Abstract
Advances in tumor immunology and Identification of tumor-associated antigens (TAAs) provide a basis for the development of novel immunotherapies to treat malignant diseases. In order to identify novel TAAs, we performed comparative microarray analysis of (heterogeneous) tissues and found regulator of G protein-signaling 1 (RGS1) extensively up-regulated in renal cell carcinoma (RCC) tissues. To examine the possible function of this molecule as a novel, broadly applicable TAA, synthetic full-length RGS1-mRNA was synthesized for the transfection of monocyte-derived dendritic cells (DCs). These modified antigen-presenting cells (APCs) were then used to induce RGS1-specific cytotoxic T cells (CTLs) in vitro. The CTLs generated from several healthy donors and a patient with chronic lymphocytic leukemia (CLL) elicited an antigen-specific and HLA-A2- and -A3-restricted cytolytic activity against tumor cells endogenously expressing the RGS1 protein including renal cell carcinomas (RCCs), melanoma, ovarian carcinoma and the primary autologous CLL-blasts. In conclusion, our study demonstrates that the in vitro induction of RGS1-specific CTLs by RNA-transfected DCs is feasible and highly effective. Since this molecule is (over-) expressed in a broad variety of malignancies it might represent an interesting novel TAA in the context of cancer vaccines designed to target RGS1 expressing tumor cells.
Keywords: Renal Cell Carcinoma, Acute Myeloid Leukemia, Chronic Lymphocytic Leukemia, Chronic Lymphocytic Leukemia Patient, RGS1 Protein
Introduction
Tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs) can evoke humoral and/or cellular immunoresponses in tumor patients. Consequently, cytotoxic T cells (CTLs) specific for the autologous tumor cells are detectable in these individuals. In vitro, those lymphocytes can kill tumor cells but frequently fail to do so in vivo [4].
Dendritic cells (DCs) play a key role in the regulation of cell-mediated immunity. They are the most specialized antigen-presenting cells (APCs), which are able to initiate primary immune responses. By directing naïve lymphocyte development into defined sub-classes of effector cells, e. g. T helper (Th) 1 lymphocytes, Th2, or regulatory T cells (Treg) they control and regulate the type of T cell responses [18, 31]. Importantly, DCs have the special ability to ingest virus-infected or tumor cells and present antigens from these cells on MHC class I molecules, a process called “cross-presentation”. As adjuvants, in the context of immunotherapies, they became a major focus of interest in recent years.
In order to identify genes selectively expressed or over-expressed in tumor cells we performed comparative expression profiling of tumor tissues and the corresponding normal tissue using DNA oligonucleotide microarray technology [16, 17, 29, 30, 36].
By applying this approach to renal cell carcinoma (RCC) tissues RGS1 was found to be over-expressed in 9 of the 11 tumors tested (more than 18-fold). Furthermore, over-expression of RGS1 mRNA was recently demonstrated in four of six adult T-cell leukemia (ATL) patients as compared with PHA blasts from healthy controls making this antigen an interesting candidate to be targeted in immunotherapeutic approaches [15].
G-protein-linked receptors form the largest family of cell-surface receptors and are found in all eukaryotes. The G proteins are attached to the cytoplasmatic face of the cellular plasma membrane, where they serve as relay molecules, functionally coupling the receptors to enzymes or ion channels in the membrane [8, 12].
The function of regulators of G protein signaling (RGS) is to inhibit the biological activity of G proteins. Through the binding to G proteins’ α subunits they stimulate their intrinsic GTPase activity. The hydrolysis of GTP to GDP thereby is accelerated, the inactive heterotrimer more rapidly restored and the signaling loop terminated [13, 28].
RGS1 was cloned first as a B cell activating factor (BL34). It is induced rapidly after in vitro B cell activation, especially in the germinal center of lymphoid tissues but it can not be detected in primary T cells [14]. Studies in mice have demonstrated that over-expressed RGS1 in antigen-stimulated B cells impaired chemotaxis to some chemokines, such as stromal cell-derived factor-1 (SDF-1), platelet-activating factor (PAF), and B lymphocyte chemoattractant (BLC) [27]. Consequently, RGS1−/− mice possess B cells that respond aberrantly to chemokines. Immunization of these animals leads to excessive germinal center formation, partial disruption of the normal structure of the spleen and Peyer’s patches, and aberrant trafficking of immunoglobulin-producing cells [20]. Furthermore, Han et al. showed that cells obtained from such mice stick better to lymph node high endothelial venules, home better to lymph nodes, and move more rapidly within lymph node follicles than do wild-type B cells [9]. Based on these observations, Agenes et al. recently proposed that over-expression of RGS1 by regulatory T cells may account for their hypo-responsiveness to chemokines CCL19 and CCL21 [1].
We applied an RNA-based approach to examine the possible function of this molecule as a novel TAA. It was shown that DCs transfected with RNA coding for a TAA or even whole tumor mRNA are able to induce potent antigen and tumor-specific T-cell responses directed against multiple epitopes [2, 7, 23–25]. Therefore, the RGS1-cDNA was sub-cloned into a transcription vector. In a second step, full-length RGS1-cDNA that coded for the entire RGS1-protein was in vitro transcribed and utilized for the transfection of monocyte-derived DCs. These modified cells were used as APCs for the in vitro induction of antigen-specific CTLs.
