Abstract
Cytokine shedding by tumor cells into the local microenvironment modulates host immune response, tumor growth, and metastasis. The study aimed to verify the hypothesis that the immunological microenvironment of pancreatic carcinoma exists in a prevalently immunosuppressive state, influencing survival. We analyzed expression profiles of pro-inflammatory (IL-1β, IL-2, IL-6, IL-8, IL-12 p40, IL-18 and IFN-γ) and anti-inflammatory (IL-10, IL-11, IL-13 and TGF-β isoforms) cytokines. The study was performed both in vitro, in five pancreatic carcinoma cell lines (real time RT-PCR), and in specimens from 65 patients, comparing tumoral versus non-tumoral pancreatic tissues (real time RT-PCR and immunohistochemistry). Furthermore, cytokines were measured in supernatants and sera (from patients and controls) by ELISA. All cell lines expressed IL-8, IL-18, TGF-β1, TGF-β2 and TGF-β3, but not IFN-γ and IL-2 transcripts. Expression of IL-1β, IL-6, IL-10, IL-11, IL-13 and IL-12 mRNA was variable. All the above cytokines were detected as soluble proteins in supernatants, except IL-13. Tumor tissues overexpressed IL-1β, IL-6, IL-8, IL-10, IL-11, IL-12 p40, IL-18, IFN-γ, TGF-β1, TGF-β2 and TGF-β3 at the mRNA level and IL-1β, IL-18, TGF-β2 and TGF-β3 also at the protein level. Conversely, non-tumor tissues had stronger RNA and protein expression of IL-13. Survival was significantly longer in patients with high IL-1β and IL-11 and moderate IL-12 expression. Serum IL-8, IL-10, IL-12, IL-18, TGF-β1 and TGF-β2 were higher in patients than in controls, as opposed to IL-1β and IL-13. Patients with low circulating levels of IL-6, IL-18 and TGF-β2 survived longer. Pancreatic cancer is characterized by peculiar cytokine expression patterns, associated with different survival probabilities.
Keywords: Cytokines, Pancreatic carcinoma, Tumor immunity
Introduction
Carcinoma of the exocrine pancreas is usually associated with a poor prognosis [19]. Among the causes of its aggressive behavior, several passive and active strategies appear to be adopted by tumor cells to circumvent anti-tumor immune defenses. They include altered expression of major histocompatibility complex (MHC) Class I and II antigens [45], which may impair interactions between malignant cells and potentially tumor cytotoxic T lymphocytes (CTLs), and resistance to apoptosis through the Fas receptor pathway coupled with aberrant expression of the ligand, which may be considered part of the “counterattack” of tumor cells against immune effector cells [4, 57, 59]. Pancreatic carcinoma cells have also been shown to spontaneously secrete immunosuppressive cytokines, such as Interleukin (IL)-10 and transforming growth factor (TGF)-β, that down-regulate the host immune system and contribute to a systemic T helper (h) 2 immune phenotype in vivo [5, 60].
Cytokines play a pivotal role in the induction of cell-mediated and humoral immunity. A better understanding of the basis of molecular cross-talk between tumor cells and the immune system would be helpful in developing immunotherapeutic approaches to pancreatic cancer, motivated by the dearth of conventional therapeutic options for these patients. Thus the main goals of the present study were to analyze in depth the cytokine expression patterns in pancreatic carcinoma, both in vitro and in vivo, and to identify those patterns associated with better patient survival.
Materials and methods
Human pancreatic cell lines
Five human pancreatic cell lines: BxPC-3, Capan-2, PANC-1 and MIAPaCa-2 (American Type Culture Collection, Rockville, MD) and PT-45 (kindly provided by Dr. M.F. Di Renzo, Department of Biomedical Sciences and Human Oncology, University of Turin, Italy) were grown in RPMI 1640 medium (Life Technologies, Inc., Grand Island, NY, USA) supplemented with 10% fetal calf serum (Life Technologies). All cell lines were routinely screened for mycoplasma contamination, using the Hoechst dye H33258 (Sigma, St. Louis, MO, USA).
Tumor and non-tumor pancreatic tissue specimens
The study group comprised 65 patients who received a diagnosis of pancreatic carcinoma at the Department of Medical-Surgical Disciplines (University of Turin, Turin, Italy) between January 1999 and December 2002. None underwent anti-cancer treatment before entering the study, which was conducted under strict observance of the principles of the Declaration of Helsinki.
Twenty-four patients had advanced non-resectable pancreatic carcinoma and received only supportive treatment. For them, a diagnosis of pancreatic carcinoma was based on typical radiographic findings at ultrasonography, computed tomography, endoscopic retrograde cholangiopancreatography, and/or endoscopic ultrasonography.
Pancreatic carcinoma tissue samples were obtained from 41/65 patients who underwent surgical resection of the tumor. Tumors were histopathologically confirmed primary pancreatic duct adenocarcinomas and classified by the UICC Staging System [47]. Aliquots of fresh pancreatic cancer (n=41) and tumor-free pancreatic tissue samples (n=9) were fixed in formalin and paraffin-embedded for immunohistochemical analysis or placed in liquid nitrogen prior to mRNA extraction. Frozen sections (6 μm) were taken from blocks of tumor tissue and, starting with the first section, every fifth section was routinely stained with hematoxylin and eosin and evaluated histopathologically by two independent, experienced pathologists. Sections were pooled for cytokine mRNA analysis from areas estimated to have at least 90% malignant cells. In the same way, tumor-free pancreatic tissue samples were examined by the pathologists and defined as “normal” tissue areas.
Informed consent was obtained from patients for experimental use of blood and surgical specimens, as per the hospital’s ethical guidelines. Clinical and demographics characteristics of the patients and details of which analysis each patient’s biological material was used for are shown in Table 1.
Table 1.
Clinical, demographic and pathological features of the study population
| Therapy | Total | ||
|---|---|---|---|
| Resective | Conservative | ||
| Number of patients | 41 (63) | 24 (37) | 65 (100) |
| Male gender | 23 (56) | 14 (58) | 37 (57) |
| Age | 65 (50–77) | 64.5 (50–87) | 65 (50–87) |
| Survivala, months | 19.5 (5.2–31.9) | 9.5 (0.8–32.9) | 14.1 (0.8–32.9) |
| Stage grouping | |||
| IIAb | 10 (100) | 10 (100) | |
| IIBc | 6 (100) | 6 (100) | |
| IIId | 16 (84) | 3 (16) | 19 (100) |
| IVe | 9 (30) | 21 (70) | 30 (100) |
| Cytokine studies | |||
| Real-time PCR | 41 (63) | 0 (0) | 41(63) |
| Immunohistochemistry | 41 (63) | 0 (0) | 41 (63) |
| ELISA | 41 (63) | 24 (37) | 65 (100) |
Data are presented as medians (range) for continuous variables, and as frequencies (%) for categoric variables.
aat the end of follow-up (see text for details)
b T3 N0 M0, c T1-T3 N1 M0, d T4 Any N M0, eany T any N M1
Sufficient follow up data were available for 49 patients. Among them, 13 patients (27%) were still alive when the study terminated, whereas 36 patients (73%) had died. The median follow-up period of surviving patients was 14 months (range 6–29 months): four patients were disease free or with stable disease, whereas nine had progressive disease.
RNA extraction and reverse transcription
RNA extraction from frozen tissue specimens, cell lines and appropriate positive controls, consisting of Phytohaemoagglutinin (PHA) or Staphylococcus Aureus Cowan I (SAC) activated-normal peripheral blood mononuclear cells (PBMC), IL-2 plus IL-12 activated natural killer (NK) cells, bone marrow stroma cells (BMSC), and macrophage-like cell line U937, was performed using TRIzol Reagent (Invitrogen, Life Technologies, Gaithersburg MD) following the manufacturer’s instructions. To remove traces of genomic DNA, total RNAs (1 μg) were treated with DNase I (Invitrogen) and reverse-transcribed to cDNAs using SuperScript II (Invitrogen) as described elsewhere [7]
Real time quantitative RT-PCR
Real-time quantitative RT-PCR analysis was performed on iCycler iQ system (Bio-Rad, Hercules, CA) by SYBR green I dye detection. Amplification of β-actin and cytokines was performed in duplicate in a PCR optical 96-well reaction plate (Bio-Rad). 25 μl of the PCR mixture in each well contained 5 μl of cDNA (corresponding to 100 ng of total RNA), 2.5 μl of each sequence-specific primer (150 nM for β-actin, 600 nM for IL-11 and 300 nM for others cytokines), 12.5 μl of 1X iQ SYBR Green Supermix (Bio-Rad) and 2.5 μl of nuclease-free water. Primer sequences were designed to be cDNA specific and to work under equivalent reaction conditions using Beacon Designer 2 Software (Bio-Rad); primers were synthesized by Invitrogen and reconstituted in nuclease-free water before use. Primer sequences and reaction efficiency are listed in Table 2. A negative PCR control without cDNA template and a positive control sample with a known Ct value were included in each assay. Optimized thermal cycling conditions were as follow: 5 min at 95 °C followed by 40 cycles of 15 s at 95°C and 1 min at 60 °C (two step PCR). Specificity of the PCR products was confirmed by the melting curve program at the end of reaction (55–95°C with a heating rate of 0.5°C/10 s and continuous fluorescence measurements). PCR efficiency (E) was determined using the iCycler iQ software and the method described by Ramakers et al. [40]. For each sample the cycle threshold value (Ct) was acquired using the Fit point Method [41]. The mRNA expression data for β-actin showed no significant change compared with control and patient groups. The mean normalized gene expression (MNE) in cell lines was calculated by the following formula:
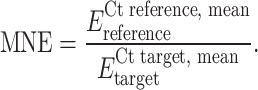 |
The relative expression ratio of the target cytokine genes was computed by the Relative Expression Software Tool (REST) [39]. This software calculates an expression ratio relative to the control group (normal pancreas tissue) normalized by a reference gene (β-actin). The expression ratio (R) is:
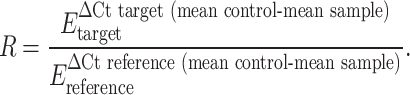 |
Table 2.
