Abstract
Dipeptidyl peptidase IV (DPP-IV), assigned to the CD26 cluster, is expressed on epithelial cells and lymphocytes and is a multifunctional or pleiotropic protein. Its peptidase activity causes degradation of many biologically active peptides, e.g. some incretins secreted by the enteroendocrine system. DPP-IV has, therefore, become a novel therapeutic target for inhibitors that extend endogenously produced insulin half-life in diabetics, and several reviews have appeared in recent months concerning the clinical significance of CD26/DPP-IV. Biological fluids contain relatively high levels of soluble CD26 (sCD26). The physiological role of sCD26 and its relation, if any, to CD26 functions, remain poorly understood because whether the process for CD26 secretion and/or shedding from cell membranes is regulated or not is not known. Liver epithelium and lymphocytes are often cited as the most likely source of sCD26. It is important to establish which tissue or organ is the protein source as well as the circumstances that can provoke an abnormal presence/absence or altered levels in many diseases including cancer, so that sCD26 can be validated as a clinical marker or a therapeutic target. For example, we have previously reported low levels of sCD26 in the blood of colorectal cancer patients, which indicated the potential usefulness of the protein as a biomarker for this cancer in early diagnosis, monitoring and prognosis. Through this review, we envisage a role for sCD26 and the alteration of normal peptidase capacity (in clipping enteroendocrine or other peptides) in the complex crosstalk between the lymphoid lineage and, at least, some malignant tumours.
Keywords: sCD26, Dipeptidyl peptidase IV, Cancer, T cells, Chemokines, Incretins
Introduction
The CD26 protein
The exoprotease dipeptidyl peptidase IV (DPP-IV, EC 3.4.14.5), also known as CD26, is a transmembrane glycoprotein of 110 kDa MW expressed constitutively in a dimeric form (220 kDa) on a variety of cell types, particularly prostate, kidney, liver and epithelial cells, predominantly in exocrine glands and absorptive epithelia [1–5], as well as on some endothelial cells of (rat) blood vessels and capillaries [3] and also on lymphocytes [4]. As a special exception, CD26 expression is low in the resting state T and NK cells but it is rapidly up-regulated upon activation of these cells [3–6].
CD26 was originally described in 1966, by Hopsu-Havu and Glenner [7], by its DPP-IV activity in human liver. In 1977, Schrader and Stacy [8] discovered the adenosine deaminase (ADA) binding or complexing protein (ADAbp, ADCP) function. In 1984, Fox et al. [4] described the protein as a leucocyte antigen because of binding of the Ta1 monoclonal antibody. In 1993, the protein was identified as CD26 independently by the groups led by Houghton and by Schlossman [9, 10]. It has also been shown to be a functional receptor for collagen and fibronectin, and to interact with the transmembrane tyrosine phosphatase CD45 (in leucocytes), with glypican-3, and with the chemokine receptor CXCR4, as reviewed in [2, 11–14]. A new model for CD26 costimulatory function [13] suggests that, at least in activated memory T cells, CD26 enhances antigen-specific T cell proliferation by engaging signalling pathways in the APCs, in particular the up-regulation of CD86, through the CD26’s caveolin-binding domain interaction with caveolin-1, transported to the APC membrane along with the peptide–MHC complex. This interaction also presumably leads to downstream signal transduction in T cell costimulation, in which the cytoplasmic tail of dimeric CD26 may bind to caspase recruitment domain-containing membrane-associated guanylate kinase protein-1 (CARMA).
Therefore, DPP-IV can act in an enzymatic activity-dependent and -independent fashion.
The DASH family
The many proteins, apart from CD26, that exhibit similar DPP-IV activity and/or varying degrees of structural homology are members of the SC clan (representing enzymes with an α/β hydrolase fold) and are known as “DPP-IV activity and/or structure homologues” [2, 15, 16]. These comprise the S9B family, i.e. proteins with DPP-IV activity, from plasmatic membrane (CD26/DPP-IV and FAP-α/seprase) or cytoplasm (DPP8 and DPP9); the S9 family, i.e. DPP6 and DPP10 plasma membrane proteins homologous to CD26 with no peptidase activity (they are involved in neuronal membrane complexes); and the S28 family, a CD26 sequence divergent protein known as DPP7/QPP (quiescent cell proline dipeptidase) or DPP-II, with DPP-IV activity in the lysosomal fraction. There are some excellent reviews on the DASH proteins [15, 16]. Some important facts to consider are (1) there is substantial overlap of substrate specificity and catalytic properties, which indicates the importance of this enzymatic activity, as well as the critical regulation of DASH expression and tissue specificity. (2) The domains unrelated to the catalytic activity are also highly conserved. Together with the fact that the plasma membrane proteins have very short cytoplasmic domains and that they are present in supramacromolecular complexes such as neuronal and lymphocyte synapses or the invadopodia of metastatic cells [17–22], these properties imply evolution of those domains to interact with other functional molecules.
In the context of this review, it is also remarkable that FAP-α, which shares a sequence identity of 50% (and many other similarities such as the size or chromosomal localization) and which may form heterodimers with DPP-IV, has very restricted expression in normal human cells (embryonic and wound healing tissues and pancreatic islet cells), but is selectively expressed on tumour stromal fibroblasts in more than 90% of human epithelial carcinomas such as pancreas, breast, lung and colorectal carcinomas. Some carcinoma cells in melanomas and sarcomas are also positive for FAP-α [21, 23, 24]. In addition to its DPP-IV-like activity, FAP-α has the endopeptidase ability to cleave denatured or unwound type I and III collagens, and therefore may be involved in regulating the extracellular matrix (ECM) of the tumour microenvironment [20–24] (Fig. 1).
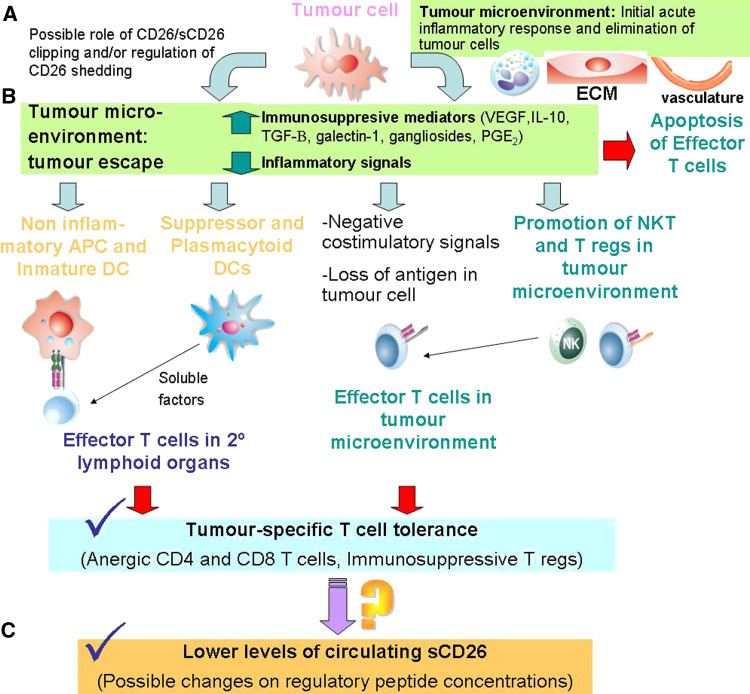
Fig. 1.
Scheme of the cancer immunoediting model in which a hypothesis explaining the lower levels of sCD26 found in serum cancer patients is included. a The tumour microenvironment (or stroma, composed of epithelial and inflammatory cells, activated fibroblasts, ECM and blood vessels) plays a critical role in tumourigenesis. DPP-IV/CD26 and other DASH proteins play a not wholly known role in the process of tumour progression to malignancy in enzyme activity-dependent and -independent fashions, as reviewed in [2, 11, 14]. b According to the cancer immunoediting hypothesis, the escape of tumour variants, which will grow into clinical apparent tumours, develops from cellular and molecular mechanisms leading to immune tolerance. Tumour cells employ a plethora of mechanisms that may act in concert to directly evade effector T cells responses, such as negative costimulatory signals, apoptosis and impairment of the antigen presentation machinery. However, the dominant mechanism which renders T cells tolerant during tumour growth is to change the tumour microenvironment through secretion of many immunosuppressive mediators and absence of some inflammatory mediators. This network leads to: problems in tumour antigen processing by APCs, particularly DCs, and later presentation to T cells in secondary lymphoid organs; the inhibition of DC maturation and differentiation; and the recruitment of different regulatory populations (NKT cells, Tregs, subsets of myeloid and plasmacytoid DCs, and others) [174, 338]. c As consequence, effector T cells become anergic after tumour-specific Ag presentation. A huge amount of information supports T cells as a major source of circulating sCD26. Serum concentration of sCD26 is significantly lower in patients of some cancers (see text). Taking into account these facts, we propose that tumour-specific tolerant cells or a subset of regulatory cells may be responsible of sCD26 lower concentrations in cancer patients. This drop in sCD26 may have further consequences
CD26 enzymatic activity
DPP-IV activity cleaves two N-terminal amino acids from peptides and small polypeptides with, usually (but not only), proline or alanine in the second position of polypeptidic chains, which are otherwise resistant to most proteases [10–14]. Its first studied role was in the process of dietary protein assimilation on the surface of enterocytes, since DPP-IV enzymatic activity is present in the gastrointestinal tract as a brush border enzyme, and CD26 was used to study the sorting of proteins to the brush border apical membranes, which in this case involved protein glycosylation and lipid microdomains [25–28].
However, many regulatory peptides also contain these sequences [29], and several chemokines, integrins and neuropeptides have been already demonstrated to be cleaved or clipped by this enzyme (clipping has been defined as the proteolytic activation or deactivation of chemokines and other chemoattractants by the removal of short N- or C-terminal peptides; see review [30]) (see Table 1 for known biologically active substrates). Clipping of chemokines by CD26 (some become inactivated and others activated) generally favours the preferential attraction of Th1 cells, and consequently, the recruitment of neutrophils and macrophages (reviewed by Boonacker and Van Noorden [11]).
Table 1.
Peptides with known physiological activity that are cleaved by dipeptidyl peptidase IV enzymatic activity
| Substrate families | Peptides | Biological effect | Species (in vitro/in vivo) | References |
|---|---|---|---|---|
| Incretins and gastrointestinal hormones | GLP-1 |
Inactivation Possible cardiovascular role of the product |
Human (in vivo) Dog (in vivo) |
[35] |
| GLP-2 | Inactivation | Human (in vivo) | [36] | |
| GIP | Inactivation | Human (in vivo) | [37, 38] | |
| Glucagon | Inactivation | Human (in vivo) | [39, 40] | |
| PACAPa | Inactivation | Human (in vitro) | [41] | |
| GRP | Not known |
Human (in vitro) Product in dogs |
[41, 42] | |
| Peptide YYa | Change in receptor preference |
Human (in vivo) Rat (in vivo, vasoactive action) |
[43–47] | |
| Vasoactive peptides | Bradykinin | Change in receptor preference or inactivation (in conjunction with APN) |
Human (in vivo, indirectly) Rat (in vivo) |
[48–51] |
| VIP | Inactivation | Human (in vitro) | [41] | |
| BNPa | Change in receptor preference or Inactivation |
Human (in vitro) Product in dogs |
[52, 53] | |
| Neuropeptides | NPYb | Change in receptor preference |
Human (in vivo, indirectly) Rat (in vivo) |
|
| Beta-casomorphins | Inactivation |
Human (in vivo, indirectly) Rat (in vivo) |
[57, 58] | |
| Endomorphins | Change in receptor preference |
Rat (in vivo) Mouse (in vivo) |
[51] |
|
| Substance P | Inactivation |
Rat (in vivo) Pig (in vivo) Human (in vitro) |
[51, 59–63] | |
| Chemokines | CCL3 (MIP-1α, LD78β) |
Enhanced activity Change in receptor preference |
Human (in vivo, indirectly) | [64, 65] |
| CCL4 (MIP-1β) | Change in receptor preference | Human (in vivo, indirectly) | [66, 67] | |
| CCL5 (RANTES) | Change in receptor preference | Human (in vitro, indirectly) | [68–70] | |
| CCL11 (Eotaxin) | Inactivation |
Human (in vitro) Rat (in vivo) |
[71, 72] | |
| CCL22 (MDC) | Change in receptor preference | Human (in vitro) | [73] | |
| CXCL6 (GCP-2) | No changes | Human (in vitro) | [68] | |
| CXCL9 (MIG) | Inactivation | Human (in vitro) | [74, 75] | |
| CXCL10 (IP-10) | Inactivation, CXCR3 antagonist | Human (in vivo, indirectly) | [74, 76] | |
| CXCL11 (I-TAC) | Inactivation, CXCR3 antagonist | Human (in vivo, indirectly) | [74, 77, 78] | |
| CXCL12 (SDF-1alpha) | Inactivation, CXCR4 antagonist | Human (in vivo) | [79–83] |
Biological effect in italics refers to hypothesis. Species (in vivo/in vitro) refers to: In vivo, the existence of studies in humans or other species with inhibitors as well as the existence of the product and well-known biological effects of peptides; indirectly refers to the lack of in vivo studies with inhibitors. In vitro, studies not fulfilling these parameters
aPeptides belong also to the neuropeptide group
bPeptides belong also to the vasoactive group
Although a peptide becomes less susceptible to cleavage by DPP-IV with increasing length (see the excellent review of Lambeir et al. [2]), the fact that several synthetic oligopeptides with sequences analogous to the amino-terminal sequence of several releasing human hormones and cytokines (e.g. chorionic gonadotropin, prolactin, aprotinin, corticotropin-like intermediate lobe peptide and (Tyr-)melanostatin [84], G-CSF, GM-CSF, TNF-β, IL-1β, IL-2, IL-3, IL-5, IL-8, IL-10, IL-11, IL-13, IL-2, thrombopoietin [85], fibrin inhibitory peptide [13, 86]) are hydrolyzed by this protein, also suggests that DPP-IV may participate physiologically not only by itself but also in orchestrated mechanisms with other proteases, particularly aminopeptidase N/CD13 (APN) [87, 88], and matrix metalloproteinases [30, 89].
Most data indicate that glycosylation of CD26 is not a prerequisite for DPP-IV activity, dimerization or ADA binding [90, 91] but that certain specific glycosylations can profoundly affect the enzyme activity [92].
In conclusion, the enzyme is currently viewed as having a dual function, depending on the tissue: in changing the functional activity of its substrates as well as a checkpoint to general proteolytic degradation [2, 11, 16].
CD26 as a therapeutic target
Many inhibitors of DPP-IV are currently under investigation (some of them reviewed in [2]). Haematopoietic stem cell transplantation [68, 83, 94] and some T cell-dependent inflammatory diseases [87, 88, 94] will soon start clinical trials. In clinical Phase II and III trials, Val-boroPro (Talabostat) was originally intended to inhibit FAP, but is a non-selective dipeptidyl peptidase inhibitor that has been tested in patients with lung, pancreas and colon cancer, and with melanoma and chronic lymphocytic leukaemia [21, 23, 24, 95]. Two inhibitors have already been approved for use in the European Union (sitagliptin, MSD and vildagliptin, Novartis), and the first also in the US, for the treatment of type 2 diabetes. This new class of drugs for the treatment of diabetes acts by enhancing the half-lives of the incretins (GIP and GLP-1), which induce insulin secretion, leading to a sustained reduction in blood glucose levels [96, 97]. The large insulin response to ingestion of a meal is mainly due to the effects of these hormones released into the circulation by K and L cells located in the gastrointestinal tract [96–98]. Both have short half-lives (5 min; active peptides, 2 min) due in part to rapid inactivation by DPP-IV, which limits the effects of GLP-1 and GIP on glucose homeostasis [96]. The inactivation may occur partially on the endothelial cells of the capillary vessels that drain the intestinal mucosa, as has been demonstrated in pigs [33], although only a tenuous DPP-IV activity and immunostaining have been described directly in human, mouse and rat intestine endothelia [2, 3, 99]; and partially by soluble protein DPP-IV existing in capillary blood [32, 99, 100], before GLP-1 enters the portal circulation [96].
No immediate adverse or secondary effects resulting from the therapeutic alteration of DPP-IV activity in controlled clinical studies have been reported by the manufacturers [except a small increase in nasopharyngitis with sitagliptin, but not with vildagliptin (product monographs for Januvia, MSD and for Galvus, Novartis)], although obviously no long-term studies have been carried out. As preclinical animal studies identified several problems, possibly due to cross-enzymatic inhibition (at least DPP8 and DPP9) [101, 102], and the enormous number of substrates (Table 1), see [48], vigilance over long-term use of inhibitors is mandatory [103, 104].
CD26 in cancer
CD26/DPP-IV has been consistently associated with cancer since it was known as ADCP, or the 2 or Large ADA isoform [105, 106]. It is important to point out that these studies with ADCP involved immunological techniques and are therefore more specific than earlier studies that measured the DPP-IV enzymatic activity in tumour tissues. The group led by Bosman found that ADCP staining was decreased in about one-third of colorectal, prostatic and renal tumours, but unaltered or even increased in the other two-thirds; significant intratumour and intertumour heterogeneity as well as differences in the cellular staining pattern with respect to the normal tissue were also reported [107–110]. It should be also pointed out that the non-enzymatic role of CD26 as an extracellular anchorage for ADA may be important in tumourigenesis. Although the presence of extracellular ADA is independent of PM CD26 expression [111–114], the ADA–CD26 complexes may participate in cell-to-cell contacts [18, 113, 115] or, more probably in this context, through the catalysis of adenosine to inosine [112–114]. Proliferating cells accumulate high extracellular concentrations of adenosine, a purine nucleoside found within the interstitial fluid of solid tumours, which may be toxic or influence the proliferative potential of a cell, depending on the relative expression, and type, of adenosine receptor (AR). Therefore, the different levels of cell surface CD26–ADA complex and relative expression of ARs on a tumour cell may lead to generation of tumour subclones as well as to participation in the well-known adenosine inhibition of cell-mediated immune responses to tumour cells [16, 114, 116–120].
Pro-oncogenic activities
In addition, it has recently been reported that CD26–ADA may form a ternary complex with plasminogen. Binding of plasminogen to cell surface receptors promotes its conversion to plasmin, which is required for proteolysis of the ECM in several physiological and pathological processes, including cell migration, tumour cell invasion and metastasis [120].
Many reviews have discussed the role of CD26/DPP-IV activity in cancer and the potential usefulness of this protein in therapeutics and diagnostics [12, 16, 116, 121]. It is important to note that early studies measured the DPP-IV enzymatic activity in tumour tissues and body fluids, as already mentioned, but some DASH proteins also use strikingly similar substrates. For example, the enhanced DPP-IV and DPP-II activity observed in lung squamous cell carcinoma as compared with that in normal lung tissue [122–124] may be related to the well-established FAP expression in that tumour [125]. In addition, glypican-3 has been recently reported as the first natural inhibitor of CD26/DPP-IV enzymatic activity, in in vitro experiments [126]. Glypican-3 is one of the six mammalian glypicans (heparan sulphate proteoglycans GPI-linked to the cell membrane) that are usually expressed during development and are basically absent from adult tissues but up-regulated in many tumour tissues [127]. Therefore, a possible glypican-3-dependent DPP-IV inhibition may also lead to levels of enzymatic activity in the tumour being independent of the actual amount of CD26. It appears that glypican-3 can modulate the activity of many growth-signalling peptides, such as insulin-like growth factor 2, and that it is related to apoptosis and confers oncogenicity to the cell [16, 128, 129].
