Abstract
T-cell trafficking is determined by expression patterns of chemokine receptors. The chemokine receptor CXCR3 is expressed on a subpopulation of type 1 T cells and plays an important role for migration of T cells into inflamed and tumor tissues. Here, we studied the chemokine receptor expression on specific T cells generated against the neoantigen keyhole limpet hemocyanin (KLH) in patients who had been immunized in the context of a tumor peptide vaccination trial with or without the adjuvant granulocyte-macrophage colony-stimulating factor (GM-CSF). In patients immunized in the presence of GM-CSF the fraction of CXCR3+ KLH-specific T cells was significantly higher than in patients immunized in the absence of GM-CSF (median 45 vs. 20%, P = 0.001). In contrast, the chemokine receptor CCR4, associated with migration to the skin was found in both cohorts on less than 10% of KLH-specific T cells. These results show that CXCR3 expression on vaccine-induced T cells can be modulated by modifying the local vaccine milieu.
Keywords: CXCR3, T cells, Vaccination, GM-CSF
Introduction
T-cell migration is a multistep process, in which chemokines and chemokine receptors play a key role. The chemokine receptor CXCR3 is expressed on a subset of differentiated CD4+ and CD8+ T cells. CXCR3 plays an important role in mediating migration of T cells into type 1-dominated inflammatory processes, where the specific ligands CXCL9 (Mig), CXCL10 (IP-10), and CXCL11 (I-TAC) are abundantly expressed [2, 13, 17, 26]. These chemokines have also been found upregulated in human immunodeficiency virus (HIV)-infected macrophages and dendritic cells and were implicated in the recruitment of T cells to HIV-infected lymph nodes and central nervous system (CNS) [5, 16]. Furthermore, CXCL9, 10, and 11 are frequently expressed in tumor tissues as shown for renal cell carcinoma and melanoma [11, 25]. CXCL9 and CXCL10 have been associated with heavy infiltration of T cells in human melanoma suggesting that CXCR3 can mediate T-cell migration into tumor tissue [11]. Transfection of CXCL11 into tumor cells resulted in increased infiltration by CXCR3+ CD8+ T cells and tumor rejection [7]. A recent study analyzing the chemokine receptor profile of melanoma-peptide stimulated T cells in melanoma patients showed that expression of CXCR3 on T cells was associated with increased survival [15]. Thus, it is of considerable interest for both cancer and infectious disease vaccine development to find out conditions to enhance the expression of CXCR3 on T cells generated by vaccination.
In vitro, CXCR3 is expressed within a few days following activation of T cells independently of the cytokine milieu. After stimulation, however, CXCR3 expression is maintained preferentially on T cells that have been activated in type 1 conditions [10, 18].
GM-CSF is frequently given as vaccine adjuvant due to its ability to enhance the immunogenicity of protein or peptide vaccines [3, 23]. GM-CSF stimulates the activation and migration of dendritic cells and induces their expression of MHC class II molecules. GM-CSF was reported to induce CXCR3 expression on CD34+ stem cells [8].
In the current study expression of CXCR3 was analyzed on specific T cells generated in melanoma patients in a peptide vaccine trial either in the presence or absence of the adjuvant GM-CSF as previously described [21]. As T-cell responses against the melanoma peptide tyrosinase were only generated in a subset of patients who received GM-CSF, we studied T-cell responses generated against the neoantigen keyhole limpet hemocyanin (KLH), which was included in the vaccine as unspecific T helper protein and against which all patients mounted a T-cell response.
Materials and methods
Patient samples and vaccination protocol
Cell samples analyzed in this study were those available from patients with stage III or IV melanoma who had been vaccinated within the context of two consecutive phase I melanoma peptide vaccine trials, which have been previously reported [12, 21]. The trials had been approved by the Institutional Ethics Committee. All patients had received six cycles of intradermal and subcutaneous injections of 4 mg KLH (Vacmun; Biosyn, Stuttgart, Germany) admixed with tyrosinase peptide(s) (Bachem, Bubendorf, Switzerland). Patients in one cohort received in addition GM-CSF (Leukomax; Essex, Munich, Germany) as adjuvant in a dose of 75 μg for 4 days injected at the same site beginning 2 days before peptide vaccination. Blood samples were obtained from each patient before and 4 weeks after the fourth and sixth vaccination. Written informed consent was obtained from all patients.
