Abstract
Background
E75, a HER2/neu immunogenic peptide, is expressed in breast cancer (BCa). We have performed clinical trials of E75 + GM-CSF vaccine in disease-free, node-positive and node-negative BCa patients at high recurrence risk and recurrences were noted in both control and vaccine groups.
Methods
Among the 186 BCa patients enrolled, 177 completed the study. Patients were HLA typed; the HLA-A2+/A3+ patients were vaccinated; HLA-A2−/A3− patients were followed as controls. Standard clinicopathological factors, immunologic response to the vaccine, and recurrences were collected and assessed.
Results
The control group recurrence rate was 14.8 and 8.3% in the vaccinated group (P = 0.17). Comparing the 8 vaccinated recurrences (V-R) to the 88 vaccinated nonrecurrent patients (V-NR), the V-R group had higher nodal stage (≥N2: 75 vs. 5%, P = 0.0001) and higher grade tumors (%grade 3: 88 vs. 31%, P = 0.003). The V-R group did not fail to respond immunologically as noted by equivalent dimer responses and post-DTH responses. Compared to control recurrent patients (C-R), V-R patients trended toward higher-grade tumors and hormone-receptor negativity. C-R patients had 50% bone-only recurrences, compared to V-R patients with no bone-only recurrences (P = 0.05). Lastly, V-R mortality rate was 12.5% compared with 41.7% for the C-R group (P = 0.3).
Conclusions
The vaccinated patients who recurred had more aggressive disease compared to V-NR patients. V-R patients had no difference in immune response to the vaccine either in vitro or in vivo. V-R patients, when compared to C-R patients, trended towards more aggressive disease, decreased recurrence rates, decreased mortality, and no bone-only recurrences.
Keywords: Vaccine, E75 peptide, Preventive, Recurrence, Pathologic patterns, Immunologic response
Introduction
Some cancer vaccines target tumor-associated antigens (TAA), which are proteins expressed by tumors capable of eliciting a specific immune response that may be protective against recurrence of disease. One of the most investigated TAA in breast cancer (BCa) is the HER2/neu protein [1]. HER2/neu is a known prognostic factor in BCa and is the target for trastuzumab (Herceptin®). The mechanism of action of this monoclonal antibody is uncertain, but our preclinical data shows trastuzumab saturates HER2/neu receptors and promotes internalization increasing sensitivity of HER2/neu-expressing tumor cells to lysis by vaccine specific T cells [2]. Data from others suggest that trastuzumab mediates an antibody-dependent cellular cytotoxicity against cancer cells over-expressing HER2/neu [3]. The use of trastuzumab has been shown to decrease recurrence rates when administered as an adjuvant in combination with chemotherapy compared to chemotherapy alone, and is now current standard of care for patients with node-positive or metastatic breast cancer which overexpress HER2/neu (meaning IHC 3+ or FISH ≥ 2.0) [4, 5].
Cancer vaccines targeting TAA are not a new idea but in the past have focused on treating patients with metastatic disease and therefore their preventive capabilities have not been fully explored. One vaccination strategy is to use immunogenic peptides from the TAA to engender and/or boost pre-existing immune responses against the tumor cells. E75 (KIFGSLAFL, HER2/neu, 369–377) is an immunodominant peptide from the HER2/neu protein and is the most extensively studied peptide from this TAA. This HLA-A2 binding nonapeptide is recognized by cytotoxic T lymphocytes (CTL) [6]. E75 has been used in multiple clinical trials as an anticancer vaccine in various forms to include: single peptide vaccine combined with different immunoadjuvants [7–9]; loaded onto autologous dendritic cells and reinfused [10]; or embedded in longer peptides capable of binding HLA class II molecules in order to recruit CD4 helper T cells [11, 12]. Each approach listed above has been shown to be safe and effective at stimulating E75-specific immunity. In our lab, we have combined the E75 peptide with immunoadjuvant, granulocyte macrophage-colony stimulating factor (GM-CSF) and tested its ability to prevent recurrence in maximally treated, disease-free BCa patients.
The first of our E75 clinical trials involved immunocompetent, disease-free, node-positive (NP) BCa patients who express some level of HER2/neu (IHC 1+–3+ or FISH > 0). After completion of the standard of care therapies (surgery, chemotherapy and radiation as clinically indicated), the patients were enrolled and HLA typed. HLA-A2+/A3+ patients were vaccinated and HLA-A2−/A3− patients were followed as prospective controls [13]. The study was designed as a two-stage safety trial with escalating doses of peptide in the initial stage and alterations of schedule in the latter stage. The vaccine was found to be safe and induced reproducible HER2/neu-specific immune responses. Notably, the induced HER2/neu immunity appeared to reduce the recurrence rate in patients with NP BCa.
A second trial with immunocompetent, disease-free, high-risk node-negative (NN) BCa patients was performed. Just as in the NP trial, patients were enrolled after completion of standard therapies and HLA typed. Of note, a difference in the NN trial was that five control (5/35) and seven vaccinated (7/51) patients had non-HER2/neu-expressing tumors (IHC 0). This was done in order to determine the feasibility of vaccinating a presumably antigen-naive host for future true prevention trials. Other than these cumulative 12 patients, the remainder of patients had some level of HER2/neu expression (IHC 1+–3+ or FISH > 0). The NN trial was designed to further delineate optimal biologic dose (OBD). As in the NP trial, the NN trial demonstrated the vaccine to be safe and capable of inducing an immune response at a variety of doses and an OBD was determined.
