Abstract
Serum vitamin D binding protein (Gc protein) is the precursor for the principal macrophage-activating factor (MAF). The MAF precursor activity of serum Gc protein of colorectal cancer patients was lost or reduced because Gc protein is deglycosylated by serum α-N-acetylgalactosaminidase (Nagalase) secreted from cancerous cells. Deglycosylated Gc protein cannot be converted to MAF, leading to immunosuppression. Stepwise treatment of purified Gc protein with immobilized β-galactosidase and sialidase generated the most potent macrophage-activating factor (GcMAF) ever discovered, but it produces no side effect in humans. Macrophages treated with GcMAF (100 pg/ml) develop an enormous variation of receptors and are highly tumoricidal to a variety of cancers indiscriminately. Administration of 100 nanogram (ng)/human maximally activates systemic macrophages that can kill cancerous cells. Since the half-life of the activated macrophages is approximately 6 days, 100 ng GcMAF was administered weekly to eight nonanemic colorectal cancer patients who had previously received tumor-resection but still carried significant amounts of metastatic tumor cells. As GcMAF therapy progressed, the MAF precursor activities of all patients increased and conversely their serum Nagalase activities decreased. Since serum Nagalase is proportional to tumor burden, serum Nagalase activity was used as a prognostic index for time course analysis of GcMAF therapy. After 32–50 weekly administrations of 100 ng GcMAF, all colorectal cancer patients exhibited healthy control levels of the serum Nagalase activity, indicating eradication of metastatic tumor cells. During 7 years after the completion of GcMAF therapy, their serum Nagalase activity did not increase, indicating no recurrence of cancer, which was also supported by the annual CT scans of these patients.
Keywords: Colorectal cancer, Macrophages, Macrophage-activating factor, Immunotherapy, Deglycosylation, α-N-acetylgalactosaminidase, Immunosuppression
Introduction
Colorectal cancer is one of the major malignant diseases. Surgical resectability is an important prognostic determinant. However, recurrent tumors are commonly noted. After curative resection of tumors in the colon and rectum, various types of recurrence occur due to the presence of residual occult disease and distant micrometastasis [3, 20, 21]. The presence of micrometastatic cells in bone marrow has been shown to correlate with a poor clinical outcome [2]. Therefore, an adjuvant treatment to improve the prognosis in these patients is desirable. There have been a number of adjuvant chemotherapy trials for colorectal cancers. However, the majority of these studies failed to show any significant advantage in the various adjuvant therapies. One of the major problems in chemotherapy of colorectal cancers is chemoresistance [14]. Therefore, therapeutic approaches capable of tumoricidal to chemoresistant cells and improving the quality of life without side effects are limited to a certain immunotherapy.
Inflammation of cancerous tissues, induced by intratumor administration of a potent inflammatory agent, BCG (Bacille Calmette Guerin) or other bacterial cells, can result in regression of local as well as metastasized tumor suggesting the development of specific immunity against the tumors [15, 47]. However, administration of BCG into noncancerous tissues results in no significant effect on the tumors. Inflamed noncancerous normal tissues release lipid metabolites, lysophosphatidylcholine (lyso-Pc) and other lysophospholipids that efficiently activate macrophages [17, 18, 19, 22]. Inflamed cancerous tissues release lysoalkylphospholipids and alkylglycerols because cancerous tissues contain alkylphospholipids [7, 22, 23, 24]. Both lysoalkylphospholipids and alkylglycerols are at least 400 times more potent macrophage-activating agents than lysophospholipids [7, 22, 23, 24]. These findings suggest that highly activated macrophages can kill cancerous cells.
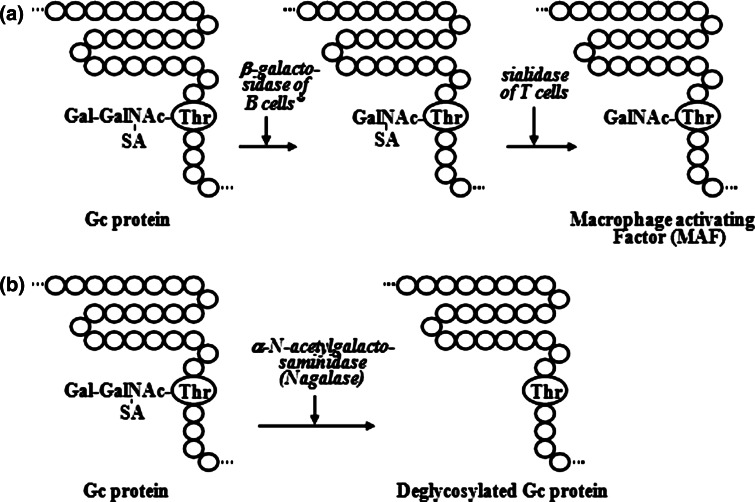
Inflammation-derived macrophage activation is the principal macrophage activation process which requires serum vitamin D3-binding protein (known as Gc protein) [8, 16, 25, 26, 27, 29] and participation of B and T lymphocytes [7, 18, 19, 22, 23, 24]. Gc protein carries one trisaccharide composed of N-acetylgalactosamine with dibranched galactose and sialic acid termini at 420 threonine residue [25, 33]. This oligosaccharide is hydrolyzed by the inducible membranous ß-galactosidase (Bgl i) of inflammation-primed (or lyso-Pc-treated) B cells and by the membranous Neu-1 sialidase of T cells to yield a macrophage-activating factor, the protein with N-acetylgalactosamine as the remaining sugar (MAF) [25, 28] (Fig. 1a). Thus, Gc protein is the precursor for the principal MAF [25, 28]. However, the MAF precursor activity of cancer patient Gc protein is lost or reduced, because their serum Gc protein is deglycosylated by serum α-N-acetylgalactosaminidase (Nagalase) secreted from cancerous cells [34, 35, 37, 39] (Fig. 1b). Deglycosylated Gc protein cannot be converted to MAF, resulting in no macrophage activation. Macrophages are the major phagocytic and antigen-presenting cells. Since macrophage activation for phagocytosis and antigen presentation to B and T lymphocytes are the first indispensable steps in the development of both humoral and cellular immunities, lack of macrophage activation leads to immunosuppression [32, 34, 35, 37, 39, 42, 44, 45]. Advanced cancer patients have high serum Nagalase activity, resulting in no macrophage activation and severe immunosuppression that explain why cancer patients die with overwhelming infection (e.g., pneumonia) [37].
