Abstract
Background: Previous work from our center has suggested a correlation between increased donor-derived Vδ1+ γδ T cells and long-term relapse-free survival following bone marrow transplantation for leukemia. Questions remain, however, as to whether this observation can be explained by a γδ T cell-based immune response against primary leukemia. Methods: We examined γδ T cell receptor (TCR) phenotype, cell proliferation, and cytolytic activity following culture with irradiated primary leukemia blasts from a haploidentical first-degree relative. Subsequently, we also studied the γδ TCR phenotype and complimentarity determining region 3 (CDR3) cDNA sequences from 17 newly diagnosed leukemia patients. Results: In 17/28 (61%) of in vitro cultures, γδ T cells proliferated in culture with primary blasts. Vδ1+ T cells were proportionally increased in all cultures and were the predominant cell population in 6/17. In the 7 cultures where cytotoxicity could be assessed, 6 (86%) showed some degree of cytotoxicity to the primary leukemia. Vδ1+ T cells were also the predominant γδ T cell subtype in pre-treatment leukemia patients principally due to loss of Vδ2+ T cells rather than expansion of Vδ1+ cells. The Vδ1 CDR3-region cDNA sequence from these patients revealed exclusive use of the Jδ1 constant region and sequence conservation in 4/11 patients. Conclusions: γδ T cells exhibit an in vitro response to primary leukemia blasts that is manifested by proliferation, an increased proportion of Vδ1+ T cells, and cytotoxicity to the primary leukemia blasts. The Vδ1+ T cell population is also predominant in newly diagnosed leukemia patients likely due to a loss of circulating Vδ2+ T cells. A small proportion of newly diagnosed patients showed Vδ1 CDR3 region similarity. These findings suggest a role for γδ T cells in the immune response to leukemia.
Keywords: Laboratory Information Management System, Primary Blast, Cytoreductive Therapy, Donor Leukocyte Infusion, Primary Leukemia
Introduction
Significant advances have been made in the treatment of acute leukemia over the past 20 years. However, patients who fail standard chemotherapy/radiotherapy protocols do not usually have a successful outcome, even following allogeneic bone hematopoietic stem cell transplantation (HSCT) and cellular immunotherapy. This is largely because immunotherapy protocols currently in use are nonspecific and rely heavily on the development of an αβ+CD8+ cytotoxic T lymphocyte (CTL)-mediated adaptive immune response [1–6] as a result of donor leukocyte infusions. Although leukemia-reactive T cell clones have been isolated and leukemia-associated antigens have been identified [7–9], progress toward the therapeutic application of specific CTL therapy for primary leukemia has been generally disappointing.
Several in vitro studies have shown γδ T cells to be potent effectors against lymphoid and myeloid malignancies [10, 11]. Although γδ T cells share many of the cell surface proteins and effector capabilities as αβ T cells, the process of ligand recognition is very different. In particular, γδ T cells respond to immunogenic stimuli through less specific mechanisms that require no prior antigen exposure or priming [12–18]. Human γδ T cells normally do not recognize processed tumor-specific antigens in the context of the MHC, although they can recognize and respond to a variety of MHC class I-like stress-induced self-antigens commonly displayed by malignant cells and lyse these cells almost immediately upon encounter. While many ligands for γδ T cells remain unknown or uncharacterized, there are several known tumor-associated stimulatory ligands for γδ T cells that are differentially expressed on leukemias, lymphomas, and lymphoid cell lines including HSP-60 [19], CD30 [20], and MIC proteins A and B [21].
Our laboratory has previously shown a significant association between an increase in donor-derived γδ T cells following allogeneic bone marrow transplantation (BMT) and improved relapse-free survival [22, 23]. Preliminary work has also shown that allogeneic γδ T cells can lyse primary leukemia blasts in vitro [10]. Consistent with what is known about the mechanisms by which γδ T cells recognize various stimulatory ligands, these γδ T cells appear to respond to leukemia in a manner that is independent of a purely allogeneic response as they neither proliferate in response to nor do they lyse normal allogeneic mononuclear cells [24].
In this study, we have expanded our previous findings of in vitro allogeneic γδ T cell responses against primary leukemia and have investigated whether an autologous γδ T cell immune response is evident in pre-treatment leukemia patients.
Methods
Patient accrual and cell preparation
Pediatric and adult patients presenting with newly diagnosed ALL or AML were entered consecutively following informed consent. Blasts were extensively characterized by morphologic examination, flow cytometry, and cytogenetics. Peripheral blood was obtained and mononuclear cells (MNC) were separated on a density gradient and cryopreserved in AIM-V medium (Gibco Invitrogen; Grand Island, NY, USA) containing 10% DMSO and 20% pooled human serum (C-Six; Milwaukee, WI, USA).
