Abstract
To establish the prognostic value of B7-H4 expression by tumor cells in gastric cancer patients, we evaluated the association of B7-H4 expression with clinicopathologic factors and overall survival of gastric cancer patients. A retrospective cohort study including 156 gastric cancer patients was performed in the present report. Immunohistochemical assay was used to evaluate the expression of B7-H4 in the surgical specimens of gastric cancer tissues. Multi-univariate COX model was then used to evaluate the association of B7-H4 expression with the patients’ survival and clinicopathological parameters. B7-H4 expression in the gastric cancer cells was observed in about 44.9% gastric cancer specimens. Univariate analysis demonstrated that there was no correlation between B7-H4 expression and sex, age, histological type, pathological grade or tumor size. In contrast, B7-H4 expression correlated positively with cancer invasiveness and lymph node metastasis. In addition, the median overall survival time of patients with lower B7-H4 expression was 13 months longer than that of patients with higher expression (χ2 = 12.38, P < 0.0001), and the median disease-free survival time of patients with lower B7-H4 expression was significantly longer than that of patients with higher expression (33 vs. 16 months, χ2 = 14.977, P < 0.0001). After adjustment for other confounding factors, the COX model analysis indicated that the death risk was significantly higher in patients with higher B7-H4 expression than those with lower expression (RR = 1.85, 95% CI = 1.15–2.96). The present study demonstrated that higher B7-H4 expression in cancer cells was associated with poor prognosis of gastric cancer patients. This is consistent with the idea that B7-H4 promotes cancer progression, likely via inhibition of anti-tumor immune responses.
Keywords: Gastric carcinoma, B7-H4, Chronic gastritis, Gastric adenoma, Prognosis
Introduction
Gastric cancer is a leading cause of cancer-related death worldwide [1], especially in China [2]. Despite the availability of various treatment modalities such as surgery [3], surgery in combination with radiotherapy [4], chemotherapy [5] and immunotherapy [6], the 5-year survival rate for patients suffering from gastric cancer is still poor [7]. It has been suggested that application of adjuvant immunotherapy could be an important way to increase patients’ 5-year survival rate [8]. Therefore, it is critical to further understand the immune environment of gastric cancer for developing immune-based therapy.
B7-H4 is a member of B7 family and thought to inhibit tumor-specific T cell-mediated immunity [9]. It inhibits T cell proliferation [10, 11], cytokine secretion and development of cytotoxicity [12, 13]. Therefore, the expression of B7-H4 in the tumor microenvironment can greatly suppress the function of anti-tumor T cells, and thereby promote tumor progression and metastasis. In addition, it can potentially limit the efficacy of immune therapy [9]. It has been reported that B7-H4 is highly expressed in many cancer tissues, such as primary or metastatic breast carcinoma [14], lung cancer [15, 16], ovarian cancer [17, 18] and many other diseases [19–21]. Besides cancer cells, B7-H4 is also induced in ovarian cancer-associated macrophages [10, 11].
In the present study, we studied the B7-H4 expression in tumor specimens from a large cohort of gastric cancer patients. We then determined the correlation between tumor B7-H4 expression and various clinicopathological parameters as well as patient survival. Our studies establish a strong association between B7-H4 expression in cancer tissues with gastric cancer progression and patient outcome.
Materials and methods
Selection of patients
A total of 156 pathologically confirmed and newly diagnosed gastric cancer patients who received operation in the Third Affiliated Hospital, Soochow University, were followed up from 1 January 1998 to 31 December 2008. The characteristics of patients are shown in Table 1. Ten gastritis (7 male and 3 female, aged 62.2 ± 11.2 years old) and ten gastric adenomas (4 male and 6 female, aged 52.5 ± 10.9 years old) were chosen as controls. The present study was approved by the Ethics Committee of the Third Affiliated Hospital of Soochow University.
Table 1.
