expanded ex vivo. Recombinant human IL-2 is used in these protocols to stimulate T-cell growth in both the ex vivo expansion phase as well as in the patients after cell transfer. Thus, a strategy to substitute IL-2 with scTCR-IL2 could allow expansion and activation of the patient’s T-cells, induction of cell-surface high-affinity IL-2R and subsequent retargeting of these cells via surface bound scTCR-IL2 to the tumors displaying the peptide/HLA antigen. It has previously been shown that Ab-IL2 fusions could redirect CD8 T-cell lytic activity against tumors bearing antigens recognized by the Ab domain [18], and the c264scTCR-IL2 fusion protein may provide comparable effector activity against tumors presenting p53 (aa264–272)/HLA-A2 complexes. Additionally, our adoptive transfer studies with c264scTCR-IL2 treated nude mouse splenocytes demonstrate that ex vivo incubation with scTCR-IL2 fusion protein allows targeting of other immune effector cells, including NK cells, to the tumor sites. Based on recent strategies for treating poor-prognosis leukemia patients with autologous NK cell infusions and systemic rhIL-2 [20], it is possible that an approach to direct NK cell activity against solid tumors using a targeted scTCR-IL-2 fusion protein may also have therapeutic utility. We are actively exploring the application of the scTCR-IL2 fusion proteins in such an NK cell-based approach.
Disclosures
The authors, except for J.L.W., are employees and equity holders of Altor BioScience Corporation.
Acknowledgments
This work is supported by National Institutes of Health Small Business Innovation Research grant: 2R44CA097550-03 (to H.C.W.). We thank Drs. Richard Morgan and Steven Rosenberg (National Cancer Institute) for kindly providing the MART-1 (aa27–35)/HLA-A2.1-specific TCR alpha and beta genes. We are grateful to Dr. Jeffrey Weber (H. Lee Moffitt Cancer Center) and Dr. Richard A. Morgan for their thoughtful comments and critical review of this manuscript.
References
- 1.Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clin Cancer Res. 2004;10:6342S–6346S. doi: 10.1158/1078-0432.CCR-040029. [DOI] [PubMed] [Google Scholar]
- 2.Becker JC, Pancook JD, Gillies SD, Furukawa K, Reisfeld RA. T cell-mediated eradication of murine metastatic melanoma induced by targeted interleukin 2 therapy. J Exp Med. 1996;183:2361–2366. doi: 10.1084/jem.183.5.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmont HJ, Price-Schiavi S, Liu B, Card KF, Lee HI, Han KP, Wen J, Tang S, Zhu X, Merrill J, Chavillaz PA, Wong JL, Rhode PR, Wong HC. Potent antitumor activity of a tumor-specific soluble TCR/IL-2 fusion protein. Clin Immunol. 2006;121:29–39. doi: 10.1016/j.clim.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Card KF, Price-Schiavi SA, Liu B, Thomson E, Nieves E, Belmont H, Builes J, Jiao JA, Hernandez J, Weidanz J, Sherman L, Francis JL, Amirkhosravi A, Wong HC. A soluble single-chain T-cell receptor IL-2 fusion protein retains MHC-restricted peptide specificity and IL-2 bioactivity. Cancer Immunol Immunother. 2004;53:345–357. doi: 10.1007/s00262-003-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang DZ, Wu Z, Ciardelli TL. A point mutation in interleukin-2 that alters ligand internalization. J Biol Chem. 1996;271:13349–13355. doi: 10.1074/jbc.271.23.13349. [DOI] [PubMed] [Google Scholar]
- 6.Christ O, Seiter S, Matzku S, Burger C, Zoller M. Efficacy of local versus systemic application of antibody-cytokine fusion proteins in tumor therapy. Clin Cancer Res. 2001;7:985–998. [PubMed] [Google Scholar]
- 7.Dela Cruz JS, Huang TH, Penichet ML, Morrison SL. Antibody-cytokine fusion proteins: innovative weapons in the war against cancer. Clin Exp Med. 2004;4:57–64. doi: 10.1007/s10238-004-0039-y. [DOI] [PubMed] [Google Scholar]
- 8.Demaison C, Fiette L, Blanchetiere V, Schimpl A, Theze J, Froussard P. IL-2 receptor {alpha}-chain expression is independently regulated in primary and secondary lymphoid organs. J Immunol. 1998;161:1977–1982. [PubMed] [Google Scholar]
- 9.Depper JM, Leonard WJ, Drogula C, Kronke M, Waldmann TA, Greene WC. Interleukin 2 (IL-2) Augments transcription of the IL-2 receptor gene. PNAS. 1985;82:4230–4234. doi: 10.1073/pnas.82.12.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimm EA, Mazumder A, Zhang HZ, Rosenberg SA. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med. 1982;155:1823–1841. doi: 10.1084/jem.155.6.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann TK, Nakano K, Elder EM, Dworacki G, Finkelstein SD, Appella E, Whiteside TL, DeLeo AB. Generation of T cells specific for the wild-type sequence p53(264–272) peptide in cancer patients: implications for immunoselection of epitope loss variants. J Immunol. 2000;165:5938–5944. doi: 10.4049/jimmunol.165.10.5938. [DOI] [PubMed] [Google Scholar]
- 13.Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D, Robinson BW, Nelson DJ. IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2. J Immunol. 2003;171:5051–5063. doi: 10.4049/jimmunol.171.10.5051. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME, Robbins PF, Rosenberg SA. Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes. J Immunol. 2006;177:6548–6559. doi: 10.4049/jimmunol.177.9.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavergne E, Combadiere B, Bonduelle O, Iga M, Gao JL, Maho M, Boissonnas A, Murphy PM, Debre P, Combadiere C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003;63:7468–7474. [PubMed] [Google Scholar]
