Abstract
It is becoming increasingly apparent that the majority of tumours display defects in the MHC class I antigen processing pathway, particularly low levels of the transporters-associated with antigen processing (TAP) and tapasin. Thus, immunotherapy approaches targeting such tumours with CD8+ cytotoxic T lymphocytes (CTL) requires strategies to overcome these defects. Previously we had identified an antigen processing pathway by which cytosolically derived hydrophobic peptides could be presented in the absence of TAP. Here we show in the tapasin-negative cell line 721.220 that a number of these hydrophobic TAP-independent peptides can also be presented in a tapasin-independent manner. Yet when these experiments were extended to tumour cell lines derived from small cell lung cancer (SCLC), which we show to be tapasin deficient in addition to TAP-negative, the TAP-, tapasin-independent peptides were not presented. This lack of presentation could be rectified by pre-treatment of SCLC cells with IFNγ. Alternatively, by directing the TAP-, tapasin-independent peptides into the endoplasmic reticulum (ER) via an ER signal sequence, these peptides were presented efficiently by SCLC cells. We infer from this data that the TAP-independent pathway for presentation of hydrophobic peptides generates a low concentration of peptide in the ER and, for tumour cells which also lack tapasin, this concentration of antigenic peptide is insufficient to load onto MHC class I molecules. Thus, for immunotherapeutic approaches to target SCLC and other tumours with defects in the MHC class I antigen processing pathway it will be important to consider strategies that address tapasin-defects.
Keywords: Antigen presentation, Tumour cells, TAP, Tapasin, Small cell lung cancer
Introduction
Cancer immunotherapy utilises the immune response to recognise and destroy tumour cells. One important approach is the targeting of tumours with CD8+ cytotoxic T lymphocytes (CTL) [19]. This process requires that the antigenic peptides, for which the CTLs are specific, are efficiently processed and presented by the MHC class I antigen processing pathway within the tumour cell. MHC class I antigen processing involves degradation of endogenously expressed antigen, primarily by the proteasome, to release short antigenic peptides in the cytosol. The transporter-associated with antigen processing (TAP) transports these peptides into the endoplasmic reticulum (ER), where they are loaded onto appropriate MHC class I molecules. Within the ER, MHC class I heavy chains associate with β2-microglobulin and together with the chaperone calreticulin and the MHC class I accessory proteins tapasin and ERp57, form the peptide loading complex (PLC). Tapasin plays an important role in bridging the PLC to TAPs to ensure efficient loading of high affinity peptides onto MHC class I molecules. Once peptides are loaded, the MHC molecules are released and migrate to the cell surface to display their cargo to circulating CD8+ CTL [22]. Both TAP and tapasin play critical roles in the generating peptide-loaded MHC molecules, as mutant cell lines deficient in either of these components are defective in conventional MHC class I antigen processing and presentation [21, 32].
It is well documented that in many tumour cells there is down regulation of peptide-loaded MHC class I molecules on the cell surface [26], and in many of these instances this is a result of defects in expression of TAP and/or tapasin [4, 9, 17, 20, 26, 28]. These defects mean that such cancer cells are generally insensitive to killing by CD8+ CTL. Therefore, if these tumours are to be targeted with CD8+ CTL, additional strategies are required to overcome this barrier, for example pre-treatment with IFNγ or targeting antigenic peptides which can be presented in the absence of TAP and/or tapasin. There are a number of examples of presentation of antigenic peptides on MHC class I molecules by TAP-independent pathways, most characterised using the TAP1/2-negative T2 cell line [25]. Such TAP-independent peptides are primarily generated post-ER and are derived from signal sequences or from processing of antigens in the ER or further secretory vesicles [10, 12, 31, 33]. Cell lines deficient in tapasin can also present antigenic peptides on MHC class I molecules and this appears to be related to the HLA presenting allele rather than specific properties of the antigenic peptide or protein from which it is derived. Thus, HLA alleles A2 and B*2705 have been shown to be capable of presenting antigenic peptides in the absence of tapasin, whereas HLA-A1, B8 and B44 required tapasin function [11, 16, 23].
By studying the processing of antigens from the human herpesvirus Epstein-Barr virus (EBV), we have previously identified a pathway by which antigenic peptides generated in the cytosol via proteasomal degradation, can access MHC class I molecules in the absence of TAP [15]. This TAP-independent presentation was linked to the hydrophobicity of the peptide. Although the precise mechanism by which these antigenic peptides access the ER is unknown, it has been suggested that such TAP-independent peptides may prove to be useful targets for immunotherapy of TAP-negative tumours [14]. However, it was not clear how efficiently these peptides would be presented in cells which were also defective for tapasin. Here we show that HLA A2-restricted TAP-independent antigenic peptides are also presented in the tapasin-negative 721.220 cell line. Yet when tested in small cell lung cancer (SCLC) tumour cell lines, defective in TAP [24, 30] and as shown here tapasin, none of these peptides when expressed in the cytosol were presented on the cell surface to CD8+ CTL. However, directly targeting a TAP-independent, tapasin-independent peptide to the ER of SCLC cells overcomes this block. Thus, the critical element for presentation of antigenic peptides in tumour cells with an antigen processing defective background is the ability of the peptide to be presented in the absence of tapasin rather than TAP.
Materials and methods
Cell lines and peptides
Standard EBV-transformed B-lymphoblastoid cell lines (B-LCLs), and the TAP-negative T2 cell line [25] were grown in RPMI media supplemented with 10% foetal calf serum (FCS), 2 mM glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin (growth medium). The tapasin-negative 721.220 cell line (.220) and the tapasin-transfectant of 721.220 (.220:Tpn) [21] were provided by P. Lehner (Cambridge, UK) and maintained in growth medium supplemented with 0.5 mg/ml G418. The SCLC cell lines NCI-H69, NCI-H345, GLC-19, U2020, Lu-165 were sourced as previously described [7] and cultured in RPMI 1640 supplemented with 10% FCS. The HLA type of all five SCLC cell lines was determined using sequence-specific PCR by the Department of Immunology, Royal Liverpool University Hospital, UK. Synthetic peptides were purchased from Alta Bioscience (University of Birmingham, Birmingham, UK).