Correlation between B7-H4 expression and patients’ clinical characteristics
| Clinical features | Cases | B7-H4 expression | χ2 | P value | |
|---|---|---|---|---|---|
| Higher (%) | Lower (%) | ||||
| Sex | |||||
| Men | 122 | 55 (45.1) | 67 (54.9) | 0.010 | 0.920 |
| Women | 34 | 15 (55.9) | 19 (44.1) | ||
| Age (years) | |||||
| ≤45 | 14 | 5 (35.7) | 9 (64.3) | Z = 1.361a | 0.087 |
| 46–60 | 71 | 29 (40.9) | 42 (59.1) | ||
| >60 | 71 | 36 (50.7) | 35 (49.3) | ||
| Tumor location | |||||
| Gastric cardia | 58 | 36 (62.1) | 22 (37.9) | 10.934 | 0.004 |
| Gastric body | 64 | 23 (35.9) | 41 (64.1) | ||
| Gastric antrum | 36 | 12 (33.3) | 24 (66.7) | ||
| Tumor size | |||||
| <5 cm | 76 | 31 (40.8) | 45 (59.2) | 0.723 | 0.396 |
| ≥5 cm | 56 | 27 (48.2) | 29 (51.8) | ||
| Histology differentiation | |||||
| Differentiated | 52 | 19 (36.5) | 33 (63.5) | 3.725 | 0.054 |
| Poorly differentiated | 94 | 50 (53.2) | 44 (46.8) | ||
| Invasion | |||||
| Yes | 112 | 55 (49.1) | 57 (50.9) | 8.468 | 0.004 |
| No | 24 | 4 (16.7) | 20 (83.3) | ||
| Lymph node metastasis | |||||
| With | 100 | 54 (54.0) | 46 (46.0) | 17.339 | <0.0001 |
| Without | 36 | 5 (13.9) | 31 (86.1) | ||
| Recurrence | |||||
| With | 98 | 53 (54.1) | 45 (45.9) | 9.038 | 0.003 |
| Without | 58 | 17 (29.3) | 41 (70.7) | ||
| Pathological gradeb | |||||
| 1 | 2 | 1 (50.0) | 1 (50.0) | Z = 0.191a | 0.424 |
| 2 | 27 | 12 (44.4) | 15 (45.6) | ||
| 3 | 92 | 42 (45.6) | 50 (54.4) | ||
| 4 | 35 | 15 (42.9) | 20 (57.1) | ||
| UICC stageb | |||||
| I | 14 | 2 (14.3) | 12 (85.7) | Z = 1.504a | 0.066 |
| II | 22 | 12 (54.5) | 10 (45.5) | ||
| III | 102 | 47 (46.1) | 55 (53.9) | ||
| IV | 18 | 9 (50.0) | 9 (50.0) | ||
aCochran–Armitage test, b Sobin LH, Wittekind C, eds. TNM Classification of Malignant Tumours (6th edited). UICC (International Union Against Cancer) 0.65–68, 2002
The eligibility criteria were as follows: primary gastric cancer patients aged 30–78 years who had undergone a curative gastric resection with well-preserved tissue paraffin blocks. The exclusion criteria for eligibility were: patients who did not meet the above conditions, or suffered from other tumors at the same time. Standard questionnaires were designed to document general information of each gastric cancer patient, including demographic characteristics, operative time, tumor size, tumor site, pathological type, and tumor stage and metastasis. All patients received oxaliplatin program [22]: oxaliplatin 120 mg/m2 D1, 5-Fu 400 mg/m2 CIV 24 h D 1-5, CF 200 mg/m2 D 1-5 and adjusted dose according to toxicity and side effects. Chemotherapy was continued until disease progression, development of unacceptable toxicity or completion of six cycles. The curative effects were evaluated after each treatment.
Immunohistochemical staining
Immunohistochemical staining was performed by using the ElivsionTM method. Paraffin sections were deparaffinized and hydrated. Antigen retrieval was done in a citrate buffer (10 mmol/l, pH 6.0) at 100°C for 30 min. After cooling, sections were incubated in 0.3% H2O2 solution for 30 min to block endogenous peroxidase. After washing three times with PBS (pH 7.4) for 5 min each, sections were incubated with the primary antibodies (B7-H4 mouse anti-human polyclonal antibody, USCNLIFE, USA, diluted 1:400) in a humid chamber at 4°C overnight. Negative controls were carried out by substituting PBS for the primary antibody. After washing three times with PBS (pH 7.4) for 5 min each, sections were incubated with the secondary antibody (mouse/rabbit general second antibody, Maixin Biotechnology Co. Ltd, Fuzhou) at room temperature for 30 min. After washing again with PBS, the sections were visualized by incubation with DAB, counterstained with hematoxylin and differentiated with 0.1% hydrochloric acid alcohol. The sections were dehydrated in graded alcohol and mounted with a neutral resin.