We have recently reported the combined safety and efficacy results of the combined NP and NN trials [14, 15]. These trials have allowed us to develop an optimal dosing regimen, but more importantly, the combined results demonstrate that the E75 vaccine significantly reduced the recurrence rate in vaccinated patients compared to the prospective controls at a median of 20 months follow-up. However, without booster inoculations, the statistical significance was lost at 26 months median follow-up, and the question remains how best to maintain long-term immunity and protection against BCa recurrence with a CTL epitope vaccine.
Taken together, our NP and NN clinical trials with the E75 peptide vaccine represent one of the largest BCa prevention immunotherapeutic trials performed thus far. Here, we report a detailed analysis of the patients that recurred in both arms of the trials assessing their clinicopathologic prognostic factors, immune responses, and patterns of recurrence.
Materials and methods
Patient characteristics and clinical protocol
The NP and NN trials were approved by the Institutional Review Boards and conducted at Walter Reed Army Medical Center (WRAMC), Washington, DC and the Joyce Murtha Breast Care Center, Windber, PA under an investigational new drug application (BB-IND#9187). All patients had histologically confirmed BCa, and had completed a standard course of surgery, chemotherapy, and radiation therapy (as required) before enrollment. Patients on hormonal therapy were continued on their specific regimen. All patients expressed varying levels of HER2/neu, except 12 patients in the NN trial who had HER2/neu negative tumors (IHC 0). After proper counseling and consenting, the patients were enrolled and HLA typed. The HLA-A2+ patients were vaccinated as E75 binds primarily HLA-A2+ and the HLA-A2-patients were unvaccinated controls. Since 40–50% of the general population is HLA-A2+, we expected equivalent numbers of patients in each group [16]. Subsequently, HLA-A3+ patients (6 NP patients, 5 NN patients) were vaccinated as well, based on two observations. First binding affinity data from two commonly used HLA-peptide binding algorithms: BIMAS (http://bimas.dcrt.nih.gov/molbio/hla_bind/) and SYFPEITHI (http://www.syfpeithi.de/) [17, 18]. Second, our own pre-clinical evaluation demonstrated that E75-stimulated HLA-A3+ CTL could lyse HLA-A3+ HER2/neu-expressing cancer cells [19].
Vaccine
The E75 peptide was commercially produced in good manufacturing practices grade by NeoMPS, Inc. (San Diego, CA). Peptide purity (>95%) was verified by high-performance liquid chromatography and mass spectrometry, and the amino acid content was determined by amino acid analysis. Lyophilized peptide was reconstituted in sterile saline at 100, 500, or 1,000 μg in 0.5 ml. This peptide was mixed with GM-CSF (Berlex, Seattle, WA) in 0.5 ml, and the 1.0 ml inoculation was split and given intradermally at two sites 5 cm apart. All inoculations were given in the same extremity.
Vaccination series
The NP trial was designed as a two-stage safety trial with escalating doses of peptide in the initial stage and alterations of the schedule in the latter stage. Details of the vaccine series have been previously published [13, 14]. Briefly, three to six patients were each assigned to receive four or six monthly injections of 100, 500, or 1,000 μg of E75 peptide mixed with 0.5 mL of GM-CSF. Groups were ultimately expanded in order to determine and confirm optimal dosing in NP patients, accounting for the larger number of patients in the latter dose groups.
The NN trial was designed to further delineate the OBD by varying the dose of GM-CSF and altering the inoculation schedule. A total of 12 patients with non-HER2/neu-expressing tumors (IHC 0) were allowed in this trial to determine the feasibility of vaccinating a presumably antigen-naive host. Ten patients were assigned to each dose group to receive three, four or six monthly injections of constant peptide dose of 500 μg, and varying doses of 125 or 250 μg of GM-CSF.
Toxicity
Patients were observed 1 h post-vaccination for immediate hypersensitivity and returned 48–72 h later to have their injection sites measured and questioned about toxicities. Toxicities were graded by the NCI Common Terminology Criteria for Adverse Events, v3.0 and reported on a 0–5 scale. Progression from one dose group to the next occurred only if no significant toxicity occurred in the lower dose group. Patient-specific results are reported based on maximal local and systemic toxicity occurring during the series.
Peripheral blood mononuclear cell isolation and cultures
Blood was drawn before each vaccination and at one (post-vaccine) and six months (long-term) after vaccine series completion. Fifty ml of blood was drawn and Peripheral blood mononuclear cell (PBMC) isolated. PBMC were washed and re-suspended in culture medium and used as a source of lymphocytes as previously described [13, 14].
HLA-A2:immunoglobulin dimer assay
The presence of CD8+ E75-specific T cells in freshly isolated PBMC from patients was directly assessed by using the dimer assay as previously described [20]. Briefly, the HLA-A2:immunoglobulin (Ig) dimer (PharMingen, San Diego, CA) was loaded with the E75 or control peptide (E37, folate binding protein (25–33) RIAWARTEL) by incubating 1 μg of dimer with an excess (5 μg) of peptide and 0.5 μg of β2-microglobulin (Sigma, St. Louis, MO) at 37°C overnight then stored at 4°C until used. PBMC was washed and re-suspended in PharMingen Stain Buffer (PharMingen) and added at 5 × 105 cells/100 μl per tube in 5 ml round-bottom polystyrene tubes (Becton Dickinson, Mountain View, CA) and stained with the loaded dimers and antibodies. In each patient the level of CD8+ E75-specific T cells was determined in response to each successive vaccination, and average post-inoculation levels were compared to their pre-inoculation level.
Delayed type hypersensitivity
In both trials, a delayed type hypersensitivity (DTH) reaction was assessed with 100 μg of E75 in 0.5 ml of normal saline (without GM-CSF) and 0.5 ml normal saline as a volume control 1 month after completion of the vaccine series as described previously. The DTH reaction was measured in two dimensions at 48–72 h by using the sensitive ballpoint-pen method and reported as the orthogonal mean and compared to control [21]. Patients in the NN group also had DTH testing prior to vaccination.