FAP-α also appears to be pro-oncogenic and its collagenolytic or gelatinase activity at the invadopodia supramolecular complex, implicated in ECM remodelling during tumour invasion, metastasis or angiogenesis, is well established [15, 16, 20–24, 130]. Therapies targeting FAP inhibit tumour growth [95, 131], and FAP is selectively expressed on stromal fibroblasts of various epithelial carcinomas, such as pancreas, breast, lung, esophagic, gastric and colon cancers, as well as in melanoma, cervical and brain tumours, as commented [15, 16, 20–24, 130, 132, 133]. The possible usefulness of FAP as a marker of (poor) patient prognosis in cervical, colon and ovarian cancer has recently been well reviewed by Sedo et al. [16].
CD26, also present at the invadopodia, together with other ectoproteases and metalloproteases [21, 130, 134], can participate in malignant transformation and cancer progression through its ability to bind collagen and fibronectin [20–22, 116, 121, 130, 135]. MMPs, including MMP-2 and MMP-9, and FAP digestion of ECM components will permit passage of the malignant cells through basement membranes and stromal barriers. This pro-oncogenic behaviour is thus consistent with the non-enzymatic interactions with cell surface ADA–plasminogen and glypican-3 mentioned above, and the formation of FAP–CD26 heterodimers, although it is not known whether these heterodimers have a different function from homodimers (in fact they are not co-expressed in tumour stromal fibroblasts or sarcomas) [130, 136]. A clue to later discussion is that glypican-3 may inhibit the enzymatic activity of CD26 in this context, i.e. in a particular tumourigenic niche [137].
The up-regulated CD26 expression associated with the aggressiveness of T and B lymphomas and leukaemias [138–141], thyroid follicular, papillary carcinomas, astrocytic tumours and gastrointestinal stromal tumour [142–145] are consistent with these findings. Very recently, CD26 expression was evaluated in various peripheral B-cell lymphoid tumours: CD26 expression was absent or barely detectable in follicular and mantle cell lymphomas, high in multiple myelomas and hairy cell leukaemias, and variable in chronic lymphocytic leukaemias, in CD5(neg) B-cell chronic lymphoproliferative diseases and in diffuse large cell lymphomas. In B-CLL, CD26 expression on cell surface (analysed by flow cytometry [146]) and CD26 gene overexpression (analysed by microarray technology [147]) may identify subsets of patients with an unfavourable clinical outcome, thus suggesting its potential role as a prognostic marker of progressive disease. The enhanced DPP-IV activity (not necessarily CD26 specific) found in early lung squamous cell and skin basal cell (and precancerous dermatosis) carcinomas, prostatic tumours and hepatocellular carcinomas [148–150], which is then lost in later phases (probably due to a transition from early invasive stages), as well as the higher CD26 expression in benign melanoma (compared with a decrease in malignant melanomas) [151], and the demonstrated use of mAb anti-CD26 as treatment for mesothelial and renal cancers [152, 153] may also support these findings.
Anti-oncogenic activities
There is a fundamental difference between CD26 and the other proteases involved in cancer development and progression as executors of ECM degradation: CD26 is constitutively expressed in the tissues mentioned at the beginning of this article, and its enzymatic activity regulates the biological activity of regulatory peptides, growth factors and chemokines. If glypican-3-dependent local DPP-IV inhibition can be confirmed in a physiological context, this indicates a natural protective role for the enzyme that should be blocked in the tumourigenic process.
This idea was proposed early on following the immunohistological studies cited above [107–110], which reported a loss of CD26 expression in some tumour tissues, and from other studies that found significant decreases in DPP-IV activity [16, 117], not only in the tumour microenvironment but also in the systemic circulation (see further below). In addition to some prostatic, colorectal, haematological and renal tumours [107–110, 154–157], a decrease in CD26 was found to accompany the progression of melanoma lesions, and CD26 was absent from metastatic tumours [151, 158]. An inverse correlation between CD26 expression and the grade of tumour was also observed in endometrial cancers [159, 160]. Interestingly, in advanced stages from prostate and lung and skin squamous cell carcinomas [161, 162], CD26 expression is lower than in early stages, or may even be absent.
The protective role was first contrasted in 1999 by the Houghton’s group [163–166] when they reexpressed wild-type or mutant CD26 transfected in human melanoma cells at levels comparable to those found in normal melanocytes and observed reversions of the malignant phenotype: an enzymatic activity-dependent loss of tumourigenicity and abrogation of a block in differentiation, as well as enzymatic activity-independent re-emergence of dependence on exogenous growth factors for cell survival. They found similar or additional tumour suppressor functions for CD26 reexpressed in vitro in cell lines from non-small cell lung and prostatic carcinomas [167, 168], and concluded that DPP-IV regulates the activities of (unidentified) locally produced mitogenic peptides involved in cancer development. Similar changes in morphology, as well as decreased growth, migration and adhesion, and changes in E-cadherin and MMPs and TIMPs cell surface expressions were recently described for ovarian carcinoma and glioma cells [169–171].
Together these data—differences in the cellular staining pattern with respect to the normal tissue, significant intratumour heterogeneity and changes of CD26 expression linked to the transition of tumour stages—indicate a quite complex situation in the physiological microenvironment of cancer niches. The possibility that the tumourigenic process may manipulate the functions of CD26/DPP-IV, e.g. evading the immune system by modifying local chemokine gradients (and therefore, the immune cell homing), and by modulating cytokines and angiogenic or immunosuppressive factors (Table 1) [89, 172–176] deserves to be studied in more detail. Nevertheless, some diagnostic and prognostic uses for tumour CD26 expression have been proposed (and reviewed in [2, 16, 119]).
Serum CD26
Significant levels of DPP-IV activity have been shown to occur in body fluids such as plasma, serum, cerebrospinal and synovial fluids, semen and urine (see reviews [2, 11–13, 16]). Serum DPP-IV activity was discovered in 1968 by Nagatsu’s group in Japan [177]. It is very important to note that there is no direct correlation between serum CD26 (or soluble, in contrast to transmembrane) (sCD26) protein concentrations and serum enzymatic activity assays, for three reasons: (1) There are some circulating proteins other than CD26 with DPP-IV activity, as will be discussed below. (2) Sialylation (a type of glycosylation) of sCD26 [2, 11–14, 92] is strongly enhanced in elderly individuals [178], and the recent finding that a certain type of hypersialylation can inhibit DPP-IV activity [93] is consistent with the fact that serum/plasma DPP-IV enzymatic activity tends to decrease with age [2]. The slight but significant decrease of the serum sCD26 protein levels we have observed in a large cohort (data submitted) is not enough to explain the decrease in activity. (3) It has recently been suggested that the serum protein attractin, which has a CUB domain (a motif of around 110 amino acids residues present in multiple plasma membrane-associated proteins) that enhances the enzymatic activity of tolloid proteases [179, 180], may regulate the DPP-IV activity of CD26/sCD26 in the same way [16]. Attractin is the product of the mahogany (human ATRN) gene involved in control of pigmentation, energy metabolism, immune status and neurodegeneration [181]. Once thought to have DPP-IV activity itself [182–184], serum attractin has both secreted (with isoforms) and membrane forms that result from an alternative splicing that it is differentially regulated at least in lymphoid tissues [185], and is actually frequently co-purified with sCD26 [16, 182–184].
The protein
Within normal plasma/serum, some 90–95% of DPP-IV activity has been associated with a relatively high concentration of sCD26 in human serum (~600 μg L−1) (Table 2) [2, 16, 186–188]. Since sCD26 is heavily glycosylated, its molecular weight is similar to that of transmembrane CD26 [186, 188] although it lacks transmembrane and cytoplasmic domains (the sequence starting at the 39th position) [187]. Iwaki-Egawa et al. [187, 189, 190] suggested that sCD26 must be shed from any plasma membrane on CD26 expressing cells that are in contact with blood, by proteolytic cleavage, which is analogous to the findings of some differently processed derivatives of other PM proteases such as APN (lacking 59 or 68 amino acid residues) in normal and maternal or pregnant serum. Other natural or recombinant sCD26 proteins are similar, but not equal, to the naturally occurring soluble form (reviewed by Gorrell et al. [14]).
Table 2.
Studies showing physiological and pathophysiological levels of sCD26 concentration measured by ELISA in human serum (sometimes plasma)
| Disease | Concentration ± SD (μg L−1)a | Cohort (n) | References |
|---|---|---|---|
| Healthy | 591 ± 179 | 38 |
Bender MedSystems Chemicon/Millipore insert |
| Healthy | 415 ± 96 | 36 | R&D Systems insert |
| Healthy/ | 560 ± 126 | 52 | [241] (Bender MedSystems) |
| Colorectal cancer/ | 262 ± 138 | 110 | |
| Gastric cancer | 585 ± 148 | 9 | |
| Healthy/ | 557 ± 181 | 2,673 | [243] (Bender MedSystems) |
| Colorectal cancer | 312.9 ± 102.4 | 12 | |
| Healthy/ | 2,270 ± 770 | 45 | [234] (rabbit Ab) |
| Oral cancer (SCC) | 1,500 ± 350 | 25 | |
| Healthy/ | 590 ± 81 | 11 | [246] (Bender MedSystems) |
| Rheumatoid arthritis | 505 ± 142 | 13 inactive RA | |
| 403 ± 97 | 16 active RA | ||
| Healthy/ | 113 | – | [247] (Chemicon/Millipore) |
| Rheumatoid arthritis | 100 | 22 MTX-nonresp | |
| 95/73 | 8/4 MTX-resp | ||
| Healthy/ | 1,030 (median) | 25 | [92, 248] (monoclonal Ab BA5) |
| Rheumatoid arthritis/ | 850 (median) | 25 | |
| Lupus erythematosus/ | 420 (median) | 10 | |
| Sjögren syndrome/ | 450 (median) | 10 | |
| Myocardial infarction | 1,900 (median) | 10 | |
| Healthy/ | – | [7] (Bender MedSystems) | |
| Osteoarthritis | ~600 (from figure) | 26 | |
| Rheumatoid arthritis | ~450 (from figure) | 41 | |
| Healthy/ | 15,600 ± 2,400 | 54 | [249] (monoclonal Ab 1F7 and 5F8) |
| Systemic lupus erythematosus | 7,900 ± 2,400 | 12 active | |
| 11,300 ± 3,300 | 41 inactive | ||
| Healthy/ | 182 ± 29 | 26 | [250] (Bender MedSystems) |
| Scleroderma | 160 ± 53 | 30 limited sclerosis | |
| 126 ± 40 | 26 diffuse sclerosis | ||
| Healthy/ | 398 ± 100 | 35 | [251] (Bender MedSystems) |
| Allergic asthmatics | 526 ± 120 | 51 | |
| Healthy/ | 540 | 12 | [252] (Bender MedSystems) |
| Atopic dermatitis | 710 | 88 (do not change with exacerbation nor therapy) | |
| Healthy/ | 411 | 15 | [253] (Bender MedSystems) |
| ANCA-associated vasculitides | 258 | 15 WG (G) active | |
| 295 | 15 WG (G) recession | ||
| 316 | 6 WG (localized) | ||
| 188 | 16 CSS active | ||
| 257 | 17 CSS recession | ||
| 283 | 7 MPA active | ||
| 228 | 14 MPA recession | ||
| Healthy/ | 14.9 ± 3.1 mg L−1 | 79 | [254] (monoclonal Ab 1F7) |
| HIV-1 | 15.6 ± 7 mg L−1 | 90 | |
| Healthy/ | 200.6 ± 60.3 | 20 | [255] (Bender MedSystems) |
| HCV (chronic) | 140.4 ± 63.9 | 33 | |
| 115.9 ± 32.9 | 33 | ||
| Healthy/ | ~140 (from figure) | 10 | [256] (Chemicon/Millipore) |
| HCV | ~185 (from figure) | 19 | |
| Healthy/ | 630 | 27 (mean 6 years old) | [257] (Bender MedSystems) |
| Visceral leishmaniasis | 890 active | 33 (mean 3.6 years old) | |
| 995 asymptomatic | 15 (mean 6 years old) | ||
| Healthy/ | 627 | 24 (mean 17 years old) | [258] (Bender MedSystems) |
| Cutaneous leishmaniasis | 693 acute | 41 (mean 20 years old) | |
| 1,003 non-healing | 22 (mean 14 years old) |
Data with “(from figure)” mean that numbers were calculated from article’s figures as they were not cited in the text. In the References column, the manufacturer of commercial ELISAs or non-commercial antibodies used for the concentration measurements are cited in order to comparison
WG Wegener’s granulomatosis, G generalized, CSS Churg-Strauss syndrome, MPA microscopic polyangiitis, PBC primary biliary cirrhosis, SCC squamous cell carcinoma, RA rheumatoid arthritis, MTX methotrexate, HAV, HBV, HCV hepatitis virus, EBV Epstein-Barr virus, ANCA antineutrophil cytoplasmic antibodies
aExcept where indicated
A diverse range of PM proteins of Type 1 or Type II topology that also occur as a circulating, soluble form are derived from the membrane by a group of enzymes referred to collectively as ‘secretases’ or ‘sheddases’ [191]. The facts that only one CD26 mRNA form is usually reported [146, 192, 193], and that it is transported from its site of synthesis in the rough endoplasmic reticulum to the microvillar membrane of enterocytes and some cell lines in a membrane-bound state [11, 14, 27, 28, 194], also suggest that it is not secreted. It is important to point out that the shedding of most integral membrane proteins is regulated, often by a PKC-dependent mechanism [30, 195, 196].
However, CD26 has been found to be soluble in the lumen of secretory granules. In endocrine pancreatic A cells, accompanying glucagon, both undergo exocytosis to the interstitial space where sCD26 may act on secretory products of neighbouring islet cells [197, 198]. In this case, another possible mechanism for the release of CD26 from the membrane is autolysis of the protein (as observed in vitro) [199] by the acidic pH conditions found inside the granules [198]. In addition, as the amount of sCD26 decreases during granule maturation, this suggests that the protein is sorted (a pathway already observed with other secreted proteins such as the insulin C-peptide) [198], which is another example of the particularity of CD26 gene expression, i.e. it is mostly regulated at the posttranslational level. Although some regulation at the transcriptional level has been found in recent times, particularly in B and T cells, in spite of the housekeeping features of the gene promotor [11, 192, 200–202], the first striking result was found in T cells by Mattern et al. [203]: only around half of the T cell population expresses cell surface CD26 despite the fact that both CD26+ and CD26− have similar CD26 mRNA and intracellular protein concentrations, and 4–8 h after T cell stimulation most or all cells express surface CD26 (the intracellular pool is translocated to the cell surface). We also described cytokine-dependent CD26 translocation to the T cell surface related to glycosylation events, and interestingly, observed two different mRNA transcripts by Northern blot in these immune cells [204]. It has recently been shown that rotavirus infection of enterocytes inhibits translation of CD26 but not mRNA transcription [205]. Furthermore, as already mentioned, sCD26 and membrane CD26 present a considerable and tissue-specific molecular heterogeneity originated mainly but not exclusively from different glycosylations [85, 194, 206, 207]. At least in T cells, mitogenic stimulation changed the enzymatic and immunoreactive patterns of molecular CD26 as well as the subcellular localization of the distinct forms [85] and CD26 has been found in endosomes in a process of recycling [208, 209].
Another possibility not yet fully explored and related to the intracellular sorting is the secretion of soluble proteins through MMP-dependent shedding from exosomes. Exosomes are small membrane vesicles derived from intracellular multivesicular bodies (MVBs, formed from the late endosomal compartment) that can undergo constitutive and regulated secretion from cells upon fusion with the PM and have been particularly well studied within the immune system [208–210]. Exosomes with CD26/DPP-IV have been found in human saliva, released at the basolateral surface of enterocytes, and in ram epididymal fluid [211–213].
As it is not known to which CD26 functions regulation of this proteolytic or secretory process is related, the physiological role of soluble CD26 in biological fluids with respect to the transmembrane CD26 remains poorly understood. Current data support three potential biological functions for sCD26 refined in recent years, and which may be partly responsible for the different roles of CD26 in various clinical settings. (1) Involvement in the activation–deactivation of some chemokines and therefore in inflammatory processes. Extracellular proteases, many shed or ripped [30], which alter the chemokine gradients, participate in this crucial early step of the immune response. For CD26, the modulation of SDF-1 and the CXCR4 axis of cell homing has been particularly well studied [214, 215]. (2) Circulating sCD26 may also participate in the clipping or inactivation of the biologically still active blood substrates such as vascular regulatory peptides (substance P or bradykinin) [48], growth factors or hormones (e.g. 20% of incretins GLP-1 and GIP, originated in the gastrointestinal duct, are still active in the blood pool) [96, 97]. (3) In the case of oncogenic processes, in addition to possible involvement in both immunosuppressor [115, 177] and angiogenic mechanisms [48–56], the process of shedding may initiate or dampen the CD26 involvement in cell-adhesion processes through fibronectin, ADA or collagen binding, depending on its initial pro- or anti-oncogenic role in the tumourigenic niche or in a later metastatic process [110–114, 117, 118, 122, 127, 177].
Furthermore, sCD26 can participate in the immune response of T cell activation by APCs and CD86-dependent APC activation (CD86 is up-regulated in a caveolin-1/CD26 binding-dependent fashion), although this process should occur in the lymph node [13].
Other serum proteins with DPP-IV activity
The facts that around 10% of serum DPP-IV activity is not associated with sCD26 [2, 16, 187–189] and that CD26 gene knockouts or deficient animals still retain the same percentage of blood DPP-IV-like activity [43, 93, 216–218] suggest that other DASH proteins are present in systemic circulation.
DPP-II is possibly involved, as indicated by studies of enzymatic activity [175, 219, 220], although the use of preferential (but not specific) substrates and different pH (not fully discriminating) cannot prevent overlap amongst DPP-II, -IV or other DPPs [2, 16, 221, 222]. DPP-II/DPP7/QPP has a ubiquitous distribution and a limited range of substrates (tripeptides), and a housekeeping role in the final steps of peptide degradation in lysosomes has been suggested [221–224]. However, QPP has also been located in the cytoplasm and other non-lysosomal vesicules, it contains a leucine zipper motif that may endow it with extraenzymatic functions through protein–protein binding [225–227], and it may be involved in the apoptotic process [228, 229].
Although neither the physiological role of DPP-II nor whether it is secreted [230] have been elucidated [221, 222], its intracellular activity is increased in squamous cell lung carcinoma [124], and varies with the progression of B-cell chronic lymphocytic leukaemia [231, 232]; moreover, its serum activity in patients with oral squamous cancer and hepatic cancer as well as in patients with lupus erythematosus and rheumatoid arthritis is higher than in healthy subjects [150, 233, 234].
FAP-α, which may form heterodimers with CD26, is also involved. Although it has a very restricted expression in normal human cells as a transmembrane protein [20–23], it has very recently been demonstrated that the circulating antiplasmin-cleaving enzyme (APCE) is a soluble derivative of FAP present in human plasma [235–237], and seprase activity (the other enzymatic specificity of FAP) has been purified from bovine plasma [238]. APCE circulating in human plasma appears to have a role in making α2AP (antiplasmin) more efficient for protection of extravascular fibrin, which forms as a host response to staunch haemorrhage [236]. However, although cultured endothelial cells have been reported to express FAP mRNA, translation of FAP protein was not documented [239], and the origin and additional functions of membrane-bound or soluble FAP under normal conditions remain enigmatic. Importantly, cancer patients probably have increased serum levels of this shortened form of FAP [20, 240].
