Abstract
Immunotherapy, including the use of cytokines and/or modified tumour cells immune stimulatory cytokines, can enhance the host anti-tumour immune responses. Interleukin-23 (IL-23) is a relative novel cytokine, which consists of a heterodimer of the IL-12p40 subunit and a novel p19 subunit. IL-23 has biological activities similar to but distinct from IL-12. IL-23 can enhance the proliferation of memory T cells and the production of IFN-γ, IL-12 and TNF-α from activated T cells. IL-23 activates macrophages to produce TNF-α and nitric oxide. IL-23 can also act directly on dendritic cells and possesses potent anti-tumour and anti-metastatic activity in murine models of cancer. IL-23 can also induce a lower level of IFN-γ production compared with that induced by IL-12. This may make IL-23 an alternative and safer therapeutic agent for cancer, as IL-12 administration can lead to severe toxic side effects because of the extremely high levels of IFN-γ it induces.
Keywords: IL-23, Immune response, Antitumour activity
Introduction
Tumours are now one of the most serious diseases threatening human health. Despite treatments with surgery, chemotherapy and radiation, many cancers will recur, metastasize or remain resistant to conventional therapies. Development of cancer is believed to be influenced by defects in the host immunosurveillance system and a number of escape mechanisms exist by which tumour can elude host immune responses. Advances in cellular and molecular immunology have greatly improved our understanding of the interactions between tumours and immune cells, which show that immunotherapy, the stimulation of the immune system to preferentially kill tumour cells while having less toxicity on normal cells, may be an appropriate strategy for cancer treatment.
Cytokines can have pleiotropic effects and mediate systemic and local biological actions, which affect cell growth and differentiation, immune function and inflammation. Systemic administrations of some cytokines such as interleukin-2 (IL-2) [1–3], interferon α (IFN-α) [4, 5] and IL-12 [6, 7] have resulted in clinical responses in several types of cancer. However, severe toxicities limit them in clinical applications. Therefore, studies have looked for more effective and less toxic cytokines and novel routes of administration. Cytokine gene therapy is one of the approaches of immunotherapy, in which cytokine genes in vectors are transduced into cellular vehicles such as tumour cells, dendritic cells (DCs) or fibroblasts. These cellular vehicles express cytokines locally and induce immune responses against the tumour, overcoming the drawbacks of systemic administration of cytokines. Tumour cells and DCs are naturally loaded with tumour antigens which can provide the necessary tumour antigens to induce specific anti-tumour responses. Almost all of the cytokine genes, including IL-2 [8–10], IL-4 [11, 12], IL-6 [13, 14], IL-12 [15–17], IL-15 [18, 19], IL-18 [20, 21], IL-21 [22], tumour necrosis factor α (TNF-α) [23, 24], IFN-α [25], IFN-β [26], IFN-γ [27, 28] and granulocyte-macrophage colony stimulating factor (GM-CSF) [29, 30], have been evaluated for their anti-tumour effects in animal models. IL-23 is one of the newly reported cytokines [31]. In this symposium report, we discuss the background and our studies on the effects of IL-23 on the immune system and its anti-tumour activity.
Structure and production of IL-23
Interleukin-23 is comprised of the IL-12p40 subunit and a novel p19 subunit [32]. The p19 subunit is closely related in structure to IL-6, G-CSF, and IL-12p35, and there is 70% homology between the mouse and human forms of IL-23p19 [32]. In the same way that IL-12p35 is biologically inactive by itself, so is IL-23p19. It requires coexpression of the p40 subunit within the same cell to form the biologically active IL-23 molecule [32]. IL-23 is secreted by activated DCs [32, 33], activated macrophages [34, 35] and activated monocytes [36].
IL-23 receptor and signal transduction
The IL-12 receptor (IL-12R) complex is composed of two subunits, IL-12Rβ1 and IL-12Rβ2 [37–39]. IL-23 was found to bind to the IL-12Rβ1 subunit but not the IL-12Rβ2 subunit [32]. In fact, IL-23 binds a receptor composed of IL-12Rβ1 and a novel subunit termed IL-23R [40]. IL-23R is expressed on T cells, NK cells, NKT cells, monocytes, macrophages and DCs [40]. IL-23 activates the same spectrum of Jak/Stat molecules as IL-12: Jak2, Tyk2, Stat1, Stat3, Stat4 and Stat5 [40]. However, in contrast to IL-12, the most prominent Stat induced by IL-23 is Stat3 rather than Stat4, and different Stat complexes appear to be involved in DNA-binding [40]. IL-12 induces a DNA-binding complex containing only Stat4, while IL-23 induces several complexes containing Stat1, Stat3, Stat4, and possibly Stat3/Stat4 [40].
