Abstract
The microenvironment produced by solid tumors is inhibitory to the immune system, inducing dendritic cell (DC) alterations, but there is a paucity of information regarding haematological malignances. The aim of this study was to investigate DC differentiation under the influence of leukemic cell products. Monocytes from healthy volunteers were cultured in the presence of IL-4 and GM-CSF for the generation of immature DCs. Supernatants from leukemic cultures were added to monocyte cultures during differentiation. The lineages used were K562, a chronic myeloid leukemia, HL-60, a promyelocytic leukemia and DAUDI, originated from Burkitt lymphoma. It was observed that the expression of CD14 remained high and the CD1a was low in the presence of tumor supernatants, while non-malignant supernatants did not affect these parameters. Furthermore, IL-1β and TNF-α production by monocytes during differentiation was increased by the presence of tumor supernatants. The modifications on CD14 and CD1a expressions could be mimicked by the addition of exogenous IL-1β and partially inhibited by the neutralization of IL-1β. These results suggest that soluble products from leukemic cells interfere with DC differentiation and, in the present work, this effect could be mediated by monocyte-derived IL-1β in response to tumor supernatants.
Keywords: Dendritic cell differentiation, Leukemic cell products, IL-1β, TNF-α
Introduction
The microenvironment surrounding tumor growth presents paradoxical features. Despite the infiltration of immune cells and the inflammatory characteristics at the site of the tumor [1–3], there are evidences that substances released by tumor cells and inflammatory cells may have immunosuppressive activities [4–6]. Furthermore, during tumor growth and inflammation, the cytokine environment produced by tumor cells and macrophages may stimulate the release of other cytokines leading to a complex situation where local modulation of the response may even favor cancer development and progression [7].
There are evidences that prostaglandin 2 (PGE2), interleukin 6 and 10 (IL-6, IL-10) and transforming growth factor-β (TGF-β) present in many supernatants of tumor cell cultures may inhibit macrophage cytotoxic activity while inducing suppressor activity [8]. Moreover, the cytokines IL-6 and IL-10 are known to suppress human dendritic cell (DC) differentiation [9, 10], whereas PGE2 is important in the maturation process [11]. DCs are professional antigen-presenting cells (APCs) involved in the capture and processing of antigens and capable of inducing primary responses of T cells [12]. In humans, DCs represent less than 1% of circulating cells in peripheral blood [13]. These cells can originate directly from bone marrow CD34+ cells or from monocytes [14]. It is possible, in vitro, using a combination of cytokines to differentiate, to mature and to study the function of DCs [15].
Using CD34+ cells, the generation of DCs was inhibited by the serine protease (PSA) produced by prostatic tumors [16]. Moreover, other solid tumor-derived factors, such as gangliosides, also contribute to the inhibition of DC differentiation, as shown by Shurin group [17].
Similarly, the release of cytokines by solid tumors has been shown to regulate the differentiation pathway of monocytes. It has been demonstrated that IL-6 and macrophage colony-stimulating factor (M-CSF) secreted by renal cell carcinoma and macrophages inhibit DC differentiation while stimulating macrophage differentiation by increasing the expression of M-CSF receptor on monocytes [18]. Besides, IL-6 and granulocyte colony-stimulating factor (G-CSF) exogenous seem to inhibit DC differentiation and maturation [19]. The ability of tumor necrosis factor (TNF-α) to promote tumor growth has been demonstrated in several studies. TNF-α, produced by tumor cells or inflammatory cells in a tumor microenvironment, promotes the survival of tumor cells through induction of antiapoptotic genes of molecules dependent on nuclear factor κB (NF-κB). Furthermore, TNF-α seems to promote angiogenesis and metastasis in some cancers [20]. Apte and collaborators [21], in 2006, suggested that interleukin-1α (IL-1α) expressed on the surface of tumor cells stimulates an antitumour response, whereas the secreted form, interleukin-1β (IL-1β), derived from tumor cells or inflammatory cells promotes invasiveness and immunosuppression. Conversely, cytokines, such as TNF-α and IL-1β, are capable of activating immature DCs [22, 23], although it has been demonstrated that TNF-α inhibits DC differentiation from monocytes [24].
Some studies have proposed mechanisms by which tumor products interfere with DC differentiation. Sombroek and co-workers [25] showed that primary tumors, including colon, breast, renal cell carcinoma, and melanoma, negatively modulate DC development from monocytes and DC activity via cyclooxygenase (COX)-1- and -2-regulated factors. In addition, Kiertscher et al. demonstrated that CD14+ monocytes responded to tumor culture supernatant (lung, breast, renal cell carcinomas and melanoma) by increasing the expression of APC surface markers, up-regulating nuclear translocation of RelB, and developing allostimulatory activity. Although displaying these characteristics of mature DC, these cells lacked the capacity to produce IL-12, did not acquire full allostimulatory activity, and rapidly underwent apoptosis [26]. Furthermore, colon adenocarcinoma was also shown to affect the generation of DCs that become incapable of producing interleukin-12 (IL-12) and this has been attributed to IL-10 production by tumor cells and consequently down-regulation of CD40 in DCs [27].
Most studies looking at the influence of tumors on the immune response focused on soluble factors produced by solid tumors. However, less is known regarding haematological malignances and this knowledge needs to be expanded and the mechanisms involved better understood. Leukemia cell factors are released in an environment where monocytes are present and may regulate their differentiation and function. The aim of this work was to study the effect of leukemic cell factors during the process of DC differentiation from human blood monocytes.