Evaluation of B7-H4 positive staining
The slides were examined by two pathologists, and the sections were evaluated according to the immunohistochemical scores (IHS) [23]. In brief, five high-power fields (200×) were randomly selected and observed under light microscope (Leica DM2500). Staining intensity and percentage of positive cells were assessed by the software Leica QWin Plus version 3.5.1 (Leica Microsystems, Switzerland). The extent of the staining was semi-quantitatively categorized to: 0 (<5% positive cells), 1 (6–25% positive cells), 2 (26–50% positive cells), 3 (51–75% positive cells) and 4 (>75% positive cells) [24]. The staining intensity in the cytoplasm and membrane was also semi-quantitatively evaluated on a scale of 0–3 as follows: 0 (negative), 1 (weakly positive), 2 (moderately positive) and 3 (strongly positive). Multiplication of the intensity and the percentage scores became the final staining score: 0 (negative), weakly positive (1–4), moderately positive (5–8) and strongly positive (9–12). Coloring cytoplasm as well as membrane of tumor cells was considered as a positive reaction for B7-H4. In this study, the B7-H4 expression is considered as weak positive (lower expression) when score is less than 9, and positive (higher expression) when score is equal to or more than 9.
Statistical analysis
All data were imported into Epidata3.0 database with double-check, and were analyzed by the SAS 9.13 software package (version 9.13; SAS Institute, Cary, NC USA). Quantitative and qualitative data were expressed as means ± SD or rate, respectively. ANOVA/Dunnett test and χ2 test were used to compare the difference for means or rates between two or more than two groups. Cochran–Armitage trend test was used to test the trend changes of more than two groups if the group variable was ordinal. Survival data were analyzed by Kaplan–Meier method and log-rank test. Multivariate COX model was performed to evaluate prognosis factors for gastric cancer. The RR and 95% CI were estimated for the prognosis factors associated with gastric cancer death risk.
Results
Determination of B7-H4 expression by immunohistochemical analysis
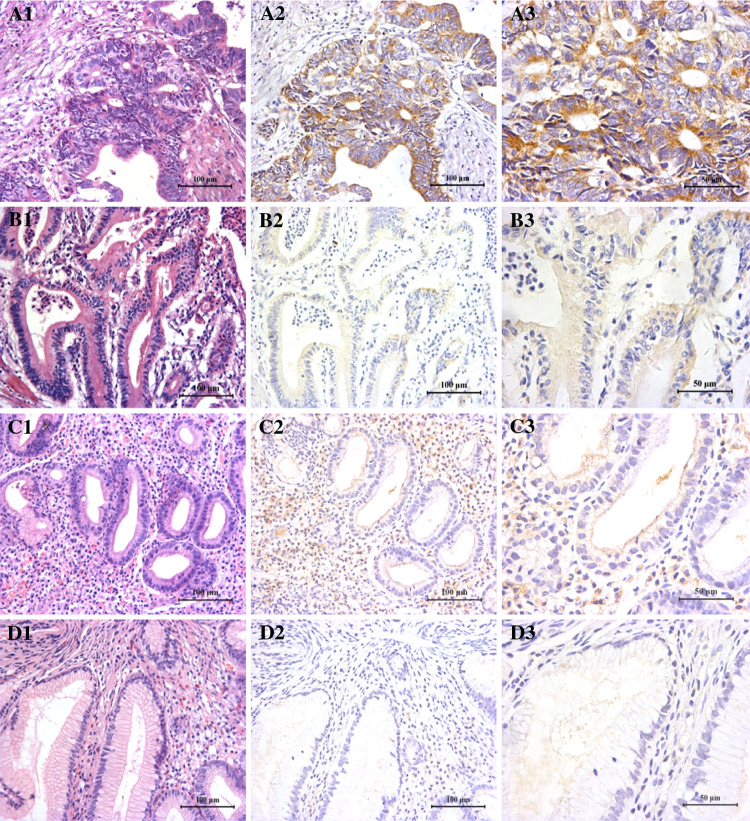
Immunohistochemistry analysis demonstrated that B7-H4 was highly expressed in many cancerous cells of gastric cancer tissues (Fig. 1, A1–A3, B1–B3), whereas there was no or very weak B7-H4 staining in the gastric polyp tissues (Fig. 1, D1–D3) or adjacent normal tissues (data not shown). Interestingly, although B7-H4 was not expressed in the epithelial cells of gastritis tissues, it was highly expressed in the infiltrating mononuclear cells in these tissues (Fig. 1, C1–C3), which is consistent with its role during active inflammation [25]. The positive staining of B7-H4 was found in 70 out of 156 gastric cancer specimens (the positive rate was 44.9%). The mean B7-H4 staining scores among gastric cancer tissues (higher and lower expressers), gastritis epithelia and gastric polyp tissues were significantly different (F = 376.88, P < 0.0001) and the mean staining scores of gastric cancerous cells were significantly higher than those of the epithelial cells of non-cancer tissues (Dunnett test, P < 0.05) (Fig. 2). These data suggest that B7-H4 is highly expressed in many gastric cancer cells.