Immunophenotyping
Whole peripheral blood aliquots of 100 μl were labeled with flourochromes-conjugated antibodies specific for T-lymphocytes (CD3, CD4, CD8, TCR-γδ, Vδ1–Vδ3), and prepared using a commercially available erythrocyte-lysis procedure. Acquisition and analysis were performed using a FACS Calibur flow cytometer and CellQuest Pro acquisition and analysis software (BD Biosciences, San Jose, CA, USA).
Mixed lymphocyte cultures
Primary leukemia blasts were obtained from patients prior to initiation of cytoreductive therapy, purified, and cryopreserved. Peripheral blood was also obtained from a haploidentical first degree relative, in most cases a parent with the exception of one unrelated matched (#4) and one sibling (#9). Mononuclear cells (MNC) were separated by density gradient centrifugation on Ficoll (Life Technologies; Rockville, MD, USA). The γδ T cells were enriched by immunomagnetic depletion of CD4+ and CD8+ T cells (Dynal; Oslo, Norway). Depletion of >90% αβ+ T cells was verified by flow cytometry. Irradiated blasts and CD4/CD8 depleted MNC were both adjusted to a concentration of 1×105/ml in AIM-V Lymphocyte Culture Medium (Life Technologies; Rockville, MD, USA) supplemented with 15% pooled human AB serum (C-Six Corporation). Aliquots of 1 ml each/well were placed in 24 well tissue culture plates and incubated at 37°C in 5% CO2. Controls consisted of CD4/CD8 depleted PBMNC alone, blasts alone, and CD4/CD8 depleted or T cell replete PBMNC cultured with third-party irradiated PBMNC. The cultures were refreshed with thawed blasts after 1 week. Cell proliferation was assessed daily and recorded weekly by visual inspection and automated cell counting (Beckman Coulter; Miami, FL, USA). Lymphocyte and T cell subsets were also assessed weekly. Every culture experiment was repeated in its entirety to verify the result.
Cytotoxicity assays
Freshly thawed primary leukemia blasts were labeled with the membrane dye PKH26 (Sigma; St. Louis, MO, USA) using the manufacturer’s instructions. The blasts were then placed in aliquots of 1×105 in 3 ml polypropylene tubes. Expanded γδ T cells specific from the procedure described above were then added to the blasts at ratios of 0:1 (Background), 3.125:1 6.25:1, 12:1, 25:1 and 50:1 effectors/blast targets. The tubes were then briefly centrifuged to pellet the cells and resuspended in 100 μl AIM-5 supplemented with 15% pooled human AB serum. The tubes were incubated for 4 h at 37°C and 5% CO2. The tubes were then washed ×1 in HBSS and resuspended in 1 ml HBSS. Propidium iodide solution (Sigma) 20 μl was added to each tube immediately prior to acquisition on the flow cytometer. Cytotoxicity was calculated as follows:
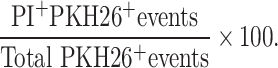 |
Polymerase chain reaction (PCR) amplification of γδ transcripts
Peripheral blood was obtained from patients newly diagnosed with leukemia prior to cytoreductive therapy. Total RNA was extracted from MNC using TrizolTM (Life Technologies). First-strand cDNA was synthesized using the Qiagen one-step RT-PCR kit with 0.2 mM of each dNTP, 25 pmol of 5′ Vδ1 (5′-TCTGGATACAAGTGTGGC-3′), Vδ2 (5′TCTGGGCAGGAGTCATGT 3′), and Vδ3 (5′ GCGAGTGGCAGTGAGGTG 3′) sense primers, 25 pmol of 3′ constant region gene antisense primer Cδ (5′-TTCACCAGACAAGCGACA-3′) and 2.5 U Hot Starttm Taq DNA polymerase (Qiagen; Valencia, CA, USA). Reaction mixtures were amplified for 35 cycles at 95°C for 1 min, 50°C for 1 min and 72°C for 1 min after an initial incubation at 95°C.
Cloning of PCR products
TA cloning was used as a standardized method (Invitrogen; Carlsbad, CA, USA). The cDNA described above was cloned into TOP10F′ cells using the pCR 2.1 vector, plated on LB agar with 1 μg/ml ampicillin and selected for by the β-galactosidase colony assay. Transformed colonies were expanded in 5 ml cultures and the plasmids purified using the Eppendorf PerfectPrepTM Plasmid Mini kit (Eppendorf; Westbury, NY, USA). Cloned CDR3 cDNA was amplified using the same primers to provide a pure template for sequencing.