Materials and methods
DC differentiation
Peripheral blood was obtained from healthy volunteers using sodium heparin (Roche, Brazil) as an anticoagulant. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque (GE, USA) density gradient centrifugation. PBMC were seeded at a concentration of 5 × 106 cells per well (1 mL final volume) for 2 h in 24-well plates (TPP, Switzerland), at 37°C. Afterwards, non-adherent cells were removed by extensive washing. Adherent cells were cultured in RPMI 1640 (Sigma Chemical Co., USA) supplemented with 10% fetal bovine serum (FBS) (500 μL final volume) with or without 50 ng/mL GM-CSF, 50 ng/mL IL-4 (PeproTech, USA) and 0.2, 0.8, 3.2, 10 ng/mL IL-1β (R&D Biosystems, USA), for 5 days. In some experiments, tumor supernatants were added at the beginning of the culture and remained throughout the culture period (5 days).
This project has been approved by local Ethical Committee.
Tumor supernatants and lymphocyte supernatants
The cell lines K562 (chronic myeloid leukemia) [28], HL-60 (promyelocytic leukemia) [29] and DAUDI (Burkitt lymphoma) [30] cultured in vitro were used to obtain tumor supernatants. K562 and HL-60 lineages were cultured in RPMI 1640 supplemented with 10% FBS for 3 days. DAUDI was cultured in RPMI 1640 supplemented with 20% FBS for 3 days. Lymphocytes from healthy volunteers were obtained by Ficoll-Paque density gradient followed by 2 h adhesion in 24-well plates. The non-adherent cells were cultured in vitro without stimulation for 3 days in RPMI 1640 supplemented with 10% FBS. After this period, supernatants were collected after centrifugation (200×g for 7 min), filtered (0.2 μm filters) and added to monocyte cultures. The volume of supernatants added to monocyte cultures was 25, 50 or 100 μL, respectively, 5, 10 or 20% final volume.
Phenotypical analysis by flow cytometry
To determine CD14 and CD1a expressions, after DC differentiation, cells were collected and incubated for 10 min with phosphate-buffered saline (PBS) with 5% FBS. Cells were stained for 30 min at 4°C with FITC-conjugated anti-CD14 (from BD Biosciences, USA) and PE-conjugated anti-CD1a (from BD Biosciences, USA or E Bioscience, USA). After the incubation period, cells were washed with PBS and analyzed by flow cytometry (FACSCalibur; Becton and Dickinson). Data analyses were performed via the software Summit 4.3.
In some experiments, 3 μg/mL monoclonal anti-IL-1β (from R&D Biosystems) was added to monocytes in the first day of culture and in the third day to neutralize IL-1β.
Cytokine production
DCs were differentiated in 0.5 mL medium with 10% FBS with or without tumor supernatants. DC culture supernatants were collected and stored at −20°C until use. IL-1β, IL-6, IL-10 and TNF-α concentrations were measured with enzyme-linked immunosorbent assay (Duo-Set kits purchased from R&D Biosystems) according to the manufacturer’s instructions. Optical density was read at 450 nm in a microplate reader Sunrise Basic (Tecan, Austria).
Statistical analysis
Statistical analyses were performed using paired one-tailed t test in GraphPad Prim. Values of P ≤ 0.05 were considered statistically significant.
Results
Effect of K562 supernatants on CD14 and CD1a expressions
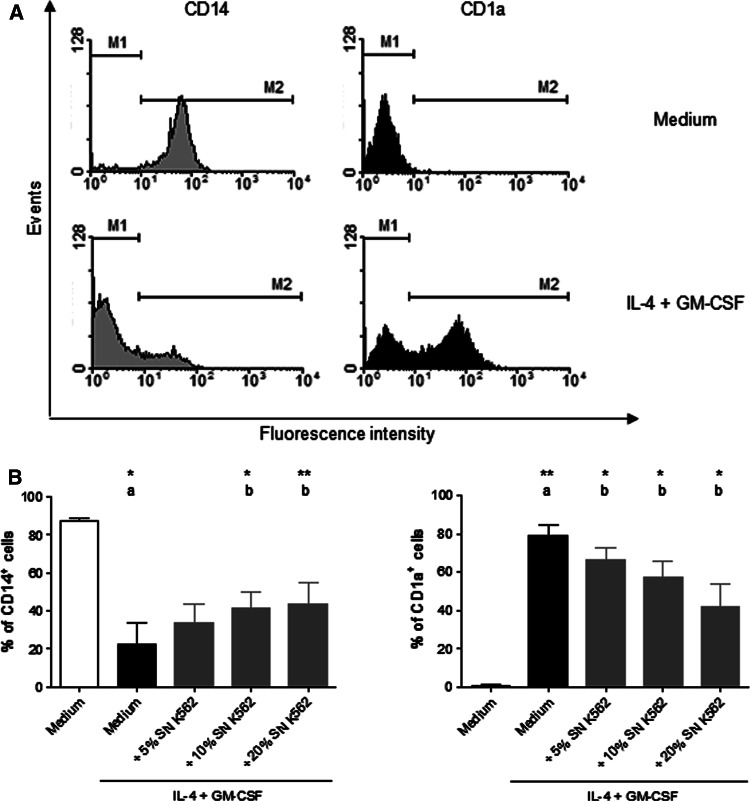
Monocytes express high levels of CD14. When stimulated with IL-4 and GM-CSF, monocytes differentiate into immature DCs, which lose CD14 expression, but start to express CD1a [13, 24]. To investigate a possible influence of products secreted by K562 lineage on DC differentiation, monocytes were cultured with IL-4, GM-CSF, with the addition of different amounts: 5, 10 and 20% (final culture volume) of K562 supernatants. After 5 days, CD14 and CD1a expressions were evaluated.