T-cell response assessment by Interferon γ (IFNγ) flow cytometry analysis
Peripheral blood mononuclear cells (PBMC) were thawed and after overnight resting incubated with 1 mg/ml KLH and without antigen as negative control for 18 h. After 2 h, 10 μg/ml brefeldin A (Sigma, Deisenhofen, Germany) were added. In case PBMCs were stimulated with 10 μg/ml peptide tyrosinase 368–376, 370D [22, 29], influenza matrix protein, 58–66 (Thermo BioSciences, Ulm, Germany) or 100 ng/ml phorbol myristate acetate (PMA) and 1 ng/ml ionomycin (Sigma-Aldrich, Munich, Germany) incubation times were 6 h, and brefeldin A was added after 1 h. In peptide stimulation experiments, HIV reverse transcriptase 476-84 (Sigma-Genosys, Cambridge, United Kingdom) was used as negative control. PBMC were then stained extracellularly with fluorescence-conjugated monoclonal antibodies against CD4, CD8, CD3 (BD Bioscience, Heidelberg, Germany) and intracellularly with IFNγ fluorescence-conjugated monoclonal antibody (BD Bioscience). Staining with the fluorescence-conjugated monoclonal antibodies against CXCR3, CCR4 and CCR9 (BD Bioscience, and R&D, Wiesbaden, Germany) was performed prior to the antigen incubation. Data acquisition was performed on FACSCalibur and analyzed using Cellquest Software (BD Bioscience). For calculation of percentages of KLH-specific T cells CD3+CD4+ IFNγ+ T cells counted in the absence of antigen were subtracted from those counted in the presence of KLH.
Statistical analysis
The Mann–Whitney U test was used to determine whether there was a statistically significant difference in the percentage of chemokine receptor positive T cells between the two patient cohorts.
Results
Assessment of CXCR3 on antigen-specific T-cells ex vivo
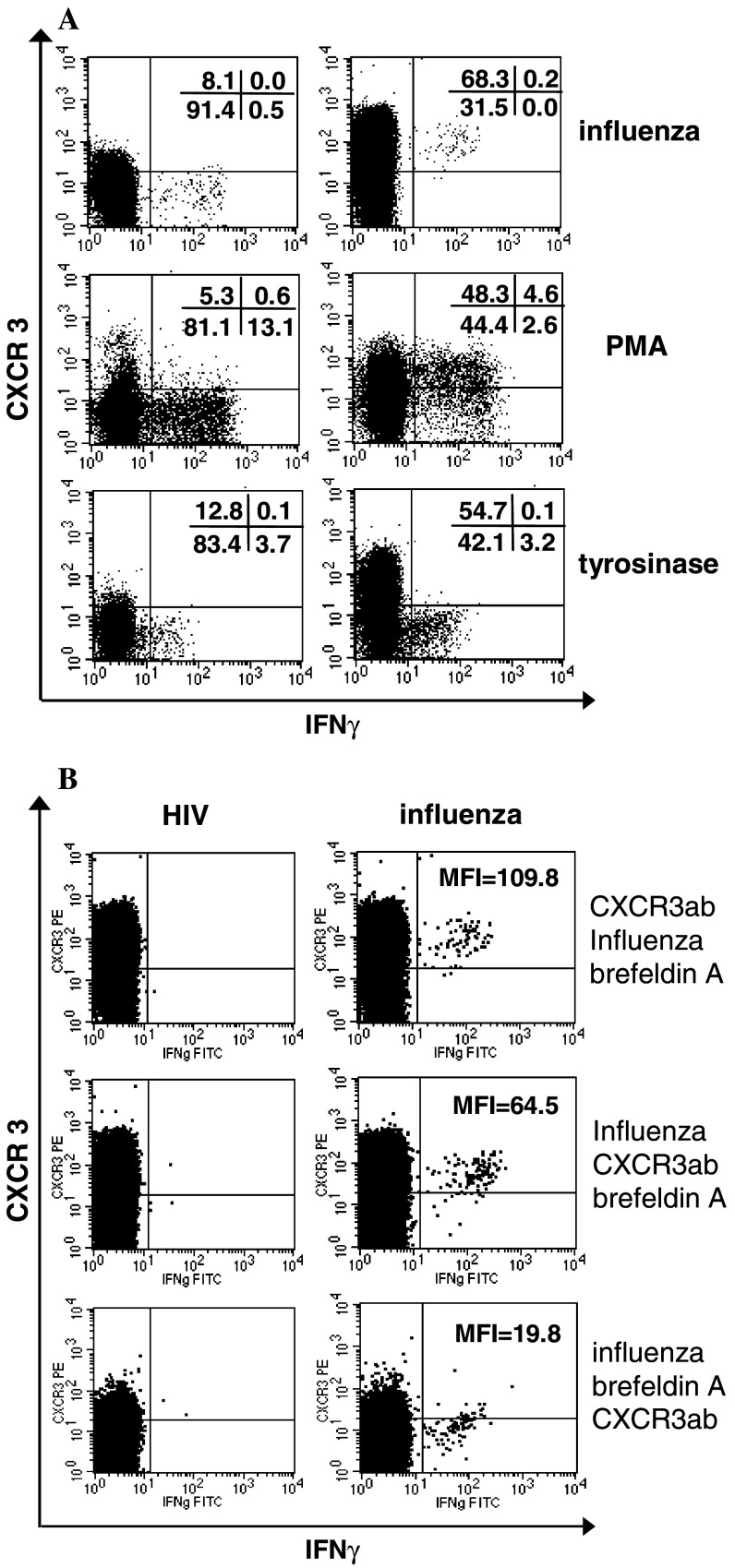