Primer sequences for cytokine quantification by real-time RT-PCR
| Primer set | GenBank accession # | Primer sequence (5′
 3′) 3′) |
RT-PCR E (%) |
|---|---|---|---|
| β −actin | NM_001101 |
FW -GCG AGA AGA TGA CCC AGA TC- RW -GGA TAG CAC AGC CTG GAT AG- |
96 |
| IL-1β | M15330 |
FW -TGA TGG CTT ATT ACA GTG GCA ATG- RW -GTA GTG GTG GTG GGA GAT TCG- |
93 |
| IL-2 | U25676 |
FW -CAA GAA TCC CAA ACT CAC CAG- RW -CGT TGA TAT TGC TGA TTA AGT CC- |
100.9 |
| IL-6 | M54894 |
FW -GTG TTG CCT GCT GCC TTC- RW -AGT GCC TCT TTG CTG CTT TC- |
101.3 |
| IL-8 | Y00787 |
FW -GAC ATA CTC CAA ACC TTT CCA C- RW -CTT CTC CAC AAC CCT CTG C- |
85.3 |
| IL-10 | M57627 |
FW -GAA CCA AGA CCC AGA CAT C- RW -CAT TCT TCA CCT GCT CCA C- |
98.4 |
| IL-11 | M57765 |
FW -GGA CTG CTG CTG CTG CTG AAG- RW -CAC GGA AGG ACT GTC TCT AAC- |
88.9 |
| IL-12 p40 | M65272 |
FW -TCG GCA GGT GGA GGT CAG C- RW -CGC AGA ATG TCA GGG GA AGT AGG- |
94.30 |
| IL-13 | NM_002188 |
FW -AAC ATC ACC CAG AAC CAG AAG- RW -CAG AAT CCG CTC AGC ATC C- |
92.5 |
| IL-18 | D49950 |
FW -CCT CCT GGC TGC CAA CTC T- RW -GAA GCG ATC TGG AAG GTC TGA G- |
97 |
| IFN-γ | M29383 |
FW -TGT GGA GAC CAT CAA GGA AGA C- RW -TGC TTT GCG TTG GAC ATT CAA G- |
96.7 |
| TGF-β1 | X02812 |
FW - GAC ACC AAC TAT TGC TTC AG- RW -CAG GCT CCA AAT GTA GGG- |
112.1 |
| TGF-β2 | M19154 |
FW -GCG AGA GGA GCG ACG AAG AG- RW -TGT AGA AAG TGG GCG GGA TGG- |
98.8 |
| TGF-β3 | J03241 |
FW -CGC CTC AAG AAG CAG AAG- RW -TGT CGG AAG TCA ATG TAG AG- |
115.3 |
FW = forward primer; RW = reverse primer; E = efficiency deducted from the slope (s) of the standard curve based on E=eln 10/-s- 1
Immunohistochemical detection of cytokines
The characteristics, working conditions and internal controls of antibodies used for immunohistochemistry are listed in Table 3. Briefly, formalin-fixed, paraffin-embedded sections were dewaxed and rehydrated through decreasing alcohol series up to distilled water. Endogenous peroxidase activity was suppressed by incubation with 3% solution of H2O2 for 5 min. Sections were subjected to heat-induced epitope retrieval (Target Retrieval Solution, DAKO, Carpinteria, CA, USA) for 10 min at 100 °C. Immunostaining for IL-1β, IL-6, IL-10, IL-11, IL-12, IL-18, IFN-γ, TGF-β1, TGF-β2 and TGF-β3 was done with peroxidase-based visualization DAKO LSAB registered kit, following the manufacturer’s recommendations. Immunostaining for IL-2, IL-8, and IL-13 used peroxidase-based visualization DAKO EnVision TM kit, following the manufacturer’s recommendations. Diaminobenzidine tetrahydrochloride was used as chromogen. The slides were then counterstained with Mayer hematoxylin for 5 s, dehydrated and mounted in Clarion (Biomeda, Foster City, CA, USA).
Table 3.
Antibodies and immunohistochemical procedures
| Reactivity | Antibody species/clones | Source/donor | Antibody dilutions | Positive internal control |
|---|---|---|---|---|
| IL-1β | Rabbit | Santa Cruz Biotech., Santa Cruz, CA | 1:20 | Macrophages |
| IL-2 | Rabbit | Santa Cruz | 1:20 | T cells |
| IL-6 | Goat | Santa Cruz | 1:200 | Macrophages |
| IL-8 | Rabbit | Santa Cruz | 1:30 | Macrophages |
| IL-10 | Rat (IgG2a)clones JES3-12G8 | BD Pharmingen, San Diego, CA, | 1:1000 | T cells |
| IL-11 | Goat | Santa Cruz | 1:20 | Fibroblasts |
| IL-12 (p40/p70) | Mouse (IgG1)clone C8.6 | a | 1:200 | Macrophages |
| IL-13 | Rabbit | Santa Cruz | 1:40 | T cells |
| IL-18 | Mouse (IgG1)clone 125-2H | MBL, Nagoya, Japan | 1:100 | Macrophages T cells |
| IFN-γ | mouse IgG1Clones B133.1, B133.5 | a | 1:100 | T cells |
| TGF-β1 | Rabbit | Santa Cruz | 1:20 | Macrophages |
| TGF-β2 | Rabbit | Santa Cruz | 1:40 | Macrophages |
| TGF-β3 | Rabbit | Santa Cruz | 1:20 | Macrophages |
aKindly provided by Giorgio Trinchieri, Schering-Plough Laboratory for Immunological Research, Dardilly, France.
To determine unspecific staining, either peptides provided by the manufacturer that blocked polyclonal antibody binding, or non-immune mouse serum, was used. Positivity for the different cytokines was evaluated as a brown stain in the cytoplasm of normal and neoplastic pancreatic cells. The occasional positivity observed in the scanty interstitial inflammatory infiltrate was used as internal positive control. The degree of immunostaining (IRS) was evaluated as follows: IRS = SI (staining intensity) × PP (percentage of positive cells). SI was classified as 0 = negative; 1 = weak; 2 = moderate; and 3 = strong. PP was defined as 0 = negative; 1 = 1–20% positive cells; 2 = 21–50% positive cells; and 3 = 51–100% positive cells. Immunostaining was evaluated independently by two observers (FM and GB).
Cytokine detection
Cell free-supernatants from cell lines and venous serum samples, collected from patients prior to surgery and/or chemotherapy (n=65) and, for comparison, from 30 healthy donors (13 m, 17 f, median age 40, range 24–65 years), were assayed for IL-1β, IL-6, IL-8, IL-10, IL-12 total p40, and IL-13, using commercially available ELISA kits from Euroclone (Paignton, Devon, UK), and for IL-18, IL-11, TGF-β1 and TGF-β2 using commercially available ELISA kits from R&D Systems (Abingdon, UK). The minimum detectable cytokine concentrations were 5 pg/ml, 0.8, 25, 5, 20, 1.5, 12.5, 31.2, 50, and 7 pg/ml, respectively.
Statistical analysis
Statistical analysis of data was performed using the biomedical statistical software package BMDP Dynamic, Rel. 7.0 (Statistical Solutions, Cork, Ireland). The compatibility of data from continuous variables with a normal distribution was checked by means of Shapiro and Wilk’s W test. Throughout the paper, means and SD are given as measures of central tendency and dispersion for data with normal distribution, medians and range otherwise. Differences between continuous variables were analyzed by the Student’s t test or one-way analysis of variance when data were normally distributed, and by means of the Mann–Whitney or the Kruskal–Wallis test otherwise. Associations between categorical variables were explored by means of Fisher’s exact test and, when appropriate, the chi-square test for linear trend. Survival data for patients still alive at the end of the study were calculated to the time of the last follow-up. The log-rank (Mantel-Cox) test was used to test the null hypothesis of similarity of survival times among patients with different IHC cytokine patterns. Survival probabilities were plotted as Kaplan–Meier curves. The hazard ratio (R), which gives the relative event rates in the groups, and the 95% confidence interval of R were calculated to measure relative survival. The statistical significance of differences in cytokine mRNA expression examined in patients and controls was analyzed using the REST, which allows group-wise comparison and statistical analysis of relative expression results in real-time PCR [39].
A level of 0.05 (two tailed) was taken to indicate statistical significance.
