Abstract
Objectives
Patients with renal cell carcinomas (RCC) have few treatment options, underscoring the importance of developing new approaches such as immunotherapy. However, few tumor associated antigens (TAA), which can be targeted by immunotherapy, have been identified for this type of cancer. von Hippel-Lindau clear cell RCC (VHL−/−RCC) are characterized by mutations in the VHL tumor suppressor gene. Loss of VHL function causes the overexpression of transforming growth factor (TGF)-α, leading us to hypothesize that TGF-α could be a potential TAA for immunotherapy of kidney cancer, which was evaluated in this study.
Methods and results
We first confirmed the absent or weak expression of TGF-α in important normal tissues as well as its overexpression in 61% of renal tumors in comparison to autologous normal kidney tissues. In addition, we demonstrated the immunogenicity of TGF-α, by expanding many T cell lines specific for certain TGF-α peptides or the mature TGF-α protein, when presented by major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells. Interestingly, some of these TGF-α-specific T cells were polyfunctionals and secreted IFN-γ, TNF-α and IL-2.
Conclusion
We have shown that TGF-α is a valid candidate TAA, which should allow the development of a targeted immunotherapy.
Keywords: Transforming growth factor (TGF)-α, Clear cell renal cell carcinoma, Tumor antigen, Cancer immunotherapy, Polyfunctional antigen-specific T lymphocytes
Introduction
Renal cell carcinomas (RCC) account for 90–95% of all kidney cancer types [1]. Surgery is the treatment of choice for patients with localized RCC, but 20–30% of them will develop metastases [2]. Although 5-year survival is 60% overall for kidney cancer, it drops to 10% for metastatic disease [2]; alternative approaches are clearly required to improve survival, since very few therapeutic regimens are available. Immunotherapy is an alternative approach, and, currently, high-dose interleukin (IL)-2 and interferon (IFN)-α are deployed clinically but objective clinical responses have been reported in only 10–23% of cases [3, 4]. Furthermore, a specific immune response can be elicited against tumor-associated antigens (TAA), but only few have been identified for RCC. Specifically, carbonic anhydrase IX (CA IX) [5, 6], C-Met [7], cyclin D1 [8] and renal tumor antigen (RAGE-1) [9] have been identified as relevant TAA for RCC, and may be of clinical interest. However, to be highly suitable for immunotherapy, a TAA needs to: (1) be expressed by tumor cells; (2) be absent from important normal tissues; (3) demonstrate immunogenicity, and ideally; (4) be involved in tumor progression. When considering the last point, very few TAA for RCC are available. Given the central role of transforming growth factor (TGF)-α in RCC progression [10, 11], we studied the possibility of targeting this protein as a TAA.
The vast majority of clear cell RCC (CCRCC) bear an inactivated von Hippel-Lindau (VHL) tumor suppressor gene leading to stabilized and constitutive expression of hypoxia-inducible factor (HIF)-1α and HIF-2α proteins [12–16]. Such abnormal expression results in the accumulation of different proteins involved in angiogenesis, such as vascular endothelial growth factor (VEGF). Many therapies have been tested based on inhibition of VEGF including: recombinant human monoclonal antibody against VEGF (bezacizumab) [17], and VEGF tyrosine kinase receptor inhibitors (sorafenib [18] and sunitinib [19]). Phase II and III clinical trials have shown that these new drugs increase objective response rates and progression-free survival, but patients still do not achieve complete remission. Constitutive expression of HIF-1α and HIF-2α proteins also results in the accumulation of growth factors, such as glucose transporter-1 (Glut-1) [20] and TGF-α [11]. When considering this pathway, TGF-α has emerged as a compelling TAA candidate because of its potentially weak expression in normal tissues and its direct involvement in tumor progression. However, its expression pattern needs to be extensively defined in the context of TAA, and its potential immunogenicity in RCC patients should be explored.
The current study investigates TGF-α expression in a panel of normal tissues and numerous human kidney cancer specimens. In addition, T lymphocytes specific to TGF-α could be detected from tumor-infiltrating lymphocytes (TIL) and peripheral blood mononuclear cells (PBMC) obtained from RCC patients. Based on these data, we believe that TGF-α could be a valuable candidate TAA.
Materials and methods
Clinical specimens
Kidney tumors and normal kidney tissues were obtained after total or partial nephrectomy from patients recruited by Dr. Simon Tanguay at the Montreal General Hospital (McGill University Health Centre). Tumor histology, grade, and staging (TNM) are presented in Table 1. Blood was drawn from kidney cancer patients at the time of surgery. All study subjects signed an informed patient consent form and the project has been reviewed and approved by the Ethics Committee at both CHUM-Notre-Dame Hospital and Montreal General Hospital. Staging (pTNM) was undertaken according to the AJCC Cancer Staging Manual (http://www.cancerstaging.org).
Table 1.
Clinicopathological characteristics of kidney cancer patients
| Patients | Tumor histology | Grade | pTNM |
|---|---|---|---|
| 1 | CCRCC | 2 | pT2 |
| 2 | CCRCC | 2 | pT1a |
| 3 | CCRCC | n/a | pT1a |
| 4 | RCC chromophobe | – | pT1b |
| 5 | RCC papillary | – | pT1b |
| 6 | RCC chromophobe | – | pT3a |
| 7 | RCC chromophobe | – | pT1a |
| 8 | CCRCC | 2 | pT2 |
| 9 | CCRCC | 2 | pT3a |
| 10 | CCRCC | 2 | pT2 |
| 11 | CCRCC | 3 | pT1b |
| 12 | CCRCC | 3 | pT1b |
| 13 | CCRCC | 2 | pT1b |
| 14 | CCRCC | 2 | pT1a |
| 15 | CCRCC | 1 | pT1a |
| 16 | CCRCC | 3 | pT1a |
| 18 | RCC papillary | – | pT1b |
| 19 | CCRCC | 3 | pT2 |
| 20 | CCRCC | 2 | pT3b |
| 21 | CCRCC | 2 | pT1a |
| 22 | CCRCC | 1 | pT1a |
| 23 | CCRCC | 2 | pT2 |
| 24 | RCC chromophobe | – | pT3a |
| 25 | CCRCC | 2 | pT1a |
| 27 | CCRCC | 1 | pT1a |
| 28 | CCRCC | 3 | pT3a |
| 29 | Angiomyolipoma | – | – |
CCRCC clear cell renal cell carcinoma
Cell culture
A498, 786-0, and KTCL140 cell lines are VHL−/−RCC. The A498 + VHL cell line is derived from A498 cells stably transfected with hemagglutinin (HA)-tagged VHL. All these cell lines were prepared as described elsewhere [21]. The HeLa cell line is a cervical cancer cell line containing wild-type VHL. All cell lines were obtained from the American Type Culture Collection (Manassas, VA). They were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wisent, St-Bruno, Québec, Canada) supplemented with 10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA and Wisent), 2 mM l-glutamine, 100 μg/ml penicillin/streptomycin and 10 μg/ml gentamicin (all from Invitrogen).
Peripheral blood mononuclear cells (PBMC) from kidney cancer patients or normal donors were enriched with lymphocyte separation medium (Cellgro, Herndon, VA and Wisent). PBMC were cryopreserved in 90% calf serum (Wisent)/10% DMSO (Sigma, St-Louis, MO), and stored in liquid nitrogen.