Specific T-cell responses to protein antigens can be assessed in unstimulated PBMC samples by antigen-induced intracellular accumulation of IFNγ. In order to facilitate the chemokine receptor expression on antigen-specific T cells we first analyzed conditions how to determine chemokine receptor expression on T cells after short time stimulation with antigen. It had been shown previously, that T-cell receptor stimulation can result in transient CXCR3 downregulation [19, 20]. When we incubated T cells with peptide or protein antigens for 6 or 18 h in the presence of brefeldin A and stained T cells thereafter for CXCR3 expression, all T cells specifically secreting IFNγ in response to various antigens or stimulation with PMA/Ionomycin were CXCR3-negative (Fig. 1a, left column). When the CXCR3 antibody was, however, added prior to stimulation, both CXCR3-positive and CXCR3-negative antigen-specific T cell responses could be detected (Fig. 1a, right column). To further study the underlying mechanisms we stained antigen-specific T cells either before antigen stimulation, after antigen stimulation but before addition of brefeldin A, and after addition of brefeldin A (Fig. 1b). As shown in Fig. 1b (middle dot plots) stimulation with influenza for 1 h led already to marked CXCR3 downregulation in accordance to previous studies [19, 20]. CXCR3 completely disappeared following addition of brefeldin A for 5 h (Fig. 1b, lower dot plots). Brefeldin A is known to inhibit the transport of immunoglobulin receptors to the cell surface [1], it probably inhibits reexpression of CXCR3 on the cell surface. As a control CCR9 expression was analyzed which was always negative on influenza-reactive T cells irrespective of the staining sequence (data not shown). In our series of experiments analyzing the chemokine receptor profile of KLH-specific T cells we therefore assessed chemokine receptor expression by adding the antibodies prior to antigen stimulation.
Fig. 1.