Results
Expression of pro- and anti-inflammatory cytokines in pancreatic carcinoma cell lines, tumor and non-tumor pancreatic tissues
Pro-(IL-1β, IL-2, IL6, IL-8, IL-12p40, IL-18, IFN-γ) and anti-(IL-10, IL-11, IL-13, TGF-β1, TGF-β2, TGF-β3) inflammatory cytokine gene expression was quantitatively assessed in human pancreatic carcinoma cell lines as well as in primary tumors and in control samples (normal pancreas) using real-time RT-PCR.
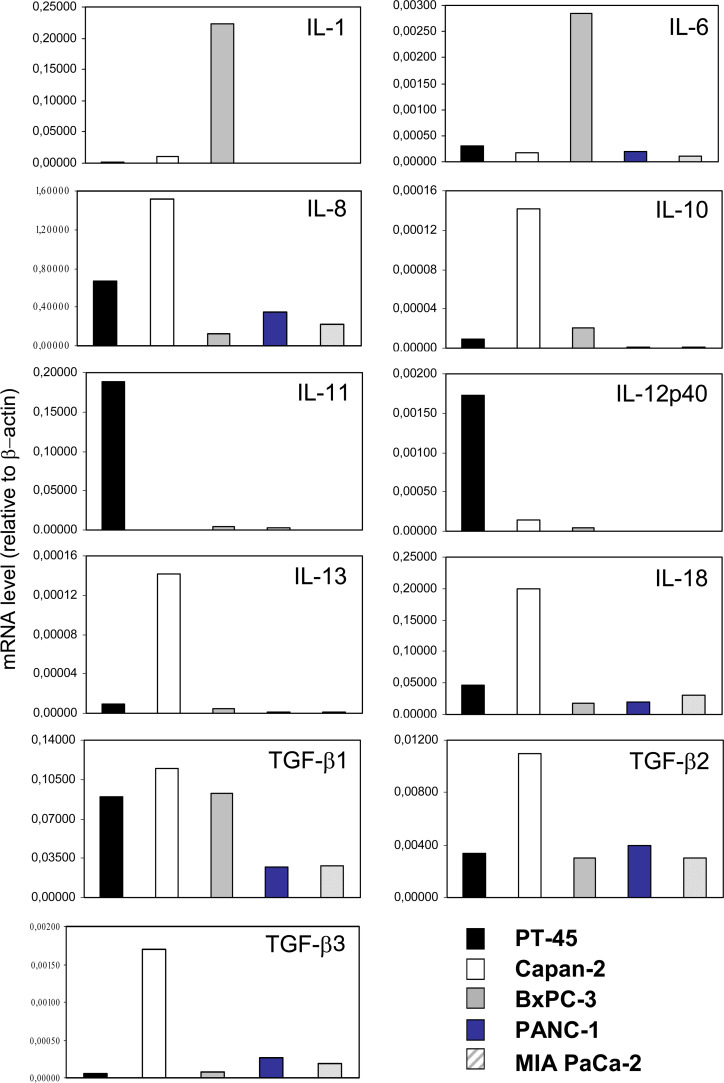
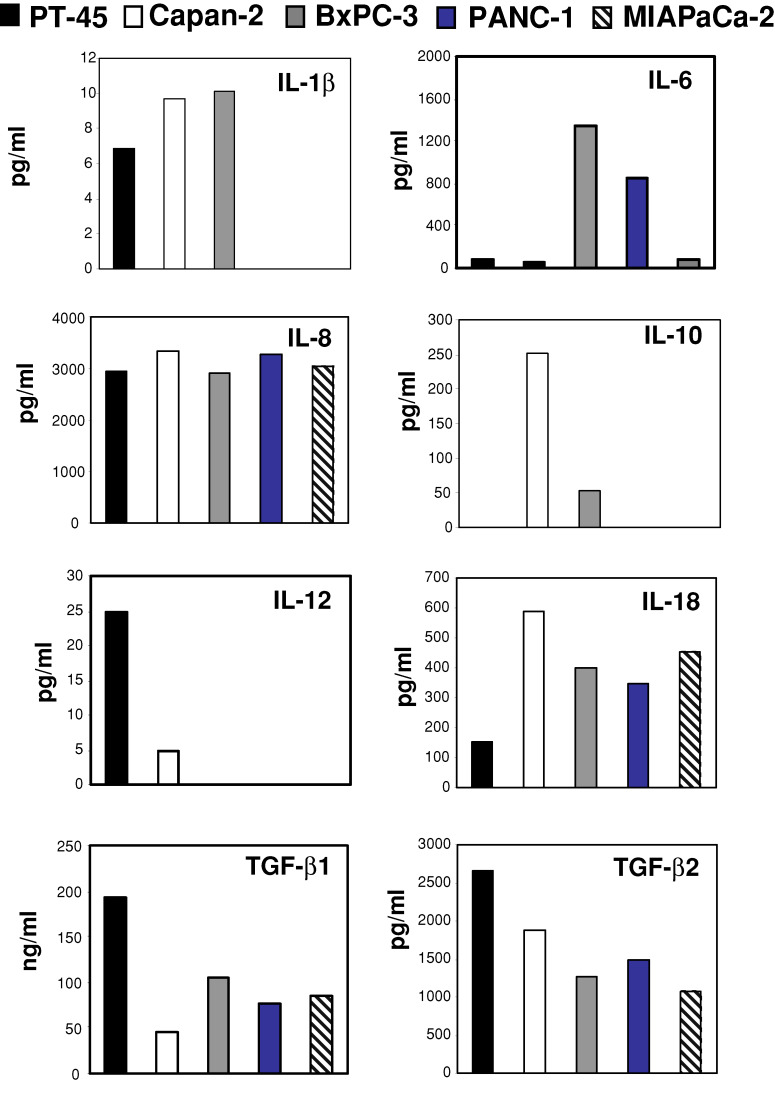
Normalized gene expression was compared across cell lines. As shown in Fig. 1, PT-45 cells expressed IL-8, IL-11, IL-12p40, TGF-β1 and TGF-β2 at relatively high levels, while they expressed lower levels of IL-6, IL-18 and TGF-β3, and minimal levels of IL-1β, IL-10 and IL-13. IL-8, IL-10, IL-18, TGF-β isoforms were expressed at relatively high levels by Capan-2 cells, while IL-1β, IL-6, IL-12p40 and IL-13 were minimally expressed; IL-11 mRNA was not found. BxPC-3 expressed IL-1β, IL-6, TGF-β1 at relatively high levels, while they expressed lower levels of IL-8, IL-18 and TGF-β2, and minimal levels of IL-10, IL-11, IL-12p40, IL-13 and TGF-β3. PANC-1 and MIAPaCa-2 cells exhibited low IL-6, IL-8, IL-18, TGF-β1, TGF-β2, TGF-β3, mRNA expression and were almost negative for IL-1β, IL-10, IL-11, IL-12p40 and IL-13. No cell lines expressed IL-2 or IFN-γ transcripts (data not shown).
Fig. 1.
Levels of pro- (IL-1β, IL-6, IL-8, IL-12 p40, IL-18) and anti-inflammatory (IL-10, IL-11, IL-13 and TGF-β isoforms) cytokine mRNA in PT-45, Capan-2, BxPC-3, PANC-1 and MIAPaCa-2 cell lines. mRNA levels were assessed via real-time RT-PCR, and normalized to β-actin mRNA levels. MNE values are shown
Moreover, quantitative PCR results showed that IL-1β mRNA was present in 86% of malignant specimens, IL-8, IL-10 and IL-12p40 mRNA in 78%, IL-6 and IL-11 in 64%, IL-2 mRNA in 57%, IFN-γ mRNA in 28%, IL-13 mRNA in 14%. All tumor specimens expressed IL-18, TGF-β1, TGF-β2, and TGF-β3 messages. As shown in Table 4, which reports cytokine gene expression ratios in relation to normal pancreatic tissue, in pancreatic carcinoma specimens TGF-β1, IL-8 and TGF-β3 were strongly up-regulated, by a median factor of 474.4, 213.4 and 173.9, respectively (P<0.001). TGF-β2, IL-6, IL-10, IL-11, IL-1β and IL-18 mRNAs were also overexpressed, although to different extents (median factors: 83, 62.4, 56.5, 54.6, 28.5, and 12.9, respectively, P<0.001). Lower up-regulation was observed for IFN-γ (median factor 5.6, P=0.004) and IL-12p40 messages (median factor 3.7, P=0.01). No significant differences in IL-2 mRNA expression were found between tumor and non-tumors samples. The only cytokine down-regulated in pancreatic carcinoma compared with its normal counterpart was IL-13 (median factor 0.03, P<0.001).
Table 4.
Cytokine gene expression ratios in relation to normal tissue in pancreatic carcinoma specimens
| Gene expression ratioamedian (range, min-max) | P valueb | |
|---|---|---|
| IL-1β | 28.5 (2.3–237.9) | <0.001 |
| IL-2 | 1.9 (0.14–54) | 0.2 |
| IL-6 | 62.4 (0.8–2313.9) | <0.001 |
| IL-8 | 213.4 (2.8–810.8) | <0.001 |
| IL-10 | 56.5 (0.3–208.3) | <0.001 |
| IL-11 | 54.6 (0.7–635.5) | <0.001 |
| IL-12 p40 | 3.7 (0.4–29.5) | 0.01 |
| IL-13 | 0.03 (0.006–0.98) | <0.001 |
| IL-18 | 12.9 (3.9–28.4) | <0.001 |
| IFN-γ | 5.6 (0.22–85.6) | 0.004 |
| TGF-β1 | 474.4 (44.9–2907.1) | <0.001 |
| TGF-β2 | 83 (8.6–260.4) | <0.001 |
| TGF-β3 | 173.9 (18.2.1–2171.6) | <0.001 |
aCalculated according to the REST-software, bMann–Whitney test
When patients were divided between those with locally extended tumors (UICC stages II and III) and those with metastatic tumors (UICC stage IV), significantly higher IL-8 mRNA expression was observed in higher tumor stages (P=0.01).
