Abstract
We reported previously that pigeon cytochrome c-derived peptides (Pan-IA), which bind broad ranges of MHC class II molecules efficiently, activate T helper (Th) function in mice. In an experimental model, Pan-IA DNA vaccines augmented antitumor immunity in tumor antigen-immunized mice. To elicit more potent antitumor immunity and to eradicate tumors in a therapeutic setting, Pan-IA-loaded dendritic cells (DCs) were inoculated in combination with vaccines including ovalbumin (OVA) antigen DNA in tumor-bearing mice. Seventy percent of the immunized mice survived tumor-free for at least 4 months after treatment. In contrast, mice vaccinated with OVA DNA, either with or without naïve DCs, did not eliminate the tumors and died within 5 weeks. Only in mice vaccinated with OVA DNA and Pan-IA-loaded DCs were both cytotoxic and helper responses specific for OVA induced at the spleen and tumor sites as well as at the vaccination sites. Furthermore, accumulation of OVA-specific CD4+ and CD8+ T lymphocytes and interferon-gamma-mediated anti-angiogenesis were observed in the tumors of these mice. Thus, the combined vaccination primed both tumor-specific cytotoxicity and helper immunity resulting in augmented tumor lysis ability and anti-angiogenic effects. This is the first report to show that most established tumors were successfully eradicated by collaboration of potent antitumor immunity and anti-angiogenic effects by vaccination with tumor antigens and helper-activating analogs. This novel vaccination strategy is broadly applicable, regardless of identifying helper epitopes in target molecules, and contributes to the development of therapeutic cancer vaccines.
Keywords: Cancer vaccine, Pan-MHC class II peptide, T helper function, Dendritic cells, Anti-angiogenesis
Introduction
CD4+ Th cells play a critical role in the successful priming of tumor-specific CD8+ CTLs in cancer immunotherapy by providing activation signals to CTLs via molecular interactions and Th1 cytokines [1–6]. We previously reported that simultaneous inoculation with naïve DCs and tumor antigen DNA prolonged the survival of tumor-bearing mice [7]. We also reported that combined vaccination with DNA encoding tumor antigens and DNA encoding a helper-activator, Pan-IA, elicited potent antitumor immunity and augmented the suppression of tumor growth in mice [8]. In these two models, activated Th function was essential in the augmentation of tumor-specific CTL activity capable of suppressing tumor growth.
In order to efficiently activate Th cells reactive with target antigens in cancer vaccines, helper epitopes derived from the same or different molecules in which the CTL epitopes are located have been used [9–12]. Ideally, a single helper epitope would enhance any antigen-specific CTL activity in vaccine immunotherapy. We reported that a 16-amino acid peptide analog (Pan-IA), AEGFSYTVANKAKGIT, which is derived from pigeon cytochrome c (p43–58), activated Th function without any MHC class II restrictions in mice [13, 14]. In an experiment with tumor-bearing mice that received combined vaccination with DNA encoding OVA and DNA encoding Pan-IA, growth of established tumors with OVA expression were significantly suppressed and survival of the treated mice was prolonged when compared with that of mice vaccinated with OVA DNA, either with or without naïve DCs. Accumulation of both antigen-specific CTLs and CD4+ T cells was confirmed in the tumor sites of the immunized mice.
In this study, we utilized the Pan-IA presented by professional APCs and DCs, in order to increase the efficacy of antigen-specific Th activation in combination with OVA vaccines in a therapeutic setting. Here, we examine and compare cellular responses to the OVA-derived CTL and helper epitopes and the Pan-IA analog at the vaccination site, spleen, and tumors of treated mice. We also address the involvement of IFNγ-mediated anti-angiogenic effects in tumor suppression.
Materials and methods
Mice and cells
Female C57BL/6J mice 6–8 weeks of age were purchased from CLEA Japan Inc. (Tokyo, Japan), maintained under specific pathogen-free conditions, and studied in compliance with institutional guidelines. Murine lymphoma cell lines EL-4 and E.G7, which was generated by transducing the chicken OVA gene into EL-4 cells, were purchased from American Type Culture Collection (Manassas, VA, USA). P12 is a transfected derivative of the DAP.3 subline of L cell fibroblasts that expresses high levels of I-Ab, B7.1, and ICAM-1 (gift from Dr. Germain RN, Laboratory of Immunology, National Institute of Allergy and Infectious Diseases, Bethesda, MA, USA) [15].
Synthetic peptides and plasmid DNA
Pan-IA peptide analog (AEGFSYTVANKAKGIT) derived from pigeon cytochrome c (p43–58) having a two-residue substitution (D–V at 50 and N–A at 54), the H-2Kb-restricted CTL epitope in OVA, SIINFEKL (257–264), and the I-Ab-restricted helper epitope in OVA, ISQAVHAAHAEINEAGR (323–339) [16], were synthesized commercially by Hokkaido System Science Co., Ltd. (Sapporo, Japan). An expression vector, pcDNA3.1/OVA, was prepared by cloning the full-length OVA cDNA, which was obtained from pAc-neo-OVA (gift from Dr. Yukio Koide, Department of Microbiology and Immunology, Hamamatsu University School of Medicine, Hamamatsu, Japan) by digestion with EcoRI (Toyobo Inc., Osaka, Japan) into the pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA).
DC preparation
DCs were generated from bone marrow cells as described previously [7]. Briefly, bone marrow cells from the femurs of C57BL/6J mice were cultured for 7 days in the presence of recombinant mouse GM-CSF and IL-4 (Genzyme Techne Corporation, Minneapolis, MN, USA). Induced immature DCs were matured by culture for another 24 h in the presence of 0.5 μg/ml LPS (Nacalai Tesque, Inc. Kyoto, Japan). Nonadherent cells were harvested, washed in PBS, and used for inoculation as mature DCs. Flow cytometric analysis showed expression of CD11c, CD86, and MHC class II molecules in 80–90% of mature DCs (date not shown).
Vaccination
Mice were injected with 80 μg of pcDNA3.1/OVA or pcDNA3.1 dissolved in 25 μl of PBS in both hind-leg quadriceps muscles. For DC inoculation, 1 × 106 mature DCs loaded with 1 μM Pan-IA at 37°C for 2 h or nonpulsed DCs were suspended in 25 μl of PBS and simultaneously injected at the same site of DNA vaccination. One week after the first vaccination, the mice were again vaccinated using the same protocol.