Biological activities of IL-23
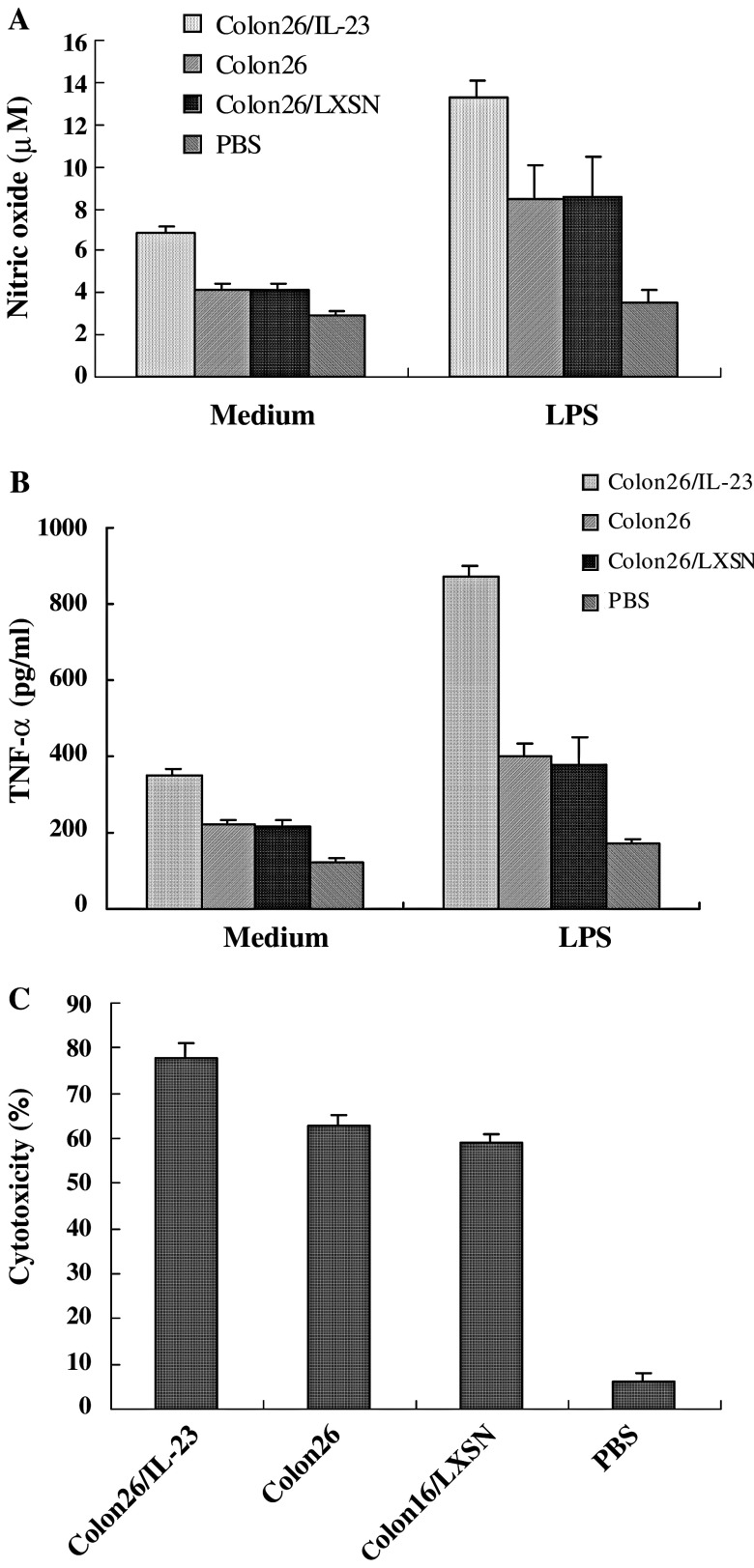
Interleukin-23 has biological activities that are similar to but distinct from IL-12 [32]. The effects of IL-23 and IL-12 on murine DCs have been studied using cytokine-immunoglobulin (Ig) fusion proteins [41]. IL-23 acts on both CD8α- and CD8α+ splenic DCs and enhances both DC subsets to present P815AB tumour peptide, while IL-12 can only bind CD8α- splenic DCs leading to the same effect [41]. IL-23 induces the production of IL-12 and IFN-γ by DCs, and together with IL-12 promotes the production of higher levels of IL-12 and IFN-γ compared with that induced by either IL-12 or IL-23 alone [41]. Macrophages are classified into two types, named M1 and M2, according to their functional activities. M1 macrophages are characterized by the spontaneous secretion of low levels of TGF-β1 and by their response to LPS or IFN-γ leading to an elevated nitric oxide (NO) production [42]. In contrast, M2 macrophages spontaneously secrete large amounts of TGF-β1 and respond to LPS or IFN-γ by the production of low levels of NO [42]. Our unpublished data showed that the peritoneal macrophages of mice injected with murine colon carcinoma cells (Colon26) which had been transduced with the IL-23 gene could produce high levels of NO. In addition NO production was further enhanced in the presence of LPS compared with that of control mice (P < 0.01, Fig. 1a). Although the effects of IL-23 on the regulation of TGF-β1 production have yet to be evaluated in our study, we propose that IL-23 may promote differentiation of macrophages to the M1 type. Evaluation of this hypothesis is the focus of current future studies. It has been shown that intraperitoneal administration of IL-23 into mice induced the production of IL-1β and TNF-α by peritoneal macrophages, even in the presence of neutralizing antibodies to IFN-γ [43]. DCs are important antigen presenting cells (APC) and are able to bridge both the innate and adaptive arms of the immune system. Macrophages are also APCs and important cells in the innate immune system. Therefore, IL-23 may play a critical role in bridging innate and adaptive responses in a similar way to IL-12.
Fig. 1.
Effects of IL-23 on macrophages. Peritoneal cells were collected from mice 5 days after i.p. injection, washed in PBS and plated onto a plastic surface for 1 h in serum-free culture medium. Adherent cells were collected as peritoneal macrophages. a Evaluation of the capacity of peritoneal macrophages to produce NO. Triplicate samples of peritoneal macrophages were cultured in medium alone or medium with LPS (2 × 105 cells/microtiter well). Following a 48 h incubation (in 37°C, 5% CO2 incubator), NO in culture supernatants was quantified using Griess reagent. b Production of TNF-α of peritoneal macrophages. Triplicate samples of macrophages (2 × 105 cells/well) were cultured in 96-well microtiter plates with or without LPS for 48 h, then culture supernatants were collected and TNF-α levels were assessed by ELISA. c Cytotoxic activity of macrophages in vitro. Colon26 cells in their exponential growth phase were incubated for 24 h with medium containing 1.5 μCi/ml [3H-methyl]thymidine. The macrophages (1 × 105 cells/well) and 3H-labelled Colon26 cells (1 × 104 cells/well) were plated into wells of 96-well microtiter plates. After 48 h incubation, the wells were washed twice with PBS, and adherent viable cells were lysed with 0.1 ml 0.1 N NaOH. The lysates were harvested and [3H-methyl]thymidine levels counted using a liquid scintillation counter. The percentage of cytotoxicity was calculated by formula: cytotoxicity (%) = (A − B)/A × 100, where A is cpm in the culture of target cells alone, and B is the cpm in the test culture
Transgenic mice overexpressing the p19 subunit of IL-23 develop severe multiorgan inflammation, runting, infertility and premature death with elevated production of TNF-α and IL-1 [44]. In addition, they show increased numbers of circulating neutrophils and constitutive expression of acute phase proteins throughout their lives [44].
In the presence of anti-CD3 mAb, murine IL-23 induces the proliferation of memory T cells but not naive T cells. In contrast, IL-12 has an effect on naive T cells but not memory T cells [32]. In the presence of anti-CD3 and anti-CD28 antibodies, human IL-23 induces human memory T cells to produce high levels of IFN-γ, but induces naive T cells to produce modest amounts of IFN-γ only after a prolonged incubation period [32]. The levels of IFN-γ induced by IL-23 are far lower than that induced by IL-12 [32]. These data suggest that IL-23 plays an important role in memory responses. In addition, IL-23 can stimulate activated CD4+ T cells to produce IL-17, but IL-12 has only marginal effects on IL-17 production [45]. During adaptive immune responses, IL-23 and IL-12 exert their effects on two complementary T-cell pathways. One is the classical Th1-type response and the other is the novel IL-23/IL-17 immune pathway [45, 46]. In autoimmunity, as well as intracellular microbial infection, IL-12 and IL-23 appear to have different activities. IL-12, but not IL-23, is indispensable for intracellular parasitic infection. In contrast, IL-23, but not IL-12, is essential for the induction and maintenance of brain and joint inflammation autoimmune diseases [46].