Cytokine detection in pancreatic carcinoma tissue specimen in situ
The cytoplasmatic protein expression pattern is presented in Table 5. To compare immunoreactivity scores (IRS) of tumoral and non-tumoral tissues, IRS were grouped as low (scores 0 and 1) or high (scores 2 and 3), except for IL-18 and TGF-β isoforms (grouped as moderate, scores 1–2, or high, score 3). Figures 2 and 3 show the immunohistochemical staining of representative non-tumoral and tumoral pancreas tissue samples.
Table 5.
Cytoplasmatic cytokine expression patterns
| Cytokine expression | Tumor | Normal exocrine | Normal endocrine | P valuea | |||
|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | ||
| Pro-inflammatory | |||||||
| IL-1β | 15b | 26 | 8 | 1 | 1 | 8 | |
| IL-2 | 29 | 12 | 9 | 0 | 3 | 6 | NS |
| IL-6 | 15 | 26 | 2 | 7 | 2 | 7 | NS |
| IL-8 | 34 | 7 | 3 | 6 | 9 | 0 | 0.006 |
| IL-12 total p40 | 10 | 31 | 9 | 0 | 9 | 0 | <0.0001 |
| IL-18c | 8 | 33 | 9 | 0 | 9 | 0 | <0.0001 |
| IFN-γ | 0 | 0 | 0 | 0 | 9 | 0 | – |
| Anti-inflammatory | |||||||
| IL-10 | 15 | 26 | 9 | 0 | 9 | 0 | 0.0005 |
| IL-11 | 15 | 26 | 9 | 0 | 9 | 0 | 0.0005 |
| IL-13 | 41 | 0 | 2 | 7 | 9 | 0 | <0.0001 |
| TGF-β1c | 8 | 33 | 2 | 7 | 4 | 4 | NS |
| TGF-β2c | 0 | 41 | 2 | 7 | 9 | 0 | 0.03 |
| TGF-β3c | 1 | 40 | 3 | 6 | 9 | 0 | 0.01 |
aFisher’s exact test (two tails)
bIRS
cSee Results for details
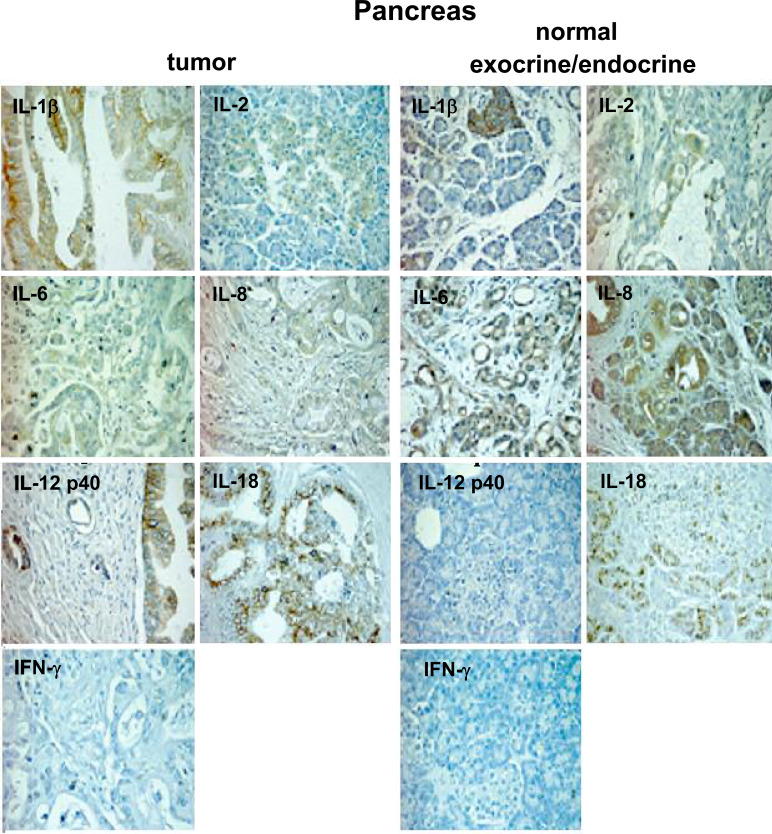
Fig. 2.
Detection of pro-inflammatory cytokines in non-tumoral and tumoral pancreatic tissue specimens as determined by immunohistochemistry. Specimens are representative examples of nine non-tumoral and forty one tumoral pancreas tissue samples (original magnification 250×)
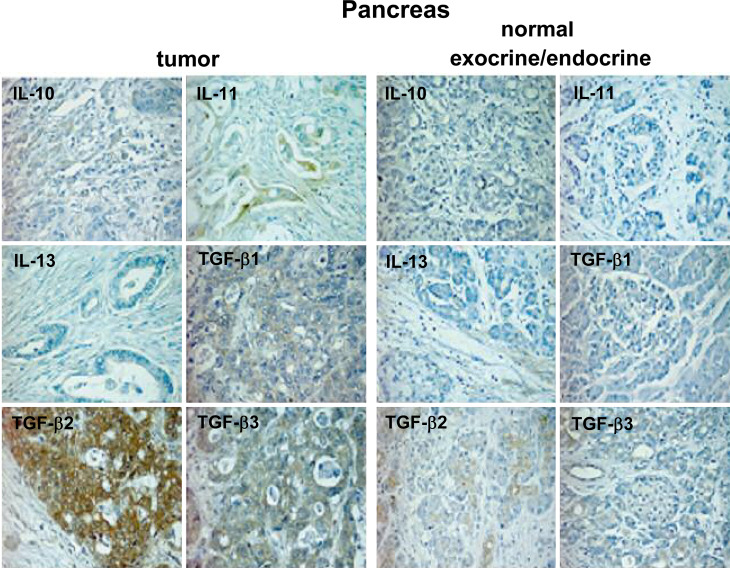
Fig. 3.
Detection of anti-inflammatory cytokines in non-tumoral and tumoral pancreatic tissue specimens as determined by immunohistochemistry. Specimens are representative examples of nine non-tumoral and 41 tumoral pancreas tissue samples (original magnification 250×)
Among pro-inflammatory cytokines (Fig. 2), those that were more expressed in tumoral than in non-tumoral tissue samples included IL-1β (26/41 vs. 1/9, P=0.007), IL-12 total p40 (31/41 vs. 0/9, P<0.0001) and IL-18 (33/41 vs. 0/9, P<0.0001); conversely, IL-8 was less expressed in tumoral than in non-tumoral tissues (7/41 vs. 6/9, P=0.006). Staining for IFN-γ was not observed in either type of tissue sections.
Regarding anti-inflammatory cytokines (Fig. 3), a higher expression in tumoral than in non-tumoral tissues was observed for IL-10 (26/41 vs. 0/9, P=0.0005), IL-11 (26/41 vs. 0/9, P=0.0005), TGF-β2 (41/41 vs. 7/9, P=0.03) and TGF-β3 (40/41 vs. 6/9, P=0.01); IL-13 expression was observed only in non-tumoral tissues (0/41 vs. 7/9, P<0.0001).
Non-tumoral endocrine pancreatic tissue showed different patterns of positivity for IL-1β IL-2, IL-6 and TGF-β1, and was completely negative for all other cytokines.
Correlation between immunostaining score and clinical parameters
When specimens were categorized in the order—normal pancreatic tissue - tumoral tissues from stage II–stage III–stage IV patients—a linear trend for increased cytokine immunostaining was observed with regard to IL-1β (P=0.014), IL-10 (P=0.001), IL-11 (P=0.004), IL-12p40 (P=0.0007), IL-18 (P=0.0002), TGF-β2 (P=0.009) and TGF-β3 (P=0.004).
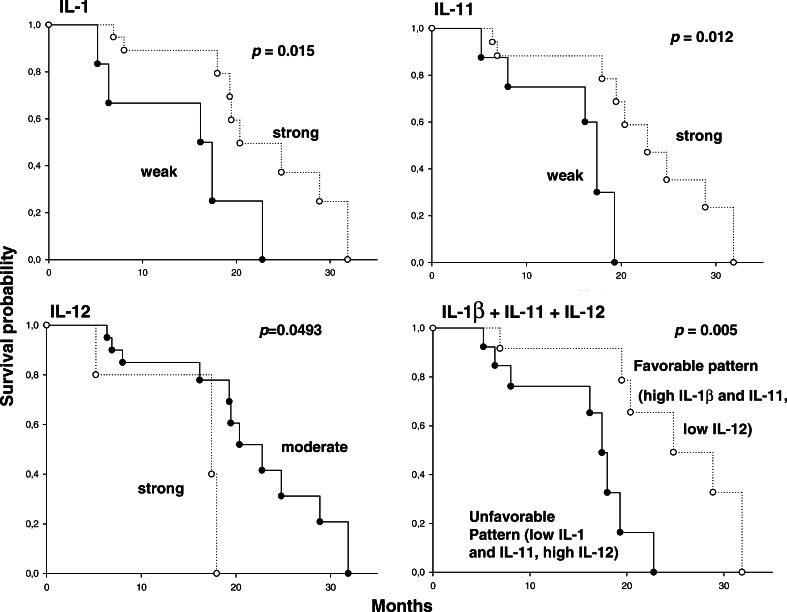
An association was found between protein expression (using an IRS score cutoff point of 1) and survival (Fig. 4), with regard to IL-1 (P=0.015; hazard ratio 3.41, 95% C.I. 1.44–32.66) and IL-11 (P=0.012; hazard ratio 3.33, 95% C.I. 1.59–42.39); in both cases shorter survival times were associated with lower expression levels. A weaker, inverse association existed between IL-12 expression and survival (P=0.049, hazard ratio 2.03, 95% C.I. 0.54–7.2), with moderate (IRS=2) tissue expression being associated with longer survival times than high (IRS=3) expression. When patients with all three “protective” features (high IL-1β and IL-11, and moderate IL-12 expression) were compared to all remaining patients, a stronger survival advantage emerged (P=0.0055, hazard ratio 0.2, 95% C.I. 0.02–0.37).