Clinical recurrences
All patients were observed for clinical recurrence per standard of care cancer screening as dictated by the patient’s primary oncologist. A patient was considered recurrent if biopsy proven or if treated for recurrence by the primary oncology team.
Statistical analysis
Recurrence rates were compared between groups using survival analysis by the Kaplan–Meier method, and the proportion of subjects who had recurrences were compared using log-ranked analysis. P values for clinicopathologic factors were calculated using Wilcoxon, Fisher’s exact test or χ2 as appropriate. P values for comparing pre-vaccine and post-vaccine dimer levels were calculated using Wilcoxon and for DTH using unpaired, two-tailed Student’s t test.
Results
Recurrence rates and clinicopathological prognostic factors of patients in the NP and NN trials
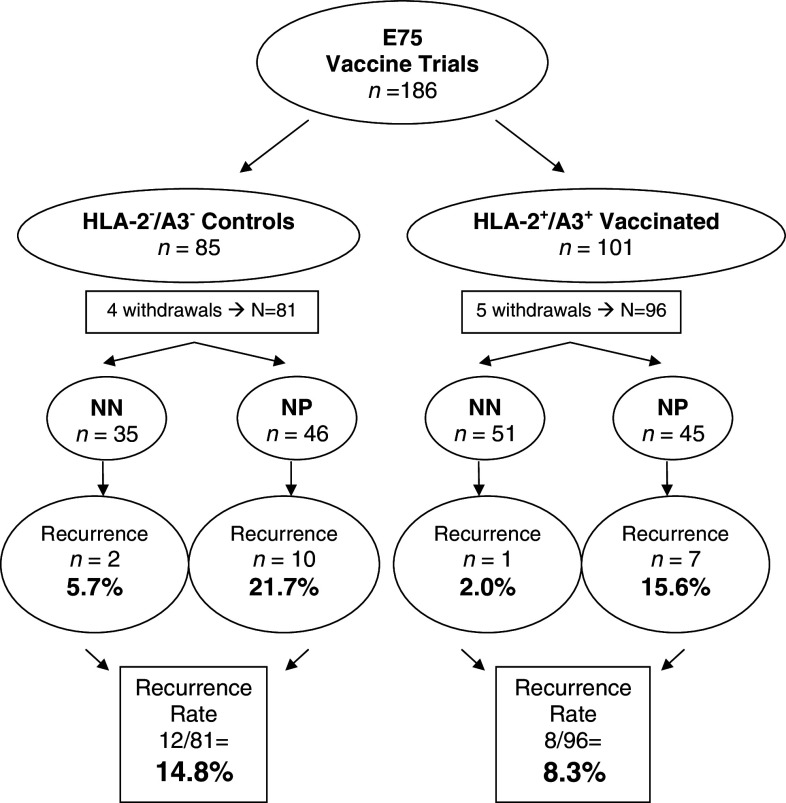
To better understand why vaccinated patients experienced BCa disease recurrence, we analyzed the number of patients with a disease recurrence in the vaccinated and control (non-vaccinated) groups. Totally 186 patients were enrolled in the two studies; 9 withdrew (4 control patients and 5 vaccinated patients—none withdrew due to toxicity) resulting in 177 completing the trials. There were 96 patients receiving the E75 vaccine (45 NP and 51 NN patients) and 81 controls (46 NP and 35 NN patients). The E75 vaccinated group had eight recurrences (7 NP and 1 NN patients). The control group had 12 recurrences (10 NP and 2 NN patients). Overall, the recurrence rate for E75 vaccinated patients at a median of 26 months was 8.3% and the control group recurrence rate was 14.8% (P = 0.17) (Fig. 1).
Fig. 1.
Diagram of all patients in trial (n = 186). Overall, enrollment withdrawals and recurrences are per vaccinated and control groups. Disease-free breast cancer patients were HLA typed; excluding withdrawals HLA-A2−/A3− patients were followed prospectively as controls (n = 81), and HLA-A2+/A3+ patients were vaccinated (n = 96). Recurrence rate in control and vaccinated groups were 14.8 and 8.3%, respectively (P = 0.17)
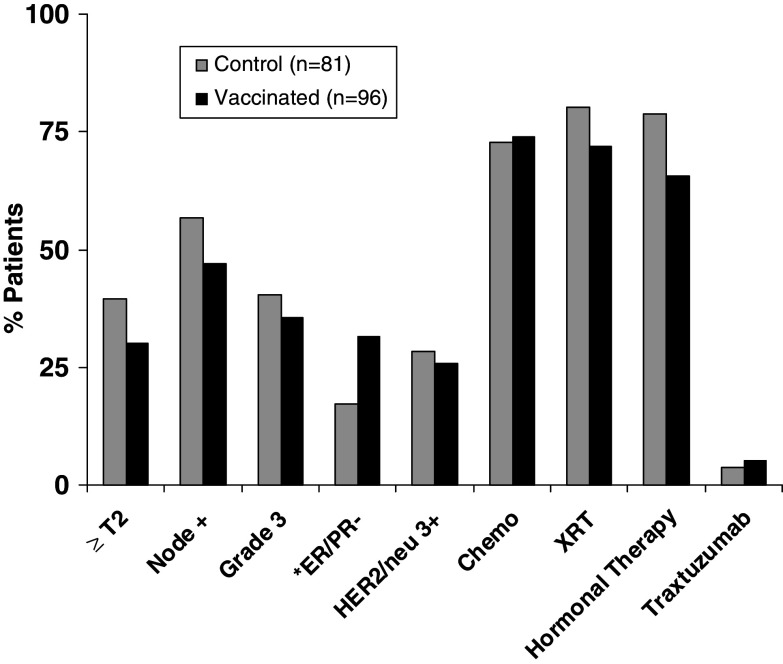
Due to the similarities of these parallel trials using the same vaccine in disease-free BCa patients, we combined the NP and NN results for an overall analysis of the safety and effectiveness of the E75 vaccine. To validate the comparison of the combined control arms to the vaccinated arms, we analyzed clinicopathological factors of the two groups. Clinically relevant prognostic factors assessed include tumor size, nodal status, grade, hormone-receptor status, and degree of HER2/neu expression (Fig. 2). Control and vaccinated patients had similar prognostic and treatment factors, except a higher percentage of vaccinated patients had hormone-receptor negative tumors (32 vs. 17%, P = 0.03). We also evaluated the treatment profile of our patients in terms of receiving chemotherapy, radiation therapy, hormonal therapy, and/or Trastuzumab (Fig. 2). Since fewer patients in the vaccine group had hormone-receptor positive tumors, fewer vaccinated patients received adjuvant hormonal therapy, known to reduce the risk of recurrence [15].