High-throughput sequencing and data analysis
Vδ1 CDR3 region cDNA sequencing was performed by Agencourt Bioscience Corporation (Madison, WI, USA). Fosmid libraries were picked into 384-well format plates containing 2× LB culture media with glycerol. High copy plasmid shotgun clones were sequenced using BigDye Version 3.1 reactions on ABI3730xl instruments (Foster City, CA, USA). Thermal cycling was performed using 384-well thermal cycling blocks. Sequencing reactions were purified using Agencourt’s CleanSeq dye-terminator removal kit. Sample sheets for the sequencing instruments were automatically generated from the barcode-associated data, and the resulting sequence traces automatically uploaded and processed to identify vector sequence and other possible contaminants (e.g., E. coli, phage sequences, etc). The data were then subjected to quality clipping, processed using the Phred or KB base calling software, and the resulting files are stored in the Laboratory Information Management System (LIMS). All reads and associated data (library information, sample plates, project history, sequence traces, etc.) were tracked though an Oracle 9i driven LIMS. Every clone was tracked on an individual well-by-well basis throughout the storage, DNA prep, clean up, sequencing and rearray processes. Sequence acquisition, alignment, translation, and statistical analysis were performed using SeqMan IITM software (DNA Star; Madison, WI, USA).
Results
Primary blasts stimulate γδ T cell expansion
For these co-culture experiments, αβ-T cell depleted mononuclear cells from normal haploidentical donors were cultured as responders with irradiated primary leukemic blasts obtained prior to cytoreductive therapy as stimulators. A total of 28 patients and donors were accrued for this series of experiments (Table 1). In 17 of 28 cell cultures (61%) γδ T cells expanded between approximately 20 and 100-fold when cultured for 2 weeks with primary leukemic lymphoblasts. Expansion of other lymphocyte subsets was negligible, although a modest expansion of residual CD4+ T cells was noted in about one-third of the cultures. When present, CD4+ T cells did not appear to influence γδ T cell expansion or cytotoxicity relative to cultures in which no CD4+ T cell expansion occurred. Immunophenotypic analysis from these cultures revealed a proportional increase in Vδ1+ T cells (>10%) in all cultures, and Vδ1+ T cells were the predominant population in 6/17 (35%) of cultures. This is in contrast to most healthy volunteers, where the Vδ2+ population is predominant and Vδ1+ cells represent <10% of total γδ T cells. Expansion of γδ T cells was not observed in cultures established from 11 patients (35% of total). No obvious linkage between leukemia linage, phenotype, or cytogenetics and γδ expansion or phenotype was seen; however; there were an insufficient number of experiments for meaningful statistical analysis.
Table 1 .
Patient and culture characteristics
| Study # | Disease | Sex donor/recipient | HLA status | Cytogenetics | γδ expansion | %Vδ1 | %Vδ2 | %Vδ3 |
|---|---|---|---|---|---|---|---|---|
| 1 | ALL | F/F | Haplo | t(4:11) | N | NM* | NM | NM |
| 2 | AML | F/Ua | Haplo | Normal | N | NM | NM | NM |
| 3 | ALL | F/M | Haplo | Normal | N | NM | NM | NM |
| 4 | AML | M/M | MUD | ND* | N | 61 | 26 | 11 |
| 5 | ALL | M/M | Haplo | Ph+ tri8 | Y | 65 | 26 | 15 |
| 6 | AML | F/M | Haplo | Normal | Y | 38 | 60 | 2 |
| 7 | ALL | F/M | Haplo | Normal | Y | 87 | 13 | 0 |
| 9 | T-ALL | M/M | 1Ag M/M | Multiple | Y | 46 | 35 | 19 |
| 10 | ALL | F/M | Haplo | Multiple | Y | 91 | 4 | 3 |
| 12 | ALL | M/F | Haplo | Ph+ | Y | 33 | 78 | 0 |
| 14 | T-ALL | F/M | Haplo | ND | N | 52 | 29 | 20 |
| 17 | AML | F/F | Haplo | Ph+ | Y | 46 | 52 | 3 |
| 19 | APL | F/F | Haplo | ND | Y | 20 | 70 | 4 |
| 22 | ALL | F/M | Haplo | Normal | N | NM | NM | NM |
| 23 | ALL | M/F | Haplo | Hyperdiploid | N | NM | NM | NM |
| 24 | ALL | M/F | Haplo | Normal | Y | 29 | 77 | 5 |
| 25 | ALL | M/M | Haplo | ND | Y | 48 | 45 | 6 |
| 26 | ALL | F/M | Haplo | Trisomy 8 | Y | 42 | 52 | 5 |
| 27 | AML | F/M | Haplo | Trisomy 8 | Y | 60 | 11 | 13 |
| 28 | ALL | M/M | Haplo | Normal | Y | 34 | 56 | 0 |
| 32 | AML | F/F | Haplo | Trisomy 8 | Y | 9 | 93 | 1 |
| 33 | AML | F/M | Haplo | Multiple | N | NM | NM | NM |
| 35 | ALL | F/M | Haplo | Normal | Y | 73 | 15 | 12 |
| 36 | AML | F/M | Haplo | Normal | Y | 12 | 82 | 2 |
| 37 | T-ALL | M/F | Haplo | Normal | Y | 11 | 87 | 0 |
| 38 | ALL | F/F | Haplo | Normal | N | NM | NM | NM |
| 39 | ALL | F/M | Haplo | Acquired derivative 19 | N | NM | NM | NM |
| 43 | ALL | M/F | Haplo | ND | N | NM | NM | NM |
| 47 | APL* | ND | ND | ND | ND | ND | ND | ND |