To generate CD40-activated B-cell cultures (CD40-B) [22], PBMC were cultured in complete B cell medium defined as Iscove’s Modified Dulbecco’s medium (Invitrogen) supplemented with 7.5% human serum (heat-inactivated, prepared from 3 normal donors recruited by Dr Jean-Pierre Routy at the Royal Victoria Hospital (McGill University Health Centre), Montréal, Québec, Canada), 2 mM l-glutamine, 100 U/ml penicillin/streptomycin and 10 μg/ml gentamicin. Soluble trimeric CD40L (500 ng/ml) (Immunex Corporation, Seattle, WA), recombinant human IL-4 (250 U/ml) (Peprotech, Rocky Hill, NJ) and cyclosporin A (0.66 μg/ml) (Calbiochem, San Diego, CA) were added to PBMC on the first day. Fresh complete medium containing 250 U/ml IL-4 and 250 ng/ml CD40L was added on day 3. After the first round of proliferation (days 5–8), cells were either frozen for future use or re-stimulated every 2–3 days when the culture reached a density of 1.5–2 × 106 cells/ml. B lymphocytes immortalized by the Epstein–Barr virus (EBV-B) were generated, as described previously [23].
RNA extraction and reverse transcription: polymerase chain reaction (RT-PCR)
Kidney tumors and normal tissues were stabilized in RNAlater (Sigma) and homogenized with a Medimachine™ (Dako Cytomation, Glostrup, Denmark) according to the manufacturer’s instructions and as described elsewhere [24]. RNA was prepared with Qiazol reagent (QIAGEN GmbH, Hilden, Germany), followed by a cleanup and concentration procedure using RNeasy™ Mini or Micro kits (QIAGEN) according to the manufacturer’s instructions [24].
For quantitative RT-PCR (qRT-PCR) analysis, cDNA was synthesized from RNA (1 μg) with random hexamer primers (Operon Biotechnologies, Huntsville, AL) and Omniscript Reverse Transcriptase kit (QIAGEN). TGF-α amplification was performed by QuantiTect custom assay (QIAGEN) and QuantiTect Probe PCR Master mix (QIAGEN). Primer and probe sequences were: TGF-α forward: 5′-CAGATTCCCACACTCAGTT-3′; TGF-α reverse: 5′-TACCCAGAATGGCAGACACA-3′; TGF-α internal probe: 5′-(Eclispe Dark Quencher)-GCAGGAGGACAAGCCAG-(FAM)-3′. The 18S subunit of the ribosome was amplified by QuantiTect Gene Expression Assay (QIAGEN) and QuantiTect Probe PCR Master mix (QIAGEN). Cycling conditions were implemented according to the manufacturer’s instructions. Amplification was performed in a Rotor Gene (Corbett Research, Sydney, Australia), and relative TGF-α expression was established according to the following mathematical formula [25, 26] :
 |
The relative TGF-α expression ratio over the 18S subunit of the ribosome was reported in relation to A498 cell line expression established at a value of 1. The equation takes into account the PCR efficiencies (E) of both genes and the difference (Δ) between the moment at which the fluorescence of a given sample versus A498 crosses the threshold (CP). Standard curves were generated in every run with serial dilutions of cDNA from the 786-0 cell line. All samples were tested in duplicate in at least two independent runs.
Pulsing of antigen presenting cells (APCs) with exogenous antigens
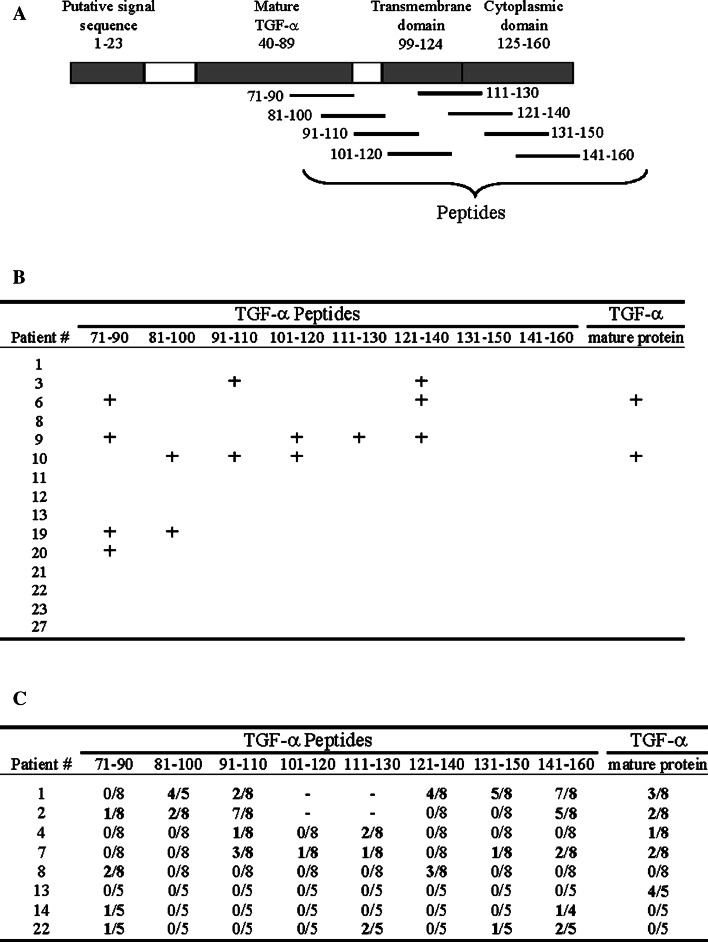
Eight synthetic 20-residue-long peptides overlapping by 10 amino acids derived from the TGF-α sequence were prepared (Fig. 3a) (Alpha Diagnostic International, San Antonio, TX; Service de synthèse de peptides de l’Est du Québec, Centre hospitalier de l’Université Laval, Canada (peptide 81–100 only). Peptides were reconstituted at a concentration of 25 mM in DMSO, except for peptides 91–110 and 111–130, which were reconstituted at 12.5 mM, and for peptide 101–120 which was reconstituted at 6.25 mM, because of lower solubility. Cells were plated at 1 × 106 cells/ml in complete B cell medium containing 500 ng/ml of soluble CD40L for CD40-B cells and in RPMI 1640 complete medium for EBV-B cells. Recombinant, mature TGF-α (amino acids 40–89 of the precursor TGF-α protein; Fig. 3a) (PeproTech, Ottawa, Ontario, Canada) or synthetic peptides were added to B cells at a final concentration of 10 μg/ml for the mature protein, and from 6 to 115 μM for the peptides depending on their solubility. Cells were incubated for 16–20 h, then washed to remove unloaded protein or peptide. In some recognition assays, CD40-B cells were previously incubated for 20 min with 40–80 μg/ml of blocking antibodies specific for either major histocompatibility complex (MHC) class I (clone W6/32), MHC class II (clone IV A12), or HLA-DR (clone HB55). CD40-B cells were used for recognition assays and for stimulation assays, while immortalized EBV-B cells were employed for recognition assays only.
Fig. 3.