Anti-tumour activity of IL-23
To investigate the anti-tumour effects of IL-23, murine tumour cell lines were transduced with the murine IL-23 gene in a retroviral vector, and injected into mice [47]. IL-23 has potent anti-tumour and anti-metastatic effects in murine CT26 and B16F1 melanoma models giving similar results to IL-12. Most animals (70%) completely rejected IL-23-transduced tumours and remained tumour free for the entire 120-day observation period [47]. However, the anti-tumour responses induced by IL-23 and IL-12 were different, IL-23-mediated tumour suppression was only evident at later time points after tumour inoculation, while IL-12 induced tumour suppression was obvious in an earlier time frame [47]. The lack of an early anti-tumour response in the presence of IL-23 may be due to its inability to induce the higher levels of IFN-γ that is associated with IL-12 secreting tumour cells [32, 47]. This study also evaluated the anti-tumour mechanisms of IL-23 in immunocompromised mice and in animals selectively depleted of specific lymphocyte populations. The data suggested that CD8+ T cells play an important role in IL-23-mediated anti-tumour activity, because the protective effects were completely lost in T cell-deficient SCID mice and in wild-type mice depleted of CD8+ T cells [47]. Immunohistochemical examination confirmed CD8+ T cells infiltration at the time tumours started to regress, and that the number of infiltrating CD8+ T cells increased further in the later phase of tumour regression [47]. In this study, a slower rate of tumour growth was observed with IL-23-transduced cells in CD4+ T cell-depleted mice than in control mice, indicating that CD4+ T cells were apparently not required for the anti-tumour activity of IL-23 [47]. The fact that NK cell depletion did not affect either the growth rate of IL-23-transduced cells or the percentage of tumour-free animals suggested that NK cells are not needed for the anti-tumour activity of IL-23 at least in the CT26 tumour model [47]. Mice that rejected IL-23-transduced tumours could also reject subsequent wild-type tumour challenge, and a tumour-specific CTL response was induced in mice that had previously rejected IL-23-transduced tumours. All these results indicated that IL-23 induced an anti-tumour T cell memory response [47]. Other studies in mouse and human tumour models provide similar results as described here, and showed that all animals inoculated with IL-23-transduced murine tumour cells could completely reject their tumours [48–50]. Wang et al. [48] examined the secretion of IFN-γ from the spleen cells of mice injected with IL-23-transduced tumour cells. Following the rejection of IL-23-transduced tumours, spleen cells were cultured with irradiated wild-type tumour cells and irrelevant tumour cells. Higher levels of IFN-γ were secreted from spleen cells cultured with irradiated wild-type tumour cells than those incubated with irradiated irrelevant tumour cells, suggesting that IL-23 secretion by IL-23-transduced tumour cells induced antigen-specific cellular responses [48]. IFN-γ induction is known to induce anti-tumour effects mediated by the immune system. However, Lo et al. [47] reported that the anti-tumour properties of IL-23 were not affected in mice depleted of IFN-γ. Liebau et al. [51] transfected UMR 108 osteosarcoma cells with different plasmids encoding IL-12, IL-23, pro IL-18 and ICE (interleukin-1β converting enzyme). Local secretion of IL-23 by UMR 108 osteosarcoma cells generated a lower level of production of IFN-γ compared with IL-12 or IL-18 [51]. This data demonstrated that the lower level of IFN-γ induced by IL-23 did not play a key role in the anti-tumour activity of IL-23. Therefore, we propose that other immune mechanisms may be involved in the anti-tumour activity of IL-23.
To try to determine the immune mechanism of action of IL-23, we transduced Colon26 cells with the retroviral vector LXSN containing murine IL-23 cDNA (provided by Dr. M. Tagawa, Division of Pathology, Chiba Cancer Center Research Institute, Chiba, Japan). The transduced cells were s.c injected into BALB/c mice. We examined the effects of IL-23 on cytokine production by spleen cells and DCs. To examine the effects of IL-23 on macrophages we i.p inoculated BALB/c mice with Colon26 cells genetically modified to express IL-23. The mice injected with Colon26 cells, Colon26/LXSN cells (transduced with LXSN vector alone) or PBS alone were used as controls. Our unpublished data showed that the spleen cells from the mice injected with IL-23-transduced tumour cells produced higher levels of IFN-γ, IL-12 and TNF-α but not IL-4 compared with control mice at day 30 post-inoculation (P < 0.01, Fig. 2). Flow cytometry showed that the percentage of DCs (CD11c positive cells) in the spleen of mice injected with IL-23-transduced tumour cells were increased compared with that of control mice. However, the effect of IL-23 on the activity of DCs in tumour bearing mice has yet to be determined. On day 5 after inoculation with IL-23 transduced tumour cells peritoneal macrophages from all mice were examined and shown to produce higher levels of NO and TNF-α compared with control mice (P < 0.01, Fig. 1b). The cytotoxicity of the peritoneal macrophages of these mice was also enhanced compared with that of control mice (P < 0.01, Fig. 1c). The survival of the mice injected s.c. or i.p. with IL-23-transduced tumour cells (> 250 days, 54 ± 20 days) were prolonged compared with that of the mice injected with Colon26/LXSN cells (50 ± 3 days, 15 ± 3 days, P < 0.01) and Colon26 cells (51 ± 3 days, 14 ± 3 days, P < 0.01). Together, these results suggest that macrophages, DCs, IL-12, TNF-α, and NO all contribute to the antitumour activity of IL-23.