Fig. 4.
Kaplan–Meier estimates of survival in pancreatic carcinoma patients, stratified by in situ cytokine expression pattern
Cytokine levels in supernatants of pancreatic carcinoma cell lines and in patients’ serum samples
Constitutive production of high levels of IL-8, IL-18, TGF-β1 and TGF-β2, and of lower levels of IL-1β, was detected in the supernatants of all cell lines (Fig. 5). IL-6 was produced especially by BxPC-3 and Panc-1 cells, while IL-10 was produced only by Capan-2 and BxPC-3. IL-12 total p40 was detected in PT-45 supernatants. IL-13 not detected in any specimen.
Fig. 5.
Secretion of cytokines by pancreatic carcinoma cell lines as determined by ELISA in supernatants
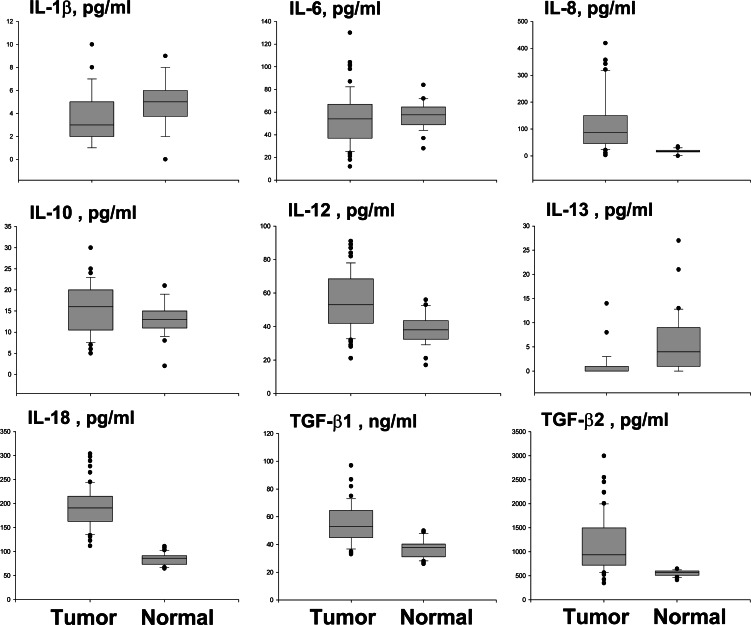
Figure 6 presents a comparison of serum cytokine levels in pancreatic carcinoma patients versus healthy blood donors. IL-8 (median 87.6 pg/ml, range 3.3–419.5 vs. 17.5 pg/ml, range 0–35, P<0.0001), IL-10 (mean 15.5±5.7 pg/ml vs. 13.5±3.7 pg/ml, P=0.04), IL-12 p40/p70 (mean 55.6±16.9 pg/ml vs. 38.5±9 pg/ml, P<0.0001), IL-18 (mean 192.8±42.6 pg/ml 84.7±12.5 pg/ml, P<0.0001), TGF-β1 (mean 55.2±13.9 ng/ml vs. 37.9±6.6 ng/ml, P<0.0001) and TGF-β2 (median 934 pg/ml, range 345–2993 vs. 568 pg/ml range 413–645, P<0.0001) were detected at higher concentrations in patients than in controls. Conversely, IL-1β (mean 4.04±2.15 pg/ml vs. 5.35±2 pg/ml, P=0.005) and IL-13 levels (median 0 pg/ml, range 0–14.6, vs. 4.4 pg/ml range 0–27.4, P<0.0001) were lower in patients than in controls. IL-6 levels were similar in patients and controls. Circulating IL-11 levels were in all cases below the detection limit of the assay.
Fig. 6.
Serum cytokine concentration in patients and controls by ELISA. Median, 10th, 25th, 75th and 90th percentiles are presented as vertical boxes with error bars. Dots indicate outliers
Correlation between serum cytokine levels and clinical parameters
When circulating cytokine levels assessed in patients with different tumor UICC stages (II, III and IV) were compared, statistically significant differences were found for IL-1β (mean values respectively 2.3±0.8 pg/ml vs. 4±1.2 pg/ml vs. 4.9±2.5 pg/ml, P=0.0001), IL-6 (means 45.5±23.5 pg/ml vs. 43.6±17.3 pg/ml vs. 65.4±22.2 pg/ml, P=0.0009), IL-10 (means 11.8±5.2 pg/ml vs. 16.1±5.1 pg/ml vs. 17.8±5.2 pg/ml, P=0.001), IL-12 (means 42.4±13.4 pg/ml vs. 48.8±11.8 pg/ml vs. 66.9±13.9 pg/ml, P<0.0001), TGF-β1 (mean 43.2±7.5 ng/ml vs. 57.2±16 ng/ml vs. 60.4±11.3 ng/ml, P<0.0001) and TGF-β2 (means 667.2±156.3 pg/ml vs. 1340.4±645.7 pg/ml vs. 1249.9±554 pg/ml, P=0.0004).
When patients were divided into those with locally extended tumors (UICC stages II and III) and those with metastatic tumors (UICC stage IV), significant differences were observed for IL-1β (mean values respectively 3.2±1.4 pg/ml vs. 4.9±2.5 pg/ml, P=0.001), IL-6 (means 44.5±20.1 pg/ml vs. 65.4±22.2 pg/ml, P=0.0001), IL-10 (means 14.1±5.5 pg/ml vs. 17.8±5.2 pg/ml, P=0.008), IL-12 (means 45.9±12.8 pg/ml vs. 66.9±13.9 pg/ml, P<0.0001) and TGF-β1 means 50.8±14.5 pg/ml vs. 60.4±11.3 pg/ml, P=0.004).
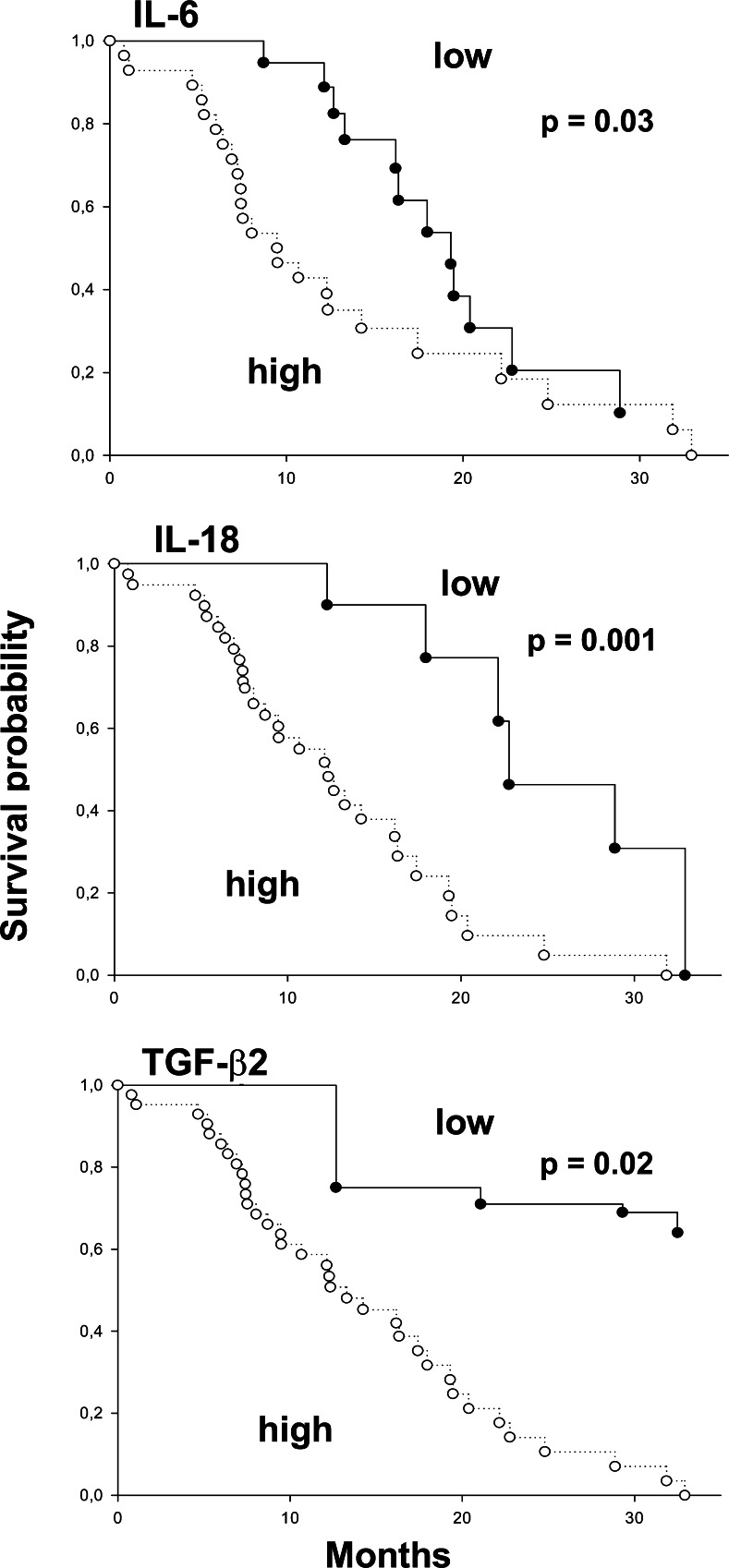
Categorizing circulating cytokine concentrations as low versus high (with median values taken as cutoff points) a survival advantage was observed for patients with low IL-6 (P=0.03), IL-18 (P=0.001) and TGF-β2 (P=0.02). The corresponding Kaplan–Meier curves are in Fig. 7. There was a trend for IL-12 to be associated with longer survival (Mantel-Cox P=0.051).