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; APL, acute promyelocytic leukemia; ND, no data; NM, not measured; U, unidentified-NMDP unrelated donor (MUD); Haplo, haploidentical donor
a No culture data; pre-treatment Vδ repertoire and sequencing studies only
Expanded γδ T cells from related donors are cytotoxic to the primary leukemia
Cytotoxicity assays of expanded donor γδ T cells versus patient blasts were performed in seven patient–donor cultures in which an increase in γδ T cells was documented. We were unable to perform this assay using the remaining ten cultures due to the lack of a sufficient number of expanded γδ T cells and/or frozen blasts (n=7), or failure of the blasts to load PKH26 (n=3) sufficiently to be reliably distinguished from effectors. In 6/7 experiments γδ T cells were cytotoxic to primary leukemia at a maximum of 20–58% lysis (Fig. 1). In many cases, the γδ T cell killing could be augmented by stimulating the cultured γδ T cells with pan-δ antibody bound to the culture plate (data not shown). Expanded γδ T cells were not cytotoxic to third party MNC.
Fig. 1.

γδ T cells were expanded in culture with primary leukemic blasts, harvested, and placed into a 4 h cytotoxicity assay against the respective blasts with which they were cultured. Four representative patient/donor pairs identified by their unique patient number (UPN) are shown at E:T ratio of 6.25:1 to 50:1. The bar chart shows maximum cytotoxicity achieved by γδ T cells against their respective primary leukemic blasts. Maximum cytotoxicity was achieved at an E:T ratio of 50:1 with the exceptions of UPN 27 (25:1) and UPN 37 (12:1). Refer to Table 1 for patient and culture characteristics
Peripheral blood flow cytometric and molecular phenotyping of γδ T cells from leukemia patients reveal differences in the γδ T cell phenotype and repertoire from healthy volunteers
Seventeen patients with a diagnosis of ALL, on AML, were accrued to this series of experiments (Table 1). Of these, we were able to detect a sufficient number of γδ T cells (minimum of 100 events) to allow statistically relevant determination of differences in δ-chain expression in 12 patients. The large number of blasts in many of the pretreatment specimens prevented the collection of sufficient γδ events for analysis in five patients. In each case where sufficient γδ T cells could be collected, the absolute number was not increased, although in 9/12 (75%) the Vδ1+ subset was predominant and in 6/12 patients the presence of circulating Vδ2+ cells could not be documented (Fig. 2).
Fig. 2.
Representative flow cytometric dot plots showing Vδ1 and Vδ2 expression obtained from a healthy volunteer designated C3 and b a B-ALL patient #11. Note that expression of Vδ2 is dominant in the healthy volunteer but is substantially reduced in the leukemia patient. Non-quantitative agarose electrophoresis gel plots of Vδ chain PCR (c) are shown from healthy volunteers (C1–C3) and selected patients that also suggest a reduction of Vδ2 expression and predominance of Vδ1 transcription at diagnosis
Sequence analysis from the Vδ1 CDR3 region revealed that Vδ1 transcripts expressed in healthy volunteers consist of a small number of heterogeneous clones as well as heterogeneous J chain usage not shown. Patient transcripts used the Jδ1 chain exclusively (Fig. 3).
Fig. 3.
Pt patient number, DIS disease type, FREQ frequency of expression of the CDR3 region sequence, GL germline sequence. Sequences with Jδ2 expression are shown in italics. CDR3 region sequences are shown from the six patients in Fig. 2c. Patients are in the same order the same as shown on Fig. 2. Note that although the CDR3 regions are generally heterogeneous between patients, every patient sequence shows exclusive use of the Jδ1 region, while the controls show use of both Jδ1 and Jδ2. In addition, two identical CDR3 sequences were found among transcripts derived from the γδ T cells of three different patients (patients #11, 47, and 14; and patients 19, 47, and 14; see blue and red boxes). Three nearly identical sequences, closely related to the recurring sequence shown in blue, were also found in patient #47, and are marked with green boxes
The Vδ1 CDR3 region from leukemia patients was generally heterogeneous, although N2 region similarities were present in several sequences in two APL patients (19 and 47) as well as in one T-ALL patient (14). Specifically, two identical sequences were found among γδ T cells from three of these patients (see red and blue boxes, Fig. 3). In addition, three other nearly identical sequences from one APL patient were also noted (green boxes in Fig. 3), which are very closely related to one of the two recurring identical sequences (blue box sequence), and all are in the same reading frame sequence.