Fig. 1.
Immunohistochemical staining of B7-H4. The B7-H4 immunohistochemical staining in gastric cancer (a, higher expression; b, lower expression), gastritis (c) and gastric polyp tissues (d) is shown. Left panels show HE staining. Middle and right panels show B7-H4 staining at lower and higher magnifications, respectively
Fig. 2.
Differential B7-H4 expression in the gastric cancer tissue (higher and lower expression), gastritis and gastric polyp tissues. Intensity of expression of B7-H4 was evaluated according to the immunohistochemical score as described in “Materials and methods”. *p < 0.05; **p < 0.001
Correlation between B7-H4 expression and patients’ clinical characteristics
Because B7-H4 is highly expressed in cancer tissues from a subgroup of gastric cancer patients, we decided to determine whether its expression correlates with any clinicopathological parameters. As shown in Table 1, single factor analysis demonstrated that B7-H4 positive expression rate was significantly higher in the gastric cancer patients with myometrial invasion than in patients with non-invasive cancer (49.1% vs. 16.7%, χ2 = 8.467, P = 0.004). In addition, B7-H4 positive expression was significantly higher in patients with lymph node metastasis than in those without lymph node metastasis (χ2 = 17.339, P < 0.0001). The rate of B7-H4 higher expressing specimens for patients with recurrence was also significantly higher than for patients without recurrence (54.1% vs. 29.3, χ2 = 9.038, P = 0.003) (Table 1).
Relationship between B7-H4 expression and gastric cancer patient survival
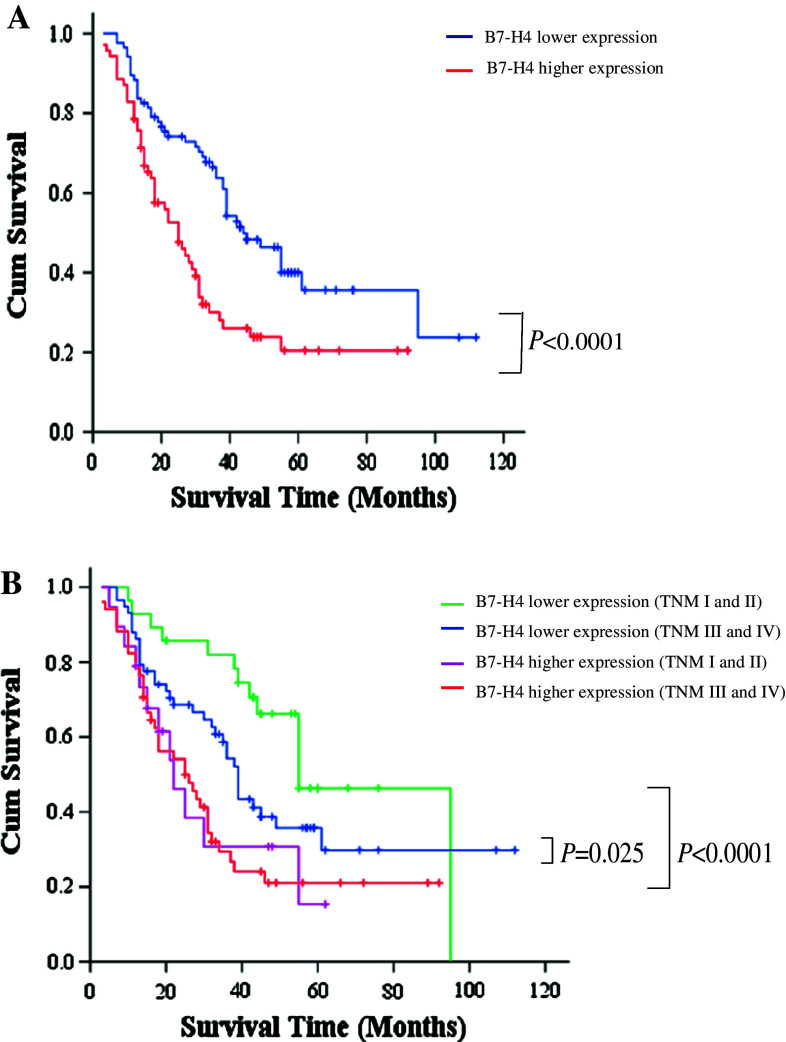
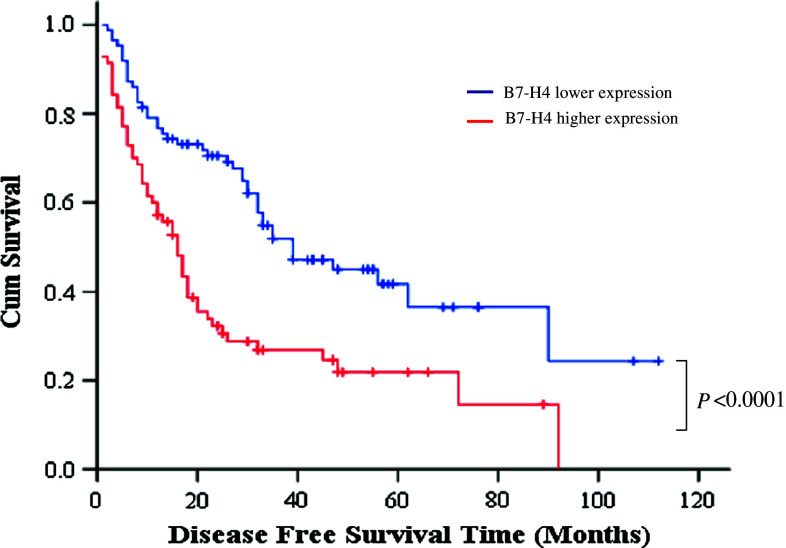
Further analysis revealed that there was a significant correlation in the survival time of gastric patients with different levels of B7-H4 expression. The median overall survival time of patients with lower and higher expression of B7-H4 were 38 and 25 months, respectively (Fig. 3a and Table 2) (χ2 = 12.38, P < 0.0001), and the median disease-free survival time of patients with lower B7-H4 expression