Abstract
“Cancer-germline” genes such as those of the MAGE family are expressed in many tumors and in male germline cells, but are silent in other normal tissues. They encode tumor specific antigens that are used in cancer immunotherapy trials. MAGE-4 antigens represent promising targets for cancer immunotherapy because gene MAGE-4 is expressed in more than 50% of carcinomas of the esophagus, lung, bladder, and head and neck. To identify new MAGE-4 antigenic peptides, we have folded HLA–A*2402 soluble molecules with candidate peptide NYKRCFPVI, which corresponds to amino acids 143 to151 of the MAGE-4 protein. A24/MAGE-4 multimers were used to isolate a cytolytic T cell clone that recognized the MAGE-4 peptide from the blood cells of a donor without cancer. This clone lysed specifically A24 carcinoma cells expressing MAGE-4. The antigenic peptide is processed more efficiently in tumor cells pre-treated with IFN-γ. This MAGE-4 peptide could represent an interesting target for immunotherapy because it is presented by HLA–A24 molecules, which are widely expressed in different ethnic groups.
Keywords: Carcinoma, CTL, HLA–A24, MAGE-4, Peptide, Tumor
Introduction
There is now ample evidence for recognition of cancer cells by the autologous human host and numerous antigenic peptides recognized on human tumors by cytolytic T lymphocytes (CTL) have been identified in the last 15 years [4, 27]. For the purpose of cancer immunotherapy, an interesting category of antigenic peptides are those encoded by cancer germline genes, comprising members of the MAGE, BAGE, GAGE, LAGE, and SSX gene families [3, 6, 7, 10, 16, 20, 25, 26, 28]. These genes are expressed in various tumors but not in normal tissues except male germline cells, and placenta for some of them. Because germline cells are devoid of MHC molecules, they do not display antigenic peptides at their surface and, therefore, antigenic peptides encoded by cancer-germline genes are strictly tumor specific [11]. A number of clinical trials of therapeutic vaccination have been initiated, based on the use of defined antigens encoded by cancer-germline genes. In the clinical trials performed with short peptides, a minority of patients showed regression of metastatic tumors after vaccination [2, 12, 18, 22, 23]. In order to improve the efficacy of vaccination, formulations with combinations of selected peptides could be tested.
The design of vaccines comprising several antigens will be facilitated by the identification of additional antigenic peptides presented by HLA molecules expressed by a large number of patients. We describe here the identification of such a new antigenic peptide, encoded by gene MAGE-4 and presented by HLA–A24 molecules.
Material and methods
Cell lines, media, and reagents
Epstein Barr Virus-transformed B (EBV-B) cell lines and tumor cell lines were cultured in IMDM (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% fetal calf serum (Life Technologies). COS-7 cells were maintained in DMEM (Life Technologies) supplemented with 5% fetal calf serum. All media were supplemented with 0.24 mM l-asparagine, 0.55 mM l-arginine, 1.5 mM l-glutamine (AAG), 100 U/ml penicillin and 100 μg/ml streptomycin. Human recombinant IL-2 was purchased from Eurocetus (Amsterdam, The Netherlands), IL-6, IL-7, IL-12, and IFN-γ from Peprotech (Rocky Hill, NJ, USA), GM-CSF (Leucomax) from Schering-Plough (Brinny, Ireland), geneticin from Life Technologies, and gentamicin from ICN (Esxchwege, Germany). IL-4 was produced in our laboratory.
Dendritic cells
Hemochromatosis patients require regular blood removal to reduce excess iron. Several blood samples can therefore easily be obtained from the same individual. Donor LB2348 was typed HLA-A*0201, -A*2402, -B51, -B55, -Cw3, -Cw15, -DRB1 14, and -DRB1 15, and peripheral blood was obtained as standard buffy coat preparation, which was laid down on a 15-ml Lymphoprep layer (Axis-Shield PoCAS, Oslo, Norway) in 50-ml LEUCOSEP tubes (Greiner, Frickenhausen, Germany). The tubes were centrifuged at 2,200 rpm for 20 min at room temperature. The interphase containing the PBMC was harvested and washed three times in cold phosphate buffer solution with 2 mM EDTA to eliminate the remaining platelets. To generate dendritic cells, PBMC were left to adhere for 1 h at 37°C in culture flasks (FALCON, Becton Dickinson) at a density of 2×106 cells per cm2 in RPMI 1640 supplemented with Hepes (2.38 g/l), AAG, antibiotics, and 1% autologous plasma that was heat inactivated at 56°C for 30 min (hereafter referred to as complete RPMI medium). Nonadherent cells were frozen and adherent cells were cultured in the presence of IL-4 (200 U/ml) and GM-CSF (70 ng/ml) in complete RPMI medium. Cultures were fed on days 2 and 4 by removing one-third of the volume and adding fresh medium with cytokines. Cells were frozen on day 5 and thawed 1 day before being loaded with peptide and used as stimulator cells.