Fig. 7.
Kaplan–Meier estimates of survival in pancreatic carcinoma patients, stratified by circulating serum cytokine pattern
Discussion
The present study shows for the first time that both pro- and anti-inflammatory cytokine mRNAs and proteins are expressed by pancreatic carcinoma cells in vitro, and can also be detected in situ in the tumor microenvironment and systemically in patients with pancreatic carcinoma, importantly suggesting potential significant consequences for tumor progression and patient disease outcome.
The data indicate that, in patients with pancreatic carcinoma, TGF-β isotypes are overexpressed both in the tumor microenvironment and systemically. Moreover, all cell lines tested expressed the three TGF-β isotypes. In epithelial cells, TGF-β blocks cell proliferation [21]. However, inactivating mutations of receptor genes or alterations in TGF-β signal transduction pathway occurring in tumor cells, including pancreatic carcinoma cells, may render them resistant to TGF-β’s anti-proliferative effects [13, 17]. In addition, TGF-β may act as an immunosuppressant, similarly to and in synergy with the typical type-2 cytokine IL-10, also found here overexpressed, subverting local attempts to elicit a type-1 immune reaction to a tumor tolerogenic response [28]. The presence of IL-10 at the site of growing tumors has been reported in a variety of human cancers, including melanoma, non-small cell lung carcinoma, renal cell carcinoma and bladder cancer [14, 18, 20, 33, 44]. Moreover, increased IL-10 serum levels have been reported in patients with melanoma, renal cell carcinoma, gastric and colon carcinoma [11]. In a previous study we showed that pancreatic carcinoma cell-derived IL-10 inhibited, in an additive fashion with TGF-β both proliferation and development of type-1 immune responses in PBMC from normal donors, and that activated PBMC derived from pancreatic carcinoma patients preferentially displayed a type-2-like cytokine expression pattern compared to normal controls [5].
To the best of our knowledge, this is the first demonstration of the local presence of up-regulated IL-11 mRNA and protein in primary pancreatic cancer tissues, in comparison with normal counterparts. IL-11 has recently been considered to be a cytokine with anti-inflammatory capacity [53], expressed in several tumors, including thyroid carcinoma cells, breast carcinomas and melanomas [37, 48, 51]. Its role in cancer is not fully elucidated; however, since IL-11 led to immune response impairment in a tumor model, a paracrine function of this cytokine during tumor progression has been proposed [52]. Surprisingly, pancreatic carcinoma cells in vivo did not produce the Th2 cytokine IL-13, in contrast to their non-tumor counterparts, and IL-13 serum levels in patients were lower than in healthy subjects. Nevertheless, given that IL-13 can exhibit both pro- and anti-tumorigenic activity [50], the exact role of IL-13 must be carefully dissected.
One major finding in this study is that pancreatic carcinoma cells produce IL-12 and IL-18, and that higher serum levels of both cytokines were detected in patients than were found in normal subjects. Bioactive heterodimer IL-12 p70, composed of p35 and p40 subunits, is a potent Th1-related cytokine clearly involved in the anti-tumor response [54]. In contrast, the p40 homodimer, and to a lesser extent the p40 monomer, have been shown to compete, both in mice and in man, at the IL-12 receptor level [30], inhibiting IL-12-dependent immune functions in vitro and in vivo [24]. All pancreatic carcinoma cells expressed IL-12 p35 mRNA (data not shown), but only PT-45 and Capan-2 cells expressed IL-12 p40. In supernatants, only IL-12 total p40 was detected, but not IL-12 p70 (data not shown). In situ, the cytokine appeared to be more expressed in tumor than normal samples, and IL-12 total p40 serum levels in patients were found to be above the normal level, while IL-12 p70 levels were undetectable (data not shown). These findings strongly suggest that, in pancreatic carcinoma, free IL-12 p40 can act as an IL-12 antagonist during development of the anti-tumor immune response.
Like IL-12 and in synergy with it, biologically active IL-18, elaborated by proteolytic cleavage of a cytoplasmic 24-kDa precursor (pro-IL-18) [12], induces IFN-γ and up-regulates cytotoxic activities of NK cells and CTLs [35]. We recently demonstrated that, in basal conditions, pancreatic carcinoma cells preferentially produce the inactive form of the cytokine [7]. Thus it seems that, in spite of elevated local and circulating IL-18 levels, tumor activity is not effectively suppressed. This finding is not so unexpected: several recent studies have shown that a wide range of tumors express IL-18 in situ [22, 29, 32, 36, 38, 61, 64]. It is conceivable that, in vivo, tumor-associated IL-18 might compete with the active isoforms, allowing malignant cells to escape from immune-mediated control. On the other hand, IL-18, but not IL-12, may potentially act as a cofactor of both Th1 and Th2 cell development, depending on the surrounding cytokine milieu [34]. Thus the role of this cytokine in the anti-tumor response remains elusive.
Cytokines with immunoregulatory activities, such as IL-1, IL-6, and IL-8, have also been suggested to be involved, via autocrine or paracrine mechanisms, in the carcinogenesis and progression of some types of tumor, by attracting inflammatory cells, promoting new vessel formation in growing tumors, and altering cell adhesion molecules and chemotaxis. We show here that cytokines are overexpressed in situ at the pancreatic tumor site and that some of them are increased in patients’ sera. IL-1, regulating key enzymes of the extracellular matrix and inducing secretion by fibroblasts of neutral proteases such as collagenase, elastase, stromolysin, and plasminogen activator [9], may provide the advantage the tumor needs to invade the basement membrane [2]. IL-6, as a secondary proinflammatory cytokine, has been found to be “bifunctional" in a variety of human tumors, in that it can switch from behaving as a paracrine growth inhibitor to behaving as an autocrine growth stimulator with the same cells during malignant tumor cell proliferation [26]. In addition, as a tumor product, it causes severe cachexia [3]. IL-8, in addition to being a chemotactic factor for leukocytes, has recently been shown to contribute to human cancer progression through its functions as mitogenic, angiogenic, and motogenic factor [23].
Given the role of cytokines in tumor biology, our attention was also focused on the clinical application of our findings on in situ cytokine expression and circulating cytokine levels, for predicting clinical outcome. However, it is important to remember that prognosis overall for pancreatic cancer is very poor, with 1-year survival rate being below approximately 20% [62]. This dim prognosis is confirmed here, and is outlined by the skewed distribution observed in the Kaplan–Meier plots. Nevertheless, a “protective” cytokine immunoreactivity pattern, consisting in high IL-1β and IL-11, and moderate IL-12 expression, appears to exist and confers some survival advantage. Low levels of IL-1β expression and association with an increased risk for cancer-specific death in bladder cancer patients and elevated glial expression of IL-1 with improved survival of glioblastoma multiforme patients have been reported [8, 46]. IL-1β, as IL-18, is produced as a leaderless prohormone that requires specific enzymatic activating cleavage [10]. Thus, the apparent paradox of improved survival in the presence of enhanced IL-1β might be due to competition between its active and its inactive forms.
Finally, we found that elevated serum levels of IL-6, IL-18, TGF-β2, and, to a lesser extent, of IL-12, were correlated with poor survival. This is in accordance with reports showing a correlation between increased serum levels of proinflammatory cytokines, such as IL-6, IL-12 and IL-18, and advanced stage, metastatic disease and poor outcome in other types of cancer [1, 6, 15, 25, 31, 42, 49, 55, 56]. Moreover, elevated TGF-β serum levels were found to be correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma and nasopharyngeal carcinomas [43, 63]. The demonstration of high IL-6, IL-12, IL-18 and TGF-β serum levels in pancreatic carcinoma might thus be a useful indicator of poor prognosis.