Fig. 2.
Production of cytokines by spleen cells. Spleen cells were collected from mice at day 30 after inoculation. The spleen cells (1 × 106 cells/ml) were cultured with mitocycin C-treated Colon26 cells (1 × 105 cells/ml) in culture medium. Culture supernatants were collected 48 h later and the concentration of secreted cytokines examined using standard ELISA assays
Footnotes
This article is a symposium paper from the Annual Meeting of the “International Society for Cell and Gene Therapy of Cancer”, held in Shenzhen, China, on 9–11 December 2005.
References
- 1.Lotze MT, Chang AE, Seipp CA, Simpson C, Vetto JT, Rosenberg SA. High-dose recombinant interleukin-2 in the treatment of patients with disseminated cancer response, treatment-related morbidity and histological finding. JAMA. 1986;256:3117–3124. doi: 10.1001/jama.256.22.3117. [DOI] [PubMed] [Google Scholar]
- 2.Pizza G, Viaz D, Devince C, Vichi-Pascuuchi JM, Busutti L, Bergami T. Intralymphatic administration of interleukin-2 (IL-2) in cancer patients: a pilot study. Cancer Res. 1988;7:46–48. [PubMed] [Google Scholar]
- 3.Sama G, Collins J, Figlin R, Robertson P, Altrock B, Abels R. A pilot study of intralymphatic interleukin-2 II clinical and biological effects. J Biol Response Modif. 1990;9:81–86. [PubMed] [Google Scholar]
- 4.Zinzani PL, Lauria F, Salvucci M, Rondelli D, Raspadori D, Bendandi M, Magagnoli M, Tura S. Hairy-cell leukemia and alpha-interferon treatment: long-term responders. Hematoligica. 1997;88:152–155. [PubMed] [Google Scholar]
- 5.Ozer H, Wiernik PH, Giles F, Tendler C. Recombinant interferon-alpha therapy in patients with follicular lymphoma. Cancer. 1998;82:1821–1830. doi: 10.1002/(SICI)1097-0142(19980515)82:10<1821::AID-CNCR3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Rakhit A, Schqartz LH, Olencki T, Malone TM, Sandstrom K, Nadeau R, Parmar H, Bukowski R. Phase I trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res. 1998;4:1183–1191. [PubMed] [Google Scholar]
- 7.Robertson MJ, Cameron C, Atkins MM, Gordon MS, Lotze MT, Sherman ML, Ritz J. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin Cancer Res. 1999;5:9–16. [PubMed] [Google Scholar]
- 8.Gansbacher B, Zier K, Daniels B, Cronin K, Bannedi R, Gilboa E. Interleukin-2 gene transfer into tumour cells abrogates tumourigenicity and induce protective immunity, J Exp Med. 1990;172:1217–1223. doi: 10.1084/jem.172.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karp SE, Farber A, Salo JC, Hwu P, Jaffe G, Asher AL, Shiloni E, Restifo N, Mule JJ, Rosenberg SA. Cytokine secretion by genetically modified non-immunogenic murine fibrosarcoma. Tumour inhibition by IL-2 but not tumour necrosis factor. J Immunol. 1993;150:896–908. [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara H, Koide Y, Sugaya M, Gunji Y, Asano T, Ochiai T, Takeganak, Sakiyama S, Tagawa M. Antitumour responses of genetically engineered IL-2 expression to human esophageal carcinoma cells in mature T cell-defective condition. Int J Cancer. 1998;53:471–477. doi: 10.3892/ijo.13.6.1217. [DOI] [PubMed] [Google Scholar]
- 11.Golumbek PT, Lazenby A, Levitsky HI, Jaffee LM, Karasuyama H, Baker M, Pardoll DM. Treatment of established renal cancer by tumour cells engineered to secrete interleukin-4. Science. 1991;254:713–716. doi: 10.1126/science.1948050. [DOI] [PubMed] [Google Scholar]
- 12.Sturlan S, Beinhauer BG, Oberhuber G, Huang L, Aasen AO, Roay MA. In vivo gene transfer of murine interleukin-4 inhibits colon26-mediated cancer cachexia in mice. Anticancer Res. 2002;22:2547–2554. [PubMed] [Google Scholar]