was significantly longer than that of patients with higher B7-H4 expression (Fig. 4 and Table 3) (33 vs. 16 month, χ2 = 14.977, P < 0.0001). Considering both the univariate analysis results and the clinical issues, in addition to the B7-H4 expression variable, we introduced invasion, lymph node metastasis, sex, and age factors into the COX model. After adjusting the confounding factors such as age, sex, depth of invasion and lymph node metastasis, the death risk of gastric cancer patients in the higher B7-H4 expression group was significantly greater (RR = 1.85, 95% CI = 1.15–2.96) (Table 4), which means that B7-H4 expression is an independent negative factor affecting the survival time of gastric cancer patients.
Fig. 3.
Overall survival of gastric cancer patients with different levels of B7-H4 expression. a All patients were divided into higher and lower B7-H4 expression groups. Survival data were analyzed by Kaplan–Meier method and log-rank test. b Patients in stages I and II were divided into higher and lower B7-H4 expression groups and survival data were analyzed by Kaplan–Meier method and log-rank test. Patients in stages III and IV were also divided into higher and lower B7-H4 expression groups and survival data were analyzed by Kaplan–Meier method and log-rank test
Table 2.
Patients’ median survival time (MST)
| B7-H4 expression | MST (months) and 95% CI | Log-rank test χ2-value | P value |
|---|---|---|---|
| Lower | 38 (34.9–53.1) | ||
| Higher | 25 (18.7–31.3) | 12.38 | <0.0001 |
Fig. 4.
Disease-free survival curve of gastric cancer patients with lower and higher B7-H4 expression. The median disease-free survival time of patients with lower B7-H4 expression was significantly longer than that of patients with higher B7-H4 expression (33 vs. 16 months, χ2 = 14.977, P < 0.0001)
Table 3.
Patients’ median disease-free survival time (MDFST)
| B7-H4 expression | MDFST (months) and 95% CI | Log-rank test χ2-value | P value |
|---|---|---|---|
| Lower | 33 (23.5–50.4) | ||
| Higher | 16 (12.2–19.8) | 14.977 | <0.0001 |
Table 4.