Multimer production and labeling with multimers
Recombinant HLA-A*2402 molecules were folded in vitro with β1142 -microglobulin and peptide NYKRCFPVI from MAGE-4, or peptide LYVDSLFFL from PRAME [13]. They were purified by gel filtration, biotinylated, and mixed as described [1] with Streptavidin-PE (Sigma, St Louis, MI, USA) for the HLA–A24/MAGE-4 multimer, or streptavidin-APC (BD-Pharmingen, San Diego, CA, USA) for the A24/PRAME control multimer [13]. Concentrations of the fluorescent HLA–peptide complexes refer to the concentrations of the HLA–peptide monomers in the sample. For staining, the frozen non-adherent fraction of PBMC of donor LB2348 was thawed. After overnight incubation, T cells were isolated by rosetting with sheep red blood cells. The T cells were washed, resuspended at 20×106 cells per ml in Hank’s solution modified for flow cytometry with 1% human serum [15], and incubated for 15 min at room temperature with HLA–A24 multimers loaded with MAGE-4 peptide (20 nM). To stain the cells of the microcultures on day 26 and the CTL clone 13, cells were stained for 15 min at room temperature with A24/MAGE-4 and A24/PRAME multimers and, subsequently, anti-CD8 antibodies coupled to FITC (SK1 at 1/50, BD-Pharmingen) were added for a further incubation of 15 min.
MACS sorting and culture conditions of the sorted cells
Multimer-labeled cells (25×106 cells/80 μl) were incubated at 4°C with anti-PE microbeads (20 μl) according to the instructions of the manufacturer (Miltenyi Biotec, Bergisch Gladbach, Germany), washed and sorted through a separation column inserted to a magnet in an AUTOMACS at 0.5 ml/min (Miltenyi Biotec). The fraction of cells obtained after magnetic sorting was distributed at 4,700 cells per well in 51 U-bottomed microwells, and cultured in 200 μl of IMDM supplemented with gentamicin (15 μg/ml), AAG, 10% human serum, IL-2 (10 U/ml), IL-6 (1,000 U/ml), and IL-12 (10 ng/ml). T cells were stimulated with irradiated (100 Gray) autologous peptide-pulsed dendritic cells (2,000 cells/well). On day 12, 13,000 stimulator cells were added to each microwell together with IL-2 (10 U/ml) and IL-7 (5 ng/ml). To prepare the stimulator cells, monocyte-derived immature dendritic cells were incubated for 6 h with 5 μg/ml of peptide NYKRCFPVI, IL-4 (200 U/ml), GM-CSF (70 ng/ml), in the presence of 1 μg/ml of ribomunyl (INAVA, Pierre Fabre Medicament Production, Boulogne, France), and washed. On day 26, approximately 105 cells from each microculture were stained in 50 μl with both multimers and anti-CD8.FITC, and analyzed by flow cytometry.
Transfection of COS cells and recognition assay based on IFN-γ production
COS-7 cells (1.5×104) were distributed in flat-bottom microwells. They were cotransfected 1 day later with pcDNAI/Amp (50 ng) (Invitrogen) containing either a MAGE-4a cDNA or a MAGE-1 cDNA, and pcDNA3 (50 ng) containing an HLA-A*2402 cDNA. The cells were always transfected with a total of 100 ng of DNA and 1 μl of Lipofectamine (Invitrogen, Merelbeke, Belgium). If needed, 50 ng of pcDNA3.1/His-B/LacZ (Invitrogen) was added to the DNA coding for a MAGE or an HLA protein. Transfected cells were incubated for 24 h at 37°C and 8% CO2. In total, 3,000 CTL were added in the microwells containing the transfected cells, in a total volume of 150 μl of complete IMDM supplemented with 25 U/ml of IL-2. After 24 h, IFN-γ released in the supernatant was measured by ELISA using reagents from Medgenix Diagnostics-Biosource (Fleurus, Belgium).