Lymphocyte proliferation assay
One week after the second immunization, mice were sacrificed, and spleen cells or draining lymph node (LN) cells (2 × 105 cells/well) were incubated in 96-well culture plates (Corning Inc., Corning, NY, USA) with irradiated (100 Gy) EL-4 (2 × 104 cells/well) that had been pulsed with various concentrations of OVA-Kb peptides or P12 cells (2 × 104 cells/well) that had been pulsed with various concentrations of OVA323–339 or Pan-IA peptides for 48 h. Sixteen hours before termination of culture, 1 μCi of [3H] thymidine (Perkin-Elmer Life Science, Boston, MA, USA) was added to each well. Cells were harvested from the wells, and [3H] thymidine uptake was measured using a scintillation counter.
Cytotoxicity assay
Spleen cells from the mice in which established tumors were eliminated following vaccination were cultured in complete medium containing 0.1 μM OVA-Kb peptides and 1 ng/ml recombinant mouse IL-2 (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 5 days. After in vitro stimulation, spleen cells were incubated with 1 × 104 EL4 or E.G7 cells that were labeled with Na512CrO4 (Perkin-Elmer Life Science) at various effector/target (E/T) ratios in 96-well culture plates for 6 h. The amount of 51Cr released from lysed target cells in the supernatants was estimated using Auto Gamma Counting Systems (Packard Instrument Company, Meriden, CT, USA). The ratio (percentage) of 51Cr release was calculated using the following formula: percentage of specific lysis = {(experimental release−spontaneous release)/(maximal release−spontaneous release)} × 100. To deplete CD8+, CD4+, or NK1.1+ cells, spleen cells from vaccinated mice were incubated with 10 μg/ml anti-mouse CD8, CD4, or NK1.1 antibodies (bioscience Inc., San Diego, CA, USA), respectively, on ice for 30 min. After washing with PBS, cells were incubated with rabbit complement (Wako Pure Chemical Industries, Ltd.) diluted at 1:20 in PBS at 37°C for 45 min. Flow cytometric analysis showed that over 90% of CD8+, CD4+, or NK1.1+ cells were depleted from the treated spleen cells (data not shown).
Intracellular IFNγ staining and flow cytometric analysis
One week after the second immunization, spleen cells from vaccinated mice were cultured in the presence or absence of 1 μM OVA-Kb or OVA323–339 peptides for 16 h. Monensin (eBiosciences Inc.) was added to a final concentration of 2 μM, 5 h before termination of culture. Cells were harvested, washed in PBS, and subsequently incubated with PE-labeled anti-mouse CD8 or CD4 antibodies (eBiosciences Inc.) at room temperature for 20 min. After washing with PBS, cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% saponin (Nacalai Tesque, Inc.) followed by staining with FITC-labeled anti-mouse IFNγ antibody (eBiosciences Inc.) for 20 min. Cells were washed in PBS and examined for surface expressions of CD4 and CD8 molecules and intracellular IFNγ using a FACScan flow cytometer (Becton Dickinson Immunocytometry system, Mountain View, CA, USA). Data are presented as dot blots produced using CellQuest software (Becton Dickinson Immunocytometry system).
Tumor challenge experiment
Mice received s.c. inoculations in the right flank of 1 × 105 E.G7 cells. On day 7, when tumors 4–5 mm in diameter were detected initially, the mice were vaccinated with 80 μg OVA DNA or control DNA, with or without inoculation with 1 × 106 Pan-IA-loaded or naïve DCs, into both hind-leg quadriceps muscles. Vaccination was repeated four times weekly. Tumor sizes were monitored once a week and tumor volume was calculated using the following formula: (length) × (width)2/2.
Matrigel implantation and assay for angiogenesis and IFNγ concentration
One week after the second immunization, mice received s.c. inoculations, in both flanks, of 0.5 ml of growth factor-reduced Matrigel (BD Biosciences, Bedford, MA, USA) containing 10 ng/ml basic fibroblast growth factor (bFGF) (PeproTech, Rocky Hill, NJ, USA) and 50 U/ml heparin mixed with 5 × 105 E.G7 or EL-4 cells. One week after implantation, Matrigel plugs were harvested and the surrounding tissue was dissected. Subsequently, plugs were liquefied by incubation in PBS overnight at 4°C. To quantify angiogenesis, hemoglobin content of the liquefied plugs was measured by Drabkin’s method (Sigma Diagnostics, St. Louis, MO, USA), as described previously [17]. Concentration of IFNγ in liquefied plugs was measured using a mouse IFNγ ELISA kit (BioSource International Inc., Camarillo, CA, USA).
Immunohistochemistry
Matrigel plugs harvested from the mice were frozen, sectioned (10 μm), and fixed with 50% acetone in methanol. Sections were incubated in 2% normal goat serum in order to block nonspecific binding for 20 min, washed in PBS, and incubated with anti-von Willebrand factor antibody (DAKO, Kyoto, Japan) diluted at 1:200 overnight at 4°C. Sections were then washed in PBS and incubated with biotinylated anti-rabbit immunogloblins diluted at 1:500 for 2 h followed by incubation with horse radish peroxidase-conjugated avidin solution (Vector Laboratories, Inc., Burlingame, CA, USA) for 1.5 h at room temperature. Sections were stained by incubation with 0.1 mg/ml 3.3′-diaminobenzidine (Wako Pure Chemical Industries, Ltd.) for 10 min at room temperature, washed in tap water, and counterstained with 0.1% hematoxylin solution.
Characterization of Matrigel-infiltrating lymphocytes
Matrigel plugs harvested from mice were incubated in complete medium containing 2,000 μ/ml dispase (Godo Shusei Co. Ltd., Tokyo, Japan) at 37°C for 3 h. Cells infiltrating Matrigel were collected by centrifugation and cultured with 0.1 μM OVA-Kb peptide for 3 days. Cells were harvested and then incubated with FITC-labeled anti-mouse CD8 antibody and PE-labeled H-2Kb/OVA257–264 complex (MBL Co., Ltd., Nagoya, Japan) at room temperature for 30 min. After washing with PBS, cells were examined by flow cytometry in order to quantify OVA-specific CTLs. To study cellular responses of T cells with OVA antigens or Pan-IA, cells collected from the Matrigel were examined by lymphocyte proliferation assay as described above.
Statistical analysis
The statistical significance of differential findings between experimental and control groups was determined by Student’s t test and P values < 0.05 were considered statistically significant.