CXCR3 is downregulated in vitro following antigen stimulation and incubation with brefeldin A. a CXCR3/IFNγ profile of CD3+CD8+ gated lymphocytes stimulated with influenza peptide, phorbol myristate acetate/Ionomycin or tyrosinase peptide are shown. CXCR3 was stained after antigen stimulation and brefeldin A incubation (left dot plots) or prior to antigen stimulation (respective right dot plots). T cells reactive with tyrosinase peptide were from a HLA-A2+ melanoma patient who exhibited a spontaneous high-frequency specific T-cell response [28]. b PBMCs of a HLA-A2+ healthy donor were stimulated with HIV (left dot plots) and influenza (respective right dot plots) peptides. The CXCR3/IFNγ profile of CD3+CD8+ gated lymphocytes are shown. CXCR3 staining was either performed before antigen stimulation (b, upper dot plots), or after antigen stimulation but before addition of brefeldin A (b, middle dot plots) or after antigen stimulation and addition of brefeldin A (b, lower dot plots). MFI mean fluorescence intensity of CXCR3 expression of influenza-specific IFNγ-producing T cells, ab antibody
Chemokine receptor profile of KLH-specific T-cell responses
PBMC samples of 15 patients who had received repeated immunizations with tyrosinase peptides and KLH either in the presence (n = 8) or absence of GM-CSF (n = 7) were available for this study. Specific T-cell responses to KLH were detected in all patients after vaccination (median 0.10%, range 0.05–0.32% with GM-CSF, median 0.27%, range 0.05–0.69% without GM-CSF), in contrast to T-cell responses to tyrosinase, which were only detectable in patients immunized in the presence of GM-CSF [21]. Before vaccination, no T cells in response to KLH were detected [21]. KLH-reactive T cells were costained with antibodies against the chemokine receptors CXCR3, CCR4 and CCR9. CCR4 has been associated with trafficking to the skin, and is predominately expressed on type 2 T cells, but also on a subpopulation of CXCR3+ T cells [9]. CCR9, which is expressed on T cells primed in the small intestine [27], was used as negative control. The analyses of CCR4 and CCR9 were done in a subset of samples only due to paucity of clinical samples. Figure 2a shows a representative dot plot of the CXCR3/IFNγ profile of KLH-reactive T cells in two patients. The fractions of total CD4+ T cells expressing CXCR3, CCR4 and CCR9 were similar in patients immunized in the presence or absence of GM-CSF (Fig. 2b–d). The percentage of KLH-specific CD4+ T cells expressing CXCR3 was significantly higher in patients vaccinated in the