According to control differentiation, approximately 20% of monocytes cultured with IL-4 and GM-CSF presented CD14 molecules and 65% of them expressed CD1a molecules (Fig. 1a). The presence of K562 supernatant partially blocked the loss of CD14 expressions and also the appearance of CD1a (Fig. 1b). This effect was proportional to the concentration of supernatant in culture. When cultured in the presence of 10% final volume of K562 supernatant, there was 20% increase in the amount of cells that remained expressing CD14 and not expressing CD1a. This volume of supernatant was chosen to continue this study.
Fig. 1.
CD14 and CD1a expressions by monocytes in the presence or absence of K562 supernatants. Monocytes isolated from healthy volunteers were incubated with IL-4 and GM-CSF, in the presence or absence of 5, 10 and 20% final volume of K562 supernatant (SN K562) for 5 days. After this period, CD14 and CD1a expressions were analyzed by flow cytometry. Representative histogram (a) shows CD14 and CD1a expressions in a control differentiation. Data in bars (b) show CD14 and CD1a expressions of monocytes cultured in medium (white bar), with IL-4 and GM-CSF (black bar) and in the presence of SN K562 (gray bars). The result shows the mean ± SEM of at least three independent experiments. a Compared with monocytes without IL-4 and GM-CSF, b compared with monocytes cultured with IL-4 and GM-CSF. *Significantly different (P < 0.05), **Significantly different (P < 0.01)
Effect of K562, HL-60 and DAUDI supernatants on CD14 and CD1a expressions
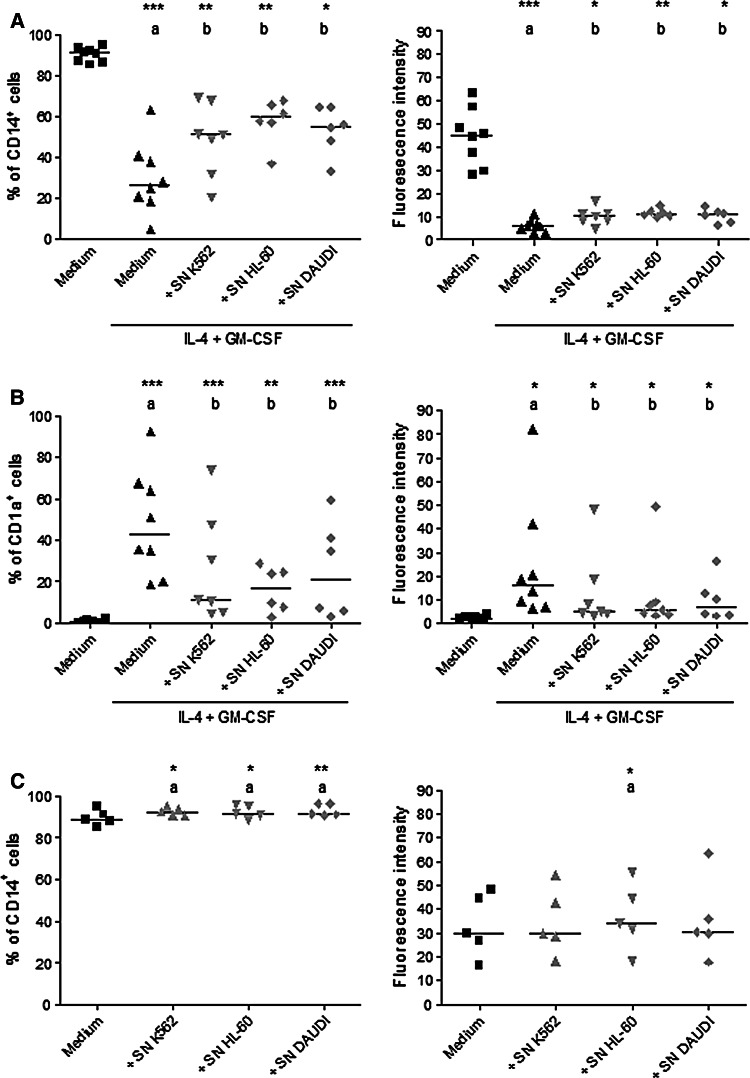
The influence of K562 supernatants on DC differentiation was suggested by their effect on CD14 and CD1a expressions. To investigate if other leukemias had a similar effect on DC differentiation, two different cell lines were also studied: HL-60 and DAUDI. Supernatants were added (10% final volume) to the monocyte culture. After 5 days, CD14 and CD1a expressions were evaluated.
According to Fig. 2, tumor supernatants significantly affected the number of cells expressing CD14 (Fig. 2a) and CD1a (Fig. 2b). About 20% more cells remained expressing CD14 and there was a 30% increase of cells not expressing CD1a in the presence of any tumor supernatant tested. Using tumor supernatants, there was also an increase in the amount of CD14 and a decrease in the amount of CD1a expression by each cell (fluorescence intensity).