Results
Antigen-specific immunity induced by vaccination
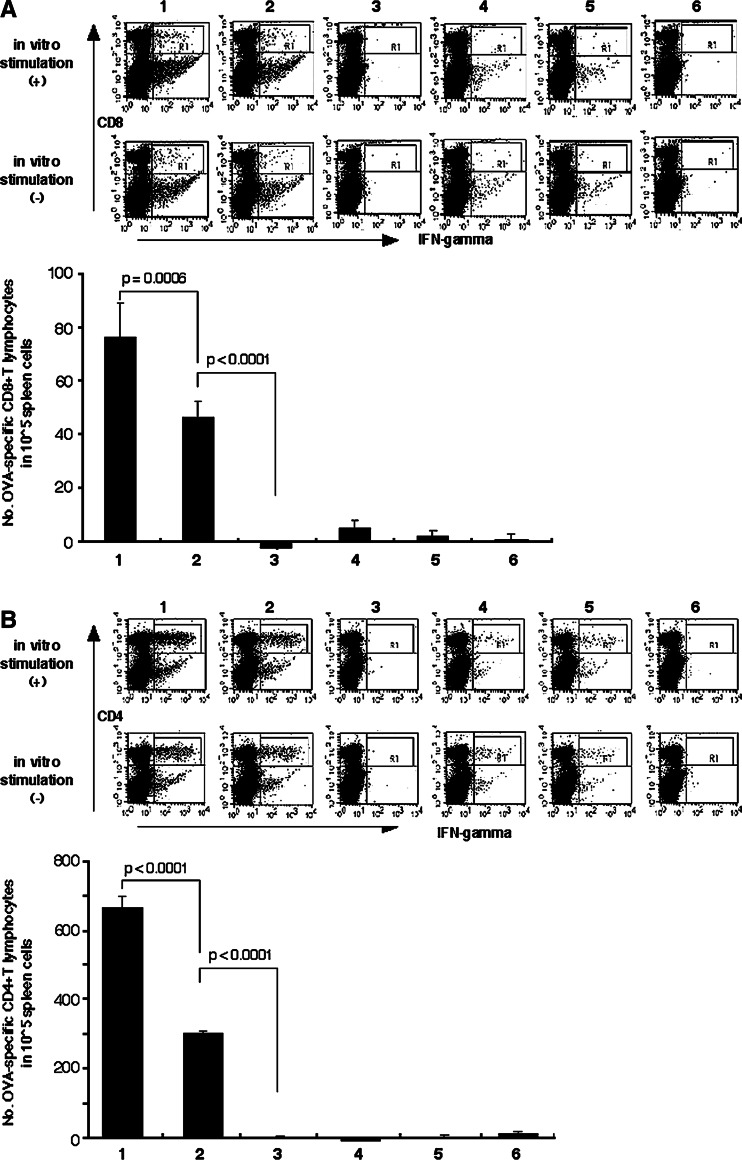
In order to address the effects of DC inoculation on host T cell response to OVA antigens, spleen cells from vaccinated mice were examined for IFNγ production in response to OVA antigens. The number of CD8+ T cells reactive with OVA257–264 peptides was significantly increased by combined vaccination with OVA DNA and DCs, with or without Pan-IA-loading, when compared to vaccination with DNA alone or in other combinations (75.67 ± 12.97 CD8+/IFNγ+ cells in 105 spleen cells with OVA + Pan-IA DC or 46.0 ± 5.59 cells with OVA + DC vs. −2.67 ± 0.95 cells by OVA alone: P < 0.0001, Fig. 1a). When the number of OVA-specific CTLs was compared between mice inoculated with Pan-IA-loaded DCs and naïve DCs in combination with OVA DNA vaccination, Pan-IA-loaded DCs significantly increased the number of OVA-specific CTLs in treated mice (P < 0.0006). As seen in CD8+ T cell responses, the number of CD4+ T cells reactive with OVA323–339 peptides was markedly increased in both mice vaccinated with OVA DNA plus Pan-IA-loaded and naïve DCs (663.7 ± 31.17 CD4+/IFNγ+ cells in 105 spleen cells with OVA + Pan-IA DC or 299.3 ± 5.40 cells with OVA + DC vs. −2.3 ± 4.01 cells with OVA alone: P < 0.0001, Fig. 1b). There was a significant difference in the numbers of OVA-reactive CD4+ T cells between mice vaccinated with OVA DNA plus Pan-IA-loaded DCs and OVA DNA plus naïve DCs (P < 0.0001). The number of CD4+ T cells producing IFNγ was approximately ninefolds higher than that of CD8+ T cells producing IFNγ in mice that received OVA DNA and Pan-IA-loaded DC vaccination (Fig. 1). Note that about half of the CD4+ and CD8+ T cells producing IFNγ in response to OVA antigens were already activated to produce IFNγ without in vitro stimulation in mice vaccinated with OVA DNA and DCs, with or without Pan-IA-loading. Since we cannot exclude the possibility that spleen cells producing IFNγ without in vitro stimulation are nonspecifically activated, the number of OVA-specific CTLs or helper T cells were calculated by subtracting the number of IFNγ-producing cells without restimulation from that of IFNγ-producing cells after restimulation.
Fig. 1.
Production of IFNγ by spleen T cells in response to OVA peptides. Spleen cells from mice vaccinated with OVA or mock DNA with or without DC inoculation were incubated with OVA257–264 or OVA323–339 peptides, stained with PE-labeled anti-CD8 or -CD4 antibody and FITC-labeled anti-IFNγ antibody and subsequently examined by flow cytometry. The numbers of the CD8+ and CD4+ T cells producing IFNγ in 105 splenocytes are indicated in the lower panels of a and b, respectively. 1 OVA DNA plus Pan-IA DC, 2 OVA DNA plus naïve DC, 3 OVA DNA alone, 4 mock DNA plus Pan-IA DC, 5 mock DNA plus naïve DC, 6 mock DNA alone
Based on examination of humoral immunity in treated mice, OVA-specific antibodies were shown to be elicited in mice following vaccination with OVA DNA, with or without DC inoculation, but not following vaccination with mock DNA, with or without DC inoculation (data not shown). Additional effects of DC inoculation on titers of anti-OVA antibody in sera were not observed among OVA DNA-vaccinated mice.