Altered levels of serum sCD26/DPP-IV in diseases
By use of immunodetection, we have reported reduced levels of sCD26 in the serum of colorectal cancer (CRC) patients, compared with healthy donors, particularly in early stages of the disease, which suggested the potential usefulness of this molecule for early diagnosis of CRC [241, 242]. Later case-finding and case–control studies allowed us to obtain accurate clinical values that suggest that a serum CD26 test is an improvement on current non-invasive screening tests recommended for the detection of colorectal polyps and cancer [243]. Additional data [241–244] also support the usefulness of serum sCD26 levels for patient monitoring and prognosis. By use of an enzymatic activity assay, other authors found, however, increased DPP-IV activity in a similar cohort of colorectal cancer patients [245].
In the same way, in myocardial infarction patients treated with streptokinase, the concentration of enzyme is reduced to more than 50% after 90 days of therapy, while measurements of DPP-IV enzymatic activity did not change during that period [248].
On the contrary, the same authors found that there was no change in sCD26 concentrations but a lower enzymatic activity, with respect to the healthy donors, in rheumatoid arthritis and lupus erythematosus [92].
As mentioned above, these discrepancies can now be explained by putative changes in the glycosylation pattern (leading to a lack of immunorecognition of sCD26), the putative presence of DPP-IV activator attractin, or the secretion of DPP-II or, perhaps soluble FAP. These hypotheses require urgent research to validate sCD26/DPP-IV as a clinical marker.
As many studies have demonstrated altered serum levels of enzymatic DPP-IV activity (Tables 3, 4) and soluble CD26 protein (Table 2) in several diseases, the above factors should be taken into account. Although reference values of DPP-IV specific activity have been reported for serum (and plasma, with no difference) from a relevant group of healthy adults (Table 3) [2, 256, 261], most reports neither use the same assay conditions nor the same definition of specific activity, the same applies to the units of catalytic activity, making it difficult to compare these results, even from the same authors. In addition, some studies show contradictory results, probably related to the stage of disease considered (or a particular patient has been recruited) [92, 241–245, 249].
Table 3.
Studies showing physiological and pathophysiological levels of human serum (sometimes plasma) DPP-IV activity in patients with diseases not related to the immune response (type I diabetes is included for comparison) and with tumours
| Disease | Catalytic activity/specific activity (±SD) (U L−1)a | Cohort (n) | References |
|---|---|---|---|
| Healthy | 27.5 ± 6.1 women | 481 | [2, 259] |
| 32.3 ± 6.4 men | |||
| Healthy | 58 ± 16 | 64 | [260] |
| Healthy/ | 22.6 ± 0.9 | 40 | [261] |
| 21.8 ± 1.1 | 29 younger (<50) | ||
| 24.1 ± 1.4 | 18 young male | ||
| 18.2 ± 1.1 | 11 young female | ||
| 24.5 ± 1.5 | 11 elder | ||
| 23.8 ± 2.4 | 6 elderly male | ||
| 25.4 ± 2.0 | 5 elderly female | ||
| Gastric cancer | 15.1 ± 1.1 | 27 | |
| Pancreatic cancer | 11.9 ± 2.8 | 2 | |
| Gastric ulcer | 17.4 ± 2.3 | 3 | |
| Pancreatitis | 21.0 ± 2.5 | 2 | |
| Bile duct cancer | 42.6 ± 10.5 | 2 | |
| Acute hepatitis | 37.4 ± 6.4 | 8 | |
| Chronic hepatitis | 33.4 ± 1.8 | 3 inactive | |
| 29.5 ± 4.2 | 5 active | ||
| Cirrhosis | 35.5 ± 3.6 | 11 | |
| Healthy/ | 60.4 ± 3.2 | 12 males | [262] |
| Early hypertensive | 81.0 ± 3.9 | 20 (without drug treatment) | |
| Fixed hypertensive | 89.3 ± 3.7 | 17 (with drug treatment) | |
| Healthy/ | 51.6 ± 9.7 | 61 | [263] |
| Umbilical blood | 32.7 ± 5.9 | 65 | |
| Gastric cancer | 33.0 ± 7.8 | 9 | |
| Blood cancers | 38.8 ± 11.9 | 22 ALL | |
| 52.1 ± 24.11 | 62 AML | ||
| 49.4 ± 23.7 | 28 CML | ||
| 36.3 ± 5.6 | 11 lymphosarcoma | ||
| 35.6 ± 11.6 | 5 Hodgkin’s disease | ||
| Healthy/ | 77.5 ± 17.1 | 100 (automated) | [264] |
| Hepatocellular carcinoma | 198 ± 110.4 | 53 (+6 metastatic) | |
| Healthy/ | 70.1 ± 11.4 | 1,117 | [265] |
| Gastric carcinoma | 67.2 ± 20.4 | 10 early | |
| 51.1 ± 11.4 | 13 advanced | ||
| Healthy/ | 43.9 ± 1.1 | 21 | [266] |
| Miscellaneous cancers (<10 patients) | ~32 (figure) | AML | |
| ~28 (figure) | ALL | ||
| ~43 (figure) | CML | ||
| ~31 (figure) | Malignant lymphoma | ||
| ~32 (figure) | Multiple myeloma | ||
| ~38 (figure) | Oesophagus | ||
| ~28 (figure) | Colorectum | ||
| ~48 (figure) | Liver | ||
| ~43 (figure) | Gall bladder | ||
| ~46 (figure) | Leiosarcoma | ||
| Healthy/ | 43.1 ± 4.8 | 45 | [234] |
| Oral cancer (SCC) | 28.6 ± 12.7 | 25 | |
| Healthy/ | 55.1 ± 16.4 | 66 | [267, 268] |
| Oral SCC | 31.6 ± 12.4 | 51 (similar in all stages) | |
| Healthy/ | 11.6%Ab | 7 | [215] |
| CTCL (NH lymphoma) | 7.4 | 11 Sézary syndrome | |
| 8.6 | 7 mycosis fungoides | ||
| Healthy/ | 54 ± 0.9 | 120 | [270] |
| 54.1 | 60 men | ||
| 57.7 | 30 younger (<50) | ||
| 50.4 | 30 older | ||
| 53.9 | 60 women | ||
| 54 | 30 younger (<50) | ||
| 53.8 | 30 older | ||
| Osteoporotic | 70.7 ± 1.8 | 30 | |
| Healthy/ | 6.9 ± 1.4 | 10 infants | [273] |
| 5.9 ± 1.5 | 50 children | ||
| 4.5 ± 1.5 | 50 adults | ||
| Liver diseases | 50.2 ± 12.2 | 7 Biliary atresia (paediatric) | |
| 9.4 ± 4.2 (GGT < 500) | 8 Hepatitis syn. (paediatric) | ||
| 36.9 ± 12.8 (GGT > 500) | 8 Hepatitis syn. (paediatric) | ||
| 6.5 ± 1.7 | 5 Jaundice pers. (paediatric) | ||
| 17.1 ± 5.2 | 24 cirrhosis | ||
| 25 ± 5.1 | 8 Mech icterus (tumour) | ||
| 22.5 ± 4.8 | 6 Mech icterus (cholelith.) | ||
| 13.6 ± 3.2 | 4 toxic liver | ||
| 5.2 ± 1.1 | 5 chronic hepatitis | ||
| 21.5 ± 9.4 | 23 primary biliary cirrhosis | ||
| 28.1 ± 11.1 | 16 (primary biliary cirrhosis, late stages) | ||
| Healthy/ | 12.4 ± 1.8 | 24 | [275] |
| Liver diseases (PBC) | 21.4 ± 1.8 | 42 (increases with stages) | |
| Healthy/ | 43.6 ± 10.6 | 17 | [276] |
| Liver diseases (non-alcoholic steatohepatitis) | 57.3 ± 7.8 | 31 | |
| Healthy/ | 0.241 ± 0.015 (ΔOD/20 min) | 9 (middle aged) | [277] |
| 0.223 ± 0.019 | 9 (elderly) | ||
| Diabetes (II)/ | 0.179 ± 0.017 | 12 (middle aged) | |
| 0.173 ± 0.017 | 19 (elderly) | ||
| Healthy/ | 34.5 ± 11.8 | 29 | [278] |
| Diabetes (I)/ | 36.2 ± 11.7 | 29 medicated for years | |
| Diabetes (II) | 27.7 ± 7.1 | 31 HbA1C >8.5%; >1 year | |
| 22.1 ± 6.0 | 31 HbA1C <7.5%; n. diag. | ||
| 18.8 ± 8.8 | 31 IGT | ||
| ~20 (figure) | 62 NGT |
Data with “(figure)” mean that numbers were calculated from article’s figures as they were not cited in the text
AML acute myelocytic leukaemia, ALL acute lymphocytic leukaemia, CML chronic myelocytic leukaemia, CTCL chronic T cell lymphoma, HAV, HBV, HCV hepatitis virus, EBV Epstein-Barr virus
aExcept where indicated
bAbsorbance
Table 4.
Studies showing physiological human serum (sometimes plasma) DPP-IV activity and pathophysiological levels in patients with immune response related diseases (including psychologically related disorders)
| Disease | Catalytic activity/specific activity (±SD) (U L−1)a | Cohort (n) | References |
|---|---|---|---|
| Osteoarthritis | ~38 (figure) | 26 | [7] |
| Rheumatoid arthritis | ~28 (figure) | 41 | |
| Healthy/ | 1.6 mmol/min mol (median) | 25 | [92] |
| Rheumatoid arthritis/ | 1.2 (median) | 25 | |
| Lupus erythematosus/ | 1.7 (median) | 10 | |
| Sjögren syndrome/ | 2 (median) | 10 | |
| Healthy/ | 41.3 ± 4.8 | 25 | [269] |
| Rheumatoid arthritis/ | 34.5 ± 3.2 | 21 | |
| Lupus erythematosus/ | 29.9 ± 64.6 | 21 | |
| Healthy/ | 54 ± 0.9 | 120 | [270] |
| Osteoporotic | 54.1 | 60 men | |
| 57.7 | 30 younger (<50) | ||
| 50.4 | 30 older | ||
| 53.9 | 60 women | ||
| 54 | 30 younger (<50) | ||
| 53.8 | 30 older | ||
| 70.7 ± 1.8 | 30 | ||
| Healthy/ | ~54 (figure) | 22 | [271] |
| Systemic lupus erythematosus | ~43 (figure) | 21 inactive disease | |
| ~39 (figure) | 18 moderate disease | ||
| ~37 (figure) | 8 active disease | ||
| Healthy/ | 9.3 ± 1.3 | 54 | [249] |
| Systemic lupus erythematosus | 5.8 ± 1.8 | 53 | |
| Healthy/ | 0.95 ± 0.13 nmol/min/μg | 79 | [255] |
| HIV-1 | 0.82 ± 0.14 | 90 | |
| Healthy/ | 0.77 ± 0.6 | 20 | [272] |
| Sepsis | 0.39 ± 0.15 | 15 moderate | |
| 0.26 ± 0.15 | 15 severe | ||
| Healthy/ | 21.4(median) | 12 | [274] |
| Liver diseases | 40 (median) | 10 primary biliary cirrhosis | |
| 29.8 (median) | 36 HCV | ||
| 26.5 (median) | 10 HBV | ||
| 35.5 (median) | 10 HAV | ||
| 37.2 (median) | 10 EBV | ||
| Healthy/ | 71.9 ± 18.4 | 28 | [279, 280] |
| Inflammatory bowel diseases | 52.8 ± 16.9 | 63 Crohn’s disease | |
| 55.7 ± 15.1 | 47 ulcerative colitis | ||
| Healthy/ | 63.3 ± 15.1 | 28 | [281] |
| Crohn’s disease | 55.8 ± 17.7 | 48 remission | |
| 47.1 ± 14.1 | 23 active | ||
| Healthy/ | ~24 (figure) | 10 | [282] |
| Smokers | ~18 | 9 | |
| Allergic asthmatics | ~26 | 31 (do not change with corticoid therapy) | |
| Healthy breast-fed infants/ | 92.6 ± 4.8 | 13 | [283] |
| Allergic (atopic dermatitis) | 64.2 ± 13.2 | 23 | |
| Healthy/ | 76.9 ± 23.1 | 50 children | [284] |
| Celiac disease (acute) | 83.4 ± 20.2 | 48 children | |
| Healthy/ | 51.2 ± 15.6 | 52 | [285] |
| Depression | 49.2 ± 14.2 | 14 | |
| Healthy/ | 40.8 ± 6.4 | 12 | [285, 286] |
| Abstinent alcohol dependent | 28.9 ± 7.3 | 12 (without liver damage) | |
| Stress | 39 ± 7.7/38.8 ± 8.4 (after) | 30 | |
| Anxiety | 37 ± 7.6 (changes in males) | 22 (males and females) | |
| Healthy/ | 46.8 | 15 | [287, 288] |
| Major depressed | 36.9 | 36 (antidepressant resistant) | |
| Healthy/ | 19.4 ± 1.8 | 25 | [289] |
| Major depressed | 10.2 ± 1.1 | 18 (no change in additional 12 minor depressed) | |
| Healthy/ | 80.3 | 20 females | [290] |
| Hyporectic disorders | 108.1 | 34 anorexia | |
| 91.1 | 11 bulimia | ||
| Healthy/ | 34.4 ± 7.8 | 19 females | [291] |
| Hyporectic disorders | 27.3 ± 14.7 | 21 anorexia | |
| 22.7 ± 11.4 | 21 bulimia | ||
| Healthy/ | 29.4 ± 9.4 | 13 children | [292] |
| Tonsil diseases | 39.8 ± 6.5 hypertrophy | 19 before tonsillectomy | |
| 29.9 ± 7.3 hypertrophy | 14 after tonsillectomy | ||
| 19.9 ± 7.8 | 36 young adult | ||
| 19.2 ± 2.7 recurring tonsillitis | 13 before tonsillectomy | ||
| 12.7 ± 5.2 recurring tonsillitis | 8 after tonsillectomy |
Data with “(figure)” mean that numbers were calculated from figures in the article because they were absent from the text
RA rheumatoid arthritis; HAV, HBV, HCV hepatitis virus; EBV Epstein-Barr virus
aExcept where indicated
However, it is very interesting that the amount of sCD26 antigen found in normal serum with the most commonly used commercial ELISA kit (Bender MedSystems), corresponds well with the expected values based on the specific activity of purified serum DPP-IV [2, 256]. Together these findings support the use of immunodetection techniques for the quantification of these molecules because they are more specific.
We have included in Tables 2, 3 and 4 all major studies (if the disease is well represented, studies with all statistical groups of n < 10 are not included, although cited) published in English. Interestingly, many studies have used CD26 as a cell surface of Th1 cellular immune activation and sCD26 as a soluble marker, together with sCD30 and sometimes sCD23 as markers of Th2 (humoral response) [252, 253, 255, 257, 258]. Although there was no inverse correlation between increased sCD30 (see review [293]) and decreased sCD26 in many diseases, or vice versa, sCD30 is also shed (from hematopoietic cells) and, together with other surface antigens, it appear to suppress an appropriate T cell-dependent immune response, allowing tumour cells to escape immunosurveillance, resulting in progression of the tumour and spread of the disease [293]. It must also be remembered that DPP-IV activity has also been detected in other body fluids such as urine or synovial fluid, and its altered levels in some diseases has also been proposed as a clinically useful marker [273, 294].
The concentration of sCD26 increases in HIV-1 patients and leishmaniasis as well as in myocardial infarction and atopic dermatitis, does not change in asthmatics, osteoarthritis and gastric cancers, and decreases, apart from in CRC, in rheumatoid arthritis and particularly lupus erythematosus and Sjögren syndrome (Table 2). Results from hepatitis C virus (HCV) are not consistent.
DPP-IV enzymatic activity is high in patients with hepatic cancer, hepatitis, osteoporosis (in which it probably determines the severity of the disease), cholestasis and other liver diseases (Table 3), and in psychologically related eating disorders such as anorexia or bulimia (Table 4). In contrast with protein levels, it is also increased in CRC, rheumatoid arthritis, lupus erythematosus and Sjögren syndrome, according to some studies and in disagreement with others [92, 241–245, 249, 295, 296]. DPP-IV levels remain unchanged in metastatic bone disease, oesophagus, gall bladder, chronic myelocytic leukaemia or leiomyosarcoma cancers (Table 3), and in allergic asthma (with or without treatment with inhaled glucocorticoids, although it changes in allergic infants) and in celiac disease (Table 4). In adult T cell leukaemia, although serum DPP-IV is strongly correlated with the percentage of CD26+ T cells, no apparent change in the mean value of activity was found [297]. However, decreased levels of DPP-IV are observed in patients with some blood (particularly acute lymphocytic leukaemia), thyroid [298] and oral cancer, advanced gastric carcinoma, and in particular, colorectal cancer (in accordance with our data) (Table 3). Lower levels were also found in HCV infections, in inflammatory bowel diseases, in healthy smokers, in pregnancy [299] and in type II diabetes, and in alcoholics and patients suffering from major depression. A reduction in DPP-IV activity has been related to symptoms of depression and anxiety under certain circumstances, with contradictory results in bulimia and anorexia (Table 4).
In summary, low levels of DPP-IV/sCD26 occur concurrently with impaired immune status, including some haematological and solid malignancies, whereas increased levels occur in inflammatory and infectious diseases (enhanced immune status), other haematological tumours, and liver diseases.
The source of sCD26
In order to validate a candidate clinical or tumour marker, or a therapeutic target, it is important to know how it is distributed in cells, tissues or systems, as well as the circumstances that provoke its abnormal presence/absence. However, the origin of serum CD26 is unknown.
The hepatobiliary system was the first to be suggested as the sCD26 source by the group of Nagatsu [261] because serum enzyme activity levels in hepatitis and cirrhosis patients were correlated with the serum group of enzymes that are present at high levels in patients with obstructive jaundice and infiltrative disease of the liver. However, they were cautious because electrophoresis revealed the presence of novel isoforms in the sera from those patients, and lower activities in patients suffering from gastric or pancreatic cancer and normal levels in patients with pancreatitis were observed. Liver epithelium is often cited as the most likely potential source of serum CD26 as hypothesized from hepatocellular carcinoma studies, in which loss of CD26 from membrane is accompanied by increased DPP-IV activity in patients [106, 107, 116, 264, 273], and similar results were also observed in studies of hepatic regeneration [14, 300, 301]. However, the increased enzyme activity could not be explained by CD26 expressed in hepatocytes [14], and CD26 is predominantly located in the bile canaliculi [273, 275, 302]. At least in some conditions, sCD26 originates from the brush border of hepatocytes [273], but a recent study found that in chronic hepatitis C and other liver viral infections DPP-IV activity levels were not correlated with several markers of bile duct injury or hepatocyte injury [274]. The authors, therefore, suggested that the increased activity in these diseases may originate directly from shedding from the peripheral blood T cells involved in the control of viral infections or, in the case of HCV infection which takes place with a weak T cell response during the chronic phase of the disease, indirectly by stimulating other cells such as hepatic stellate cells. The involvement of T cells in the enhancement of sCD26 levels has already been suggested by the group of Gorrell and McCaughan [14, 303] in studies of liver regeneration, where T cell activation is known to occur.