Cytotoxicity assay
Epstein Barr Virus-B cells and tumor cells were labeled with 100 μCi of Na(51Cr)O for 1 h, washed and pulsed, if indicated, for 5 min with peptide NYKRCFPVI. CTL were then added and chromium release was measured after incubation at 37°C for 4 h.
Results and discussion
HLA–A24 multimers folded with MAGE-4 peptide NYKRCFPVI
MAGE-1 peptide NYKHCFPEI was reported to be presented to CTL by HLA–A24 molecules [9]. We observed that peptide NYKRCFPVI, encoded by the homologous region of MAGE-4, contains potential anchor residues for HLA–A24, namely Y in position 2 and I at the carboxy terminus [19]. NYKRCFPVI corresponds to amino acids 143–151 of both the MAGE-4a and the MAGE-4b protein, which differ by a single amino acid at position 173. HLA–A2402 molecules and β2-microglobulin, produced in E. coli, were successfully refolded with the MAGE-4 peptide, demonstrating that the peptide binds efficiently to HLA–A2402 (data not shown). The HLA–peptide complexes were biotinylated and multimerized with avidin conjugated to phycoerythrin (PE). These multimers will be referred to as A24/MAGE-4 multimers.
Isolation of a CD8 T cell clone directed against the MAGE-4 peptide
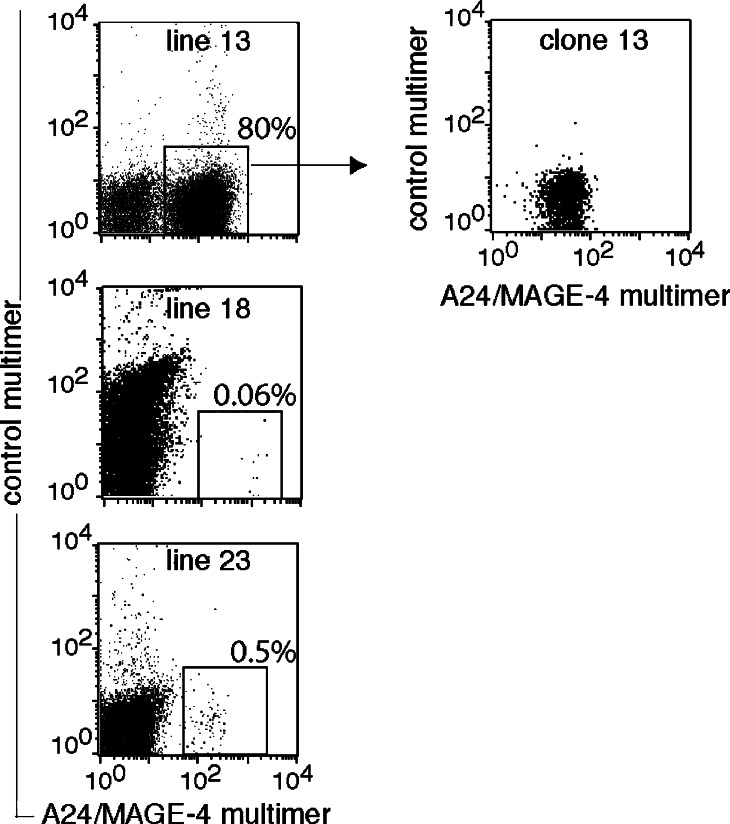
In total, 180 million T cells obtained from an HLA–A*2402 blood donor without cancer were incubated with A24/MAGE-4 multimers conjugated to PE. The cells were then incubated with an anti-PE antibody coupled to magnetic beads. The 2.4×105 cells selected by magnetic sorting were distributed at 4,700 cells per well and were stimulated on days 0 and 12 with peptide-pulsed autologous dendritic cells. On day 26, three out of the 51 microcultures contained A24/MAGE-4 multimer-positive cells, which represented between 0.06 and 80% of the CD8 fraction (Fig. 1). CD8+ cells were isolated by magnetic sorting from microculture 13 and stimulated with the antigen. The resulting CTL line stained with A24/MAGE-4 multimers (Fig. 1) and the clonality of the CTL line, hereafter referred to as clone 13, was verified by RT-PCR amplification of the β chain of the T cell receptor (TCR) and sequencing of the PCR products. Only one Vβ chain was identified, encoding the following CDR3 region: Vβ20-1*06 (or 07) S SRGVGSP TGELFFG Jβ2-2*01.