Tumor challenge test
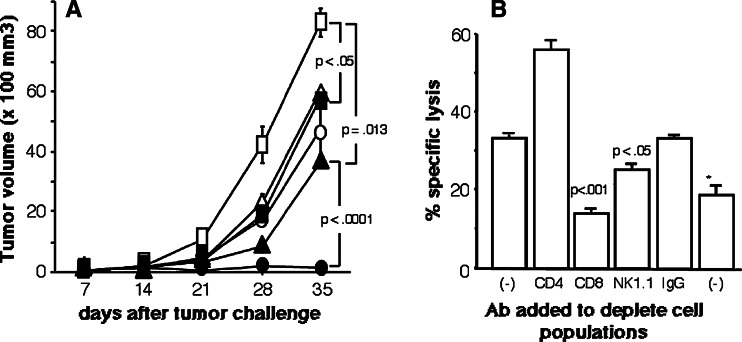
C57BL/6J mice that had been challenged with OVA-expressing tumor cells, E.G7, received vaccination with OVA or mock DNA, either with or without DC inoculation, at intervals of 1 week. Tumor growth was significantly suppressed in mice vaccinated with either OVA DNA or DCs when compared to mice vaccinated with mock DNA alone (P < 0.05, Fig. 2a). In particular, established tumors were only eliminated by combined vaccination with OVA DNA and Pan-IA-loaded DCs (14 of 20 vaccinated mice rejected the tumor, data not shown). All mice in which established tumors were eradicated survived tumor-free for at least 4 months. Among other groups of mice, all died within 5 weeks of tumor challenge (data not shown). Spleen cells from mice receiving this combination showed strong cytotoxic activity against OVA-expressing targets and CD8+ splenic T cells were responsible for target cell lysis (Fig. 2b). In this assay, spleen cells were incubated with target cells as responder cells after depletion of CD4−, CD8−, or NK1.1-positive cells at an E/T ratio of 50:1. Therefore, the number of CD8+ cells was increased in the CD4+ cell-depleted spleen cells and the cytolytic activity by the CD8+ cells was thought to be raised. In other groups of mice, spleen cells from none of the mice exhibited cell lysis activity against OVA-expressing targets (data not shown).
Fig. 2.
Suppressive effects of vaccines on tumor growth and effector cells responsible for tumor cell lysis. a Mice challenged with E.G7 cells were vaccinated with OVA or mock DNA with or without DC inoculation, at intervals of 1 week. Tumor sizes were monitored once a week after vaccination. The number of mice that received both OVA DNA and Pan-IA-loaded DCs was ten and the numbers of mice in the other groups were five (circles DNA plus Pan-IA DC, triangles DNA plus naïve DC, squares DNA alone, closed symbols OVA DNA, open symbols mock DNA). b Spleen cells from mice in which the challenged E.G7 cells were eradicated after vaccination with OVA DNA and Pan-IA-loaded DCs were stimulated in vitro with OVA-Kb peptides for 5 days. After incubation with anti-CD4, -CD8, or -NK1.1 antibody followed by incubation with rat serum to deplete CD4, CD8, or NK1.1-positive cells, respectively, cells were incubated with OVA-Kb peptide-pulsed EL-4 cells at E/T ratios of 50:1 for 6 h (asterisks target cells were EL-4 cells that were not pulsed with peptides). Results are representative of experiments that were repeated three times. Established E.G7 tumors were completely eliminated in 14 (70%) of the 20 mice that received combined vaccination with OVA DNA and Pan-IA-loaded DCs
IFNγ-mediated anti-angiogenesis in the tumor
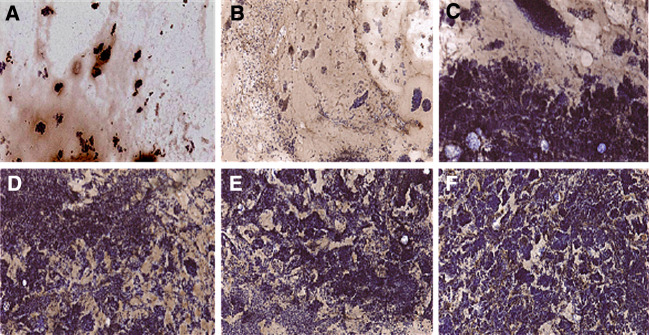
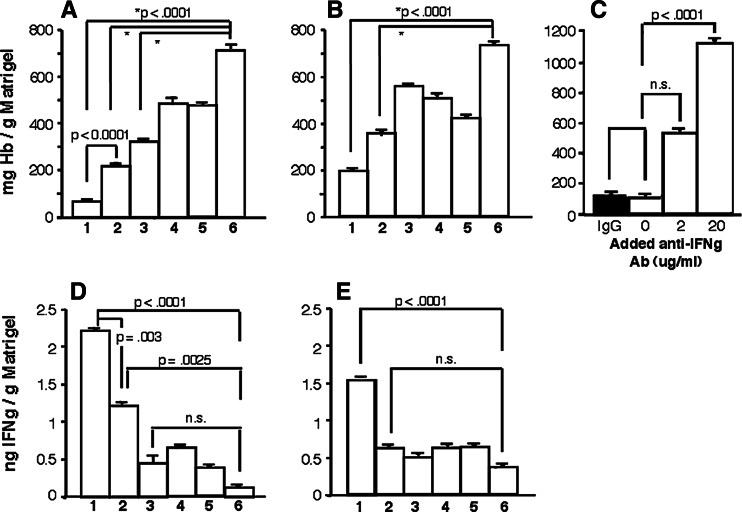
Matrigel plugs mixed with E.G7 or EL4 cells that had been implanted in vaccinated mice were examined for tumor vasculature by immunohistochemistry using antibodies directed against von Willebrand factor, which is expressed in vascular endothelial cells. Matrigel mixed with E.G7 cells from mice vaccinated with OVA DNA and DCs demonstrated markedly reduced numbers of tumor cells and cells expressing von Willebrand factors, regardless of Pan-IA-loading (Fig. 3). In contrast, neither tumor growth nor tumor vasculature was suppressed in Matrigel mixed with E.G7 cells from mice vaccinated with OVA DNA alone or mock DNA plus DCs (Fig. 3) or in any of Matrigel mixed with EL-4 cells (data not shown). To compare tumor angiogenesis quantitatively among the groups of vaccinated mice, hemoglobin contents in implanted Matrigel were measured, as described in Materials and methods. As seen in the immunohistochemical study, tumor angiogenesis in Matrigel mixed with E.G7 cells was significantly suppressed by vaccination with OVA DNA, with or without DC inoculation, when compared with mock DNA vaccination (P < 0.0001, Fig. 4a). In particular, tumor angiogenesis was almost completely inhibited by combined vaccination with OVA DNA and Pan-IA-loaded DCs (Figs. 3, 4a). Even in Matrigel mixed with EL-4 cells, hemoglobin contents were markedly reduced by OVA DNA and DC vaccination to half or one-third of those shown in control mice (P < 0.0001, Fig. 4b). Concentrations of IFNγ in implanted Matrigel were also measured. Production of IFNγ was significantly increased by OVA DNA and DC vaccination, regardless of Pan-IA-loading, when compared to vaccination with control DNA (P < 0.0025, Fig. 4d). Significant increases in IFNγ level were not observed in mice that received vaccination with OVA DNA alone when compared to mice that received mock DNA vaccination. IFNγ levels in mice that received OVA DNA plus Pan-IA-loaded DC vaccination were twofold higher than those in mice that received OVA DNA plus naïve DC vaccination (P = 0.003). In Matrigel mixed with EL-4 cells, only vaccination with OVA DNA plus Pan-IA-loaded DCs resulted in elevated IFNγ levels (Fig. 4e). To determine whether IFNγ is involved in suppression of tumor angiogenesis, EG.7 cells that were mixed in Matrigel containing anti-IFNγ antibody were implanted in mice vaccinated with both OVA DNA and Pan-IA-loaded DCs. Anti-IFNγ antibody blocked the anti-angiogenic effects induced by combined vaccination (Fig. 4c).