Interestingly, the events observed in hepatocarcinoma—loss of membrane CD26 and elevation of DPP-IV levels—are not seen in CRC. Almost all CRC patients show reduced serum levels of sCD26 [241–243], whereas loss of membrane CD26 expression only occurs in 11% of colorectal tumours [108], i.e. in CRC, sCD26 is not correlated with cell proliferation, or with the alteration of CD26 expression in CRC tumour cells. There is no direct correlation between sCD26 levels and tumour location, degree of histological differentiation, type of metastasis or Dukes’ stages of CRC [241], which may affect the hepatic production of sCD26. As in hepatic regeneration but in the opposite direction, immunity involved in CRC as an immune defective anti-tumour response, including a deficiency in IL-12 production [304] which is a well-known CD26 up-regulator in T lymphocytes [305], has been described.
A possible origin of sCD26 from the immune system, as well as from spleen, was first suggested in 1984 by Kasahara et al. [306], as these authors observed a significant correlation between the normal serum DPP-IV activity and the peripheral blood lymphocyte count, although they also identified serum isoforms from liver, spleen or kidney. Kidney, an obvious potential source because it contains large amounts of CD26, was rejected early on [187] because anephric individuals have normal amounts of sCD26, and because sCD26 contains approximately twice as much sialic acid as kidney CD26.
Several data suggest that serum CD26 is at least partly shed from T cells. Much data come from glycosylation and electrophoretic mobility studies. The sialylation of CD26 appears to be cell-type specific: the sialic acid content of vascular and circulating CD26 is higher than that of the kidney and intestinal brush border enzyme [85, 307, 308]. Sialylation of CD26 is increased in peripheral blood mononuclear cells (PBMCs) of healthy elderly individuals (by 80 years of age, sialylation of T cell DPP-IV/CD26 is three to five times that of a healthy 20-year old); hypersialylation is more extreme in HIV-positive individuals with AIDS, and these glycosylation states coincide with the forms found in the corresponding serums [178]. Sialylation may prevent removal from the circulation by the liver asialoglycoprotein receptors and increased sialylation may be related to impaired immunocompetence [92, 178, 307, 308]. However, these data do not preclude the possibility of sCD26 shed being from the endothelium of venules or the capillary bed of several organs such as lung, myocardium and striated muscles, spleen and pancreas [3, 201, 202, 307, 309–313].
Other data include the changes in sCD26 levels in relation to physiological or pathophysiological processes. As already commented, most changes (Tables 2, 3, 4) occur concurrently with the immune status, sCD26 levels decrease generally in disease unless a liver injury or extensive lymphocyte proliferation is involved [2, 11–14]. In relation to this, reduced concentrations of the peptidase in healthy smokers, alcoholics and severely depressed patients (Table 2) are consistent with impaired immune response. Interestingly, anti-TNFα treatment (adalimumab) of patients with rheumatoid arthritis augmented DPP-IV activity [314]. Also, that sCD26 may be valuable as prognostic marker in CRC [241] fit this hypothesis, because patients with activated immune systems (higher sCD26 levels) may show a better chance of survival than those with lower sCD26 levels.
The most important data correspond firstly to in vitro studies [305, 312, 315, 316] which demonstrate secretion/shedding of sCD26 from lymphocytes to culture media, whereas no data exist for other cell types; interestingly, TGF-β1 down-regulates CD26/DPP-IV expression in T cells, which is accompanied by decreased DPP-IV activities in the supernatants of cultured cells [316]. Secondly, many in vivo studies found a correlation between changes in serum DPP-IV activity and the numbers of PBL, T lymphocytes, CD26+ T cells and the amount of CD26 in T lymphocyte plasma membranes, in patients with adult T cell leukaemia [297], oral cancer [315], gastric and colorectal carcinoma [317–319], with mycosis fungoides and Sézary syndrome (two major variants of the cutaneous T cell lymphoma, a type of non-Hodgkin lymphoma) [215], with the autoimmune diseases systemic lupus erythematosus [271], with arthritis rheumatoid [247, 249, 306] and inflammatory bowel (Crohn’s disease and ulcerative colitis) [279, 281], with adult allergic asthmatics [251] and in human miscarriage [320]. In addition, cell surface CD26 [88, 305, 321–326] and serum sCD26 are markers of Th1 cellular immune activation together with CD30 and sCD30 as markers of Th2 (humoral) response, although an inverse correlation between increases in sCD30 and decreases in sCD26 or vice versa was not always found in diseases, as already mentioned [252, 253, 255, 257, 258, 293, 327–332].
Although these changes in cell surface and soluble forms may be associated with regulation of the enzymatic cleavage of CD26 from the T cell surface, the observation of anti-CD26 autoantibodies in rheumatoid arthritis and systemic lupus erythematosus patients and the fact that its levels are correlated with an increased clearance rate of the circulating CD26 [92, 248] suggest an alternative, but also immune-related, hypothesis for the regulation of sCD26 levels. It will be interesting to look for these autoantibodies in cancer patients to test this hypothesis. In fact, an association between chronic inflammatory conditions and eventual development of cancer was described several years ago [333, 334].
In summary, a fraction of serum CD26 originates from immune system cells, and this sCD26 fraction can be regulated and, therefore, causes an imbalance amongst specific sCD26 isoforms in the serum of patients.
On the role of sCD26 in cancer: conclusion
Complex crosstalk between the lymphoid lineage and malignant tumours in vivo has been discussed since the days of Paul Ehrlich a century ago; the ability to suppress the immune response is essential for tumours to develop [116, 174, 333–336]. Taking the example of CRC and the lower levels of sCD26 in these patients, we will suggest a framework to research the hypothetic role of sCD26 in crosstalk between the immune system and carcinogenesis. This should be investigated because drugs that inhibit DPP-IV activity [96–98, 101–104] may exacerbate development of tumours and/or immune diseases and, consequently, a follow-up of diabetics under this therapy should be done. In a similar way, it has been already proposed that genetic or environmental factors that decrease DPP-IV activity might increase the risk of ACE (angiotensin converting enzyme) inhibitor-associated angioedema [43, 337].
It is well known that tumour cells secrete immunosuppressive factors such as TGF-β1. TGF-β1 acts on many immune cells such as APCs, T cells and NKT cells, thus participating in the induction of T tolerance to tumour antigens [174, 338], and down-regulates in vitro CD26/DPP-IV expression in T cells as well as culture supernatant sCD26 levels [316]. Consequently, it is possible that TGF-β1 also down-regulates production of circulating sCD26 in cancer patients, which may reflect tumour-induced T cell tolerance (Fig. 1). The facts that inhibitors of CD26/DPP-IV activity up-regulate TGF-β1 secretion by T cells in vivo [172, 175], that anti-TNF-α therapy augments DPP-IV activity in patients with rheumatoid arthritis [314] (TNF-α is pivotal in inflammatory reactions and is frequently detected in human cancers), and that an immune defective IL-12 production (a well-known CD26 up-regulator [17, 204, 305] has been described in CRC [304] also support this link.
By lowering CD26 expression, sCD26 and therefore the DPP-IV activity, tumour cells will alter one or more of the physiological functions of that protein. For example, immune cell homing through chemokine clipping, as already mentioned (Table 1) [11], i.e. accumulation of plasmacytoid dendritic cells found inside ovarian and head and neck SCC, was attributed to SDF-1/CXCL12 secreted by malignant cells [339, 340]; also, Tregs were recruited to the tumour site under the influence of CCL22 [341]. Another consequence may be to increase the active levels of incretins GLP-1, -2 and GIP, and in doing so, to improve insulin secretion. This argument has already been used by Meneilly et al. to explain the lower DPP-IV levels in elderly obese patients with diabetes as an adaptation of the body to enhance the incretin-mediated insulin secretion [241]. A diverse body of evidence that relates higher levels of insulin to elevated risk of colon cancer and carcinogenesis in general, through direct and/or IGF-1-dependent mechanisms, has been impressively reviewed [342]. Moreover, GLP-2 has direct tumour-promoting effects on intestinal cancer cells (and perhaps it may be generalized to other cancers) [343].
Therefore, the lymphocyte count, subset distribution and other immune parameters of patients, as well as the levels of entero- and neuropeptides, should be recorded in future studies of CRC and other carcinomas. As suggested in a study with rats [344], in which the antidiabetic agents metformin and pioglitazone (that improve insulin sensitivity) reduced serum DPP-IV activity, in direct relation to reductions in glycosylated haemoglobin and increases in GLP-1 levels, it should be investigated whether glycemic control regulates the release of DPP-IV from the different cell types proposed as its source; the isoform patterns of sCD26 and CD26 in those tissues should also be studied. In vitro and in vivo studies also showed cyclosporin A down-regulation of CD26 and sCD26 [204, 345].
DPP-IV is also an important regulator of NPY-induced angiogenesis in the Ewing’s sarcoma family of tumours [54–56], and can be up-regulated by hypoxia in endothelial cells [346]. This up-regulation may explain the increased levels of the protein and the poorer behaviour of sCD26 as a marker, in late-stage CRC Duke’s D patients (and tumour angiogenesis) with respect to earlier stages [241]. However, the effect of pathophysiologically lower sCD26 concentrations on the NPY-YRs system, including vasoconstriction, is not known [347].
Another important feature, in relation to the value of sCD26 as a biomarker, is the timing of sCD26 alterations in the plasma of patients with tumours and/or immune system-related diseases. If further studies confirm this hypothetic immunosuppressive mechanism mediated by tumour cells (Fig. 1), then they would have identified another target to be used to overcome immunological tolerance and promote tumour regression in combination with other conventional strategies.
Current clinical trials (basically vaccination strategies and adoptive transfer of effector cells) aimed at harnessing the immune system to eliminate tumours already include sometimes immunomodulatory agents that support Th1 responses, such as IL-2, -12, -18, -21, and IFN-γ [348–352], which may enhance the sCD26 levels [252, 253, 255, 258, 259, 293, 327–332]. The effectiveness of anti-tumour responses is usually restricted by inhibitory signals from the tumour microenvironment, and some progress has been made in the direction to combining strategies involving blockade of these signals [175, 348]. Anti-TGF-β treatment should also lead to higher sCD26 levels [173, 176, 316]. Treatment with high exogenous concentration of TNF has some anti-tumour effects but TNF produced endogenously, by several types of tumour cells (including colorectal) and by many immunotherapy treatments that induce higher levels of TNF, also favours the development and progression of cancer [353]. Therefore, the use of anti-TNF therapy has been proposed for cancer therapy and employed in some clinical trials, with no need to avoid TNF administration in cases of organ-confined solid tumours, if required [348, 354]. Millions of patients with autoimmune/inflammatory disorders have been treated with antibodies against TNF, which enhance sCD26 levels [314]. In conclusion, the proposed immunosuppressive mechanism may be easily short circuited by a combination of stimulatory therapy, anti-TGF-β and anti-TNF treatment.
Finally, sCD26 levels are higher in patients at late and metastatic stages [241, 275] as well as in patients with liver tumours in general and other liver diseases [261, 264, 266, 273, 276]. Consequently, drugs that inhibit DPP-IV activity or anti-CD26 Ab [153] may be helpful in these cases.
Acknowledgments
We thank M Páez de la Cadena and FJ Rodríguez-Berrocal for their suggestions and comments. This work was supported by grants (Ref.) PGIDT05PXIB20001PR and BFU2006-09717 from the Spanish administrations of the Xunta de Galicia (Secretaría Xeral de Investigación e Desenvolvemento) and the Ministerio de Educación y Ciencia (Dirección General de Investigación), respectively.
Abbreviations
- DPP-IV
Dipeptidyl peptidase IV
- ADA
Adenosine deaminase
- ADCP
Adenosine deaminase complexing protein
References
- 1.Dinjens WN, et al. Distribution of adenosine deaminase complexing protein (ADCP) in human tissues. J Histochem Cytochem. 1989;37(12):1869–1875. doi: 10.1177/37.12.2573631. [DOI] [PubMed] [Google Scholar]
- 2.Lambeir AM, et al. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003;40(3):209–294. doi: 10.1080/713609354. [DOI] [PubMed] [Google Scholar]
- 3.Hartel S, et al. Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry. Histochemistry. 1988;89(2):151–161. doi: 10.1007/BF00489918. [DOI] [PubMed] [Google Scholar]
- 4.Fox DA, et al. Ta1, a novel 105 KD human T cell activation antigen defined by a monoclonal antibody. J Immunol. 1984;133(3):1250–1256. [PubMed] [Google Scholar]
- 5.Hegen M, et al. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990;144(8):2908–2914. [PubMed] [Google Scholar]
- 6.Yamabe T, et al. Induction of the 2B9 antigen/dipeptidyl peptidase IV/CD26 on human natural killer cells by IL-2, IL-12 or IL-15. Immunology. 1997;91(1):151–158. doi: 10.1046/j.1365-2567.1997.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hopsu-Havu VK, Glenner GG. A new dipeptide naphthylamidase hydrolyzing glycyl-prolyl-beta-naphthylamide. Histochemie. 1966;7(3):197–201. doi: 10.1007/BF00577838. [DOI] [PubMed] [Google Scholar]
- 8.Schrader WP, Stacy AR. Purification and subunit structure of adenosine deaminase from human kidney. J Biol Chem. 1977;252(18):6409–6415. [PubMed] [Google Scholar]
- 9.Morrison ME, et al. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med. 1993;177(4):1135–1143. doi: 10.1084/jem.177.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameoka J, et al. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- 11.Boonacker E, Van Noorden CJF. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003;82(2):53–73. doi: 10.1078/0171-9335-00302. [DOI] [PubMed] [Google Scholar]
- 12.De Meester I, et al. CD26, let it cut or cut it down. Immunol Today. 1999;20(8):367–375. doi: 10.1016/S0167-5699(99)01486-3. [DOI] [PubMed] [Google Scholar]
- 13.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: CD26 and its molecular mechanisms in T cell function. Trends Immunol. 2008;29(6):295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Gorrell MD, et al. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001;54(3):249–264. doi: 10.1046/j.1365-3083.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 15.Busek P, Malík R, Sedo A. Dipeptidyl peptidase IV activity and/or structure homologues (DASH) and their substrates in cancer. Int J Biochem Cell Biol. 2004;36(3):408–421. doi: 10.1016/S1357-2725(03)00262-0. [DOI] [PubMed] [Google Scholar]
- 16.Sedo A, et al. Dipeptidyl peptidase-IV and related molecules: markers of malignancy? Expert Opin Med Diagn. 2008;2(6):1–13. doi: 10.1517/17530059.2.6.677. [DOI] [PubMed] [Google Scholar]
- 17.Salgado FJ, et al. A role for interleukin-12 in the regulation of T cell plasma membrane compartmentation. J Biol Chem. 2003;278(27):24849–24857. doi: 10.1074/jbc.M212978200. [DOI] [PubMed] [Google Scholar]
- 18.Pacheco R, et al. CD26, adenosine deaminase, and adenosine receptors mediate costimulatory signals in the immunological synapse. Proc Natl Acad Sci USA. 2005;102(27):9583–9588. doi: 10.1073/pnas.0501050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ren X, et al. Transmembrane interaction mediates complex formation between peptidase homologues and Kv4 channels. Mol Cell Neurosci. 2005;29(2):320–332. doi: 10.1016/j.mcn.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Ghersi G, et al. The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res. 2006;66(9):4652–4661. doi: 10.1158/0008-5472.CAN-05-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen W-T, Kelly T. Seprase complexes in cellular invasiveness. Cancer Metastasis Rev. 2003;22(2–3):259–269. doi: 10.1023/A:1023055600919. [DOI] [PubMed] [Google Scholar]
- 22.Cheng HC, Abdel-Ghany M, Pauli BU. A novel consensus motif in fibronectin mediates dipeptidyl peptidase IV adhesion and metastasis. J Biol Chem. 2003;278(27):24600–24607. doi: 10.1074/jbc.M303424200. [DOI] [PubMed] [Google Scholar]
- 23.Henry LR, et al. Clin Cancer Res. 2007;13(6):1736–1741. doi: 10.1158/1078-0432.CCR-06-1746. [DOI] [PubMed] [Google Scholar]
- 24.Cheng JD, et al. Abrogation of fibroblast activation protein enzymatic activity attenuates tumor growth. Mol Cancer Ther. 2005;4(3):351–360. doi: 10.1158/1535-7163.MCT-04-0269. [DOI] [PubMed] [Google Scholar]
- 25.Morita A, et al. Intestinal assimilation of a proline-containing tetrapeptide. Role of a brush border membrane postproline dipeptidyl aminopeptidase IV. J Clin Invest. 1983;72(2):610–616. doi: 10.1172/JCI111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tiruppathi C, et al. Genetic evidence for role of DPP IV in intestinal hydrolysis and assimilation of prolyl peptides. Am J Physiol. 1993;265(1 Pt 1):G81–G89. doi: 10.1152/ajpgi.1993.265.1.G81. [DOI] [PubMed] [Google Scholar]