Fig. 2.
CD14 and CD1a expressions by monocytes after induction of differentiation in the presence of K562, HL-60 and DAUDI supernatants. Monocytes isolated from healthy volunteers were incubated with or without IL-4 and GM-CSF in the presence of K562, HL-60 and DAUDI supernatants (10% final volume). After 5 days, CD14 and CD1a expressions were analyzed by flow cytometry. Data show the percentage of cells stimulated with IL-4 and GM-CSF that express CD14 (left) and mean of fluorescence intensity of CD14 (right) (a); the percentage of cells stimulated with IL-4 and GM-CSF that express CD1a (left) and mean of fluorescence intensity of CD1a (right) (b); the percentage of cells not stimulated with IL-4 and GM-CSF that express CD14 (left) and mean of fluorescence intensity of CD14 (right) (c). Each symbol per group represents one individual assessed under the different conditions. The horizontal lines represent the median of at least five independent experiments. a Compared with monocytes without IL-4 and GM-CSF, b compared with monocytes cultured with IL-4 and GM-CSF. *Significantly different (P < 0.05), **Significantly different (P < 0.01), ***Significantly different (P < 0.001)
To investigate the possibility that tumor supernatants may have affected monocytes before differentiation, monocytes were cultured only in the presence of tumor supernatants (K562, HL-60 and DAUDI) for 5 days. The presence of tumor supernatants promoted a slight increase in the percentage of cells expressing CD14 (Fig. 2c). HL-60 supernatant also increased the amount of CD14 by each cell (fluorescence intensity). CD1a expression was not found in monocytes cultured only with tumor supernatants (data not shown).
Effect of lymphocyte supernatants on CD14 and CD1a expressions
To investigate if products from normal cells could also interfere with DC differentiation, lymphocyte supernatants were used as non-malignant cell control. Lymphocytes were maintained in culture without stimulation for 3 days and the supernatant was collected after this period. Monocytes were cultured for 5 days in the presence of lymphocyte supernatants (10% final volume) and their expression of CD14 and CD1a evaluated. The presence of lymphocyte supernatants did not affect CD14 and CD1a expressions (data not shown), suggesting that only malignant cell cultures suppress DC differentiation.
Effect of K562 supernatants on CD14 and CD1a expressions during the differentiation
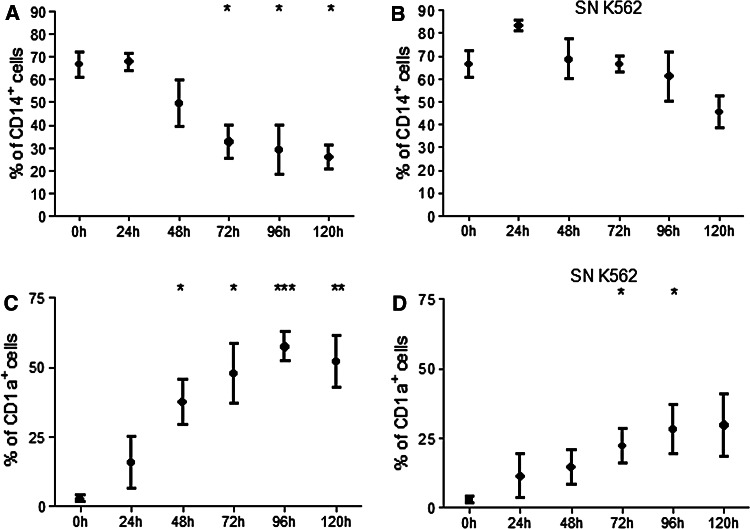
The next step was to study the kinetics of CD14 loss and CD1a appearance in the presence or absence of K562 supernatants. To answer this question, monocytes were stimulated with IL-4, GM-CSF and K562 supernatants (10% final volume) added at 0 h. The expression of CD14 and CD1a was evaluated thereafter at 24 h intervals.
During the first evaluation (0 h), monocytes were not stimulated. Therefore, CD14 levels observed at 0 h represent the expression found in monocytes immediately after adhesion (Fig. 3a). Subsequent evaluations represented the period under stimulation with IL-4, GM-CSF and K562 supernatants (Fig. 3).
Fig. 3.
CD14 and CD1a expressions by monocytes during differentiation in the presence or absence of K562 supernatants. Monocytes isolated from healthy volunteers were incubated with IL-4 and GM-CSF in the presence or absence of K562 supernatant (SN K562). CD14 and CD1a expressions were analyzed in 24 h intervals by flow cytometry. Data show the percentage of cells expressing CD14 and CD1a in a control differentiation (a, c) and in the presence of SN K562 (b, d). The result shows the mean ± SEM of at least three independent experiments. All comparisons were made with monocytes at 0 h. *Significantly different from the control (P < 0.05), **Significantly different from the control (P < 0.01), ***Significantly different from the control (P < 0.001)
At 0 h, almost 70% of monocytes expressed CD14 (Fig. 3a) and practically no cells expressed CD1a (Fig. 3c). After 24 h, monocytes cultured with IL-4 and GM-CSF remained expressing CD14 with almost no expression of CD1a. After 48 h, about 50% of cells expressed CD14 and, at the same time, about 35% of cells expressed CD1a. After 72 h, the percentage of cells expressing CD14 decreased to 30% and the percentage of cells expressing CD1a increased to almost 50%. This profile was not significantly affected in 96 h when compared with 72 h. Finally, at 120 h, about 25% of cells remained expressing CD14 and 60% expressing CD1a (Fig. 3a, c). The period between 24 and 72 h was the most important for the loss of CD14 and appearance of CD1a.