We demonstrate that the CTLs generated from several healthy donors and a patient with CLL elicited an antigen-specific and HLA-A2- or -A3-restricted cytolytic activity against tumor cells endogenously expressing the RGS1 protein including RCCs, melanoma, ovarian carcinoma and primary autologous CLL-blasts. RGS1 might therefore represent interesting novel TAA in the context of cancer vaccines designed to target tumor cells expressing this protein.
Materials and methods
Tumor cell lines
MCF-7 (breast cancer, RGS1 −, HLA-A2+); A498 and MZ1774 (RCC cell lines, RGS1 +, HLA-A2+; kindly provided by Prof. A. Knuth, Zürich, Switzerland); U266 (multiple myeloma, RGS1 +, HLA-A2+); Mel1479 (malignant melanoma, RGS1 +, HLA-A3+; kindly provided by Prof. G. Pawelec, Tübingen, Germany); SKOV-3 (ovarian cell line, RGS1 +, HLA-A3+; kindly provided by O.J. Finn, PA, USA); Caki-1 (RCC, RGS1 +); CROFT (EBV-immortalized B-cell line, RGS1 +, HLA-A2+; kindly provided by O.J. Finn); SD-1 (human peripheral blood B lymphoblastoid cells, RGS1 +); THP-1 (RGS1 −, human acute monocytic leukemia); NT-2 (NTERA-2, pleuripotent, embryonal carcinoma cells, RGS1 +); KASUMI-1 (AML, RGS1 +); WERI-RB-1 (human retinoblastoma, RGS1-); HL-60 (human AML, RGS1 +). All cell lines were grown in RP10 medium (RPMI 1640 with glutamax I, (Invitrogen, Karlsruhe, Germany) supplemented with 10% heat-inactivated FCS, 2 mmol/l, L-glutamine, 50 μmol/l 2-mercaptoethanol, and antibiotics) at 37°C and 5% CO2.
Generation of monocyte-derived DCs from adherent peripheral blood mononuclear cells [3]
PBMCs were isolated by Ficoll/Paque (Biochrom, Berlin, Germany) density gradient centrifugation of blood obtained from buffy coats of healthy volunteers from the blood bank of the University of Tübingen. 1 × 108 cells were seeded into 75 cm2 cell culture flasks (BD-Falcon, Heidelberg, Germany) in serum-free X-VIVO 20 medium (Lonza Verviers, Verviers, Belgium). After 1.5 h of incubation at 37°C/5% CO2, non-adherent cells were removed and cryopreserved at −80°C to be used later for cell isolation or re-stimulations. Human recombinant granulocyte macrophage-colony stimulating factor (GM-CSF; 100 ng/ml; Leukine Liquid Sargramostim; Bayer HealthCare Pharmaceuticals, USA) and interleukin-4 (20 ng/ml; R&D Systems, Wiesbaden-Nordenstadt, Germany) were added every second day starting at day 0 of culture to generate immature DCs. Maturation was induced on day 6 by adding Poly I:C (TLR3L, 50.0 μg/ml; Sigma, Deisenhofen, Germany) and R848 (TLR7/8L, 2.0 μg/ml; InvivoGen, San Diego, USA) for 24 h. Phenotypic analysis were done by FACS.
Gene expression analysis by high-density oligonucleotide microarrays
Frozen fragments of tumors RCC0044, RCC0068, RCC0070, RCC0073, RCC0075, RCC0098, and RCC0103 were homogenized with mortar and pestle under liquid nitrogen. Total RNA was prepared from these samples using TRIzol (Invitrogen) according to the manufacturer’s protocol, followed by a cleanup with RNeasy (Qiagen, Hilden, Germany). Quality and quantity were assessed on an Agilent 2100 Bioanalyzer (Agilent, Waldbronn, Germany) using the RNA 6000 Pico LabChip kit (Agilent). Gene expression analysis of RNA samples from RCC0044, RCC0068, RCC0070, RCC0073, RCC0075, RCC0098, and RCC0103 was done by Affymetrix Human Genome U133A oligonucleotide microarrays (Affymetrix, Buckinghamshire, UK). For all other samples, HG-U133 Plus 2.0 was used. The same normal kidney sample was hybridized to both array types to achieve comparability (data not shown). All steps were carried out according to the Affymetrix manual.
Briefly, double-stranded cDNA was synthesized from 5 to 8 μg total RNA, using SuperScript RTII (Invitrogen) and the oligo-dT-T7 primer (MWG Biotech, Ebersberg, Germany) as described in the manual. In vitro transcription was done with the BioArray High Yield RNA Transcript Labeling kit (ENZO Diagnostics Inc., Farmingdale, USA) for the U133A arrays or with the GeneChip IVT Labeling kit (Affymetrix) for the U133 Plus 2.0 arrays, followed by cRNA fragmentation, hybridization, and staining with streptavidin-phycoerythrin and biotinylated anti-streptavidin antibody (Molecular Probes, Invitrogen). Images were scanned with the Agilent 2500A GeneArray Scanner (U133A) or the Affymetrix GeneChip Scanner 3000 (U133 Plus 2.0), and data were analyzed with the MAS 5.0 (U133A) or GCOS (U133 Plus 2.0) software (Affymetrix), using default settings for all variables. Pairwise comparisons were calculated using the respective normal kidney array as baseline. For normalization, 100 housekeeping genes provided by Affymetrix were used. Relative expression values were calculated from the signal log ratios given by the software, and the normal kidney sample was arbitrarily set as 1.