presence of the adjuvant GM-CSF (median 45.3%, range 30.0–76.7%) than in patients who did not receive GM-CSF (median 20.0%, range 1.3–37.0%, P = 0.001, Fig. 2a, b). In contrast, no obvious difference in the percentages of CCR4+ KLH-specific CD4+ T cells was found among the two patient cohorts (median 0%, range 0–42.4%, versus median 7.7%, range 0–20.4%, P = 0.33, Fig. 2c). Less than 1% of KLH-specific CD4+ T cells did express CCR9 in both groups (Fig. 2d).
Fig. 2.
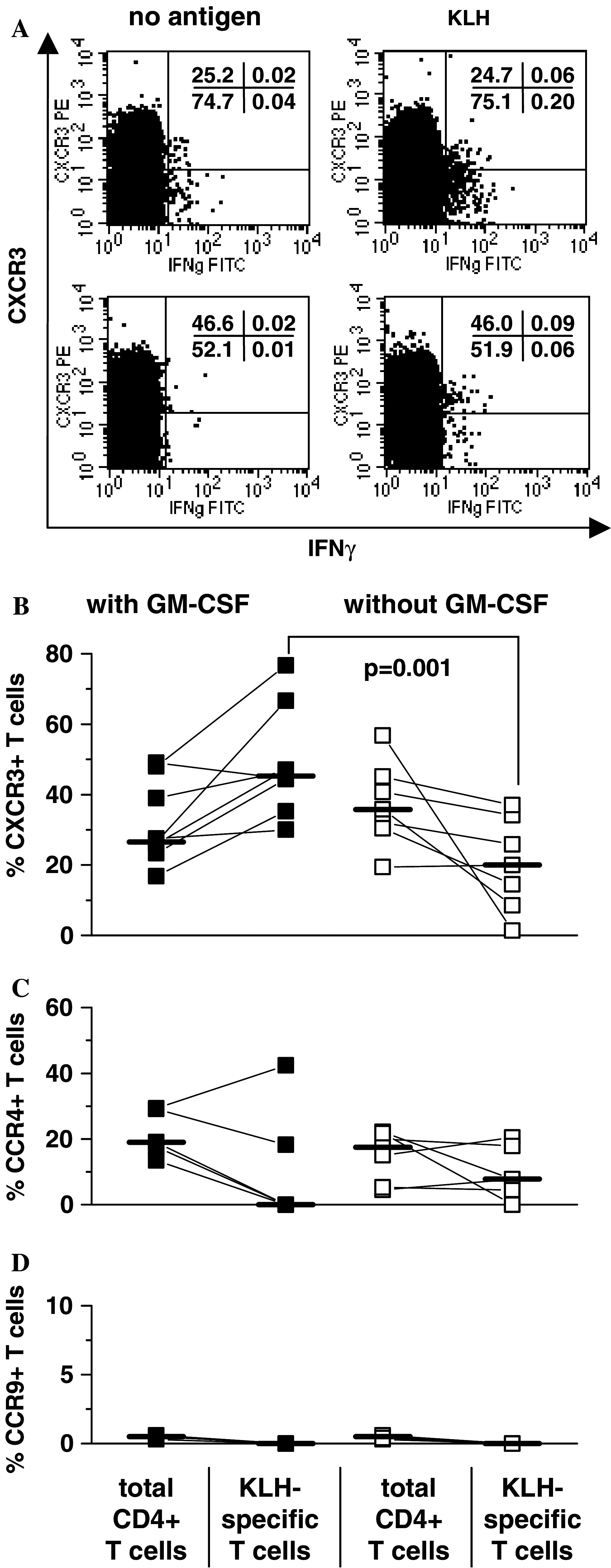
Chemokine receptor expression by KLH-specific T cells in patients immunized in the presence or absence of GM-CSF. a CXCR3/IFNγ profile of CD3+CD4+ gated lymphocytes in unstimulated (left) or KLH-exposed (right) PBMCs in two patients immunized in the absence (upper dot plots) or presence of GM-CSF (lower dot plots) are shown. The percentages of total CD3+CD4+ T cells and KLH-specific CD3+CD4+ T cells are displayed expressing CXCR3 (b), CCR4 (c) or CCR9 (d) in patients immunized in the presence (n = 8, filled cycles) or absence (n = 7, open squares) of GM-CSF. In each patient, chemokine receptor expression of all CD3+CD4+ T cells was compared with the respective chemokine receptor expression of the KLH-specific CD3+CD4+ T cells (connecting line). CCR4 and CCR9 were only determined in a subset of samples (GM-CSF cohort: CCR4 n = 5; CCR9: n = 4; non GM-CSF cohort: CCR4 n = 6, CCR9 n = 7) due to paucity of material. Bars indicate the median
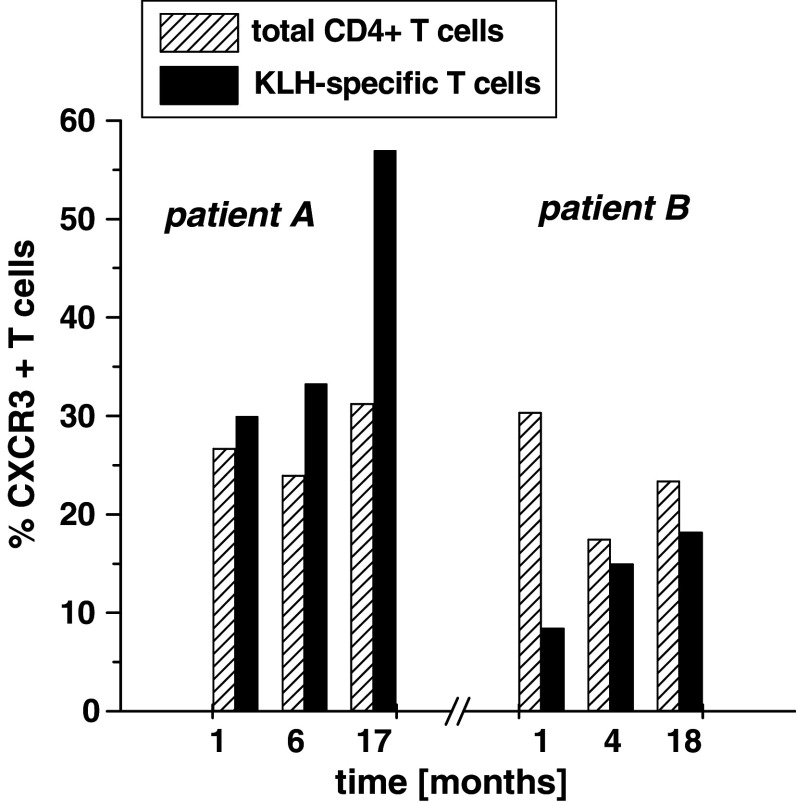
In two patients from whom consecutive post-vaccination samples were available the percentages of CXCR3+ KLH-specific T cells were repeatedly analyzed in samples 1, 6/4 and 17/18 months post-vaccination (Fig. 3). In patient A who had been immunized in the presence of GM-CSF the percentage of CXCR3+ T cells among the KLH-specific CD4+ T-cell fraction was always higher than the percentage of CXCR3+ T cells among the total CD4+ T-cell fraction. In contrast, in patient B, who had been immunized without GM-CSF, frequencies of CXCR3+ T cells among the KLH-specific CD4+ T-cell fraction remained lower compared to frequencies of CXCR3+ cells among total CD4+ T cells at all time points.
Fig. 3.
Course of CXCR3 profile of KLH-specific T cells in two patients. Analysis of PBMCs was performed in two patients (patient A: GM-CSF cohort, patient B: non GM-CSF cohort) 1, 6/4 and 17/18 months after immunization. The percentages of CXCR3+ T cells among the KLH-specific CD4+ T-cell fraction (black columns) and the total CD4+ T-cell fraction (hatched columns) are shown
Discussion