K562 supernatant affected CD14 and CD1a expressions already in the first 24 h of culture (Fig. 3b, d). In the presence of K562 supernatant, more cells remained expressing CD14 without CD1a expression at all times studied.
Tumor production of IL-1β, IL-6, IL-10 and TNF-α
The next step was to analyze leukemic cell products present in tumor supernatants. IL-1β, IL-6 and TNF-α are proinflammatory cytokines produced by some tumor cells and related with tumor development and progression. IL-10 is classically characterized as an immunosuppressive and anti-inflammatory cytokine [20]. In some instances, the expression of IL-10 by tumor cells is associated with tumor survival [31]. To clarify if leukemic cells produced some proinflammatory and anti-inflammatory cytokines, IL-1β, IL-6, IL-10 and TNF-α were quantified in 22 supernatants, including K562, HL-60 and DAUDI. The cytokines IL-1β, IL-10 and TNF-α were not found in any of the tumor supernatants tested. IL-6 was found in 5 out of 22 tumor supernatants. In these supernatants, the average concentration was 15.5 pg/mL (data not shown).
Monocyte production of TNF-α and IL-1β
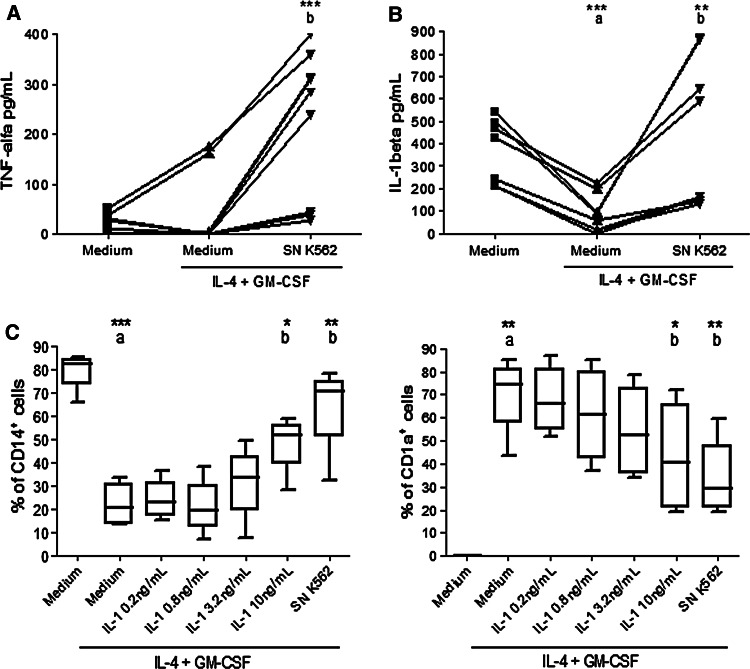
In spite of the paucity of some proinflammatory cytokines produced by leukemic cells, their products could stimulate the production of these cytokines by monocytes during differentiation. To investigate this hypothesis, monocyte supernatants were collected after 5 days in culture with or without IL-4 and GM-CSF and K562 supernatants to quantify TNF-α and IL-1β production.
According to Fig. 4a, monocytes without stimulation secreted approximately 30 pg/mL of TNF-α. When monocytes were cultured with IL-4 and GM-CSF, TNF-α production remained comparable to that of control monocytes. When cultured in the presence of K562 supernatant, monocytes produced about 200 pg/mL of TNF-α.
Fig. 4.
TNF-α and IL-1β production by monocytes in the presence of K562 supernatants and CD14 and CD1a expressions by monocytes in the presence of IL-1β. Monocytes isolated from healthy volunteers were incubated with IL-4 and GM-CSF in the presence of K562 supernatant (SN K562). After 5 days, the supernatant of monocytes culture was removed and TNF-α and IL-1β secretion quantified by ELISA assay. Data in a show TNF-α production. Data in b show IL-1β production. Monocytes were also incubated with IL-4, GM-CSF and different concentrations of IL-1β (0.2, 0.8, 3.2, 10 ng/mL). After 5 days, CD14 and CD1a were analyzed by flow cytometry. Data in c show the percentage of cells expressing CD14 (left) and CD1a (right). At least four independent experiments are represented. Each symbol per group represents one individual assessed under the different conditions (a, b). The median is represented by central horizontal lines (c). a Compared with monocytes without IL-4 and GM-CSF, b compared with monocytes cultured with IL-4 and GM-CSF. *Significantly different (P < 0.05), **Significantly different (P < 0.01), ***Significantly different (P < 0.001)
IL-1β is a characteristic cytokine produced by monocytes; therefore, quantification of IL-1β production after differentiation is another way to assess DC differentiation. As shown in Fig. 4b, monocytes in culture without stimulation secreted about 300 pg/mL of IL-1β. When stimulated with IL-4 and GM-CSF, monocytes produced half this amount compared with control monocytes. The presence of K562 supernatant increased IL-1β production by monocytes in culture. In this case, IL-1β production by monocytes in culture with IL-4, GM-CSF and K562 supernatants was equivalent to that observed in monocyte cultures not stimulated to differentiate into DC.