Fig. 1.
Schematic illustration of formation of macrophage-activating factor (a) and deglycosylation of Gc protein (b). * Inflammation-primed B cells: B cells can be treated with an inflamed membranous lipid metabolite, e.g., lysophosphatidylcholine
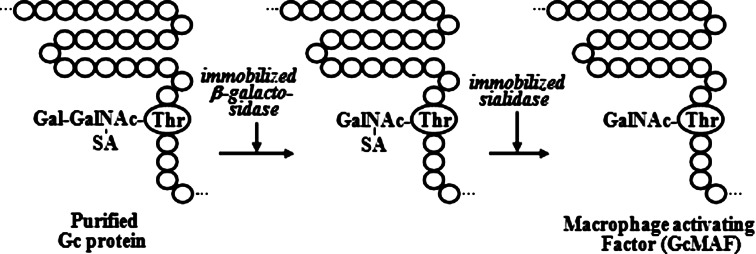
Stepwise treatment of highly purified Gc protein with immobilized ß-galactosidase and sialidase generates probably the most potent macrophage-activating factor (termed GcMAF) [11, 16, 28, 31, 33, 36, 38, 41, 46] (Fig. 2), which produces no side effects in humans [33, 39, 41, 43]. Administration of 100 nanogram (ng) GcMAF to humans results in the maximal activation of macrophages with 30-fold increased ingestion index and 15-fold increased superoxide-generating capacity [33] in 3.5 h. GcMAF also has a potent mitogenic capacity to act on the myeloid progenitor cells, resulting in a 40-fold increase in systemic macrophage cell counts in 4 days [33, 40]. Such highly activated systemic macrophages are chemotactically recruited to inflamed lesions by 180-fold increase of the macrophage cell counts [40]. Macrophages activated by GcMAF develop an enormous variation of receptors that recognize the abnormality in cancerous cell surface and kill cancerous cells [16, 31, 36, 41, 43, 46]. A series of glycolipid, glycoprotein and mucin antigens have been identified and designated as tumor-associated antigen (TAA) on the cell surface of a variety of tumor cells [48]. When human macrophages were treated in vitro with 100 picogram (pg) GcMAF/ml for 3 h, the macrophages were highly activated. The activated macrophages can bind and kill a variety of cancerous cells indiscriminately [39, 41, 43, 46]. Time course study of cell death was performed with effector/target ratio of 1.5. In 4 h, 51% of prostate cancer cells LNCaP and 60% of breast cancer cells MCF-7 were killed. In 18 h, 82% of LNCaP cells and 86% of MCF-7 cells were killed [43, 46]. The administration of 100 nanogram (ng)/week to metastatic breast and prostate cancer patients eradicated their tumors within 25 weeks [43, 46]. This tumoricidal capacity of macrophages activated by GcMAF led us to investigate the therapeutic efficacy of GcMAF for colorectal cancer. Although carcinoembryonic antigen (CEA) [6] and CA19-9 [12] have been used as diagnostic and prognostic indices, more precision for prognostic index is desirable for GcMAF therapy of colorectal cancer. Serum Nagalase was found to be the most accurate tumor marker [11, 34–36, 37, 39, 41, 46].
Fig. 2.
Stepwise treatment of Gc protein with immobilized ß-galactosidase and sialidase to generate GcMAF
Materials and methods
Chemicals and reagents
Phosphate-buffered saline (PBS) contained 1 mM sodium phosphate and 0.15 M NaCl. When peripheral blood monocytes adhere to vessel substratum, they behave like macrophages, which show increased synthesis of hydrolases. For manipulation in vitro and cultivation of peripheral blood mononuclear cells (PBMCs) containing monocytes/macrophages (macrophages for short) and lymphocytes (B and T cells), medium RPMI-1640 supplemented with 0.1% egg albumin (EA medium) was used. Sera for isolation of Gc protein were donated by members of the institute and routinely screened to check if they were free of viruses, using ELISA assays for antibodies against HIV, hepatitis B and C viruses (Cambridge Biotech and Abbott Laboratories). Human Gc protein was purified by vitamin D-affinity chromatography [13, 41]. ß-Galactosidase and sialidase were purchased from Boehringer Mannheim Biochemicals, Indianapolis, IN, USA and immobilized on Sepharose beads [28, 34, 38]. Lysophosphatidylcholine (lyso-Pc) and p-Nitrophenyl N-acetyl-a-d-galactosaminide were purchased from Sigma Chemical Co. (St.Louis, MO, USA).
Procedure for preparation of GcMAF
The serum was heat inactivated at 60°C 1 h and mixed with 30% saturated ammonium sulfate that precipitates Gc protein fraction [30]. The precipitate was dissolved in PBS (pH 7.4) containing 0.5% Triton-X 100 and 0.3% tri-n-butyl phosphate, and kept at room temperature overnight to resolve lipid-containing microbial contaminants including enveloped viruses if any. The treated samples were precipitated by 30% saturated ammonium sulfate, dissolved in citrate buffer at pH 4.0 and kept overnight. Gc protein was purified using 25-hydroxyvitamin D3-affinity chromatography [13, 41]. This chromatographic specificity to Gc protein yields highly pure Gc protein and eliminates all possible contaminations of macromolecules. Electrophoretic analysis proved purity of Gc protein (MW 52,000). Stepwise incubation of the purified Gc protein with immobilized ß-galactosidase and sialidase yielded the most potent macrophage-activating factor (termed GcMAF) [16, 29, 33, 38] (Fig. 2). The final product, GcMAF, was filtered through low protein-binding filter, Millex-HV; 0.45 μm (Millipore Corp., Bedford, MA, USA) for sterilization.