In this study we investigated whether GM-CSF given as vaccine adjuvant can modulate the chemokine receptor expression of vaccine-induced T cells. Results from our study show that vaccination in the presence of the adjuvant GM-CSF promotes the generation of enhanced frequencies of KLH-specific CD4+ T cells expressing CXCR3. In contrast, few KLH-specific T cells beared CCR4 despite intradermal injection of the vaccine and no obvious difference between the cohorts was observed. CCR4 is, however, predominantly expressed on type 2-cytokine producing T cells, which we have not analyzed in this study and this may explain our failure to detect generation of CCR4+ KLH-specific T cells.
The influence of GM-CSF on CXCR3 expression during the priming of naïve T cells has not been studied in vitro or animal models yet. Although GM-CSF has been shown to be able to induce CXCR3 expression on CD34+ stem cells [8], GM-CSF was unable to directly induce CXCR3 expression on T cells (data not shown). GM-CSF is known to enhance recruitment of dendritic cells to the injection site [3, 14], which may promote induction of CXCR3 on KLH-specific T cells. In vitro activation of T cells by dendritic cells induces CXCR3 expression independently of the cytokine milieu [10]. However, T cells primed in the presence of IL-4 loose CXCR3 expression within 7 days while it is maintained stably expressed on T cells that have been activated in the presence of interleukin 12 [18]. As we have found CXCR3 expression on KLH-specific T cells 4 weeks after the last vaccination this suggests that GM-CSF promotes conditions under which T cells stably expressing CXCR3 are primed.
A prerequisite for effective T-cell therapy is the migration of antigen-specific T cells into the tumor. Very little is known so far about the migratory characteristics of vaccine-induced T cells. In experimental models the failure of adoptively transferred T cells to migrate to tumor tissues was shown [6]. CXCR3 expression on vaccine-induced T cells may enhance their ability to migrate into tumors as CXCR3 ligands were shown to be frequently expressed in tumors [11, 25].
Addition of GM-CSF to peptide-, protein- or gene transfer-based vaccination has resulted in the augmentation of antitumor immune responses by enhancing T-cell responses in several studies [3, 4]. Findings from clinical trials suggest that GM-CSF given alone or as vaccine adjuvant may result in improved clinical outcome [23, 24]. Our findings suggest that GM-CSF as vaccine adjuvant can also qualitatively enhance the T-cell response to vaccination.