Fig. 2.
Clinicopathological prognostic factors and treatment profiles of all patients completing the study (n = 177). Control patients (n = 81) were compared to vaccinated patients (n = 96) for known pathologic prognostic factors and treatment related factors. The asterisk denotes statistical significant number of patients in the vaccinated group were ER/PR negative compared to the control group (32 vs. 17%; P = 0.03)
Clinicopathological features in vaccinated patients who recurred
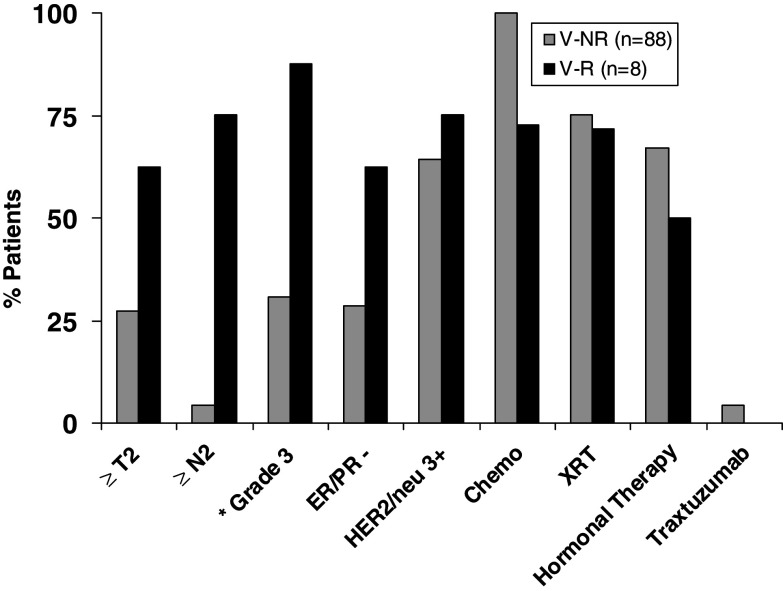
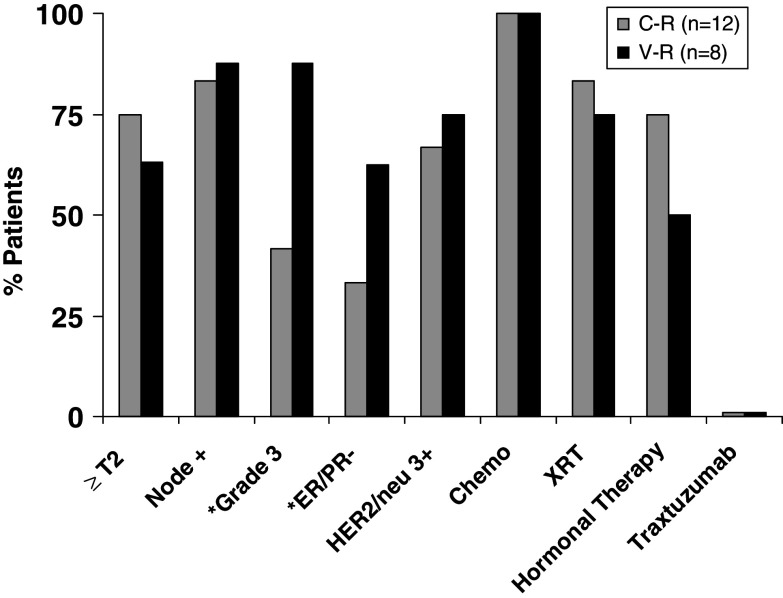
In order to confirm the impact of disease aggressiveness on recurrence, we analyzed the clinicopathological factors in the vaccinated patients and compared the results between the recurrent (V-R) and nonrecurrent (V-NR) vaccinated groups of patients (Fig. 3). As expected, the recurrent group had worse prognostic factors with higher nodal stage (% ≥ N2 = 75 vs. 5%, P = 0.0001) and higher grade tumors (% grade 3 = 88 vs. 31%, P = 0.003) compared to the V-NR group. Additionally, the V-R group showed a trend toward larger tumors (% ≥ T2 = 62.5 vs. 27.3%, P = 0.09) and hormone-receptor negativity (% ER/PR− = 62.5 vs. 27.7%, P = 0.11) compared to the V-NR group.
Fig. 3.