- 27.Alfalah M, Jacob R, Naim HY. Intestinal dipeptidyl peptidase IV is efficiently sorted to the apical membrane through the concerted action of N- and O-glycans as well as association with lipid microdomains. J Biol Chem. 2002;277(12):10683–10690. doi: 10.1074/jbc.M109357200. [DOI] [PubMed] [Google Scholar]
- 28.Alfalah M, et al. A novel type of detergent-resistant membranes may contribute to an early protein sorting event in epithelial cells. J Biol Chem. 2005;280(52):42636–42643. doi: 10.1074/jbc.M505924200. [DOI] [PubMed] [Google Scholar]
- 29.Vanhoof G, et al. Proline motifs in peptides and their biological processing. FASEB J. 1995;9(9):736–744. [PubMed] [Google Scholar]
- 30.Murphy G, Murthy A, Khokha R. Clipping, shedding and RIPping keep immunity on cue. Trends Immunol. 2008;29(2):75–82. doi: 10.1016/j.it.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 31.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–835. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 32.Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995;136(8):3585–3596. doi: 10.1210/en.136.8.3585. [DOI] [PubMed] [Google Scholar]
- 33.Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995;80(3):952–957. doi: 10.1210/jc.80.3.952. [DOI] [PubMed] [Google Scholar]
- 34.Hansen L, et al. Glucagon-like peptide-1-(7–36)amide is transformed to glucagon-like peptide-1-(9–36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140(11):5356–5363. doi: 10.1210/en.140.11.5356. [DOI] [PubMed] [Google Scholar]
- 35.Nikolaidis LA, et al. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289(6):H2401–H2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 36.Jeppesen PB, et al. Teduglutide (ALX-0600), a dipeptidyl peptidase IV resistant glucagon-like peptide 2 analogue, improves intestinal function in short bowel syndrome patients. Gut. 2005;54(9):1224–1231. doi: 10.1136/gut.2004.061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deacon CF, et al. Degradation of endogenous and exogenous gastric inhibitory polypeptide in healthy and in type 2 diabetic subjects as revealed using a new assay for the intact peptide. J Clin Endocrinol Metab. 2000;85(10):3575–3581. doi: 10.1210/jc.85.10.3575. [DOI] [PubMed] [Google Scholar]
- 38.Deacon CF, et al. Dipeptidyl peptidase IV inhibition reduces the degradation and clearance of GIP and potentiates its insulinotropic and antihyperglycemic effects in anesthetized pigs. Diabetes. 2001;50(7):1588–1597. doi: 10.2337/diabetes.50.7.1588. [DOI] [PubMed] [Google Scholar]
- 39.Pospisilik JA, et al. Metabolism of glucagon by dipeptidyl peptidase IV (CD26) Regul Pept. 2001;96(3):133–141. doi: 10.1016/S0167-0115(00)00170-1. [DOI] [PubMed] [Google Scholar]
- 40.Hinke SA, et al. Dipeptidyl peptidase IV (DPIV/CD26) degradation of glucagon. Characterization of glucagon degradation products and DPIV-resistant analogs. J Biol Chem. 2000;275(6):3827–3834. doi: 10.1074/jbc.275.6.3827. [DOI] [PubMed] [Google Scholar]
- 41.Lambeir AM, et al. Kinetic study of the processing by dipeptidyl-peptidase IV/CD26 of neuropeptides involved in pancreatic insulin secretion. FEBS Lett. 2001;507(3):327–330. doi: 10.1016/S0014-5793(01)02982-9. [DOI] [PubMed] [Google Scholar]
- 42.Reeve JR., Jr Amino acid sequences of three bombesin-like peptides from canine intestine extracts. J Biol Chem. 1983;258(9):5582–5588. [PubMed] [Google Scholar]
- 43.Ballantyne GH. Peptide YY(1–36) and peptide YY(3–36). Part II. Changes after gastrointestinal surgery and bariatric surgery. Obes Surg. 2006;16(6):795–803. doi: 10.1381/096089206777346619. [DOI] [PubMed] [Google Scholar]
- 44.Ballantyne GH. Peptide YY(1-36) and peptide YY(3-36). Part I. Distribution, release and actions. Obes Surg. 2006;16(5):651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 45.Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase IV inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol. 2008;35(1):29–34. doi: 10.1111/j.1440-1681.2007.04737.x. [DOI] [PubMed] [Google Scholar]
- 46.Medeiros MD, Turner AJ. Processing and metabolism of peptide-YY: pivotal roles of dipeptidylpeptidase-IV, aminopeptidase-P, and endopeptidase-24.11. Endocrinology. 1994;134(5):2088–2094. doi: 10.1210/en.134.5.2088. [DOI] [PubMed] [Google Scholar]
- 47.Mentlein R. Dipeptidyl-peptidase IV (CD26)—role in the inactivation of regulatory peptides. Regul Pept. 1999;85(1):9–24. doi: 10.1016/S0167-0115(99)00089-0. [DOI] [PubMed] [Google Scholar]
- 48.Byrd JB, et al. Dipeptidyl peptidase IV in angiotensin-converting enzyme inhibitor associated angioedema. Hypertension. 2008;51(1):141–147. doi: 10.1161/HYPERTENSIONAHA.107.096552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fryer RM, et al. Effect of bradykinin metabolism inhibitors on evoked hypotension in rats: rank efficacy of enzymes associated with bradykinin-mediated angioedema. Br J Pharmacol. 2008;153(5):947–955. doi: 10.1038/sj.bjp.0707641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pesquero JB, et al. Bradykinin metabolism pathway in the rat pulmonary circulation. J Hypertens. 1992;10(12):1471–1478. doi: 10.1097/00004872-199210120-00006. [DOI] [PubMed] [Google Scholar]
- 51.Mentlein R, Roos T. Proteases involved in the metabolism of angiotensin II, bradykinin, calcitonin gene-related peptide (CGRP), and neuropeptide Y by vascular smooth muscle cells. Peptides. 1996;17(4):709–720. doi: 10.1016/0196-9781(96)00066-6. [DOI] [PubMed] [Google Scholar]
- 52.Brandt I, et al. Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem. 2006;52(1):82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 53.Vanderheyden M. Clinical importance of BNP truncation by DPPIV. Clin Chem Lab Med. 2008;46(4):A18. [Google Scholar]
- 54.Abe K, Tilan JU, Zukowska Z. NPY and NPY receptors in vascular remodeling. Curr Top Med Chem. 2007;7(17):1704–1709. doi: 10.2174/156802607782340948. [DOI] [PubMed] [Google Scholar]
- 55.Kuo LE, et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat Med. 2007;13(7):803–811. doi: 10.1038/nm1611. [DOI] [PubMed] [Google Scholar]
- 56.Kitlinska J, et al. Differential effects of neuropeptide Y on the growth and vascularization of neural crest-derived tumors. Cancer Res. 2005;65(5):1719–1728. doi: 10.1158/0008-5472.CAN-04-2192. [DOI] [PubMed] [Google Scholar]
- 57.Jarmołowska B, et al. Serum activity of dipeptidyl peptidase IV (DPPIV; EC 3.4.14.5) in breast-fed infants with symptoms of allergy. Peptides. 2007;28(3):678–682. doi: 10.1016/j.peptides.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Tiruppathi C, et al. Hydrolysis and transport of proline-containing peptides in renal brush-border membrane vesicles from dipeptidyl peptidase IV-positive and dipeptidyl peptidase IV-negative rat strains. J Biol Chem. 1990;256(3):1476–1483. [PubMed] [Google Scholar]
- 59.Guieu R, et al. CD26 modulates nociception in mice via its dipeptidyl-peptidase IV activity. Behav Brain Res. 2006;166(2):230–235. doi: 10.1016/j.bbr.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Bagosi Z, et al. The effects of endomorphins and diprotin A on striatal dopamine release induced by electrical stimulation—an in vitro superfusion study in rats. Neurochem Int. 2006;49(7):665–668. doi: 10.1016/j.neuint.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 61.Karl T, et al. Extreme reduction of dipeptidyl peptidase IV activity in F344 rat substrains is associated with various behavioral differences. Physiol Behav. 2003;80(1):123–134. doi: 10.1016/S0031-9384(03)00229-4. [DOI] [PubMed] [Google Scholar]
- 62.Grouzmann E, et al. Loss of dipeptidylpeptidase IV activity in chronic rhino sinusitis contributes to the neurogenic inflammation induced by substance P in the nasal mucosa. FASEB J. 2002;16(9):1132–1134. doi: 10.1096/fj.01-0939fje. [DOI] [PubMed] [Google Scholar]
- 63.Busek P, et al. Modulation of substance P signaling by dipeptidyl peptidase-IV enzymatic activity in human glioma cell lines. Physiol Res. 2008;57(3):443–449. doi: 10.33549/physiolres.931231. [DOI] [PubMed] [Google Scholar]
- 64.Proost P, et al. Cleavage by CD26/dipeptidyl peptidase IV converts the chemokine LD78beta into a most efficient monocyte attractant and CCR1 agonist. Blood. 2000;96(5):1674–1680. [PubMed] [Google Scholar]
- 65.Struyf S, et al. Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol. 2001;31(7):2170–2178. doi: 10.1002/1521-4141(200107)31:7<2170::AID-IMMU2170>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 66.Guan E, Wang J, Norcross MA. Amino-terminal processing of MIP-1beta/CCL4 by CD26/dipeptidyl-peptidase IV. J Cell Biochem. 2004;92(1):53–64. doi: 10.1002/jcb.20041. [DOI] [PubMed] [Google Scholar]
- 67.Campbell TB, Broxmeyer HE. CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front Biosci. 2008;13:1795–1805. doi: 10.2741/2800. [DOI] [PubMed] [Google Scholar]
- 68.Oravecz T, et al. Regulation of the receptor specificity and function of the chemokine RANTES (regulated on activation, normal T cell expressed and secreted) by dipeptidyl peptidase IV (CD26)-mediated cleavage. J Exp Med. 1997;186(11):1865–1872. doi: 10.1084/jem.186.11.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schols D, et al. CD26-processed RANTES(3-68), but not intact RANTES, has potent anti-HIV-1 activity. Antiviral Res. 1998;39(3):175–187. doi: 10.1016/S0166-3542(98)00039-4. [DOI] [PubMed] [Google Scholar]
- 70.Proost P, et al. Amino-terminal truncation of chemokines by CD26/dipeptidyl-peptidase IV. Conversion of RANTES into a potent inhibitor of monocyte chemotaxis and HIV-1-infection. J Biol Chem. 1998;273(13):7222–7227. doi: 10.1074/jbc.273.13.7222. [DOI] [PubMed] [Google Scholar]
- 71.Struyf S, et al. CD26/dipeptidyl-peptidase IV downregulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J Immunol. 1999;162(8):4903–4909. [PubMed] [Google Scholar]
- 72.Forssmann U, et al. Inhibition of CD26/dipeptidyl peptidase IV enhances CCL11/eotaxin-mediated recruitment of eosinophils in vivo. J Immunol. 2008;181(2):1120–1127. doi: 10.4049/jimmunol.181.2.1120. [DOI] [PubMed] [Google Scholar]
- 73.Proost P, et al. Truncation of macrophage-derived chemokine by CD26/dipeptidyl-peptidase IV beyond its predicted cleavage site affects chemotactic activity and CC chemokine receptor 4 interaction. J Biol Chem. 1999;274(7):3988–3993. doi: 10.1074/jbc.274.7.3988. [DOI] [PubMed] [Google Scholar]
- 74.Proost P, et al. Amino-terminal truncation of CXCR3 agonists impairs receptor signaling and lymphocyte chemotaxis, while preserving antiangiogenic properties. Blood. 2001;98(13):3554–3561. doi: 10.1182/blood.V98.13.3554. [DOI] [PubMed] [Google Scholar]
- 75.Proost P, et al. Natural posttranslational modifications of chemokines. Biochem Soc Trans. 2006;34(6):997–1001. doi: 10.1042/BST0340997. [DOI] [PubMed] [Google Scholar]
- 76.Proost P, et al. Coexpression and interaction of CXCL10 and CD26 in mesenchymal cells by synergising inflammatory cytokines: CXCL8 and CXCL10 are discriminative markers for autoimmune arthropathies. Arthritis Res Ther. 2006;8(4):R107. doi: 10.1186/ar1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ludwig A, et al. Dipeptidyl peptidase IV (CD26) on T cells cleaves the CXC chemokine CXCL11 (I-TAC) and abolishes the stimulating but not the desensitizing potential of the chemokine. J Leukoc Biol. 2002;72(1):183–191. [PubMed] [Google Scholar]
- 78.Proost P, et al. Proteolytic processing of CXCL11 by CD13/aminopeptidase N impairs CXCR3 and CXCR7 binding and signaling and reduces lymphocyte and endothelial cell migration. Blood. 2007;110(1):37–44. doi: 10.1182/blood-2006-10-049072. [DOI] [PubMed] [Google Scholar]
- 79.Ohtsuki T, et al. Negative regulation of the anti-human immunodeficiency virus and chemotactic activity of human stromal cell-derived factor 1alpha by CD26/dipeptidyl peptidase IV. FEBS Lett. 1998;431(2):236–240. doi: 10.1016/S0014-5793(98)00763-7. [DOI] [PubMed] [Google Scholar]
- 80.Proost P, et al. Processing by CD26/dipeptidyl-peptidase IV reduces the chemotactic and anti-HIV-1 activity of stromal-cell derived factor-1alpha. FEBS Lett. 1998;432(1–2):73–76. doi: 10.1016/S0014-5793(98)00830-8. [DOI] [PubMed] [Google Scholar]
- 81.Shioda T, et al. Anti-HIV-1 and chemotactic activities of human stromal cell-derived factor 1alpha (SDF-1alpha) and SDF-1beta are abolished by CD26/dipeptidyl peptidase IV-mediated cleavage. Proc Natl Acad Sci USA. 1998;95(11):6331–6336. doi: 10.1073/pnas.95.11.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Struyf S, et al. Regulation of the immune response by the interaction of chemokines and proteases. Adv Immunol. 2003;81:1–44. doi: 10.1016/S0065-2776(03)81001-5. [DOI] [PubMed] [Google Scholar]
- 83.Sun YX, et al. CD26/dipeptidyl peptidase IV regulates prostate cancer metastasis by degrading SDF-1/CXCL12. Clin Exp Metastasis. 2008;25(7):765–776. doi: 10.1007/s10585-008-9188-9. [DOI] [PubMed] [Google Scholar]
- 84.Nausch I, et al. The degradation of bioactive peptides and proteins by dipeptidyl peptidase IV from human placenta. Biol Chem Hoppe Seyler. 1990;371(11):1113–1118. doi: 10.1515/bchm3.1990.371.2.1113. [DOI] [PubMed] [Google Scholar]
- 85.Kahne T, et al. Dipeptidyl peptidase IV: a cell surface peptidase involved in regulating T cell growth. Int J Mol Med. 1999;4(1):3–15. doi: 10.3892/ijmm.4.1.3. [DOI] [PubMed] [Google Scholar]
- 86.Banbula A, et al. Emerging family of proline-specific peptidases of Porphyromonas gingivalis: purification and characterization of serine dipeptidyl peptidase, a structural and functional homologue of mammalian prolyl dipeptidyl peptidase IV. Infect Immun. 2000;68(3):1176–1182. doi: 10.1128/IAI.68.3.1176-1182.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Reinhold D, et al. Dual inhibition of dipeptidyl peptidase IV and aminopeptidase N suppresses inflammatory immune responses. Ann N Y Acad Sci. 2007;1110:402–409. doi: 10.1196/annals.1423.042. [DOI] [PubMed] [Google Scholar]
- 88.Reinhold D, et al. DP IV/CD26, APN/CD13 and related enzymes as regulators of T cell immunity: implications for experimental encephalomyelitis and multiple sclerosis. Front Biosci. 2008;13:2356–2363. doi: 10.2741/2849. [DOI] [PubMed] [Google Scholar]
- 89.Wolf M, Albrecht S, Märki C. Proteolytic processing of chemokines: implications in physiological and pathological conditions. Int J Biochem Cell Biol. 2008;40(6–7):1185–1198. doi: 10.1016/j.biocel.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 90.Weihofen WA, et al. Crystal structure of CD26/dipeptidyl-peptidase IV in complex with adenosine deaminase reveals a highly amphiphilic interface. J Biol Chem. 2004;279(41):43330–43335. doi: 10.1074/jbc.M405001200. [DOI] [PubMed] [Google Scholar]
- 91.Aertgeerts K, et al. N-linked glycosylation of dipeptidyl peptidase IV (CD26): effects on enzyme activity, homodimer formation, and adenosine deaminase binding. Protein Sci. 2004;13(1):145–154. doi: 10.1110/ps.03352504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cuchacovich M, et al. Characterization of human serum dipeptidyl peptidase IV (CD26) and analysis of its autoantibodies in patients with rheumatoid arthritis and other autoimmune diseases. Clin Exp Rheumatol. 2001;19(6):673–680. [PubMed] [Google Scholar]
- 93.Christopherson KW, II, et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science. 2004;305(5686):1000–1003. doi: 10.1126/science.1097071. [DOI] [PubMed] [Google Scholar]
- 94.Thielitz A, et al. The ectopeptidases dipeptidyl peptidase IV (DP IV) and aminopeptidase N (APN) and their related enzymes as possible targets in the treatment of skin diseases. Front Biosci. 2008;13:2364–2375. doi: 10.2741/2850. [DOI] [PubMed] [Google Scholar]
- 95.Narra K, et al. Phase II trial of single agent Val-boroPro (Talabostat) inhibiting Fibroblast Activation Protein in patients with metastatic colorectal cancer. Cancer Biol Ther. 2007;6(11):1691–1699. doi: 10.4161/cbt.6.11.4874. [DOI] [PubMed] [Google Scholar]
- 96.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 97.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 98.Baggio LL, Drucker DJ. Therapeutic approaches to preserve islet mass in type 2 diabetes. Annu Rev Med. 2006;57:265–281. doi: 10.1146/annurev.med.57.110104.115624. [DOI] [PubMed] [Google Scholar]
- 99.Dinjens WN, et al. Distribution of adenosine deaminase-complexing protein in murine tissues. J Biol Chem. 1989;264(32):19215–19220. [PubMed] [Google Scholar]
- 100.Deacon CF, et al. Both subcutaneously and intravenously administered glucagon-like peptide 1 are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995;44(9):1126–1131. doi: 10.2337/diabetes.44.9.1126. [DOI] [PubMed] [Google Scholar]
- 101.Lankas GR, et al. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005;54(10):2988–2994. doi: 10.2337/diabetes.54.10.2988. [DOI] [PubMed] [Google Scholar]
- 102.Chyan YJ, Chuang LM. Dipeptidyl peptidase IV inhibitors: an evolving treatment for type 2 diabetes from the incretin concept. Recent patents on endocrine. Metab Immune Drug Discov. 2007;1:15–24. [Google Scholar]
- 103.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298(2):194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 104.Grouzmann E, et al. Adverse effects of incretin therapy for type 2 diabetes. JAMA. 2007;298(15):1759–1760. doi: 10.1001/jama.298.15.1759-b. [DOI] [PubMed] [Google Scholar]
- 105.Trotta PP, Balis ME. Characterization of adenosine deaminase from normal coton and colon tumors. Evidence for tumor specific variants. Biochemistry. 1978;77(2):270–277. doi: 10.1021/bi00595a013. [DOI] [PubMed] [Google Scholar]
- 106.Ten Kate J, et al. Quantitative changes in adenosine deaminase isoenzymes in human colorectal adenocarcinomas. Cancer Res. 1984;44(10):4688–4692. [PubMed] [Google Scholar]
- 107.Ten Kate J, et al. Immunohistochemical localization of adenosine deaminase complexing protein in intestinal mucosa and in colorectal adenocarcinoma as a marker for tumour cell heterogeneity. Histochem J. 1985;17(1):23–31. doi: 10.1007/BF01003400. [DOI] [PubMed] [Google Scholar]