In summary, our study is the first in humans suggesting that the local vaccine milieu can modulate the chemokine receptor expression on specific T cells generated by vaccination. Upregulation of CXCR3 may facilitate migration of vaccine-generated T cells into inflammatory and tumor sites.
Acknowledgment
This work was supported by grant 10-2148-Ke3 from the Deutsche Krebshilfe, Mildred-Scheel-Stiftung.
References
- 1.Apodaca G, Aroeti B, Tang K, Mostov K. Brefeldin-A inhibits the delivery of the polymeric immunoglobulin receptor to the basolateral surface of MDCK cells. J Biol Chem. 1993;268:20380–20385. [PubMed] [Google Scholar]
- 2.Cole K, Strick C, Paradis T, Ogborne K, Loetscher M, Gladue R, Lin W, Boyd J, Moser B, Wood D, Sahagan B, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC), a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disis M, Bernhard H, Shiota F, Hand S, Gralow J, Huseby E, Gillis SMAC. Granulocyte-macrophage colony-stimulating factor: an effective adjuvant for protein and peptide-based vaccines. Blood. 1996;88:202–210. [PubMed] [Google Scholar]
- 4.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan R. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley J, Yu C, Solow R, Yacobucci M, Peden K, Farber J. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV-1-infected monocyte-derived macrophages, dendritic cells, and lymph nodes. J Immunol. 2005;174:4892–4900. doi: 10.4049/jimmunol.174.8.4892. [DOI] [PubMed] [Google Scholar]
- 6.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 7.Hensbergen PJ, Wijnands PG, Schreurs MW, Scheper RJ, Willemze R, Tensen CP. The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis. J Immunother. 2005;28:343–351. doi: 10.1097/01.cji.0000165355.26795.27. [DOI] [PubMed] [Google Scholar]
- 8.Jinquan T, Quan S, Jacobi HH, Jing C, Millner A, Jensen B, Madsen HO, Ryder LP, Svejgaard A, Malling HJ, Skov PS, Poulsen LK. CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood. 2000;96:1230–1238. [PubMed] [Google Scholar]
- 9.Kim C, Rott L, Kunkel E, Genovese M, Andrew D, Wu L, Butcher E. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI200113543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim C, Nagata K, Butcher E. Dendritic cells support sequential reprogramming of chemoattractant receptor profiles during naive to effector T cell differentiation. J Immunol. 2003;171:152–158. doi: 10.4049/jimmunol.171.1.152. [DOI] [PubMed] [Google Scholar]
- 11.Kunz M, Toksoy A, Goebeler M, Engelhardt E, Brocker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 12.Letsch A, Keilholz U, Fluck M, Nagorsen D, Asemissen A, Schmittel A, Thiel E, Scheibenbogen C. Peptide vaccination after repeated resection of metastases can induce a prolonged relapse-free interval in melanoma patients. Int J Cancer. 2005;114:936–941. doi: 10.1002/ijc.20819. [DOI] [PubMed] [Google Scholar]
- 13.Liao F, Rabin R, Yannelli J, Koniaris L, Vanguri P, Farber J. Human Mig chemokine, biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mach N, Gillessen S, Wilson S, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 15.Mullins I, Slingluff C, Lee J, Garbee C, Shu J, Anderson S, Mayer M, Knaus W, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 16.Poluektova L, Moran T, Zelivyanskaya M, Swindells S, Gendelman H, Persidsky Y. The regulation of alpha chemokines during HIV-1 infection and leukocyte activation: relevance for HIV-1-associated dementia. J Neuroimmunol. 2001;120:112–128. doi: 10.1016/S0165-5728(01)00413-1. [DOI] [PubMed] [Google Scholar]