Fig. 1.
Labeling with A24/MAGE-4 multimers of T cell lines and a T cell clone obtained from microculture 13. Cells were labeled for 15 min at room temperature with the A24/MAGE-4 multimer conjugated to PE. The control multimer is an HLA–A24 multimer conjugated to APC and containing a PRAME peptide [13]
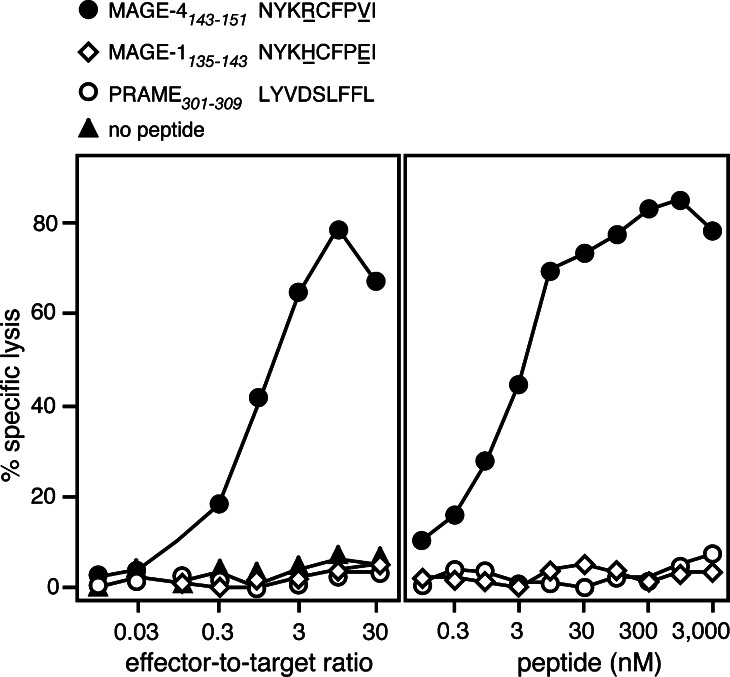
CTL 13 was able to lyse HLA–A24 cells loaded with MAGE-4 peptide NYKRCFPVI (Fig. 2a). Titration of the peptide revealed that half maximal lysis of target cells was obtained at a peptide concentration of 3 nM (Fig. 2b). This is in the range of concentration observed for the previously identified MAGE antigenic peptides, for which values ranging from 0.05 to 200 nM were observed [5, 14, 17, 21, 24, 29]. Longer and shorter MAGE-4 peptides were much less efficient in sensitizing target cells to lysis by CTL 13 (data not shown). The MAGE-1 peptide, NYKHCFPEI, which was shown to bind to HLA–A24 molecules [9], and the PRAME peptide, which was used to fold the control multimer, were not recognized by CTL 13 (Fig. 2).
Fig. 2.