Fig. 3.
Immunohistochemical study of tumor angiogenesis in vaccinated mice. Matrigel mixed with E.G7 cells were implanted in vaccinated mice. One week after implantation, Matrigel was removed, frozen, and examined for angiogenesis using monoclonal antibodies directed against von Willebrand factor. a OVA DNA plus Pan-IA DC, b OVA DNA plus naïve DC, c OVA DNA alone, d mock DNA plus Pan-IA DC, e mock DNA plus naïve DC, f mock DNA alone
Fig. 4.
Concentrations of hemoglobin and IFNγ in Matrigel implanted in vaccinated mice. Matrigel mixed with E.G7 or EL-4 cells were implanted in vaccinated mice. One week after implantation, Matrigel was removed, liquefied, and examined for concentrations of hemoglobin (a, b, c) and IFNγ (d, f), as described in Materials and methods . 1 OVA DNA plus Pan-IA DC, 2 OVA DNA plus naïve DC, 3 OVA DNA alone, 4 mock DNA plus Pan-IA DC, 5, mock DNA plus naïve DC, 6 mock DNA alone. Results are representative of triplicate experiments
Frequency of OVA-specific CTLs at tumor site
The number of CD8+ T cells reactive with OVA257–264 peptides that infiltrated Matrigel mixed with E.G7 cells were counted by flow cytometry using a tetramer comprising H-2Kb, β2-micoglobulin, and OVA257–264 peptide. The number of OVA-specific CTLs at the tumor site was significantly increased only by vaccination with both OVA DNA and Pan-IA-loaded DCs (Fig. 5a, b).
Fig. 5.
Frequency of CTLs reactive with OVA-Kb peptide/H-2Kb complex. Matrigel mixed with E.G7 cells was implanted in vaccinated mice. One week after implantation, cells infiltrating Matrigel were collected and cultured with 0.1 μM OVA-Kb peptide for 3 days. Cells were then incubated with FITC-labeled anti-mouse CD8 antibody and PE-labeled H-2Kb/OVA257–264 complex (MBL Co., Ltd., Nagoya, Japan) and assayed for quantifying OVA-specific CTLs by flow cytometry. In the left panel, each dot on the graph represents a spleen cell. The cell number in the upper right quadrant of each graph was counted as CD8-positive CTLs reactive with OVA-Kb peptide/H-2Kb and indicated in the right panel. a OVA DNA plus Pan-IA DC, b OVA DNA plus naïve DC, c OVA DNA alone, d mock DNA plus Pan-IA DC, e mock DNA plus naïve DC, f mock DNA alone. Results are representative of triplicate experiments
Cellular responses to antigens at different sites of immunized mice
Mice were twice vaccinated with OVA or mock DNA, with or without DC inoculation. One week after the second vaccination, cells from the spleen, lymph node (LN) at the vaccination site, or implanted Matrigel of the immunized mice were examined by the proliferation assay for cellular responses to OVA-Kb, OVA-helper peptides, or Pan-IA. In the spleen, concentration-dependent cellular responses to both OVA257–264 and OVA323–339 peptides were observed only in mice vaccinated with OVA DNA and Pan-IA-loaded DCs (Fig. 6a, b). Spleen cells from mice vaccinated with OVA DNA and naïve DCs showed proliferative responses to OVA323–339 peptides, but no concentration-dependent responses to OVA257–264 peptides (Fig. 6a). In contrast, proliferative responses to the Pan-IA were observed in none of the mice (Fig. 6c). In draining lymph nodes, cells from all of the OVA DNA-vaccinated mice proliferated in response to OVA-Kb peptides (Fig. 6d). Of the three groups of mice vaccinated with OVA DNA, mice that received vaccination with OVA DNA plus Pan-IA-loaded DCs showed the strongest response (versus OVA plus naïve DC, P = 0.0388). Furthermore, only mice that received vaccination with OVA DNA plus Pan-IA-loaded DCs showed proliferative responses to OVA-helper epitopes (Fig. 6e). Cellular responses to Pan-IA were observed in mice vaccinated with Pan-IA-loaded DCs (Fig. 6f). At the tumor site, only mice vaccinated with both OVA DNA and Pan-IA-loaded DCs demonstrated specific cellular responses to OVA-Kb, OVA-helper epitopes, and Pan-IA (Fig. 6g, h, i).
Fig. 6.
Cellular responses to OVA antigens or Pan-IA by spleen cells, LN cells, and tumor-infiltrating cells from vaccinated mice. Mice were twice vaccinated with OVA or mock DNA, with or without Pan-IA-loaded or naïve DCs inoculation, at an interval of 1 week. Seven days after immunization, spleen cells (a, b, c), draining LN cells (d, e, f), or cells that infiltrated Matrigel mixed with E.G7 cells (g, h, i) were cultured with EL-4 cells pulsed with various concentrations of OVA-Kb (A, D, G) or with P12 cells pulsed with various concentrations of OVA-helper peptides (b, e, h) or Pan-IA (c, f, i) for 48 h. Circles DNA plus Pan-IA DC, triangles DNA plus naïve DC, squares DNA alone, closed symbols OVA DNA, open symbols mock DNA. Results are representative of triplicate experiments
Discussion
CD4+ Th cells play a central role in both cellular and humoral immunity [18–20]. To elicit potent antitumor immunity capable of suppressing tumor growth, cancer vaccines must activate both CD8+ CTLs and CD4+ Th1 cells. Th1 cells that exhibit cytokine-producing profiles for IFNγ and IL-2 are involved in priming tumor-specific CTLs via cytokine-mediated signals and in activating APCs via cytokine-mediated signals and molecular interactions [1, 2, 21–24]. To efficiently activate tumor-specific Th1 function, approaches such as identification and utilization of target antigen-derived Th epitopes and administration of adjuvant or cytokines capable of priming Th cells have been attempted [25–30]. It has been reported that activated tumor-specific Th cells augmented CTL activity elicited by immunization with target antigens. However, Th epitopes have not been identified for every tumor antigen, while administration of adjuvant at the vaccination site is not always available in cell-based therapy. If helper activators that can prime any target antigen-specific CTLs can be identified, these would be ideal for use in combination with antigen-immunization.