The optimal human dose of GcMAF, to achieve phagocytic capacity by 30-fold increased ingestion index and 15-fold increased superoxide-generating capacities of peripheral blood monocytes/macrophages, was found to be approximately 100 ng/human. Since the molecular structure of GcMAF is identical to that of the native human MAF, GcMAF (even 5-fold higher therapeutic dosage) produced no side effects on humans [31, 33, 41, 43, 46]. Numerous administrations (more than 10 times for 3–6 month period) of GcMAF (100–500 ng/human) to 12 humans (incl. four physicians) showed no signs of side effect. Quality control of the preparation of GcMAF was performed for activity, sterility and safety tests.
GcMAF therapy of colorectal cancer patients
Participants
Patients with colorectal carcinoma already treated with the conventional therapies, tumor resection and chemotherapy or a combination of chemotherapy and radiation, were eligible for GcMAF therapy. Although their serum Nagalase activities indicated that they have significant amounts of metastasized tumor cells, CT did not detect metastasized tumor lesions in other organs. A group of eight nonanemic colorectal cancer patients was included in this study. Patients received GcMAF therapy exclusively, therefore excluding combination with erythropoiesis induction. Thus, anemic cancer patients were not eligible in the program. The study was approved by the Institutional Research and Ethic Committees of Nagasaki Immunotherapy Group, Nagasaki, Japan, and by the Institutional Review Board of Hyogo Immunotherapy Group, Hyogo, Japan. The participants gave written informed consent before entering the study.
GcMAF administration
Because the half-life of the activated macrophages is approximately 6 days [23, 24, 41], 100 ng GcMAF was administered intramuscularly once a week.
Procedures to be used for clinical study and study parameters
Serum samples (>2 ml) were periodically (weekly or biweekly) collected immediately prior to each GcMAF administration and used for prognostic analysis. Assessment of patient response to each GcMAF administration was performed by determining both the MAF precursor activity of serum Gc protein and serum Nagalase activity. Since serum Nagalase activity is proportional to tumor burden [11, 37, 46], assessment of curative response to GcMAF therapy was performed by determining serum Nagalase activity as a prognostic index.
Assay for the MAF precursor activity of patient serum Gc protein
Blood samples of healthy humans were collected in tubes containing EDTA to prevent coagulation. Five milliliter blood sample and 5 ml saline (0.9% NaCl) was mixed and gently laid on a 15-ml centrifuge tube containing 3 ml Lymphoprep™ (similar to Ficoll; Polysciences, Inc., Warrington, PA, USA) and centrifuged at 800 × g for 15 min. The dense white cell band as PBMCs containing monocytes/macrophages (macrophages for short) and lymphocytes (B and T cells) was collected using a Pasteur pipette. The white cell mixture was washed twice with PBS, suspended in EA medium and placed in 16-mm wells. Incubation for 45 min in a 5% CO2 incubator at 37°C allowed adherence of macrophages to plastic surface. The mixture of lymphocytes and adherent macrophages of healthy humans was treated with 1 μg lyso-Pc/ml in EA medium for 30 min. Because of the adherence of macrophages to plastic substrata, lymphocytes and macrophages were separately washed with PBS, admixed and cultured in EA medium containing 0.1% serum of colorectal cancer patients or healthy human as a source of Gc protein. After 3 h of cultivation the macrophages were assayed for superoxide-generating capacity [34, 37]. The macrophages were washed with PBS and incubated in one ml PBS containing 20 μg cytochrome c for 10 min. Thirty minutes after addition of phorbol-12-myristate acetate (5 μg/ml), the superoxide-generating capacity of the macrophages was determined spectrophotometrically at 550 nm. The data were expressed as nmoles of superoxide produced/min/106 macrophages. These values represent the MAF precursor activity of patient serum Gc protein [34, 37]. Lost or reduced MAF precursor activity of patient serum Gc protein is expressed as a decrease in superoxide generation as compared with control healthy human Gc protein. Thus, the MAF precursor activity measures the ability of individual patient to activate macrophages and for immune potential. However, loss of MAF precursor activity results in immunosuppression.
Cultivation of the mixture of lyso-Pc-treated lymphocytes and macrophages in EA medium without containing serum results in the production of 0.5–0.85 nmole superoxide/min/106 cells [30, 46]. Thus, if patient serum (0.1%) generates <0.85 nmol superoxide/min/106 cells, the precursor activity of patient serum Gc is considered to be lost.
Determination of Nagalase activity in patient blood stream
Patient sera (300 μl) were precipitated with 70% saturated ammonium sulfate. The precipitates were dissolved in 50 mM sodium citrate buffer (pH 6.0) and dialyzed against the same buffer at 4°C for 2 h. The dialysates were made up to 1 ml in volume and assayed for Nagalase activity [34, 37]. Substrate solution (250 μl) contained 5 μm of p-nitrophenyl N-acetyl-α-d-galactosaminide in 50 mM citrate buffer (pH 6.0). The reaction was initiated by addition of 250 μl of the dialyzed samples, kept at 37°C for 60 min and terminated by adding 200 μl of 10% TCA. After centrifugation of the reaction mixture, 300 μl of 0.5 M Na2CO3 solution was added to the supernatant. The amount of released p-nitrophenol was determined spectrophotometrically at 420 nm and expressed as nmol/min/mg protein [34, 37]. Protein concentrations were estimated by the Bradford method [1].
The half-life of Nagalase activity in vivo is less than 24 h as we observed a sudden drop of Nagalase activity in 24-h post-resection of the tumor [37]. However, Nagalase in collected serum is extremely stable probably because of the presence of product inhibitor and is highly reproducible after storage of sera at 4°C for more than 6 months [37].
Healthy control sera exhibit low levels (0.35–0.65 nmol/min/mg) of the enzyme activity. This is the enzyme activity of α-galactosidase that can catabolize the chromogenic substrate (i.e., p-nitrophenyl N-acetyl-α-d-galactosaminide) for Nagalase [32, 34, 37]. A reduction in serum Nagalase activity to 0.65 nmol/min/mg or less in patients during GcMAF therapy serves as a demonstration that tumor burden has been eradicated.
Results
Therapeutic history and immunodiagnostic parameters of metastatic colorectal cancer patients
Therapeutic history of eight colorectal cancer patients prior to GcMAF therapy is summarized in Table 1. All eight patients previously received surgical resection of their tumors. They have been treated with chemotherapy alone or a combination of radiation and chemotherapy. Because the fate and staging of the malignant disease correlate with tumor burden and the degree of immunosuppression [11, 37], the potency of macrophages and tumor burden index for each patient must be determined, regardless the lapse of time after tumor resection and adjuvant therapy.