- 16.Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. doi: 10.1038/351453a0. [DOI] [PubMed] [Google Scholar]
- 17.Lode HN, Xiang R, Dreier T, Varki NM, Gillies SD, Reisfeld RA. Natural killer cell-mediated eradication of neuroblastoma metastases to bone marrow by targeted interleukin-2 therapy. Blood. 1998;91:1706–1715. [PubMed] [Google Scholar]
- 18.Lustgarten J, Marks J, Sherman LA. Redirecting effector T cells through their IL-2 receptors. J Immunol. 1999;162:359–365. [PubMed] [Google Scholar]
- 19.Melder RJ, Osborn BL, Riccobene T, Kanakaraj P, Wei P, Chen G, Stolow D, Halpern WG, Migone TS, Wang Q, Grzegorzewski KJ, Gallant G. Pharmacokinetics and in vitro and in vivo anti-tumor response of an interleukin-2-human serum albumin fusion protein in mice. Cancer Immunol Immunother. 2005;54:535–547. doi: 10.1007/s00262-004-0624-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 21.Mosquera LA, Card KF, Price-Schiavi SA, Belmont HJ, Liu B, Builes J, Zhu X, Chavaillaz PA, Lee HI, Jiao JA, Francis JL, Amirkhosravi A, Wong RL, Wong HC. In vitro and in vivo characterization of a novel antibody-like single-chain TCR human IgG1 fusion protein. J Immunol. 2005;174:4381–4388. doi: 10.4049/jimmunol.174.7.4381. [DOI] [PubMed] [Google Scholar]
- 22.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/S0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 23.Puri RK, Travis WD, Rosenberg SA. In vivo administration of interferon alpha and interleukin 2 induces proliferation of lymphoid cells in the organs of mice. Cancer Res. 1990;50:5543–5550. [PubMed] [Google Scholar]
- 24.Radny P, Caroli UM, Bauer J, Paul T, Schlegel C, Eigentler TK, Weide B, Schwarz M, Garbe C. Phase II trial of intralesional therapy with interleukin-2 in soft-tissue melanoma metastases. Br J Cancer. 2003;89:1620–1626. doi: 10.1038/sj.bjc.6601320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robb RJ, Greene WC. Internalization of interleukin 2 is mediated by the beta chain of the high-affinity interleukin 2 receptor. J Exp Med. 1987;165:1201–1206. doi: 10.1084/jem.165.4.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg SA, Lotze MT, Muul LM, Chang AE, Avis FP, Leitman S, Linehan WM, Robertson CN, Lee RE, Rubin JT, et al. A progress report on the treatment of 157 patients with advanced cancer using lymphokine-activated killer cells and interleukin-2 or high-dose interleukin-2 alone. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 27.Running Deer J, Allison DS. High-level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF-1alpha gene. Biotechnol Prog. 2004;20:880–889. doi: 10.1021/bp034383r. [DOI] [PubMed] [Google Scholar]
- 28.Strano S, Dell’Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- 29.Tarhini AA, Agarwala SS. Interleukin-2 for the treatment of melanoma. Curr Opin Investig Drugs. 2005;6:1234–1239. [PubMed] [Google Scholar]
- 30.Toomey JA, Gays F, Foster D, Brooks CG. Cytokine requirements for the growth and development of mouse NK cells in vitro. J Leukoc Biol. 2003;74:233–242. doi: 10.1189/jlb.0303097. [DOI] [PubMed] [Google Scholar]
- 31.Voss SD, Robb RJ, Weil-Hillman G, Hank JA, Sugamura K, Tsudo M, Sondel PM. Increased expression of the interleukin 2 (IL-2) receptor beta chain (p70) on CD56+ natural killer cells after in vivo IL-2 therapy: p70 expression does not alone predict the level of intermediate affinity IL-2 binding. J Exp Med. 1990;172:1101–1114. doi: 10.1084/jem.172.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiang R, Lode HN, Dolman CS, Dreier T, Varki NM, Qian X, Lo KM, Lan Y, Super M, Gillies SD, Reisfeld RA. Elimination of established murine colon carcinoma metastases by antibody-interleukin 2 fusion protein therapy. Cancer Res. 1997;57:4948–4955. [PubMed] [Google Scholar]
- 33.Xu X, Clarke P, Szalai G, Shively JE, Williams LE, Shyr Y, Shi E, Primus FJ. Targeting and therapy of carcinoembryonic antigen-expressing tumors in transgenic mice with an antibody-interleukin 2 fusion protein. Cancer Res. 2000;60:4475–4484. [PubMed] [Google Scholar]
- 34.Yu YR, Fong AM, Combadiere C, Gao JL, Murphy PM, Patel DD. Defective antitumor responses in CX3CR1-deficient mice. Int J Cancer. 2007;121:316–322. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- 35.Zhai Y, Yang JC, Kawakami Y, Spiess P, Wadsworth SC, Cardoza LM, Couture LA, Smith AE, Rosenberg SA. Antigen-specific tumor vaccines. Development and characterization of recombinant adenoviruses encoding MART1 or gp100 for cancer therapy. J Immunol. 1996;156:700–710. [PubMed] [Google Scholar]
- 36.Zhu X, Belmont HJ, Price-Schiavi S, Liu B, Lee HI, Fernandez M, Wong RL, Builes J, Rhode PR, Wong HC. Visualization of p53(264–272)/HLA-A*0201 complexes naturally presented on tumor cell surface by a multimeric soluble single-chain T cell receptor. J Immunol. 2006;176:3223–3232. doi: 10.4049/jimmunol.176.5.3223. [DOI] [PubMed] [Google Scholar]