We previously reported that Pan-IA (AEGFSYTVANKAKGIT) derived from the pigeon cytochrome c (p43–58) having a two-residue substitution (D–V at 50 and N–A at 54) can be presented by various types of mouse MHC class II molecules (I–Ab, d, q and s) and is able to efficiently activate Th function without any MHC class II-restriction [13, 14]. Indeed, in our preliminary experiments, LN cells at the vaccination sites of mice immunized with Pan-IA peptides demonstrated production of Th1-type cytokines, such as IFNγ and IL-2, but not IL-4 (data not shown). In a previous report, vaccination with DNA encoding an immunogenic tumor antigen, MUC1, led to successful rejection of antigen-expressing tumors in mice [7]. MUC1 protein was shown to be persistently expressed at the vaccination site for at least 14 days and DNA vaccination elicited both cellular and humoral immunity specific for this antigen in mice. However, the induced antitumor immunity was not sufficiently strong to suppress tumor growth in a therapeutic setting. To prime Th function in this model, combined vaccination with MUC1 DNA and naïve DCs was performed in tumor-bearing mice. As a result, tumor growth was significantly suppressed, although none of the challenged tumors were eliminated.
In order to determine whether Th function was fully activated to elicit strong antitumor immunity after vaccination with Pan-IA, combined vaccination with DNA encoding tumor antigens and DNA encoding Pan-IA was administered to tumor-bearing mice [8]. By this strategy, established tumors were eradicated in about half of the treated mice, and accumulation of activated CD8+ and CD4+ cells was confirmed at the tumor sites. We demonstrated that administered DNA was transduced in myocytes and resident cells, including APCs, at the vaccination sites of the mice [7]. These findings suggest that Pan-IA products expressed in the APCs lead to activation of Th cells at the vaccination site. We expected that more DCs presenting Pan-IA more efficiently exert Th1 function followed by enhancement of tumor-specific CTL activity.
In this study, we simultaneously inoculated tumor-bearing mice with Pan-IA-loaded DCs in combination with tumor antigen DNA vaccines at the same sites. Established tumors were completely eradicated in 70% of the mice after this combination treatment. Marked increases in the numbers of not only tumor antigen-specific CTLs but also Th1 cells were confirmed in the treated mice (Figs. 1, 6). Although increases in CTL frequency resulted in enhanced killing activity against tumor cells, CD4+ Th cells were not involved in tumor cell lysis in the effector phase, as shown in Fig. 2. Data demonstrating increased frequency of OVA-specific CTLs in the treated mice suggest that the efficacy in priming tumor-specific CTLs was improved by the increased number of CD4+ Th cells reactive with OVA. Based on immunofluorescent study with tumors implanted in vaccinated mice, a number of CD4+ and CD8+ T cells producing INFγ were shown to be accumulated at the tumor sites in mice vaccinated with both OVA DNA and Pan-IA-loaded DCs when compared with other groups of mice (data not shown). In particular, because the number of CD4+ T cells reactive with OVA was ninefold higher than that of CD8+ T cells in the treated mice, these CD4+ T cells contribute mainly to elevated IFNγ levels in the tumor. As shown in Fig. 4d, IFNγ levels in implanted Matrigel were significantly elevated in mice vaccinated with this combination when compared with those in other groups of mice. IFNγ levels at the tumor sites were inversely correlated with tumor angiogenesis (Fig. 4). The finding that anti-IFNγ antibody blocked the observed anti-angiogenic effects in implanted Matrigel demonstrated the involvement of IFNγ in anti-antiogenesis in this model. In fact, INFγ has been reported to induce two potent anti-angiogenic chemokines, monokine-induced by IFNγ (MIG), and IFN-inducible protein 10 (IP-10), which are produced by monocytes, macrophages, fibroblasts, and some tumor cells [31–34]. Our data are compatible with those reported previously [35, 36]. In addition, cells reactive with OVA-Kb and OVA-helper epitopes were observed at the tumor site and spleen as well as the vaccination site of mice that received vaccination with OVA DNA and Pan-IA-loaded DCs (Fig. 6). These findings suggest that elicitation of systemic immunity to tumor antigens can be achieved only by this combination. However, we did not expect Pan-IA-specific cells infiltrating in the tumors of mice. We need to further investigate why these cells are involved at the tumor sites in which Pan-IA is not present. Thus, combined vaccination with OVA DNA and Pan-IA-loaded DCs elicited potent tumor antigen-specific cytotoxic and helper immune responses, resulting in augmented CTL activity and anti-angiogenic effects in mice. In our preliminary experiment, NK activity was significantly augmented in mice that were inoculated with DCs regardless of Pan-IA loading in combination of OVA or mock DNA vaccination when compared to mice that were vaccinated with DNA alone (20–26% of specific lysis by DC-inoculated mice vs. 5–7% by mice vaccinated with DNA alone, P = 0.0068, data not shown). The activity was not different among the groups of DC-inoculated mice. Furthermore, the observed cytotoxicity against E.G7 cells of spleen cells from mice that received OVA DNA and Pan-IA-loaded DCs was reduced by about 30% by adding cold YAC-1 cells that were NK-sensitive targets (data not shown). Thus, although NK activity that was augmented by DC inoculation was partially involved in tumor cell lysis, tumor eradication observed in the treated mice may be done mainly by T cells. Through this novel therapeutic strategy, established tumors were completely eradicated in most of the treated mice. This animal model may contribute to the development of clinical immunotherapy for cancer.