Inflammatory infiltrates are frequently observed in pancreatic carcinomas. The finding of the presence of IL-2 and IFN-γ messages in several of our tumor tissue samples demonstrates an attempted anti-tumor immune response. However, the immunological microenvironment of pancreatic carcinoma was found to be clearly in an immunosuppressive state, as convincingly illustrated by the aberrant concomitant expression of potent anti-inflammatory cytokines, such as TGF-β and IL-10 and potentially inactive proinflammatory cytokines, such as IL-12 and IL-18. The cellular immunosuppression observed in many patients is a striking biological feature of pancreatic carcinoma [58] and attempts to expand and enhance tumor-specific immunity with biotherapy have not yet met with success, suggesting that tumor cells in vivo produce protective factors that can specifically defend them against immune attack.
The survival rate for pancreatic adenocarcimona is among the poorest of all cancers. Lack of effective early detection, high rates of metastases and lack of response to available treatment modalities contribute to this poor prognosis. New drugs, molecular targeted therapy and immunotherapy offer promise in theory [16, 27], but there is little evidence to justify their place in today’s treatment protocols. We hope that this unprecedented complete analysis of the cytokine profile, in pancreatic carcinoma, at both the tumor and the systemic level, may contribute to understanding the mechanisms of tumor escape from immune-surveillance and immune-mediated killing, hence potentially defining new strategies for effective immunomodulatory treatment intervention.
Acknowledgments
This work was supported by a grant from MIUR (Rome, Italy) (ex-60%) to GE, and in part by a grant from the Piedmontese Regional Government (Regione Piemonte) to GB. ET and AB are the recipients of an award from the Piedmontese Regional Government.
References
- 1.Alexandrakis MG, Passam FH, Pappa CA, Sfiridaki K, Tsirakis G, Damilakis J, Stathopoulos EN, Kyriakou DS. Interleukin-18 in multiple myeloma patients: serum levels in relation to response to treatment and survival. Leuk Res. 2004;28:259–266. doi: 10.1016/S0145-2126(03)00261-3. [DOI] [PubMed] [Google Scholar]
- 2.Apte RN, Voronov E. Interleukin-1–a major pleiotropic cytokine in tumor-host interactions. Semin Cancer Biol. 2002;12:277–290. doi: 10.1016/S1044-579X(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 3.Barton BE. IL-6-like cytokines and cancer cachexia: consequences of chronic inflammation. Immunol Res. 2001;23:41–58. doi: 10.1385/IR:23:1:41. [DOI] [PubMed] [Google Scholar]
- 4.Bellone G, Smirne C, Carbone A, Mareschi K, Dughera L, Farina EC, Alabiso O, Valente G, Emanuelli G, Rodeck U. Production and pro-apoptotic activity of soluble CD95 ligand in pancreatic carcinoma. Clin Cancer Res. 2000;6:2448–2455. [PubMed] [Google Scholar]
- 5.Bellone G, Turletti A, Artusio E, Mareschi K, Carbone A, Tibaudi D, Robecchi A, Emanuelli G, Rodeck U. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol. 1999;155:537–547. doi: 10.1016/s0002-9440(10)65149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berek JS, Chung C, Kaldi K, Watson JM, Knox RM, Martinez-Maza O. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol. 1991;164:1038–1042. doi: 10.1016/0002-9378(91)90582-c. [DOI] [PubMed] [Google Scholar]
- 7.Carbone A, Rodeck U, Mauri FA, Sozzi M, Gaspari F, Smirne C, Prati A, Addeo A, Novarino A, Robecchi A, Berretto O, Emanuelli E, Bellone G. Human pancreatic carcinoma cells secrete bioactive Interleukin-18 after treatment with 5-Fluorouracil: implications for anti-tumor immune response. Cancer Biol Ther. 2005;4:231–241. doi: 10.4161/cbt.4.2.1476. [DOI] [PubMed] [Google Scholar]
- 8.Cuny E, Loiseau H, Penchet G, Ellie E, Arsaut J, Vital A, Vincendeau P, Demotes-Mainard J. Association of elevated glial expression of interleukin-1beta with improved survival in patients with glioblastomas multiforme. J Neurosurg. 2002;96:294–301. doi: 10.3171/jns.2002.96.2.0294. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Interleukin-1, interleukin-1 receptors and interleukin-1 receptor antagonist. Int Rev Immunol. 1998;16:457–499. doi: 10.3109/08830189809043005. [DOI] [PubMed] [Google Scholar]
- 10.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1) J Clin Immunol. 1999;19:1–11. doi: 10.1023/A:1020506300324. [DOI] [PubMed] [Google Scholar]
- 11.Foppoli M, Gianotti L, Galli L, Citterio G, Consogno G, Gentilini O, Braga M. Increased interleukin-10 serum levels in patients with solid tumors. Cancer Lett. 1996;104:1–5. doi: 10.1016/0304-3835(96)04213-9. [DOI] [PubMed] [Google Scholar]
- 12.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Caspase-1 processes IFN-β-inducing factor and regulates LPS-induced IFN-β production. Nature. 1997;386:619–623. doi: 10.1038/386619a0. [DOI] [PubMed] [Google Scholar]
- 13.Goggins M, Shekher M, Turnacioglu K, Yeo CJ, Hruban RH, Kern SE. Genetic alterations of the transforming growth factor beta receptor genes in pancreatic and biliary adenocarcinomas. Cancer Res. 1998;58:5329–5332. [PubMed] [Google Scholar]
- 14.Huang M, Wang J, Lee P, Sharma S, Mao JT, Meissner H, Uyemura K, Modlin R, Wollman J, Dubinett SM. Human non-small cell lung cancer cells express a type 2 cytokine pattern. Cancer Res. 1995;55:3847–3853. [PubMed] [Google Scholar]
- 15.Kawabata T, Ichikura T, Majima T, Seki S, Chochi K, Takayama E, Hiraide H, Mochizuki H. Preoperative serum interleukin-18 levels as a postoperative prognostic marker in patients with gastric carcinoma. Cancer. 2001;92:2050–2055. doi: 10.1002/1097-0142(20011015)92:8<2050::AID-CNCR1544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Okada T, Akada M. Development of immunotherapy for pancreatic cancer. Pancreas. 2004;28:320–325. doi: 10.1097/00006676-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Kleeff J, Ishiwata T, Maruyama H, Friess H, Truong P, Buchler MW, Falb D, Korc M. The TGF-beta signaling inhibitor Smad7 enhances tumorigenicity in pancreatic cancer. Oncogene. 1999;18:5363–5372. doi: 10.1038/sj.onc.1202909. [DOI] [PubMed] [Google Scholar]
- 18.Kruger-Krasagakes S, Krasagakis K, Garbe C, Schmitt E, Huls C, Blankenstein T, Diamantstein T. Expression of interleukin 10 in human melanoma. Br J Cancer. 1994;70:1182–1185. doi: 10.1038/bjc.1994.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Lattime EC, McCue PA, Keely FX, Li W, Gomella LG. Expression of IL-10 mRNA in biopsies of superficial and invasive TCC of the human bladder. Proc Am Assoc Cancer Res. 1995;36:462. [Google Scholar]
- 21.Lawrence DA. Transforming growth factor-beta: a general review. Eur Cytokine Netw. 1996;7:363–374. [PubMed] [Google Scholar]
- 22.Lebel-Binay S, Thiounn N, De Pinieux G, Vieillefond A, Debre B, Bonnefoy JY, Fridman WH, Pages F. IL-18 is produced by prostate cancer cells and secreted in response to interferons. Int J Cancer. 2003;106:827–835. doi: 10.1002/ijc.11285. [DOI] [PubMed] [Google Scholar]
- 23.Li A, Dubey S, Varney ML, Dave BJ, Singh RK. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 24.Ling P, Gately MK, Gubler U, Stern AS, Lin P, Hollfelder K, Su C, Pan YC, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 25.Lissoni P, Brivio F, Rovelli F, Fumagalli G, Malugani F, Vaghi M, Secondino S, Bucovec R, Gardani GS. Serum concentrations of interleukin-18 in early and advanced cancer patients: enhanced secretion in metastatic disease. J Biol Regul Homeost Agents. 2000;14:275–277. [PubMed] [Google Scholar]
- 26.Lu C, Kerbel RS. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol. 1993;120:1281–1288. doi: 10.1083/jcb.120.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacKenzie MJ. Molecular therapy in pancreatic adenocarcinoma. Lancet Oncol. 2004;5:541–549. doi: 10.1016/S1470-2045(04)01565-7. [DOI] [PubMed] [Google Scholar]
- 28.Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol. 1996;156:73–78. [PubMed] [Google Scholar]
- 29.Martone T, Bellone G, Pagano M, Beatrice F, Palonta F, Emanuelli G, Cortesina G. Constitutive expression of interleukin-18 in head and neck squamous carcinoma cells. Head Neck. 2004;26:494–503. doi: 10.1002/hed.20011. [DOI] [PubMed] [Google Scholar]
- 30.Mattner F, Fischer S, Guckes S, Jin S, Kaulen H, Schmitt E, Rude E, Germann T. The interleukin-12 subunit p40 specifically inhibits effects of the interleukin-12 heterodimer. Eur J Immunol. 1993;23:2202–2208. doi: 10.1002/eji.1830230923. [DOI] [PubMed] [Google Scholar]