Lysis of an HLA–A24 target loaded with MAGE-4 peptide NYKRCFPVI. a HLA–A24 EBV-B cells from donor LB2348 were 51Cr-labeled for 1 h, incubated for 5 min with 1 μg/ml of one of the indicated peptides and incubated with CTL at indicated effector-to-target ratios. The MAGE-4 and the MAGE-1 peptide are very similar. The amino acid differences are underlined. Chromium release was measured after 4 h. b HLA–A24 EBV-B cells were 51Cr-labeled for 1 h, incubated for 15 min with threefold dilutions of each of the synthetic peptides. CTL was subsequently added at an effector-to-target ratio of 10. Chromium release was measured 4 h later. The concentration of peptide indicated in the figure corresponds to the concentrations during the 4 h of incubation after addition of the CTL
Recognition of HLA–A24 cells expressing MAGE-4
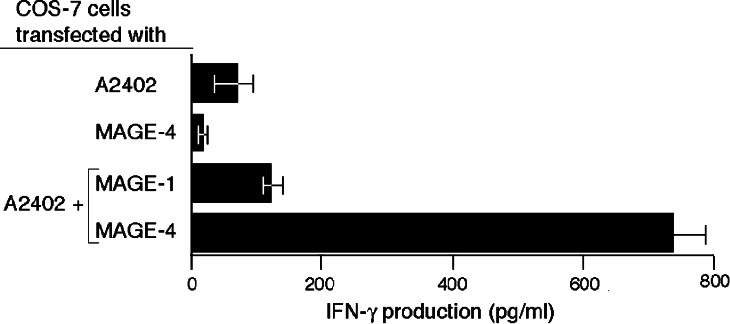
COS-7 cells transfected with both a MAGE-4a and an HLA-A*2402 cDNA construct stimulated clone 13 to produce IFN-γ, indicating that the MAGE-4 antigen can be processed in these cells (Fig. 3). MAGE-4 belongs to a family of genes and some other MAGE genes code for peptides that differ slightly from the MAGE-4 peptide NYKRCFPVI. We have therefore tested if other MAGE family members can generate a peptide recognized by CTL 13 on HLA–A24 molecules. However, cells transfected with an HLA-A*2402 cDNA and either MAGE-1 (Fig. 3) or MAGE-2, -3, -6, -8, -9, -10, -11, -12, -C1, -C2, -D1, or MAGE–D2 cDNA were not recognized by the CTL (data not shown).
Fig. 3.
Recognition of cells transfected with MAGE-4.COS-7 cells were transiently transfected with a MAGE-4a or a MAGE-1 coding sequence inserted in expression vector pcDNAI/Amp and an HLA–A24 coding sequence inserted in expression vector pcDNA3. Transfections were performed with 15,000 COS-7 cells, 50 ng of each cDNA and 1 μl of Lipofectamine. One day after transfection, 3,000 CTL 13 were added to the transfected cells. IFN-γ production was measured by ELISA after overnight co-culture
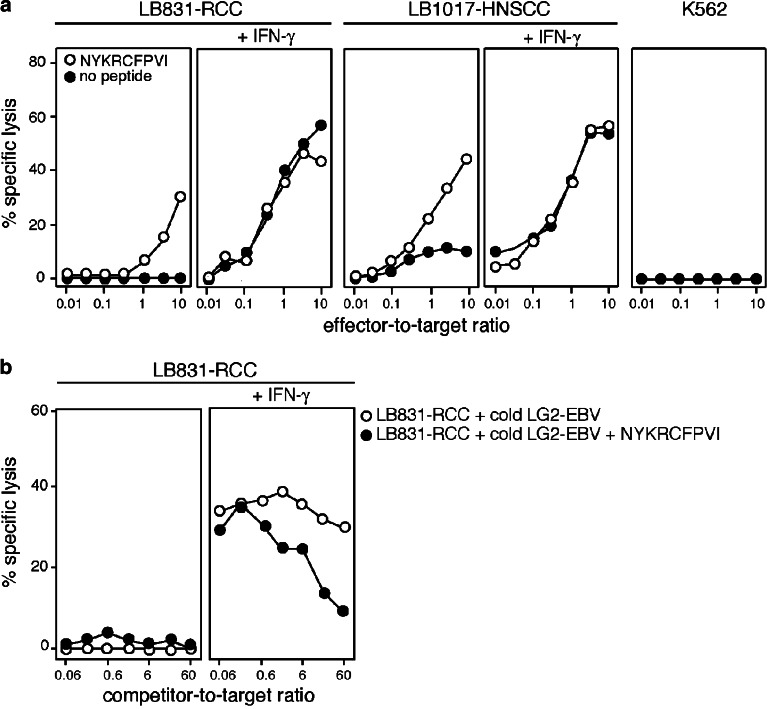
Two HLA–A24 tumor cell lines expressing MAGE-4, a renal cell carcinoma, and a head and neck squamous cell carcinoma, were tested for recognition by CTL 13 (Fig. 4a). They were efficiently lysed only after a 6-day IFN-γ treatment. Lysis of the IFN-γ-treated renal cell carcinoma line was quenched in the presence of an excess of unlabeled A24 EBV-B cells pulsed with the MAGE-4 peptide, further demonstrating that the antigen recognized on the tumor cells was peptide NYKRCFPVI (Fig. 4b). Peptide-loaded targets were more efficiently lysed after treatment with IFN-γ, most probably because of the IFN-γ-induced upregulation of HLA expression. The increased sensitivity to lysis after IFN-γ treatment of the tumor cells that were not pulsed with the peptide is partly due to a higher expression of HLA molecules. However, our results are also compatible with a better processing of the MAGE-4 antigen by the immunoproteasome compared to the standard proteasome. A definitive conclusion will require further experiments, such as the transfection of MAGE-4 in cells expressing exclusively the standard proteasome or the immunoprotaseome, and digestions of a precursor peptide by each type of the proteasome, followed by recognition assays of the digests with CTL 13. We believe that vaccination strategies against cancer should take into account the differential processing of tumor antigens by the two proteasome types. Optimal vaccine formulations could consist of a mixture of peptides efficiently produced by one or the other proteasome type.