RNA isolation for reverse transcription
Total RNA was isolated from tumor cell Iysates using RNeasy Mini anion-exchange spin columns (Qiagen) according to the protocol for isolation of total RNA from animal cells provided by the manufacturer. Quantity and purity of RNA was determined by UV spectrophotometry. RNA samples were routinely checked by formaldehyde/agarose gel electrophoresis for integrity and stored at -80°C in small aliquots.
Reverse transcription (RT)-PCR
Up to 5.0 μg total RNA was subjected to a 20-μl cDNA synthesis reaction (Transcriptor First-Strand cDNA Synthesis kit; Roche Diagnostics, Mannheim, Germany) using random primers. cDNA (1.0 μl) was used in a 50.0-μl PCR amplification reaction. To control the integrity of the RNA and the efficiency of the cDNA synthesis, 1.0 μL cDNA was amplified by an intron-spanning primer pair for the ß2-microglobulin gene. For the RGS1 and the ß2-microglobulin cDNA, the PCR temperature profiles were as follows: 5-min pretreatment at 94°C and 32 or 25 cycles, respectively, at 94°C for 30 s, annealing for 30 s and 72°C for 60 s with a final extension at 72°C for 7 min. The annealing temperatures for ß2-microglobulin and RGS1 were 55°C and 60°C, respectively. Primer sequences were deduced from published cDNA sequences: ß2-microglobulin (accession no. NM_004048), 5′-GGGTTTCATCCATCCGACAT-3′ and 5′-GATGCTGCTTACATGTCTCGA-3′; RGS1 (NM_002922), 5′-TCCCAGGTTCCTCAAATCAG-3′ and 5′-TCTGCGCCTGGATAACTTTC-3′. Reverse transcription-PCR (RT-PCR) reactions (10.0 μl) were electrophoresed through a 3.0% agarose gel and stained with ethidium bromide for visualization under UV light.
Generation of RGS1- and EGFP-in vitro transcripts
For generation of RGS1-IVT a synthetic poly(A)30-tail including a new single Ndel restriction site was inserted into the Notl site of pRc/CMV-RGS1 resulting in the construct pRc/CMV-RGS1-poly(A)30. Before in vitro transcription, this plasmid was linearized behind the poly(A)-tail by Ndel restriction enzyme digestion. For in vitro transcription under control of the T7 promoter, the T7 mMESSAGE mMACHINE kit (Ambion, Huntingdon, UK) was used according to the protocol provided by the manufacturer. EGFP-IVT was generated as described previously using the SP6 mMESSAGE mMACHINE® kit (Ambion) [7]. Purification of IVTs was performed with RNeasy Mini anion-exchange spin columns (Qiagen) according to the RNA cleanup protocol provided by the manufacturer. Quantity and purity of IVTs were determined by UV-spectrophotometry. The IVTs were routinely checked by formaldehyde/agarose gel electrophoresis for size and integrity and stored at −80°C in small aliquots.
RNA electroporation of DCs
Electroporation of DCs with RNA was performed as described previously [6, 7, 35]. Briefly, on day 7 of culture, mature DCs were harvested, washed twice with X-VIVO 20® medium (Lonza Verviers) and resuspended to a final concentration of 2 × 107 cells/ml. Subsequently, 200 μl of the cell suspension was mixed with 10 μg of RNA/IVT and electroporated in a 4-mm cuvette using an Easyject Plus® unit (EquiBio/Peqlab, Erlangen Germany). The settings were: voltage of 300 V, capacitance of 150 μF and resistance of 1,540 Ω. After electroporation, cells were immediately transferred into RP10 medium and returned to the incubator.
CTL-lnduction using DCs transfected with RNA
DCs were electroporated with different sources of RNA/IVT as described above. After transfection, DCs were incubated for 24 h in RP10 medium supplemented with GM-CSF and IL-4. For induction of specific CTLs, 5.0 × 105 electroporated DCs were washed, and incubated with 3.0 × 106 PBMCs. After 7 days of culture, cells were restimulated with RNA-electroporated DCs, and 2.0 ng/ml IL-2 (R&D Systems) was added on d 1, 3, and 5. The cytolytic activity of induced CTLs was analyzed on day 5 after the last restimulation in a standard 51Cr-release assay.
Standard 51Cr-release assays
CTL assays were performed as described previously [3]. In brief, target cells were labeled with 51Cr sodium chromate in X-VIVO 20® (Lonza Verviers) medium for 1 h at 37°C. 1.0 × 104 target cells were transferred to a well of a round-bottomed 96-well plate. Varying numbers of CTLs were added to a final volume of 200.0 μl and incubated for 4 h at 37°C. At the end of the assay, supernatants (50.0 μl/well) were harvested and counted in a beta-plate counter. The percentage of specific lysis was calculated as: 100× (experimental release—spontaneous release)I(maximal release—spontaneous release). Spontaneous and maximal releases were determined in the presence of either X-VIVO 20® (Lonza Verviers) medium or 2% Triton X-100, respectively. Inhibition of HLA class I molecules was achieved by incubating target cells for 1 h prior to the assay with the monoclonal antibody W6/32 (20.0 μg/ml) directed against HLA class I molecules. Antigen specificity of cell lysis was further determined in a cold target inhibition assay by analyzing the capacity of unlabeled PBMCs transfected with RGS1-IVT to block lysis of tumor cell lines. The spontaneous release of DCs as target cells was 15–20% throughout all experiments.