Clinicopathological prognostic factors and treatment profiles of all vaccinated patients (n = 96). Vaccinated patients who recurred (V-R) (n = 8) were compared to vaccinated patients who did not recur (V-NR). The asterisk denotes statistically significant differences between V-NR and V-R groups
Immunologic response to vaccination in patients who recurred
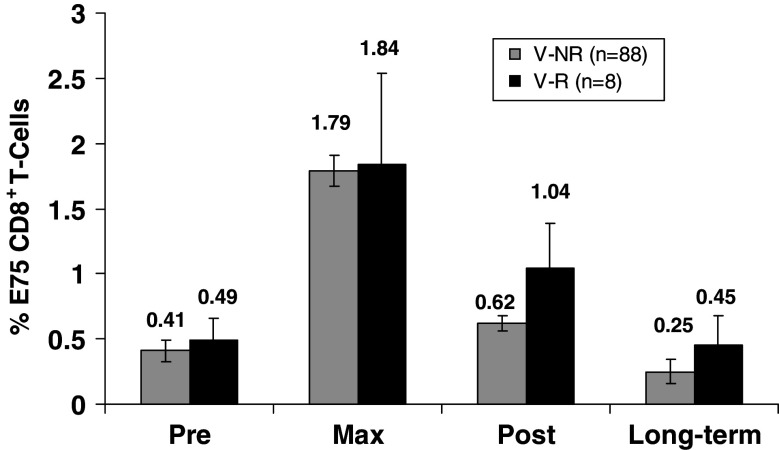
We analyzed the V-R verses V-NR groups to assess whether or not failure to respond to the vaccine contributed to recurrence. E75-specific CD8+ T cells were assessed and the results are presented as the mean dimer response pre-vaccination (pre), maximum dimer response (max), post completion of the series (post), and 6 months after last vaccine (long-term) (Fig. 4). The assessment revealed a generalized increase in the percentage of E75-specific CD8+ T cells during the vaccination series which then decreased to a plateau at the completion of the series. The results show no statistical difference in the in vitro immune response between the V-R and V-NR patients, but there is a trend that patients with recurrent disease had an increased post and long-term in vitro immune response.
Fig. 4.
All vaccinated patients’ in vitro immune responses during vaccination course. E75-specific CD8+ T cells were measured pre-vaccination (pre), maximum dimer response (max), completion of series (post), and 6 months after last vaccine (long-term), using HLA-A2:Ig dimer assay and results are reported as a percentage of all CD8+ T cells. Results are expressed as mean ± standard errors. There were no statistical differences in the dimer assays during any point between the V-R and V-NR patients
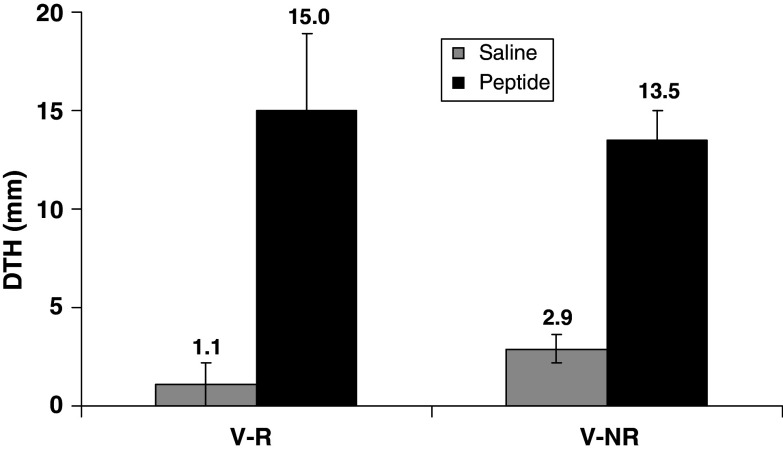
Response to vaccine was also assessed in vivo by DTH analysis at 1 month after completion of the vaccination series. The DTH measurements show no difference in the in vivo response between V-R and V-NR patients (15.0 ± 3.9 vs. 13.5 ± 1.5, P = 0.7) (Fig. 5).
Fig. 5.
All vaccinated patients’ in vivo immune responses during vaccination course. DTH depicted as mean orthogonal diameter in millimeters measured 1 month after completion of vaccination series. Results are expressed as mean ± standard errors. There was no statistical difference between the V-R and V-NR DTH measurements
Assessment of vaccine effects on patients who recurred
Since the V-R patients responded to the vaccine as measured by in vitro and in vivo responses, we compared the V-R patients to those patients who recurred in the control group (C-R) to evaluate for any impact of the vaccine on recurrences. First we compared standard clinicopathological risk factors and we found no statistically significant differences between these groups; however, the V-R patients trended toward higher-grade tumors (% grade 3 = 88 vs. 42%; P = 0.07) and the V-R patients were more hormonally insensitive (%ER/PR− = 63 vs. 33%, P = 0.10) (Fig. 6). These trends suggest that less aggressive tumors may have been prevented in the V-R patients thus accounting for the differences between the groups’ recurrence rates.
Fig. 6.
Clinicopathological prognostic factors and treatment profiles of patients with BCa recurrence (n = 20). The recurrences in the control group (C-R) (n = 12) were compared with the vaccinated patients that recurred (V-R) (n = 8). The asterisk denotes values trending towards statistical significance
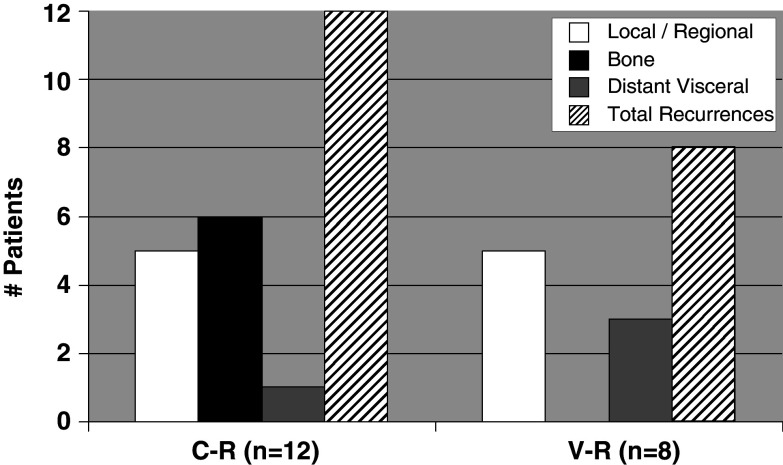
More interesting and convincing of a vaccine effect is the assessment of disease recurrence patterns. We compared the location of recurrences in the V-R and C-R groups. While the rates of loco-regional and visceral metastases were similar between the groups, the C-R patients had 50% bone-only recurrences (6/12), whereas the V-R had no bone-only recurrences (0/8) (P = 0.05) (Fig. 7).