- 108.Ten Kate J, et al. Adenosine deaminase complexing protein (ADCP) immunoreactivity in colorectal adenocarcinoma. Int J Cancer. 1986;37(4):479–485. doi: 10.1002/ijc.2910370402. [DOI] [PubMed] [Google Scholar]
- 109.Ten Kate J, et al. Adenosine deaminase complexing protein in cancer studies. Anticancer Res. 1986;6(5):983–988. [PubMed] [Google Scholar]
- 110.Dinjens WN, et al. Adenosine deaminase complexing protein (ADCP) expression and metastatic potential in prostatic adenocarcinomas. J Pathol. 1990;160(3):195–201. doi: 10.1002/path.1711600303. [DOI] [PubMed] [Google Scholar]
- 111.Martín M, et al. Expression of ecto-adenosine deaminase and CD26 in human T cells triggered by the TCR-CD3 complex. Possible role of adenosine deaminase as costimulatory molecule. J Immunol. 1995;155(10):4630–4643. [PubMed] [Google Scholar]
- 112.Cordero OJ, et al. Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. J Leukoc Biol. 2001;70(6):920–930. [PubMed] [Google Scholar]
- 113.Herrera C, et al. Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol Pharmacol. 2001;59(1):127–134. [PubMed] [Google Scholar]
- 114.Hashikawa T, et al. Regulation of adenosine receptor engagement by ecto-adenosine deaminase. FASEB J. 2004;18(1):131–133. doi: 10.1096/fj.03-0011fje. [DOI] [PubMed] [Google Scholar]
- 115.Ginés S, et al. Regulation of epithelial and lymphocyte cell adhesion by adenosine deaminase-CD26 interaction. Biochem J. 2002;361(2):203–209. doi: 10.1042/0264-6021:3610203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iwata S, Morimoto C. CD26/dipeptidyl peptidase IV in context. The different roles of a multifunctional ectoenzyme in malignant transformation. J Exp Med. 1999;190(3):301–306. doi: 10.1084/jem.190.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26(2):273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 118.Hoskin DW, et al. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells. Int J Oncol. 2008;32(3):527–535. [PubMed] [Google Scholar]
- 119.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27(4):211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 120.Gonzalez-Gronow M, et al. Dipeptidyl peptidase IV (DPP IV/CD26) is a cell-surface plasminogen receptor. Front Biosci. 2008;13:1610–1618. doi: 10.2741/2785. [DOI] [PubMed] [Google Scholar]
- 121.Havre PA, et al. The role of CD26/dipeptidyl peptidase IV in cancer. Front Biosci. 2008;13:1634–1645. doi: 10.2741/2787. [DOI] [PubMed] [Google Scholar]
- 122.Sedo A, Krepela E, Kasafírek E. Dipeptidyl peptidase IV, prolyl endopeptidase and cathepsin B activities in primary human lung tumors and lung parenchyma. J Cancer Res Clin Oncol. 1991;117(3):249–253. doi: 10.1007/BF01625433. [DOI] [PubMed] [Google Scholar]
- 123.Asada Y, et al. Expression of dipeptidyl aminopeptidase IV activity in human lung carcinoma. Histopathology. 1993;23(3):265–270. doi: 10.1111/j.1365-2559.1993.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 124.Krepela E, et al. Lysosomal dipeptidyl-peptidases I and II in human squamous cell lung carcinoma and lung parenchyma. Neoplasma. 1996;43(3):171–178. [PubMed] [Google Scholar]
- 125.Park JE, et al. Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem. 1999;274(51):36505–36512. doi: 10.1074/jbc.274.51.36505. [DOI] [PubMed] [Google Scholar]
- 126.Davoodi J, et al. The Simpson-Golabi-Behmel syndrome causative glypican-3, binds to and inhibits the dipeptidyl peptidase activity of CD26. Proteomics. 2007;7(13):2300–2310. doi: 10.1002/pmic.200600654. [DOI] [PubMed] [Google Scholar]
- 127.Baumhoer D, et al. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129(6):899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 128.Cheng W, et al. Glypican-3-mediated oncogenesis involves the insulin-like growth factor-signaling pathway. Carcinogenesis. 2008;29(7):1319–1326. doi: 10.1093/carcin/bgn091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jakubovic BD, Jothy S. Glypican-3: from the mutations of Simpson-Golabi-Behmel genetic syndrome to a tumor marker for hepatocellular carcinoma. Exp Mol Pathol. 2007;82(2):184–189. doi: 10.1016/j.yexmp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 130.O’Brien P, O’Connor BF. Seprase: an overview of an important matrix serine protease. Biochim Biophys Acta. 2008;1784(9):1130–1145. doi: 10.1016/j.bbapap.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 131.Ostermann E, et al. Effective immunoconjugate therapy in cancer models targeting a serine protease of tumor fibroblasts. Clin Cancer Res. 2008;14(14):4584–4592. doi: 10.1158/1078-0432.CCR-07-5211. [DOI] [PubMed] [Google Scholar]
- 132.Huber MA, et al. Fibroblast activation protein: differential expression and serine protease activity in reactive stromal fibroblasts of melanocytic skin tumors. J Invest Dermatol. 2003;120(2):182–188. doi: 10.1046/j.1523-1747.2003.12035.x. [DOI] [PubMed] [Google Scholar]
- 133.Goscinski MA, et al. Seprase, dipeptidyl peptidase IV and Urokinase-type plasminogen activator expression in dysplasia and invasive squamous cell carcinoma of the esophagus. A study of 229 cases from Anyang Tumor Hospital, Henan Province, China. Oncology. 2008;75(1–2):49–59. doi: 10.1159/000151741. [DOI] [PubMed] [Google Scholar]
- 134.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91(4):439–442. doi: 10.1016/S0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 135.Piazza GA, et al. Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J. 1989;262(1):327–334. doi: 10.1042/bj2620327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Scanlan MJ, et al. Molecular cloning of fibroblast activation protein alpha, a member of the serine protease family selectively expressed in stromal fibroblasts of epithelial cancers. Proc Natl Acad Sci USA. 1994;91(12):5657–5661. doi: 10.1073/pnas.91.12.5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1(6):607–611. doi: 10.1016/j.stem.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ruiz P, et al. CD26 expression and dipeptidyl peptidase IV activity in an aggressive hepatosplenic T-cell lymphoma. Cytometry. 1998;34(1):30–35. doi: 10.1002/(SICI)1097-0320(19980215)34:1<30::AID-CYTO5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 139.Dang NH, et al. T-large granular lymphocyte lymphoproliferative disorder: expression of CD26 as a marker of clinically aggressive disease and characterization of marrow inhibition. Br J Haematol. 2003;121(5):857–865. doi: 10.1046/j.1365-2141.2003.04365.x. [DOI] [PubMed] [Google Scholar]
- 140.Carbone A, et al. The expression of CD26 and CD40 ligand is mutually exclusive in human T-cell non-Hodgkins-lymphomas leukemias. Blood. 1995;86(12):4617–4626. [PubMed] [Google Scholar]
- 141.Bauvois B, et al. Constitutive expression of CD26/dipeptidylpeptidase IV on peripheral blood B lymphocytes of patients with B chronic lymphocytic leukaemia. Br J Cancer. 1999;79(7–8):1042–1048. doi: 10.1038/sj.bjc.6690167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hirai K, et al. Dipeptidyl peptidase IV (DPP IV/CD26) staining predicts distant metastasis of ‘benign’ thyroid tumor. Pathol Int. 1999;49(3):264–265. doi: 10.1046/j.1440-1827.1999.00859.x. [DOI] [PubMed] [Google Scholar]
- 143.de Micco C, et al. Utility of malignancy markers in fine-needle aspiration cytology of thyroid nodules: comparison of Hector Battifora mesothelial antigen-1, thyroid peroxidase and dipeptidyl aminopeptidase IV. Br J Cancer. 2008;98(4):818–823. doi: 10.1038/sj.bjc.6604194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stremenova J, et al. Expression and enzymatic activity of dipeptidyl peptidase-IV in human astrocytic tumours are associated with tumour grade. Int J Oncol. 2007;31(4):785–792. [PubMed] [Google Scholar]
- 145.Yamaguchi U, et al. Distinct gene expression-defined classes of gastrointestinal stromal tumor. J Clin Oncol. 2008;26(25):4100–4108. doi: 10.1200/JCO.2007.14.2331. [DOI] [PubMed] [Google Scholar]
- 146.Cro L et al (2009) CD26 expression in mature B-cell neoplasia: its possible role as a new prognostic marker in B-CLL. Hematol Oncol Feb 26 on-line [DOI] [PubMed]
- 147.Carlucci F et al (2009) A 57-gene expression signature in B-cell chronic lymphocytic leukemia. Biomed Pharmacother. doi:10.1016/j.biopha.2009.02.001 [DOI] [PubMed]
- 148.Wilson MJ, et al. Elevation of dipeptidylpeptidase IV activities in the prostate peripheral zone and prostatic secretions of men with prostate cancer: possible prostate cancer disease marker. J Urol. 2005;74(3):1124–1128. doi: 10.1097/01.ju.0000168621.84017.5c. [DOI] [PubMed] [Google Scholar]
- 149.Sedo A, Krepela E, Kasafirek E. Dipeptidyl peptidase IV, prolyl endopeptidase and cathepsin B activities in primary human lung tumors and lung parenchyma. J Cancer Res Clin Oncol. 1991;117(3):249–253. doi: 10.1007/BF01625433. [DOI] [PubMed] [Google Scholar]
- 150.Kojima J, et al. Glycylproline dipeptidyl aminopeptidase and gamma-glutamyl-transferase transpeptidase in human hepatic cancer and embryonal tissues. Clin Chim Acta. 1987;167(3):285–291. doi: 10.1016/0009-8981(87)90348-2. [DOI] [PubMed] [Google Scholar]
- 151.Moehrle MC, et al. Aminopeptidase-M and dipeptidyl peptidase-IV activity in epithelial skin tumors—a histochemical-study. J Cutan Pathol. 1995;22(3):241–247. doi: 10.1111/j.1600-0560.1995.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 152.Roesch A, et al. Loss of dipeptidyl peptidase IV immunostaining discriminates malignant melanomas from deep penetrating nevi. Mod Pathol. 2006;19(10):1378–1385. doi: 10.1038/modpathol.3800663. [DOI] [PubMed] [Google Scholar]
- 153.Inamoto T, et al. Humanized anti-CD26 monoclonal antibody as a treatment for malignant mesothelioma tumors. Clin Cancer Res. 2007;13(14):4191–4200. doi: 10.1158/1078-0432.CCR-07-0110. [DOI] [PubMed] [Google Scholar]
- 154.Inamoto T, et al. Anti-CD26 monoclonal antibody-mediated G1-S arrest of human renal clear cell carcinoma Caki-2 is associated with retinoblastoma substrate dephosphorylation, cyclin-dependent kinase 2 reduction, p27(kip1) enhancement, and disruption of binding to the extracellular matrix. Clin Cancer Res. 2006;12(11 Pt1):3470–3477. doi: 10.1158/1078-0432.CCR-06-0361. [DOI] [PubMed] [Google Scholar]
- 155.Verstovsek S, Cabanillas F, Dang NH. CD26 in T-cell lymphomas: a potential clinical role? Oncology (Huntingt) 2000;14(6 Suppl 2):17–23. [PubMed] [Google Scholar]
- 156.Jones D, et al. Absence of CD26 expression is a useful marker for diagnosis of T-cell lymphoma in peripheral blood. Am J Clin Pathol. 2001;115(6):885–892. doi: 10.1309/U1Y6-J4AG-5M4M-7AYV. [DOI] [PubMed] [Google Scholar]
- 157.Bernengo MG, et al. The relevance of the CD4+ CD26− subset in the identification of circulating Sézary cells. Br J Dermatol. 2001;144(1):125–135. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- 158.Kondo S, et al. Expression of CD26/dipeptidyl peptidase IV in adult T cell leukemia/lymphoma (ATLL) Leuk Res. 1996;20(4):357–363. doi: 10.1016/0145-2126(95)00159-X. [DOI] [PubMed] [Google Scholar]
- 159.Van den Oord JJ. Expression of CD26/dipeptidyl-peptidase IV in benign and malignant pigment-cell lesions of the skin. Br J Dermatol. 1998;138(4):615–621. doi: 10.1046/j.1365-2133.1998.02171.x. [DOI] [PubMed] [Google Scholar]
- 160.Khin EE, et al. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am J Obstet Gynecol. 2003;188(3):670–676. doi: 10.1067/mob.2003.169. [DOI] [PubMed] [Google Scholar]
- 161.Kajiyama H, et al. Expression of CD26/dipeptidyl peptidase IV in endometrial adenocarcinoma and its negative correlation with tumor grade. Adv Exp Med Biol. 2003;524:245–248. doi: 10.1007/0-306-47920-6_29. [DOI] [PubMed] [Google Scholar]
- 162.Bogenrieder T, et al. Expression and localization of aminopeptidase A, aminopeptidase N, and dipeptidyl peptidase IV in benign and malignant human prostate tissue. Prostate. 1997;33(4):225–232. doi: 10.1002/(SICI)1097-0045(19971201)33:4<225::AID-PROS1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 163.Houghton AN, et al. Cell surface antigens of human melanocytes and melanoma. Expression of adenosine deaminase binding protein is extinguished with melanocyte transformation. J Exp Med. 1988;167(1):197–212. doi: 10.1084/jem.167.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Morrison ME, et al. A marker for neoplastic progression of human melanocytes is a cell surface ectopeptidase. J Exp Med. 1993;177(4):1135–1143. doi: 10.1084/jem.177.4.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Albino AP, et al. Malignant transformation of human melanocytes: induction of a complete melanoma phenotype and genotype. Oncogene. 1992;7(11):2315–2321. [PubMed] [Google Scholar]
- 166.Wesley UV, et al. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J Exp Med. 1999;190(3):311–322. doi: 10.1084/jem.190.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wesley UV, et al. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004;109(6):855–866. doi: 10.1002/ijc.20091. [DOI] [PubMed] [Google Scholar]
- 168.Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65(4):1325–1334. doi: 10.1158/0008-5472.CAN-04-1852. [DOI] [PubMed] [Google Scholar]
- 169.Kajiyama H, et al. Prolonged survival and decreased invasive activity attributable to dipeptidyl peptidase IV overexpression in ovarian carcinoma. Cancer Res. 2002;62(10):2753–2757. [PubMed] [Google Scholar]
- 170.Kikkawa F, et al. Dipeptidyl peptidase IV in tumor progression. Biochim Biophys Acta. 2005;1751(1):45–51. doi: 10.1016/j.bbapap.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 171.Busek P, Stremenova J, Sedo A. Dipeptidyl peptidase-IV enzymatic activity bearing molecules in human brain tumors-good or evil? Front Biosci. 2008;13:2319–2326. doi: 10.2741/2846. [DOI] [PubMed] [Google Scholar]
- 172.Preller V, et al. TGF-beta1-mediated control of central nervous system inflammation and autoimmunity through the inhibitory receptor CD26. J Immunol. 2007;178(7):4632–4640. doi: 10.4049/jimmunol.178.7.4632. [DOI] [PubMed] [Google Scholar]
- 173.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Steinbrecher A, et al. Targeting dipeptidyl peptidase IV (CD26) suppresses autoimmune encephalomyelitis and up-regulates TGF-beta 1 secretion in vivo. J Immunol. 2001;166(3):2041–2048. doi: 10.4049/jimmunol.166.3.2041. [DOI] [PubMed] [Google Scholar]
- 176.Shingu K, et al. CD26 expression determines lung metastasis in mutant F344 rats: involvement of NK cell function and soluble CD26. Cancer Immunol Immunother. 2003;52(9):546–554. doi: 10.1007/s00262-003-0392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nagatsu I, Nagatsu T, Yamamoto T. Hydrolysis of amino acid beta-naphthylamides by aminopeptidases in human parotid salva and human serum. Experientia. 1968;24(4):347–348. doi: 10.1007/BF02140813. [DOI] [PubMed] [Google Scholar]
- 178.Smith RE, et al. The significance of hypersialylation of dipeptidyl peptidase IV (CD26) in the inhibition of its activity by Tat and other cationic peptides. CD26: a subvertid adhesion molecule for HIV peptide binding. AIDS Res Hum Retroviruses. 1998;14(10):851–868. doi: 10.1089/aid.1998.14.851. [DOI] [PubMed] [Google Scholar]
- 179.Blanc G, et al. Insights into how CUB domains can exert specific functions while sharing a common fold: conserved and specific features of the CUB1 domain contribute to the molecular basis of procollagen C-proteinase enhancer-1 activity. J Biol Chem. 2007;282(23):16924–16933. doi: 10.1074/jbc.M701610200. [DOI] [PubMed] [Google Scholar]
- 180.Wermter C, et al. The protease domain of procollagen C-proteinase (BMP1) lacks substrate selectivity, which is conferred by non-proteolytic domains. Biol Chem. 2007;388(5):513–521. doi: 10.1515/BC.2007.054. [DOI] [PubMed] [Google Scholar]
- 181.Gunn TM, et al. The mouse mahogany locus encodes a transmembrane form of human attractin. Nature. 1999;398(6723):152–156. doi: 10.1038/18217. [DOI] [PubMed] [Google Scholar]
- 182.Duke-Cohan JS, et al. A novel form of dipeptidylpeptidase IV found in human serum. Isolation, characterization, and comparison with T lymphocyte membrane dipeptidylpeptidase IV (CD26) J Biol Chem. 1995;270(23):14107–14114. doi: 10.1074/jbc.270.23.14107. [DOI] [PubMed] [Google Scholar]
- 183.Duke-Cohan JS, et al. Serum high molecular weight dipeptidyl peptidase IV (CD26) is similar to a novel antigen DPPT-L released from activated T cells. J Immunol. 1996;156(5):1714–1721. [PubMed] [Google Scholar]
- 184.Friedrich D, et al. Does human attractin have DP4 activity? Biol Chem. 2007;388(2):155–162. doi: 10.1515/BC.2007.017. [DOI] [PubMed] [Google Scholar]
- 185.Tang W, et al. Secreted and membrane attractin result from alternative splicing of the human ATRN gene. Proc Natl Acad Sci USA. 2000;97(11):6025–6030. doi: 10.1073/pnas.110139897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Schrader WP, Woodward FJ, Pollara B. Purification of an adenosine deaminase complexing protein from human plasma. J Biol Chem. 1979;254(23):11964–11968. [PubMed] [Google Scholar]
- 187.Iwaki-Egawa S, et al. Dipeptidyl peptidase IV from human serum: purification, characterization, and N-terminal amino acid sequence. J Biochem (Tokyo) 1998;124(2):428–433. doi: 10.1093/oxfordjournals.jbchem.a022130. [DOI] [PubMed] [Google Scholar]
- 188.Durinx C, et al. Molecular characterization of dipeptidyl peptidase activity in serum. Soluble CD26/dipeptidyl peptidase IV is responsible for the release of X-Pro dipeptides. Eur J Biochem. 2000;267(17):5608–5613. doi: 10.1046/j.1432-1327.2000.01634.x. [DOI] [PubMed] [Google Scholar]
- 189.Watanabe Y, et al. Aminopeptidase N in sera of healthy subjects is a different N-terminal processed derivative from the one obtained from maternal serum. Mol Genet Metab. 1998;63(4):289–294. doi: 10.1006/mgme.1998.2676. [DOI] [PubMed] [Google Scholar]
- 190.Watanabe Y, et al. Identification of an alanine aminopeptidase in human maternal serum as a membrane-bound aminopeptidase N. Biol Chem Hoppe Seyler. 1995;376(7):397–400. doi: 10.1515/bchm3.1995.376.7.397. [DOI] [PubMed] [Google Scholar]