Discussion
These studies developed as a result of our previous observations showing an association between in vivo expansion of donor-derived γδ T cells and long-term survival in patients receiving a haploidentical marrow graft as therapy for leukemia [22, 23, 25]. We sought to clarify this finding using an in vitro model to determine if primary leukemia could be recognized and killed by γδ T cells from a prospective HSCT donor for the particular patient from whom the primary blasts were obtained. Data from this series of experiments show that allogeneic γδ T cells from a parent or sibling will proliferate in culture with primary acute leukemia and will lyse the leukemia in a 4-h cytotoxicity assay. Others have shown that γδ T cells are cytotoxic to leukemia and lymphoma cell lines [11, 19, 26–30], but have not specifically addressed γδ T cell responses to primary leukemia from a prospective HSCT donor/recipient pair. In addition, we and others have shown that γδ T cells do not proliferate when cultured with normal allogeneic mononuclear cells [10, 31], a finding that is consistent with the innate immune properties of γδ T cells discussed above (e.g. γδ T cells do not recognize peptides presented in the context of classical MHC I and II molecules and therefore it is likely that the TCR does not interact with alloantigens). Thus, it is unlikely that the expansion and cytotoxicity of γδ T cells against primary blasts is due to allorecognition.
The use of cryopreserved blasts as stimulators in the proliferation assay and as targets in the cytotoxicity assay raises the question as to whether freezing and thawing may result in stress antigen expression that otherwise may not have been present. In our studies, several cultures were initially conducted with fresh blasts and later, in repeat experiments, with cryopreserved blasts. No differences were seen between the two, and in addition, cryopreserved/thawed normal MNC did not stimulate γδ T cell proliferation.
The optimal control for expansion and lysis of leukemia by γδ T cells is the same patient’s normal lymphocytes. However, most patients in this study presented with >90% blasts, and the logistics of separating normal cells from blasts in this setting prevented us from purifying a sufficient number of normal cells to conduct these experiments. Preparation of blasts from normal lymphocytes using mitogens such as phytohemagglutinin (PHA) or Epstein-Barr virus (EBV), could also induce expression of stress antigens that could make them vulnerable to γδ T cell recognition as previously reported [29]. In addition, we and others have shown that γδ T cells do not proliferate in co-culture with normal third-party mononuclear cells [10, 31], suggesting that γδ T cell proliferation found in this study is induced by contact with leukemic blasts.
The proportional increase in Vδ1+ T cells in our cultures where γδ T cells were expanded is also consistent with published reports. Duval [11] showed that IL-2 stimulated γδ T cells from leukemia patients expanded in culture to a greater degree than γδ T cells from healthy controls. Vδ1+ and Vδ2+ T cells both expanded, but only the Vδ1+ clones lysed the ALL cell line NALM-6. Vδ1+ T cells have also been shown to lyse Hodgkin’s lymphoma [32], EBV-transformed B cells [29], and AML [30].
Why did γδ T cells expand in some cultures and not in others? There was no specific pattern of expansion that correlated with leukemia phenotype or cytogenetics, although the small sample size prevented meaningful statistical analysis. Leukemia cells that did not provoke a proliferative response from γδ T cells may not have expressed the specific innate or stress-associated antigens at sufficient density to trigger a γδ T cell response. We had previously tested several of the leukemias for expression of MIC-A and MIC-B, but found that the absence of MIC expression did not affect the ability of the blasts to provoke a γδ T cell response (unpublished observations). Indeed, the vast majority of antigens that stimulate γδ T cells remain undiscovered. These findings also provide insight into our previous observations from long-term survivors of acute leukemia that developed increased Vδ1+ T cells early during post-transplant immune recovery as discussed above. Although approximately 70% of these patients remain relapse free at 6–9 years, 30% did succumb to relapse which also could not be correlated with leukemia phenotype or cytogenetic profile.
We also studied the γδ T cell phenotype and CDR3 region sequences from newly diagnosed leukemia patients prior to the initiation of cytoreductive therapy. These studies were designed to determine if evidence existed for an autologous γδ T cell response to leukemia. Indeed, we observed an increased proportion of Vδ1+ T cells in these patients that was principally due to loss of Vδ2+ T cells rather than an increase in Vδ1+ T cells. Depletion of circulating Vδ2+ cells has been described in patients in the late stages of AIDS [33], and patients with tuberculosis [34] and melanoma [33, 35]. The mechanism of Vδ2+ T cell loss is incompletely understood although studies [27, 33] suggest that activation of the Vδ2+ population induces apoptosis. As Vδ2+ cells are also active against hematopoietic cancers [11], their loss may result in an impaired innate immune response that may play a role in disease initiation or progression, although this will require further study. Since the Vδ1+ T cell absolute count was not increased in these patients, there was a question as to whether the Vδ1+ cells are simply left behind or if they actively participate in the immune response to the patient’s leukemia.