- 31.Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer. 2004;90:2312–2316. doi: 10.1038/sj.bjc.6601814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moore MB, Kurago ZB, Fullenkamp CA, Lutz CT. Squamous cell carcinoma cells differentially stimulate NK cell effector functions: the role of IL-18. Cancer Immunol Immunother. 2003;52:107–115. doi: 10.1007/s00262-002-0361-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagomi H, Pisa P, Pisa EK, Yamamoto Y, Halapi E, Backlin K, Juhlin C, Kiessling R. Lack of interleukin-2 (IL-2) expression and selective expression of IL-10 mRNA in human renal cell carcinoma. Int J Cancer. 1995;63:366–371. doi: 10.1002/ijc.2910630311. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 is a unique cytokine that stimulates both Th1 and Th2 responses depending on its cytokine milieu. Cytokine Growth Factor Rev. 2001;12:53–72. doi: 10.1016/S1359-6101(00)00015-0. [DOI] [PubMed] [Google Scholar]
- 35.Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, Akita K, Namba M, Tanabe F, Konishi K, Fukuda S, Kurimoto M. Cloning of a new cytokine that induces IFN-γ production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 36.Pages F, Berger A, Henglein B, Piqueras B, Danel C, Zinzindohoue F, Thiounn N, Cugnenc PH, Fridman WH. Modulation of interleukin-18 expression in human colon carcinoma: consequences for tumor immune surveillance. Int J Cancer. 1999;84:326–330. doi: 10.1002/(SICI)1097-0215(19990621)84:3<326::AID-IJC22>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Paglia D, Oran A, Lu C, Kerbel RS, Sauder DN, McKenzie RC. Expression of leukemia inhibitory factor and interleukin-11 by human melanoma cell lines: LIF, IL-6, and IL-11 are not coregulated. J Interferon Cytokine Res. 1995;15:455–460. doi: 10.1089/jir.1995.15.455. [DOI] [PubMed] [Google Scholar]
- 38.Park H, Byun D, Kim TS, Kim YI, Kang JS, Hahm ES, Kim SH, Lee WJ, Song HK, Yoon DY, Kang CJ, Lee C, Houh D, Kim H, Cho B, Kim Y, Yang YH, Min KH, Cho DH. Enhanced IL-18 expression in common skin tumors. Immunol Lett. 2001;79:215–219. doi: 10.1016/S0165-2478(01)00278-4. [DOI] [PubMed] [Google Scholar]
- 39.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl. Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakers C, Ruijter JM, Deprez RH, Moorman AF. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 41.Rasmussen R. Quantification on the LightCycler. In: Meuer S, Wittwer C, Nakagawara K, editors. Rapid cycle real-time PCR, methods and applications. Heidelberg: Springer Press; 2001. pp. 21–34. [Google Scholar]
- 42.Riedel F, Adam S, Feick P, Haas S, Gotte K, Hormann K. Mannheim Alcohol Study Group. Expression of IL-18 in patients with head and neck squamous cell carcinoma. Int J Mol Med. 2004;18:267–272. [PubMed] [Google Scholar]
- 43.Saito H, Tsujitani S, Oka S, Kondo A, Ikeguchi M, Maeta M, Kaibara N. An elevated serum level of transforming growth factor-beta 1 (TGF-beta 1) significantly correlated with lymph node metastasis and poor prognosis in patients with gastric carcinoma. Anticancer Res. 2000;20:4489–4493. [PubMed] [Google Scholar]
- 44.Sato T, McCue P, Masuoka K, Salwen S, Lattime EC, Mastrangelo MJ, Berd D. Interleukin 10 production by human melanoma. Clin Cancer Res. 1996;2:1383–1390. [PubMed] [Google Scholar]
- 45.Scupoli MT, Sartoris S, Tosi G, Ennas MG, Nicolis M, Cestari T, Zamboni G, Martignoni G, Lemoine NR, Scarpa A, Accolla RS. Expression of MHC class I and class II antigens in pancreatic adenocarcinomas. Tissue Antigens. 1996;48:301–311. doi: 10.1111/j.1399-0039.1996.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 46.Seddighzadeh M, Larsson P, Ulfgren AC, Onelov E, Berggren P, Tribukait B, Torstensson A, Norming U, Wijkstrom H, Linder S, Steineck G. Low IL-1alpha expression in bladder cancer tissue and survival. Eur Urol. 2003;43:362–368. doi: 10.1016/S0302-2838(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 47.Sobin LH, Wittekind CL (2002) (eds) International Union Against Cancer (UICC): TNM classification of malignant tumors. John Wiley & Sons Inc New York, pp. 93–96
- 48.Sotiriou C, Lacroix M, Lespagnard L, Larsimont D, Paesmans M, Body JJ. Interleukins-6 and −11 expression in primary breast cancer and subsequent development of bone metastases. Cancer Lett. 2001;169:87–95. doi: 10.1016/S0304-3835(01)00524-9. [DOI] [PubMed] [Google Scholar]
- 49.Sozen S, Coskun U, Sancak B, Bukan N, Gunel N, Tunc L, Bozkirli I. Serum levels of interleukin-18 and nitrite+nitrate in renal cell carcinoma patients with different tumor stage and grade. Neoplasma. 2004;51:25–29. [PubMed] [Google Scholar]
- 50.Terabe M, Park JM, Berzofsky JA. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunol Immunother. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tohyama K, Yoshida Y, Ohashi K, Sano E, Kobayashi H, Endo K, Naruto M, Nakamura T. Production of multiple growth factors by a newly established human thyroid carcinoma cell line. Jpn J Cancer Res. 1992;83:153–158. doi: 10.1111/j.1349-7006.1992.tb00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Torroella-Kouri M, Keith JC, Ivanova M, Lopez DM. IL-11-induced reduction of C/EBP transcription factor binding may contribute to the IL-12 downregulation in tumor-bearing mice. Int J Oncol. 2003;22:439–448. [PubMed] [Google Scholar]
- 53.Trepicchio WL, Bozza M, Pedneault G, Dorner AJ. Recombinant human IL-11 attenuates the inflammatory response through down-regulation of proinflammatory cytokine release and nitric oxide production. J Immunol. 1996;157:3627–3634. [PubMed] [Google Scholar]
- 54.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 55.Tsuboi K, Miyazaki T, Nakajima M, Fukai Y, Masuda N, Manda R, Fukuchi M, Kato H, Kuwano H. Serum interleukin-12 and interleukin-18 levels as a tumor marker in patients with esophageal carcinoma. Cancer Lett. 2004;205:207–214. doi: 10.1016/j.canlet.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Ueda T, Shimada E, Urakawa T. Serum levels of cytokines in patients with colorectal cancer: possible involvement of interleukin-6 and interleukin-8 in hematogenous metastasis. J Gastroenterol. 1994;29:423–429. doi: 10.1007/BF02361238. [DOI] [PubMed] [Google Scholar]
- 57.Ungefroren H, Voss M, Jansen M, Roeder C, Henne-Bruns D, Kremer B, Kalthoff H. Human pancreatic adenocarcinomas express Fas and Fas ligand yet are resistant to Fas-mediated apoptosis. Cancer Res. 1998;58:1741–1749. [PubMed] [Google Scholar]
- 58.Ungefroren H, Voss M, Bernstorff WV, Schmid A, Kremer B, Kalthoff H. Immunological escape mechanisms in pancreatic carcinoma. Ann N Y Acad Sci. 1999;880:243–251. doi: 10.1111/j.1749-6632.1999.tb09529.x. [DOI] [PubMed] [Google Scholar]
- 59.von Bernstorff W, Spanjaard RA, Chan AK, Lockhart DC, Sadanaga N, Wood I, Peiper M, Goedegebuure PS, Eberlein TJ. Pancreatic cancer cells can evade immune surveillance via nonfunctional Fas (APO-1/CD95) receptors and aberrant expression of functional Fas ligand. Surgery. 1999;125:73–84. doi: 10.1067/msy.2099.93570. [DOI] [PubMed] [Google Scholar]
- 60.von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H. Systemic and local immunosuppression in pancreatic cancer patients. Clin Cancer Res. 2001;7(Suppl):925s–932s. [PubMed] [Google Scholar]
- 61.Wang ZY, Gaggero A, Rubartelli A, Rosso O, Miotti S, Mezzanzanica D, Canevari S, Ferrini S. Expression of interleukin-18 in human ovarian carcinoma and normal ovarian epithelium: evidence for defective processing in tumor cells. Int J Cancer. 2002;98:873–878. doi: 10.1002/ijc.10268. [DOI] [PubMed] [Google Scholar]
- 62.Wakeman CJ, Martin IG, Robertson RW, Dobbs BR, Frizelle FA. Pancreatic cancer: management and survival. ANZ J Surg. 2004;74:941–944. doi: 10.1111/j.1445-1433.2004.03210.x. [DOI] [PubMed] [Google Scholar]
- 63.Xu J, Menezes J, Prasad U, Ahmad A. Elevated serum levels of transforming growth factor beta1 in Epstein-Barr virus-associated nasopharyngeal carcinoma patients. Int J Cancer. 1999;84:396–399. doi: 10.1002/(SICI)1097-0215(19990820)84:4<396::AID-IJC11>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 64.Zhang B, Ma XT, Zheng GG, Li G, Rao Q, Wu KF. Expression of IL-18 and its receptor in human leukemia cells. Leuk Res. 2003;27:813–822. doi: 10.1016/S0145-2126(03)00005-5. [DOI] [PubMed] [Google Scholar]