Expansion of T lymphocytes specific to TGF-α or TGF-α-derived peptides. a Schematic representation of the TGF-α precursor and localization of synthesized overlapping peptides. b Resected tumors from kidney cancers were homogenized, and T cells from infiltrating single cell suspensions were expanded with PHA for 10–15 days prior to be tested for TGF-α-specificity. The symbol + means production of IFN-γ above background. Absence means no TGF-α-specific T cell detected. c PBMC from kidney cancer patients were co-cultured in multiple duplicated wells with peptide or TGF-α-pulsed autologous CD40-B cells as in Sect. “Materials and methods”. The specificity of expanded cells in b and c was evaluated by co-culture with autologous-pulsed CD40-activated B cells in IFN-γ (ELISPOT) and GM-CSF (ELISA). In ELISPOT, a T cell line was scored positive when >10 SFC and more than twice the number of SFC were observed in the well with the relevant peptide or protein, when compared with negative controls (irrelevant peptide from TGF-α). In GM-CSF secretion assays, a line was scored positive when secretion was >50 pg/ml and was twice the amount obtained with relevant controls. SFC spot-forming cells. c Numbers indicate number of wells positive over number of tested wells
Tumor infiltrating lymphocytes (TIL) culture
After tumor resection, kidney tumors were desegregated mechanically and homogenized with a Medimachine™ (Dako) to prepare single cell suspensions, from which TIL were enriched by centrifugation in lymphocyte separation medium (Cellgro and Wisent). About 1–5 × 105 TIL were then activated [27] with 5 μg/ml phytohemaglutinnin (PHA) (Sigma) in the presence of 2.5 × 107 irradiated (5,000 rads) PBMC from three different normal donors and IL-2 (300 IU/ml) (Chiron, Emeryville, CA) in complete T cell medium consisting of AIM-V (Invitrogen) supplemented with 5% heat-inactivated AB serum (Gemini Bio-Products, Calabasas, CA), 2 mM l-glutamine, 100 U/ml penicillin/streptomycin and 10 μg/ml gentamicin. On day 2, 300 IU/ml IL-2 was added, and on day 5, the supernatant was replaced by fresh complete medium and 300 IU/ml IL-2. After the first round of proliferation (days 8–10), cells were either frozen for future use or replated at 5–8 × 105 cells/ml in complete T cell medium containing 300 IU/ml of IL-2.
Expansion of peripheral T lymphocytes specific to TGF-α or TGF-α-derived peptides
PBMC from kidney cancer patients were cultured in multiple wells in complete T cell medium supplemented with 500 ng/ml CD40L. The recombinant mature TGF-α or individual peptides were then added at a concentration of 6–10 μg/ml for the protein, and 7–60 μM for the peptides, according to their solubility. On days 8–15, cells were re-stimulated with autologous 1.7–5.5 × 104 CD40-B cells pulsed with the corresponding peptide or protein, and 150–300 IU/ml of IL-2 were added to T cells on that same day and every 2–3 days.
Recognition assays
Tumor infiltrating lymphocytes were tested on days 14–20, and peripheral T cell lines on days 16–27, for their specificity to TGF-α on the basis of cytokine secretion. Specifically, 5 × 104 TIL or peripheral T cell lines were co-cultured with 5 × 104 CD40-B or EBV-B pulsed with TGF-α peptides or mature protein. When screening many peripheral T cell lines, 100 μl of cells were used. INF-γ secretion was measured by ELISPOT, and granulocyte–macrophage-colony stimulating factor (GM-CSF) was measured by ELISA.
For ELISPOT, 96-well filtration plates (MultiScreen™-HTS; Millipore, Bedford, MA) were coated with anti-IFN-γ mAb (5 μg/ml, Mabtech, Stockholm, Sweden) and incubated for 18 h at 4°C. On the following day, plates were washed with phosphate buffered saline (PBS, Wisent), and blocked with complete T cell medium for 2 h at 37°C. T cells were then added to wells. One hour later, pulsed autologous CD40-B cells or EBV-B cells were added to T cells for 16–20 h. Supernatants were collected and frozen at −20°C for future evaluation of GM-CSF secretion by ELISA. Meanwhile, ELISPOT plates were washed with PBS/0.01% Tween 20 (Sigma) and biotinylated anti-IFN-γ filtered mAb (2 μg/ml, Mabtech) were added. After 2 h of incubation at 37°C, plates were washed and incubated for 45 min at room temperature with streptavidin-HRP (1:100 or 1:1,000 dilution, Mabtech). The plates were again washed, and spots were revealed with 3-amino-9-ethyl-carbazole substrate (Sigma). Spots were counted with the ImmunoSpot Series 3B Analyzer (Cellular Technology Ltd., Cleveland, OH). In ELISPOT, a T cell line was scored positive when >10 spot-forming cells (SFC) and more than twice the number of SFC were observed in the well with the relevant peptide or protein, when compared with negative controls (irrelevant peptide from TGF-α).
For ELISA, MaxiSorp 96-well plates (Nalge Nunc International, Rochester, NY) were coated with anti-GM-CSF mAb (0.4 μg/ml; Endogen, Rockford, IL) overnight at 4°C. Plates were washed, blocked with PBS/5%FBS, washed again, and then secondary biotinylated mAb (1.4 μg/ml; Endogen) and T cell co-culture supernatants were added. After 90 min, plates were washed and incubated with Poly HRP20-streptavidin (0.25 μg/ml; Research Diagnostics Inc, Concord, MA) for 30–45 min. Plates were then revealed with TMB substrate (Neogen, Lexington, KY), and the reaction was stopped with 2 N H2SO4. In GM-CSF secretion assays, a T cell line was scored positive when secretion was >50 pg/ml and was twice the amount obtained with relevant controls.
Flow cytometric analyses
For intracellular cytokine staining, T cell lines and pulsed autologous-antigen presenting cells were co-cultured at a ratio of 1:1 for 3 h and for an additional 12 h in the presence of brefeldin A (5 μg/ml; Sigma). Cells were stained using Pacific Blue, Alexa-700, APC-Cy7-conjugated antibodies specific for human CD3, CD4, CD8 or corresponding isotype controls (all from BD Biosciences Mississauga, ON). Dead cells were excluded by staining with the Live/Dead Fixable Dead Cell Stain Kits (Invitrogen). Cells were surface stained for 30 min at 4°C, washed with staining buffer (PBS containing 1% FBS and 0.1% NaN3) and then fixed and permeabilized in 4% (w/v) paraformaldehyde with 0.1% (w/v) saponin in Hank’s Balanced Salt Solution for 10 min at room temperature. Intracellular staining was performed by incubating cells with antibodies against IFN-γ, TNF-α, IL-2 or corresponding isotype controls (all from BD Biosciences) for 30 min on ice in staining buffer containing 0.1% (w/v) saponin. Cells were washed twice and resuspended in staining buffer. Flow cytometry data was acquired using LSRII instrument (BD Bioscience), and analyzed using FlowJo software (Tree Star, Ashton, OR).
Statistical analyses
The differential TGF-α expression in kidney tumors and adjacent normal kidney tissues was evaluated by paired t test. Mean values of TGF-α expression were compared by clinicopathological clusters by the two-tailed t test for independent samples, or ANOVA if more than two groups. Differences were considered significant when P < 0.05. The data were analyzed by SPSS 13.0 software for Windows (LEAD Technologies, Chicago, IL).