CTL clones
EBV-specific CTL clones were generated from virus-immune donors by autologous LCL stimulation as described [15]. EBV-specific CTL used in this study were specific for the following peptides: HLA A*0201-restricted peptide CLGGLLTMV (CLG) and the HLA A*2402-restricted peptide TYGPVFMCL (TYG) from the latent antigen LMP2; the HLA A*0201-restricted peptides GLCTLVAML (GLC), TLDYKPLSV (TLD) and YVLDHLIVV (YVL) from the lytic antigens BMLF1, BMRF1 and BRLF1, respectively.
Viruses
The following recombinant vaccinia viruses were used in this study: vTK-, a thymidine kinase negative virus; vA2, expressing the HLA A*0201 allele; vA24, expressing the HLA A*2402 allele; vBRLF1, expressing the EBV lytic antigen BRLF1; and the minigene recombinants vCLG, expressing the CLG peptide from EBV latent antigen LMP2; vGLC, expressing the GLC peptide from EBV lytic antigen BMLF1; vTLD, expressing the TLD peptide from EBV lytic antigen BMRF1; vL+CLG, expressing the CLG peptide fused to an ER signal sequence. All vaccinia viruses are based on the WR strain of vaccinia and have been described in detail elsewhere [15].
Plasmids
Plasmid constructs expressing the synthetic minigenes encoding the HLA A*0201-restricted peptides TLD and YVL were generated by inserting annealed overlapping oligonucleotides encoding each peptide into the Xho1 site of the plasmid pShuttle-CMV (pCMV, QBiogene). The oligonucleotides used were as follows TLD 5′-TCGATGACCCTAGACTACAAGCCTCTGAGTGTGTAATTAATTAA-3′ and 5′-TCGATTAATTAATTACACACTCAGAGGCTTGTAGTCTAGGGTCA-3′; YVL 5′-TCGATGTACGTGTTAGACCACCTGATAGTGGTCTAATTAATTAA-3′ and 5′-TCGATTAATTAATTAGACCACTATCAGGTGGTCTAACACGTACA-3′, creating the plasmids pCMV-TLD and pCMV-YVL.
Western blotting
For immunoblotting, protein extracts from SCLC cell lines were harvested at 90% confluence, or 48 h post treatment with 1,000 U/ml IFNγ, resolved by SDS-PAGE (10% gel), transferred to nitrocellulose and probed with specific antibodies followed by detection using a chemiluminescence protocol (Amersham, UK). Human TAP1/TAP2 were detected using rabbit antisera [15], Tapasin using a rabbit antisera (provided by T. Elliot, Southampton, UK) and actin using a monoclonal antibody (Sigma-Aldrich, St Louis, MO, USA).
Flow cytometry
SCLC cell lines, either untreated or pretreated with IFNγ (1,000 U/ml) for 48 h, were harvested and washed twice in phosphate-buffered saline (PBS). Cells were then stained with antibody BB7.2 (specific for HLA-A2), followed by a FITC-labelled secondary antibody and fixed in 1% paraformaldehyde before analysis on a Beckman Coulter EPICS XL Flow Cytometer. Flow Cytometry data was analysed using WinMDI Software.
Antigen presentation assays
Antigen presentation was investigated using both standard chromium release assays and by monitoring IFNγ release into culture supernatant. Chromium release assays on target cells infected overnight with recombinant vaccinia viruses were carried out as previously described [15]. IFNγ release into culture supernatant was measured by ELISA. CTL specific for the appropriate antigenic peptide were tested in triplicate microwell cultures against target cell lines treated as follows; either (1) infected with recombinant vaccinia virus (10 moi) for 5–6 h prior to extensive washing before incubation for 20 h with appropriate CTL or (2) transfected with 20 μg plasmid by electroporation as described previously [6], and harvested 48 h later for use as targets in a 20-h incubation with appropriate CTL. Where indicated, cells were pretreated with IFNγ (1,000 U/ml) for 48 h before being washed with RPMI and either infected with recombinant vaccinia viruses or transfected with plasmids as described. Numbers of T cells and target cells used per well are indicated in figure legends. After the 20-h incubation, culture supernatants were harvested and IFNγ measured by ELISA using antibody reagents from Endogen. In both types of assay, targets included cells exposed to the antigenic peptide at a final concentration of 2 × 10−8 M immediately prior to the assay, and cells exposed to an equivalent amount of DMSO solvent as a control.