Fig. 4.
Lysis of HLA–A24 tumor cells expressing MAGE-4. a LB831-RCC is an A*2403 renal cell carcinoma cell line and LB017-HNSCC an A*2402 squamous cell carcinoma of the head and neck. K562 is a target for natural killer cells. The tumor cell lines were treated for 6 days with 50 U/ml IFN-γ. Targets were 51Cr-labeled and, if indicated, pulsed for 5 min with 1 μg/ml of MAGE-4 peptide NYKRCFPVI. CTL clone 13 was added at the indicated effector-to-target ratios and chromium release was measured after 4 h. b Unlabeled LG2-EBV competitor cells were added to 1,000 51Cr-labeled targets before adding CTL 13 at an effector-to-target ratio of 10. If indicated, LG2-EBV were pulsed for 30 min at room temperature with 5 μg/ml of peptide NYKRCFPVI, and washed
A large number of tumors express MAGE-4, in particular squamous-cell carcinomas of the esophagus (74%), lung (59%), head and neck (53%), and infiltrating bladder carcinoma (45%) [30]. We had previously identified three MAGE-4 antigenic peptides recognized by CTL on HLA-A1, -A2, and -B37 molecules [8, 14, 31]. The antigenic peptide described here represents a new interesting target for cancer immunotherapy, considering that 42% of Orientals and 20% of Caucasoids express HLA–A24 molecules [19].
Acknowledgements
We thank Dr. Sophie Lucas for critical reading and Mrs. Nathalie Krack for editorial assistance. This work was supported by the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister’s Office, Science Policy Programming, and by a grant from the Fédération Belge contre le Cancer (Belgium).
References
- 1.Altman JD, Moss PAH, Goulder PJR, Barouch DH, McHeyzer-Williams MG, Bell JI, McMichael AJ, Davis MM. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 2.Bettinotti MP, Panelli MC, Ruppe E, Mocellin S, Phan GQ, White DE, Marincola FM. Clinical and immunological evaluation of patients with metastatic melanoma undergoing immunization with the HLA-Cw*0702-associated epitope MAGE-A12:170–178. Int J Cancer. 2003;105:210–216. doi: 10.1002/ijc.11045. [DOI] [PubMed] [Google Scholar]
- 3.Boël P, Wildmann C, Sensi M-L, Brasseur R, Renauld J-C, Coulie P, Boon T, van der Bruggen P. BAGE, a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167–175. doi: 10.1016/S1074-7613(95)80053-0. [DOI] [PubMed] [Google Scholar]
- 4.Boon T, Cerottini J-C, Vanden Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 5.Chaux P, Luiten R, Demotte N, Vantomme V, Stroobant V, Traversari C, Russo V, Schultz E, Cornelis GR, Boon T, van der Bruggen P. Identification of five MAGE-A1 epitopes recognized by cytolytic T lymphocytes obtained by in vitro stimulation with dendritic cells transduced with MAGE-A1 . J Immunol. 1999;163:2928–2936. [PubMed] [Google Scholar]
- 6.Chen Y-T, Scanlan MJ, Sahin U, Türeci Ö, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci USA. 1997;94:1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Backer O, Arden KC, Boretti M, Vantomme V, De Smet C, Czekay S, Viars CS, De Plaen E, Brasseur F, Chomez P, Vanden Eynde B, Boon T, van der Bruggen P. Characterization of the GAGE genes that are expressed in various human cancers and in normal testis. Cancer Res. 1999;59:3157–3165. [PubMed] [Google Scholar]