In order to gain further insight into this finding, we sequenced the Vδ1 CDR3 region from 11 leukemia patients to determine if any evidence of CDR3 region conservation was present that would suggest evidence for an antigen-driven response. Although most patients showed CDR3 region heterogeneity, four showed sequence conservation predominantly in the N2 region. All sequencing reactions were repeated at least once from the original RNA preparation to verify that these results were not a result of cross-contamination between specimens during the PCR and transfections.
Similar patterns of γδ TCR restriction have been observed in other diseases such as rheumatoid arthritis [36] and multiple sclerosis [37]. In HIV+ patients where Vδ2+ T cells have been lost, there is no difference in Vδ1 junctional diversity between HIV+ patients and HIV- controls [38], arguing against a clonal expansion of Vδ1+ T cells in this patient population. In our studies, every patient showed depletion of Vδ2+ T cells and most showed CDR3 region heterogeneity among the Vδ1+ T cells as well. Interestingly, however, several instances of CDR3 region conservation was seen in four patients, the mechanism of which remains in question. This might reflect preferential expansion of γδ T cells bearing a TCR specific for a common leukemia antigen. Indeed, among mouse γδ T cells, a defined CDR3 sequence in the delta chain has been shown to confer specificity for a particular ligand, the non-classical MHC class I molecule T22 [39]. However, in contrast to the findings of Shin et al. [39] in which the CDR3 of the delta chain was in fact found to be sufficient for the TCR specificity, the results shown here suggest that germline components (Vδ1 and Jδ1) are also required. The unusual structure of the γδ TCRs studied by Shin et al. [39], a fairly rare γδ TCR type in the mouse, may explain the lack of a requirement for germline components in that particular case, since the critical CDR3 region studied was found to protrude as an extended loop from the main structure of the TCR as described by Adams et al. [40].
Taken together with previous findings of γδ+ T cell activation, expansion, and cytotoxicity in response to leukemia, these observations point to a role for γδ T cells in the immune response to leukemia. A further understanding of the significance of these findings will require the study of a larger patient population to determine whether a discernable pattern of CDR3 region sequence conservation is present in a significant population of leukemia patients. If such patterns are present, these studies could result in the discovery of novel tumor-associated ligands for γδ T cells. Moreover, an expanded understanding of γδ T cell cytolytic activity against leukemia could soon give way to early-stage cellular therapy trials using γδ T cells to replace and enhance innate immune activity against leukemia.
Acknowledgements
Funding for this study was provided by the Leukemia and Lymphoma Society Grant #6507, NIH Grant R21 CA 76667, the National Childhood Cancer Foundation, and the South Carolina Endowment for Children’s Cancer Research. The authors appreciate the work of Dr. Jeff Welsh and Dr. Robert Best in providing morphologic and cytogenetic data in addition to that from the clinical record.
Footnotes
Paul F. Meeh and Michelle King are contributed equally to this work.
References
- 1.Cortes JE, Kantarjian HM. Acute lymphoblastic leukemia: a comprehensive review with emphasis on biology and therapy. Cancer. 1995;76:2393. doi: 10.1002/1097-0142(19951215)76:12<2393::AID-CNCR2820761203>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535. doi: 10.1056/NEJMra023001. [DOI] [PubMed] [Google Scholar]
- 3.Cornelissen JJ, Carston M, Kollman C, King R, Dekker AW, Lowenberg B, Anasetti C. Unrelated marrow transplantation for adult patients with poor-risk acute lymphoblastic leukemia: strong graft-versus-leukemia effect and risk factors determining outcome. Blood. 2001;97:1572. doi: 10.1182/blood.V97.6.1572. [DOI] [PubMed] [Google Scholar]
- 4.Porter DL, Collins RH, Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S, Flowers ME, Casper J, Leahey A, Parker P, Mick R, Bate-Boyle B, King R, Antin JH. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214. [PubMed] [Google Scholar]