Table 1.
Therapeutic history and diagnostic parameters of colorectal cancer patients
| Patient | Therapeutic history | Precursor activitya superoxide (nmol) | NaGalaseb (nmol/mg/min) | |||
|---|---|---|---|---|---|---|
| No. | Age | Sex | Surgery | Radiat./chemo. | ||
| 1 | 71 | F | Colon resect. | Chemotherapy | 1.88 | 2.72 |
| 2 | 63 | M | Rectum resect. | Radiat./chemo. | 2.01 | 2.56 |
| 3 | 68 | M | Colon resect. | Chemotherapy | 0.84 | 4.84 |
| 4 | 41 | M | Colon resect. | Chemotherapy | 0.72 | 6.71 |
| 5 | 51 | F | Colon resect | Chemotherapy | 2.29 | 1.78 |
| 6 | 79 | M | Rectum resect. | Radiat./chemo. | 2.14 | 2.57 |
| 7 | 55 | F | Colon resect. | Chemotherapy | 1.68 | 3.28 |
| 8 | 82 | M | Colon resect. | Chemotherapy | 1.29 | 3.89 |
| C | Healthy humanc | 5.12 | 0.39d | |||
a Precursor activity <0.85 is unable to support activation of macrophages and that is considered to be loss of activity
b Nagalase assayed before entering GcMAF therapy
c Average of six healthy humans
d This activity level is enzyme activity of α-galactosidase and not of Nagalase
Since macrophage activation for phagocytosis and subsequent antigen presentation is the first indispensable step for immune development [37, 41, 43, 45], the lack of macrophage activation leads to immunosuppression. Because serum Gc protein is the precursor for the principal MAF, the MAF precursor activity of patient serum Gc protein was first to be examined. As shown in Table 1, the MAF precursor activities of patient Gc protein were lost (<0.85 nmol/min) or reduced. Because loss or decrease in the MAF precursor activity of patient Gc protein results from deglycosylation of the Gc protein by serum Nagalase secreted from cancerous cells [34, 37] (Fig. 1b), patient serum Nagalase activities were examined. Patients having lower MAF precursor activities of their serum Gc protein had higher serum Nagalase activities (Table 1). Since serum Nagalase activity of cancer patients is directly proportional to their tumor burden [34, 35, 37, 39, 43, 46], the serum Nagalase activities of these tumor-resected patients estimate their total amount of metastasized tumor cells. Thus, the serum Nagalase activity of individual colorectal cancer patients should serve as a baseline control for prognostic analysis of patient serum Nagalase activity during GcMAF therapy.
Prognostic parameters for colorectal cancer during GcMAF therapy
During the course of GcMAF therapy, the MAF precursor activity and serum Nagalase activity of first five colorectal patients were analyzed. As GcMAF therapy progressed the MAF precursor activity of all five patients increased and their Nagalase activity decreased inversely as shown in Table 2. As the MAF precursor activity increased toward healthy control values, serum Nagalase activities of these patients decreased toward healthy control levels. Thus, these immunodiagnostic parameters of colorectal cancer patients served as excellent prognostic indices. Because serum Nagalase activity is proportional to the total tumor burden and kinetic decrease of serum Nagalase activity allows us to envision the curative process of the malignancy, serum Nagalase activity should be monitored during the time course study of GcMAF therapy.
Table 2.
Correlation of the MAF precursor activity of colorectal cancer patients with their serum α-N-acetylgalactosaminidase activity
| Patient no. | Time assay week | Precursor activity superoxide (nmol) | NaGalase (nmol/min/mg) |
|---|---|---|---|
| 1 | 0 | 1.88 | 2.72 |
| 1 | 2.01 | 2.43 | |
| 2 | 2.15 | 2.02 | |
| 3 | 2.21 | 1.89 | |
| 4 | 2.75 | 1.55 | |
| 8 | 3.14 | 1.15 | |
| 11 | 3.18 | 1.03 | |
| 14 | 3.20 | 1.01 | |
| 17 | 3.21 | 0.99 | |
| 20 | 3.25 | 0.97 | |
| 26 | 3.45 | 0.82 | |
| 35 | 4.08 | 0.68 | |
| 2 | 0 | 2.01 | 2.56 |
| 4 | 2.08 | 2.41 | |
| 6 | 2.25 | 2.36 | |
| 8 | 2.31 | 2.28 | |
| 9 | 2.42 | 1.97 | |
| 10 | 2.51 | 1.85 | |
| 11 | 2.91 | 1.62 | |
| 15 | 3.21 | 1.47 | |
| 19 | 3.69 | 1.03 | |
| 25 | 3.80 | 0.88 | |
| 36 | 3.93 | 0.80 | |
| 41 | 4.01 | 0.53 | |
| 45 | 4.11 | 0.44 | |
| 3 | 0 | 0.84 | 4.84 |
| 1 | 1.18 | 4.48 | |
| 3 | 1.24 | 4.02 | |
| 4 | 1.33 | 3.77 | |
| 6 | 1.44 | 3.42 | |
| 7 | 2.12 | 3.38 | |
| 10 | 2.22 | 3.02 | |
| 16 | 2.31 | 2.38 | |
| 25 | 2.44 | 1.92 | |