Fig. 7.
Location of recurrences in control (C-R) (n = 12) and vaccinated groups (V-R) (n = 8). No bone-only recurrences were observed in the V-R patients. The asterisks denote statistical significance
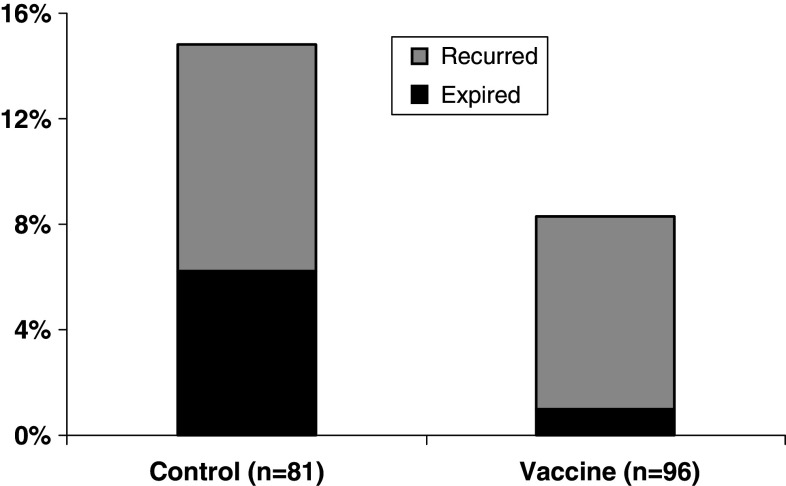
Finally, we assessed the incidence of recurrence and mortality of patients with recurrence, and the vaccinated group trended towards decreased recurrence rates, mortality per recurrence and overall mortality. As previously noted, the recurrence rate in the vaccinated patients was 8.3% (8/96) compared to the control patients with 14.8% (12/81); this difference was not statistically significant (P = 0.17). The overall mortality rate among control patients was 6.2% (5/81) compared to only 1.0% (1/96) (P = 0.1) in the vaccinated group. Among recurrent patients, the V-R and C-R groups mortality rate was 41.7 (5/12) and 12.5% (1/8), respectively (P = 0.3) (Fig. 8).
Fig. 8.
Recurrence and survival rates for all patients completing the trial (n = 177). The control and vaccinated groups had recurrence rates of 14.8 and 8.3%, respectively (P = 0.17) and mortality rates of 6.2 and 1.0%, respectively (P = 0.1). In the recurrent patients, the mortality rate for the control and vaccinated groups were 41.7 and 12.5%, respectively; P = 0.3)
Discussion
In our clinical trials, the E75 peptide vaccine statistically reduced BCa recurrences in patients with a history of NP and NN BCa at 20 months of median follow-up. After this point in the trial and without boosters, the statistical significance was lost (as was immunity), but the trend continued towards prevention of BCa recurrence [13, 14]. In this study, we have analyzed the vaccinated patients who did or did not have a recurrence of disease along with control patients who recurred. Based upon the assessment of a total of 177 patients, we have shown that V-R patients had more aggressive disease compared with V-NR patients when using known clinicopathologic prognostic and treatment factors. Importantly, the V-R patients did not fail to immunologically respond to the vaccine. In fact, it would appear that despite the presence of recurrence, the vaccine impacted the distribution of disease and perhaps even improved survival rates.
As expected the V-R group clearly had more aggressive disease when compared to the V-NR group since tumor biology will drive the nature of the disease despite treatment received [22–25]. Of much more interest to our vaccine program is whether patients recurred due to a failure to respond to the vaccine. In previous large vaccine studies like the Theratope® and Canvaxin™ trials, there have been suggestions that the reason vaccinated patients recurred was due to a failure of those vaccines to induce a meaningful immune response in those patients. Theratope® is a vaccine developed against Sialyl-Tn (STn), which is a carbohydrate associated with mucin-1 and is expressed on the cell surface of human cancer cells, to include BCa. Phase I–III trials of this vaccine have shown the vaccine to be safe and capable of generating an immune response in patients with metastatic BCa (therapeutic vaccine). There has been some concern that the vaccination has not been beneficial to all patients but subset analysis has shown that varying response rates may be due to lack of immune response. The current phase III Theratope® and hormone therapy breast cancer trial demonstrated a survival benefit in patients who could mount an adequate antibody immune response to STn [26]. CancerVax which makes Canvaxin™, a whole-cell vaccine prepared from three allogenic melanoma cell lines, is another well known cancer vaccine being studied for metatstatic melanoma that has shown promise in phase III trials (therapeutic vaccine) [27]. Overall both of these trials were negative, but subset analysis has shown that patients who immunologically responded to the respective vaccines had improved outcomes.