Results
TGF-α is expressed in CCRCC and detected marginally in normal tissues
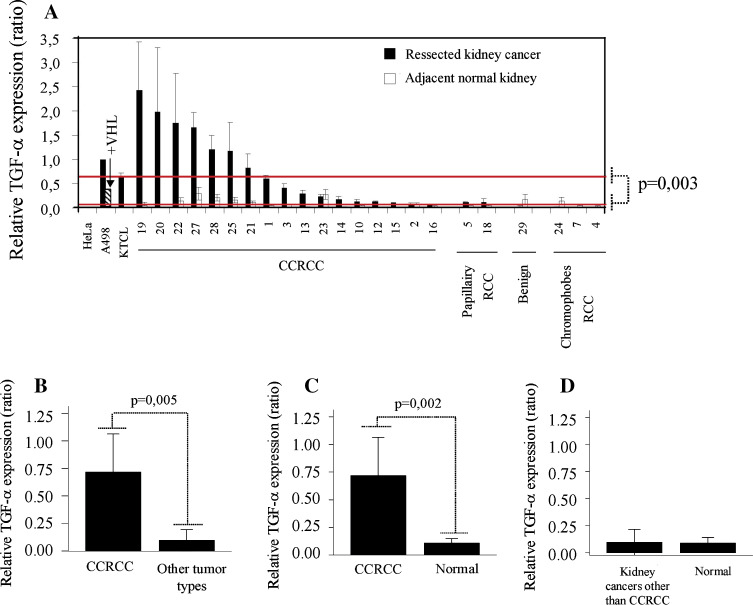
TGF-α expression has been demonstrated previously in kidney cancers [28–32]. Our previous in vitro functional experiments linked TGF-α expression to HIF accumulation which is a consequence of VHL loss specifically in CCRCC [11, 33]. Here, we exploited real time qRT-PCR to assess expression in clinical samples of CCRCC and a few other kidney cancer types. As predicted, TGF-α expression was significantly higher in multiple CCRCC compared to adjacent normal kidney tissues (Fig. 1a, c). TGF-α expression was significantly higher in CCRCC than in other kidney cancer types (Fig. 1b) and normal adjacent margins (Fig. 1c), but marginal expression was similar in kidney cancers other than CCRCC and autologous normal kidney tissues (Fig. 1d). No correlation was established between TGF-α expression and tumor size or grade (data not shown).
Fig. 1.
TGF-α expression in kidney cancers and adjacent normal tissues. a RNA and cDNA were prepared from resected kidney cancers (black bar) and normal kidney tissues adjacent to the tumor (white bar), and TGF-α expression was evaluated by qRT-PCR (relative to 18S ribosomal RNA). Number corresponds to patient number as listed in Table 1. HeLa cells were used as negative controls whereas the VHL−/−RCC lines: A498 and KTCL140 were used as positive controls. Reintroduction of VHL resulted in decreased TGF-α expression (see hatched bar). Expression levels were compared between CCRCC and other kidney cancer types (b), CCRCC and adjacent normal margins (c), and kidney cancers other than CCRCC and adjacent tissue (d)
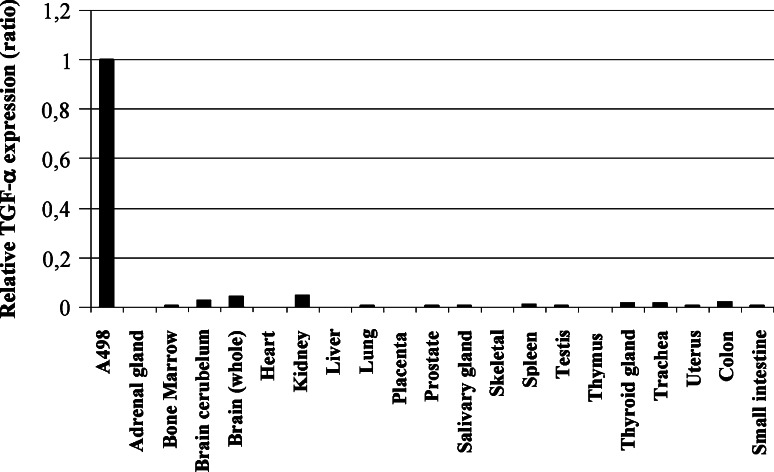
TGF-α expression was also reported in very specific situations in some normal human tissues [34–40]. Considering that a TAA must be minimally expressed from normal tissues in immunotherapies, to avoid autoimmunity and strong immune tolerance, we evaluated expression in a panel of normal tissues (n = 20; including the brain, heart, lung, and digestive tract), some being essential for survival. As illustrated in Fig. 2, relative TGF-α mRNA was barely detectable from normal tissues but was positive for the A498 CCRCC line.
Fig. 2.
Expression profile of TGF-α in normal tissues. cDNA was prepared from commercial RNA from normal human tissues, and TGF-α expression was evaluated by real-time qRT-PCR. Expression was normalized according to 18S ribosomal RNA and reported relative to the expression level of A498. The results presented are representative of at least two independent experiments
Altogether, considering its high expression from CCRCC clinical samples, and its relative absence from multiple normal tissues, TGF-α appears to be a compelling target as a TAA.
Human TGF-α-specific T cells are expanded from both tumor infiltrates and peripheral blood of RCC patients
Immunogenicity is an essential attribute for a TAA. We first evaluated whether TGF-α-specific T cells could be detected in kidney tumor infiltration. T cells were expanded by PHA stimulation in enriched cells from kidney cancers. We routinely isolated 3 × 105–2 × 106 TIL from kidney cancer specimens and expanded them to 5 × 107–1 × 108 T cells after 2 or 3 weeks in culture. The specificity of expanded T cells was examined by co-culture with mature TGF-α protein or peptide-pulsed autologous APC, and reactivity was assessed by IFN-γ release (with ELISPOT) and GM-CSF release (with ELISA). The peptides and mature TGF-α are presented in Fig. 3a. As seen in Fig. 3b, specific T cells were detected from 6 of 15 tumors analyzed. These results are based on IFN-γ secretion only since GM-CSF secretion was not detected from any of the specific T cells. There was no correlation between the frequency of specific T cells and TGF-α expression by tumors, since TGF-α-specific T cells could be detected regardless of tumor TGF-α expression levels. TGF-α-specific T cells were also expanded in vitro from peripheral blood using pulsed autologous APC in multiple wells for each TGF-α peptide or mature protein. After expansion, specificity was assessed by co-culture with APC pulsed with the same peptide/protein employed for expansion. An irrelevant peptide served as a negative control. As shown in Fig. 3c, for each patient at least one TGF-α-activation condition led to a specific response with either peptide or mature protein. Moreover, expanded TGF-α-specific T cells from different patients recognized different peptides from TGF-α, which may reflect different peptide/MHC interactions.