Effect of IL-1β on CD14 and CD1a expressions
Therefore, the next experiment was to analyze the effect of IL-1β addition on monocytes incubated with IL-4 and GM-CSF. Based on the IL-1β production observed, the lowest concentration of IL-1β used was 0.2 ng/mL and the highest was 10 ng/mL. As shown in Fig. 4c, the presence of IL-1β modulated the loss of CD14 and the appearance of CD1a. This effect was proportional to the concentration of IL-1β used. When monocytes induced to differentiate were cultured in the presence of 10 ng/mL of IL-1β, around 50% of cells expressed CD14 and CD1a. This result is significantly different from the one obtained when monocytes were cultured with IL-4 and GM-CSF only (Fig. 4c).
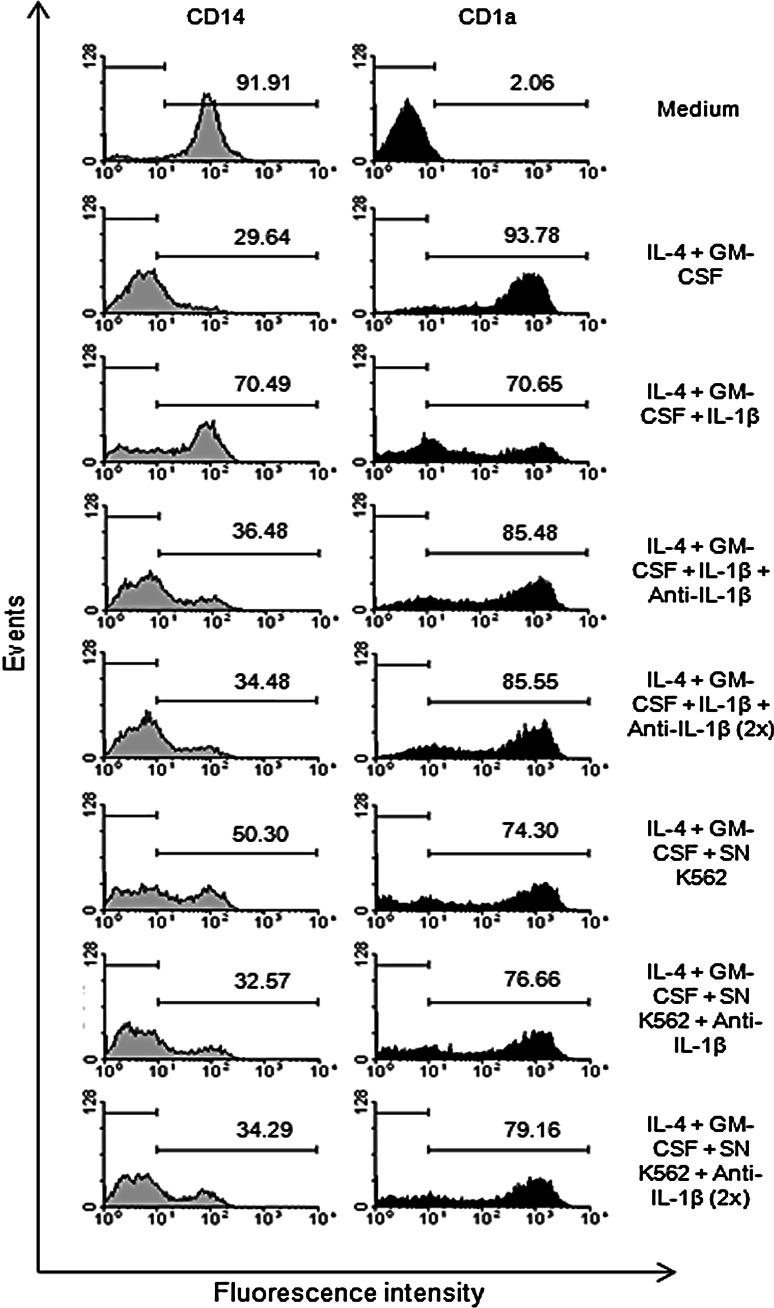
In order to confirm the effect of IL-1β on DC differentiation, a neutralizing antibody anti-IL-1β was used during the differentiation process. Monocytes were incubated with IL-4, GM-CSF, K562 supernatants and anti-IL-1β, which was added to monocyte cultures, once in the first, or twice in the first and in the third days. According to Fig. 5, the presence of anti-IL-1β reversed the effect observed when monocytes were stimulated to differentiate in the presence of exogenous IL-1β. The inhibition of the effect produced by IL-1β was proportional to the amount of anti-IL-1β used. When monocytes were induced to differentiate in the presence of K562 supernatants, the addition of anti-IL-1β partially reversed CD14 expression (Fig. 5). However, the addition of anti-IL-1β was not enough to modify the effect of K562 supernatants on the CD1a expression on monocytes (Fig. 5).
Fig. 5.