Results
Processing and presentation of TAP-independent peptides in tapasin-negative cells
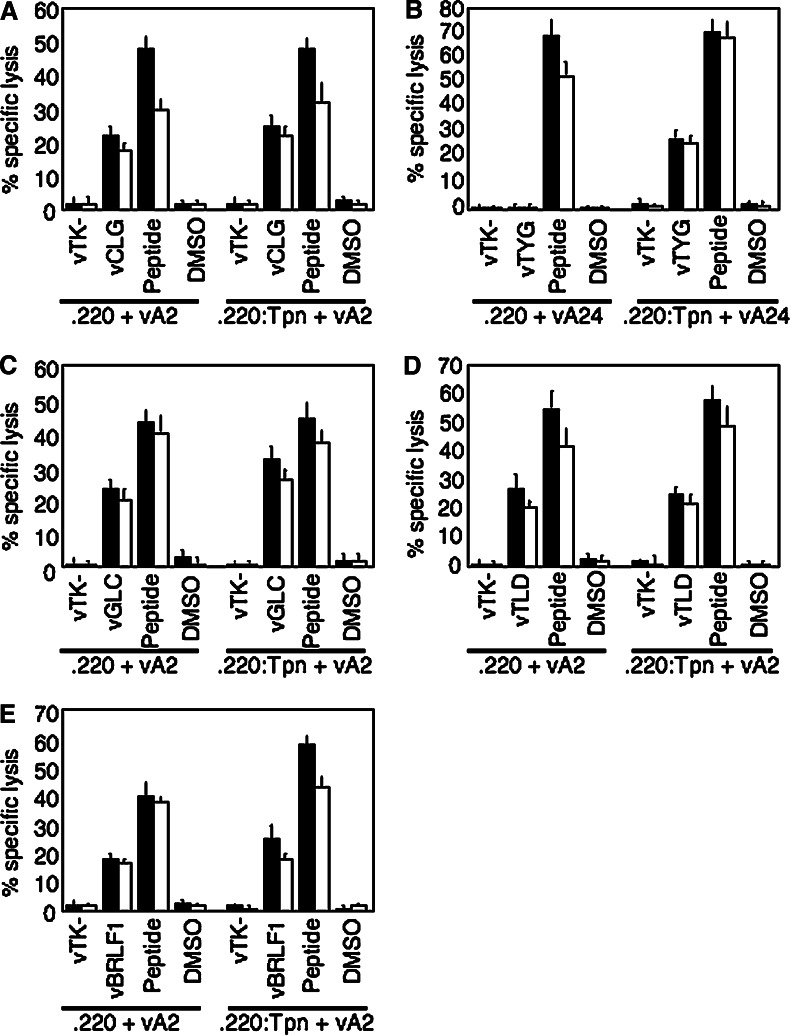
A number of antigenic peptides from a range of EBV antigens were previously shown to be presented to CD8+ CTL in a TAP-independent manner. We began this study to investigate how they were presented in cells deficient for a second MHC Class I accessory protein, tapasin. Using the tapasin-negative .220 cell line [21], we investigated the presentation of five TAP-independent antigenic peptides from the EBV antigens LMP2, BMLF1, BMRF1 and BRLF1. We first looked at two peptides from EBV LMP2 restricted through HLA-A2 and A24. When expressed as minigenes using recombinant vaccinia viruses, the A2-resticted peptide CLG was efficiently presented in .220 cells, at comparable levels to the tapasin-transfected control line .220:Tpn (Fig. 1a), whereas the A24-restricted peptide TYG was only presented in the tapasin-transfected cells (Fig. 1b). Peptide-pulsed control cells were recognised efficiently in all cases. We extended these experiments to examine three additional TAP-independent HLA A2-restricted peptides, GLC, TLD and YVL from the EBV lytic antigens BMLF1, BMRF1 and BRLF1, respectively. Both the GLC and TLD peptides expressed as minigenes in .220 were presented efficiently, again to a level similar to that in .220:Tpn cells (Fig. 1c, d). In addition, when the whole BRLF1 antigen, which contains the YVL peptide, was expressed from a recombinant vaccinia in .220 cells, this peptide was presented to CTL to comparable levels to that seen in .220:Tpn cells (Fig. 1e).
Fig. 1.
Presentation of EBV TAP-independent peptides in Tapasin-negative 721.220 cells. Data from cytotoxicity assays using as effectors CTL clones specific for the a HLA A2-restricted CLG peptide, b HLA A24-restricted TYG peptide, c HLA A2-restricted GLC peptide, d HLA A2-restricted TLD peptide and e HLA A2-restricted YVL peptide and as targets either .220 cells (Tapasin-negative) or .220:Tpn cells (Tapasin-transfectant of .220). Target cells were infected with vaccinia–minigene recombinants expressing the indicated antigenic peptide (vCLG, vTYG, vGLC and vTLD), or for YVL-specific effectors, a recombinant vaccinia expressing the whole antigen (vBRLF1). As .220 cells are not only tapasin-negative but also deficient for HLA class I alleles, target cells were also co-infected with a recombinant vaccinia expressing the relevant HLA class I restriction element (vA2 or vA24). Target cells were pre-exposed to their antigenic peptide as a positive control or to an equivalent concentration of DMSO solvent as a negative control. vTK- is a control vaccinia recombinant. Results are shown as % specific lysis in standard 5-h chromium release assays using effector:target ratios of 5:1 (black bars) and 2:1 (white bars), and represent the mean values from triplicate wells (+SD). The data shown are from one representative experiment out of at least three for each individual peptide
Thus, all HLA-A2 restricted TAP-independent peptides tested, whether expressed as minigenes or for the YVL peptide as part of a whole antigen, were also presented in cells deficient in tapasin, whereas the TAP-independent A24-restricted peptide was found to be tapasin-dependent. Importantly, the tapasin-negative cell line .220, which is derived from the .184 cell line, is deficient in MHC Class I expression (hence the need to co-express the revelant HLA allele via a second recombinant vaccinia) but does express both TAP1 and TAP2 [11]. Thus, in either a TAP-negative or a tapasin-negative background these hydrophobic HLA A2-resticted peptides are presented efficiently. However, it was not clear how well these TAP-independent, tapasin-independent peptides would be presented in a cell which is deficient for both TAP and tapasin, a scenario which is likely to be the case in a significant number of human tumours.