In this study, certain numbers of CD4+ and CD8+ T cells that produced IFNγ were found in the spleens of mice vaccinated with OVA DNA and DCs, regardless of Pan-IA loading (Fig. 1). Many of the CD4+ and CD8+ T cells were already activated without in vitro stimulation with OVA antigens, as shown in Fig. 1. In addition, in Matrigel mixed with OVA-deficient EL-4 cells from mice vaccinated with OVA DNA and Pan-IA-loaded DCs, IFNγ levels were significantly elevated when compared with those in Matrigel from other groups of mice (Fig. 4e). These CD4+ and CD8+ T cells may be adequately primed by vaccination and maintained in the activated state in the vaccinated mice. It is likely that immune responses to a xenogenic and immunogenic antigen, OVA, can be easily elicited by DC inoculation in combination with antigen-immunization but not by antigen-immunization alone, and that the previously activated T cells can be detected by flow cytometry using anti-IFNγ antibody without in vitro stimulation. We need to further study the immune response and therapeutic effects of this vaccination strategy in the transgenic mouse model in which target antigens are endogenously expressed. We were unable to determine whether all of these primed T cells were specific for OVA antigens. If they were nonspecifically primed by vaccination, this could lead to unexpected responses, such as autoimmune or nonspecific inflammatory responses. To eliminate this possibility, most of the immunized mice were sacrificed to histologically examine their organs, i.e., eye, thyroid, lung, liver, intestine, testis, and ovary. None of the mice showed any autoimmune or inflammatory findings in these organs (data not shown). In preparation of DCs and assays for cellular responses in this study, cells were cultured in medium supplemented with 10% FCS. Some of the T cells from immunized mice may have produced IFNγ in response to antigens present in FCS, such as calf albumin [37]. In fact, both CD4+ and CD8+ T cells producing IFNγ from mice vaccinated with mock DNA and DCs nearly disappeared when they were incubated in medium supplemented with rat serum (data not shown). However, we did not use rat serum in this study because T cells and DCs were not well maintained in medium supplemented with rat serum.
In conclusion, this novel vaccination strategy is able to fully activate antigen-specific CTL and Th1 function in tumor-bearing hosts. We demonstrated that most of the established tumors were completely eradicated by collaboration of potent cytotoxic immunity and anti-angiogenic effects due to fully activated Th1 function in a murine model. This strategy is broadly applicable, even against cancers in which tumor antigen-derived helper epitopes have not been identified, and contributes to the development of therapeutic cancer vaccines.
Acknowledgment
This study was supported in part by grant-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan (Nos. 13671380, 14571262, 15591340, and 16591392) and grant from YASUDA Medical Research Foundation.
Reference
- 1.Qin Z, Richter G, Schuler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nat Med. 1998;4:627–630. doi: 10.1038/nm0598-627. [DOI] [PubMed] [Google Scholar]
- 2.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med. 1998;187:693–702. doi: 10.1084/jem.187.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol. 1991;147:4069–4073. [PubMed] [Google Scholar]
- 4.Hart MK, Weinhold KJ, Scearce RM, Washburn EM, Clark CA, Palker TJ, Haynes BF. Priming of anti-human immunodeficiency virus (HIV) CD8+ cytotoxic T cell in vivo by carrier-free HIV synthetic peptide. Proc Natl Acad Sci USA. 1991;88:9448–9452. doi: 10.1073/pnas.88.21.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasarte JJ, Sarobe P, Gullon A, Prieto J, Borras-Cuesta F. Induction of cytotoxic T lymphocytes in mice against the principal neutralizing domain of HIV-1 by immunization with an engineered T-cytotoxic-T-helper synthetic peptide construct. Cell Immunol. 1992;141:211–218. doi: 10.1016/0008-8749(92)90140-K. [DOI] [PubMed] [Google Scholar]
- 6.Shirai M, Pendleton CD, Ahler J, Takeshita T, Newman M, Berzofsky JA. Helper-cytotoxic T lymphocyte (CTL) determinant linkage required for priming of anti-HIV CD8+ CTL in vivo with peptide vaccine constructs. J Immunol. 1994;152:549–556. [PubMed] [Google Scholar]
- 7.Kontani K, Taguchi O, Ozaki Y, Hanaoka J, Tezuka N, Sawai S, Inoue S, Fujino S, Maeda T, Itoh Y, Ogasawara K, Sato H, Ohkubo I, Kudo T. Novel vaccination protocol consisting of injecting MUC1 DNA and nonprimed dendritic cell at the same region greatly enhanced MUC1-specific antitumor immunity in a murine model. Cancer Gene Ther. 2002;9:330–337. doi: 10.1038/sj.cgt.7700444. [DOI] [PubMed] [Google Scholar]
- 8.Teramoto K, Kontani K, Ozaki Y, Sawai S, Tezuka N, Nagata T, Fujino S, Itoh Y, Taguchi O, Koide Y, Asai T, Ohkubo I, Ogasawara K. Deoxyribonucleic acid (DNA) encoding a pan-major histocompatibility complex class II peptide analogue augmented antigen-specific cellular immunity and suppressive effects on tumor growth elicited by DNA vaccine immunotherapy. Cancer Res. 2003;63:7920–7925. [PubMed] [Google Scholar]
- 9.Minci S, Sturniolo T, Imro MA, Hammer J, Sinigaglia F, Noppen C, Spagnoli G, Mazzi B, Bellone M, Dellabona P, Protti MP. Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi H, Wood M, Song Y, Appella E, Celis E. Defining promiscuous MHC class II helper T-cell epitopes for the HER2/neu tumor antigen. Cancer Res. 2000;60:5228–5236. [PubMed] [Google Scholar]
- 11.Zarour HM, Maillere B, Brusic V, Coval K, Williams E, Pouvelle-Moratille S, Castelli F, Land S, BennounaJ, Logan T, Kirkwood JM. NY-ESO-1 119–143 is a promiscuous major histocompatibility complex class II T-helper epitope recognized by Th1- and Th2-type tumor reactive CD4+ T cells. Cancer Res. 2002;62:213–218. [PubMed] [Google Scholar]