CD14 and CD1a expressions by monocytes after induction of differentiation in the presence of neutralizing anti-IL-1β. Monocytes isolated from healthy volunteers were incubated with IL-4 and GM-CSF in the presence of K562 supernatant (SN K562), exogenous IL-β (10 ng/mL) and neutralizing anti-IL-1β (3 μg/mL). The neutralizing antibody was added to the monocytes in the first day or in the first and in the third days (2×) of culture. After 5 days, CD14 and CD1a expressions were analyzed by flow cytometry. Representative histogram (from 5 independent experiments) shows the percentage of cells that express CD14 and CD1a molecules
According to the analysis of five independent experiments, we observed 24.38 and 36.67% reversal on CD14 expression when IL-1β and anti-IL-1β (once and twice, respectively) were present. In this case, 29.36 and 32.93% reversal were observed on CD1a expression. When K562 supernatant and anti-IL-1β (once and twice, respectively) were present, 12.55 and 20.92% reversal on CD14 expression were observed, while only 2.17 and 9.45% reversal on CD1a expression were obtained.
Discussion
In the present study, using a model of DC differentiation from monocytes stimulated by IL-4 and GM-CSF [15], we investigated parameters of differentiation that could be altered by the presence of leukemic cell products.
In an ideal condition, monocytes differentiating into DCs lose CD14 expression and start to express CD1a. However, in the present work, it was possible to demonstrate a significant alteration of this pattern, suggesting that leukemic cells secrete one or more products capable of affecting DC differentiation. Most DC differentiation measured by the dynamical changes on CD14 and CD1a expressions occurred between 24 and 72 h and these two events are apparently unrelated. After 24 h in culture with IL-4 and GM-CSF, small changes in CD14 and CD1a expressions were observed. However, in the same time interval, the presence of K562 supernatants modified the expression of these molecules. It seems that leukemic cell products act on monocytes preventing, at least in part, their differentiation into DCs. Furthermore, leukemic cell products could have a direct effect on monocytes as seen by induction of a small increase on CD14 expression by these cells.
The importance of DCs during immune responses against tumors was shown by Hillenbrand and collaborators [32] who correlated the presence of a consistent number of tumor infiltrating CD1a+ DCs with better prognosis. Furthermore, monocyte-derived CD1a+ DCs undergoing activation have been reported to induce apoptosis, as well as cell-cycle arrest, of breast cancer cells through the secretion of soluble factors [33]. In the present study, leukemic cell products inhibited the gain of CD1a molecules in monocytes-derived DC. If CD1a+ DCs are more effective in combating tumor cells, the inhibition of CD1a expression appears to be very important for tumor development.
The tumor microenvironment is rich in cytokines and factors that do not favor DC development and function, such as vascular endothelial growth (VEGF), IL-6, M-CSF, TGF-β, IL-10, COX-2, PGE2 and gangliosides [7–10, 17–19, 25, 34].
Differently from some solid tumors, leukemic cells did not produce IL-6 in large amounts. Moreover, IL-1β, IL-10 and TNF-α were not found in any of the supernatants tested, indicating that leukemic cells did not secrete these cytokines, at least in considerable amounts. Thus, this was not the mechanism used by leukemic cells to affect DC differentiation. On the other hand, leukemic cell products stimulated TNF-α and IL-1β production by monocytes induced to differentiate into DCs.
It has been demonstrated that TNF-α when incubated at the initial stage of monocyte differentiation prevents DC generation [24]. The concentration of TNF-α capable of modulating the generation of DCs was of the order of 10 ng/mL [24] higher than that induced by leukemic cell products in the present study.
Less is known regarding the effect of IL-1β during DC differentiation. Monocytes are capable of high IL-1β production and decrease this secretion when differentiate into DC. In the present study, when differentiation was stimulated in the presence of K562 supernatants, IL-1β levels remained comparable to those produced by monocytes. Moreover, IL-1β addition to monocyte cultures under differentiation partially prevented the loss of CD14 and the appearance of CD1a molecules. Thus, IL-1β affected DC differentiation, similar to leukemic cell products, suggesting this might be the underlying mechanism of the present study. However, the neutralization of IL-1β on such cultures was more effective in promoting the loss of CD14 than the appearance of CD1a, suggesting that IL-1β has an important role on CD14 expression.
It seems, therefore, that IL-1β is one of the factors induced by leukemic cells but not the only one. In this case, neutralizing just one factor might be insufficient to completely reverse the inhibitory effect of the microenvironment on DCs.
On the other hand, if the secreted form, IL-1β, stimulates and promotes tumor invasiveness and immunosuppression [21], this provides an advantage for tumor survival and progression and it must be one of the mechanisms for tumor escape.
Finally, the impairment of monocytes differentiation into DCs appears to be mediated by a number of tumor cell products, and in the present work, by soluble factors, mainly IL-1β, secreted by monocytes in response to leukemic cell products. The inhibition of DC development may be a way to ensure tumor cell survival, as the immune response becomes compromised. The development of therapies that aim to strengthen the immune system depends on DC function. Therefore, it is important to understand DC mechanisms of differentiation, maturation and regulation.
Acknowledgments
This work was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), INCT-CNPq/FAPERJ and D-MED. The authors thank Dr. Cerli Gatass and Dr. Julio Sharfstein for providing us with some reagents.