- 8.Duffour M-T, Chaux P, Lurquin C, Cornelis G, Boon T, van der Bruggen P. A MAGE-A4 peptide presented by HLA-A2 is recognized by cytolytic T lymphocytes. Eur J Immunol. 1999;29:3329–3337. doi: 10.1002/(SICI)1521-4141(199910)29:10<3329::AID-IMMU3329>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Fujie T, Tahara K, Tanaka F, Mori M, Takesako K, Akiyoshi T. A MAGE-1-encoded HLA–A24-binding synthetic peptide induces specific anti-tumor cytotoxic T lymphocytes. Int J Cancer. 1999;80:169–172. doi: 10.1002/(SICI)1097-0215(19990118)80:2<169::AID-IJC1>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 10.Gure AO, Türeci Ö, Sahin U, Tsang S, Scanlan MJ, Jäger E, Knuth A, Pfreundschuh M, Old LJ, Chen Y-T. SSX: a multigene family with several members transcribed in normal testis and human cancer. Int J Cancer. 1997;72:965–971. doi: 10.1002/(SICI)1097-0215(19970917)72:6<965::AID-IJC8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Haas GG, Jr, D’Cruz OJ, De Bault LE. Distribution of human leukocyte antigen-ABC and -D/DR antigens in the unfixed human testis. Am J Reprod Immunol Microbiol. 1988;18:47–51. doi: 10.1111/j.1600-0897.1988.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 12.Hersey P, Menzies SW, Coventry B, Nguyen T, Farrelly M, Collins S, Hirst D, Johnson H. Phase I/II study of immunotherapy with T-cell peptide epitopes in patients with stage IV melanoma. Cancer Immunol Immunother. 2005;54:208–218. doi: 10.1007/s00262-004-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda H, Lethé B, Lehmann F, Van Baren N, Baurain J-F, De Smet C, Chambost H, Vitale M, Moretta A, Boon T, Coulie PG. Characterization of an antigen that is recognized on a melanoma showing partial HLA loss by CTL expressing an NK inhibitory receptor. Immunity. 1997;6:199–208. doi: 10.1016/S1074-7613(00)80426-4. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Lonchay C, Colau D, Demotte N, Boon T, van der Bruggen P. New MAGE-4 antigenic peptide recognized by cytolytic T lymphocytes on HLA-A1 tumor cells. Tissue Antigens. 2003;62:426–432. doi: 10.1034/j.1399-0039.2003.00123.x. [DOI] [PubMed] [Google Scholar]
- 15.Lehmann F, Marchand M, Hainaut P, Pouillart P, Sastre X, Ikeda H, Boon T, Coulie PG. Differences in the antigens recognized by cytolytic T cells on two successive metastases of a melanoma patient are consistent with immune selection. Eur J Immunol. 1995;25:340–347. doi: 10.1002/eji.1830250206. [DOI] [PubMed] [Google Scholar]
- 16.Lethé B, Lucas S, Michaux L, De Smet C, Godelaine D, Serrano A, De Plaen E, Boon T. LAGE-1, a new gene with tumor specificity. Int J Cancer. 1998;76:903–908. doi: 10.1002/(SICI)1097-0215(19980610)76:6<903::AID-IJC22>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Luiten R, van der Bruggen P. A MAGE-A1 peptide is recognized on HLA-B7 human tumors by cytolytic T lymphocytes. Tissue Antigens. 2000;55:149–152. doi: 10.1034/j.1399-0039.2000.550206.x. [DOI] [PubMed] [Google Scholar]
- 18.Marchand M, van Baren N, Weynants P, Brichard V, Dréno B, Tessier M-H, Rankin E, Parmiani G, Arienti F, Humblet Y, Bourlond A, Vanwijck R, Liénard D, Beauduin M, Dietrich P-Y, Russo V, Kerger J, Masucci G, Jäger E, De Greve J, Atzpodien J, Brasseur F, Coulie PG, van der Bruggen P, Boon T. Tumor regressions observed in patients with metastatic melanoma treated with an antigenic peptide encoded by gene MAGE-3 and presented by HLA-A1. Int J Cancer. 1999;80:219–230. doi: 10.1002/(SICI)1097-0215(19990118)80:2<219::AID-IJC10>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 19.Marsh SGE, Parham P, Barber LD, editors. The HLA FactsBook. London: Academic; 2000. [Google Scholar]