- 13.Porgador A, Tzehoval E, Katz A, Vadai E, Revel M, Feldman M, Eisenbach L. Interleukin 6 gene transfection into Lewis lung carcinoma tumour cells suppresses the malignant phenotype and confers immunotherapeutic competence against parental metastatic cells. Cancer Res. 1992;52:3679–3686. [PubMed] [Google Scholar]
- 14.Cao X, Wang Q, Ju DW, Tao Q, Wang J. Efficient induction of local and systemic antitumour immune response by liposome-mediated intratumoural co-transfer of interleukin-2 gene and interleukin-6 gene. J Exp Clin Cancer Res. 1999;18:191–200. [PubMed] [Google Scholar]
- 15.Chen L, Chen D, Block E, O’Donnell M, Kufe DW, Clinton SK. Eradication of murine bladder carcinoma by intratumour injection of a bicistronic adenoviral vector carrying cDNAs for the IL-12 heterodimer and its inhibition by the IL-12 p40 subunit homodimer. J Immunol. 1997;159:351–359. [PubMed] [Google Scholar]
- 16.Satoh Y, Esche C, Gambotto A, Shurin GV, Yurkovetsky ZR, Robbins PD, Watkins SC, Todo S, Herberman RB, Lotze MT, Shurin MR. Local administration of IL-12-transfected dendritic cells induces antitumour immune responses to colon adenocarcinoma in the liver in mice. J Exp Ther Oncol. 2002;2:337–349. doi: 10.1046/j.1359-4117.2002.01050.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, DeGroot LJ. Gene therapy of a rat follicular thyroid carcinoma model with adenoviral vectors transducing murine interleukin-12. Endocrinology. 2003;144:1393–1398. doi: 10.1210/en.2002-221013. [DOI] [PubMed] [Google Scholar]
- 18.Tasaki K, Yoshida Y, Miyauchi M, Maeda T, Tagenaga K, Kouzu T, Asano T, Ochiai T, Sakiyamna S, Tagawa M. Transduction of murine colon carcinoma cells with interleukin-15 gene induces antitumour effects in immunocompetent and immunocompromised hosts. Cancer Gene Ther. 2000;7:255–261. doi: 10.1038/sj.cgt.7700112. [DOI] [PubMed] [Google Scholar]
- 19.Yoshida Y, Tasaki K, Miyauchi M, Narita M, Takenaga K, Yamamoto H, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Impaired tumourigenicity of human pancreatic cancer cells retrovirally transduced with interleukin-12 or interleukin-15 gene. Cancer Gene Ther. 2000;7:324–331. doi: 10.1038/sj.cgt.7700118. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimura K, Haxama S, Iixuka N, Yoshino S, Yamamoto K, Muraguchi M, Ohmoto Y, Noma T, Oka M. Successful immunogene therapy using colon cancer cells (colon26) transfected with plasmid vector containing mature interleukin-18 cDNA and the Igkappa leader sequence. Cancer Gene Ther. 2001;8:9–6. doi: 10.1038/sj.cgt.7700277. [DOI] [PubMed] [Google Scholar]
- 21.Nagai H, Hara I, Horikawa T, Oka M, Kamidono S, Ichihashi M. Gene transfer of secreted-type modified interleukin-18 gene to B16F10 melanoma cells suppresses in vivo tumour growth through inhibition of tumour vessel formation. J Invest Dermatol. 2002;119:541–548. doi: 10.1046/j.1523-1747.2002.01866.x. [DOI] [PubMed] [Google Scholar]
- 22.Ugai S, Shimozato O, Kawamura K, Wang YQ, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Expression of the interleukin-21 gene in murine colon carcinoma cells generates systemic immunity in the inoculated hosts. Cancer Gene Ther. 2003;10:187–192. doi: 10.1038/sj.cgt.7700552. [DOI] [PubMed] [Google Scholar]
- 23.Vanhaesebroeck B, Mareel M, Van Roy F, Grooten J, Fiers W. Expression of the tumour necrosis factor gene in tumour cells correlates with reduced tumourigenicity and reduced invasiveness in vivo. Cancer Res. 1991;51:2229–2238. [PubMed] [Google Scholar]