Multivariate COX model analysis of B7-H4 expression in correlation to patients’ prognosis
| Variables | Cases | RR (95%CI) | P value |
|---|---|---|---|
| B7-H4 expression | |||
| Lower | 77 | 1.00 (–) | |
| Higher | 59 | 1.85 (1.15–2.96) | 0.0087 |
| Sex | |||
| Men | 107 | 1.00 (–) | |
| Women | 29 | 0.80 (0.44–1.45) | 0.5157 |
| Age (years) | |||
| ≤45 | 13 | 1.00 (–) | |
| 46–60 | 63 | 0.75 (0.35–1.58) | 0.4447 |
| >60 | 60 | 1.13 (0.53–2.41) | 0.7621 |
| Trend test | P = 0.275 | ||
| Invasion | |||
| Without | 24 | 1.00 (–) | |
| With | 112 | 1.46 (0.71–2.99) | 0.3567 |
| Lymph node metastasis | |||
| Without | 36 | 1.00 (–) | |
| With | 100 | 1.21 (0.65–2.25) | 0.5324 |
We further analyzed the prognostic value of B7-H4 in early stage cancer (stage I and II) and late stage cancer (stages III and IV) patients separately. Patients with higher B7-H4 expression in stage I and II groups had much worse survival rates compared to patients with lower expression (RR = 3.033, 95%CI: 1.327–6.884) (Fig. 3b). Similarly, patients with higher B7-H4 expression in stage III and IV groups also had much worse survival rates compared to patients with lower B7-H4 expression (RR = 1.688, 95%CI: 1.055–2.701) (Fig. 3b). Collectively, these data suggest that B7-H4 is an important prognostic marker for cancer survival. It is particularly useful for identifying early stage patients with potentially worse prognosis.
Discussion
In the present study, we found that B7-H4 was expressed in about 44.9% gastric cancer specimens, but very weakly or with no expression in normal gastritis epithelia or gastric polyp tissues. We found that B7-H4 was positively associated with tumor invasiveness, nodal metastasis, and recurrence of gastric cancer. Gastric cancer recurrence and metastasis are the main causes of cancer-related death [26]. Therefore, B7-H4 expression is associated with more aggressive gastric cancer. After adjusting for some confounding factors such as age, sex, the depth of invasion and lymph node metastasis, compared to those with lower B7-H4 expression, the death risk of gastric cancer patients with higher B7-H4 expression was significantly increased, suggesting that B7-H4 is an important prognostic factor for gastric cancer patients. In fact, even among early stage cancer patients, higher B7-H4 expression is a signal for a grave outcome. Our results suggest that B7-H4 expression should be determined for early stage gastric cancer patients to assist in making treatment decisions. More aggressive treatment need to be considered for early stage cancer patients with higher expression of B7-H4. Our results also suggest that B7-H4 blockade might benefit gastric cancer patients at advanced stages, as well as some early stage patients with higher B7-H4 expression.
B7-H4 might contribute to immune tolerance during tumor progression. In vitro anti-CD3-mediated T cell activation assays with plate-bound B7-H4 Ig indicates that B7-H4 inhibits CD4+ and CD8+ T cell proliferation, cytokine production, and generation of alloreactive CTLs by arresting the cell cycle [27–29]. B7-H4 expressed on the surface of surrogate APCs also inhibits T cell proliferation [28, 29]. Likewise, in vivo blockade of endogenous B7-H4 by specific mAb promotes T cell responses, indicating an inhibitory role for B7-H4 [28]. B7-H4 overexpression might also contribute to the gastric cancer cells’ avoidance of immune attack, leading to hastened cancer progression. Our study suggests that B7-H4 blockade might benefit gastric cancer patients at advanced stages, as well as some early stage patients with higher B7-H4 expression.
The expression of B7-H4 cell surface protein is generally absent in most human normal somatic tissues, except in normal human epithelial cells of the female genital tract, kidney, lung, and pancreas [15]. Aberrant B7-H4 was detected in primary breast cancers and metastatic breast cancers, but was very low in normal breast tissues. However, there was no statistically significant relationship between the proportion of B7-H4 positive cells or staining intensity and grade, stage, or other clinicopathologic variables [14]. Additionally, B7-H4 was highly expressed in ovarian cancer cells [30], but no prognostic value was found for this cancer. Besides being expressed in the cancer cells, soluble B7-H4 can be detected in the blood of cancer patients. The serum levels of B7-H4 have been shown to be highly specific cancer markers for ovarian cancer [31] and renal cell carcinoma (RCC) [32]. Therefore, B7-H4 is a very specific marker for cancer and may be involved in cancer biology.
Aside from cancer cells, other cells in the tumor microenvironment were shown to express B7-H4. Tumor-associated macrophages are a prominent component of ovarian cancer stroma and contribute to tumor progression. It has been shown that a fraction of ovarian tumor-associated macrophages express surface B7-H4 [17, 18]. B7-H4+ tumor macrophages, but not primary ovarian tumor cells, suppress tumor-associated antigen-specific T cell immunity. B7-H4 blockade restores the T cell stimulating capacity of the macrophages. IL-6 and IL-10 stimulate B7-H4 expression in tumor-associated macrophages. However, we did not find significant expression of B7-H4 in tumor stromal cells in gastric cancer. Besides stromal cells, B7-H4 is highly expressed in endothelial cells of RCC tumor vasculature, whereas it is only expressed in 6.5% of normal adjacent renal tissue vessels [33]. However, we did not find such expression pattern in gastric cancer specimens.