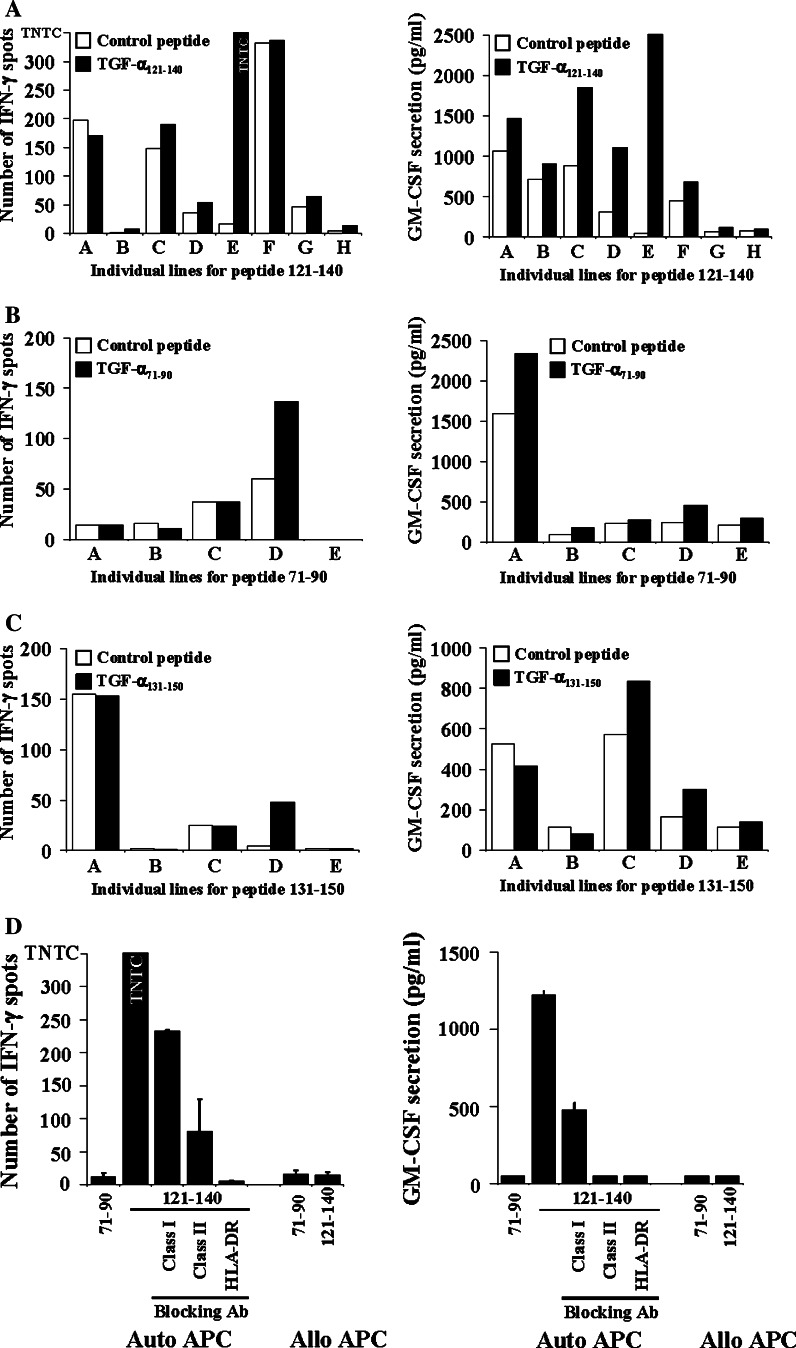
Finally, we further characterized some T cell lines to define whether TGF-α was recognized by CD4+ or CD8+ T cells and to establish the poly-functionality of these cells. Characterization of three T cell lines specific to different peptides from two patients are presented in Fig. 4. The results of the specificity assay (IFN-γ ELISPOT and GM-CSF ELISA) for T cells (T cell lines A–H) from patient #8 expanded in vitro in the presence of TGF-α peptide 121–140 are illustrated in panel a. T cell lines C and D were specific only according to the GM-CSF assay, but T cell E showed a strong specificity in both assays. The results of the specificity assay (IFN-γ ELISPOT and GM-CSF ELISA) for T cells (T cell lines A–E) from patient #22 expanded in vitro in the presence of TGF-α peptide 71–90 and peptide 131–150 are illustrated in panels b and c, respectively. TGF-α 71–90 specific response could be observed for line D (panel b) as detected by IFN-γ ELISPOT although levels of GM-CSF were low but still above control peptide. Regarding TGF-α 131–150 specific responses, line D (panel c) was also significantly above the control peptide in both assays.
Fig. 4.
Anti-TGF-α-specific T cell lines are MHC class II restricted. The specificity of T lymphocytes expanded from PBMC with TGF-α peptide 121–140 (a), 71–90 (b) and 131–150 (c) was evaluated as described in Fig. 3. Data for IFN-γ ELISPOT and GM-CSF secretion assays are presented. T cell line specificity was based on criteria described in Fig. 3 legend. D. The T cell line specific to TGF-α peptide 121–140 was co-cultured with allogeneic or autologous APC pulsed with the relevant peptide with or without antibodies blocking antigenic presentation mediated by MHC class I or class II molecules. TNTC to numerous to count, auto autologous, allo allogenic
We further characterized T cell line E TGF-α 121–140 specific for MHC restriction. Blockade of MHC class II HLA-DR or pan-specific MHC class II using blocking antibodies abrogated IFN-γ and GM-CSF secretion (Fig. 4d), while antibodies blocking MHC class I marginally decreased cytokine production.