It is important to note that contrary to the results in the Theratope® and Canvaxin™ trials, our data shows no statistical difference in the in vitro or in vivo immune responses between the V-R and V-NR patients. The immune response to the E75 peptide vaccine was measured both in vitro with a phenotypic assay to detect peptide-specific clonal expansion, and in vivo with clinical DTH reactions [28, 29]. Both of these assays showed a positive induction of E75-specific immune responses in all of the vaccinated patients. Therefore, the recurrences in the eight patients cannot be accounted for based on a failure to respond to the vaccine. Of note, the V-R patients trended towards improved post and long-term in vitro immune responses. The later were measured prior to V-R patients recurring and may be the result of immune processing of occult tumor cells in these patients who would subsequently recur.
The E75 peptide is a MHC class I vaccine and stimulates CD8+ T cells, which are often not capable of sustaining a prolonged memory immune response in the absence of continued antigen exposure and stimulation by antigen-presenting cells. The concern then becomes over time that the immune response to the vaccine may decline, leading to decreased effectiveness of the vaccine and an increased risk of recurrence. When assessing long-term immune responses, a waning of the levels of E75-specific CD8+ T cells was noted and for this reason we are now performing booster vaccinations and are assessing the prolonged immune response and recurrence rates.
Since it would appear that the vaccinated recurrent patients did respond to the vaccine, then it is important to know if the vaccine impacted their disease. Comparing the V-R and C-R patients, it is interesting to note that there is a trend towards higher grade tumors and hormone-receptor negativity in the vaccinated patients. These differences suggest that the vaccine may be impacting and thus preventing some less aggressive tumors from recurring. More likely, the vaccine may particularly limit the recurrences among patients with less aggressive disease. Expanding upon this line of investigation, we analyzed the location of BCa recurrence and found the results to be statistically significant in regards to the site of recurrence. In the V-R group, there were no patients with bone as the site of their initial recurrence, while bone-only disease was seen in half of the control patients. Bone is the most common site of breast cancer metastasis with 35–50% of initial recurrences being bone-only recurrences as seen in our control group [30]. Many studies have suggested that patients with bone as the sole site of metastatic disease having better prognosis with median survival from diagnosis of 35 months compared with 11–26 months for visceral recurrence [31]. The proposed mechanism of osteolytic bone metastasis has been recently reported to be due to the interaction of GM-CSF with nuclear factor-κB (NF-κB) as a key target leading to stimulation of osteoclast development. In that study, it was found that the expression of GM-CSF correlated with NF-κB activation in bone-metastatic tumors in patients with breast cancer [32]. However, in our study, we used GM-CSF as an immunoadjuvant with all of our vaccinations, and there were no bone-only recurrences in our vaccinated patients. This leads to the consideration that the vaccination may somehow alter the immune process leading to fewer recurrences in more indolent disease, or patients who would have gone on to have bone as their site of metastasis.
Another possibility is that the vaccine could alter the tumor recurrence site via the modulation of memory CD8+ T cells. It has recently been demonstrated that antigen specific CD8+ T cells localize in the bone marrow and persist for several months after immunization [33]. In the study by Di Rosa and Santoni, the bone marrow had a larger number of CD8+ T cells than both lymph nodes and spleen. In addition the CD8+ T cells in the bone marrow had a more prompt response to antigen than splenic CD8+ T cells, suggesting a higher state of activation in the bone marrow. So, while bone may be a favored site for metastatic recurrences, it may also be a site for maintaining immunologic memory against cancer cells. Vaccination may increase the presence of anti-tumor CD8+ T cells in the bone marrow, leading to cell-mediated killing of micro-metastatic disease.
In addition to a different recurrence pattern, we observed a difference in the survival rates between the two arms with 99.0% overall survival (OS) in the vaccinated group compared to 93.8% OS in the controls. Of the patients who do recur there is an 88% survival rate in the vaccinated group verses a 58% survival rate in the control group. The vaccine appears to improve both OS and survival rates in patients who have recurred.
Conclusion
In conclusion, our study demonstrates that the HER2/neu (E75) vaccine may lead to a decrease in the recurrence rate. Our study shows that recurrence is not due to lack of immune response to the vaccine. We also found that while prognostic factors continue to drive recurrences, induced HER2/neu immunity appears to alter recurrence and overall survival. Importantly, compared with the controls, it appears that the vaccine may limit the recurrences among patients with less aggressive disease and may alter the distribution of disease with no bone marrow metastasis. Further studies, including a multi-center, randomized, prospective phase III clinical trial are upcoming to validate the true clinical benefit of this vaccine and at the same time we will continue to optimize the vaccine regimen to promote prolonged protective immunity.
Acknowledgments
The authors thank Ms. Diane Papay and Ms. Maureen Pavlik who provided outstanding patient care and administration of the clinical trial. Also, authors thank Ms. Jennifer Pappas, CVDP Chief Regulatory Affairs Officer, for her assistance with protocols and editing.
Footnotes
Supported by the United States Military Cancer Institute and the Department of Clinical Investigation at Walter Reed Army Medical Center. Funded primarily by the Clinical Breast Care Project. The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of the Army, the Department of the Navy, or the Department of Defense. This work represents original research that has not been submitted elsewhere for publication.