For some cancers, B7-H4 expression correlated with cancer progression. RCC tumor specimens exhibited B7-H4 staining and in that tumor cell B7-H4 expression was associated with adverse clinical and pathologic features, such as advanced tumor size, grade, and stage [33]. Patients with tumors expressing B7-H4 were much more likely to die from RCC compared to patients lacking B7-H4 expression. B7-H4 is also highly expressed in prostate cancer specimens. Patients with a strong intensity of B7-H4 were significantly more likely to have disease spread at the time of surgery and at significantly higher risk of cancer recurrence and cancer-related death [34]. Our study reveals that B7-H4 is a very good prognostic marker for gastric cancer and should be used in clinics to help identify patients with advanced gastric cancer.
It has been shown that patients with RCC tumors expressing both B7-H4 and B7-H1 are at an even greater risk of death from RCC [33]. We have previously shown that B7-H3 and B7-H1 are also highly expressed in gastric cancer [35, 36]. It would be interesting in the future to study whether combining the markers of B7 family members will be of better predictive value for gastric cancer survival.
Acknowledgments
Haifeng Deng, Mingyang Lu, Bin Xu, Yan Tan, Min Li, Jian Liu, Zhengguang Li and Yi Zhou provided excellent technical assistance. This research project was supported by the National Natural Science Foundation of China (NSFC) (30950022, 30972703 and 30872176) and Society or Developing Plans, Department of Science and Technology, Changzhou (BS20092019).
Conflict of interest
The authors declare that they have no competing or financial interests regarding this paper.
Contributor Information
Binfeng Lu, Phone: +1-412-6489339, Email: binfeng@pitt.edu.
Xueguang Zhang, Phone: +86-512-65125011, Email: xueguangzh@yahoo.com.cn.
References
- 1.Hartgrink HH, Jansen EP, van Grieken NC, van de Velde CJ. Gastric cancer. Lancet. 2009;374:477–490. doi: 10.1016/S0140-6736(09)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeh JM, Kuntz KM, Ezzati M, Goldie SJ. Exploring the cost-effectiveness of Helicobacter pylori screening to prevent gastric cancer in China in anticipation of clinical trial results. Int J Cancer. 2009;124:157–166. doi: 10.1002/ijc.23864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee JH, Ryu KW, Kook MC, Lee JY, Kim CG, Choi IJ, Kim SK, Jang S, Park SR, Kim YW, Nam BH, Bae JM. Feasibility of laparoscopic sentinel basin dissection for limited resection in early gastric cancer. J Surg Oncol. 2008;98:331–335. doi: 10.1002/jso.21115. [DOI] [PubMed] [Google Scholar]
- 4.Valentini V, Cellini F, Minsky BD, Mattiucci GC, Balducci M, D’Agostino G, D’Angelo E, Dinapoli N, Nicolotti N, Valentini C, La Torre G. Survival after radiotherapy in gastric cancer: systematic review and meta-analysis. Radiother Oncol. 2009;92:176–183. doi: 10.1016/j.radonc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 5.Sun P, Xiang JB, Chen ZY. Meta-analysis of adjuvant chemotherapy after radical surgery for advanced gastric cancer. Br J Surg. 2009;96:26–33. doi: 10.1002/bjs.6408. [DOI] [PubMed] [Google Scholar]
- 6.Jiang J, Xu N, Wu C, Deng H, Lu M, Li M, Xu B, Wu J, Wang R, Xu J, Nilsson-Ehle P. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26:2237–2242. [PubMed] [Google Scholar]
- 7.Morabito A, Carillio G, Longo R. Systemic treatment of gastric cancer. Crit Rev Oncol Hematol. 2009;70:216–234. doi: 10.1016/j.critrevonc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Varadhachary G, Ajani JA. Gastric cancer. Clin Adv Hematol Oncol. 2005;3:118–124. [PubMed] [Google Scholar]
- 9.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 10.Aunoble B, Sanches R, Didier E, Bignon YJ. Major oncogenes and tumor suppressor genes involved in epithelial ovarian cancer (review) Int J Oncol. 2000;16:567–576. doi: 10.3892/ijo.16.3.567. [DOI] [PubMed] [Google Scholar]
- 11.Nicosia SV, Bai W, Cheng JQ, Coppola D, Kruk PA. Oncogenic pathways implicated in ovarian epithelial cancer. Hematol Oncol Clin North Am. 2003;17:927–943. doi: 10.1016/S0889-8588(03)00056-X. [DOI] [PubMed] [Google Scholar]