Small cell lung cancer cell lines are deficient in tapasin in addition to TAP
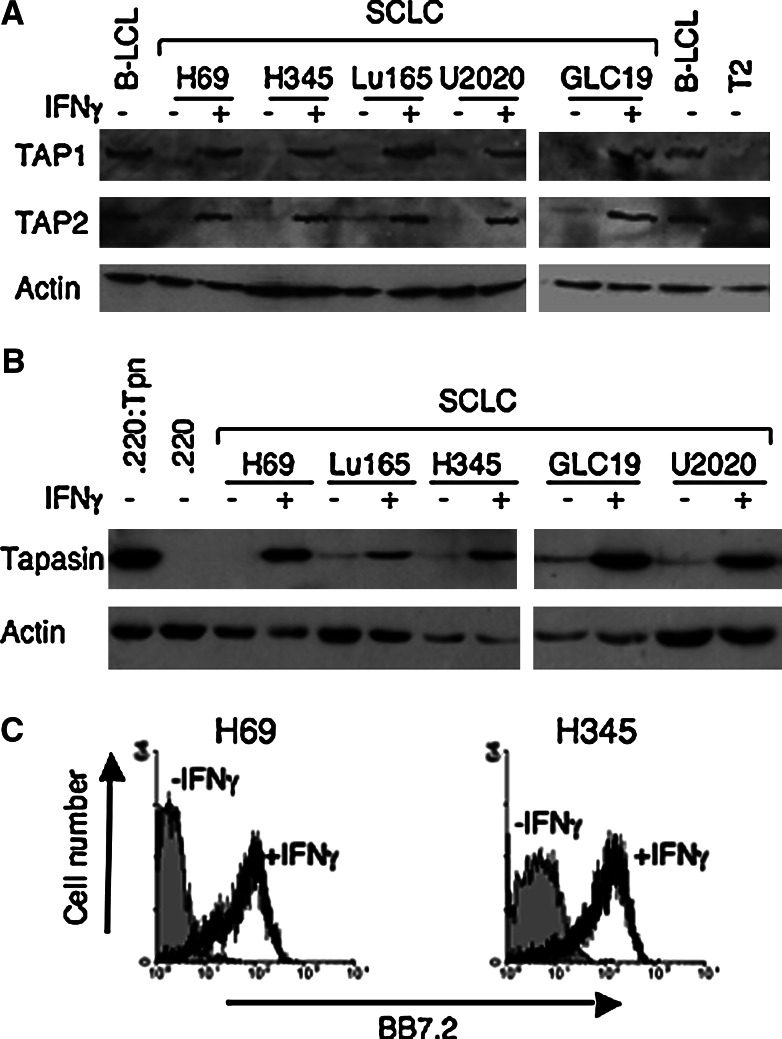
Many tumour cells have been shown to have decreased levels of TAP and increasingly have also been shown to be defective in tapasin. We speculated that it might be possible to target such tumour cells using CTL specific for TAP-, tapasin-independent peptides. SCLC tumours are well documented to have low levels of surface MHC Class I [8] and have reduced levels of both TAP1 and TAP2 [24, 30]. To test our strategy we choose to look at cell lines derived from SCLC and first investigated the level of tapasin expression. We characterised expression of antigen processing machinery in five available SCLC cell lines (NCI-H69, NCI-H345, Lu-165, U2020 and GLC-19). As shown in Fig. 2a, we confirmed that TAP1 and TAP2 were expressed at barely detectable levels, but could be restored by treatment with IFNγ, as previously reported for many other SCLC lines [24, 30]. We then investigated tapasin expression in these SCLC cell lines and found that all five expressed very little, if any, tapasin, but that again this could be restored by IFNγ treatment (Fig. 2b). Thus, of the five SCLC cell lines tested, all were deficient for both TAP and tapasin.
Fig. 2.
SCLC cells are deficient for Tapasin in addition to TAP. Protein extracts from SCLC cell lines, before and after treatment with IFNγ, were analysed by western blotting with rabbit anti-sera specific for a TAP1 and TAP2, and b Tapasin. Control cell lines included for the TAP blot, a standard TAP-positive lymphoblastoid cell line (B-LCL), and TAP-negative T2 cells, and for the Tapasin blot the Tapasin negative .220 cell line and its tapasin-transfected counterpart (.220:Tpn). Replicate blots were probed for actin as a loading control. c Flow cytometric analysis of expression of HLA-A2 on SCLC cell lines NCI-H69 and NCI-H345 before and after treatment with IFNγ, as determined by staining cells with monoclonal antibody BB7.2
The HLA type of each cell line was determined and revealed that both NCI-H69 and NCI-H345 were HLA A2-positive. This was confirmed by staining cells with the A2-specific monoclonal antibody BB7.2 pre- and post-IFNγ treatment. As expected, untreated cells showed no surface expression of HLA A2, but high levels of HLA A2 were detected after treating the cells with IFNγ (Fig. 2c). In addition, using both cell lines as targets in chromium release assays after pulsing with the HLA A*0201-restricted peptides, CLG and TLD confirmed they were capable of presenting HLA A*0201-restricted peptides to specific CTL (data not shown). Thus, the SCLC cell lines NCI-H69 and NCI-H345 are suitable tumour cell models to investigate how antigenic peptides, which can be presented efficiently in cells defective in either TAP or tapasin, are presented in cells deficient for both TAP and tapasin.