References
- 1.Emens LA, Reilly RT, Jaffee EM. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr Relat Cancer. 2005;12:1–17. doi: 10.1677/erc.1.00671. [DOI] [PubMed] [Google Scholar]
- 2.Mittendorf EA, Storrer CE, Shriver CD, et al. Investigating the combination of trastuzumab and HER2/neu peptide vaccines for the treatment of breast cancer. Ann Surg Oncol. 2006;13:1085–1098. doi: 10.1245/ASO.2006.03.069. [DOI] [PubMed] [Google Scholar]
- 3.Iannelo A, Ahmad A. Role of antibody-dependent cell-mediated cytotoxicty in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev. 2005;24:487–499. doi: 10.1007/s10555-005-6192-2. [DOI] [PubMed] [Google Scholar]
- 4.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 5.Eh Romond, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 6.Fisk B, Blevins TL, Wharton JT, et al. Identification of immunodominant peptide of the HER2/neu proto-oncogene recognized by ovarian tumor-specific CTL lines. J Exp Med. 1995;181:2109–2117. doi: 10.1084/jem.181.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]
- 8.Knutson KL, Schiffman K, Cheever MA, et al. Immunization of cancer patients with HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 9.Murray JL, Gillogly ME, Przepiorka D, et al. Toxicity, immunogenicity, and induction of E75-specific tumorlytic CTLs by HER-2 peptide E74 (369–377) combined with granulocyte macrophage colony-stimulating factor in HLA-A2 + patients with metastatic breast and ovarian cancer. Clin Cancer Res. 2002;8:3407–3418. [PubMed] [Google Scholar]
- 10.Avigan D, Vasir B, Gong J, et al. Fusion cell vaccination of patients with metastatic breast and renal cell cancer induces immunological responses. Clin Cancer Res. 2004;10:4699–4708. doi: 10.1158/1078-0432.CCR-04-0347. [DOI] [PubMed] [Google Scholar]
- 11.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER2/neu protein after active immunization with HER2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 12.Disis ML, Grabstein KH, Sleath PR, et al. Generation of immunity to the HER-2/neu oncogenic protein in patients with breast and ovarian cancer using a peptide-based vaccine. Clin Cancer Res. 1999;5:1289–1297. [PubMed] [Google Scholar]
- 13.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 14.Peoples GE, Holmes JP, Hueman MT, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 15.Peoples GE, Khoo S, Dehqanzada ZA, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for prevention of recurrence in high-risk breast cancer patients [abstract] Br Cancer Res Treat. 2006;100:3. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 16.Mori M, Beatty PG, Graves M, et al. HLA gene and haplotype frequencies in the North American population: the National Marrow Donor Registry 1. Transplantation. 1997;64:1017–1027. doi: 10.1097/00007890-199710150-00014. [DOI] [PubMed] [Google Scholar]
- 17.Parker KC, Bednarek MA, Coligan JE. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 18.Rammensee HG, Bachmann J, Emmerich NP, Bachor OA, Stevanovi S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics. 1999;50:213–219. doi: 10.1007/s002510050595. [DOI] [PubMed] [Google Scholar]
- 19.Patil R, Holmes JP, Amin A et al. (2007) Clinical and immunologic responses of HLA-A3+ breast cancer patients vaccinated with the HER2/neu-derived peptide vaccine, E75, in a phase I clinical trial [abstract]. Breast Cancer Res Treat [DOI] [PubMed]
- 20.Woll MM, Fisher CM, Ryan GB, et al. Direct measurement of peptide specific CD8+ T cells using HLA-A2:Ig dimer for monitoring the in vivo immune response to a HER2/neu vaccine in breast and prostate cancer patients. J Clin Immunol. 2004;24:449–461. doi: 10.1023/B:JOCI.0000029117.10791.98. [DOI] [PubMed] [Google Scholar]
- 21.Sokal JE. Measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 22.Kimura M, Yanagita Y, Fujisawa T, et al. Study of time-course changes in annual recurrence rates for breast cancer: data analysis of 2, 209 patients for 10 years post-surgery [abstract] Breast Cancer Res Treat. 2007;106(3):407–411. doi: 10.1007/s10549-007-9510-2. [DOI] [PubMed] [Google Scholar]
- 23.Saphner T, Tormey D, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 24.Nemoto T, Vana J, Ramez N, et al. Management and survival of female breast cancer: results of a national survey by the American College of Surgeons. Cancer. 1980;45:2917–2924. doi: 10.1002/1097-0142(19800615)45:12<2917::AID-CNCR2820451203>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 25.Ellston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 26.Holmberg LA, Sandmaier BM. Vaccination with Theratope® (STn-KLH) as treatment for breast cancer. Expert Rev Vaccines. 2004;3:655–663. doi: 10.1586/14760584.3.6.655. [DOI] [PubMed] [Google Scholar]
- 27.Faries MB. Evaluation of immunotherapy in the treatment of melanoma. Surg Oncol N Am. 2006;15:399–418. doi: 10.1016/j.soc.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Jordan T, Sunderam G, Thomas L, Reichman LB. Tuberculin reaction size measurements by the pen method compared to traditional palpation. Chest. 1987;92:234–236. doi: 10.1378/chest.92.2.234. [DOI] [PubMed] [Google Scholar]
- 29.Storrer CE, Hueman MT, Gurney JM, et al. Comparison of HLA-A2 dimer and tetramer molecules for the immunological monitoring of breast and prostate cancer vaccine clinical trials. Clin Invest Med. 2004;27:211D. [Google Scholar]
- 30.Patanaphan V, Salazar OM, Risco R. Breast cancer: metastatic patterns and their prognosis. South Med J. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 31.Jacobson AF, Shapiro CL, Van den Abbeele AD, Kaplan WD. Prognostic significance of the numbers of bone scan abnormalities at the time of initial bone metastatic recurrence in breast carcinoma. Cancer. 2001;91:17–24. doi: 10.1002/1097-0142(20010101)91:1<17::AID-CNCR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 32.Park BK, Zhang H, Zeng Q, et al. NF-κB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nature Med. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 33.Di Rosa F, Pabst R. The bone marrow: a nest for migratory memory T cells. Trends Immunol. 2005;26:360–366. doi: 10.1016/j.it.2005.04.011. [DOI] [PubMed] [Google Scholar]