- 5.Wheeler KA, Richards SM, Bailey CC, Gibson B, Hann IM, Hill FG, Chessells JM. Bone marrow transplantation versus chemotherapy in the treatment of very high-risk childhood acute lymphoblastic leukemia in first remission: results from Medical Research Council UKALL X and XI. Blood. 2000;96:2412. [PubMed] [Google Scholar]
- 6.Gajewski JL, Ho WG, Feig SA, Hunt L, Kaufman N, Champlin RE. Bone marrow transplantation using unrelated donors for patients with advanced leukemia or bone marrow failure. Transplantation. 1990;50:244. doi: 10.1097/00007890-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 7.Mori T, Sugita K, Suzuki T, Okazaki T, Manabe A, Hosoya R, Mizutani S, Kinoshita A, Nakazawa S. A novel monoclonal antibody, KOR-SA3544 which reacts to Philadelphia chromosome-positive acute lymphoblastic leukemia cells with high sensitivity. Leukemia. 1995;9:1233. [PubMed] [Google Scholar]
- 8.Dolstra H, Fredrix H, Maas F, Coulie PG, Brasseur F, Mensink E, Adema GJ, de Witte TM, Figdor CG, van de Wiel-van Kemenade E. A human minor histocompatibility antigen specific for B cell acute lymphoblastic leukemia. J Exp Med. 1999;189:301. doi: 10.1084/jem.189.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molldrem J, Dermime S, Parker K, Jiang YZ, Mavroudis D, Hensel N, Fukushima P, Barrett AJ. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450. [PubMed] [Google Scholar]
- 10.Lamb LS, Jr, Musk P, Ye Z, van Rhee F, Geier SS, Tong JJ, King KM, Henslee-Downey PJ. Human gammadelta(+) T lymphocytes have in vitro graft vs leukemia activity in the absence of an allogeneic response. Bone Marrow Transpl. 2001;27:601. doi: 10.1038/sj.bmt.1702830. [DOI] [PubMed] [Google Scholar]
- 11.Duval M, Yotnda P, Bensussan A, Oudhiri N, Guidal C, Rohrlich P, Boumsell L, Grandchamp B, Vilmer E. Potential antileukemic effect of gamma delta T cells in acute lymphoblastic leukemia. Leukemia. 1995;9:863. [PubMed] [Google Scholar]
- 12.Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, Hobby P, Sutton B, Tigelaar RE, Hayday AC. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 13.Kaminski MJ, Cruz PD, Jr, Bergstresser PR, Takashima A. Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res. 1993;53:4014. [PubMed] [Google Scholar]
- 14.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA (see comments) Science. 1999;285:727. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 15.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 16.Raulet DH. The structure, function, and molecular genetics of the g/d T cell receptor. Ann Rev Immunol. 1989;7:175. doi: 10.1146/annurev.iy.07.040189.001135. [DOI] [PubMed] [Google Scholar]
- 17.Lanier L. Unusual lymphocytes—γδ T cells and NK cells. Immunol. 1995;3:182. [Google Scholar]
- 18.Bigby M, Markowitz JS, Bleicher PA, Grusby MJ, Simha S, Siebrecht M, Wagner M, Nagler-Anderson C, Glimcher LH. Most gamma delta T cells develop normally in the absence of MHC class II molecules. J Immunol. 1993;151:4465. [PubMed] [Google Scholar]
- 19.Leca G, Vita N, Maiza H, Fasseu M, Bensussan A. A monoclonal antibody to the Hodgkin’s disease-associated antigen CD30 induces activation and long-term growth of human autoreactive gamma delta T cell clone. Cell Immunol. 1994;156:230. doi: 10.1006/cimm.1994.1167. [DOI] [PubMed] [Google Scholar]
- 20.Steinle A, Groh V, Spies T. Diversification, expression, and gamma delta T cell recognition of evolutionarily distant members of the MIC family of major histocompatibility complex class I-related molecules. Proc Natl Acad Sci USA. 1998;95:12510. doi: 10.1073/pnas.95.21.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olive C, Gatenby PA, Serjeantson SW. Evidence for oligoclonality of T cell receptor delta chain transcripts expressed in rheumatoid arthritis patients. Eur J Immunol. 1992;22:2587. doi: 10.1002/eji.1830221018. [DOI] [PubMed] [Google Scholar]
- 22.Lamb LS, Jr, Henslee-Downey PJ, Parrish RS, Godder K, Thompson J, Lee C, Gee AP. Increased frequency of TCR gamma delta + T cells in disease-free survivors following T cell-depleted, partially mismatched, related donor bone marrow transplantation for leukemia. J Hematother. 1996;5:503. doi: 10.1089/scd.1.1996.5.503. [DOI] [PubMed] [Google Scholar]