- 191.Hooper NM, Karran EH, Turner AJ. Membrane protein secretases. Biochem J. 1997;321(2):265–279. doi: 10.1042/bj3210265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bauvois B, et al. Regulation of CD26/DPPIV gene expression by interferons and retinoic acid in tumor B cells. Oncogene. 2000;19(2):265–272. doi: 10.1038/sj.onc.1203292. [DOI] [PubMed] [Google Scholar]
- 193.Schade J, et al. Regulation of expression and function of dipeptidyl peptidase 4 (DP4), DP8/9, and DP10 in allergic responses of the lung in rats. J Histochem Cytochem. 2008;56(2):147–155. doi: 10.1369/jhc.7A7319.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Danielsen EM, Cowell GM, Poulsen SS. Biosynthesis of intestinal microvillar proteins. Role of the Golgi complex and microtubules. Biochem J. 1983;216(1):37–42. doi: 10.1042/bj2160037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Arribas J, et al. Diverse cell surface protein ectodomains are shed by a system sensitive to metalloprotease inhibitors. J Biol Chem. 1996;271(19):11376–11382. doi: 10.1074/jbc.271.19.11376. [DOI] [PubMed] [Google Scholar]
- 196.Lemberg MK, Freeman M. Cutting proteins within lipid bilayers: rhomboid structure and mechanism. Mol Cell. 2007;28(6):930–940. doi: 10.1016/j.molcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 197.Grondin G, Hooper NM, LeBel D. Specific localization of membrane dipeptidase and dipeptidyl peptidase IV in secretion granules of two different pancreatic islet cells. J Histochem Cytochem. 1999;47(4):489–498. doi: 10.1177/002215549904700407. [DOI] [PubMed] [Google Scholar]
- 198.Poulsen MD, et al. Dipeptidyl peptidase IV is sorted to the secretory granules in pancreatic islet A-cells. J Histochem Cytochem. 1993;41(1):81–88. doi: 10.1177/41.1.8093256. [DOI] [PubMed] [Google Scholar]
- 199.Macnair DC, Kenny AJ. Proteins of the kidney microvillar membrane. The amphipathic form of dipeptidyl peptidase IV. Biochem J. 1979;179(2):379–395. doi: 10.1042/bj1790379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Böhm SK, et al. Human dipeptidyl peptidase IV gene promoter: tissue-specific regulation from a TATA-less GC-rich sequence characteristic of a housekeeping gene promoter. Biochem J. 1995;311(3):835–843. doi: 10.1042/bj3110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Teague TK, et al. Activation changes the spectrum but not the diversity of genes expressed by T cells. Proc Natl Acad Sci USA. 1999;96(22):12691–12696. doi: 10.1073/pnas.96.22.12691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Rogge L, et al. Transcript imaging of the development of human T helper cells using oligonucleotide arrays. Nat Genet. 2000;25(1):96–101. doi: 10.1038/75671. [DOI] [PubMed] [Google Scholar]
- 203.Mattern T, et al. Antibody-induced modulation of CD26 surface expression. Immunology. 1995;84(4):60–595. [PMC free article] [PubMed] [Google Scholar]
- 204.Salgado FJ, et al. Mechanisms of CD26/dipeptidyl peptidase IV cytokine-dependent regulation on human activated lymphocytes. Cytokine. 2000;12(7):1136–1141. doi: 10.1006/cyto.1999.0643. [DOI] [PubMed] [Google Scholar]
- 205.Beau I, Berger A, Servin AL. Rotavirus impairs the biosynthesis of brush-border-associated dipeptidyl peptidase IV in human enterocyte-like Caco-2/TC7 cells. Cell Microbiol. 2007;9(3):779–789. doi: 10.1111/j.1462-5822.2006.00827.x. [DOI] [PubMed] [Google Scholar]
- 206.Hama T, et al. Purification of dipeptidyl-aminopeptidase IV from human kidney by anti dipeptidyl-aminopeptidase IV affinity chromatography. Mol Cell Biochem. 1982;43(1):35–42. doi: 10.1007/BF00229537. [DOI] [PubMed] [Google Scholar]
- 207.Krepela E, et al. Demonstration of two molecular forms of dipeptidyl peptidase IV in normal human serum. Physiol Bohemoslov. 1983;32(6):486–496. [PubMed] [Google Scholar]
- 208.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 209.Mignot G, et al. Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med. 2006;10(2):376–388. doi: 10.1111/j.1582-4934.2006.tb00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9(6):871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gatti JL, et al. Identification, proteomic profiling, and origin of ram epididymal fluid exosome-like vesicles. Biol Reprod. 2005;72(6):1452–1465. doi: 10.1095/biolreprod.104.036426. [DOI] [PubMed] [Google Scholar]
- 212.Mallegol J, van Niel G, Heyman M. Phenotypic and functional characterization of intestinal epithelial exosomes. Blood Cells Mol Dis. 2005;35(1):11–16. doi: 10.1016/j.bcmd.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 213.Ogawa Y, et al. Exosome-like vesicles with dipeptidyl peptidase IV in human saliva. Biol Pharm Bull. 2008;31(6):1059–1062. doi: 10.1248/bpb.31.1059. [DOI] [PubMed] [Google Scholar]
- 214.Busso N, et al. Circulating CD26 is negatively associated with inflammation in human and experimental arthritis. Am J Pathol. 2005;166(2):433–442. doi: 10.1016/S0002-9440(10)62266-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Narducci MG, et al. Skin homing of Sezary cells involves SDF-1-CXCR4 signaling and down-regulation of CD26/dipeptidylpeptidase IV. Blood. 2006;107(3):1108–1115. doi: 10.1182/blood-2005-04-1492. [DOI] [PubMed] [Google Scholar]
- 216.Iwaki-Egawa S, Watanabe Y, Fujimoto Y. Is CD26/dipeptidyl peptidase IV a really important molecule in T cell activation of a certain rat strain? Immunobiology. 1995;194(4–5):429–442. doi: 10.1016/S0171-2985(11)80109-9. [DOI] [PubMed] [Google Scholar]
- 217.Marguet D, et al. Enhanced insulin secretion and improved glucose tolerance in mice lacking CD26. Proc Natl Acad Sci USA. 2000;97(12):6874–6879. doi: 10.1073/pnas.120069197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yan S, et al. Deficiency of CD26 results in a change of cytokine and immunoglobulin secretion after stimulation by pokeweed mitogen. Eur J Immunol. 2003;33(6):1519–1527. doi: 10.1002/eji.200323469. [DOI] [PubMed] [Google Scholar]
- 219.Nagatsu T, Sakai T, Kojima K. A sensitive and specific assay for dipeptidyl-aminopeptidase II in serum and tissues by liquid chromatography-fluorometry. Anal Biochem. 1985;147(1):80–85. doi: 10.1016/0003-2697(85)90011-9. [DOI] [PubMed] [Google Scholar]
- 220.Kojima K, Mihara R, Sakai T. Serum activities of dipeptidyl-aminopeptidase II and dipeptidyl-aminopeptidase IV in tumor-bearing animals and in cancer patients. Biochem Med Metab Biol. 1987;37(1):35–41. doi: 10.1016/0885-4505(87)90007-7. [DOI] [PubMed] [Google Scholar]
- 221.Maes MB, Scharpé S, De Meester I. Dipeptidyl peptidase II (DPPII), a review. Clin Chim Acta. 2007;380(1–2):31–49. doi: 10.1016/j.cca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 222.Rosenblum JS, Kozarich JW. Prolyl peptidases: a serine protease subfamily with high potential for drug discovery. Curr Opin Chem Biol. 2003;7(4):496–504. doi: 10.1016/S1367-5931(03)00084-X. [DOI] [PubMed] [Google Scholar]
- 223.Maes MB, et al. Kinetic investigation of human dipeptidyl peptidase II (DPPII)-mediated hydrolysis of dipeptide derivatives and its identification as quiescent cell proline dipeptidase (QPP)/dipeptidyl peptidase 7 (DPP7) Biochem J. 2005;386(2):315–324. doi: 10.1042/BJ20041156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Araki H, et al. Purification, molecular cloning, and immunohistochemical localization of dipeptidyl peptidase II from the rat kidney and its identity with quiescent cell proline dipeptidase. J Biochem. 2001;129(2):279–288. doi: 10.1093/oxfordjournals.jbchem.a002855. [DOI] [PubMed] [Google Scholar]
- 225.Underwood R, et al. Sequence, purification, and cloning of an intracellular serine protease, quiescent cell proline dipeptidase. J Biol Chem. 1999;274(48):34053–34058. doi: 10.1074/jbc.274.48.34053. [DOI] [PubMed] [Google Scholar]
- 226.Chiravuri M, et al. Vesicular localization and characterization of a novel post-proline-cleaving aminodipeptidase, quiescent cell proline dipeptidase. J Immunol. 2000;165(10):5695–5702. doi: 10.4049/jimmunol.165.10.5695. [DOI] [PubMed] [Google Scholar]
- 227.Chiravuri M, et al. Homodimerization via a leucine zipper motif is required for enzymatic activity of quiescent cell proline dipeptidase. J Biol Chem. 2000;275(35):26994–26999. doi: 10.1074/jbc.M005445200. [DOI] [PubMed] [Google Scholar]
- 228.Chiravuri M, Huber BT. Aminodipeptidase inhibitor-induced cell death in quiescent lymphocytes: a review. Apoptosis. 2000;5(4):319–322. doi: 10.1023/A:1009675223443. [DOI] [PubMed] [Google Scholar]
- 229.Maes MB, et al. Dipeptidyl peptidase II and leukocyte cell death. Biochem Pharmacol. 2006;72(1):70–79. doi: 10.1016/j.bcp.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 230.Struckhoff G, Heymann E. Rat peritoneal mast cells release dipeptidyl peptidase II. Biochem J. 1986;236(1):215–219. doi: 10.1042/bj2360215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Klener P, et al. Possible prognostic significance of the assessment of dipeptidylpeptidase II in peripheral blood lymphocytes of patients with chronic lymphocytic leukemia. Neoplasma. 1987;34(5):581–586. [PubMed] [Google Scholar]
- 232.Danilov AV, et al. Differential control of G0 programme in chronic lymphocytic leukaemia: a novel prognostic factor. Br J Haematol. 2005;128(4):472–481. doi: 10.1111/j.1365-2141.2004.05346.x. [DOI] [PubMed] [Google Scholar]
- 233.Urade M, et al. Serum dipeptidyl peptidase activities as a possible marker of oral cancer. Cancer. 1989;64(6):1274–1280. doi: 10.1002/1097-0142(19890915)64:6<1274::AID-CNCR2820640618>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 234.Mogi M, et al. Sandwich enzyme-immunoassay for dipeptidyl aminopeptidase IV in the serum of people with oral cancer. Arch Oral Biol. 1986;31(7):505–507. doi: 10.1016/0003-9969(86)90028-2. [DOI] [PubMed] [Google Scholar]
- 235.Lee KN, et al. A novel plasma proteinase potentiates alpha2-antiplasmin inhibition of fibrin digestion. Blood. 2004;103(10):3783–3788. doi: 10.1182/blood-2003-12-4240. [DOI] [PubMed] [Google Scholar]
- 236.Lee KN, et al. Antiplasmin-cleaving enzyme is a soluble form of fibroblast activation protein. Blood. 2006;107(4):1397–1404. doi: 10.1182/blood-2005-08-3452. [DOI] [PubMed] [Google Scholar]
- 237.Christiansen VJ, et al. The effect of a single nucleotide polymorphism on human alpha 2-antiplasmin activity. Blood. 2007;109(12):5286–5292. doi: 10.1182/blood-2007-01-065185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Collins PJ, et al. Purification, identification and characterisation of seprase from bovine serum. Int J Biochem Cell Biol. 2004;36(11):2320–2333. doi: 10.1016/j.biocel.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 239.Aimes RT, et al. Endothelial cell serine proteases expressed during vascular morphogenesis and angiogenesis. Thromb Haemost. 2003;89(3):561–572. [PubMed] [Google Scholar]
- 240.Chen D, et al. Activation of EDTA-resistant gelatinases in malignant human tumors. Cancer Res. 2006;66(20):9977–9985. doi: 10.1158/0008-5472.CAN-06-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Cordero OJ, et al. Preoperative serum CD26 levels: diagnostic efficiency and predictive value for colorectal cancer. Br J Cancer. 2000;83(9):1139–1146. doi: 10.1054/bjoc.2000.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Ayude D, et al. Clinical interest of the combined use of serum CD26 and alpha-l-fucosidase in the early detection diagnosis of colorectal cancer. Dis Markers. 2004;19(6):267–272. doi: 10.1155/2004/834309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cordero OJ, et al. Validation of serum CD26 as a screening marker for colorectal cancer. Clin Chem Lab Med. 2008;46(4):A23. [Google Scholar]
- 244.Cordero OJ, et al. How the measurements of a few serum markers can be combined to enhance their clinical values in the management of cancer. Anticancer Res. 2008;28(4):2333–2341. [PubMed] [Google Scholar]
- 245.de la Haba-Rodríguez J, et al. Soluble dipeptidyl peptidase IV (CD-26) in serum of patients with colorectal carcinoma. Neoplasma. 2002;49(5):307–311. [PubMed] [Google Scholar]
- 246.Cordero OJ, et al. Interleukin 12, interleukin 15, soluble CD26 and adenosine deaminase levels in the sera of rheumatoid arthritis patients. Rheumatol Int. 2001;21(2):69–74. doi: 10.1007/s002960100134. [DOI] [PubMed] [Google Scholar]
- 247.Ellingsen T, et al. In active chronic rheumatoid arthritis, dipeptidyl peptidase IV density is increased on monocytes and CD4(+) T lymphocytes. Scand J Immunol. 2007;66(4):451–457. doi: 10.1111/j.1365-3083.2007.01966.x. [DOI] [PubMed] [Google Scholar]
- 248.Cuchacovich M, et al. Streptokinase promotes development of dipeptidyl peptidase IV (CD26) autoantibodies after fibrinolytic therapy in myocardial infarction patients. Clin Diagn Lab Immunol. 2002;9(6):1253–1259. doi: 10.1128/CDLI.9.6.1253-1259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Kobayashi H, et al. Reduction of serum soluble CD26/dipeptidyl peptidase IV enzyme activity and its correlation with disease activity in systemic lupus erythematosus. J Rheumatol. 2002;29(9):1858–1866. [PubMed] [Google Scholar]
- 250.Tamaki Z, et al. Serum levels of soluble CD26 in patients with scleroderma. J Dermatol Sci. 2008;52(1):67–69. doi: 10.1016/j.jdermsci.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 251.Lun SW, et al. Increased expression of plasma and CD4+ T lymphocyte costimulatory molecule CD26 in adult patients with allergic asthma. J Clin Immunol. 2007;27(4):430–437. doi: 10.1007/s10875-007-9093-z. [DOI] [PubMed] [Google Scholar]
- 252.Katoh N, et al. Soluble CD30 is more relevant to disease activity of atopic dermatitis than soluble CD26. Clin Exp Immunol. 2000;121(2):187–192. doi: 10.1046/j.1365-2249.2000.01286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Schönermarck U, et al. Circulating cytokines and soluble CD23, CD26 and CD30 in ANCA-associated vasculitides. Clin Exp Rheumatol. 2000;18(4):457–463. [PubMed] [Google Scholar]
- 254.Hosono O, et al. Decreased dipeptidyl peptidase IV enzyme activity of plasma soluble CD26 and its inverse correlation with HIV-1 RNA in HIV-1 infected individuals. Clin Immunol. 1999;91(3):283–295. doi: 10.1006/clim.1999.4711. [DOI] [PubMed] [Google Scholar]
- 255.Yang SS, et al. Changes of soluble CD26 and CD30 levels correlate with response to interferon plus ribavirin therapy in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21(12):1789–1793. doi: 10.1111/j.1440-1746.2006.04677.x. [DOI] [PubMed] [Google Scholar]
- 256.Itou M, et al. Altered expression of glucagon-like peptide-1 and dipeptidyl peptidase IV in patients with HCV-related glucose intolerance. J Gastroenterol Hepatol. 2008;23(2):244–251. doi: 10.1111/j.1440-1746.2007.05183.x. [DOI] [PubMed] [Google Scholar]
- 257.Ajdary S, et al. Soluble CD26/CD30 levels in visceral leishmaniasis: markers of disease activity. Clin Exp Immunol. 2006;145(1):44–47. doi: 10.1111/j.1365-2249.2006.03105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ajdary S, et al. Soluble CD26 and CD30 levels in patients with anthroponotic cutaneous leishmaniasis. J Infect. 2007;55(1):75–78. doi: 10.1016/j.jinf.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 259.Durinx C, et al. Reference values for plasma dipeptidylpeptidase IV activity and their association with other laboratory parameters. Clin Chem Lab Med. 2001;39(2):155–159. doi: 10.1515/CCLM.2001.026. [DOI] [PubMed] [Google Scholar]
- 260.Scharpé S, et al. Assay of dipeptidyl peptidase IV in serum by fluorometry of 4-methoxy-2-naphthylamine. Clin Chem. 1988;34(11):2299–2301. [PubMed] [Google Scholar]
- 261.Hino M, et al. Glycylprolyl beta-naphthylamidase activity in human serum. Clin Chim Acta. 1975;62(1):5–11. doi: 10.1016/0009-8981(75)90273-9. [DOI] [PubMed] [Google Scholar]
- 262.Fuyamada H, et al. Serum glycylproline p-nitroanilidase activity in human hypertension. Clin Chim Acta. 1977;74(2):177–181. doi: 10.1016/0009-8981(77)90220-0. [DOI] [PubMed] [Google Scholar]
- 263.Fujita K, et al. Serum glycylproline p-nitroanilidase activity in blood cancers. Clin Chim Acta. 1977;81(2):215–217. doi: 10.1016/0009-8981(77)90014-6. [DOI] [PubMed] [Google Scholar]
- 264.Kojima J, et al. Serum glycylproline dipeptidyl aminopeptidase activity in human hepatic cancer. Clin Chim Acta. 1979;93(2):181–187. doi: 10.1016/0009-8981(79)90086-X. [DOI] [PubMed] [Google Scholar]
- 265.Yoshii Y, et al. Changes in serum dipeptidyl-aminopeptidase IV (glycylprolyl dipeptidyl-aminopeptidase) activity of patients with gastric carcinoma after surgical excision and the enzyme activity in the carcinoma tissue. Biochem Med. 1981;25(3):276–282. doi: 10.1016/0006-2944(81)90085-5. [DOI] [PubMed] [Google Scholar]
- 266.Kojima K, et al. Serum activities of dipeptidyl-aminopeptidase II and dipeptidyl-aminopeptidase IV in tumor-bearing animals and in cancer patients. Biochem Med Metab Biol. 1987;37(1):35–41. doi: 10.1016/0885-4505(87)90007-7. [DOI] [PubMed] [Google Scholar]
- 267.Fukasawa K, et al. Serum dipeptidyl peptidase (DPP) IV activities in oral cancer patients. Int J Oral Surg. 1982;11(4):246–250. doi: 10.1016/S0300-9785(82)80075-6. [DOI] [PubMed] [Google Scholar]
- 268.Urade M, et al. Serum dipeptidyl peptidase activities as a possible marker of oral cancer. Cancer. 1989;64(6):1274–1280. doi: 10.1002/1097-0142(19890915)64:6<1274::AID-CNCR2820640618>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 269.Hagihara M, Ohhashi M, Nagatsu T. Activities of dipeptidyl peptidase II and dipeptidyl peptidase IV in mice with lupus erythematosus-like syndrome and in patients with lupus erythematosus and rheumatoid arthritis. Clin Chem. 1987;33(8):1463–1465. [PubMed] [Google Scholar]
- 270.Gotoh H, et al. Activity of dipeptidyl peptidase IV and post-proline cleaving enzyme in sera from osteoporotic patients. Clin Chem. 1988;34(12):2499–2501. [PubMed] [Google Scholar]
- 271.Stancíková M, et al. Dipeptidyl peptidase IV in patients with systemic lupus erythematosus. Clin Exp Rheumatol. 1992;10(4):381–385. [PubMed] [Google Scholar]