- 17.Qin S, Rottman J, Myers P, Kassam N, Weinblatt M, Loetscher M, Koch A, Moser B, Mackay C. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabin R, Alston M, Sircus J, Knollmann-Ritschel B, Moratz C, Ngo D, Farber J. CXCR3 is induced early on the pathway of CD4+ T cell differentiation and bridges central and peripheral functions. J Immunol. 2003;171:2812–2824. doi: 10.4049/jimmunol.171.6.2812. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, Forster R, Lipp M, Lanzavecchia A. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 20.Sauty A, Colvin R, Wagner L, Rochat S, Spertini F, Luster A. CXCR3 internalization following T cell-endothelial cell contact: preferential role of IFN-inducible T cell alpha chemoattractant (CXCL11) J Immunol. 2001;167:7084–7093. doi: 10.4049/jimmunol.167.12.7084. [DOI] [PubMed] [Google Scholar]
- 21.Scheibenbogen C, Schadendorf D, Bechrakis N, Nagorsen D, Hofmann U, Servetopoulou F, Letsch A, Philipp A, Foerster M, Schmittel A, Thiel E, Keilholz U. Effects of granulocyte-macrophage colony-stimulating factor and foreign helper protein as immunologic adjuvants on the T-cell response to vaccination with tyrosinase peptides. Int J Cancer. 2003;104:188–194. doi: 10.1002/ijc.10961. [DOI] [PubMed] [Google Scholar]
- 22.Skipper JC, Hendrickson RC, Gulden PH, Brichard V, Van Pel A, Chen Y, Shabanowitz J, Wolfel T, Slingluff CL, Jr, Boon T, Hunt DF, Engelhard VH. An HLA-A2-restricted tyrosinase antigen on melanoma cells results from posttranslational modification and suggests a novel pathway for processing of membrane proteins. J Exp Med. 1996;183:527–534. doi: 10.1084/jem.183.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slingluff CJ, Petroni G, Yamshchikov G, Barnd D, Eastham SGH, Patterson J, Deacon D, Hibbitts S, Teates D, Neese P, Grosh W, Chianese-Bullock K, Woodson E, Wiernasz CMP, Gibson J, Ross M, Engelhard V. Clinical and immunologic results of a randomized phase II trial of vaccination using four melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Spitler LE, Grossbard ML, Ernstoff MS, Silver G, Jacobs M, Hayes FA, Soong SJ. Adjuvant therapy of stage III and IV malignant melanoma using granulocyte-macrophage colony-stimulating factor. J Clin Oncol. 2000;18:1614–1621. doi: 10.1200/JCO.2000.18.8.1614. [DOI] [PubMed] [Google Scholar]
- 25.Suyama T, Furuya M, Nishiyama M, Kasuya Y, Kimura S, Ichikawa T, Ueda T, Nikaido T, Ito H, Ishikura H. Up-regulation of the interferon gamma (IFN-gamma)-inducible chemokines IFN-inducible T-cell alpha chemoattractant and monokine induced by IFN-gamma and of their receptor CXC receptor 3 in human renal cell carcinoma. Cancer. 2005;103:258–267. doi: 10.1002/cncr.20747. [DOI] [PubMed] [Google Scholar]
- 26.Taub D, Lloyd A, Conlon K, Wang J, Ortaldo J, Harada A, Matsushima K, Kelvin D, Oppenheim J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uehara S, Grinberg A, Farber J, Love P. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 28.Valmori D, Scheibenbogen C, Dutoit V, Nagorsen D, Asemissen A, Rubio-Godoy V, Rimoldi D, Guillaume P, Romero P, Schadendorf D, Lipp M, Dietrich P, Thiel E, Cerottini J, Lienard D, Keilholz U. Circulating Tumor-reactive CD8(+) T cells in melanoma patients contain a CD45RA(+)CCR7(−) effector subset exerting ex vivo tumor-specific cytolytic activity. Cancer Res. 2002;62:1743–1750. [PubMed] [Google Scholar]
- 29.Wolfel T, Van Pel A, Brichard V, Schneider J, Seliger B, Meyer zum Buschenfelde KH, Boon T. Two tyrosinase nonapeptides recognized on HLA-A2 melanomas by autologous cytolytic T lymphocytes. Eur J Immunol. 1994;24:759–764. doi: 10.1002/eji.1830240340. [DOI] [PubMed] [Google Scholar]