Cytosolically expressed TAP-, tapasin-independent antigenic peptides are not presented in SCLC cell lines
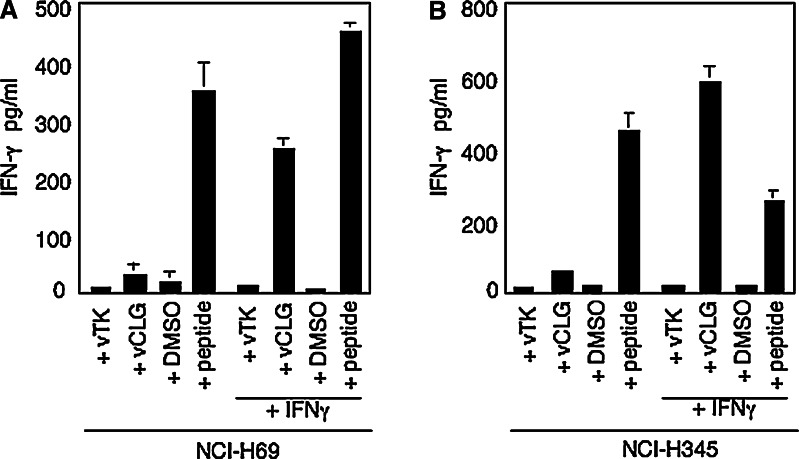
We first investigated the presentation of the HLA A2-restricted peptide CLG in NCI-H69 cells. When expressed cytosolically as a minigene using a recombinant vaccinia virus (vCLG), the CLG peptide was not presented, as there was no release of IFNγ above that seen for the control virus vTK- (Fig. 3a). Both peptide pulsed cells, and importantly cells pretreated with IFNγ prior to infection with vCLG, were efficiently recognised by CLG-specific CTL. Identical results were observed when a similar experiment was carried out using the second HLA A2-positive SCLC cell line NCI-H345 as targets (Fig. 3b). To investigate whether the lack of presentation of the TAP-independent, tapasin-independent CLG peptide in SCLC cell lines was a property specific to this peptide, we carried out experiments to look at presentation of two other HLA A2-restricted TAP-independent, tapasin-independent peptides (TLD and YVL). In this instance, we transfected both NCI-H69 and NCI-H345 with plasmid constructs expressing each peptide cytosolically as a minigene. As shown in Fig. 4a, b, neither NCI-H69 nor NCI-H345 cells were recognised by TLD-specific CTL effectors after transfection with the plasmid pCMV-TLD. Whereas, if either cell line was pretreated with IFNγ then TLD-specific effectors recognised both SCLC lines when transfected with pCMV-TLD, at levels equivalent to peptide pulsed targets. Similarly, when the YVL peptide was expressed as a minigene from the plasmid pCMV-YVL, it was not presented in either NCI-H69 or NCI-H345 cells. Whereas, both cell lines pulsed with the cognate peptide and cells which had been pretreated with IFNγ prior to transfection with pCMV-YVL were efficiently recognised by YVL-specific effectors (Fig. 4c, d). Thus, despite the fact that these hydrophobic peptides were capable of being presented in cells deficient in either TAP or tapasin, when expressed as minigenes, none of the peptides were presented efficiently in cells deficient for both TAP and tapasin.
Fig. 3.
The TAP-independent, Tapasin-independent peptide CLG is not presented in SCLC cells unless cells are pretreated with IFNγ. HLA-A*0201-positive SCLC cell lines a NCI-H69 and b NCI-H345, either untreated or pre-treated with IFNγ for 48 h, were infected with vTK-as a control or with a vaccinia–minigene recombinant expressing the TAP-independent, Tapasin-independent CLG peptide (vCLG). SCLC cells exposed to the antigenic peptide or to an equivalent concentration of DMSO solvent served as positive and negative controls. 2 × 104 target cells per well were incubated overnight with CLG-specific CTL clone WT c20 at 3,000 cells per well. Results represent the mean values of IFNγ release measured from triplicate cultures (+SD) and are from one representative experiment out of three
Fig. 4.
The TAP-independent, Tapasin-independent peptides TLD and YVL are not presented in SCLC cells unless cells are pretreated with IFNγ. HLA-A*0201-positive SCLC cell lines (a, c) NCI-H69 and (b, d) NCI-H345, either untreated or pre-treated with IFNγ for 48 h, were transfected with pCMV as a control or with a plasmid expressing the TAP-independent, Tapasin-independent TLD peptide (pCMV-TLD) (a, b) or YVL peptide (pCMV-YVL) (c, d). SCLC cells exposed to the antigenic peptide or to an equivalent concentration of DMSO solvent served as positive and negative controls. 2 × 104 target cells per well were incubated overnight with YVL-specific (IM106c75) or TLD-specific (IM113c28) CTL clone at 5,000 cells per well. Results represent the mean values of IFNγ release measured from triplicate cultures (+SD), and are from one representative experiment out of two
Peptides targeted to the ER via a signal sequence are presented in SCLC cells
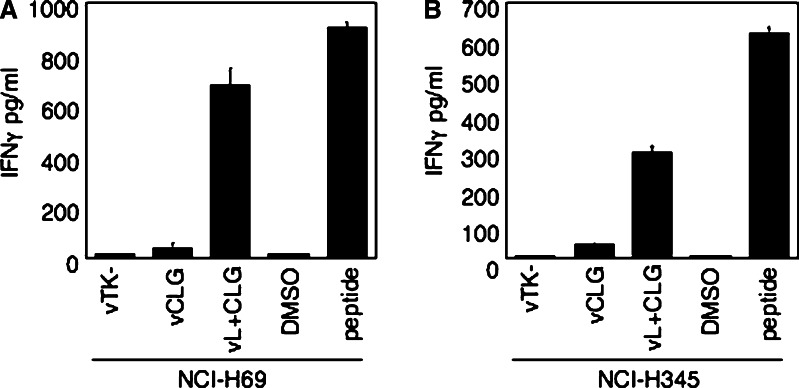
It was not clear whether the limiting step was transport across the ER membrane or loading onto MHC class I molecules. Although, as tapasin plays a dual role, both linking the PLC to TAP and optimising the loading of high affinity peptides onto MHC molecules, and even in the absence of these functions, the peptides tested were able to load onto MHC molecules it seemed likely that the limiting step in the SCLC cells was transport across the ER membrane. It was possible that, in contrast to TAP-mediated entry in .220 cells, the TAP-independent pathway utilised by these hydrophobic peptides leads to insufficient peptide accessing the ER to enable efficient loading of MHC class I molecules in the absence of tapasin. To address this issue, we utilised a recombinant vaccinia expressing the CLG peptide linked to an ER-targeting sequence (vL+CLG). This approach relies on the signal sequence to direct the antigenic peptide into the lumen of the ER, where the activity of signal peptidase generates free antigenic peptide. This has been shown to be a highly effective method at generating high concentrations of peptide in the ER for loading onto MHC class I molecules. Using the vL+CLG recombinant we investigated presentation of the hydrophobic TAP-, tapasin-independent A2-restricted CLG peptide in both NCI-H69 and NCI-H345 cell lines. Both cell lines, without pre-treatment with IFNγ, were recognised efficiently by CLG-specific effectors after infection with vL+CLG at levels significantly above that of the control virus vTK- (Fig. 5). Thus, by bypassing the TAP-independent step and actively directing the CLG peptide into the ER of tapasin negative tumour cells then it can load efficiently onto MHC class I molecules.