- 272.Bergmann A, Bohuon C. Decrease of serum dipeptidylpeptidase activity in severe sepsis patients: relationship to procalcitonin. Clin Chim Acta. 2002;321(1–2):123–126. doi: 10.1016/S0009-8981(02)00042-6. [DOI] [PubMed] [Google Scholar]
- 273.Perner F, et al. Dipeptidyl peptidase activity of CD26 in serum and urine as a marker of cholestasis: experimental and clinical evidence. J Lab Clin Med. 1999;134(1):56–67. doi: 10.1016/S0022-2143(99)90054-9. [DOI] [PubMed] [Google Scholar]
- 274.Andrieu T, et al. Similar increased serum dipeptidyl peptidase IV activity in chronic hepatitis C and other viral infections. J Clin Virol. 2003;27(1):59–68. doi: 10.1016/S1386-6532(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 275.Lakatos PL, et al. Elevated serum dipeptidyl peptidase IV (CD26, EC 3.4.14.5) activity in patients with primary biliary cirrhosis. J Hepatol. 1999;30(4):740. doi: 10.1016/S0168-8278(99)80211-6. [DOI] [PubMed] [Google Scholar]
- 276.Balaban YH, et al. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6(4):242–250. [PubMed] [Google Scholar]
- 277.Meneilly GS, et al. Effect of ageing and diabetes on glucose-dependent insulinotropic polypeptide and dipeptidyl peptidase IV responses to oral glucose. Diabet Med. 2000;17(5):346–350. doi: 10.1046/j.1464-5491.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- 278.Mannucci E, et al. Hyperglycaemia increases dipeptidyl peptidase IV activity in diabetes mellitus. Diabetologia. 2005;48(6):1168–1172. doi: 10.1007/s00125-005-1749-8. [DOI] [PubMed] [Google Scholar]
- 279.Hildebrandt M, et al. Dipeptidyl peptidase IV (DP IV, CD26) in patients with inflammatory bowel disease. Scand J Gastroenterol. 2001;36(10):1067–1072. doi: 10.1080/003655201750422675. [DOI] [PubMed] [Google Scholar]
- 280.Xiao Q, et al. Circulating levels of glucagon-like peptide-2 in human subjects with inflammatory bowel disease. Am J Physiol Regul Integr Comp Physiol. 2000;278(4):R1057–R1063. doi: 10.1152/ajpregu.2000.278.4.R1057. [DOI] [PubMed] [Google Scholar]
- 281.Rose M, et al. T-cell immune parameters and depression in patients with Crohn’s disease. J Clin Gastroenterol. 2002;34(1):40–48. doi: 10.1097/00004836-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 282.van der Velden VH, et al. Peptidase activities in serum and bronchoalveolar lavage fluid from allergic asthmatics—comparison with healthy non-smokers and smokers and effects of inhaled glucocorticoids. Clin Exp Allergy. 1999;29(6):813–823. doi: 10.1046/j.1365-2222.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 283.Jarmołowska B, et al. Serum activity of dipeptidyl peptidase IV (DPPIV; EC 3.4.14.5) in breast-fed infants with symptoms of allergy. Peptides. 2007;28(3):678–682. doi: 10.1016/j.peptides.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 284.Detel D, Persić M, Varljen J. Serum and intestinal dipeptidyl peptidase IV (DPP IV/CD26) activity in children with celiac disease. J Pediatr Gastroenterol Nutr. 2007;45(1):65–70. doi: 10.1097/MPG.0b013e318054b085. [DOI] [PubMed] [Google Scholar]
- 285.Maes M, et al. Lower activity of serum peptidases in abstinent alcohol-dependent patients. Alcohol. 1999;17(1):1–6. doi: 10.1016/S0741-8329(98)00022-6. [DOI] [PubMed] [Google Scholar]
- 286.Maes M, et al. Effects of psychological stress on serum prolyl endopeptidase and dipeptidyl peptidase IV activity in humans: higher serum prolyl endopeptidase activity is related to stress-induced anxiety. Psychoneuroendocrinology. 1998;23(5):485–495. doi: 10.1016/S0306-4530(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 287.Maes M, et al. Lower serum dipeptidyl peptidase IV activity in treatment resistant major depression: relationships with immune-inflammatory markers. Psychoneuroendocrinology. 1997;22(2):65–78. doi: 10.1016/S0306-4530(96)00040-6. [DOI] [PubMed] [Google Scholar]
- 288.Maes M, et al. Alterations in plasma dipeptidyl peptidase IV enzyme activity in depression and schizophrenia: effects of antidepressants and antipsychotic drugs. Acta Psychiatr Scand. 1996;93(1):1–8. doi: 10.1111/j.1600-0447.1996.tb10612.x. [DOI] [PubMed] [Google Scholar]
- 289.Elgün S, Keskinege A, Kumbasar H. Dipeptidyl peptidase IV and adenosine deaminase activity decrease in depression. Psychoneuroendocrinology. 1999;24(8):823–832. doi: 10.1016/S0306-4530(99)00039-6. [DOI] [PubMed] [Google Scholar]
- 290.Hildebrandt M, et al. Alterations in expression and in serum activity of dipeptidyl peptidase IV (DPP IV, CD26) in patients with hyporectic eating disorders. Scand J Immunol. 1999;50(5):536–541. doi: 10.1046/j.1365-3083.1999.00612.x. [DOI] [PubMed] [Google Scholar]
- 291.van West D, et al. Lowered serum dipeptidyl peptidase IV activity in patients with anorexia and bulimia nervosa. Eur Arch Psychiatry Clin Neurosci. 2000;250(2):86–92. doi: 10.1007/s004060070040. [DOI] [PubMed] [Google Scholar]
- 292.Vlahović P, et al. Elevated serum dipeptidyl peptidase IV activity in patients with chronic tonsillitis. Ann Clin Biochem. 2007;44(1):70–74. doi: 10.1258/000456307779595931. [DOI] [PubMed] [Google Scholar]
- 293.Gruss HJ, et al. Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today. 1997;18(4):156–163. doi: 10.1016/S0167-5699(97)84661-0. [DOI] [PubMed] [Google Scholar]
- 294.Chikuma T, et al. Purification and properties of dipeptidyl peptidase IV from human urine. Biol Chem Hoppe Seyler. 1990;371(4):325–330. doi: 10.1515/bchm3.1990.371.1.325. [DOI] [PubMed] [Google Scholar]
- 295.Haacke W, Küllertz G, Barth A. Diagnostic value of the enzyme dipeptidyl peptidase IV (DP IV) in abdominal cancers. Arch Geschwulstforsch. 1986;56(2):145–153. [PubMed] [Google Scholar]
- 296.Küllertz G, Boigk J. Dipeptidyl peptidase IV activity in the serum and synovia of patients with rheumatoid arthritis. Z Rheumatol. 1986;45(2):52–56. [PubMed] [Google Scholar]
- 297.Elgün S, et al. Serum dipeptidyl peptidase IV activity correlates with the T-cell CD26 antigen. Clin Chem Lab Med. 1999;37(8):839–840. doi: 10.1515/CCLM.1999.126. [DOI] [PubMed] [Google Scholar]
- 298.Sedo A, Hátle K, Stolba P. Changes in dipeptidyl-peptidase IV activity in human serum in pathological conditions of the thyroid gland. Cas Lek Cesk. 1985;124(51):1579–1581. [PubMed] [Google Scholar]
- 299.Krepela E, et al. An assay of dipeptidyl peptidase IV activity in human serum and serum of pregnant women with glycyl-l-proline-1-naphthylamide and other glycyl-l-proline-arylamides as substrates. Physiol Bohemoslov. 1983;32(4):334–345. [PubMed] [Google Scholar]
- 300.Bartles JR, et al. Decreases in the relative concentrations of specific hepatocyte plasma membrane proteins during liver regeneration: down-regulation or dilution? Dev Biol. 1991;143(2):258–270. doi: 10.1016/0012-1606(91)90076-F. [DOI] [PubMed] [Google Scholar]
- 301.Bartles JR, et al. Expression and compartmentalization of integral plasma membrane proteins by hepatocytes and their progenitors in the rat pancreas. J Cell Sci. 1991;98(1):45–54. doi: 10.1242/jcs.98.1.45. [DOI] [PubMed] [Google Scholar]
- 302.McCaughan GW, et al. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990;11(4):534–544. doi: 10.1002/hep.1840110403. [DOI] [PubMed] [Google Scholar]
- 303.McCaughan GW, et al. Molecular pathogenesis of liver disease: an approach to hepatic inflammation, cirrhosis and liver transplant tolerance. Immunol Rev. 2000;174:172–191. doi: 10.1034/j.1600-0528.2002.017420.x. [DOI] [PubMed] [Google Scholar]
- 304.O’Hara RJ, et al. Impaired interleukin-12 production is associated with a defective anti-tumour response in colorectal cancer. Dis Colon Rectum. 1998;41(4):460–463. doi: 10.1007/BF02235759. [DOI] [PubMed] [Google Scholar]
- 305.Cordero OJ, et al. Interleukin-12 enhances CD26 expression and dipeptidyl peptidase IV function on human activated lymphocytes. Immunobiology. 1997;197(5):522–533. doi: 10.1016/s0171-2985(97)80084-8. [DOI] [PubMed] [Google Scholar]
- 306.Kasahara Y, et al. Glycylprolyl-diaminopeptidase in human leukocytes: selective occurrence in T lymphocytes and influence on the total serum enzyme activity. Clin Chim Acta. 1984;139(3):295–302. doi: 10.1016/0009-8981(84)90275-4. [DOI] [PubMed] [Google Scholar]
- 307.Ward PE. Immunoelectrophoretic analysis of vascular, membrane-bound angiotensin I converting enzyme, aminopeptidase M, and dipeptidyl(amino) peptidase IV. Biochem Pharmacol. 1984;33(20):3183–3193. doi: 10.1016/0006-2952(84)90075-3. [DOI] [PubMed] [Google Scholar]
- 308.Mentzel S, et al. Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem. 1996;44(5):445–461. doi: 10.1177/44.5.8627002. [DOI] [PubMed] [Google Scholar]
- 309.Lojda Z. Studies on dipeptidyl(amino)peptidase IV (glycyl-proline naphthylamidase). II. Blood vessels. Histochemistry. 1979;59(3):153–166. doi: 10.1007/BF00495663. [DOI] [PubMed] [Google Scholar]
- 310.van der Velden V, et al. Expression of aminopeptidase N and dipeptidyl peptidase IV in the healthy and asthmatic bronchus. Clin Exp Allergy. 1998;28(1):110–120. doi: 10.1046/j.1365-2222.1998.00198.x. [DOI] [PubMed] [Google Scholar]
- 311.Gossrau R. Peptidases II. Localization of dipeptidylpeptidase IV (DPP IV). Histochemical and biochemical study. Histochemistry. 1979;60(2):231–248. doi: 10.1007/BF00495756. [DOI] [PubMed] [Google Scholar]
- 312.Sahara N, et al. Immunohistochemical localization of dipeptidyl peptidase IV in rat digestive organs. Acta Histochem Cytochem. 1983;16:494–501. [Google Scholar]
- 313.Pala L, et al. Dipeptidyl peptidase-IV expression and activity in human glomerular endothelial cells. Biochem Biophys Res Commun. 2003;310(1):28–31. doi: 10.1016/j.bbrc.2003.08.111. [DOI] [PubMed] [Google Scholar]
- 314.Mavropoulos JC, et al. Anti-tumor necrosis factor-alpha therapy augments dipeptidyl peptidase IV activity and decreases autoantibodies to GRP78/BIP and phosphoglucose isomerase in patients with rheumatoid arthritis. J Rheumatol. 2005;32(11):2116–2124. [PubMed] [Google Scholar]
- 315.Uematsu T, Urade M, Yamaoka M. Decreased expression and release of dipeptidyl peptidase IV (CD26) in cultured peripheral blood T lymphocytes of oral cancer patients. J Oral Pathol Med. 1998;27(3):106–110. doi: 10.1111/j.1600-0714.1998.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 316.Uematsu T, et al. Effects of oral squamous cell carcinoma-derived TGF-beta1 on CD26/DPPIV expression in T cells. Anticancer Res. 2004;24(2B):619–624. [PubMed] [Google Scholar]
- 317.Lojda Z. Proteinases in pathology. Usefulness of histochemical methods. J Histochem Cytochem. 1981;29(3A Suppl):481–493. doi: 10.1177/29.3.481. [DOI] [PubMed] [Google Scholar]
- 318.Lojda Z. The importance of protease histochemistry in pathology. Histochem J. 1985;17(10):1063–1089. doi: 10.1007/BF01002534. [DOI] [PubMed] [Google Scholar]
- 319.Lojda Z. Dipeptidyl peptidases of human lymphocytes. Czech Med. 1988;11(4):181–194. [PubMed] [Google Scholar]
- 320.Sehmsdorf US, et al. Human miscarriage is associated with increased number of CD26 decidual lymphocytes. Scand J Immunol. 2004;59(4):400–407. doi: 10.1111/j.0300-9475.2004.01406.x. [DOI] [PubMed] [Google Scholar]
- 321.Scheel-Toellner D, et al. CD26 expression in leprosy and other granulomatous diseases correlates with the production of interferon-gamma. Lab Invest. 1995;73(5):685–690. [PubMed] [Google Scholar]
- 322.Willheim M, et al. Cell surface characterization of T lymphocytes and allergen-specific T cell clones: correlation of CD26 expression with T(H1) subsets. J Allergy Clin Immunol. 1997;100(3):348–355. doi: 10.1016/S0091-6749(97)70248-3. [DOI] [PubMed] [Google Scholar]
- 323.Cavani A, et al. Human CD4+ T lymphocytes with remarkable regulatory functions on dendritic cells and nickel-specific Th1 immune responses. J Invest Dermatol. 2000;114(2):295–302. doi: 10.1046/j.1523-1747.2000.00881.x. [DOI] [PubMed] [Google Scholar]
- 324.Müller A, et al. Localized Wegener’s granulomatosis: predominance of CD26 and IFN-gamma expression. J Pathol. 2000;192(1):113–120. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH656>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 325.Boonacker EP, et al. CD26/DPPIV signal transduction function, but not proteolytic activity, is directly related to its expression level on human Th1 and Th2 cell lines as detected with living cell cytochemistry. J Histochem Cytochem. 2002;50(9):1169–1177. doi: 10.1177/002215540205000903. [DOI] [PubMed] [Google Scholar]
- 326.Schade RP, et al. Cell-surface expression of CD25, CD26, and CD30 by allergen-specific T cells is intrinsically different in cow’s milk allergy. J Allergy Clin Immunol. 2002;109(2):357–362. doi: 10.1067/mai.2002.121457. [DOI] [PubMed] [Google Scholar]
- 327.Nakao K, et al. Serum levels of soluble CD26 and CD30 in patients on hemodialysis. Nephron. 2002;91(2):215–221. doi: 10.1159/000058395. [DOI] [PubMed] [Google Scholar]
- 328.Fierro MT, et al. Expression pattern of chemokine receptors and chemokine release in inflammatory erythroderma and Sézary syndrome. Dermatology. 2006;213(4):284–292. doi: 10.1159/000096191. [DOI] [PubMed] [Google Scholar]
- 329.Krakauer M, Sorensen PS, Sellebjerg F. CD4(+) memory T cells with high CD26 surface expression are enriched for Th1 markers and correlate with clinical severity of multiple sclerosis. J Neuroimmunol. 2006;181(1–2):157–164. doi: 10.1016/j.jneuroim.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 330.Jafari-Shakib R, et al. CD26 expression on CD4+ T cells in patients with cutaneous leishmaniasis. Clin Exp Immunol. 2008;153(1):31–36. doi: 10.1111/j.1365-2249.2008.03666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Ilhan F, et al. Th1 polarization of the immune response in uveitis in Behçet’s disease. Can J Ophthalmol. 2008;43(1):105–108. doi: 10.3129/I07-179. [DOI] [PubMed] [Google Scholar]
- 332.Ma Y, et al. The CD4+ CD26− T-cell population in classical Hodgkin’s lymphoma displays a distinctive regulatory T-cell profile. Lab Invest. 2008;88(5):482–490. doi: 10.1038/labinvest.2008.24. [DOI] [PubMed] [Google Scholar]
- 333.Cordon-Cardo C, Prives C. At the crossroads of inflammation and tumorigenesis. J Exp Med. 1999;190(10):1367–1370. doi: 10.1084/jem.190.10.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Dvorak-HF Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 335.Strieter RM, et al. CXC chemokines: angiogenesis, immunoangiostasis, and metastases in lung cancer. Ann N Y Acad Sci. 2004;1028(12):351–360. doi: 10.1196/annals.1322.041. [DOI] [PubMed] [Google Scholar]
- 336.Szlosarek PW, Balkwill FR. Tumour necrosis factor alpha: a potential target for the therapy of solid tumours. Lancet Oncol. 2003;4(9):565–573. doi: 10.1016/S1470-2045(03)01196-3. [DOI] [PubMed] [Google Scholar]
- 337.Byrd JB, et al. Dipeptidyl peptidase IV deficiency increases susceptibility to angiotensin-converting enzyme inhibitor-induced peritracheal edema. J Allergy Clin Immunol. 2007;120(2):403–408. doi: 10.1016/j.jaci.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 338.Waldmann TA. Effective cancer therapy through immunomodulation. Annu Rev Med. 2006;57:65–81. doi: 10.1146/annurev.med.56.082103.104549. [DOI] [PubMed] [Google Scholar]
- 339.Zou W, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7(12):1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 340.Hartmann E, et al. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 2003;63(19):6478–6487. [PubMed] [Google Scholar]
- 341.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 342.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131(11 Suppl):3109–3120. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 343.Masur K, et al. DPPIV inhibitors extend GLP-2 mediated tumour promoting effects on intestinal cancer cells. Regul Pept. 2006;137(3):147–155. doi: 10.1016/j.regpep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 344.Lenhard JM, Croom DK, Minnick DT. Reduced serum dipeptidyl peptidase-IV after metformin and pioglitazone treatments. Biochem Biophys Res Commun. 2004;324(1):92–97. doi: 10.1016/j.bbrc.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 345.Wang CH, et al. Cyclosporine increases ischemia-induced endothelial progenitor cell mobilization through manipulation of the CD26 system. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R811–R818. doi: 10.1152/ajpregu.00543.2007. [DOI] [PubMed] [Google Scholar]
- 346.Dang DT, et al. Hypoxia-inducible factor-1 target genes as indicators of tumor vessel response to vascular endothelial growth factor inhibition. Cancer Res. 2008;68(6):1872–1880. doi: 10.1158/0008-5472.CAN-07-1589. [DOI] [PubMed] [Google Scholar]
- 347.Ghersi G, et al. Critical role of dipeptidyl peptidase IV in neuropeptide Y-mediated endothelial cell migration in response to wounding. Peptides. 2001;22(3):453–458. doi: 10.1016/S0196-9781(01)00340-0. [DOI] [PubMed] [Google Scholar]
- 348.Thompson JA, et al. Phase I study of recombinant interleukin-21 in patients with metastatic melanoma and renal cell carcinoma. J Clin Oncol. 2008;26(12):2034–2039. doi: 10.1200/JCO.2007.14.5193. [DOI] [PubMed] [Google Scholar]
- 349.Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 350.Rolle CE, Carrio R, Malek TR. Modeling the CD8+ T effector to memory transition in adoptive T-cell antitumor immunotherapy. Cancer Res. 2008;68(8):2984–2992. doi: 10.1158/0008-5472.CAN-07-3040. [DOI] [PubMed] [Google Scholar]
- 351.Duan X, et al. Murine bone marrow-derived mesenchymal stem cells as vehicles for interleukin-12 gene delivery into Ewing sarcoma tumors. Cancer. 2009;115(1):13–22. doi: 10.1002/cncr.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Park S, Cheon S, Cho D. The dual effects of interleukin-18 in tumor progression. Cell Mol Immunol. 2007;4(5):329–335. [PubMed] [Google Scholar]
- 353.Mocellin S, Nitti D. TNF and cancer: the two sides of the coin. Front Biosci. 2008;13:2774–2783. doi: 10.2741/2884. [DOI] [PubMed] [Google Scholar]
- 354.Mocellin S, et al. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev. 2005;16(1):35–53. doi: 10.1016/j.cytogfr.2004.11.001. [DOI] [PubMed] [Google Scholar]