- 12.Carreno BM, Collins M. BTLA: a new inhibitory receptor with a B7-like ligand. Trends Immunol. 2003;24:524–527. doi: 10.1016/j.it.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Wang S, Chen L. Co-signaling molecules of the B7-CD28 family in positive and negative regulation of T lymphocyte responses. Microbes Infect. 2004;6:759–766. doi: 10.1016/j.micinf.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 15.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 17.Kryczek I, Zou L, Rodriguez P, Zhu G, Wei S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, Lackner A, Alvarez X, Ochoa A, Chen L, Zou W. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 19.Xue Q, Luan XY, Gu YZ, Wu HY, Zhang GB, Yu GH, Zhu HT, Wang M, Dong W, Geng YJ, Zhang XG. The negative co-signaling molecule B7-H4 is expressed by human bone marrow-derived mesenchymal stem cells and mediates its T-cell modulatory activity. Stem Cells Dev. 2010;19:27–38. doi: 10.1089/scd.2009.0076. [DOI] [PubMed] [Google Scholar]
- 20.Yuan CL, Xu JF, Tong J, Yang H, He FR, Gong Q, Xiong P, Duan L, Fang M, Tan Z, Xu Y, Chen YF, Zheng F, Gong FL. B7-H4 transfection prolongs beta-cell graft survival. Transpl Immunol. 2009;21:143–149. doi: 10.1016/j.trim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Liu J, Cui X. Clinical significance of expression of B7-H4 in colon and rectal cancer tissues. J Shandong Univ (Health Sci) 2006;44:590–592. [Google Scholar]
- 22.De Vita F, Orditura M, Matano E, Bianco R, Carlomagno C, Infusino S, Damiano V, Simeone E, Diadema MR, Lieto E, Castellano P, Pepe S, De Placido S, Galizia G, Di Martino N, Ciardiello F, Catalano G, Bianco AR. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer. 2005;92:1644–1649. doi: 10.1038/sj.bjc.6602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soslow RA, Dannenberg AJ, Rush D, Woerner BM, Khan KN, Masferrer J, Koki AT. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer. 2000;89:2637–2645. doi: 10.1002/1097-0142(20001215)89:12<2637::AID-CNCR17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 24.Hao L, Zhang C, Qiu Y, Wang L, Luo Y, Jin M, Zhang Y, Guo TB, Matsushima K, Zhang Y. Recombination of CXCR4, VEGF, and MMP-9 predicting lymph node metastasis in human breast cancer. Cancer Lett. 2007;253:34–42. doi: 10.1016/j.canlet.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Kamimura Y, Kobori H, Piao J, Hashiguchi M, Matsumoto K, Hirose S, Azuma M. Possible involvement of soluble B7-H4 in T cell-mediated inflammatory immune responses. Biochem Biophys Res Commun. 2009;389:349–353. doi: 10.1016/j.bbrc.2009.08.144. [DOI] [PubMed] [Google Scholar]
- 26.Aparicio T, Boige V, Sabourin JC, Crenn P, Ducreux M, Le Cesne A, Bonvalot S. Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol. 2004;30:1098–1103. doi: 10.1016/j.ejso.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 28.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 29.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tringler B, Liu W, Corral L, Torkko KC, Enomoto T, Davidson S, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 31.Simon I, Liu Y, Krall KL, Urban N, Wolfert RL, Kim NW, McIntosh MW. Evaluation of the novel serum markers B7-H4, Spondin 2, and DcR3 for diagnosis and early detection of ovarian cancer. Gynecol Oncol. 2007;106:112–118. doi: 10.1016/j.ygyno.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Thompson RH, Zang X, Lohse CM, Leibovich BC, Slovin SF, Reuter VE, Cheville JC, Blute ML, Russo P, Kwon ED, Allison JP. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68:6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG. Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis. World J Gastroenterol. 2006;12:457–459. doi: 10.3748/wjg.v12.i3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C, Zhu Y, Jiang J, Zhao J, Zhang XG, Xu N. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem. 2006;108:19–24. doi: 10.1016/j.acthis.2006.01.003. [DOI] [PubMed] [Google Scholar]