Fig. 5.
Directing TAP-independent, Tapasin-independent peptides directly to the ER sensitizes SCLC cell lines for recognition by peptide-specific CTL. HLA-A*0201-positive SCLC cell lines a NCI-H69 and b NCI-H345, were infected with vTK-as a control or with a vaccinia–minigene recombinant expressing the TAP-independent, Tapasin-independent CLG peptide (vCLG) or with a vaccinia–minigene construct containing the CLG peptide attached to an ER signal sequence (vL + CLG). SCLC cells exposed to the antigenic peptide or to an equivalent concentration of DMSO solvent served as positive and negative controls. 2 × 104 target cells per well were incubated overnight with CLG-specific CTL clone WT c20 at 3,000 cells per well. Results represent the mean values of IFNγ release measured from triplicate cultures (+SD), and are from one representative experiment out of two
Discussion
A major caveat of immunotherapeutic approaches that utilize CD8+ CTL to target human tumours is the ability of the tumour cell to present antigenic peptides in the context of MHC class I molecules on the cell surface. The fact that many tumours have defects in the MHC class I antigen presentation pathway means that strategies are required to circumvent this problem. The defect seen as the most important to address has been the low expression of TAP and there are a number of approaches that have been proposed to overcome the lack of TAP function in tumour cells. These include IFNγ treatment [13], transduction of cells with TAP-expressing constructs [2], use of signal sequences to direct peptides into the ER [29] or the targeting of TAP-independent peptides such as the hydrophobic peptides described in this report [14]. While IFNγ can upregulate all components of the MHC class I antigen processing pathway, it has drawbacks due to the occurrence of IFNγ-resistant tumour cells [1, 34]. None of the other approaches take into account the increasingly apparent problem that other defects in antigen presentation are found in tumour cells, most notably downregulated tapasin levels. Low tapasin expression has been observed in many different tumours including renal cell carcinoma, astrocytic tumours and squamous cell carcinoma of head and neck [9, 17, 28, 27]. Indeed, we show here that five TAP-deficient SCLC cell lines also have low levels of tapasin expression, supporting data from a recent study by Jefferies et al. who reported low levels of tapasin in nine SCLC tumour biopsies [18].
We thus chose to investigate the role of tapasin in the presentation of antigenic peptides which we had shown previously could, due to their hydrophobic nature, be presented in TAP-negative cell lines. The data from this study show that four out of the five hydrophobic TAP-independent peptides, when analysed in the tapasin-negative cell line 721.220 were also found to be tapasin-independent. All four tapasin-independent peptides were presented by the HLA A2 allele. As reported previously, different HLA alleles vary in their requirement for tapasin-mediated loading of antigenic peptide. Thus, of the alleles studied to date, both HLA A2, confirmed here for the peptides used in this report, and HLA B*2705, can load peptide in the absence of tapasin. In contrast, HLA B*4002 and B8 were dependent on tapasin [11, 16, 23]. We can now add HLA A*2402 to the alleles that appear to rely on tapasin for efficient loading. However, to obtain a truly reflective picture of potentially useful tapasin-independent peptides from a range of tumour antigens, the testing of individual peptides across a range of alleles will be required.
The four HLA A2-restricted peptides tested here are also TAP-independent. The tapasin negative .220 cell line however is TAP-positive, so it was difficult to assess the contribution of TAP-independent transport in this system. To get a true indication of the possibility of using such TAP-, tapasin-independent peptides to sensitize tumour cells, we switched our attention to SCLC cell lines which we showed to be tapasin-deficient in addition to TAP-deficient. For all TAP-, tapasin-independent peptides tested and expressed as minigenes in the cytosol, none were able to sensitize SCLC cells for recognition by peptide-specific T cells. As shown by the pre-treatment of target cells with IFNγ this was not due to the inability of the cell lines to present peptide. It has been suggested that tapasin is required for the optimal loading of TAP-independent A2-restricted peptides. Barber et al. monitored the levels of stable HLA A2 molecules in tapasin-deficient cells and contrasted this with the high level found in the TAP-negative, tapasin-positive T2 line [5]. The source of TAP-independent peptide in this case is mainly derived from signal peptides of endogenous levels of protein expression. In this study we have overexpressed the hydrophobic TAP-independent peptides as minigenes in the cytosol of TAP-negative, tapasin-negative cells. This approach generates extremely high levels of free peptide in the cytosol [3], although it is not clear how much of this peptide is actually able to access the ER via the TAP-independent route. In the original studies characterising these peptides as TAP-independent, the cell line used was the TAP-negative, tapasin-positive T2 line. From previous work it was clear that if TAP was reintroduced into these cells then there was no significant increase in the presentation and recognition of cytosolically expressed peptide over that observed in the original TAP-deficient background (G.L. and N.B., unpublished). Thus, implying that when tapasin was present the TAP-independent route generated concentrations of peptide in the ER that were capable of loading onto MHC class I molecules to an equivalent level to that seen when TAP was present. However, despite the fact that these hydrophobic peptides can be loaded onto MHC molecules effectively in a TAP-independent manner, the data presented here would suggest that this is not an effective route for peptide to access the ER in sufficient concentration to load onto MHC molecules in the absence of tapasin. When we utilised an ER signal sequence to target a TAP-independent, tapasin-independent peptide directly into the ER, this clearly sensitized the TAP-negative, tapasin-negative SCLC cell lines for recognition by peptide-specific CTL. The use of an ER signal sequence results in a vast increase of antigenic peptide within the ER, it has been estimated that this can lead to up to a 2000-fold increase in peptide presentation [3]. Thus, in the absence of tapasin it is probable that for certain peptides, restricted through specific HLA alleles (e.g. HLA A2), a high concentration is required in the lumen of the ER for effective loading onto MHC molecules, which can then be released from the ER to be presented on the cell surface. This effective concentration of peptide can be achieved either by TAP-mediated transport or, as we show here in the absence of TAP, by use of an ER-targeting sequence. It is important to note that not all peptides if preceded by an ER signal sequence will be presented in a tapasin-independent fashion. A TAP-dependent epitope from the EBV antigen LMP2 restricted through HLA B*2704 which is also tapasin-dependent, still required tapasin for effective presentation to T cells even when directed to the ER by use of a signal sequence (G.L., unpublished).