| 32 | 2.55 | 1.75 | |
| 40 | 3.98 | 0.99 | |
| 46 | 4.01 | 0.62 | |
| 65 | 4.21 | 0.40 | |
| 4 | 0 | 0.72 | 6.71 |
| 1 | 1.06 | 5.48 | |
| 2 | 1.31 | 4.92 | |
| 4 | 1.34 | 4.82 | |
| 6 | 1.35 | 4.77 | |
| 8 | 1.37 | 4.68 | |
| 11 | 1.34 | 4.40 | |
| 16 | 1.41 | 4.09 | |
| 19 | 1.49 | 3.86 | |
| 23 | 1.52 | 3.32 | |
| 27 | 1.55 | 3.68 | |
| 30 | 2.11 | 2.34 | |
| 34 | 2.34 | 1.76 | |
| 43 | 3.10 | 1.25 | |
| 52 | 3.62 | 0.63 | |
| 64 | 3.83 | 0.50 | |
| 5 | 0 | 2.29 | 1.78 |
| 1 | 2.41 | 1.62 | |
| 2 | 2.40 | 1.55 | |
| 5 | 3.21 | 1.33 | |
| 9 | 3.32 | 0.85 | |
| 13 | 3.42 | 0.78 | |
| 15 | 3.81 | 0.66 | |
| 25 | 4.29 | 0.55 |
Time course study of serum Nagalase activity of metastatic colorectal cancer patients during GcMAF therapy
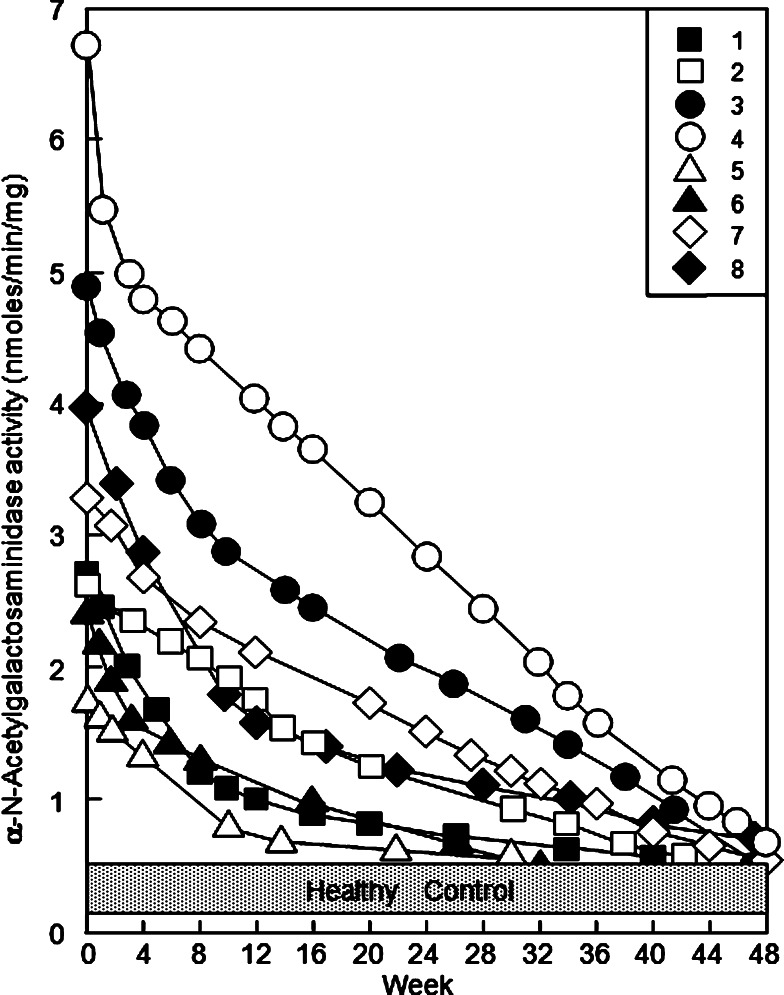
These colorectal cancer patients had the initial Nagalase activities ranging from 1.78 to 6.71 nmol/min/mg. As shown in Fig. 3, the serum Nagalase activities of all eight patients rapidly decreased in the first 4–8 weeks and followed by slow decrease of serum Nagalase activity. After about 32–50 administrations (32–50 weeks) of 100 ng GcMAF, all eight colorectal cancer patients had very low serum Nagalase activity levels equivalent to those of healthy control values ranging from 0.37 to 0.69 nmol/mg/min as shown in Fig. 3. Since these healthy control values are those of α-galactosidase and not of malignant-specific serum Nagalase activity [34, 37], the results suggest that all eight patients are free of cancerous cells. During 7 years of observation after completion of GcMAF therapy, these patients showed no increase in their serum Nagalase activities, indicating no recurrence of colorectal cancer. Furthermore, the annual CT scans evidenced that these patients were free of tumor recurrence for 7 years after GcMAF therapy.
Fig. 3.
Time course study of GcMAF therapy of colorectal cancer patients with serum α-N-acetylgalactosaminidase (Nagalase) as a prognostic index. The numbered symbols in the upper right frame correspond to the patient number in Table 1
Curative rate of GcMAF therapy of cancer depends on the degree of cell surface abnormality
Poorly differentiated (termed undifferentiated) cancer cells should have more abnormality in cell surface than moderately/immediately differentiated (differentiated for short) cancer cells [46]. Since the macrophages activated by GcMAF efficiently recognize and kill cancer cells having more abnormality, the activated macrophages kill undifferentiated cells more rapidly than differentiated cells [46]. Thus, the rapidly decreasing serum Nagalase activities during GcMAF therapy imply more abnormality in undifferentiated cells [46]. As shown in the time course study of GcMAF therapy in Fig. 3, the serum Nagalase activity of patient no. 4 decreased sharply in the first 4 weeks followed by a slow decrease during the remaining therapeutic period (>40 weeks). The serum Nagalase activity of all seven other patients decreased rapidly in the first several weeks (up to 8 weeks) followed by a very slow decrease during the remaining therapeutic period. These diphasic tumor regression graphs suggest that undifferentiated cells are mixed with differentiated cells. Thus, undifferentiated cells were killed rapidly during the first few to several weeks, and the differentiated cells were killed slowly in the remaining GcMAF therapeutic period. These mixed cell populations appeared to be developed by differentiation during growth of undifferentiated tumor cells. Similar results were also observed during GcMAF therapy of metastatic breast cancer patients [46].
Discussion
As we reported previously, GcMAF has a potent antiangiogenesis activity [9]. Thus, GcMAF has another antitumor activity via antiangiogenesis. These findings were confirmed by the group of Folkman [10]. This antiangiogeneic effect of GcMAF on malignant tumors is growth inhibition of the tumors. In contrast, GcMAF is the most potent macrophage-activating factor that can greatly activate macrophages to eradicate a variety of cancers [46]. For the assessment of therapeutic efficacy of GcMAF for metastatic colorectal cancer patients, GcMAF was used as a single remedy modality. Therefore, anemic cancer patients were excluded from GcMAF therapy.