- 24.Lasek W, Maxhiewicz A, Czajka A, Switaj T, Golb J, Wiznerowicz M, Korczak-Kawalska G, Bakowiec-Iskra EZ, Gryska K, Ixycki D, Jakobisiak M. Antirumor effects of the combination therapy with TNF-alpha gene-modified tumour cells and interleukin 12 in a melanoma model in mice. Cancer Gene Ther. 2000;7:1581–1590. doi: 10.1038/sj.cgt.7700263. [DOI] [PubMed] [Google Scholar]
- 25.Ohashi M, Yoshida K, Kushida M, Miura Y, Ohnami S, Ikaraki Y, Kitade Y, Yoshida T, Aoki K. Adenovirus-mediated interferon alpha gene transfer induces regional direct cytotoxicity and possible systemic immunity against pancreatic cancer. Br J Cancer. 2005;93:441–449. doi: 10.1038/sj.bjc.6602713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilderman MJ, Sun J, Jassar AS, Kapoor V, Khan M, Vachani A, Suzuki E, Kinniry PA, Sterman DH, Ksiser LR, Albelda SM. Intrapulmonary IFN-beta gene therapy using an adenoviral vector is highly effective in a murine orthotopic model of bronchogenic adenocarcinoma of the lung. Cancer Res. 2005;65:8379–8387. doi: 10.1158/0008-5472.CAN-05-0920. [DOI] [PubMed] [Google Scholar]
- 27.Gansbacher B, Bannerji R, Daniels B, Zier K, Cronin K, Gilboa E. Retroviral vector-mediated gamma-interferon gene transfer into tumour cells genetates potent and long lasting antitumor immunity. Cancer Res. 1990;50:713–716. [PubMed] [Google Scholar]
- 28.Dummer R, Hassel JC, Fellenberg F, Eichmuller S, Maier T, Slos P, Acres B, Bleuzen P, Bataille V, Squiban P, Burg G, Urosevic M. Adenovirus-mediated intralesional interferon-gamma gene transfer induces tumour regression in cutaneous lymphomas. Blood. 2004;104:1631–1638. doi: 10.1182/blood-2004-01-0360. [DOI] [PubMed] [Google Scholar]
- 29.Ju DW, Cao X, Acres B. Intratumour injection of GM-CSF gene encoded recombinant vaccinia virus elicits antitumour response in a mixture melanoma model. Cancer Gene Ther. 1997;4:139–144. [PubMed] [Google Scholar]
- 30.Hogge GS, Burkholder JK, Culp J, Albertini MR, Dubielzig RR, Yang NS, MacEwen EG. Preclinical development of human granulocyte-macrophage colony-stimulating factor-transfected melanoma cell vaccine using established canine cell lines and normal dogs. Cancer Gene Ther. 1999;6:26–36. doi: 10.1038/sj.cgt.7700015. [DOI] [PubMed] [Google Scholar]
- 31.Hunter CA. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- 32.Oppmann B, Lesley R, Blom B, Timans JC, Xu Y, Hunte B, Vega F, Yu N, Wang J, Singh K, Zonin F, Vaisberg E, Churakova T, Liu M, Gorman D, Wagner J, Zurawski S, Liu YJ, Abrams JS, Moore KW, Rennick D, de Waal-Malefyt R, Hannum C, Bazan JF, Kastelein RA. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity. 2000;13:715–725. doi: 10.1016/S1074-7613(00)00070-4. [DOI] [PubMed] [Google Scholar]
- 33.Sheibanie AF, Tadmori I, Jing H, Vassiliou E, Ganea D. Prostaglandin E2 induces IL-23 production in bone marrow-derived dendritic cells. FASEB J. 2004;18:1318–1320. doi: 10.1096/fj.03-1367fje. [DOI] [PubMed] [Google Scholar]
- 34.Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Sttenhoff TH. Human IL-23-producing type1 macrophages promote but IL-10-producing type2 macrophages subvert immunity to (myco) bacteria. Proc Natl Acad Sci USA. 2004;101:4560–4565. doi: 10.1073/pnas.0400983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol. 2002;169:5673–5678. doi: 10.4049/jimmunol.169.10.5673. [DOI] [PubMed] [Google Scholar]
- 36.Lee E, Trepicchio WL, Oestreicher JL, Pittman D, Wang F, Chamian F, Dhodapkar M, Krueger JG. Increased expression of interleukin 23 p19 and p40 in lesional skin of patients with psoriasis vulgaris. J Exp Med. 2004;199:125–130. doi: 10.1084/jem.20030451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chua AO, Chizzonite R, Desai BB, Truitt TP, Nunes P, Minetti LT, Warrier RR, Presky DH, Levine JF, Gately MK, et al. Expression cloning of a human IL-12 receptor component: a new member of the cytokine receptor superfamily with strong homology to gp130. J Immunol. 1994;153:128–136. [PubMed] [Google Scholar]