We propose that the concentration of peptide accessing the ER is critical for loading onto MHC class I molecules in tapasin-negative cells, and that via the TAP-independent pathway for hydrophobic peptides the concentration is insufficient to load MHC class I molecules in the absence of tapasin. With the tapasin-independent antigenic peptides studied here it is clear that if enough peptide can be directed into the ER either by the normal TAP route (in .220 cells) or by an ER translocation sequence (in SCLC cells), then tapasin-negative cells can be sensitized for recognition by peptide-specific CTL. Thus, the choice of immunotherapeutic strategy for targeting tumours with defects in MHC class I antigen processing pathways should be focused on identification of tapasin-independent peptides and the use of approaches to increase the concentration of such peptides in the ER or the identification of strategies to effectively restore expression of tapasin within tumour cells.
Acknowledgments
This work was supported by grants from North West Cancer Research Fund and Cancer Research UK. We thank the Department of Immunology (RLBUHT, Liverpool, UK) for HLA typing, and Drs A. Hislop (Birmingham, UK), T. Elliott (Southampton, UK) and P. Lehner (Cambridge, UK) for providing valuable reagents.
Abbreviations
- ER
Endoplasmic reticulum
- EBV
Epstein-Barr virus
- CTL
Cytotoxic T lymphocyte
- PLC
Peptide loading complex
- SCLC
Small cell lung cancer
- TAP
Transporters associated with antigen processing
References
- 1.Abril E, Mendez RE, Garcia A, Serrano A, Cabrera T, Garrido F, Ruiz-Cabello F. Characterization of a gastric tumor cell line defective in MHC class I inducibility by both alpha- and gamma-interferon. Tissue Antigens. 1996;47:391–398. doi: 10.1111/j.1399-0039.1996.tb02574.x. [DOI] [PubMed] [Google Scholar]
- 2.Alimonti J, Zhang QJ, Gabathuler R, Reid G, Chen SS, Jefferies WA. TAP expression provides a general method for improving the recognition of malignant cells in vivo. Nat Biotechnol. 2000;18:515–520. doi: 10.1038/75373. [DOI] [PubMed] [Google Scholar]
- 3.Anton LC, Yewdell JW, Bennink JR. MHC class I-associated peptides produced from endogenous gene products with vastly different efficiencies. J Immunol. 1997;158:2535–2542. [PubMed] [Google Scholar]
- 4.Atkins D, Ferrone S, Schmahl GE, Storkel S, Seliger B. Down-regulation of HLA class I antigen processing molecules: an immune escape mechanism of renal cell carcinoma. J Urol. 2004;171:885–889. doi: 10.1097/01.ju.0000094807.95420.fe. [DOI] [PubMed] [Google Scholar]
- 5.Barber LD, Howarth M, Bowness P, Elliott T. The quantity of naturally processed peptides stably bound by HLA-A*0201 is significantly reduced in the absence of tapasin. Tissue Antigens. 2001;58:363–368. doi: 10.1034/j.1399-0039.2001.580604.x. [DOI] [PubMed] [Google Scholar]
- 6.Coulson JM, Stanley J, Woll PJ. Tumour-specific arginine vasopressin promoter activation in small- cell lung cancer. Br J Cancer. 1999;80:1935–1944. doi: 10.1038/sj.bjc.6690623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coulson JM, Edgson JL, Woll PJ, Quinn JP. A splice variant of the neuron-restrictive silencer factor repressor is expressed in small cell lung cancer: a potential role in derepression of neuroendocrine genes and a useful clinical marker. Cancer Res. 2000;60:1840–1844. [PubMed] [Google Scholar]
- 8.Doyle A, Martin WJ, Funa K, Gazdar A, Carney D, Martin SE, Linnoila I, Cuttitta F, Mulshine J, Bunn P, Minna J. Markedly decreased expression of class I histocompatibility antigens, proteins and mRNA in human small-cell lung cancer. J Exp Med. 1985;161:1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Facoetti A, Nano R, Zelini P, Morbini P, Benericettti E, Ceroni M, Campoli M, Ferrone S. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Can Res. 2005;11:8304–8311. doi: 10.1158/1078-0432.CCR-04-2588. [DOI] [PubMed] [Google Scholar]
- 10.Gil-Torregrosa BC, Raul Castano A, Del Val Major histocompatibility complex class I viral antigen processing in the secretory pathway defined by the trans-Golgi network protease furin. J Exp Med. 1998;188:1105–1116. doi: 10.1084/jem.188.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenwood R, Shimizu Y, Sekhon GS, DeMars R. Novel allele-specific, post-translational reduction in HLA class I surface expression in a mutant human B cell line. J Immunol. 1994;153:5525–5536. [PubMed] [Google Scholar]
- 12.Henderson RA, Michel H, Sakaguchi K, Shabanowitz J, Appella E, Hunt DF, Engelhard VH. HLA-A2.1-associated peptides from a mutant cell line: a second pathway of antigen presentation. Science. 1992;255:1264–1266. doi: 10.1126/science.1546329. [DOI] [PubMed] [Google Scholar]