- 20.Ruault M, van der Bruggen P, Brun ME, Boyle S, Roizes G, De Sario A. New BAGE (B melanoma antigen) genes mapping to the juxtacentromeric regions of human chromosomes 13 and 21 have a cancer/testis expression profile. Eur J Hum Genet. 2002;10:833–840. doi: 10.1038/sj.ejhg.5200891. [DOI] [PubMed] [Google Scholar]
- 21.Schultz ES, Zhang Y, Knowles R, Tine J, Traversari C, Boon T, van der Bruggen P. A MAGE-3 peptide recognized on HLA-B35 and HLA-A1 by cytolytic T lymphocytes. Tissue Antigens. 2001;57:103–109. doi: 10.1034/j.1399-0039.2001.057002103.x. [DOI] [PubMed] [Google Scholar]
- 22.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, Hibbitts S, Grosh WW, Chianese-Bullock KA, Bissonette EA, Barnd DL, Deacon DH, Patterson JW, Parekh J, Neese PY, Woodson EM, Wiernasz CJ, Merrill P. Immunologic and clinical outcomes of vaccination with a multiepitope melanoma peptide vaccine plus low-dose interleukin-2 administered either concurrently or on a delayed schedule. J Clin Oncol. 2004;22:4474–4485. doi: 10.1200/JCO.2004.10.212. [DOI] [PubMed] [Google Scholar]
- 23.Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den Driesch P, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Traversari C, van der Bruggen P, Luescher IF, Lurquin C, Chomez P, Van Pel A, De Plaen E, Amar-Costesec A, Boon T. A nonapeptide encoded by human gene MAGE-1 is recognized on HLA-A1 by cytolytic T lymphocytes directed against tumor antigen MZ2-E. J Exp Med. 1992;176:1453–1457. doi: 10.1084/jem.176.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tureci O, Sahin U, Schobert I, Koslowski M, Scmitt H, Schild HJ, Stenner F, Seitz G, Rammensee H-G, Pfreundschuh M. The SSX-2 gene, which is involved in the t(X;18) translocation of synovial sarcomas, codes for the human tumor antigen HOM-MEL-40. Cancer Res. 1996;56:4766–4772. [PubMed] [Google Scholar]
- 26.Vanden Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. A new family of genes coding for an antigen recognized by autologous cytolytic T lymphocytes on a human melanoma. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van den Eynde B, van der Bruggen P (2004) Peptide database of T-cell defined tumor antigens. Cancer Immunity http://www.cancerimmunity.org/peptidedatabase/Tcellepitopes.htm:
- 28.van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Vanden Eynde B, Knuth A, Boon T. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 29.van der Bruggen P, Szikora J-P, Boël P, Wildmann C, Somville M, Sensi M, Boon T. Autologous cytolytic T lymphocytes recognize a MAGE-1 nonapeptide on melanomas expressing HLA-Cw*1601. Eur J Immunol. 1994;24:2134–2140. doi: 10.1002/eji.1830240930. [DOI] [PubMed] [Google Scholar]
- 30.van der Bruggen P, Zhang Y, Chaux P, Stroobant V, Panichelli C, Schultz ES, Chapiro J, Vanden Eynde BJ, Brasseur F, Boon T. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. doi: 10.1034/j.1600-065X.2002.18806.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Stroobant V, Russo V, Boon T, van der Bruggen P. A MAGE-A4 peptide presented by HLA-B37 is recognized on human tumors by cytolytic T lymphocytes. Tissue Antigens. 2002;60:365–371. doi: 10.1034/j.1399-0039.2002.600503.x. [DOI] [PubMed] [Google Scholar]