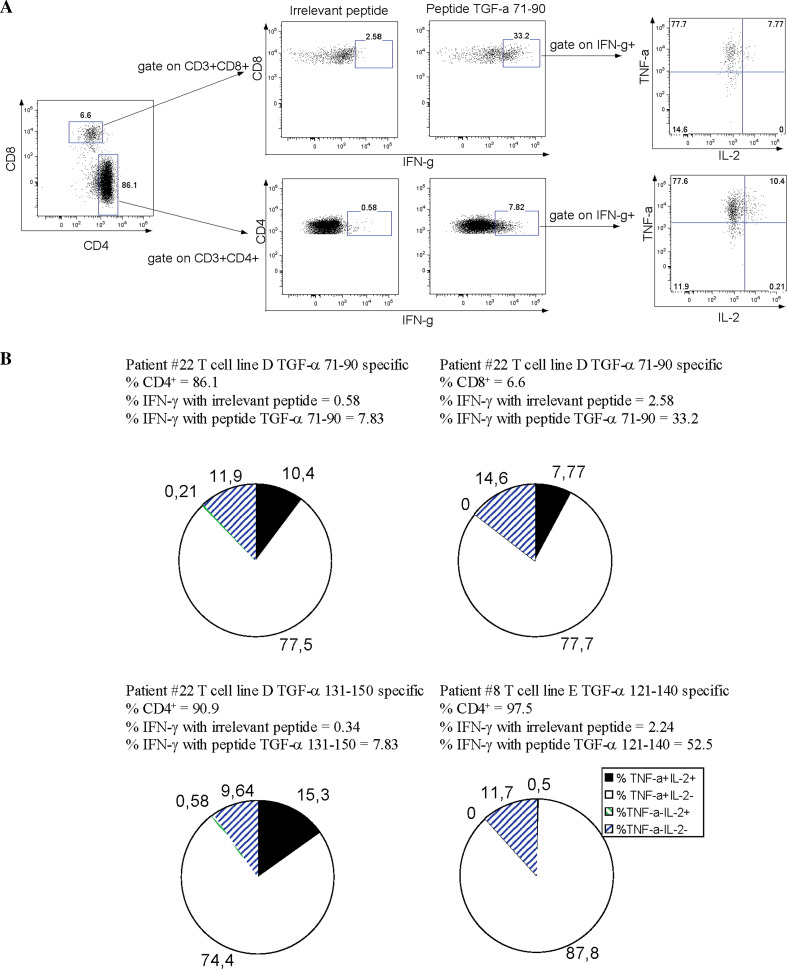
There have been numerous reports on distinct poly-functional properties in human T cells especially in the context of HIV infection [41]. To determine such poly-functional profiles of our TGF-α-specific T cell lines, we co-cultured our T cell lines in the presence of autologous APC loaded with either the specific peptide or an irrelevant peptide, and assessed the T cell subsets CD4 versus CD8 and the cytokine production looking by intracellular staining for IFN-γ, IL-2, and TNF-α simultaneously. A typical example is illustrated in Fig. 5 panel a. Results obtained from three T cell lines are illustrated in panel b (patient #22 T cell line D TGF-α 71–90 specific, patient #22 T cell line D TGF-α 131–150 specific and patient #8 T cell line E TGF-α 121–140 specific). The patient #22 T cell line D TGF-α 71–90 specific contained mainly CD4 T cells (86.1%) although both CD4+ and CD8+ T cells specifically produced IFN-γ (CD4, 0.58% for irrelevant peptide vs. 7.83% for TGF-α 71–90; CD8, 2.58% for irrelevant peptide vs. 33.2% for TGF-α 71–90). These data confirmed our IFN-γ ELISPOT data. The other two cell lines tested (patient #22 T cell line D TGF-α 131–150 specific and patient #8 T cell line E TGF-α 121–140 specific) were mainly composed of CD4+ T cells (90.9% and 97.5%) and a similar pattern was observed with a significant IFN-γ secretion. The results obtained from patient #8 T cell line E TGF-α 121–140 specific are in line with those obtained in Fig. 4d, meaning that peptide 121–140 is MHC-class II restricted and recognized by CD4+ T cells. In addition to secreting IFN-γ, 74.4–87.8% of these specific T cells also secreted TNF-α and only 0.5–15.3% of them secreted TNF-α and IL-2. In summary, we raised anti-TGF-α-specific T cells which are poly-functional based on cytokine secretion and a typical Th1/Tc1 profile being positive for both IFN-γ and TNF-α.
Fig. 5.
Anti-TGF-α-specific T cell lines are poly-functional. a Flow cytometry analysis of cytokine production by T cell line specific for peptide 71–90 from patient #22. b Summary of cytokine production by T cell lines
In summary, we have demonstrated that poly-functional T lymphocytes from blood and infiltrating tumors can recognize TGF-α-derived peptides or the mature protein when pulsed on autologous APC.
Discussion
In this report, we have demonstrated that TGF-α is a valuable TAA candidate, considering its distinct expression in CCRCC, its very low level or absence from important organs, and its potential immunogenicity as evaluated in PBMC and TIL obtained from kidney cancer patients.
Among previously-identified TAA for kidney cancer, most are expressed in a low proportion of tumor samples, and some are shared with normal tissues crucial for survival, such as CA IX, C-Met, cyclin D1 and RAGE-1. As reported here, TGF-α mRNA appears to be marginally expressed from the normal tissues as evaluated by sensitive, state-of-the-art qRT-PCR. Actually, the highest detected expression was in normal kidney samples, and it was still 22 times less than the referenced A498 kidney cancer line (Fig. 2). These data further corroborate previous studies reporting weak TGF-α expression from normal tissues [28, 34, 36, 37, 39, 40]. Normal brain tissue also expressed TGF-α at low levels similar to normal kidney tissue, but autoimmunity mediated by TGF-α-specific T cells is not likely to occur because of the decreased trafficking of lymphocytes through the blood–brain barrier. This should be taken into consideration before designing future clinical trials. Conversely, TGF-α was expressed at least three times more in 13/17 (77%) of CCRCC compared with autologous normal kidney tissues, which confirms previous reports [28–30], and further illustrates divergence between CCRCC and other kidney cancers for TGF-α expression. Moreover, at least six CCRCC expressed levels higher than our positive control A498 cells. Interestingly, HIF-2α, which regulates TGF-α expression [11, 33], also occurs in pre-malignant multicellular islets in VHL-deficient nephrons [20]. It is thus possible that TGF-α production is initiated at such an early pre-cancer state and persists during CCRCC development.
Considering its relevant expression profile as a candidate TAA, TGF-α was evaluated for potential immunogenicity, and T cells reacting against several peptides and mature TGF-α protein were found in blood samples and even in TILs obtained from RCC patients. We observed expected variations from patient to patient, which may reflect HLA polymorphisms, but all tested patients had T cells specific for at least one epitope of TGF-α. The selection of 20-mers peptides spanning the TGF-α protein, may have favored MHC class II-restricted presentation and responses since 20-mers are too long to fit into the MHC class I pocket. The use of shorter peptides, such as 9–10-mers, might show a higher frequency of spontaneous CD8+ T cell immunity to TGF-α. We further evaluated the polyfunctionality of the TGF-α-specific T cells and observed that a large proportion of the IFN-γ+ cells also secreted TNF-α, but not IL-2. In HIV infection, CD4+ and CD8+ T cells secreting only IFN-γ have a weaker proliferative capacity than IFN-γ+IL-2+ and IFN-γ-IL-2+ T cells [42–44]. If this is the case with TGF-α-specific T cells, which is yet to be determined, the low frequency of IL-2 secreting cells might have a negative impact on the proliferation and differentiation of antigen-specific T cells. In contrast, given its important role for regulatory T cells, low levels of IL-2 might limit their proliferation and consequently favor an effective anti-tumor immune response.
Potential other cytokines include Th2 cytokines such as IL-4, and Th17 cytokines such as IL-17. Although TGF-α-specific CD8+ T cells were detected only in one T cell line, and this maybe due to the use of 20 amino acid peptides, the cytotoxic capacity of these cells should also be evaluated. In addition, studies in HIV infection have shown that production of MIP-1β is more important than IFN-γ for enumerating the total number of antigen-specific CD8+ T cells [41]. Several studies on viral infections have correlated higher T cell polyfunctionality with lower antigen loads [41, 42, 44]. However, it is unknown if higher T cell polyfunctionality correlates with lower TAA load, such as early tumor development versus tumor progression and metastasis.
Finally, although TGF-α-specific T cells in peripheral blood and TIL exist, tumors still progress in RCC patients, which could be explained by several hypotheses. First, TGF-α-specific T cells infiltrating tumors could be functional but not in sufficient numbers to control the rapid growth of tumor cells. Second, tumors cells could develop strategies to evade the immune system. Indeed, many mechanisms have been described, such as decreased expression of MHC class I and II by tumor cells [45], natural selection of tumor cells refractory to the immune system [46], secretion of inhibitory cytokines by tumor cells such as TGF-β and IL-10[47], and finally the absence of co-activation signals on APC leading to T cell anergy [47]. It is thus possible that one or many of these mechanisms could be responsible for tumor progression instead of its elimination by tumor-specific T cells.