Results
Comparative analysis of gene expression in RCC
We performed high-density oligonucleotide microarray analysis to assess the expression of RGS1 mRNA in human RCCs compared to the corresponding normal renal tissues. By applying this approach we found RGS1 consistently overexpressed (9 of the 11 tumors tested with a more than 18-fold overexpression; not shown [17]).
Expression Analysis of RGS1 by RT-PCR
To assess the possible use of RGS1 as broadly applicable target antigen for the development of vaccination therapies, RT-PCR analysis was performed on human primary leukemic cells and established tumor cell lines. As demonstrated in Fig. 1, RGS1-mRNA expression was found in the following: SD-1, CCRF7, SKOV-3, U266, Caki-1, KASUMI-1, NT-2, A498, HL-60, MZ1774, Mel1479 and CROFT tumor cell lines (a) as well as in three primary chronic lymphocytic leukemia (CLL), two out of five acute myeloid leukemia (AML), one out of five chronic myelogenous leukemia (CML) and two out of five acute lymphoblastic leukemia (ALL) tumor samples analyzed (b).
Fig. 1.
Expression analysis of RGS1. RT-PCR using RGS1-specific primers was performed to analyze the mRNA expression of RGS1 in human tumor cell lines (a) as well as in primary tumor cells (b). β2-microglobulin (β2m)-specific primers were used as controls. M: Roche Molecular Weight Marker VIII
These experiments indicated that RGS1 is expressed in a broad variety of human malignancies of various origins.
RGS1-specific CTLs induced by DCs electroporated with RGS1-IVT can lyse tumor cells of epithelial and hematologic origin
In order to induce RGS1-specific T cells, full-length RGS1-mRNA (RGS1-IVT) was in vitro transcribed from a vector including a T7 promoter and utilized for the transfection of monocyte-derived DCs. In the first series of experiments, the DCs were generated from healthy HLA-A2+/HLA-A3− donors.
Various tumor cell lines or DCs transfected with RGS1-IVT were used as target cells. As shown in the standard 51Cr-release assay the CTLs did lyse cells electroporated with RGS1-IVT (Fig. 2a) or the RGS1 +/HLA-A2+ MZ1774 and A498 tumor cells (Fig. 2a,b, respectively). In contrast, the in vitro-induced CTLs did not recognize RGS1 +/HLA-A3+ SKOV-3 tumor cells or DCs electroporated with irrelevant EGFP-IVT as a control (Fig. 2a). Furthermore, K-562 cells were spared indicating that the cytotoxic activity was not NK cell-mediated (Fig. 2a).
Fig. 2.
Induction of RGS1-specific CTLs using DCs electroporated with RGS1-IVT. Immature monocyte-derived DCs generated from a healthy HLA-A2+ donor were electroporated with pure RGS1-IVT and used as APCs for the induction of antigen-specific CTLs. The cytolytic activity of the generated CTLs was determined after several weekly restimulations in a standard 51Cr-release assay. a DCs transfected with RGS5-IVT as well as tumor cell lines MZ1774 (HLA-A2+), or SKOV-3 (HLA-A3+) were used as target cells. DCs transfected with an irrelevant EGFP-IVT served as control. K-562 cells were included to determine the NK cell activity. b Tumor cell line A498 (HLA-A2+) served as target cells. Inhibition of the recognition of HLA class I or class II was performed by incubating target cells prior to the assay with anti-HLA class I or II antibodies
The HLA specificity of the induced CTLs was further confirmed by the addition of monoclonal antibodies directed against HLA molecules. As shown in Fig. 2b, the specific lysis of the target cells could be blocked using an antibody directed against HLA class I molecules indicating that the elicited T cell responses were HLA class I-restricted. Consequently, the antibody directed against HLA class II molecules did not inhibit the lysis of the tumor cells.
These data demonstrated that DCs electroporated with an excess of pure full length RGS1-IVT could induce RGS1-specific class I restricted CTLs that recognize tumor cells endogenously expressing RGS1 in an antigen-specific and HLA-restricted manner in vitro.
In the next series of experiments we generated monocyte-derived DCs from the PBMCs of healthy HLA-A3+/ HLA-A2− donors and transfected them with an excess of pure full-length RGS1-IVT. As shown in the standard 51Cr-release assay the induced CTLs did lyse cells electroporated with RGS1-IVT (Fig. 3a) or RGS1 +/HLA-A3+ SKOV-3 or Mel1479 tumor cells (Fig. 3a). In contrast, the in vitro-induced T cells did not recognize RGS1 +/HLA A2+ A498 tumor cells or DCs electroporated with irrelevant EGFP-IVT as a control (Fig. 3a). Furthermore, K-562 cells were spared indicating that the cytotoxic activity was not NK cell-mediated (Fig. 3a).
Fig. 3.