Approximately 85% of patients diagnosed with colorectal cancer can have an operation intended for cure [2]. However, micrometastasis and recurrent tumors are commonly noted. Micrometastatic cells in bone marrow have been shown to correlate with a poor clinical outcome [2]. Micrometastasis in bone marrow lead to the recurrence and cause anemia. Tumor resection of anemic patients aborts anemia. Then, they became eligible for GcMAF therapy. Even patients deemed to have incurable cancer should have palliative resection of tumors to abort anemia, prevent obstruction, and reduce the risk of invasion to adjacent organs such as bladder [4]. As soon as they became nonanemic, they should be eligible for GcMAF therapy.
Administration of GcMAF to cancer patients is a very effective procedure for cancer therapy in spite of the presence of high Nagalase activity in patient sera [46]. Although Gc protein is efficiently deglycosylated by serum Nagalase secreted from cancerous cells [11, 34, 36, 37] (Fig. 1b), serum Nagalase has no effect on GcMAF. Thus, the capacity of the enzyme to remove the trisaccharide of the glycoprotein indicates that serum Nagalase is endo-Nagalase [35]. When GcMAF was added to cancer patient serum containing a high Nagalase activity and incubated for 4 h at 37°C, the potency of GcMAF activity did not decrease [37]. These results indicate that Nagalase under colloidal serum environment, known as oncotic pressure [46], acts as endo-Nagalase but not as an exo-enzyme because Nagalase in serum is unable to deglycosylate a monosaccharide, N-acetylgalactosamine (GalNAc), of GcMAF. Administration of GcMAF (100 ng) to healthy humans and advanced cancer patients results in the same extent of macrophage activation, confirming that serum Nagalase has no effect on the potency of GcMAF. However, under nononcotic salt-buffered medium, Nagalase activity can be measured as an exo-enzyme activity with a readily available chromogenic substrate, p-nitrophenyl N-acetyl-α-d-galactosaminide. This Nagalase assay procedure allows us to use it for prognosis of all types of malignant diseases.
Since the half-life of the activated macrophages is approximately 6 days [23, 24], weekly administrations of 100 ng GcMAF were found to be the most effective among the therapies for cancers. Macrophages activated by GcMAF develop an enormous variation of receptors [46] that recognize a variety of bacteria, viruses and abnormalities in cell surface of cancerous cells. This fundamental nature of macrophages to recognize the abnormality of cells is universal to all types of cancers. In fact, administration of GcMAF (100 ng/week) to cancer patients showed curative effects on a variety of cancers indiscriminately [11, 35, 36, 39, 41]. Types of cancer so far tested are prostate, breast, stomach, liver, lung (including mesothelioma), uterus, ovary, head/neck, brain, skin, fibrosarcoma and various leukemias [39, 41]. Efficacy of GcMAF therapy and curative rates of various cancers by GcMAF therapy depend on the degree of cell membrane abnormality, which corresponds to the grade of differentiation of the malignant cells.
Efficacy of GcMAF therapy of colorectal cancer was evaluated by kinetic decrease of tumor burden with measurement of serum Nagalase activity. Because the levels of other tumor markers, CEA and CA19-9, of tumor-resected patients are close to those of healthy controls, kinetic decrease of the levels of these tumor markers during GcMAF therapy is not feasible. Precise measuring of Nagalase activity allowed us to determine the degree of cell-surface abnormality by the curative rate during GcMAF therapy. Undifferentiated tumor cells are killed more efficiently than differentiated cells because the activated macrophages carry larger numbers of receptors for undifferentiated cells than those for differentiated cells. In fact adenocarcinoma such as breast and prostate cancer cells are undifferentiated and killed rapidly by the activated macrophages, whereas well-differentiated cancer cells such as squamous carcinoma cells are slowly killed by the activated macrophages. Faster curative rates, requiring less than 25 weeks, were always observed during GcMAF therapy of adenocarcinomas (e.g., prostate and breast cancers) [46]. In contrast, GcMAF therapy of well-differentiated squamous cell carcinomas such as head/neck cancers requires more than 75 weeks. Thus, the faster curative rate of adenocarcinomas is due to efficient macrophage recognition of the abnormality of the adenocarcinoma cell surface. However, a variety of cancers contain the mixed population of undifferentiated and differentiated cells within a tumor (e.g., breast cancer [46] and colorectal cancer). This type of fine differentiation in prostate cancer has been known for many years. In 1977, Gleason [5] separated histologic patterns of prostate cancer into a grading 1 to 5 patterns of decreasing differentiation, tumor pattern grade 1 being most differentiated and pattern grade 5 being least differentiated (poorly differentiated or undifferentiated). Tumor pattern grade 3 (Gleason grade 3) is the most common histologic pattern and is considered moderately well differentiated. However, one can readily interpret the histologic pattern of grade 3 (schematic diagram developed by Gleason) as a mixture of differentiated cells (grade 1) and least differentiated (undifferentiated) cells (grade 5). This can explain the diphasic tumor regression graphs of GcMAF therapy for prostate cancer being a mixture of differentiated and undifferentiated cells (N. Yamamoto, H. Suyama, Y. Koga, unpublished observation). In the present study, similar diphasic tumor curative rates were demonstrated for GcMAF therapy of colorectal cancer being a mixture of differentiated and undifferentiated cells.
The curative rate measurements of tumors during GcMAF therapy and the estimation of the degree of tumor differentiation have been possible because of the availability of precision measurement of serum Nagalase. Therefore, the significance of GcMAF therapy of cancers has been greatly enhanced by the discovery of cancer cell-specific Nagalase that can accurately monitor the rate of tumor regression during GcMAF therapy.
Acknowledgment
This investigation was supported in part by US Public Health Service Grant AI-32140 and Elsa U. Pardee Foundation Grant to N. Y.
Abbreviations
- Gc
Human vitamin D3 binding protein
- GcMAF
Enzymatically generated Gc-derived macrophage-activating factor
- Nagalase
α-N-acetylgalactosaminidase
Footnotes
This article has been retracted by the journal's co-Editors-in-Chief in conjunction with the publisher (Springer) due to irregularities in the Institutional Review Board documentation.