With these experiments, we believe that TGF-α is a valuable candidate TAA, considering its relevant expression profile, its involvement in tumor progression, and its potential immunogenicity involving polyfunctional specific T cells. Work is currently underway to integrate TGF-α in different experimental immunotherapy models to further evaluate it as a rejection TAA in CCRCC. In addition, TGF-α as a TAA could also be transposed to other cancer types in view of its shared overexpression with colon cancer [36], lung cancer [48, 49], pancreatic cancer [50], colorectal cancer [51], and others.
Acknowledgments
The editorial assistance of Ovid Da Silva, Support Office, Research Centre, CHUM, is acknowledged. We also thank Robert Boileau for statistical analyses. N.A. was supported by a Senior Research Fellowship from the Canadian Institutes of Health Research and now holds a Donald Paty Career Development Award from the Multiple Sclerosis Society of Canada and a Chercheur-Boursier from the Fonds de la Recherche en Santé du Québec. R.L. is the recipient of a “Fond pour la recherche en santé du Québec” scholarship.
Conflict of interest statement
The authors have nothing to disclose.
Abbreviations
- APC
Antigen presenting cells
- CCRCC
Clear cell renal cell carcinoma
- CD40-B
CD40-activated B lymphocytes
- EBV-B
Immortalized B lymphocytes with the Epstein–Barr virus
- GM-CSF
Granulocyte/macrophage-colony stimulating factor
- HIF
Hypoxia inducible factor
- HLA
Human leukocyte antigen
- IFN-γ
Interferon-γ
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cells
- TAA
Tumor-associated antigen
- TIL
Tumor-infiltrating lymphocytes
- TGF-α
Transforming growth factor-α
- VHL
von Hippel-Lindau
References
- 1.Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Can J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Schrader AJ, Varga Z, Hegele A, Pfoertner S, Olbert P, Hofmann R. Second-line strategies for metastatic renal cell carcinoma: classics and novel approaches. J Can Res Clin Oncol. 2006;132:137–149. doi: 10.1007/s00432-005-0058-4. [DOI] [PubMed] [Google Scholar]
- 3.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, Seipp CA, Rogers-Freezer L, Morton KE, White DE, Liewehr DJ, Merino MJ, Rosenberg SA. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, Kirkwood JM, Gordon MS, Sosman JA, Ernstoff MS, Tretter CPG, Urba WJ, Smith JW, Margolin KA, Mier JW, Gollob JA, Dutcher JP, Atkins MB. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 5.Oosterwijk E, Debruyne FMJ, Schalken JA. The use of monoclonal-antibody G250 in the therapy of renal-cell carcinoma. Semin Oncol. 1995;22:34–41. [PubMed] [Google Scholar]
- 6.Grabmaier K, Vissers JLM, de Weijert MCA, Oosterwijk-Wakka JC, Van Bokhoven A, Brakenhoff RH, Noessner E, Mulders PA, Merkx G, Figdor CG, Adema GJ, Oosterwijk E. Molecular cloning and immunogenicity of renal cell carcinoma-associated antigen G250. Int J Cancer. 2000;85:865–870. doi: 10.1002/(SICI)1097-0215(20000315)85:6<865::AID-IJC21>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 7.Fischer J, Palmedo G, von Knobloch R, Bugert P, Prayer-Galetti T, Pagano F, Kovacs G. Duplication and overexpression of the mutant allele of the MET proto-oncogene in multiple hereditary papillary renal cell tumours. Oncogene. 1998;17:733–739. doi: 10.1038/sj.onc.1201983. [DOI] [PubMed] [Google Scholar]
- 8.Hedberg Y, Davoodi E, Roos G, Ljungberg B, Landberg G. Cyclin-D1 expression in human renal-cell carcinoma. Int J Cancer. 1999;84:268–272. doi: 10.1002/(SICI)1097-0215(19990621)84:3<268::AID-IJC12>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Oehlrich N, Devitt G, Linnebacher M, Schwitalle Y, Grosskinski S, Stevanovic S, Zoller M. Generation of RAGE-1 and MAGE-9 peptide-specific cytotoxic T-lymphocyte lines for transfer in patients with renal cell carcinoma. Int J Cancer. 2005;117:256–264. doi: 10.1002/ijc.21200. [DOI] [PubMed] [Google Scholar]
- 10.Paulsen N, Brychzy A, Fournier MC, Klausner RD, Gnarra JR, Pause A, Lee S. Role of transforming growth factor-alpha in von Hippel-Lindau (VHL)(−/−) clear cell renal carcinoma cell proliferation: a possible mechanism coupling VHL tumor suppressor inactivation and tumorigenesis. Proc Natl Acad Sci USA. 2001;98(4):)1387–1392. doi: 10.1073/pnas.031587498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunaratnam L, Morley M, Franovic A, de Paulsen N, Mekhail K, Parolin DAE, Nakamura E, Lorimer IAJ, Lee S. Hypoxia inducible factor activates the transforming growth factor-alpha/epidermal growth factor receptor growth stimulatory pathway in VHL−/− renal cell carcinoma cells. J Biol Chem. 2003;278:44966–44974. doi: 10.1074/jbc.M305502200. [DOI] [PubMed] [Google Scholar]
- 12.Ivan M, Kondo K, Yang HF, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG. HIF alpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O-2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 13.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1 alpha and HIF-2 alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–5443. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 14.Bonicalzi ME, Groulx I, de Paulsen N, Lee S. Role of exon 2-encoded beta-domain of the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2001;276:1407–1416. doi: 10.1074/jbc.M008295200. [DOI] [PubMed] [Google Scholar]
- 15.Clifford SC, Cockman ME, Smallwood AC, Mole DR, Woodward ER, Maxwell PH, Ratcliffe PJ, Maher ER. Contrasting effects on HIF-1 alpha regulation by disease-causing pVHL mutations correlate with patterns of tumourigenesis in von Hippel-Lindau disease. Hum Mol Gen. 2001;10:1029–1038. doi: 10.1093/hmg/10.10.1029. [DOI] [PubMed] [Google Scholar]
- 16.Wiesener MS, Munchenhagen PM, Berger I, Morgan NV, Roigas J, Schwiertz A, Jurgensen JS, Gruber G, Maxwell PH, Loning SA, Frei U, Maher ER, Grone HJ, Eckardt KU. Constitutive activation of hypoxia-inducible genes related to overexpression of hypoxia-inducible factor-1 alpha in clear cell renal carcinomas. Can Res. 2001;61:5215–5222. [PubMed] [Google Scholar]
- 17.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, Chevreau C, Filipek M, Melichar B, Bajetta E, Gorbunova V, Bay JO, Bodrogi I, Jagiello-Gruszfeld A, Moore N. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–2111. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 18.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 19.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 20.Mandriota SJ, Turner KJ, Davies DR, Murray PG, Morgan NV, Sowter HM, Wykoff CC, Maher ER, Harris AL, Ratcliffe PJ, Maxwell PH. HIF activation identifies early lesions in VHL kidneys: evidence for site-specific tumor suppressor function in the nephron. Cancer Cell. 2002;1:459–468. doi: 10.1016/S1535-6108(02)00071-5. [DOI] [PubMed] [Google Scholar]