Induction of RGS1-specific CTLs using DCs electroporated with RGS1-IVT. Immature monocyte derived DCs generated from a healthy HLA-A3+ donor were electroporated with pure RGS1-IVT and used as APCs for the induction of antigen-specific CTLs. The cytolytic activity of the generated CTLs was determined after several weekly restimulations in a standard 51Cr-release assay. a DCs transfected with RGS5-IVT as well as tumor cell lines SKOV-3 (HLA-A3+), Mel1479 (HLA-A3+) or 498 (HLA-A2+) were used as target cells. DCs transfected with an irrelevant EGFP-IVT served as control. K-562 cells were included to determine the NK cell activity. b For cold target inhibition experiments, unlabeled PBMCs transfected with RGS-IVT or with irrelevant EGFP-IVT as a control were added to 51Cr labeled SKOV-3 cells at the cold/labeled target ratio of 20:1
The antigen specificity of cell lysis was further determined in a cold target inhibition assay by analyzing the capacity of unlabeled autologous PBMCs transfected with RGS1-IVT to block lysis of tumor cell lines [34]. As shown in Fig. 3b PBMCs transfected with RGS1-IVT efficiently inhibited the lysis of the RGS1 +/HLA-A3+ SKOV-3 cells. In contrast, PBMCs electroporated with an excess of irrelevant EGFP-IVT could not inhibit the recognition of tumor cell derived RGS1 epitopes.
Taken together, these experiments confirmed that the in vitro induced CTLs are specific for RGS1-derived epitopes and recognize target cells in an antigen-specific and HLA-restricted manner.
Next, we repeated the previous experiments using PBMCs of a healthy HLA-A2+/A3+ donor for the generation of monocyte-derived DCs. These cells were transfected with an excess of pure full-length RGS1-IVT as above and used as APCs for the in vitro induction of RGS1-specific CTLs. As shown in the standard 51Cr-release assay these CTLs did lyse cells electroporated with RGS1-IVT (Fig. 4a). To further confirm the antigen specificity of cell lysis we performed cold target inhibition assays. As shown in Fig. 4b, PBMCs transfected with RGS1-IVT efficiently inhibited the lysis of both, the RGS1 +/HLA-A2+ A498 cells and the RGS1 +/HLA-A3+ SKOV-3 cells. In contrast, PBMCs electroporated with an irrelevant EGFP-IVT could not inhibit the recognition of RGS1 +/HLA-A2+ or HLA-A3+ tumor cells.
Fig. 4.
Induction of RGS1-specific CTLs using DCs electroporated with RGS1-IVT. Immature monocyte derived DCs generated from a healthy HLA-A2+/−A3+ donor were electroporated with pure RGS1-IVT and used as APCs for the induction of antigen specific CTLs. The cytolytic activity of the generated CTLs was determined after several weekly restimulations in a standard 51Cr-release assay. a DCs transfected with RGS5-IVT were used as target cells. DCs transfected with an irrelevant EGFP-IVT served as control. K-562 cells were included to determine the NK cell activity. b For cold target inhibition experiments, unlabeled PBMCs transfected with RGS-IVT or with irrelevant EGFP-IVT as a control were added to 51Cr labeled SKOV-3 (HLA-A3+) or A498 (HLA-A2+) cells at the cold/labeled target ratio of 20:1
These experiments demonstrated that transfection with RGS1-IVT leads to presentation of RGS1-derived epitopes on both, HLA-A2+- and A3+-molecules and induction of RGS1-specific, HLA-A2+- or A3+-restricted CTLs.
RGS1-specific CTLs can lyse autologous CLL-blasts
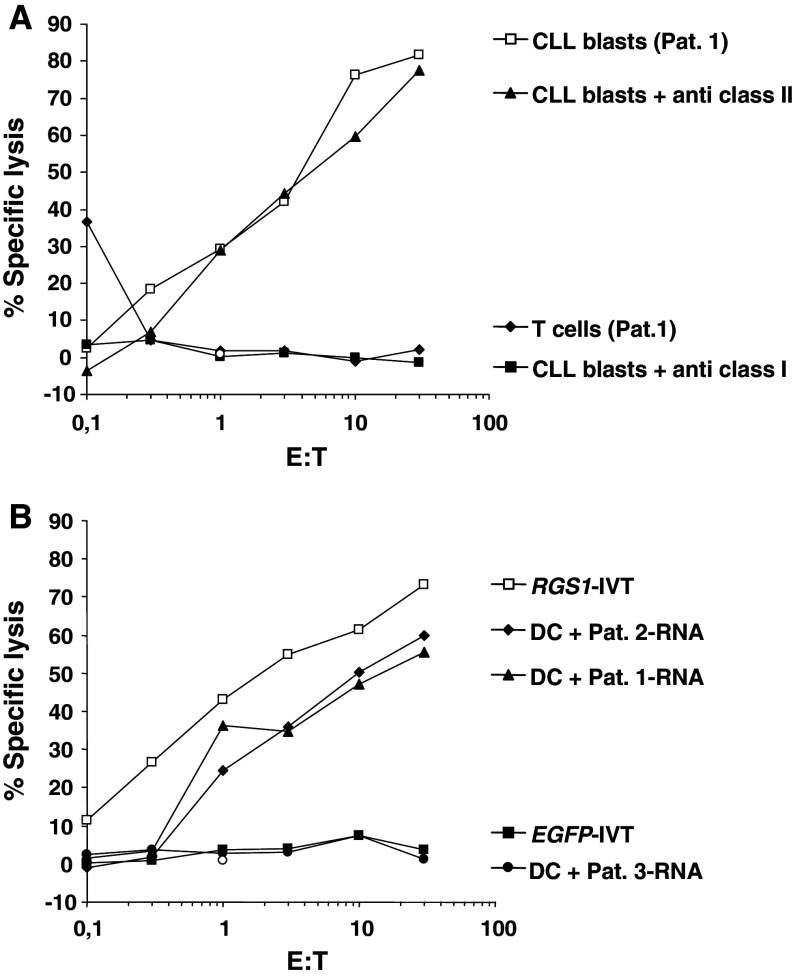
Expression analysis demonstrated that all CLL blood samples analyzed by RT-PCR exhibited RGS1-expression (Fig. 1b). In a last series of experiments, we therefore wanted to test whether our previous findings could be applied to CLL-blasts in an autologous setting. To achieve this, we generated monocyte-derived DCs from a CLL patient (Pat. 1) in complete remission after fludarabine containing chemotherapy and used them as APCs for CTL induction after transfection with RGS1-IVT.