- 23.Lamb LSHL, Musk P, et al. Influence of T cell depletion method on circulating γδ+ T cell reconstitution and potential role in the graft-versus-leukemia effect. Cytotherapy. 1999;1:7. doi: 10.1080/0032472031000141295. [DOI] [PubMed] [Google Scholar]
- 24.Lamb L, Meeh P, Muga S, O’Brien R, Marsh J, Wofford C, Neuberg R. Gamma delta T cells from acute leukemia patients show restricted CDR3 rearrangements suggestive of a directed immune response. Blood. 2003;11:384a. [Google Scholar]
- 25.Godder K, Henslee-Downey PJ, Chiang KY, Abhyankar SA, Bridges K, Mehta J, Park B, Lamb LS. Long-term disease free survival in acute leukemia patients with high gamma delta T cells following partially mismatched related donor stem cell transplantation. Proc Am Soc Clin Oncol. 2003;22:833. [Google Scholar]
- 26.Wright A, Lee JE, Link MP, Smith SD, Carroll W, Levy R, Clayberger C, Krensky AM. Cytotoxic T lymphocytes specific for self tumor immunoglobulin express T cell receptor delta chain. J Exp Med. 1989;169:1557. doi: 10.1084/jem.169.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kunzmann V, Bauer E, Feurle J, Weissinger F, Tony HP, Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384. [PubMed] [Google Scholar]
- 28.Orsini D, van Gils M, Kooy Y, et al. Functional and molecular characterization of B cell-responsive Vd1+ γδ T cells. Eur J Immunol. 2004;24:3199. doi: 10.1002/eji.1830241243. [DOI] [PubMed] [Google Scholar]
- 29.Hacker G, Kromer S, Falk M, Heeg K, Wagner H, Pfeffer K. V delta 1+ subset of human gamma delta T cells responds to ligands expressed by EBV-infected Burkitt lymphoma cells and transformed B lymphocytes. J Immunol. 1992;149:3984. [PubMed] [Google Scholar]
- 30.Dolstra H, Fredrix H, van der Meer A, de Witte T, Figdor C, van de Wiel-van Kemenade E. TCR gamma delta cytotoxic T lymphocytes expressing the killer cell-inhibitory receptor p58.2 (CD158b) selectively lyse acute myeloid leukemia cells. Bone Marrow Transpl. 2001;27:1087. doi: 10.1038/sj.bmt.1703043. [DOI] [PubMed] [Google Scholar]
- 31.Schilbach KE, Geiselhart A, Wessels JT, Niethammer D, Handgretinger R. Human gammadelta T lymphocytes exert natural and IL-2-induced cytotoxicity to neuroblastoma cells. J Immunother. 2000;23:536. doi: 10.1097/00002371-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Sathiyaseelan T, Naiman B, Welte S, Machugh N, Black SJ, Baldwin CL. Immunological characterization of a gammadelta T-cell stimulatory ligand on autologous monocytes. Immunology. 2002;105:181. doi: 10.1046/j.0019-2805.2001.01356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li B, Bassiri H, Rossman MD, Kramer P, Eyuboglu AF, Torres M, Sada E, Imir T, Carding SR. Involvement of the Fas/Fas ligand pathway in activation-induced cell death of mycobacteria-reactive human gamma delta T cells: a mechanism for the loss of gamma delta T cells in patients with pulmonary tuberculosis. J Immunol. 1998;161:1558. [PubMed] [Google Scholar]
- 34.Argentati K, Re F, Serresi S, Tucci MG, Bartozzi B, Bernardini G, Provinciali M. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- 35.Ferrarini M, Heltai S, Toninelli E, Sabbadini MG, Pellicciari C, Manfredi AA. Daudi lymphoma killing triggers the programmed death of cytotoxic V gamma 9/V delta 2 T lymphocytes. J Immunol. 1995;154:3704. [PubMed] [Google Scholar]
- 36.Hvas JOJ, Fernando R, Steinman L, Bernard CA. γδ T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J Neuroimmunol. 1993;46:225. doi: 10.1016/0165-5728(93)90253-U. [DOI] [PubMed] [Google Scholar]
- 37.Stinissen P, Vandevyver C, Medaer R, Vandegaer L, Nies J, Tuyls L, Hafler DA, Raus J, Zhang J. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J Immunol. 1995;154:4883. [PubMed] [Google Scholar]
- 38.Hinz T, Wesch D, Frise K, Reckziegel A, Arden B, Kabelitz D. T cell receptor γδ repertoire in HIV-1 infected individuals. Eur J Immunol. 1994;24:3044. doi: 10.1002/eji.1830241219. [DOI] [PubMed] [Google Scholar]
- 39.Shin S, El-Diwany R, Schaffert S, Adams EJ, Garcia KC, Pereira P, Chien YH. Antigen recognition determinants of gammadelta T cell receptors. Science. 2005;308:252. doi: 10.1126/science.1106480. [DOI] [PubMed] [Google Scholar]
- 40.Adams EJ, Chien YH, Garcia KC. Structure of a gammadelta T cell receptor in complex with the nonclassical MHC T22. Science. 2005;308:227. doi: 10.1126/science.1106885. [DOI] [PubMed] [Google Scholar]