- 21.Iliopoulos O, Kibel A, Gray S, Kaelin WG. Tumor suppression by the human von Hippel-Lindau gene-product. Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 22.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Can Res. 2003;63:2836–2843. [PubMed] [Google Scholar]
- 23.Topalian SL, Rivoltini L, Mancini M, Markus NR, Robbins PF, Kawakami Y, Rosenberg SA. Human CD4+ T cells specifically recognize a shared melanoma-associated antigen encoded by the tyrosinase gene. Proc Natl Acad Sci USA. 1994;91:9461–9465. doi: 10.1073/pnas.91.20.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forget MA, Turcotte S, Beauseigle D, Godin-Ethier J, Pelletier S, Martin J, Tanguay S, Lapointe R. The Wnt pathway regulator DKK1 is preferentially expressed in hormone-resistant breast tumours and in some common cancer types. Br J Cancer. 2007;96:646–653. doi: 10.1038/sj.bjc.6603579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nuc Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST (c)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nuc Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topalian S, Muul L, Solomon D, Rosenberg SA. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J Immunol Methods. 1987;102:127–141. doi: 10.1016/S0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 28.Gomella LG, Sargent ER, Wade TP, Anglard P, Linehan WM, Kasid A. Expression of transforming growth factor alpha in normal human adult kidney and enhanced expression of transforming growth factors alpha and beta 1 in renal cell carcinoma. Cancer Res. 1989;49:6972–6975. [PubMed] [Google Scholar]
- 29.Petrides PE, Bock S, Bovens J, Hofmann R, Jakse G. Modulation of pro-epidermal growth-factor, pro-transforming growth factor-alpha and epidermal growth-factor receptor gene-expression in human renal carcinomas. Cancer Res. 1990;50:3934–3939. [PubMed] [Google Scholar]
- 30.Mydlo JH, Michaeli J, Cordoncardo C, Goldenberg AS, Heston WDW, Fair WR. Expression of transforming growth factor-alpha and epidermal growth-factor receptor messenger-rna in neoplastic and nonneoplastic human-kidney tissue. Cancer Res. 1989;49:3407–3411. [PubMed] [Google Scholar]
- 31.Lager DJ, Slagel DD, Palechek PL. The expression of epidermal growth-factor receptor and transforming growth-factor-alpha in renal-cell carcinoma. Modern Pathol. 1994;7:544–548. [PubMed] [Google Scholar]
- 32.Uhlman DL, Nguyen P, Manivel JC, Zhang G, Hagen K, Fraley E, Aeppli D, Niehans GA. Epidermal growth factor receptor and transforming growth factor alpha expression in papillary and nonpapillary renal cell carcinoma: correlation with metastatic behavior and prognosis. Clin Cancer Res. 1995;1:913–920. [PubMed] [Google Scholar]
- 33.Smith K, Gunaratnam L, Morley M, Franovic A, Mekhail K, Lee S. Silencing of epidermal growth factor receptor suppresses hypoxia-inducible factor-2-driven VHL−/− renal cancer. Cancer Res. 2005;65:5221–5230. doi: 10.1158/0008-5472.CAN-05-0169. [DOI] [PubMed] [Google Scholar]
- 34.Kudlow JE, Leung AW, Kobrin MS, Paterson AJ, Asa SL. Transforming growth factor-alpha in the mammalian brain. Immunohistochemical detection in neurons and characterization of its mRNA. J Biol Chem. 1989;264:3880–3883. [PubMed] [Google Scholar]
- 35.Liscia DS, Merlo G, Ciardiello F, Kim N, Smith GH, Callahan R, Salomon DS. Transforming growth factor-alpha messenger-RNA localization in the developing adult-rat and human mammary-gland by in situ hybridization. Dev Biol. 1990;140:123–131. doi: 10.1016/0012-1606(90)90059-R. [DOI] [PubMed] [Google Scholar]
- 36.Liu C, Woo A, Tsao MS. Expression of transforming growth factor-alpha in primary human colon and lung carcinomas. Br J Cancer. 1990;62:425–429. doi: 10.1038/bjc.1990.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomas DM, Nasim MM, Gullick WJ, Alison MR. Immunoreactivity of transforming growth factor alpha in the normal adult gastrointestinal tract. Gut. 1992;33:628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gottlieb AB, Chang CK, Posnett DN, Fanelli B, Tam JP. Detection of transforming growth factor-alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988;167:670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffey RJ, Jr, Derynck R, Wilcox JN, Bringman TS, Goustin AS, Moses HL, Pittelkow MR. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. Nature. 1987;328:817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- 40.Madtes DK, Raines EW, Sakariassen KS, Assoian RK, Sporn MB, Bell GI, Ross R. Induction of transforming growth factor-alpha in activated human alveolar macrophages. Cell. 1988;53:285–293. doi: 10.1016/0092-8674(88)90390-X. [DOI] [PubMed] [Google Scholar]
- 41.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyasere C, Tilton JC, Johnson AJ, Younes S, Yassine-Diab B, Sekaly RP, Kwok WW, Migueles SA, Laborico AC, Shupert WL, Hallahan CW, Davey RT, Jr, Dybul M, Vogel S, Metcalf J, Connors M. Diminished proliferation of human immunodeficiency virus-specific CD4+ T cells is associated with diminished interleukin-2 (IL-2) production and is recovered by exogenous IL-2. J Virol. 2003;77:10900–10909. doi: 10.1128/JVI.77.20.10900-10909.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerli SC, Harari A, Cellerai C, Vallelian F, Bart PA, Pantaleo G. HIV-1-specific IFN-gamma/IL-2-secreting CD8 T cells support CD4-independent proliferation of HIV-1-specific CD8 T cells. Proc Natl Acad Sci USA. 2005;102:7239–7244. doi: 10.1073/pnas.0502393102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seliger B, Cabrera T, Garrido F, Ferrone S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin Cancer Biol. 2002;12:3–13. doi: 10.1006/scbi.2001.0404. [DOI] [PubMed] [Google Scholar]
- 46.Khong HT, Restifo NP. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat Immunol. 2002;3(11):999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2:293–299. doi: 10.1038/86297. [DOI] [PubMed] [Google Scholar]
- 48.Tateishi M, Ishida T, Mitsudomi T, Kaneko S, Sugimachi K. Immunohistochemical evidence of autocrine growth-factors in adenocarcinoma of the human lung. Cancer Res. 1990;50:7077–7080. [PubMed] [Google Scholar]
- 49.Liu C, Tsao MS. Invitro and in vivo expressions of transforming growth factor-alpha and tyrosine kinase receptors in human non-small-cell lung carcinomas. Am J Pathol. 1993;142:1155–1162. [PMC free article] [PubMed] [Google Scholar]
- 50.Barton CM, Hall PA, Hughes CM, Gullick WJ, Lemoine NR. Transforming growth factor-alpha and epidermal growth-factor in human pancreatic-cancer. J Pathol. 1991;163:111–116. doi: 10.1002/path.1711630206. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka S, Imanishi K, Yoshihara M, Haruma K, Sumii K, Kajiyama G, Akamatsu S. Immunoreactive transforming growth factor-alpha is commonly present in colorectal neoplasia. Am J Pathol. 1991;139:123–129. [PMC free article] [PubMed] [Google Scholar]