As demonstrated in Fig. 5a, the CLL-blasts were efficiently lysed by the RGS1-specific CTLs. Additionally, we included autologous T cells in these assays. As shown in Fig. 5a, these cells were spared. The specificity of the induced CTLs, was further confirmed by the addition of monoclonal antibodies directed against HLA molecules. The specific lysis of the target cells could be blocked using an antibody directed against HLA class I molecules indicating that the elicited T cell responses were HLA class I-restricted (Fig. 5a).
Fig. 5.
Induction of RGS1-specific CTLs using DCs electroporated with RGS1-IVT. Immature monocyte derived DCs generated from a patient with CLL in complete remission after chemotherapy (Pat. 1). a The RGS1-specific CTLs were used as effectors against the autologous malignant CLL blasts frozen at the time of diagnosis Normal T cells were included as targets in the standard 51Cr-release assay. b DCs electroporated with total RNA purified from a second RGS1 + CLL patient (Pat. 2) or RGS1-negative AML patient (Pat. 3) were included as targets. DCs transfected with RGS5-IVT or with an irrelevant EGFP-IVT served as controls
To further confirm the RGS1-specificity of the induced CTLs we included DCs electroporated with total RNA isolated from the CLL-blasts of patient 1 (Fig. 5b, Pat. 1), with total RNA from a second RGS1 + CLL patient (Fig. 5b, Pat. 2) and total RNA purified from RGS1 − AML-blasts (Fig. 5b, Pat. 3) as targets in the standard 51Cr-release assay. In these experiments only the DCs electroporated with the RGS1 + RNA were lysed by the CTLs, whereas cells transfected with RGS1 − AML-RNA were spared. As controls we included DCs transfected with RGS1-IVT, which were efficiently lysed. In contrast, DCs transfected with irrelevant EGFP-IVT were spared.
In summary, these experiments demonstrated the feasibility to induce RGS1-specific CTLs in CLL patients in complete remission after chemotherapy that efficiently lyse the autologous blasts in an antigen-specific and HLA-restricted manner.
Discussion
One of the challenges in the development of immunotherapeutic approaches is the identification of relevant TAAs with the ability to induce tumor T cell-responses. In addition, these antigens should represent target structures that are pivotal for tumor development, growth and hematogenic spread.
Gene expression profiling of normal kidney and samples from RCCs recently revealed that RGS1 is highly overexpressed in RCC tissues. Moreover, using RT-PCR analysis, we could detect expression of RGS1 in several established human tumor cell lines and primary samples from patients with acute and chronic leukemia, indicating that this gene is expressed in a broad variety of human malignancies.
G-protein-coupled receptors constitute a large and diverse family of proteins whose function is to transduce extracellular stimuli into the cell. It includes light-activated receptors (rhodopsins) in the eye, odorant receptors in the mammalian nose or receptors for various hormones and neurotransmitters. The binding of a ligand to these receptors leads to the exchange of GDP with GTP at the cytosolic bound G-protein. This exchange leads to the dissociation of the α and βγ subunits which both serve as second messengers. The function of RGS proteins is to activate the GTPase activity of the α subunit and therefore the restoration of the inactive trimeric αβγ molecule. Thus, RGS proteins function as negative feedback regulators in such signaling loops.
The fact that G-proteins and RGS participate in many cellular signaling cascades, leads to the assumption that the over-expression of RGS1 in malignant tissues, ascertained in this work, is in some way causative involved in the cancerous phenotype.
The induction of antigen-specific immune responses is critically dependent on the way antigens are delivered and processed. It was shown that DCs transfected with RNA coding for a TAA or whole tumor RNA are able to induce potent antigen- and tumor-specific T cell responses directed against multiple epitopes. The feasibility and efficacy of this approach was tested and analyzed in different settings by administration of RNA-transfected-DCs in patients with cancer [11, 21, 26, 32, 33]. We therefore utilized this RNA-based approach to analyze the induction of RGS1 specific CTLs and mediation of tumor cell lysis in vitro.
The in vitro-induced RGS1-specific CTLs were able to lyse tumor cells including RCCs, melanoma and ovarian carcinoma endogenously expressing the RGS1 protein in an antigen-specific and HLA-restricted manner.