References
- 1.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;172:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 2.Braun S, Pantel K. Micrometastatic bone marrows involvement detection and prognostic significance. Med Oncol. 1999;16:154–165. doi: 10.1007/BF02906127. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AM, Shank B, Friedman MA. Colorectal cancer. In: Devita VT Jr, Hellman S, Rosenberg SA, editors. Cancer, principle and practice of oncology, 3. Philadelphia: J.B. Lippincott; 1989. pp. 895–964. [Google Scholar]
- 4.DeCosse JJ, Tsioulias GJ, Jacobson JS. Colorectal cancer: detection, treatment and rehabilitation. CA Cancer J Clin. 1994;44:27–42. doi: 10.3322/canjclin.44.1.27. [DOI] [PubMed] [Google Scholar]
- 5.Gleason DF. The Veteran,s Administration Cooperative Urological Research Group: histological grading and clinical staging of prostate carcinoma. In: Tannenbaum M, editor. Urologic pathology: the prostate. Philadelphia: Lea and Febiger; 1977. pp. 171–198. [Google Scholar]
- 6.Gold P, Freedman SQ. Demonstration of tumor specific antigens in human colonic carcinomata by immunological tolerance and absorption techniques. J Exp Med. 1965;121:439–462. doi: 10.1084/jem.121.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Homma S, Yamamoto N. Activation process of macrophages after in vitro treatment of mouse lymphocytes with dodecylglycerol. Clin Exp Immunol. 1990;79:307–313. doi: 10.1111/j.1365-2249.1990.tb05195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Homma S, Yamamoto M, Yamamoto N. Vitamin D binding protein (group-specific component, Gc) is the sole serum protein required for macrophage activation after treatment of peritoneal cells with lysophosphatidylcholine. Immunol Cell Biol. 1993;71:249–257. doi: 10.1038/icb.1993.29. [DOI] [PubMed] [Google Scholar]
- 9.Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N. Vitamin D-binding protein-derived macrophage activating factor, GcMAF, has an antiangiogenic activity both in vivo and in vitro. J Natl Cancer Inst. 2002;94:1311–1319. doi: 10.1093/jnci/94.17.1311. [DOI] [PubMed] [Google Scholar]
- 10.Kisker O, Onizuka S, Becker CM, Fannon M, Flynn E, D’Amato R, Zetter B, Folkman J, Ray R, Swamy N, Pirie-Shepherd S. Vitamin D binding protein-macrophage activating factor (DBP-maf) inhibits angiogenesis and tumor growth in mice. Neoplasia. 2003;5:32–40. doi: 10.1016/S1476-5586(03)80015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga Y, Naraparaju VR, Yamamoto N. Antitumor effects of vitamin D3-binding protein-derived macrophage activating factor on Ehrlich tumor bearing mice. Proc Soc Exp Biol Med. 1999;220:20–26. doi: 10.3181/00379727-220-44339. [DOI] [PubMed] [Google Scholar]
- 12.Koprowski H, Herlyn M, Steplewski Z, Sears HF. Specific antigen in serum of patients with colon carcinoma. Science. 1981;212:53–55. doi: 10.1126/science.6163212. [DOI] [PubMed] [Google Scholar]
- 13.Link RP, Perlman KL, Pierce EA, Schnoes HK, DeLuca HF. Purification of human serum vitamin D-binding protein by 25-hydroxyvitamin D3-Sepharose chromatography. Anal Biochem. 1986;157:262–269. doi: 10.1016/0003-2697(86)90624-X. [DOI] [PubMed] [Google Scholar]
- 14.Maehara Y, Kohnoe S, Sugimachi K. Chemosensitivity test for carcinoma of digestive organs. Semin Surg Oncol. 1990;6:42–47. doi: 10.1002/ssu.2980060109. [DOI] [PubMed] [Google Scholar]
- 15.Morton D, Eibler FR, Malmgren RA, Wood WC. Immunological factors which influence response to immunotherapy in malignant melanoma. Surgery. 1970;68:158–164. [PubMed] [Google Scholar]
- 16.Naraparaju VR, Yamamoto N. Roles of ß-galactosidase of B lymphocytes and sialidase of T lymphocytes in inflammation-primed activation of macrophages. Immunol Lett. 1994;43:143–148. doi: 10.1016/0165-2478(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 17.Ngwenya BZ, Yamamoto N. Activation of peritoneal macrophages by lysophosphatidylcholine. Biochim Biophys Acta. 1985;839:9–15. doi: 10.1016/0304-4165(85)90175-8. [DOI] [PubMed] [Google Scholar]
- 18.Ngwenya BZ, Yamamoto N. Effects of inflammation products on immune systems: lysophosphatidylcholine stimulates macrophages. Cancer Immunol Immunother. 1986;21:174–182. doi: 10.1007/BF00199358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngwenya BZ, Yamamoto N. Contribution of lysophosphatidylcholine treated nonadherent cells to mechanism of macrophage activation. Proc Soc Exp Biol Med. 1990;193:118–124. doi: 10.3181/00379727-193-43011. [DOI] [PubMed] [Google Scholar]
- 20.Olson RM, Perencevich NP, Malcolm AW, Chaffey JT, Wilson RE. Pattern of recurrence following curative resection of adenocarinoma of the colon and rectum. Cancer. 1980;45:2969–2974. doi: 10.1002/1097-0142(19800615)45:12<2969::AID-CNCR2820451214>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317–1329. doi: 10.1002/1097-0142(19831001)52:7<1317::AID-CNCR2820520731>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Yamamoto N, Ngwenya BZ. Activation of macrophages by lysophospholipids, and ether derivatives of neutral lipids and phospholipids. Cancer Res. 1987;47:2008–2013. [PubMed] [Google Scholar]
- 23.Yamamoto N, Ngwenya BZ, Pieringer PA. Activation of macrophages by ether analogues of lysophospholipids. Cancer Immunol Immunother. 1987;25:185–192. doi: 10.1007/BF00199146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto N, St. Claire DA, Homma S, Ngwenya BZ. Activation of mouse macrophages by alkylglycerols, inflammation products of cancerous tissues. Cancer Res. 1988;48:6044–6049. [PubMed] [Google Scholar]