Footnotes
J. M. Motta and C. R. Nascimento contributed equally to this work.
References
- 1.Biemer JJ. Malignant lymphomas associated with immunodeficiency states. Ann Clin Lab Sci. 1990;20:175–191. [PubMed] [Google Scholar]
- 2.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 3.Shunyakov L, Ryan CK, Sahasrabudhe DM, Khorana AA. The influence of host response on colorectal cancer prognosis. Clin Colorectal Cancer. 2004;4:38–45. doi: 10.3816/CCC.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 4.Costello RT, Gastaut JA, Olive D. Mechanisms of tumor escape from immunologic response. Rev Med Interne. 1999;20:579–588. doi: 10.1016/S0248-8663(99)80107-6. [DOI] [PubMed] [Google Scholar]
- 5.Algarra I, García-Lora A, Cabrera T, Ruiz-Cabello F, Garrido F. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immunother. 2004;53:904–910. doi: 10.1007/s00262-004-0517-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shurin M, Gabrilovich D. Regulation of the dendritic cell system by tumor. Cancer Res Ther Control. 2001;11:65–78. [Google Scholar]
- 7.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5:263–274. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 8.Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol. 1998;64:275–290. doi: 10.1002/jlb.64.3.275. [DOI] [PubMed] [Google Scholar]
- 9.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1:510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 10.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, Mantovani A. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28:359–369. doi: 10.1002/(SICI)1521-4141(199801)28:01<359::AID-IMMU359>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Kalinski P, Schuitemaker JH, Hilkens CM, Kapsenberg ML. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol. 1998;161:2804–2809. [PubMed] [Google Scholar]
- 12.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 13.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JH, Gieseler R, Thiele B, Steinbach F. Dendritic cells: from ontogenetic orphans to myelomonocytic descendants. Immunol Today. 1996;17:273–278. doi: 10.1016/0167-5699(96)80544-5. [DOI] [PubMed] [Google Scholar]
- 15.Romani N, Reider D, Heuer M, Ebner S, Kämpgen E, Eibl B, Niederwieser D, Schuler G. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 16.Aalamian M, Tourkova IL, Chatta GS, Lilja H, Huland E, Huland H, Shurin GV, Shurin MR. Inhibition of dendropoiesis by tumor derived and purified prostate specific antigen. J Urol. 2003;170:2026–2030. doi: 10.1097/01.ju.0000091264.46134.b7. [DOI] [PubMed] [Google Scholar]
- 17.Shurin GV, Shurin MR, Bykovskaia S, Shogan J, Lotze MT, Barksdale EM., Jr Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 18.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]
- 19.Bharadwaj U, Li M, Zhang R, Chen C, Yao Q. Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res. 2007;67:5479–5488. doi: 10.1158/0008-5472.CAN-06-3963. [DOI] [PubMed] [Google Scholar]
- 20.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apte RN, Dotan S, Elkabets M, White MR, Reich E, Carmi Y, Song X, Dvozkin T, Krelin Y, Voronov E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis and tumor-host interactions. Cancer Metastasis Rev. 2006;25:387–408. doi: 10.1007/s10555-006-9004-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco P, Palucka AK, Pascual V, Banchereau J. Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine Growth Factor Rev. 2008;19:41–52. doi: 10.1016/j.cytogfr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD. Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol. 2002;168:4333–4343. doi: 10.4049/jimmunol.168.9.4333. [DOI] [PubMed] [Google Scholar]
- 26.Kiertscher SM, Luo J, Dubinett SM, Roth MD. Tumors promote altered maturation and early apoptosis of monocyte-derived dendritic cells. J Immunol. 2000;164:1269–1276. doi: 10.4049/jimmunol.164.3.1269. [DOI] [PubMed] [Google Scholar]
- 27.Shurin MR, Yurkovetsky ZR, Tourkova IL, Balkir L, Shurin GV. Inhibition of CD40 expression and CD40-mediated dendritic cell function by tumor-derived IL-10. Int J Cancer. 2002;101:61–68. doi: 10.1002/ijc.10576. [DOI] [PubMed] [Google Scholar]
- 28.Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45:321–334. [PubMed] [Google Scholar]
- 29.Gallagher R, Collins S, Trujillo J, Mccredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 30.Adams A, Strander H, Cantell K. Sensitivity of the Epstein–Barr virus transformed human lymphoid cell lines to interferon. J Gen Virol. 1975;28:207–217. doi: 10.1099/0022-1317-28-2-207. [DOI] [PubMed] [Google Scholar]
- 31.Ogden CA, Pound JD, Batth BK, Owens S, Johannessen I, Wood K, Gregory CD. Enhanced apoptotic cell clearance capacity and B cell survival factor production by IL-10-activated macrophages: implications for Burkitt’s lymphoma. J Immunol. 2005;174:3015–3023. doi: 10.4049/jimmunol.174.5.3015. [DOI] [PubMed] [Google Scholar]
- 32.Hillenbrand EE, Neville AM, Coventry BJ. Immunohistochemical localization of CD1a-positive putative dendritic cells in human breast tumours. Br J Cancer. 1999;79:940–944. doi: 10.1038/sj.bjc.6690150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joo HG, Fleming TP, Tanaka Y, Dunn TJ, Linehan DC, Goedegebuure PS, Eberlein TJ. Human dendritic cells induce tumor-specific apoptosis by soluble factors. Int J Cancer. 2002;102:20–28. doi: 10.1002/ijc.10656. [DOI] [PubMed] [Google Scholar]
- 34.Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, Kavanaugh D, Carbone DP. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2:1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]