These results could be reproduced for HLA-A2-, -A3- or -A2-/-A3-positive donors, confirming our previous studies, by which we could demonstrate that transfection of DCs with specific or whole cell RNA results in the generation of polyclonal T cell responses recognizing epitopes presented on different HLA-molecules and has therefore potential for a broad clinical application [5, 7, 10, 19, 22–24].
Finally, we tested the ability of our RNA approach to elicit CTL responses in patients with malignant diseases. Therefore, CTL lines were generated from the PBMCs of a patient with CLL in complete remission by in vitro immunization with RGS1-IVT transfected autologous DCs. The in vitro-induced CTLs efficiently lysed the autologous leukemic cells in MHC-restricted and antigen-specific manner, but spared normal T cells or DCs electroporated with total RNA purified from RGS1-negative AML blasts. These results demonstrated that RGS1-specific CTLs that are able to recognize primary autologous tumor cells in an antigen-specific manner could be generated in patients with malignant diseases.
In conclusion, our findings manifest that RGS1 is a tumor rejection antigen expressed in many solid and hematopoietic malignancies and can be used for the design of cancer vaccines.
Acknowledgments
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (SFB 685).
Footnotes
F. Grünebach and S. Erndt contributed equally to this work.
References
- 1.Agenes F, Bosco N, Mascarell L, Fritah S, Ceredig R. Differential expression of regulator of G-protein signalling transcripts and in vivo migration of CD4+ naive and regulatory T cells. Immunology. 2005;115:179–188. doi: 10.1111/j.1365-2567.2005.02146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brossart P, Grünebach F, Stuhler G, Reichardt VL, Mohle R, Kanz L, Brugger W. Generation of functional human dendritic cells from adherent peripheral blood monocytes by CD40 ligation in the absence of granulocyte-macrophage colony-stimulating factor. Blood. 1998;92:4238–4247. [PubMed] [Google Scholar]
- 4.Gilboa E. The makings of a tumor rejection antigen. Immunity. 1999;11:263–270. doi: 10.1016/S1074-7613(00)80101-6. [DOI] [PubMed] [Google Scholar]
- 5.Grünebach F, Kayser K, Weck MM, Müller MR, Appel S, Brossart P. Co-transfection of Dendritic Cells with RNA coding for HER-2/neu and 4-1BBL increases the Induction of Tumor Antigen specific cytotoxic T Lymphocytes. Cancer Gene Ther. 2005;12:749–756. doi: 10.1038/sj.cgt.7700842. [DOI] [PubMed] [Google Scholar]
- 6.Grünebach F, Müller MR, Brossart P. RNA transfection of dendritic cells. Methods Mol Med. 2005;109:47–54. doi: 10.1385/1-59259-862-5:047. [DOI] [PubMed] [Google Scholar]
- 7.Grünebach F, Müller MR, Nencioni A, Brossart P. Delivery of tumor-derived RNA for the induction of cytotoxic T-lymphocytes. Gene Ther. 2003;10:367–374. doi: 10.1038/sj.gt.3301901. [DOI] [PubMed] [Google Scholar]
- 8.Hamm HE. The many faces of G protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 9.Han SB, Moratz C, Huang NN, Kelsall B, Cho H, Shi CS, Schwartz O, Kehrl JH. Rgs1 and Gnai2 regulate the entrance of B lymphocytes into lymph nodes and B cell motility within lymph node follicles. Immunity. 2005;22:343–354. doi: 10.1016/j.immuni.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Heine A, Grünebach F, Holderried T, Appel S, Weck MM, Dorfel D, Sinzger C, Brossart P. Transfection of dendritic cells with in vitro-transcribed CMV RNA induces polyclonal CD8+- and CD4+-mediated CMV-specific T cell responses. Mol Ther. 2006;13:280–288. doi: 10.1016/j.ymthe.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 11.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, Dahm P, Niedzwiecki D, Gilboa E, Vieweg J. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepler JR, Gilman AG. G proteins. Trends Biochem Sci. 1992;17:383–387. doi: 10.1016/0968-0004(92)90005-T. [DOI] [PubMed] [Google Scholar]
- 13.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54:527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 14.Hong JX, Wilson GL, Fox CH, Kehrl JH. Isolation and characterization of a novel B cell activation gene. J Immunol. 1993;150:3895–3904. [PubMed] [Google Scholar]
- 15.Koga H, Imada K, Ueda M, Hishizawa M, Uchiyama T. Identification of differentially expressed molecules in adult T-cell leukemia cells proliferating in vivo. Cancer Sci. 2004;95:411–417. doi: 10.1111/j.1349-7006.2004.tb03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer BF, Schoor O, Kruger T, Reichle C, Müller M, Weinschenk T, Hennenlotter J, Stenzl A, Rammensee HG, Stevanovic S. MAGED4-Expression in Renal Cell Carcinoma and Identification of an HLA-A*25-Restricted MHC Class I Ligand from Solid Tumor Tissue. Cancer Biol Ther. 2005;4:943–948. doi: 10.4161/cbt.4.9.1907. [DOI] [PubMed] [Google Scholar]