- 38.Chua AO, Wilkinson VL, Presky DH, Gubler U. Cloning and characterization of a mouse IL-12 receptor-beta component. J Immunol. 1995;155:4286–4294. [PubMed] [Google Scholar]
- 39.Presky DH, Yang H, Minetti LJ, Chua AO, Nabavi N, Wu CY, Gately MK, Gubler U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc Natl Acad Sci USA. 1996;93:14002–14007. doi: 10.1073/pnas.93.24.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parham C, Chirica M, Timans J, Vaisberg E, Travis M, Cheung J, Pflanz S, Zhang R, Singh KP, Vega F, To W, Wagner J, O’Farrell AM, McClanahan T, Zurawski S, Hannum C, Gorman D, Rennick DM, Kastelein RA, de Waal Malefyt R, Moore KW. A receptor for the heterodimeric cytokine IL-23 composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J Immunol. 2002;168:5699–5708. doi: 10.4049/jimmunol.168.11.5699. [DOI] [PubMed] [Google Scholar]
- 41.Bellassonna ML, Renauld JC, Bianchi R, Vacca G, Fallarino F, Orabona C, Fioretti MC, Grohmann U, Puccetti P. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J Immunol. 2002;168:5448–5454. doi: 10.4049/jimmunol.168.11.5448. [DOI] [PubMed] [Google Scholar]
- 42.Bastos KRB, Marinho CRF, Barboza R, Russo M, Ălvarez JM, D’Império Lima MR. What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect. 2004;6:630–636. doi: 10.1016/j.micinf.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Cau DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 44.Wiekowski MT, Leach MW, Evans EW, Sullivanl L, Chen SC, Vassileva G, Bazan JF, Gorman DM, Kastelein RA, Narula S, Lira SA. Ubiquitous transgenic expression of the IL-23 subunit p19 induces multiorgan inflammation, runting, infertility and premature death. J Immunol. 2001;166:7563–7570. doi: 10.4049/jimmunol.166.12.7563. [DOI] [PubMed] [Google Scholar]
- 45.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of Interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 46.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 47.Lo CH, Lee SC, Wu PY, Pan WY, Su J, Cheng CW, Roffler SR, Chiang BL, Lee CN, Wu CW, Tao MH. Antitumour and antimetastatic activity of IL-23. J Immunol. 2003;171:600–607. doi: 10.4049/jimmunol.171.2.600. [DOI] [PubMed] [Google Scholar]
- 48.Wang YQ, Ugai S, Shimozato O, Yu L, Kawamura K, Yamamoto H, Yamaguchi T, Saisho H, Tagawa M. Induction of systemic immunity by expression of interleukin-23 in murine colon carcinoma cells. Int J Cancer. 2003;105:820–824. doi: 10.1002/ijc.11160. [DOI] [PubMed] [Google Scholar]
- 49.Ugai S, Shimozato S, Yu L, Wang YQ, Kawamura K, Yamamoto H, Yamaguchi T, Saisho H, Sakiyama S, Tagawa M. Transduction of the IL-21 and IL-23 genes in human pancreatic carcinoma cells produces natural killer cell-dependent and -independent antitumour effects. Cancer Gene Ther. 2003;10:771–778. doi: 10.1038/sj.cgt.7700630. [DOI] [PubMed] [Google Scholar]
- 50.Shan BE, Yu L, Shimozato O, Li QX, Tagawa M. Expression of interleukin-21 and -23 in human esophageal tumours produced antitumour effects in nude mice. Anticancer Res. 2004;24:79–82. [PubMed] [Google Scholar]
- 51.Liebau C, Rosesl C, Schmidf S, Karreman C, Prisack JB, Bojar H, Merk H, Wolfram N, Baltzer AW. Immunotherapy by gene transfer with plasmids encoding IL-12/IL18 is superior to IL-23/IL-18 gene transfer in a rat osteosarcoma model. Anticancer Res. 2004;24:2861–2867. [PubMed] [Google Scholar]