- 25.Yamamoto N, Homma S. Vitamin D3 binding protein (group-specific component, Gc) is a precursor for the macrophage activating signal from lysophosphatidylcholine-treated lymphocytes. Proc Natl Acad Sci USA. 1991;88:8539–8543. doi: 10.1073/pnas.88.19.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto N, Homma S, Haddad JG, Kowalski MN. Vitamin D3 binding protein required for in vitro activation of macrophages after dodecylglycerol treatment of mouse peritoneal cells. Immunol. 1991;74:420–424. [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto N, Homma S, Millman I. Identification of the serum factor required for in vitro activation of macrophages: role of vitamin D binding protein (group-specific component, Gc) in lysophospholipid activation of mouse peritoneal macrophages. J Immunol. 1991;147:273–280. [PubMed] [Google Scholar]
- 28.Yamamoto N (1993) In vitro enzymatic conversion of glycosylated human vitamin D binding protein to a potent macrophage activating factor. US Patent number: 5,177,002
- 29.Yamamoto N, Kumashiro R. Conversion of vitamin D3 binding protein (group-specific component) to a macrophage activating factor by the stepwise action of ß-galactosidase of B cells and sialidase of T cells. J Immunol. 1993;151:2794–2902. [PubMed] [Google Scholar]
- 30.Yamamoto N, Kumashiro R, Yamamoto M, Willett NP, Lindsay DD. Regulation of inflammation-primed activation of macrophages by two serum factors, vitamin D3-binding protein and albumin. Infect Immun. 1993;61:5388–5891. doi: 10.1128/iai.61.12.5388-5391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto N (1994) Macrophage activating factor from vitamin D binding protein. US Patent Number: 5,326,749
- 32.Yamamoto N, Naraparaju VR, Srinivasula SM. Structural modification of serum vitamin D3-binding protein and immunosuppression in HIV-infected patients. AIDS Res Human Retrovirus. 1995;11:1373–1378. doi: 10.1089/aid.1995.11.1373. [DOI] [PubMed] [Google Scholar]
- 33.Yamamoto N. Structural definition of a potent macrophage activating factor derived from vitamin D3 binding protein with adjuvant activity for antibody production. Mol Immunol. 1996;33:1157–1164. doi: 10.1016/S0161-5890(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto N, Naraparaju VR, Asbell SO. Deglycosylation of serum vitamin D-binding protein and immunosuppression in cancer patients. Cancer Res. 1996;56:2827–2831. [PubMed] [Google Scholar]
- 35.Yamamoto N (1997) Diagnostic and prognostic indices for cancer and AIDS. US Patent Number: 5,620,846
- 36.Yamamoto N, Naraparaju VR. Immunotherapy of BALB/c mice bearing Ehrlich ascites tumor with vitamin D-binding protein-derived macrophage activating factor. Cancer Res. 1997;57:2187–2191. [PubMed] [Google Scholar]
- 37.Yamamoto N, Naraparaju VR, Urade M. Prognostic utility of serum a-N-acetylgalactosaminidase and immunosuppression resulted from deglycosylation of serum Gc protein in oral cancer patients. Cancer Res. 1997;57:295–299. [PubMed] [Google Scholar]
- 38.Yamamoto N. Vitamin D and the immune system. In: Delves PJ, Roitt I, editors. Encyclopedia of immunology. 2. London: Academic; 1998. pp. 2494–2499. [Google Scholar]
- 39.Yamamoto N (1998) Diagnostic and prognostic ELISA assays of serum or plasma a-N-acetylgalactosaminidase for cancer. US Patent number: 5,712,104
- 40.Yamamoto N, Naraparaju VR. Structurally well-defined macrophage activating factor derived from vitamin D3-binding protein has a potent adjuvant activity for immunization. Immunol Cell Biol. 1998;76:237–244. doi: 10.1046/j.1440-1711.1998.00748.x. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto N (2002) Preparation of potent macrophage activating factors derived from cloned vitamin D binding protein and its domain and their therapeutic usage for cancer, HIV-infection and osteopetrosis. US Patent no. 6,410,269
- 42.Yamamoto N, Ueda M. Eradication of HIV by treatment of HIV-infected/AIDS patients with vitamin D-binding protein (Gc protein)-derived macrophage activating factor (GcMAF). Immunology 2000. Bolonia: Medmond Ltd; 2004. pp. 197–200. [Google Scholar]
- 43.Yamamoto N, Ueda M. Therapeutic efficacy of vitamin D-binding protein (Gc protein)-derived macrophage activating factor (GcMAF) for prostate and breast cancers. Immunology 2000. Bolonia: Medmond Ltd; 2004. pp. 201–204. [Google Scholar]
- 44.Yamamoto N, Urade M. Pathogenic significance of a-N-acetylgalactosaminidase found in the hemagglutinin of influenza virus. Microbes Infect. 2005;7:674–681. doi: 10.1016/j.micinf.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto N. Pathogenic significance of a-N-acetylgalactosaminidase found in the envelope glycoprotein gp160 of human immunodeficiency virus type 1. AIDS Res Human Retrovirus. 2006;22:262–271. doi: 10.1089/aid.2006.22.262. [DOI] [PubMed] [Google Scholar]
- 46.Yamamoto N, Suyama H, Yamamoto N-Y, Ushijima N. Immunotherapy of metastatic breast cancer patients with vitamin D-binding protein-derived macrophage activating factor (GcMAF) Int J Cancer. 2008;122:461–467. doi: 10.1002/ijc.23107. [DOI] [PubMed] [Google Scholar]
- 47.Zbar B, Tanaka T. Immunotherapy of cancer: regression of tumors after intralesional injection of living Mycobacterium bovis . Science. 1971;172:271–273. doi: 10.1126/science.172.3980.271. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Zhang HS, Cordon-Cardo C, Ragupathi G, Livingston PO. Selection of tumor antigens as targets for immune attack using immunohistochemistry. III. Protein antigen. Clin Cancer Res. 1998;4:2669–2676. [PubMed] [Google Scholar]