- 17.Krüger T, Schoor O, Lemmel C, Kraemer B, Reichle C, Dengjel J, Weinschenk T, Müller M, Hennenlotter J, Stenzl A, Rammensee HG, Stevanovic S. Lessons to be learned from primary renal cell carcinomas: novel tumor antigens and HLA ligands for immunotherapy. Cancer Immunol Immunother. 2005;54:826–836. doi: 10.1007/s00262-004-0650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/S0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 19.Milazzo C, Reichardt VL, Müller MR, Grünebach F, Brossart P. Induction of myeloma-specific cytotoxic T cells using dendritic cells transfected with tumor-derived RNA. Blood. 2003;101:977–982. doi: 10.1182/blood-2002-04-1273. [DOI] [PubMed] [Google Scholar]
- 20.Moratz C, Hayman JR, Gu H, Kehrl JH. Abnormal B-cell responses to chemokines, disturbed plasma cell localization, and distorted immune tissue architecture in Rgs1-/- mice. Mol Cell Biol. 2004;24:5767–5775. doi: 10.1128/MCB.24.13.5767-5775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y, Boczkowski D, Proia A, Neidzwiecki D, Clavien PA, Hurwitz HI, Schlom J, Gilboa E, Lyerly HK. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341–349. doi: 10.1081/CNV-120018224. [DOI] [PubMed] [Google Scholar]
- 22.Müller MR, Grünebach F, Kayser K, Vogel W, Nencioni A, Brugger W, Kanz L, Brossart P. Expression of her-2/neu on acute lymphoblastic leukemias: implications for the development of immunotherapeutic approaches. Clin Cancer Res. 2003;9:3448–3453. [PubMed] [Google Scholar]
- 23.Müller MR, Grünebach F, Nencioni A, Brossart P. Transfection of dendritic cells with RNA induces CD4- and CD8-mediated T cell immunity against breast carcinomas and reveals the immunodominance of presented T cell epitopes. J Immunol. 2003;170:5892–5896. doi: 10.4049/jimmunol.170.12.5892. [DOI] [PubMed] [Google Scholar]
- 24.Müller MR, Tsakou G, Grünebach F, Schmidt SM, Brossart P. Induction of chronic lymphocytic leukemia (CLL)-specific CD4- and CD8-mediated T-cell responses using RNA-transfected dendritic cells. Blood. 2004;103:1763–1769. doi: 10.1182/blood-2003-06-2097. [DOI] [PubMed] [Google Scholar]
- 25.Nair SK, Boczkowski D, Morse M, Cumming RI, Lyerly HK, Gilboa E. Induction of primary carcinoembryonic antigen (CEA)-specific cytotoxic T lymphocytes in vitro using human dendritic cells transfected with RNA. Nat Biotechnol. 1998;16:364–369. doi: 10.1038/nbt0498-364. [DOI] [PubMed] [Google Scholar]
- 26.Nair SK, Morse M, Boczkowski D, Cumming RI, Vasovic L, Gilboa E, Lyerly HK. Induction of tumor-specific cytotoxic T lymphocytes in cancer patients by autologous tumor RNA-transfected dendritic cells. Ann Surg. 2002;235:540–549. doi: 10.1097/00000658-200204000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reif K, Cyster JG. RGS molecule expression in murine B lymphocytes and ability to down-regulate chemotaxis to lymphoid chemokines. J Immunol. 2000;164:4720–4729. doi: 10.4049/jimmunol.164.9.4720. [DOI] [PubMed] [Google Scholar]
- 28.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt SM, Schag K, Müller MR, Weck MM, Appel S, Kanz L, Grünebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt SM, Schag K, Müller MR, Weinschenk T, Appel S, Schoor O, Weck MM, Grünebach F, Kanz L, Stevanovic S, Rammensee HG, Brossart P. Induction of adipophilin-specific cytotoxic T lymphocytes using a novel HLA-A2-binding peptide that mediates tumor cell lysis. Cancer Res. 2004;64:1164–1170. doi: 10.1158/0008-5472.CAN-03-2538. [DOI] [PubMed] [Google Scholar]
- 31.Steinman RM. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 32.Su Z, Dannull J, Heiser A, Yancey D, Pruitt S, Madden J, Coleman D, Niedzwiecki D, Gilboa E, Vieweg J. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63:2127–2133. [PubMed] [Google Scholar]
- 33.Su Z, Dannull J, Yang BK, Dahm P, Coleman D, Yancey D, Sichi S, Niedzwiecki D, Boczkowski D, Gilboa E, Vieweg J. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. J Immunol. 2005;174:3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 34.Teufel R, Carralot JP, Scheel B, Probst J, Walter S, Jung G, Hoerr I, Rammensee HG, Pascolo S. Human peripheral blood mononuclear cells transfected with messenger RNA stimulate antigen-specific cytotoxic T-lymphocytes in vitro. Cell Mol Life Sci. 2005;62:1755–1762. doi: 10.1007/s00018-005-5067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Tendeloo VF, Ponsaerts P, Lardon F, Nijs G, Lenjou M, Van Broeckhoven C, Van Bockstaele DR, Berneman ZN. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98:49–56. doi: 10.1182/blood.V98.1.49. [DOI] [PubMed] [Google Scholar]
- 36.Weinschenk T, Gouttefangeas C, Schirle M, Obermayr F, Walter S, Schoor O, Kurek R, Loeser W, Bichler KH, Wernet D, Stevanovic S, Rammensee HG. Integrated functional genomics approach for the design of patient-individual antitumor vaccines. Cancer Res. 2002;62:5818–5827. [PubMed] [Google Scholar]