- 13.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 14.Khanna R. Tumour surveillance: missing peptides and MHC molecules. Immunol Cell Biol. 1998;76:20–26. doi: 10.1046/j.1440-1711.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- 15.Lautscham G, Mayrhofer S, Taylor G, Haigh T, Leese A, Rickinson A, Blake N. Processing of a multiple membrane spanning Epstein-Barr virus protein for CD8+ T cell recognition reveals a proteasome-dependent, transporter associated with antigen processing-independent pathway. J Exp Med. 2001;194:1053–1068. doi: 10.1084/jem.194.8.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewis JW, Sewell A, Price D, Elliott T. HLA-A*0201 presents TAP-independent peptide epitopes to cytotoxic T lymphocytes in the absence of tapasin. Eur J Immunol. 1998;28:3214–3220. doi: 10.1002/(SICI)1521-4141(199810)28:10<3214::AID-IMMU3214>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 17.Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL. Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol. 2006;176:3402–3409. doi: 10.4049/jimmunol.176.6.3402. [DOI] [PubMed] [Google Scholar]
- 18.Lou Y, Vitalis TZ, Basha G, Cai B, Chen SS, Choi KB, Jeffries AP, Elliot WM, Atkins D, Seliger B, Jefferies WA. Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific responses and survival. Cancer Res. 2005;65:7926–7933. doi: 10.1158/0008-5472.CAN-04-3977. [DOI] [PubMed] [Google Scholar]
- 19.Morris EC, Bendle GM, Stuass HJ. Prospects for immunotherapy of malignant disease. Clin Exp Immunol. 2003;131:1–7. doi: 10.1046/j.1365-2249.2003.02055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogino T, Bandoh N, Hayashi T, Miyokawa N, Harabuchi Y, Ferrone S. Association of tapasin and HLA class I antigen down-regulation in primary maxillary sinus squamous cell carcinoma lesions with reduced survival of patients. Clin Can Res. 2003;9:4043–4051. [PubMed] [Google Scholar]
- 21.Ortmann B, Copeman J, Lehner PJ, Sadasivan B, Herberg JA, Grandea AG, Riddell SR, Tampe R, Spies T, Trowsdale J, Cresswell P. A critical role for tapasin in the assembly and function of multimeric MHC class I-TAP complexes. Science. 1997;277:1306–1309. doi: 10.1126/science.277.5330.1306. [DOI] [PubMed] [Google Scholar]
- 22.Pamer E, Cresswell P. Mechanisms of MHC class I-restricted antigen processing. Ann Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Peh CA, Burrows SR, Barnden M, Khannna R, Cresswell P, Moss DJ, McCluskey J. HLA-B27-restricted antigen presentation in the absence of tapasin reveals polymorphism in mechanisms of HLA class I peptide loading. Immunity. 1998;8:531–542. doi: 10.1016/S1074-7613(00)80558-0. [DOI] [PubMed] [Google Scholar]
- 24.Restifo NP, Esquivel F, Kawakami Y, Yewdell JW, Mule JJ, Rosenberg SA, Bennink JR. Identification of human cancers deficient in antigen processing. J Exp Med. 1993;177:262–272. doi: 10.1084/jem.177.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salter RD, Cresswell P. Impaired assembly and transport of HLA-A and -B antigens in a mutant TxB cell hybrid. EMBO J. 1986;5:943–949. doi: 10.1002/j.1460-2075.1986.tb04307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;211:455–464. doi: 10.1016/S0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 27.Seliger B, Schreiber K, Delp K, Meissner M, Hammers S, Reichert T, Pawlischko K, Tampe R, Huber C. Downregulation of the constitutive tapasin expression in human tumor cells of distinct origin and it upregulation by cytokines. Tissue Antigens. 2001;57:39–45. doi: 10.1034/j.1399-0039.2001.057001039.x. [DOI] [PubMed] [Google Scholar]
- 28.Seliger B, Atkins D, Bock M, Ritz U, Ferrone S, Huber C, Storkel S. Characterization of human lymphocyte antigen class I antigen-processing machinery defects in renal cell carcinoma lesions with special emphasis on transported-associated with antigen-processing down-regulation. Clin Cancer Res. 2003;9:1721–1727. [PubMed] [Google Scholar]
- 29.Sherritt M, Cooper L, Moss DJ, Kienzle N, Altman J, Khanna R. Immunization with tumor-associated epitopes fused to an endoplasmic reticulum translocation signal sequence affords protection against tumors with down-regulated expression of MHC and peptide transporters. Int Immunol. 2001;13:265–271. doi: 10.1093/intimm/13.3.265. [DOI] [PubMed] [Google Scholar]
- 30.Singal DP, Ye M, Qiu X. Molecular basis for lack of expression of HLA class I antigens in human small-cell lung carcinoma cell lines. Int J Cancer. 1996;68:629–636. doi: 10.1002/(SICI)1097-0215(19961127)68:5<629::AID-IJC13>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 31.Snyder HL, Bacik I, Bennink JR, Kearns G, Behrens TW, Bachi T, Orlowski M, Yewdell JW. Two novel routes of transporter associated with antigen processing (TAP)-independent major histocompatibility complex class I antigen processing. J Exp Med. 1997;186:1087–1098. doi: 10.1084/jem.186.7.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spies T, Cerundolo V, Colonna M, Cresswell P, Townsend A, DeMars R. Presentation of viral antigen by MHC class I is dependent on a putative peptide transporter heterodimer. Nature. 1992;355:644–646. doi: 10.1038/355644a0. [DOI] [PubMed] [Google Scholar]
- 33.Wolfel C, Drexler I, Van Pel A, Thres T, Leister N, Herr W, Sutter G, Huber C, Wolfel T. Transporter (TAP)- and proteasome-independent presentation of a melanoma-associated tyrosinase epitope. Int J Cancer. 2000;88:432–438. doi: 10.1002/1097-0215(20001101)88:3<432::AID-IJC16>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Wong LH, Krauer KG, Hatzinisiriou I, Estcourt MJ, Hersey P, Tam ND, Edmondson S, Devenish RJ, Ralph SJ. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3γ. J Biol Chem. 1997;272:28779–28785. doi: 10.1074/jbc.272.45.28779. [DOI] [PubMed] [Google Scholar]