- 12.Casares N, Lasarte JJ, de Lopez-Diaz Cerio L, Sarobe P, Ruiz M, Melero I, Prieto J, Borras-Cuesta F. Immunization with a tumor-associated CTL epitope plus a tumor-related or unrelated Th1 helper peptide elicits protective CTL immunity. Eur J Immunol. 2001;31:1780–1789. doi: 10.1002/1521-4141(200106)31:6<1780::AID-IMMU1780>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Itoh Y, Ogasawara K, Takami K, Gotohda T, Naruse H, Good RA, Onoe K. Determination of amino acids on agretopes of pigeon cytochrome c-related peptides specifically bound to I-A allelic products. Eur J Immunol. 1994;24:76–83. doi: 10.1002/eji.1830240113. [DOI] [PubMed] [Google Scholar]
- 14.Itoh Y, Kajino K, Ogasawara K, Katoh M, Namba K, Iwabuchi K, Braunstein NS, Onoe K. Determination of the allele-specific antigen-binding site on I-Ak and I-Ab molecules. Eur J Immunol. 1996;26:1314–1321. doi: 10.1002/eji.1830260621. [DOI] [PubMed] [Google Scholar]
- 15.Beatty GL, Paterson Y. IFN-γ-dependent inhibition of tumor angiogenesis by tumor-infiltrating CD4+ T cells requires tumor responsiveness to IFN-γ. J Immunol. 2001;166:2276–2282. doi: 10.4049/jimmunol.166.4.2276. [DOI] [PubMed] [Google Scholar]
- 16.Qin Z, Schwartzkopff J, Pradera F, Kammertoens, Seliger B, Pircher H, Blankenstein T. A critical requirement of interferon γ-mediated angiogenesis for tumor rejection by CD8+ T cells. Cancer Res. 2003;63:4095–4100. [PubMed] [Google Scholar]
- 17.Toldbod HE, Agger R, Bolund L, Hokland M. Potent influence of bovine serum proteins in experimental dendritic cell-based vaccination protocol. Scand J Immunol. 2003;58:43–50. doi: 10.1046/j.1365-3083.2003.01267.x. [DOI] [PubMed] [Google Scholar]
- 18.Ronchese F, Brown MA, Germain RN. Structure-function analysis of the abm 12 beta mutation using site-directed mutagenesis and DNA-mediated gene transfer. J Immunol. 1987;139:629–638. [PubMed] [Google Scholar]
- 19.Robertson JM, Jensen PE, Evavold BD. DO11.10 and OT-II T cells recognize a c-terminal ovalbumin. J Immunol. 2000;164:4706–4712. doi: 10.4049/jimmunol.164.9.4706. [DOI] [PubMed] [Google Scholar]
- 20.Passaniti A., Taylor RM, Pili R, Guo Y, Long PV, Haney JA, Pauly RR, Grant DS, Martin GR. A simple quantitative method for assessing angiogenesis and antiangiogenic agents using reconstituted basement membrane, heparin and fibroblast growth factor. Lab Invest. 1992;67:519–528. [PubMed] [Google Scholar]
- 21.Restifo NP, Spiess PJ, Karp SE, Mule JJ, Rosenberg SA. A nonimmunologenic sarcoma transduced with the cDNA for interferon γ elicits CD8+ T cells against the wild-type tumor: correlation with antigen presentation capability. J Exp Med. 1992;175:1423–1431. doi: 10.1084/jem.175.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1996;76:241–251. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 23.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 24.Ridge JP, Rosa FD, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–477. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 25.Bennett SR, Carbone FR, Karamalis F, Flavell RA, Miller JF, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature. 1998;393:478–479. doi: 10.1038/30996. [DOI] [PubMed] [Google Scholar]
- 26.Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ. T-cell help for cytotoxic lymphocytes is mediated by CD40-CD40L interaction. Nature. 1998;393:480–482. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 27.den Haan JM, Bevan MJ. A novel helper role for CD4 T cells. Proc Natl Acad Sci USA. 2000;97:12950–12952. doi: 10.1073/pnas.97.24.12950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulmer JB, DeWitt CM, Chastain M, Friedman A, Donnelly JJ, McClements WL, Caulfield MJ, Bohannon KE, Volkin DB, Evans RK. Enhancement of DNA vaccine potency using conventional aluminum adjuvants. Vaccine. 1999;8:18–28. doi: 10.1016/S0264-410X(99)00151-6. [DOI] [PubMed] [Google Scholar]
- 29.Ebhardt MB, Shive CL, Guardia R, Gapin L, Boehm BO, Forsthuber TG. Immunological adjuvants efficiently induce antigen-specific T cell responses in mice: implication for vaccine adjuvant development in aged individuals. Cell Immunol. 2002;215:87–97. doi: 10.1016/S0008-8749(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA, Yang JC, Schwartzentruber DJ, Hwu P, Marincola FM, Topalian SL, Restifo NP, Schwartz M, Spiess PJ, Wunderlich JR, Seipp CA, Einborn JH, Rogers-Freezer L, White DE. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1995;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 31.Tatsumi T, Takehara T, Kanto T, Miyagi T, Kazushita N, Sugimoto Y, Jinushi M, Kasahara A, Sasaki Y, Hori M, Hayashi N. Administration of interleukin-12 enhances the therapeutic efficacy of dendritic cell-based tumor vaccines in mouse hepatocellular carcinoma. Cancer Res. 2001;61:7563–7567. [PubMed] [Google Scholar]
- 32.Shimizu K, Thomas EK, Giedlin M, Mule JJ. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001;61:2618–2624. [PubMed] [Google Scholar]
- 33.Zaliauskiene L, Kang S, Sparks K, Zinn KR, Schwiebert LM, Weaver CT, Lollawn JF. Enhancement of MHC class II-restricted responses by receptor-mediated uptake of peptide antigens. J Immunol. 2002;169:2337–2345. doi: 10.4049/jimmunol.169.5.2337. [DOI] [PubMed] [Google Scholar]
- 34.Angiolillo AL, Sgadari C, Taub DD, Liao F, Faber JM, Maheshwari S, Kleinman HK, Reaman GH, Tosato G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–162. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arenberg DA, Kunkel SL, Polverini PJ, Morris SB, Burdick MD, Glass MC, Taub DT, Iannettoni MD, Whyte RI, Strieter RM. Interferon-γ-inducible protein 10 (IP-10) is an angiostatic factor that inhibits human non-small cell lung cancer (NSCLC) tumorigenesis and spontaneous metastasis. J Exp Med. 1996;184:981–992. doi: 10.1084/jem.184.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sgadari C, Farber JM, Angiolillo AL, Liao F, Teryua-Feldstein J, Burd PR, Yao L, Gupta G, Kanegane C, Tosato G. Mig, the monokine induced by interferon-γ promotes tumor necrosis in vivo. Blood. 1997;89:2635–2643. [PubMed] [Google Scholar]
- 37.Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokine as regulators of angiogenesis. Shock. 1995;4:155–160. doi: 10.1097/00024382-199509000-00001. [DOI] [PubMed] [Google Scholar]